Abstract
Background: There has been limited characterization of biological variables that impact vitamin K metabolism. This gap in knowledge can limit the translation of data obtained from preclinical animal studies to future human studies.
Objective: The purpose of this study was to determine the effects of diet, sex, and housing on serum, tissue, and fecal vitamin K concentrations and gene expression in C57BL6 mice during dietary vitamin K manipulation.
Methods: C57BL6 4-mo-old male and female mice were randomly assigned to conventional or suspended-wire cages and fed control [1400 ± 80 μg phylloquinone (PK)/kg] or deficient (31 ± 0.45 μg PK/kg) diets for 28 d in a factorial design. PK and menaquinone (MK) 4 plasma and tissue concentrations were measured by HPLC. Long-chain MKs were measured in all matrices by LC-atmospheric pressure chemical ionization-mass spectrometry. Gene expression was quantified by reverse transcriptase-polymerase chain reaction in the liver, brain, kidney, pancreas, and adipose tissue.
Results: Male and female mice responded differently to dietary manipulation in a tissue-dependent manner. In mice fed the control diet, females had ∼3-fold more MK4 in the brain and mesenteric adipose tissue than did males and 100% greater PK concentrations in the liver, kidney, and mesenteric adipose tissue than did males. In mice fed the deficient diet, kidney MK4 concentrations were ∼4-fold greater in females than in males, and there were no differences in other tissues. Males and females differed in the expression of vitamin K expoxide reductase complex 1 (Vkorc1) in mesenteric adipose tissue and the pancreas and ubiA domain–containing protein 1 (Ubiad1) in the kidney and brain. There was no effect of housing on serum, tissue, or fecal concentrations of any vitamin K form.
Conclusions: Vitamin K concentrations and expression of key metabolic enzymes differ between male and female mice and in response to the dietary PK concentration. Identifying factors that may impact study design and outcomes of interest is critical to optimize study parameters examining vitamin K metabolism in animal models.
Keywords: coprophagy; Ggcx, long-chain menaquinones; menaquinone-4; phylloquinone; suspended wire cages; Ubiad1, vitamin K; Vkorc1; Vkorc1l1
Introduction
Vitamin K is a fat-soluble vitamin characterized by the 2-methyl-1,4-naphthoquinone ring that exhibits anti-hemorrhagic activity and an isoprenoid side chain. The major dietary form, phylloquinone (PK)7, is found in dark green leafy vegetables and plant oils (1) and provides the basis for current dietary requirements (1, 2). Menaquinones (MKs) are a group of vitamin K derivatives that are named after the number of isoprenoid units in the side chain (3). Whereas evidence suggests MK4 is formed from dietary PK by means of tissue-specific conversion by the enzyme ubiA prenyltransferase domain-containing 1 (Ubiad1) and may have unique functions among the known vitamin K forms (4–7), longer-chain MKs are a product of bacterial synthesis in the intestine (8, 9). Bacterially produced MKs act as electron carriers in cell respiration, transporting molecules across plasma membranes and acting as an antioxidant preventing lipid oxidation (10). Dietary sources of MKs include dairy and meats, as well as fermented food products (3, 10). The contribution of bacterially synthesized MKs to overall vitamin K status in animal models is still uncertain (11), and the impact of MKs to human vitamin K requirements and status remains controversial.
There is growing awareness that there are sex-specific differences in response to specific interventions in animal models used for biomedical research (12). The majority of current preclinical studies in the vitamin K field rely on the use of a single-sex rodent model (13–18); few studies, to our knowledge, have included both male and female rodents (19, 20). However, in rats, females are reported to be more resistant to vitamin K deficiency than males (21, 22), which has been hypothesized to be attributable to increased coprophagy in females (23), and increased vitamin K requirement in males (21, 24), specific effects of sex hormones on vitamin K metabolism (25, 26), and differences in the biosynthesis of MKs by gut bacteria and corresponding absorption (26). The increased use of genetically modified mouse models and diet manipulation studies necessitates characterization of the biological variables affecting mouse models of vitamin K nutriture. This includes understanding sex-specific differences in vitamin K metabolism to gain insight into mechanistic activity of vitamin K and its alternative functions.
Coprophagy is a unique consideration in animal studies of vitamin K nutrition because of the high concentrations of bioavailable MKs produced by gut bacteria. To control for unanticipated MK intake through coprophagy, suspended wire caging in combination with vitamin K–deficient diets have been effectively used to create a vitamin K deficiency as defined by a decrease in prothrombin time (23, 27). With NIH mandates to improve animal welfare (12, 28), these caging options need to be evaluated to determine whether they indeed account for differences in vitamin K metabolism across both sexes.
Our group has developed novel methods, to our knowledge, for accurately manipulating vitamin K in the mouse diet to match the predominant forms in the human diet (29), for measuring all known MK forms (30), and for characterizing the effect(s) of diet and nondietary biological factors, such as sex, on expression of genes involved in vitamin K recycling and function (31). This study used these techniques to determine the effects of sex, diet, and caging on serum, tissue, and fecal vitamin K concentrations and vitamin K metabolism–related gene expression in C57BL6 mice, a common animal model for genetic manipulation. We hypothesized that vitamin K concentrations in serum, liver, and extrahepatic tissues would be substantially different between housing types. Additionally, we hypothesized a sex-specific difference in response to the dietary vitamin K manipulation between caging types.
Methods
Animals and diets.
