Abstract
Background: The impact of the reproductive state on vitamin D metabolism and requirements is uncertain in part because of a lack of studies with controlled dietary intakes of vitamin D and related nutrients.
Objective: We aimed to quantify the impact of the reproductive state on a panel of vitamin D biomarkers among women of childbearing age consuming equivalent amounts of vitamin D and related nutrients.
Methods: Nested within a feeding study providing 2 doses of choline, healthy pregnant (26–29 wk gestation; n = 26), lactating (5 wk postpartum; n = 28), and control (nonpregnant/nonlactating; n = 21) women consumed a single amount of vitamin D (511 ± 48 IU/d: 311 ± 48 IU/d from diet and 200 IU/d as supplemental cholecalciferol) and related nutrients (1.6 ± 0.4 g Ca/d and 1.9 ± 0.3 g P/d) for 10 wk. Vitamin D biomarkers were measured in blood obtained at baseline and study end, and differences in biomarker response among the reproductive groups were assessed with linear mixed models adjusted for influential covariates (e.g., body mass index, season, race/ethnicity).
Results: At study end, pregnant women had higher (P < 0.01) circulating concentrations of 25-hydroxyvitamin D [25(OH)D; 30%], 1,25-dihydroxyvitamin D [1,25(OH)2D; 80%], vitamin D binding protein (67%), and C3 epimer of 25(OH)D3 (100%) than control women. Pregnant women also had higher (P ≤ 0.04) ratios of 25(OH)D to 24,25-dihydroxyvitamin D [24,25(OH)2D; 40%] and 1,25(OH)2D to 25(OH)D (50%) than control women. In contrast, no differences (P ≥ 0.15) in vitamin D biomarkers were detected between the lactating and control groups. Notably, the study vitamin D dose of 511 IU/d achieved vitamin D adequacy in most participants (95%) regardless of their reproductive state.
Conclusions: The higher concentrations of vitamin D biomarkers among pregnant women than among control women suggest that metabolic adaptations, likely involving the placenta, transpire to enhance vitamin D supply during pregnancy. The study findings also support the adequacy of the current vitamin D RDA of 600 IU for achieving serum 25(OH)D concentrations ≥50 nmol/L among women differing in their reproductive state. This trial was registered at clinicaltrials.gov as NCT01127022.
Keywords: pregnancy; lactation; vitamin D; 25-hydroxyvitamin D; 1,25-dihydroxyvitamin D; 24,25-dihydroxyvitamin D; 25(OH)D:24,25(OH)2D ratio; free 25(OH)D; Epi-25(OH)D3; vitamin D requirements
Introduction
Worldwide vitamin D inadequacy is common among pregnant and lactating women (1–3), and it is linked in some epidemiologic studies to adverse reproductive outcomes, including impaired fetal/neonatal growth, preeclampsia, and immune disorders (4, 5). In 2011, the Institute of Medicine (IOM)4 established a vitamin D RDA of 600 IU for women of childbearing age. This recommendation was based on the vitamin D intake projected to achieve circulating concentrations of 50 nmol/L for 25-hydroxyvitamin D [25(OH)D], the primary indicator of vitamin D status. Reproductive state was not considered in the formulation of this RDA because of an absence of 25(OH)D dose-response data among pregnant and/or lactating women (1, 6).
Interestingly, pregnancy is characterized by robust increases in circulating 1,25-dihydroxyvitamin D [1,25(OH)2D] (7–10), the biologically active form of vitamin D, and vitamin D binding protein (DBP) (8, 11, 12), a carrier of vitamin D metabolites in blood. However, the effect of pregnancy on circulating 25(OH)D is less clear with studies reporting no effects (7, 11, 13), increases (9, 14, 15), or decreases (10, 16). These mixed findings across studies may arise from intake differences of vitamin D and relevant nutrients (e.g., calcium and phosphorus), which were either unknown or incompletely assessed. Some studies have shown that circulating concentrations of 1,25(OH)2D, DBP, and 25(OH)D decline during lactation (9, 11, 17, 18); however, it is unclear if vitamin D status differs from that of a nonpregnant, nonlactating woman under conditions of comparable vitamin D intake. Furthermore, little is known about the effect of reproductive state on several additional biomarkers of vitamin D metabolism, including 24,25-dihydroxyvitamin D [24,25(OH)2D], a catabolite of vitamin D; the ratio of 25(OH)D to 24,25(OH)2D [25(OH)D:24,25(OH)2D], a newly proposed sensitive indicator of vitamin D status (19–22); free 25(OH)D (16, 23); and the C3 epimer of 25-hydroxyvitamin D3 [epi-25(OH)D3], a recently identified metabolite (24, 25) with unknown biological function.
To advance understanding of the effects of reproductive state on vitamin D status and metabolism, we used samples obtained during a feeding study that provided a single amount of vitamin D and related nutrients (e.g., calcium and phosphorus) to pregnant, lactating, and control (nonpregnant, nonlactating) women across a 10-wk time period. Our aims were as follows: 1) to quantify the impact of reproductive state on a comprehensive panel of blood vitamin D biomarkers; 2) to examine interrelations among biomarkers of vitamin D metabolism; and 3) to ascertain the adequacy of the current RDA for achieving target circulating 25(OH)D concentrations of 50 nmol/L among pregnant and lactating women.
Methods
Study participants
Pregnant women entering their third trimester (26–29 wk gestation), lactating women (5 wk postpartum), and control women of reproductive age (nonpregnant and nonlactating) were recruited from Ithaca, New York (latitude 42.4°N) between January 2009 and October 2010 as previously described (26, 27). Inclusion criteria included the following: 1) age of 21–40 y; 2) healthiness as assessed by health-related questionnaire, a blood chemistry profile, and a complete blood count; 3) normal liver and kidney function; 4) willingness to comply with the study protocol; 5) singleton pregnancy without pregnancy-associated complications (pregnant women only); and 6) willingness to breastfeed exclusively during the study period (lactating women only). Exclusion criteria included use of tobacco, drug, or alcohol and use of prescription medications known to affect liver function. The study protocol was reviewed and approved by the Institutional Review Board for Human Study Participant Use at Cornell University and the Cayuga Medical Center (the hospital where pregnant participants delivered their infants). Informed consent was obtained from all study participants before study entry. This trial was registered at clinicaltrials.gov as NCT01127022.
