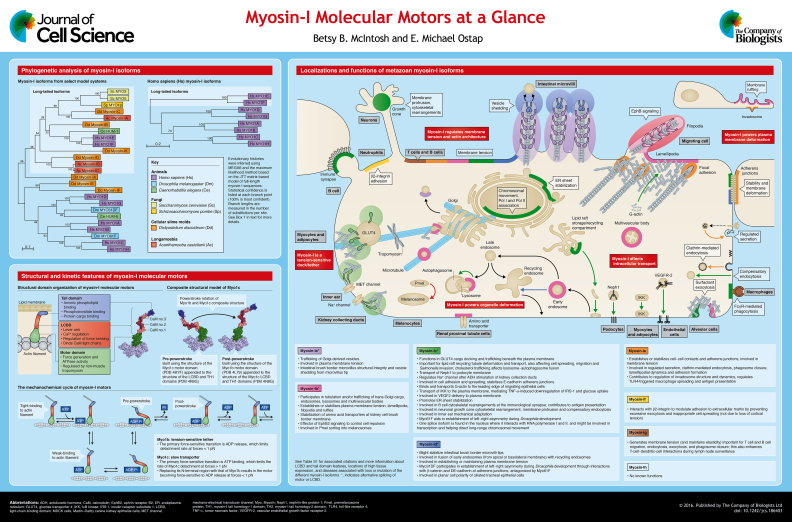
ABSTRACT
Myosin-I molecular motors are proposed to play various cellular roles related to membrane dynamics and trafficking. In this Cell Science at a Glance article and the accompanying poster, we review and illustrate the proposed cellular functions of metazoan myosin-I molecular motors by examining the structural, biochemical, mechanical and cell biological evidence for their proposed molecular roles. We highlight evidence for the roles of myosin-I isoforms in regulating membrane tension and actin architecture, powering plasma membrane and organelle deformation, participating in membrane trafficking, and functioning as a tension-sensitive dock or tether. Collectively, myosin-I motors have been implicated in increasingly complex cellular phenomena, yet how a single isoform accomplishes multiple types of molecular functions is still an active area of investigation. To fully understand the underlying physiology, it is now essential to piece together different approaches of biological investigation. This article will appeal to investigators who study immunology, metabolic diseases, endosomal trafficking, cell motility, cancer and kidney disease, and to those who are interested in how cellular membranes are coupled to the underlying actin cytoskeleton in a variety of different applications.
KEY WORDS: Myosin-I, Molecular motors, Membrane tension, Intracellular trafficking, Tension-sensitivity, Membrane deformation
Summary: Here, we review and illustrate the proposed cellular functions of metazoan myosin-I molecular motors by examining the structural, biochemical, mechanical and cell biological evidence for their proposed molecular roles.
Introduction
Myosin-I proteins were first discovered in lower eukaryotes (Pollard and Korn, 1973), but are recognized to be widely expressed. Higher vertebrates express eight different myosin-I genes Myo1a–Myo1h, with the corresponding proteins named Myo1a–Myo1h (for a discussion of myosin-I gene and protein nomenclature, see Gillespie et al., 2001) (see poster and Box 1 for more information about phylogenetic sorting). Myosin-I molecular motors are comprised of a motor domain that binds to and interacts with actin in response to ATPase cycling, a light-chain-binding domain (LCBD) that binds one to six Ca2+-sensitive calmodulin or calmodulin-like light chains and functions as a lever arm (Bähler et al., 1994; Köhler et al., 2005; Lin et al., 2005; Manceva et al., 2007; McConnell and Tyska, 2010; Ruppert et al., 1993; Sherr et al., 1993; Sielski et al., 2014; Stöffler and Bähler, 1998; Swanljung-Collins and Collins, 1991), and a tail domain (see Structural and kinetic features panel on poster). The tail domain is composed of a myosin-I family tail homology 1 (TH1) domain, which includes a pleckstrin homology (PH) domain known to bind a variety of anionic phospholipids (Adams and Pollard, 1989; Doberstein and Pollard, 1992; Feeser et al., 2010; Hayden et al., 1990; Hokanson et al., 2006; Miyata et al., 1989). Myosin-I motors are generally classified into short-tailed (Myo1a, Myo1b, Myo1c, Myo1d, Myo1g and Myo1h) or long-tailed (Myo1e, Myo1f) groups based on the presence of additional glycine-rich (TH2) and SH3 (TH3) domains in the long-tailed isoforms. The eight isoforms evolved in pairs from four precursor motors, grouping Myo1e and Myo1f, Myo1d and Myo1g, Myo1a and Myo1b, and Myo1c and Myo1h (see phylogenetic tree panel on poster).
Box 1. Generation of statistically relevant evolutionary trees of the myosin-I molecular motor family in eukaryotes and Homo sapiens.