C57BL/6NCrl VAF/Plus mice (4 mo of age, n = 64) obtained from Charles River Laboratory were acclimated with the AIN-93G diet (TD.94045, Envigo) in conventional caging for 1 wk. We used a 23 factorial design to evaluate sex, diet, and cage effects. Male and female mice were weight-matched and randomly assigned to individual conventional [20.3-cm × 31.8-cm × 20.3-cm Zyfone plastic caging supplied with Biofresh cellulose bedding (Absorption Co.)] or suspended wire caging (20.3 cm × 25.4 cm × 19.1 cm). Within each cage and sex group, mice were randomly assigned to a control diet containing 1400 ± 80 μg PK/kg diet or a deficient diet containing 31 ± 0.45 μg PK/kg diet (TD.120060, Envigo) for 4 wk ad libitum, resulting in 8 groups of 8 mice each. The experimental diet is a modification of TD.97053 with replacement of regular corn oil with tocopherol-stripped corn oil and a PK source (Supplemental Table 1) (32). Tocopherol-stripped corn oil is used due because of nutrient-nutrient interaction between vitamin K and vitamin E as reported by Tovar et al. (33). This study demonstrated rats fed a vitamin E–supplemented diet had significantly lower extrahepatic tissue concentrations of vitamin K. To eliminate potential nutrient interactions in our study, the diet was prepared with tocopherol-stripped corn oil. Body weights were measured weekly. If significant weight loss was observed and mice showed clinical signs of dehydration, fluid replacement was initiated with 1 cc of Ringer’s lactate solution injected subcutaneously. Mice were maintained in Association for Assessment and Accreditation of Laboratory Animal Care International–accredited facilities with an environmentally controlled atmosphere (22°C, 45% relative humidity, 15 air changes of 100% fresh HEPA-filtered air/h and a 12/12-h light/dark cycle, 0700 on). Mice were observed daily for clinical signs of distress or disease. At the end of the experiment, they were euthanized with carbon dioxide with secondary euthanasia ensured by subsequent cervical dislocation, followed by blood and tissue collection. Tissues of interest (the brain, liver, kidney, pancreas, and mesenteric adipose tissue) were harvested and frozen immediately in liquid nitrogen and stored at −70°C until time of analysis. All protocols were approved by the Human Nutrition Research Center on Aging at Tufts University Animal Care and Use Committee.
Vitamin K analysis.
Tissues (0.10–0.20 g of wet weight) were homogenized in PBS by use of a Powergen homogenizer (Fisher Scientific). Plasma and tissue homogenate PK and MK4 were measured by use of reversed-phase HPLC (34). Longer chain MK (MK5–MK13) concentrations were quantified in tissue and fecal samples by LC-atmospheric pressure chemical ionization-MS (30).
Gene expression.
We profiled the expression of the following genes encoding enzymes involved in vitamin K metabolism (1): vitamin K epoxide reductase complex subunit 1 (Vkorc1) (2), vitamin K epoxide reductase complex subunit 1-like 1 (Vkorc1l1) (3), γ glutamyl carboxylase (Ggcx), and (4) UbiA prenyl transferase domain-containing 1 (Ubiad1). Total RNA was isolated from tissues (the kidney, liver, brain, mesenteric adipose tissue, and pancreas) by use of TRIzol reagent and cDNA synthesized by use of Superscript III reverse transcriptase (Life Technologies). Pancreas tissue samples were not stored with RNAse inhibitor, increasing risk of degraded RNA and further lack of detectable expression. Real-time PCR was performed by use of SYBR green master mix and an ABI7300 thermocycler (Applied Biosystems). Primer sequences were obtained from qPrimerDepot (35) or NCBI Primer Blast (Supplemental Table 2) (36). Relative expression was calculated by use of the 2-ΔΔCt method, and statistical analyses were performed on ΔCt values. Gapdh was used as the control gene.
Tail clip bleeding assay (in vivo bleeding assay).
Tail bleeding time was measured by an in vivo bleeding assay on day 28 of the dietary intervention. Bleed time was defined as the start of bleeding to the cessation of bleeding (37).
Corticosterone assay.
Physiologic responses to stress lead to the release of glucocorticoids, specifically corticosterone in mouse models (38). As a measure of stress in response to different caging conditions, plasma total corticosterone concentrations were measured by ELISA kit (Alpco). The intra- and interassay coefficients of variation were 5.9% and 7.5%, respectively.
Power calculations and statistical analyses.
Sample size calculations were based on the work of Metta and Johnson (22), who compared prothrombin time among male and female rats allowed coprophagy and not allowed coprophagy. Power analysis was performed based on 2-sample comparisons on the main effects for cage and sex. We determined that 4 mice/group would provide 80% power to detect a difference in liver vitamin K status between mice in conventional (allowed coprophagy) and wire-bottomed cages (not allowed coprophagy), and 6 mice/group would provide 90% power to detect a sex-specific difference in liver vitamin K status at α = 0.05. In each group, 2 extra mice were added in case of death during the study.
The effects of cage, sex, and diet on concentrations of PK and MK4 were analyzed by a 3-factor ANOVA model with interactions and a fixed effect for tissue PK and MK4 measurements. Results of the full model led to subgroup analysis within each specific tissue for the effects of sex and diet on PK and MK4 concentrations, whereas we used a 2-factor ANOVA and Tukey’s honestly significant difference to examine sex-by-diet interactions. Pairwise comparisons of interest included male control compared with male deficient; female control compared with female deficient; male control compared with female control; and male deficient compared with female deficient. ANOVA was used to examine fluid replacement, change in body weight, and bleeding time between males and females in conventional and suspended wire cages. We assessed the ANOVA model using diagnostics for assumptions of homogeneity of variance. No outliers were found that influence significance in the full model. Data were tested for normality by the Shapiro-Wilk test. Significance was determined by P < 0.01, and all analyses were carried out by use of SAS version 9.4. Data are reported as means ± SEMs.
Results
Cage effect.
Overall, independent effects of diet (P < 0.01) and cage (P < 0.01) on body weight change in both male and female mice (sex effect P = 0.37) were observed. The deficient diet and wire-bottomed cages were both associated with lower weight gain, but the 3-way interaction between the effects of sex, cage type, and diet on weight change was not significant (P-interaction = 0.76) (Table 1). There was no diet-by-cage interaction (P-interaction = 0.92). Of the 32 mice housed in suspended wire cages, 31 (97% of mice) required fluid replacement during the 28-d experimental period (P < 0.01) (Supplemental Table 3). In comparison, only 2 mice in the conventional cages required fluid replacement.