Study design, diet, and supplements
This study used biological samples obtained during a feeding study in which healthy pregnant (n = 26), lactating (n = 28), and control (n = 21) women were randomly assigned to 480 or 930 mg choline/d for ≥10 wk (26, 27). Throughout the feeding period, consumption of all essential micronutrients was strictly controlled and equivalent across reproductive groups.
A mean total of 511 ± 48 IU vitamin D/d was consumed by participants throughout the study period. Of this total, a mean of 311 ± 48 IU vitamin D/d was provided by the 7-d rotational menu as estimated with the use of the USDA National Nutrient Database for Standard Reference Release 28 (Supplemental Table 1), and 200 IU cholecalciferol/d came from a daily prenatal multivitamin supplement (Pregnancy Plus; Fairhaven Health LLC). For calcium and phosphorus, mean intakes were estimated to be 1.6 ± 0.4 g Ca/d and 1.9 ± 0.3 g P/d (USDA National Nutrient Database for Standard Reference Release 28). In addition to the prenatal multivitamin supplement, all women consumed a docosahexaenoic acid supplement (200 mg/d; Neuromins; Nature’s Way Products) and a potassium and magnesium supplement (3 times/wk; General Nutrition Corp).
Meals were prepared in the Human Metabolic Research Unit (HMRU) at Cornell University. The pregnant and control women consumed ≥1 meal/d throughout the week at the HMRU, whereas the lactating women consumed 1 meal/d thrice weekly to lessen the burden of travel to and from the HMRU while caring for an infant. Supplements were consumed with the onsite meal under the supervision of study personnel. All other meals and supplements were provided as carry-out and were consumed off site. Participants were required to 1) refrain from consuming food, beverages (except water), and supplements outside of those provided by study personnel; 2) complete daily checklists of menu items and supplements; and 3) return empty food containers and disposables of their carry-out meals and supplements.
Sample collection and processing
Fasting (10-h) venous blood samples and 24-h urine samples were obtained at study baseline (week 0) and study end (defined as week 10), processed, and stored at −80°C (26).
Analytical measurements
25(OH)D.
Serum 25(OH)D was quantified with an isotope dilution LC-MS/MS method (24) that was validated in part by our laboratory’s achieving the performance target set by the Vitamin D External Quality Assessment Scheme for the October 2012 distribution (i.e., within 14% of All Laboratory Trimmed Mean; within 4% of the values for Vitamin D External Quality Assessment Scheme samples analyzed by the National Institute of Standards and Technology). 25(OH)D was extracted from 150-μL serum samples, calibrators, and control samples (24). The calibrators for 25(OH)D2, 25(OH)D3, and epi-25(OH)D3 were made by diluting ethanol stock solutions with PBS-4% albumin. Internal standard (100 μL) that contained 75 nmol d3-25(OH)D3/L and 50 nmol d3-25(OH)D2/L (IsoSciences) was then added to all of the samples. Extracts (65 μL) were injected onto a PFP column (PFP Accucore 2.1 × 100, 2.6 mm) with matching guard column at 45°C and separated by an LC-MS/MS system that consisted of a Surveyer HPLC system (pump and autosampler) and a TSQ Quantum Ultra mass spectrometer operated with XCalibur (2.2 SP1.48) software (ThermoElectron Corp). The analytes of interest were eluted from the column at a flow rate of 150 μL/min under the following conditions: 73% methanol and 27% water (0–9th min), linear gradient from 73% to 100% methanol (9th–11th min), 100% methanol (11th–12th min), linear gradient from 100% to 73% methanol (12th–13th min), and 73% methanol (13th–18th min). To prevent deposit build-up in the mass spectrometer, the flow was directed into the spectrometer only between the 9th and 16th min. Atmospheric pressure chemical ionization in the positive ion mode with the use of selected reaction monitoring was used for detection. Transition pairs for the analytes of interest were the same as previously described (24). Intra- and inter-assay CVs were 2.1% and 4.7%, respectively, from our in-house control duplicates that consisted of 3 human sera with 25(OH)D concentrations spanning the range of the calibration curve, a bovine serum rich in 25(OH)D2, and National Institute of Standards and Technology SRM 2972 (within the range of certified values). We herein refer to the sum of 25(OH)D2 and 25(OH)D3 as “25(OH)D” which does not include epi-25(OH)D3.
DBP and 1,25(OH)2D.
DBP and 1,25(OH)2D in plasma samples were quantified with ELISA kits [R&D Systems for DBP; Immunodiagnostic Systems, Inc. for 1,25(OH)2D], according to the manufacturers’ instructions. Intra- and inter-assay CVs for the DBP assays were 5.7% and 5.6%, respectively, from our in-house controls of human plasma with 3 different DBP concentrations. In the 1,25(OH)2D assays, both intra- and inter-assay CVs were <10% (5.2% and 7.8%, respectively) from kit controls of low and high concentrations. In addition, all measured concentrations of the kit controls fell within the acceptable ranges provided by the manufacturer.
24,25(OH)2D.
Quantification of circulating 24,25(OH)2D was performed with the LC-MS/MS method (20) with modifications from our instrumentation. Briefly, 24,25(OH)2D3 calibration curves were created by diluting ethanol stock solutions of 24,25(OH)2D3 with PBS-4% albumin. All plasma samples, calibrators, and control samples were mixed with 5 μL of internal standard solution that contained 1.5 pmol d6-24,25(OH)2D3/L (Toronto Research Chemicals Inc.). Next, 24,25(OH)2D3 was extracted from 200-μL samples, calibrators, and controls with the use of a liquid-liquid extraction, and the upper layer was dried under nitrogen before derivatization with DMEQ-TAD (20). Extracts (65 μL) were resuspended in 60:40 methanol/water solution and injected onto a PFP column (PFP Accucore 2.1 × 100, 2.6 mm) with matching guard column at 45°C and separated by the LC-MS/MS system described for serum 25(OH)D quantification. 24,25(OH)2D3 was eluted from the column at a flow rate of 200 μL/min under the following conditions: 40% acetonitrile and 60% water (0–3rd min), linear gradient from 40% to 60% acetonitrile (3rd–5th min), 60% acetonitrile (5th–10th min), linear gradient from 50% to 70% acetonitrile (10th–12th min), linear gradient 70–80% acetonitrile (12th–15th min), returning to 40% acetonitrile (15th–17th min). To prevent deposit build-up, the flow directed into the mass spectrometer was limited from the 2nd to 10th min. Atmospheric pressure chemical ionization in the positive ion mode with selected reaction monitoring was used for 24,25(OH)2D3 detection. Two transition pairs were used for 24,25(OH)2D3 [m/z 762.5 > 468.2 (762.5 > 247.1 qualifier)], whereas a single transition pair was used for d6-24,25(OH)2D3 (m/z 768.5 > 247.1). Intra- and inter-assay CVs were 5.7% and 4.9%, respectively, from duplicate measurements of our in-house control samples (human plasma with 2 different 24,25(OH)2D3 concentrations).