The statistically relevant evolutionary histories shown on the poster (‘Phylogenetic analysis’ panel) were inferred using the program MEGA6, by using the Maximum Likelihood method based on the JTT matrix-based model (Jones et al., 1992; Tamura et al., 2013). The trees with the highest log likelihood (left, −20410.6144; right, −14298.3803) are shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches, where values closer to 100% represent higher statistical confidence. Initial tree(s) for the heuristic search were obtained by applying the neighbor-joining method to a matrix of pairwise distances estimated using a JTT model. The trees are drawn to scale, with branch lengths measured in the number of substitutions per site. All positions containing gaps and missing data were eliminated. For the tree including multiple eukaryotic isoforms, the analysis involved 25 full-length amino acid sequences including all myosin-I isoforms from Homo sapiens (Hs) (MYO1A–MYO1H), Drosophila melanogaster (Dm) (Myo31DF, Myo61F), Caenorhabditis elegans (Ce) (HUM-1, HUM-5), Saccharomyces cerevisae (Sc) (Myo3, Myo5), Schizosaccharomyces pombe (Sp) (Myo1), Dictyostelium discoideum (Dd) (MyoA–MyoF, MyoK) and Acanthamoeba castellani (Ac) (Myosin-IA, Myosin-IB, Myosin-IC). There were a total of 569 positions compared in the final dataset. For the Homo sapiens MYO1 tree, the analysis involved the eight full-length Homo sapiens myosin-I isoform amino acid sequences, comparing a total of 946 positions in the final data set. Sequences used in this comparison were MYO1A (GenBank NP_001242970.1), MYO1B motor a (GenBank NP_001123630.1), MYO1C isoform a (GenBank NP_001074248.1), MYO1D isoform 1 (GenBank NP_056009.1), MYO1E (GenBank AAH98392.1), MYO1F (GenBank NP_036467.2), MYO1G (GenBank NP_149043.2) and MYO1H (GenBank NP_001188784.1).
The cellular localization of myosin-I isoforms depends both on the preference of their motor domains for different actin filament populations, as well as for specific anionic phospholipids found on different cellular membranes (Ruppert et al., 1995) (see Box 2 and Table S1 for information about tissue localization of different isoforms). In vitro and in vivo data suggests that myosin-I motors avoid tropomyosin-coated actin filaments (Collins et al., 1990; Kee et al., 2015; McIntosh et al., 2015; Tang and Ostap, 2001) and instead prefer Arp2/3-nucleated (Almeida et al., 2011) and non-tropomyosin-coated cytoskeletal actin. Although Myo1a, Myo1b, Myo1c and Myo1g have been found to preferentially bind to phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], all characterized myosin-I isoforms can bind to anionic phospholipids to some extent (Adams and Pollard, 1989; Dart et al., 2012; Doberstein and Pollard, 1992; Feeser et al., 2010; Hayden et al., 1990; Hokanson and Ostap, 2006; Hokanson et al., 2006; Komaba and Coluccio, 2010; McKenna and Ostap, 2009; Miyata et al., 1989; Patino-Lopez et al., 2010). Thus, these preferences of the motor domains and tail domains result in the predominant localization of myosin-I to the plasma membrane, with additional binding to intracellular organelles. However, it is unclear how specific myosin-I isoforms establish their individual localizations. One hypothesis is that subcellular localizations are determined in part by protein-binding partners. Although individual binding partners have been discovered (see below), there is little evidence that these proteins indeed direct myosin-I localization or have any isoform specificity. In this Cell Science at a Glance article, we focus on metazoan myosin-I research, with a particular emphasis on the molecular roles of the myosin-I isoforms in each case.
Box 2. Myosin-I tissue expression and disease phenotypes.