TABLE 1.
Weight gain in male and female mice housed in either suspended or conventional caging and fed a control or vitamin K–deficient diet for 28 d1
| Male, g/28 d |
Female, g/28 d |
||||||||||
| Conventional |
Suspended wire |
Conventional |
Suspended wire |
P |
|||||||
| Diet | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Day 0 | Day 28 | Cage | Sex | Diet |
| Control | 32.8 ± 2.8 | 39.6 ± 4.1 | 28.8 ± 2.6 | 32.0 ± 1.3 | 27.6 ± 3.5 | 32.7 ± 4.7 | 29.2 ± 3.9 | 31.3 ± 3.3 | <0.01 | 0.37 | <0.01 |
| Deficient | 31.3 ± 2.0 | 35.3 ± 3.9 | 33.0 ± 2.9 | 32.7 ± 0.9 | 27.1 ± 3.6 | 30.4 ± 4.6 | 24.9 ± 1.4 | 25.6 ± 1.7 | <0.01 | 0.37 | <0.01 |
Values are means ± SEMs, n = 8 mice/group. Overall P values reflect ANOVA for calculated changes in body weight with cage, sex, and diet as factors.
Analysis of PK and MK4 tissue concentrations in the full statistical model showed no overall cage effect for any vitamin K form (main effect cage: PK P = 0.09; MK4 P = 0.29, long-chain MKs all P values > 0.02). There was also no significant difference in bleeding time between suspended wire and conventional caging regardless of sex (data not shown, P > 0.10).
Because of the differences observed in weight gain between caging and required fluid replacement of mice housed in suspended wire cages, we examined the effect of these environmental stressors as assessed by plasma corticosterone concentrations. Although mice in suspended wire caging showed differences in weight with the different diets, there was no significant difference in corticosterone concentrations between suspended wire cages or conventional cages (data not shown, P = 0.87).
Diet and sex effects.
Because there was no effect of cage on concentrations of any vitamin K form, data were combined for cage types for all other analyses presented (Table 2). Results of the overall statistical model showed a significant difference of PK and MK4 concentrations related to tissue type (P < 0.001). Thus we chose to examine each tissue independently to examine the effect of sex and diet on PK and MK4 concentrations. For all tissues, there was an observed diet effect in PK and MK4 concentrations (main effect of diet all P values < 0.01), except for PK concentrations in the pancreas, which showed no effect (P = 0.05). There was an overall sex effect observed for PK and MK4 concentrations in each tissue (main effect of sex all P values < 0.01). The response to the diets differed by sex in most tissues (for all sex-by-diet interactions P < 0.01), with mice consuming the control diet having higher tissue concentrations of PK and MK4. The only exception was in PK concentrations in the pancreas (sex-by-diet interaction P = 0.25). Serum PK concentrations did not differ between males and females within either diet (main effect of sex P = 0.91; sex-by-diet interaction P = 0.91), PK concentrations did not differ between males and females consuming the deficient diet in any tissue (P > 0.02). MK4 concentrations were significantly different between males and females consuming the deficient diet in the kidney only (P < 0.01) with female mice having higher concentrations. In contrast, females consuming the control diet had significantly higher PK and MK4 concentrations in all tissues (all P values < 0.01). Of the tissues analyzed, only kidney, liver, and fecal samples contained long-chain MKs (Tables 2 and 3). Within the deficient diet, there were no significant differences in MK9, MK10, or MK11 in the kidney or liver between males and females (all P values > 0.02). Surprisingly, males had higher kidney MK9 and MK11 concentrations (all P values < 0.01), and in the liver, females had significantly higher MK11 concentrations than did males (P < 0.01). Long-chain MKs in the kidney and liver were unaffected by diet (main effect of diet all P values > 0.12); however, there was a significant sex-by-diet interaction in the liver MK11 (P < 0.01).
TABLE 2.
Serum and tissue PK, MK4, and detectable long-chain MK concentrations of male and female mice fed a vitamin K control or vitamin K–deficient diet for 28 d1
| Deficient diet |
Control diet |
P |
||||||||
| Tissue | Vitamin K form | Male | Female | P | Male | Female | P | Sex | Diet | Sex × diet interaction |
| Serum, pmol/mL | PK | ND | ND | — | 0.80 ± 0.2 | 0.90 ± 0.2 | 0.88 | 0.91 | <0.01† | 0.91 |
| MK4 | ND | ND | — | ND | ND | — | — | — | — | |