Free 25(OH)D.
Free 25(OH)D concentrations were estimated with a previously described equation (28, 29) in which quantified circulating concentrations of 25(OH)D [i.e., 25(OH)D2 and 25(OH)D3], albumin, and DBP were computed. Because of limited availability of serum samples, plasma DBP concentrations were measured and then entered in the equation.
Total calcium.
Serum total calcium was quantified by an automated chemistry analyzer (Dimension Xpand Plus; Siemens Healthcare Diagnostics).
Genotyping.
The GC (vitamin D binding protein gene) rs7041 G > T, CYP2R1 (25-hydroxylase gene) rs12794714 A > G, and CYP2R1 rs10741657 A > G genotypes were determined by Endpoint Genotyping on a Roche LightCycler 480 with the use of the Applied Biosystems TaqMan Genotyping Assays (Life Technologies) after DNA extraction and purification with the Qiagen DNeasy kit.
Statistical analysis
To test for differences in baseline characteristics among the reproductive groups, we used 1-factor ANOVA for normally distributed continuous variables, Kruskal-Wallis test for non-normally distributed continuous variables, and χ2 tests or Fisher exact tests for categorical variables. To examine the effect of reproductive state on biomarkers of vitamin D metabolism at both study baseline and study end, we used linear mixed-effects models (LMMs). For each biomarker, the LMM included reproductive state, time, and their interaction term as fixed effects, and a random participant identifier factor. In addition, each LMM considered the following covariates: age, ethnicity/race, prepregnancy/baseline BMI (in kg/m2), education, season of study entry, multivitamin supplement use before study entry, genetic variants in vitamin D metabolism, and choline intake (480 or 930 mg/d). Covariates that achieved statistical significance (P < 0.05) were retained in the final models, and the Bonferroni correction was used for post hoc comparisons. Finally, relations among the biomarkers of vitamin D were assessed with Pearson correlation analysis.
All analyses were performed with JMP Pro 11 (SAS Institute, Inc.). Data that did not meet the normality and homogeneity of variance criteria were ln-transformed. Two influential outliers with studentized residuals >3 were excluded from free 25(OH)D analysis. Because epi-25(OH)D3 had values below the limit of detection of 1.0 nmol/L, the limit of detection was used in place of “not detectable” among 47% of control, 31% of pregnant, and 54% of lactating women in the statistical models. Data are presented as arithmetic means ± SDs or geometric means (95% CI), unless otherwise specified. P values were 2-tailed and considered significant at <0.05. Data derived from the LMMs are “predicted mean concentrations (or ratios)” of the vitamin D biomarkers and account for influential covariates.
Results
Participant characteristics and study baseline measurements (ANOVA)
The characteristics of the study participants (26 pregnant, 28 lactating, and 21 control women) and their study baseline (week 0) measures are shown in Table 1. No differences in age and prepregnancy/baseline BMI were observed among the reproductive groups. In addition, the distributions of ethnicity/race, season of study entry, and education were balanced across the groups as were the distributions of the vitamin D-related genetic variants, GC rs7041 G > T and CYP2R1 rs12794714 A > G polymorphisms. In contrast, CYP2R1 rs10741657 A > G polymorphism distribution differed (P = 0.03) among the groups with the pregnant women having a lower prevalence of the variant GG genotype than the control and lactating women. Multivitamin supplement use before study entry also differed (P < 0.001) among the reproductive groups, with higher use in the pregnant and lactating women than in the control women.
TABLE 1.
Baseline characteristics and concentrations of vitamin D metabolites among the pregnant, lactating, and control women1
| Pregnant (n = 26) | Lactating (n = 28) | Control (n = 21) | P | |
| Age, y | 28 ± 3 | 29 ± 5 | 29 ± 5 | 0.82 |
| Ethnicity, n | 0.81 | |||
| White | 16 | 20 | 14 | |
| African American | 1 | 1 | 2 | |
| Hispanic | 4 | 3 | 2 | |
| Asian | 4 | 1 | 1 | |
| Other | 1 | 3 | 2 | |
| BMI,2 kg/m2 | 23 [21, 26] | 25 [21, 32] | 24 [21, 25] | 0.45 |
| Season at study entry, n | 0.81 | |||
| April–September | 14 | 17 | 11 | |
| October–March | 12 | 11 | 10 | |
| Multivitamin supplement use before study entry, n | 22 | 21 | 7 | <0.001 |
| Education, n | 0.30 | |||
| ≤High school | 4 | 8 | 6 | |
| College | 11 | 13 | 5 | |
| >College | 11 | 17 | 10 | |
| GC rs7041 G > T polymorphism, n | 0.35 | |||
| GG | 9 | 3 | 6 | |
| GT | 11 | 16 | 9 | |
| TT | 6 | 8 | 6 | |
| CYP2R1 rs10741657 A > G polymorphism, n | 0.03 | |||
| AA | 2 | 3 | 2 | |
| AG | 20 | 12 | 8 | |
| GG | 4 | 13 | 11 | |
| CYP2R1 rs12794714 A > G polymorphism, n | 0.15 | |||
| AA | 1 | 6 | 4 | |
| AG | 20 | 13 | 11 | |
| GG | 5 | 9 | 6 | |
| Serum 25(OH)D, nmol/L | 89 ± 29a | 73 ± 23a,b | 64 ± 25b | 0.006 |
| Plasma DBP, μg/mL | 405 (319, 515)a | 165 (136, 199)b | 204 (164, 254)b | <0.001 |
| Plasma 1,25(OH)2D, pmol/L | 283 (232, 344)a | 125 (104, 152)b | 151 (129, 178)b | <0.001 |
| Plasma 24,25(OH)2D, nmol/L | 9.6 (7.5, 12.4) | 12.7 (10.2, 15.9) | 9.1 (6.7, 12.5) | 0.13 |
| 25(OH)D:24,25(OH)2D | 8.7 (7.5, 10.1)a | 5.4 (4.8, 6.1)b | 6.7 (5.8, 7.8)b | <0.001 |
| 1,25(OH)2D:25(OH)D | 3.4 (2.7, 4.2)a | 1.8 (1.5, 2.2)b | 2.6 (2.1, 3.2)a | <0.001 |
| Serum epi-25(OH)D3, nmol/L | 3.2 ± 2.1a | 1.5 ± 0.9b | 1.9 ± 1.2b | 0.003 |
| Free 25(OH)D, pmol/L | 17.3 (13.8, 21.5)b | 28.8 (24.1, 34.4)a | 19.5 (14.9, 25.5)b | 0.002 |
| Serum total calcium, mg/dL | 8.7 ± 0.4c | 9.4 ± 0.3a | 9.2 ± 0.3b | <0.001 |
Values are geometric means (95% CIs), means ± SDs, or medians [IQRs]. Values in a row with a superscript letter indicate significant differences in the vitamin D metabolite among the reproductive groups (i.e., a>b>c), P < 0.05. CYP2R1, 25-hydroxylase gene; DBP, vitamin D binding protein; epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; GC, vitamin D binding protein gene; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 1,25(OH)2D:25(OH)D, ratio of 1,25-dihydroxyvitamin D to 25-hydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D:24,25(OH)2D, ratio of 25-hydroxyvitamin D to 24,25-dihydroxyvitamin D.