Myosin-I isoforms show varying expression levels across different tissues (see supplemental table). Myo1b, Myo1c, and Myo1e are widely expressed and found in most cell types (Krendel et al., 2009; Ruppert et al., 1993; Sherr et al., 1993; Sielski et al., 2014; Skowron et al., 1998; Tyska et al., 2005). Loss of Myo1c or Myo1e is associated with kidney disease (Arif et al., 2011; Bi et al., 2013; Krendel et al., 2009; Mele et al., 2011; Wagner et al., 2005), in addition to deafness associated with Myo1c loss (Batters et al., 2004; Holt et al., 2002) and arteriosclerosis upon loss of Myo1e (Inouye et al., 2012). Mutations in Myo1a are also associated with hearing loss (Donaudy et al., 2003), although Myo1a is most highly expressed in the small intestines and colon where it is associated with colon cancer (Mazzolini et al., 2012, 2013; Skowron et al., 1998). Myo1d is highly expressed in the central and peripheral nervous system (Bähler et al., 1994; Benesh et al., 2012; Cahoy et al., 2008; Nielsen et al., 2006; Sherr et al., 1993), liver and small intestines (Bähler et al., 1994; Benesh et al., 2010), and has been linked to autism spectrum disorders (Stone et al., 2007). Myo1f and Myo1g are predominantly expressed in hematopoietic cells, where Myo1e is also found (Diakonova et al., 2002; Hao et al., 2008; Kim et al., 2006; Nebl et al., 2002; Olety et al., 2010; Wenzel et al., 2015). Myo1f is the third myosin-I isoform that has been associated with deafness (Baek et al., 2012; Chen et al., 2001) and also linked to acute monocytic leukemia (Taki et al., 2005). Myo1h is highly expressed in the testis, with considerably lower expression in adipocytes and the heart (Fishilevich et al., 2016), and has been implicated as a marker associated with the mandibular prognathism phenotype (Tassopoulou-Fishell et al., 2012). Although loss of myosin-I motors is associated with many disorders, there is evidence that partial rescue and overlapping functions by closely related myosin-I isoforms minimizes the observed cellular and whole-animal knockdown phenotypes (Tyska et al., 2005), consistent with well-documented examples in lower eukaryotes (Jung et al., 1996; Novak et al., 1995; Ostap and Pollard, 1996).
Myosin-I regulates membrane tension, cell adhesion, and actin architecture
The presence of a lipid-binding region in the tail and an actin-binding region in the motor domain equips myosin-I motors for cellular roles that link membranes to the actin cytoskeleton. Notably, seminal work with lower eukaryotes has demonstrated a crucial role for myosin-I in setting resting cortical tension through this membrane–actin link (Dai et al., 1999). Additionally, all vertebrate myosin-I isoforms, except Myo1h, have been studied with regard to cellular membrane tension owing to their contribution to membrane–cytoskeleton adhesion (see poster). For instance, the individual overexpression of Myo1a, Myo1b, Myo1c, Myo1d, or Myo1e causes an increase in the force required to pull a tether from the plasma membrane of NIH 3T3 fibroblasts using optical tweezers, whereas overexpression of a membrane-binding dominant-negative construct of Myo1a decreases the tether force required (Nambiar et al., 2009). This method of pulling on the plasma membrane probes the tension of the membrane as established by lipid and protein composition, as well as links to the cortical actin network (Gauthier et al., 2012). These data are supported by whole-animal studies. For example, the deletion of Myo1a in knockout mice results in membrane herniation in intestinal epithelial cells due to this decreased membrane–actin attachment (Tyska et al., 2005), and the loss of Myo1b leads to increased plasma membrane blebbing, reduced cell movement directionality and reduced net speed in the developing mesoderm of zebrafish embryos (Diz-Muñoz et al., 2010).
Myo1g, which is highly expressed in T cells, has also been shown to have a role in the maintenance of membrane tension, which is important for T cell migration and enhanced interaction between T cells and dendritic cells during lymph node surveillance (Gérard et al., 2014). Additionally, by utilizing an atomic force microscope to investigate cortical and membrane tension, researchers have found that loss of Myo1g in B-lymphocytes leads to decreased cell stiffness due to a loss of cortical tension, again affecting cell adhesion, spreading, phagocytosis and endocytosis (López-Ortega et al., 2016). Surprisingly, another study has found that depletion of Myo1f did not result in decreased cortical tension of neutrophils, as measured by micropipette aspiration, which is the first example of a myosin-I motor that is not involved in membrane and/or cortical tension (Kim et al., 2006). It is interesting that Myo1e, the other long-tailed myosin-I isoform, is required for plasma membrane tension, whereas the closely related Myo1f isoform is not involved in cortical tension; cortical tension is often one of the main determinants of plasma membrane tension generation and maintenance (Gauthier et al., 2012). The structural features that give rise to these functional differences between myosin-I isoforms are still unclear.