| Liver, pmol/g | PK | 8.3 ± 2.1 | 15.4 ± 3.9 | 0.04 | 26.0 ± 6.5 | 51.0 ± 12.7 | <0.01* | <0.01† | <0.01† | <0.01† |
| MK4 | ND | ND | — | 1.25 ± 0.3 | 10.8 ± 2.7 | <0.01* | <0.01† | <0.01† | <0.01† | |
| MK9 | 33.1 ± 8.3 | 5.8 ± 1.4 | 0.05 | 37.1 ± 9.3 | 15.8 ± 3.9 | 0.14 | 0.02 | 0.48 | 0.72 | |
| MK10 | 60.8 ± 15.2 | 40.8 ± 10.2 | 0.24 | 57.3 ± 14.3 | 68.8 ± 17.2 | 0.50 | 0.72 | 0.31 | 0.19 | |
| MK11 | 351 ± 87.8 | 200 ± 49.9 | 0.02 | 244 ± 60.9 | 401 ± 100 | 0.01* | 0.95 | 0.28 | <0.01† | |
| Kidney, pmol/g | PK | ND | ND | — | 3.3 ± 0.8 | 5.6 ± 1.4 | <0.01* | <0.01† | <0.01† | <0.01† |
| MK4 | 2.0 ± 0.5 | 10.4 ± 2.6 | <0.01* | 16.3 ± 4.1 | 122 ± 30.6 | <0.01* | <0.01† | <0.01† | <0.01† | |
| MK9 | 32.6 ± 8.1 | 18.2 ± 4.5 | 0.13 | 50.2 ± 12.6 | 19.2 ± 4.8 | 0.01* | <0.01† | 0.17 | 0.22 | |
| MK10 | 26.5 ± 6.6 | 15.1 ± 3.8 | 0.22 | 34.7 ± 8.7 | 27.8 ± 7.0 | 0.46 | 0.17 | 0.12 | 0.73 | |
| MK11 | 48.3 ± 12.1 | 28.9 ± 7.2 | 0.02 | 52.2 ± 13.1 | 25.0 ± 6.2 | <0.01* | <0.01† | 0.99 | 0.48 | |
| Brain, pmol/g | PK | ND | ND | — | ND | ND | — | — | — | — |
| MK4 | 12.3 ± 3.1 | 19.5 ± 4.9 | 0.19 | 27.2 ± 6.8 | 111 ± 27.7 | <0.01* | <0.01† | <0.01† | <0.01† | |
| Mesenteric adipose tissue, pmol/g | PK | 3.75 ± 0.9 | 7.0 ± 1.8 | 0.29 | 10.1 ± 2.5 | 25.0 ± 6.2 | <0.01* | <0.01† | <0.01† | 0.01† |
| MK4 | 6.75 ± 1.7 | 8.4 ± 2.1 | 0.72 | 10.2 ± 2.5 | 44.4 ± 11.1 | <0.01* | <0.01† | <0.01† | <0.01† | |
| Pancreas, pmol/g | PK | 12.1 ± 3.0 | 26.2 ± 6.4 | 0.02 | 14.5 ± 3.6 | 36.1 ± 9.0 | <0.01* | <0.01† | 0.05 | 0.25 |
| MK4 | 40.6 ± 10.1 | 46.7 ± 11.7 | 0.89 | 127 ± 31.7 | 337 ± 84.3 | <0.01* | <0.01† | <0.01† | <0.01† | |
Values are means ± SEMs, n = 16 mice/group. Concentration was below lower limit of detection for PK and MK4 (0.01 pmol/g) by use of an HPLC assay. *Significant difference in Tukey’s honestly significant difference test between male and female mice within diet group at P < 0.01 adjusted α level. †Significant test within the ANOVA model for specific tissue and vitamin K form at P < 0.01 adjusted significance level. MK, menaquinone; ND, nondetectable; PK, phylloquinone.
TABLE 3.
Fecal MK concentrations of male and female mice fed a control or vitamin K–deficient diet for 28 d1
| Deficient diet |
Control diet |
P |
|||||||
| Vitamin K form, pmol/g | Male | Female | P | Male | Female | P | Sex | Diet | Sex × diet interaction |
| PK | 28.1 ± 9.95 | 20.3 ± 7.2 | 0.98 | 1920 ± 678 | 2410 ± 853 | 0.02 | 0.08 | <0.01† | 0.08 |
| MK4 | 859 ± 304 | 568 ± 201 | 0.88 | 1080 ± 381 | 1190 ± 421 | 0.67 | 0.68 | <0.01† | 0.84 |
| MK5 | ND | ND | — | ND | ND | — | — | — | — |
| MK6 | 142 ± 50.3 | 202 ± 71.5 | 0.94 | 79.3 ± 28.1 | 383 ± 135 | 0.01* | 0.07 | 0.75 | 0.06 |
| MK7 | 44.4 ± 15.7 | 55 ± 19.4 | 0.95 | 29.1 ± 10.3 | ND | — | 0.53 | 0.06 | 0.48 |
| MK8 | 125 ± 44.2 | 219 ± 77.2 | 0.85 | 54.6 ± 19.3 | 212 ± 74.8 | 0.02 | 0.08 | 0.10 | 0.13 |
| MK9 | 376 ± 133 | 180 ± 63.4 | 0.28 | 196 ± 69.2 | 282 ± 99.6 | 0.22 | 0.90 | 0.65 | 0.11 |
| MK10 | 804 ± 284 | 796 ± 282 | 0.57 | 581 ± 205 | 1210 ± 429 | 0.01* | 0.13 | 0.82 | 0.02 |
| MK11 | 224 ± 79.3 | 274 ± 96.8 | 0.93 | 166 ± 58.7 | 400 ± 142 | 0.01* | 0.07 | 0.92 | 0.05 |
| MK12 | 13.8 ± 4.9 | 23.3 ± 8.2 | 0.52 | 15.2 ± 5.4 | 32.8 ± 11.6 | 0.07 | 0.08 | 0.59 | 0.39 |
| MK13 | 78.1 ± 27.6 | 59.4 ± 21 | 0.87 | 60.3 ± 21.3 | 104 ± 36.8 | 0.19 | 0.41 | 0.40 | 0.30 |
Values are means ± SEMs, n = 8 mice/group. Concentration was below lower limit of detection for MK5 and MK7 (5.0 pmol/g) by use of an LC-MS assay. *Significant difference in Tukey’s honestly significant difference test between male and female mice within diet group at P < 0.01 adjusted α level. †Significant test within the ANOVA model for specific tissue and vitamin K form at P < 0.01 adjusted significance level. MK, menaquinone; ND, nondetectable; PK, phylloquinone.
Fecal PK, MK4, and detectable long-chain MK concentrations are presented in Table 3. For longer chain MKs (MK6–MK13), there was no effect of sex (all P values > 0.07) or diet (all P values > 0.06). There was no significant difference in MK concentrations between males and females consuming the deficient diet (all P values > 0.28). In comparison, females consuming the control diet had significantly higher MK6, MK10, and MK11 than did males (all P values < 0.01). For all other MKs, there was no difference in fecal concentrations by sex or diet (P > 0.05).
Gene expression.