Self-reported prepregnancy BMI of the pregnant and lactating women and baseline BMI of the control women.
Most vitamin D metabolites varied by reproductive state at baseline (Table 1). The pregnant women exhibited 40% higher (P = 0.01) serum 25(OH)D concentrations than the control women, and 100–150% higher (P < 0.001) DBP concentrations than the control and lactating women. Similarly, the pregnant women had 90% and 130% higher (P < 0.001) 1,25(OH)2D concentrations than the control and lactating women, respectively. Although 24,25(OH)2D concentrations did not differ across the groups at baseline, 25(OH)D:24,25(OH)2D was higher in the pregnant women than in the control (30%; P = 0.03) and lactating (61%; P < 0.001) women, which did not differ from each other. The pregnant women also had 70% higher (P = 0.048) serum epi-25(OH)D3 concentrations than those of the control women and almost double (P = 0.004) the concentrations of the lactating women.
The lactating women differed from the control and pregnant women in several variables. The 1,25(OH)2D:25(OH)D was lower in the lactating women than in the control (−31%; P = 0.04) and pregnant (−47%; P < 0.001) women, which did not differ from each other. In addition, the lactating women showed higher concentrations of free 25(OH)D than the control (47%; P = 0.03) and pregnant (67%; P = 0.002) women, which did not differ from each other. Finally, the lactating women had higher (P < 0.03) serum calcium concentrations than the control and pregnant women, which differed from each other with lower (P < 0.001) serum calcium concentrations among the pregnant women.
Effect of reproductive state on blood biomarkers of vitamin D metabolism (covariate-adjusted LMM)
Serum 25(OH)D.
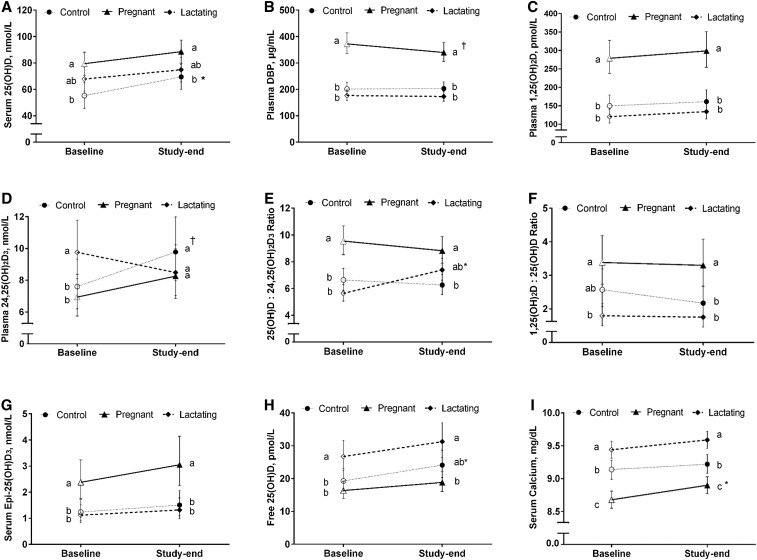
Reproductive state did not interact with time (P = 0.48) to affect serum 25(OH)D concentrations. However, a 25% increase (P = 0.02) in 25(OH)D concentrations was observed among the control women but not among the pregnant and lactating women whose concentrations remained stable (P ≥ 0.21) throughout the study (Figure 1A). Similar to study baseline, the pregnant women had ∼30% higher (P < 0.01) concentrations of 25(OH)D (89 nmol/L) than the control women (69 nmol/L) at study end (Figure 1A), whereas serum 25(OH)D concentrations in the lactating women (75 nmol/L) did not differ (P ≥ 0.15) from the pregnant and control women. At study end, predicted mean serum concentrations for all reproductive groups were above the estimated average requirement and RDA target values of 40 nmol/L and 50 nmol/L, respectively (Figure 1A). In addition, all participants, except for 2 pregnant and 2 control women, had unadjusted 25(OH)D concentrations >50 nmol/L.
FIGURE 1.
Circulating vitamin D metabolites [25(OH)D (A), DBP (B), 1,25(OH)2D (C), 24,25(OH)2D (D), 25(OH)D:24,25(OH)2D (E), 1,25(OH)2D:25(OH)D (F), epi-25(OH)D3 (G), free 25(OH)D (H), and total calcium (I)] among the pregnant (n = 26), lactating (n = 28), and control (n = 21) women who consumed equivalent amounts of vitamin D and related nutrients (e.g., calcium and phosphorus) for 10 wk. All concentrations are predicted geometric means (95% CIs), except 25(OH)D (predicted arithmetic mean), derived from the covariate-adjusted linear mixed models. Means at a time without a common letter differ, P < 0.05. *,†Significantly different from baseline: *P < 0.05; †P ≤ 0.07. DBP, vitamin D binding protein; epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 1,25(OH)2D:25(OH)D, ratio of 1,25-dihydroxyvitamin D to 25-hydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D:24,25(OH)2D, ratio of 25-hydroxyvitamin D to 24,25-dihydroxyvitamin D.
Plasma DBP.
Reproductive state did not interact with time (P = 0.13) to influence circulating DBP concentrations that did not change (P > 0.9) among the lactating and control women but tended to decrease (−9%; P = 0.07) among the pregnant women (Figure 1B). Similar to study baseline, the pregnant women had 67% higher (P < 0.001) DBP concentrations (340 μg/mL) than the control women (203 μg/mL) and almost 100% higher (P < 0.001) concentrations than the lactating women (173 μg/mL) at study end. DBP concentrations in the lactating and control women did not differ (P = 0.42) from each other (Figure 1B).
Plasma 1,25(OH)2D.
Reproductive state did not interact with time (P = 0.88) to affect circulating 1,25(OH)2D response with stable (P ≥ 0.52) concentrations of 1,25(OH)2D among all the reproductive groups throughout the study (Figure 1C). Similar to study baseline, the pregnant women had 80% and 120% higher (P < 0.001) 1,25(OH)2D concentrations (299 pmol/L) than the control (162 pmol/L) and lactating (134 pmol/L) women at study end, respectively (Figure 1C). 1,25(OH)2D concentrations in the lactating and control women did not differ from each other (P > 0.9).
Plasma 24,25(OH)2D.
Reproductive state interacted with time (P = 0.003) to affect circulating 24,25(OH)2D concentrations. Although no changes (P ≥ 0.31) in 24,25(OH)2D concentrations were observed within the pregnant and lactating women throughout the study, 24,25(OH)2D tended to increase (22%; P = 0.06) among the control women (Figure 1D). Interestingly, despite 42% higher (P < 0.03) concentrations of 24,25(OH)2D in the lactating women than the pregnant women at baseline, no differences (P > 0.9) were detected among the pregnant (8.3 nmol/L), lactating (8.5 nmol/L), and control (9.8 nmol/L) groups at study end (Figure 1D).
25(OH)D:24,25(OH)2D.
Reproductive state and time interacted (P < 0.001) to influence 25(OH)D:24,25(OH)2D, with increases (P < 0.001) observed among the lactating women (Figure 1E) but not among the pregnant and control women (P > 0.9). Similar to study baseline, the pregnant women exhibited a 40% higher ratio (P < 0.001) than the control women (8.8 compared with 6.3) at study end. However, in contrast to the higher ratio (P < 0.001) among the pregnant (compared with lactating) women at study baseline, no difference (P = 0.17) between the 2 groups (8.8 compared with 7.4) was detected at study end (Figure 1E).
1,25(OH)2D:25(OH)D.
Reproductive state did not interact with time (P = 0.36) to influence 1,25(OH)2D:25(OH)D that remained stable (P ≥ 0.56) among all the reproductive groups across study time points. At study end, the pregnant women had a 50% higher ratio (P = 0.04) than the control women (3.3 compared with 2.2), despite no differences (P = 0.53) in this ratio between the 2 groups at study baseline (Figure 1F). The pregnant women also had an 83% higher (P < 0.001) 1,25(OH)2D:25(OH)D than the lactating women (3.3 compared with 1.8) at study end, which was similar to study baseline (Figure 1F).
Serum epi-25(OH)D3.
Reproductive state did not interact with time (P = 0.92) to influence serum epi-25(OH)D3 concentrations that did not change (P > 0.9) among all reproductive states throughout the study. Similar to study baseline, epi-25(OH)D3 concentrations in the pregnant group (3.05 nmol/L) were twice (P < 0.004) those of the control (1.51 nmol/L) and lactating (1.31 nmol/L) groups at study end (Figure 1G), which did not differ from each other (P > 0.9).
Free 25(OH)D.
Reproductive state did not interact with time (P = 0.65) to affect free 25(OH)D concentrations that remained stable (P ≥ 0.1) among the lactating and control women over the course of the study and increased slightly (10%; P = 0.02) among the pregnant women (Figure 1H). Although the lactating women tended to have higher (P = 0.05) concentrations of free 25(OH)D than the control women at study baseline, no difference (P = 0.15) was observed among these groups at study end (31.3 and 24.1 pmol/L for lactating and control women, respectively). Similar to study baseline, the lactating women had 66% higher (P < 0.001) concentrations of free 25(OH)D than those of the pregnant women (18.8 pmol/L) at study end, which did not differ from the control women (P = 0.18).
Serum total calcium.
Reproductive state did not interact with time (P = 0.46) to influence serum calcium concentrations that was stable (P ≥ 0.5) among the lactating and control women but increased slightly (3%; P = 0.04) in the pregnant women throughout the study (Figure 1I). Similar to study baseline, the lactating women (9.6 mg/dL) had higher calcium concentrations than those of the control (9.2 mg/dL; P = 0.003) and pregnant women (8.9 mg/dL; P < 0.001) at study end. In addition, the pregnant women showed lower (P = 0.01) serum calcium concentrations than the control women at study end.
Associations among circulating vitamin D metabolites in all participants throughout the study
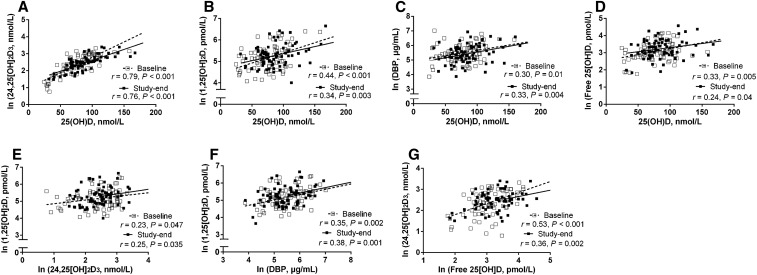
Among all study participants, circulating 25(OH)D correlated positively with 24,25(OH)2D (Figure 2A), 1,25(OH)2D (Figure 2B), DBP (Figure 2C), and free 25(OH)D (Figure 2D) at both baseline and study end. 1,25(OH)2D and 24,25(OH)2D were correlated throughout the study (Figure 2E) as was 1,25(OH)2D with DBP (Figure 2F). No correlations were detected between 1,25(OH)2D and free 25(OH)D at either baseline (P = 0.68) or study end (P = 0.29); however, free 25(OH)D correlated positively with 24,25(OH)2D at both study time points (Figure 2G).
FIGURE 2.