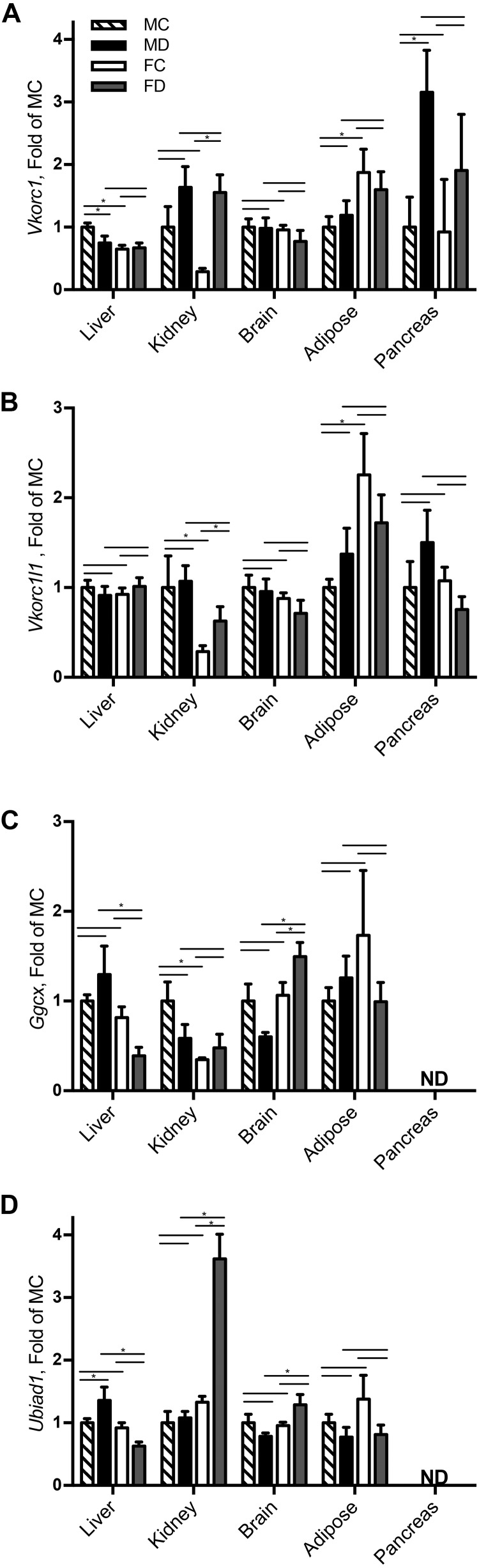
Gene expression across different tissues varied and may be indicative of tissue-specific regulation (Figure 1). Vkorc1 expression was significantly different between males and females in mesenteric adipose tissue and the pancreas (P < 0.03) and in response to diet in the pancreas and kidney (P < 0.01). Interestingly, Vkorc1 in the pancreas and kidney of both males and females had increased relative expression compared with liver Vkorc1, which was consistently expressed across all groups. Vkorc1l1 expression only had a significant sex and diet effect in the kidney and mesenteric adipose tissue, (P < 0.01). Expression of Ggcx only had a significant sex-by-diet interaction in the brain and liver (P < 0.02). Ubiad1 expression was significantly different between males and females in the kidney and brain (P < 0.04). There was a significant sex-by-diet interaction in the liver (P < 0.03) and a diet effect observed in mesenteric adipose tissue (P < 0.04). Similar to Ggcx, Ubiad1 was not expressed in the pancreas.
FIGURE 1.
Gene expression of Vkorc1 (A), Vkorc1l1 (B), Ggcx (C), and Ubiad1 (D) in tissues of male and female C57BL6 mice fed a phylloquinone-deficient or control diet for 28 d. Data are displayed as expression relative to the MC group and are presented as means ± SEMs, n = 16. Bars represent 2-group comparisons of interest (MC vs. MD, FC vs. FD, MC vs. FC, and MD vs. FD) with the endpoint of each bar indicating the 2 groups being compared. *P < 0.05. FC, female control; FD, female deficient; Ggcx, γ glutamyl carboxylase; LLD, lower limit of detection; LOQ, limit of quantification; MC, male control; MD, male deficient; ND, not detected with assay LLD < 20 copies/reaction in a 50-μL reaction, and LOQ at 98 copies/reaction; Ubiad1, ubiA domain–containing protein 1; Vkorc1, vitamin K epoxide reductase complex 1; Vkorc1l1, vitamin K epoxide reductase complex 1-like 1.
Discussion
In this study of young C57BL6 mice, there were significant sex-specific differences in concentrations of PK and MK4 in all tissues examined, and in several long-chain MK forms detected in the liver and kidney. These results and supporting gene expression data indicate that males and females respond differently to dietary manipulation of vitamin K. As such, this study provides critical information about biological variables that could influence the function of vitamin K and its metabolites.
Sex-specific differences in the tissue distribution of vitamin K forms have been documented in rats (24, 39–42). However, there is limited information available regarding the sex-specific responses to vitamin K dietary manipulation or vitamin K metabolism in mice. The C57BL6 genetic background is the most commonly used strain for transgenic models, and although transgenic mouse models for studying key enzymes in vitamin K metabolism and vitamin K–dependent proteins have been developed, none have compared responses between sexes (14, 19, 20). We saw no difference in concentrations of PK and MK4 between males and females consuming the deficient diet in any tissue except the pancreas. There may be a minimum vitamin K concentration for the individual tissues achieved with intakes sufficient to sustain coagulation that is similar for males and females, whereas only the higher intakes reveal sex-specific differences in tissue concentrations.
Collectively, these observations challenge the theories put forth based on rat studies that females are more resistant to vitamin K deficiency than are males (21, 22) because of possible increased vitamin K requirements in males (21, 24). Instead, it is more plausible that the higher tissue concentrations observed in females in response to high vitamin K intakes may be attributed to the role of estrogen and potential vitamin K requirements for reproduction. Sex-specific differences have not been well characterized in humans. Although there is a suggestive effect of sex, the data are inconsistent (43), which further supports the need to use both sexes in vitamin K studies by use of preclinical models.