Relations among circulating vitamin D metabolites in all participants (n = 75) by study time point: 25(OH)D and 24,25(OH)2D (A), 25(OH)D and 1,25(OH)2D (B), 25(OH)D and DBP (C), free 25(OH)D and 25(OH)D (D), 1,25(OH)2D and 24,25(OH)2D (E), 1,25(OH)2D and DBP (F), and free 25(OH)D and 24,25(OH)2D (G). All metabolites were ln-transformed, except for 25(OH)D. DBP, vitamin D binding protein; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Associations among circulating vitamin D metabolites by reproductive state at study end
At study end, circulating 25(OH)D correlated positively with 1,25(OH)2D among the control women (Table 2) and tended to correlate among the pregnant women (r = 0.36, P = 0.07). 25(OH)D was also highly correlated with 24,25(OH)2D, and with epi-25(OH)D3 in all the reproductive groups (Table 2). Although 1,25(OH)2D correlated positively with 24,25(OH)2D among the control women, no relation between these metabolites was observed among the pregnant or lactating women.
TABLE 2.
Correlations among circulating vitamin D metabolites in pregnant, lactating, and control women at study end1
| Pregnant (n = 26) |
Lactating (n = 28) |
Control (n = 21) |
||||
| Correlations between vitamin D metabolites | r | P | r | P | r | P |
| 25(OH)D, 1,25(OH)2D | — | NS | — | NS | 0.48 | 0.03 |
| 25(OH)D, 24,25(OH)2D | 0.85 | <0.001 | 0.67 | <0.001 | 0.89 | <0.001 |
| 25(OH)D, DBP | — | NS | — | NS | — | NS |
| 25(OH)D, Epi-25(OH)D3 | 0.72 | <0.001 | 0.63 | <0.001 | 0.59 | 0.007 |
| 25(OH)D, Free 25(OH)D | — | NS | — | NS | — | NS |
| 1,25(OH)2D, 24,25(OH)2D | — | NS | — | NS | 0.64 | 0.002 |
| 1,25(OH)2D, DBP | — | NS | — | NS | — | NS |
| 1,25(OH)2D, Free 25(OH)D | 0.47 | 0.02 | — | NS | — | NS |
| 1,25(OH)2D, Epi-25(OH)D3 | — | NS | — | NS | — | NS |
| Free 25(OH)D, 24,25(OH)2D | 0.46 | 0.02 | — | NS | 0.48 | 0.03 |
| Free 25(OH)D, Epi-25(OH)D3 | 0.49 | 0.03 | — | NS | 0.42 | 0.03 |
| Epi-25(OH)D3, DBP | 0.40 | 0.04 | — | NS | — | NS |
| Epi-25(OH)D3, 24,25(OH)2D | 0.56 | 0.003 | 0.58 | 0.001 | 0.49 | 0.03 |
Metabolites were ln-transformed, except 25(OH)D and epi-25(OH)D3. NS, P ≥ 0.05. DBP, vitamin D binding protein; epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Epi-25(OH)D3 correlated positively with 24,25(OH)2D in all the reproductive groups and with DBP in the pregnant women at study end (Table 2). In addition, free 25(OH)D correlated with epi-25(OH)D3 and with 24,25(OH)2D among the pregnant and control women at study end (Table 2). However, only the pregnant women showed correlations of free 25(OH)D with 25(OH)D (r = 0.38, P = 0.057) and 1,25(OH)2D (Table 2).
Discussion
To the best of our knowledge, this is the first feeding study to control intakes of vitamin D and related nutrients such as calcium and phosphorus that can affect vitamin D status and metabolism. Three main findings emerged as follows: 1) pregnancy induces alterations in vitamin D metabolism, including increases in 25(OH)D, 25(OH)D:24,25(OH)2D, and epi-25(OH)D3; 2) reproductive state modulates the interrelations among circulating vitamin D metabolites; and 3) the RDA for vitamin D is likely adequate for most women of reproductive age, including pregnant and lactating women.
Pregnancy increases the circulating pool of vitamin D.
Circulating concentrations of 1,25(OH)2D, DBP, and 1,25(OH)2D:25(OH)D were significantly higher among third-trimester pregnant women than the control women, confirming previous reports (7–12). The pregnant women also exhibited significantly higher circulating concentrations of 25(OH)D than the control women even after adjusting for season, prestudy supplement use, ethnicity/race, BMI, and genetic variants that influence 25(OH)D concentrations. This elevation in 25(OH)D paralleled the rise in DBP among the pregnant women, suggesting that DBP-bound 25(OH)D, rather than free 25(OH)D (which did not differ between the pregnant and control women), contributed to the higher total circulating concentrations of this metabolite. DBP-bound 25(OH)D may be favored over the free form because it is more stable and is taken up in a regulated manner by tissues that express megalin-cubilin receptors such as the placenta (30).
No differences in circulating concentrations of 24,25(OH)2D, a major catabolite of 25(OH)D (22), were detected between the pregnant and control women. However, 25(OH)D:24,25(OH)2D was significantly higher among the pregnant women than the control women, indicating attenuation of vitamin D catabolism in this reproductive state. In addition, the pregnant women had higher concentrations of epi-25(OH)D3 than the control women which may be a consequence of the elevated maternal vitamin D pool or could imply a possible role for this metabolite in maternal and fetal health.
In contrast to the robust effects of pregnancy on biomarkers of vitamin D metabolism, no differences were detected at study end between the lactating (15 wk postpartum) and control women. These findings are consistent with previous reports that examined calcium homeostasis during lactation (9, 11, 31). Of note, circulating 25(OH)D:24,25(OH)2D increased throughout the study among lactating women because of a decrease in their 24,25(OH)2D. Thus, lactating women may achieve vitamin D pools that are similar to nonlactating women by reducing 24-hydoxylase (CYP24A1) activity.
Reproductive state influenced the interrelations among circulating vitamin D metabolites.
Data from the present study showed strong positive correlations between 25(OH)D and 24,25(OH)2D at study end among the pregnant, lactating, and control women, supporting the catabolism of 25(OH)D to 24,25(OH)2D as a means to maintain homeostasis. These findings are consistent with previous reports in healthy adults (19, 20, 22) and pregnant women at term (7), but they deviate somewhat from a study in lactating women which reported significant correlations of 25(OH)D and 24,25(OH)2D at 1 wk postpartum but not at later time points (18). In addition, although 25(OH)D tended (n = 26; r = 0.36; P = 0.07) to be correlated with the bioactive 1,25(OH)2D metabolite among the pregnant women at study end, the linear relation between these 2 metabolites became significant when the pregnant women with serum 25(OH)D concentrations <100 nmol/L were examined separately (n = 33; r = 0.39; P = 0.02). This finding supports prior work to suggest that substrate-dependent 1,25(OH)2D production from 25(OH)D reaches a plateau at ∼100 nmol 25(OH)D/L in pregnant women (32). Pregnant women also exhibited a higher number of significant correlations between free 25(OH)D, a metabolite recently linked to bone health (33, 34), and other metabolites. Specifically, free 25(OH)D was associated with 4 metabolites among pregnant women [i.e., total 25(OH)D, 1,25(OH)2D, 24,25(OH)2D, and epi-25(OH)D3], 2 metabolites among control women [i.e., 24,25(OH)2D and epi-25(OH)D3], and zero metabolites among lactating women. This suggests that free 25(OH)D may be a useful indicator of vitamin D status during pregnancy but not during lactation. Notably, the bioactive metabolite 1,25(OH)2D, was associated with 24,25(OH)2D concentrations in control women but not in pregnant and lactating women. This finding supports the notion that feedback inhibition of 1,25(OH)2D production (by CYP24A1) is uncoupled during pregnancy (35, 36) and lactation, possibly because of a higher demand for 1,25(OH)2D in these reproductive states.
Vitamin D intake approximating the current RDA achieved adequacy in ≥90% of the study participants within each reproductive group.
This study provided a mean of 511 IU vitamin D/d, falling between the current estimated average requirement (400 IU/d) and the RDA (600 IU/d), through a mixed diet and a prenatal supplement. Although a simulated dose-response curve of serum 25(OH)D concentrations generated by the IOM did not include pregnant and lactating populations (6), it is notable that the study dose readily achieved 25(OH)D concentrations above both the RDA-targeted value and the IOM cutoff for vitamin D adequacy (≥50 nmol/L) in 95% of the participants: 92% in pregnant, 100% in lactating, and 90% in control women after a 10-wk period of controlled feeding. In addition, on examining 25(OH)D:24,25(OH)2D, all participants had values <20 (unadjusted ratios) at study end, corresponding to vitamin D sufficiency (20). As such, the RDA of 600 IU/d, which is higher than the vitamin D dose of the present study, would be expected to meet vitamin D requirements of these populations as defined by the IOM (5), although additional dose-response studies are warranted.
Study limitations.
The provision of a single dose of vitamin D is the main limitation of this study. We cannot exclude the possibility that the effect of reproductive state on vitamin D biomarkers and their relations might differ under conditions of lower or higher vitamin D intakes. In addition, oral contraceptive use of the control women was not considered in our statistical models. However, inclusion of this variable would be expected to accentuate the difference between the pregnant and control women (8 of whom used oral contraceptives), secondary to the positive relation between oral contraceptive use and serum 25(OH)D concentrations (37).
Conclusions.
Pregnancy increases circulating pools of vitamin D metabolites in a manner that is independent of dietary intake, supplement use, season, ethnicity/race, and BMI. The factors contributing to this increase are unclear, but they may involve the placenta that expresses the vitamin D machinery required for the synthesis of 25(OH)D and 1,25(OH)2D (30, 38, 39). The study findings also show that interrelations among vitamin D metabolites are modified by reproductive state, particularly during pregnancy. Finally, our data support the adequacy of the vitamin D RDA (600 IU) for achieving serum 25(OH)D concentrations of 50 nmol/L among women of childbearing age, including those who are pregnant or breastfeeding.
Acknowledgments
HP, PMB, and MAC designed the research; HP, AAW, JY, XJ, CAP, and OVM conducted the research; HP analyzed the data; HP and MAC wrote the manuscript; PMB and SM contributed to the methodology development and data interpretation; and MAC had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: CYP2R1, 25-hydroxylase gene; CYP24A1, 24-hydoxylase; DBP, vitamin D binding protein; epi-25(OH)D3, C3 epimer of 25-hydroxyvitamin D3; free 25(OH)D, free 25-hydroxyvitamin D; GC, vitamin D binding protein gene; HMRU, Human Metabolic Research Unit; IOM, Institute of Medicine; LMM, linear mixed-effects model; 1,25(OH)2D, 1,25-dihydroxyvitamin D; 1,25(OH)2D:25(OH)D, ratio of 1,25-dihydroxyvitamin D to 25-hydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; 25(OH)D:24,25(OH)2D, ratio of 25-hydroxyvitamin D to 24,25-dihydroxyvitamin D.
References
- 1.Brannon PM, Picciano MF. Vitamin D in pregnancy and lactation in humans. Annu Rev Nutr 2011;31:89–115. [DOI] [PubMed] [Google Scholar]
- 2.De-Regil LM, Palacios C, Ansary A, Kulier R, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev 2012:CD008876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saraf R, Morton SM, Camargo CA, Grant CC. Global summary of maternal and newborn vitamin D status - a systematic review. Matern Child Nutr 2015;25:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner CL, Taylor SN, Johnson DD, Hollis BW. The role of vitamin D in pregnancy and lactation: emerging concepts. Womens Health (Lond Engl) 2012;8:323–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O’Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ 2013;346:f1169. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Valle HD, (editors). Dietary reference intakes for calcium and vitamin D. Washington (DC): National Academies Press; 2011. [PubMed] [Google Scholar]
- 7.Papapetrou PD. The interrelationship of serum 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D in pregnancy at term: a meta-analysis. Hormones (Athens) 2010;9:136–44. [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD, Gee E, Halloran B, Haddad JG. Free 1,25-dihydroxyvitamin D levels in serum from normal subjects, pregnant subjects, and subjects with liver disease. J Clin Invest 1984;74:1966–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning : a longitudinal. Am J Clin Nutr 1995;61:514–23. [DOI] [PubMed] [Google Scholar]
- 10.Ardawi MS, Nasrat HA, BA’Aqueel HS. Calcium-regulating hormones and parathyroid hormone-related peptide in normal human pregnancy and postpartum: a longitudinal study. Eur J Endocrinol 1997;137:402–9. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Van Loan MD, Cann CE, King JC. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. Am J Clin Nutr 1998;67:693–701. [DOI] [PubMed] [Google Scholar]
- 12.Wilson SG, Retallack RW, Kent JC, Worth GK, Gutteridge DH. Serum free 1,25-dihydroxyvitamin D and the free 1,25-dihydroxyvitamin D index during a longitudinal study of human pregnancy and lactation. Clin Endocrinol (Oxf) 1990;32:613–22. [DOI] [PubMed] [Google Scholar]