The extent to which bacterially produced MKs contribute to vitamin K requirements and function in the liver is still unclear. In our study, it is assumed that the origin of hepatic and renal long-chain MKs is attributable to coprophagy because phylloquinone was the exclusive dietary source of vitamin K, and colonic absorption of bacterially synthesized MK was likely low. Despite characterizing 8 bacterially produced MKs in feces, we were only able to measure 3 long-chain MK forms in peripheral tissues and only in the liver and kidney. Females had overall higher bacterially produced MK tissue contents than did males consuming the control diet, which may be indicative of sex-specific differences in gut microbiota and/or coprophagy, as reported for other species (23, 26).
In our study, suspended wire caging had no effect on concentrations of tissue vitamin K forms or measures of vitamin K function. Mice can access cecal pellets and ingest the pellets as they are being excreted. Prevention of cecal pellet consumption requires alternative methods such as Elizabethan collars (44). Suspended wire caging however, resulted in poor health in the mice as indicated by more fluid replacement and less body weight gain. That there were no advantages to use of suspended wire cages in terms of limiting coprophagy lends further support for eliminating this caging type in mouse studies, because it does not significantly reduce or inhibit coprophagic behavior.
Vitamin K status reflects adequacy of requirements to support the biological function of posttranslational modification of vitamin K-dependent proteins, which include proteins involved in coagulation and bone metabolism. Vkorc1 is the rate-limiting step in the classic vitamin K cycle of protein post-translational modification. Vkorc1l1, the isomer to Vkorc1 has demonstrated differential regulation of expression, tissue-specific expression, and differences in vitamin K antagonist warfarin sensitivity (20, 45–47), indicating that although the 2 enzymes are structurally similar, their functionality and regulation may be independent of one another. Our results suggest tissue-specific differences in expression and possible independent regulation of the 2 paralogs, reflected by the diet and sex effects on expression in different tissues. The consistent expression of Vkorc1 in the liver, regardless of diet, may be indicative of a minimum threshold of vitamin K required to maintain coagulation. In contrast, the upregulation of Vkorc1 expression observed in the kidney and pancreas of both males and females consuming the deficient diet may suggest a compensatory effect by extrahepatic tissues for lower vitamin K intake.
A recently identified enzyme involved in vitamin K metabolism is the Ubiad1 gene coding for a prenyltransferase that enzymatically converts menadione to MK4 (6, 48). The conversion is proposed to be a multistep process involving side chain cleavage of PK to the ring structure menadione, which is then readily converted to MK4 by ubiad1. The enzyme involved in side chain cleavage has yet to be identified, but it is thought to be localized to the intestine (5). The unique tissue distribution of PK and MK4 infers possible differences in regulation of conversion at the level of Ubiad1. Although expression of Ubiad1 has been reported in the mouse pancreas (48), we were unable to detect Ubiad1 in this tissue. Interestingly, the pancreas also does not express Ggcx, an enzyme critical to the vitamin K cycle. The lack of expression of Ubiad1 in the pancreas observed in our study suggests that this tissue may not have the ability to produce or convert PK to MK4. However, the pancreas has high RNase activity, to the extent that this tissue has been excluded in other studies examining vitamin K-related gene expression (20). Our sample preparation may have led to significant RNA degradation resulting in undetectable levels of expression. Thus the origin of MK4 in the pancreas cannot be determined solely by examining the expression of Ubiad1.
Our data are consistent with prior studies that have demonstrated tissue-specific differences in MK4 and PK concentrations (4, 41, 49). A strength of this study is the exclusive use of PK as the dietary source of vitamin K, which mimics the human condition. Use of radiolabeled or stable isotopes in animal models has indicated that menadione, which is the predominant provitamin K form in unpurified diet for rodents, also is converted to MK4 (29, 50, 51). Menadione in the unpurified diet is continually being utilized in the conversion of MK4, which results in high MK4 concentrations in the liver. In contrast, studies in animals that are exclusively fed PK result in accumulation of PK in the liver (4, 52, 53). Of note, menadione is not present in the food supply, nor is it allowed in the human diet because of concerns of hepatic toxicity (2). The purpose of this study was to examine the effect of inducing vitamin K deficiency by use of housing techniques and diet manipulation. We only used young mice in this study to determine whether vitamin K deficiency was inducible. It is plausible that age is an important biological variable contributing to differences in response to dietary intervention; thus future studies may consider the use of aging mouse models to assess the role of age as a biological factor influencing vitamin K status. The inclusion of both male and female mice in our study is a substantial strength and is important in regard to preclinical models and the translatability to human conditions. Recent NIH mandates support the use of both sexes in animal studies to identify differences in response to various interventions and treatments (12). Our data support the need to use both sexes in vitamin K studies.
In conclusion, there are significant sex-specific differences in tissue concentrations of PK, MK4, and some long-chain MKs. These results and concurrent sex-specific differences in gene expression of vitamin K–related genes indicate that male and female mice respond differently to dietary vitamin K manipulation. Recent discoveries of novel, to our knowledge, vitamin K–dependent proteins that may function in multiple tissues other than the liver highlight a need to understand tissue-specific differences in vitamin K metabolism and identify potential sex-specific differences in vitamin K–dependent protein function or location. To test the hypothesis that tissue specificity may be indicative of vitamin K form-specific function, future research must consider sex-specific differences in preclinical animal models of vitamin K nutrition and metabolism.
Acknowledgments
SGH, XF, DS, and SLB designed the research; SGH, XF, JPK, DS, and XS conducted the research; SGH and KB analyzed the data; SGH and SLB wrote the paper; XF, JPK, KB, SL-F, AK, and ASG reviewed the data, aided in the interpretation of the results, and reviewed the manuscript; and SLB had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations: Ggcx, γ glutamyl carboxylase; MK, menaquinone; PK, phylloquinone; Vkorc1, vitamin K expoxide reductase complex 1; Vkorc1l1, vitamin K epoxide reductase complex 1-like 1; Ubiad1, ubiA domain–containing protein 1.
References
- 1.USDA ARS. USDA National Nutrient Database for Standard Reference, Release 26 [Internet]. 2013. [cited 2015 May 25] Available from: http://www.ars.usda.gov/nutrientdata.
- 2.Institute of Medicine Panel on Micronutrients. Dietary reference intakes for vitamin K, arsenic, chromium, copper, iodine, iron, manganese, molybdenum, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. [PubMed]
- 3.Beulens JWJ, Booth SL, van den Heuvel EGHM, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr 2013;110:1357–68. [DOI] [PubMed] [Google Scholar]
- 4.Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr 2012;142:841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirota Y, Tsugawa N, Nakagawa K, Suhara Y, Tanaka K, Uchino Y, Takeuchi A, Sawada N, Kamao M, Wada A, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem 2013;288:33071–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickerson ML, Bosley AD, Weiss JS, Kostiha BN, Hirota Y, Brandt W, Esposito D, Kinoshita S, Wessjohann L, Morham SG, et al. The UBIAD1 prenyltransferase links menaquinone-4 [corrected] synthesis to cholesterol metabolic enzymes. Hum Mutat 2013;34:317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegarty JM, Yang H, Chi NC. UBIAD1-mediated vitamin K2 synthesis is required for vascular endothelial cell survival and development. Development 2013;140:1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conly JM, Stein K, Worobetz L, Rutledge-Harding S. The contribution of vitamin K2 (menaquinones) produced by the intestinal microflora to human nutritional requirements for vitamin K. Am J Gastroenterol 1994;89:915–23. [PubMed] [Google Scholar]
- 9.Groenen-van Dooren MM, Ronden JE, Soute BA, Vermeer C. Bioavailability of phylloquinone and menaquinones after oral and colorectal administration in vitamin K-deficient rats. Biochem Pharmacol 1995;50:797–801. [DOI] [PubMed] [Google Scholar]
- 10.Walther B, Karl JP, Booth SL, Boyaval P. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr 2013;4:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev 1981;45:316–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014;509:282–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanham SA, Cagampang FR, Oreffo ROC. Maternal high-fat diet and offspring expression levels of vitamin K-dependent proteins. Endocrinology 2014;155:4749–61. [DOI] [PubMed] [Google Scholar]
- 14.Lacombe J, Karsenty G, Ferron M. In vivo analysis of the contribution of bone resorption to the control of glucose metabolism in mice. Mol Metab 2013;2:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poon CCW, Li RWS, Seto SW, Kong SK, Ho HP, Hoi MPM, Lee SMY, Ngai SM, Chan SW, Leung GPH, et al. In vitro vitamin K2 and 1α,25-dihydroxyvitamin D3 combination enhances osteoblasts anabolism of diabetic mice. Eur J Pharmacol 2015;767:30–40. [DOI] [PubMed] [Google Scholar]
- 16.Azuma K, Shiba S, Hasegawa T, Ikeda K, Urano T, Horie-Inoue K, Ouchi Y, Amizuka N, Inoue S. Osteoblast-specific γ-glutamyl carboxylase-deficient mice display enhanced bone formation with aberrant mineralization. J Bone Miner Res 2015;30:1245–54. [DOI] [PubMed] [Google Scholar]
- 17.Butschkau A, Wagner N-M, Bierhansl L, Genz B, Vollmar B. Protein Z-deficiency is associated with enhanced neointima formation and inflammatory response after vascular injury in mice. Int J Clin Exp Pathol 2014;7:6064–71. [PMC free article] [PubMed] [Google Scholar]
- 18.Shiba S, Ikeda K, Azuma K, Hasegawa T, Amizuka N, Horie-Inoue K, Inoue S. γ-Glutamyl carboxylase in osteoblasts regulates glucose metabolism in mice. Biochem Biophys Res Commun 2014;453:350–5. [DOI] [PubMed] [Google Scholar]
- 19.Ferron M, Lacombe J, Germain A, Oury F, Karsenty G. GGCX and VKORC1 inhibit osteocalcin endocrine functions. J Cell Biol 2015;208:761–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspers M, Czogalla KJ, Liphardt K, Müller J, Westhofen P, Watzka M, Oldenburg J. Two enzymes catalyze vitamin K 2,3-epoxide reductase activity in mouse: VKORC1 is highly expressed in exocrine tissues while VKORC1L1 is highly expressed in brain. Thromb Res 2015;135:977–83. [DOI] [PubMed] [Google Scholar]
- 21.Mameesh MS, Johnson B. Production of dietary vit. K deficiency in the rat. Proc Soc Exp Biol Med 1959;101:467–8. [DOI] [PubMed] [Google Scholar]
- 22.Metta VC, Johnson B. Effect of feeding vitamin K-deficient diets to female rats. J Nutr 1960;72:455–8. [DOI] [PubMed] [Google Scholar]
- 23.Barnes RH, Fiala G. Effects of the prevention of coprophagy in the rat. J Nutr 1959;68:603–14. [DOI] [PubMed] [Google Scholar]
- 24.Huber AM. Gender differences in hepatic phylloquinone and menaquinones in the vitamin K-deficient and -supplemented rat. Biochim Biophys Acta 1999;1426:43–52. [DOI] [PubMed] [Google Scholar]
- 25.Matschiner JT, Bell RG. Effect of sex and sex hormones on plasma prothrombin and vitamin K deficiency. Proc Soc Exp Biol Med 1973;144:316–20. [DOI] [PubMed] [Google Scholar]
- 26.Jolly DW, Craig C, Nelson TE. Estrogen and prothrombin synthesis: effect of estrogen on absorption of vitamin K1. Am J Physiol 1977;232:H12–7. [DOI] [PubMed] [Google Scholar]
- 27.Barnes RH. Nutritional implications of coprophagy. Nutr Rev 1962;20:289–91. [DOI] [PubMed] [Google Scholar]
- 28.Office of Laboratory Animal Welfare. Public Health Service Policy on Humane Care and Use of Laboratory Animals [Internet]. National Institutes of Health: Office of Extramural Research. 2002 [cited 2014 Oct 20]. Available from: http://grants.nih.gov/grants/olaw/references/phspol.htm.