- 13.More C, Bettembuk HP, Bhattoa P, Balogh A. The effects of pregnancy and lactation on bone mineral density. Osteoporos Int 2001;12:732–7. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez PA, Idrisa A, Bobzom DN, Airede A, Hollis BW, Liston DE, Jones DD, Dasgupta A, Glew RH. Calcium and vitamin D status of pregnant teenagers in Maiduguri, Nigeria. J Natl Med Assoc 1997;89:805–11. [PMC free article] [PubMed] [Google Scholar]
- 15.Morley R, Carlin JB, Pasco JA, Wark JD. Maternal 25-hydroxyvitamin D and parathyroid hormone concentrations and offspring birth size. J Clin Endocrinol Metab 2006;91:906–12. [DOI] [PubMed] [Google Scholar]
- 16.Zhang JY, Lucey AJ, Horgan R, Kenny LC, Kiely M. Impact of pregnancy on vitamin D status: a longitudinal study. Br J Nutr 2014;112:1081–7. [DOI] [PubMed] [Google Scholar]
- 17.Sowers M, Zhang D, Hollis BW, Shapiro B, Janney CA, Crutchfield M, Anthony Schork M, Stanczyk F, Randolph J. Role of calciotrophic hormones in calcium mobilization of lactation. Am J Clin Nutr 1998;67:284–91. [DOI] [PubMed] [Google Scholar]
- 18.Hoogenboezem T, Degenhart HJ, De Muinck Keizer-Schrama SM, Bouillon R, Grose WF, Hackeng WH, Visser HK. Vitamin D metabolism in breast-fed infants and their mothers. Pediatr Res 1989;25:623–8. [DOI] [PubMed] [Google Scholar]
- 19.Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, Sidhom G, Rousseau D, Cole DEC, Vieth R. The ratio of serum 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 is predictive of 25-hydroxyvitamin D3 response to vitamin D3 supplementation. J Steroid Biochem Mol Biol 2011;126:72–7. [DOI] [PubMed] [Google Scholar]
- 20.Kaufmann M, Gallagher JC, Peacock M, Schlingmann KP, Konrad M, DeLuca HF, Sigueiro R, Lopez B, Mourino A, Maestro M, et al. Clinical utility of simultaneous quantitation of 25-hydroxyvitamin D and 24,25-dihydroxyvitamin D by LC-MS/MS involving derivatization with DMEQ-TAD. J Clin Endocrinol Metab 2014;99:2567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cashman KD, Hayes A, Galvin K, Merkel J, Jones G, Kaufmann M, Hoofnagle AN, Carter GD, Durazo-Arvizu RA, Sempos CT. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: a double-edged sword? Clin Chem 2015;61:636–45. [DOI] [PubMed] [Google Scholar]
- 22.Berg AH, Powe CE, Evans MK, Wenger J, Ortiz G, Zonderman AB, Suntharalingam P, Lucchesi K, Powe NR, Karumanchi SA, et al. 24,25-Dihydroxyvitamin D3 and vitamin D status of community-dwelling black and white Americans. Clin Chem 2015;61:877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz JB, Lai J, Lizaola B, Kane L, Markova S, Weyland P, Terrault NA, Stotland N, Bikle D. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. J Clin Endocrinol Metab 2014;99:1631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schleicher RL, Encisco SE, Chaudhary-Webb M, Paliakov E, McCoy LF, Pfeiffer CM. Isotope dilution ultra performance liquid chromatography-tandem mass spectrometry method for simultaneous measurement of 25-hydroxyvitamin D2, 25-hydroxyvitamin D3 and 3-epi-25-hydroxyvitamin D3 in human serum. Clin Chim Acta 2011;412:1594–9. [DOI] [PubMed] [Google Scholar]
- 25.Cashman KD, Kinsella M, Walton J, Flynn A, Hayes A, Lucey A, Seamans KM, Kiely M. The 3 epimer of 25-hydroxycholecalciferol is present in the circulation of the majority of adults in a nationally representative sample and has endogenous origins. J Nutr 2014;144:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan J, Jiang X, West AA, Perry CA, Malysheva OV, Devapatla S, Pressman E, Vermeylen F, Stabler SP, Allen RH, et al. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am J Clin Nutr 2012;95:1060–71. [DOI] [PubMed] [Google Scholar]
- 27.West AA, Yan J, Perry CA, Jiang X, Malysheva OV, Caudill MA. Folate-status response to a controlled folate intake in nonpregnant, pregnant, and lactating women. Am J Clin Nutr 2012;96:789–800. [DOI] [PubMed] [Google Scholar]
- 28.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin. J Clin Endocrinol Metab 1986;63:954–9. [DOI] [PubMed] [Google Scholar]
- 29.Bolland MJ, Grey AB, Ames RW, Horne AM, Mason BH, Wattie DJ, Gamble GD, Bouillon R, Reid IR. Age-, gender-, and weight-related effects on levels of 25-hydroxyvitamin D are not mediated by vitamin D binding protein. Clin Endocrinol (Oxf) 2007;67:259–64. [DOI] [PubMed] [Google Scholar]
- 30.Lundgren S, Carling T, Hjälm G, Juhlin C, Rastad J, Pihlgren U, Rask L, Akerström GHP. Tissue distribution of human gp330/megalin, a putative Ca(2+)-sensing protein. J Histochem Cytochem 1997;45:383–92. [DOI] [PubMed] [Google Scholar]
- 31.Kalkwarf HJ, Specker BL, Heubi JE, Vieira NE, Yergey AL. Intestinal calcium absorption and after weaning. Am J Clin Nutr 1996;63:526–31. [DOI] [PubMed] [Google Scholar]
- 32.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powe CE, Ricciardi C, Berg AH, Erdenesanaa D, Collerone G, Ankers E, Wenger J, Karumanchi SA, Thadhani R, Bhan I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J Bone Miner Res 2011;26:1609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnsen MS, Grimnes G, Figenschau Y, Torjesen PA, Almås B, Jorde R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand J Clin Lab Invest 2014;74:177–83. [DOI] [PubMed] [Google Scholar]
- 35.Novakovic B, Sibson M, Ng HK, Manuelpillai U, Rakyan V, Down T, Beck S, Fournier T, Evain-Brion D, Dimitriadis E, et al. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. J Biol Chem 2009;284:14838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys 2012;523:37–47. [DOI] [PubMed] [Google Scholar]
- 37.Møller UK, Streym Sv, Jensen LT, Mosekilde L, Schoenmakers I, Nigdikar S, Rejnmark L. Increased plasma concentrations of vitamin D metabolites and vitamin D binding protein in women using hormonal contraceptives: a cross-sectional study. Nutrients 2013;5:3470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am J Pathol 2002;161:105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab 2012;303:E928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]