- 29.Fu X, Booth SL, Smith DE. Vitamin K contents of rodent diets: a review. J Am Assoc Lab Anim Sci 2007;46:8–12. [PubMed] [Google Scholar]
- 30.Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2014;963:128–33. [DOI] [PubMed] [Google Scholar]
- 31.McCabe KM, Booth SL, Fu X, Shobeiri N, Pang JJ, Adams MA, Holden RM. Dietary vitamin K and therapeutic warfarin alter the susceptibility to vascular calcification in experimental chronic kidney disease. Kidney Int 2013;83:835–44. [DOI] [PubMed] [Google Scholar]
- 32.Booth SL, Peterson JW, Smith D, Shea MK, Chamberland J, Crivello N. Age and dietary form of vitamin K affect menaquinone-4 concentrations in male Fischer 344 rats. J Nutr 2008;138:492–6. [DOI] [PubMed] [Google Scholar]
- 33.Tovar A, Ameho CK, Blumberg JB, Peterson JW, Smith D, Booth SL. Extrahepatic tissue concentrations of vitamin K are lower in rats fed a high vitamin E diet. Nutr Metab (Lond) 2006;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davidson KW, Sadowski JA. Determination of vitamin K compounds in plasma or serum by high-performance liquid chromatography using postcolumn chemical reduction and fluorimetric detection. Methods Enzymol 1997;282:408–21. [DOI] [PubMed] [Google Scholar]
- 35.Cui W. qPrimerDepot: a quantitative real time PCR primer database [Internet]. National Cancer Institute, National Institutes of Health. [cited 2015 Aug 10]. Available from: http://mouseprimerdepot.nci.nih.gov/.
- 36.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milanov P, Ivanciu L, Abriss D, Quade-Lyssy P, Miesbach W, Alesci S, Tonn T, Grez M, Seifried E, Schüttrumpf J. Engineered factor IX variants bypass FVIII and correct hemophilia A phenotype in mice. Blood 2012;119:602–11. [DOI] [PubMed] [Google Scholar]
- 38.Garrido P, de Blas M, Del Arco A, Segovia G, Mora F. Aging increases basal but not stress-induced levels of corticosterone in the brain of the awake rat. Neurobiol Aging 2012;33:375–82. [DOI] [PubMed] [Google Scholar]
- 39.Knauer TE, Siegfried CM, Matschiner JT. Vitamin K requirement and the concentration of vitamin K in rat liver. J Nutr 1976;106:1747–51. [DOI] [PubMed] [Google Scholar]
- 40.Yardimci M, Sevgiler Y, Rencuzogullari E, Arslan M, Buyukleyla M, Yilmaz M. Sex-, tissue-, and exposure duration-dependent effects of imidacloprid modulated by piperonyl butoxide and menadione in rats. Part I: oxidative and neurotoxic potentials. Arh Hig Rada Toksikol 2014;65:387–98. [DOI] [PubMed] [Google Scholar]
- 41.Thijssen HH, Drittij-Reijnders MJ, Fischer MA. Phylloquinone and menaquinone-4 distribution in rats: synthesis rather than uptake determines menaquinone-4 organ concentrations. J Nutr 1996;126:537–43. [DOI] [PubMed] [Google Scholar]
- 42.Huber AM, Davidson KW, O’Brien-Morse ME, Sadowski JA. Tissue phylloquinone and menaquinones in rats are affected by age and gender. J Nutr 1999;129:1039–44. [DOI] [PubMed] [Google Scholar]
- 43.Truong JT, Fu X, Saltzman E, Al Rajabi A, Dallal GE, Gundberg CM, Booth SL. Age group and sex do not influence responses of vitamin K biomarkers to changes in dietary vitamin K. J Nutr 2012;142:936–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang Y, Park YE, Yun C-W, Kim D-H, Chung H. The vest-collar as a rodent collar to prevent licking and scratching during experiments. Lab Anim 2015. Oct 8 (Epub ahead of print; DOI: 10.1177/0023677215610971). [DOI] [PubMed] [Google Scholar]
- 45.Hammed A, Matagrin B, Spohn G, Prouillac C, Benoit E, Lattard V. VKORC1L1, an enzyme rescuing the vitamin K 2,3-epoxide reductase activity in some extrahepatic tissues during anticoagulation therapy. J Biol Chem 2013;288:28733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Westhofen P, Watzka M, Marinova M, Hass M, Kirfel G, Müller J, Bevans CG, Müller CR, Oldenburg J. Human vitamin K 2,3-epoxide reductase complex subunit 1-like 1 (VKORC1L1) mediates vitamin K-dependent intracellular antioxidant function. J Biol Chem 2011;286:15085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oldenburg J, Watzka M, Bevans CG. VKORC1 and VKORC1L1: why do vertebrates have two vitamin K 2,3-epoxide reductases? Nutrients 2015;7:6250–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010;468:117–21. [DOI] [PubMed] [Google Scholar]
- 49.Koivu-Tikkanen TJ, Schurgers LJ, Thijssen HH, Vermeer C. Intestinal, hepatic, and circulating vitamin K levels at low and high intakes of vitamin K in rats. Br J Nutr 2000;83:185–90. [PubMed] [Google Scholar]
- 50.Martius C. The metabolic relationships between the different K vitamins and the synthesis of the ubiquinones. Am J Clin Nutr. Am J Clin Nutr 1961;9:97–103. [DOI] [PubMed] [Google Scholar]
- 51.Davidson RT, Foley AL, Engelke JA, Suttie JW. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J Nutr 1998;128:220–3. [DOI] [PubMed] [Google Scholar]
- 52.Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem 2008;283:11270–9. [DOI] [PubMed] [Google Scholar]
- 53.Thijssen HH, Drittij-Reijnders MJ. Vitamin K distribution in rat tissues: dietary phylloquinone is a source of tissue menaquinone-4. Br J Nutr 1994;72:415–25. [DOI] [PubMed] [Google Scholar]