Myo1c, Myo1d and Myo1e have been found to contribute to the stability of cell–cell adhesion at adherens junctions, and Myo1e has been found at focal adhesions (Bi et al., 2013; Gupta et al., 2013; Hegan et al., 2015; Oh et al., 2013; Petzoldt et al., 2012; Spéder et al., 2006; Stöffler and Bähler, 1998; Stöffler et al., 1995; Tokuo and Coluccio, 2013). Myo1c localizes to E-cadherin-rich areas in cell–cell contacts between Madin-Darby canine kidney epithelial (MDCK) cells and contributes to the stability of these junctions, although it is unclear how Myo1c functionally establishes and/or maintains this stability (Tokuo and Coluccio, 2013). The Drosophila homolog of Myo1d, Myo31DF, has been shown to localize to adherens junctions, where it binds to both β-catenin and DE-cadherin (Petzoldt et al., 2012; Spéder et al., 2006). Here, Myo31DF functions with its antagonist Myo61F, the Drosophila Myo1c homologue, to pattern left–right visceral asymmetry (Petzoldt et al., 2012; Spéder et al., 2006). Interestingly, mouse Myo1c has been shown to power asymmetric actin filament gliding in an in vitro motility assay, which has been provocatively suggested to be related to this organ-patterning asymmetry (Pyrpassopoulos et al., 2012). In rats, Myo1d function at adherens junctions influences the establishment and/or maintenance of rotational planar cell polarity in ciliated tracheal and ependymal epithelial cells, but does not do so in all tissues that exhibit planar cell polarity (Hegan et al., 2015). Myo1e localizes to sites of actin polymerization and adhesion in lamellipodia, thereby influencing adhesion formation and actin dynamics through localization of its binding partners (Gupta et al., 2013; Stöffler et al., 1995). Myo1e also localizes to and regulates slit junctions, the highly specialized cell–cell contacts found in the glomerulus of kidney podocyte cells, suggesting why its absence results in kidney disease (Bi et al., 2013; Mele et al., 2011). Overall, it is still unclear whether these myosin-I-mediated adhesion functions can be separated from the roles of the same isoforms in the generation and maintenance of membrane and/or cortical tension.
The expression of Myo1a, Myo1c, Myo1d and Myo1e has been shown to affect actin filament architecture. Myo1a knockdown in mice results in intestinal microvilli of irregular length (Tyska et al., 2005). Myo1d localizes to tips of microvilli; however, it is unclear whether Myo1d directly influences the length, composition and/or integrity of actin filaments in this location (Benesh et al., 2010). Myo1c more directly regulates actin architecture by influencing cytoskeletal rearrangement in the neuronal growth cone (Wang et al., 2003), in B cells at the immunological synapse (Maravillas-Montero et al., 2011), and possibly by facilitating G-actin transport to the leading edge of migrating epithelial cells (Fan et al., 2012). Myo1e has been found to be a core component of cancer invadosomes, actin-rich adhesions structures important for degradation and invasion of extracellular matrix; here, Myo1e recruitment to newly forming invadosomes precedes that of actin and paxillin (Ouderkirk and Krendel, 2014). Finally, Myo1e has been proposed to be involved in clathrin-mediated endocytosis by recruiting the actin-polymerization factors neural Wiskott–Aldrich syndrome protein (N-WASP, also known as WASL), WASP-interacting Protein (WIP, also known as WIPF1), and WASP-binding protein (WIRE, also known as WIPF2), as well as other clathrin-mediated endocytic proteins, such as synaptojanin-1 and dynamin (Cheng et al., 2012; Krendel et al., 2007). Thus, the ability of myosin-I motors to influence actin dynamics might be both direct, through binding to the motor domain and transport of G-actin, and indirect, by recruiting factors that are involved in nucleation, polymerization and stabilization of actin filaments.
Myosin-I and intracellular trafficking
In addition to affecting membrane tension, cell adhesion and actin dynamics, Myo1a, Myo1b, Myo1c, Myo1e and Myo1g have been found to participate in exocytosis, endocytosis, intracellular membrane trafficking and nuclear organization (see poster). Myo1a is present on the cytoplasmic side of Golgi-derived vesicles where it might be operating as a transporter near the Golgi or within microvilli (Fath and Burgess, 1993; Fath et al., 1994; Kravtsov et al., 2012; Skowron et al., 1998). Myo1e has been proposed to have a role in the regulated secretion of cortical granules in Xenopus oocytes (Schietroma et al., 2007). Myo1c has been found to play a role in the exocytosis of VEGF2 (Tiwari et al., 2013), IκB kinase (IKK) (Nakamori et al., 2006) and Neph1 (Arif et al., 2011), and in the recycling of lipid raft cargo toward the plasma membrane (Brandstaetter et al., 2012). In what might prove to be an entirely distinct role, Myo1c splice variants have been found in the nucleus where they are proposed to interact with RNA polymerase I and II and either directly or indirectly participate in signaling to enhance long-range chromosomal movement (Chuang et al., 2006; Kyselá et al., 2005; Percipalle et al., 2006; Pestic-Dragovich et al., 2000; Philimonenko et al., 2004).
Myo1b localizes to multivesicular sorting bodies, endosomes and lysosomes (Almeida et al., 2011; Cordonnier et al., 2001; Raposo et al., 1999; Salas-Cortes et al., 2005). Myo1b has also been found to be involved in the sorting of Pmel, which is important for melanosome maturation (Salas-Cortes et al., 2005). Myo1c is important for compensatory endocytosis in Xenopus oocytes, where it is thought to couple force-generating actin filaments to the plasma membrane (Sokac et al., 2006). Recently, both Myo1b and Myo1c have been shown to play a role in actin coat compression where they are recruited to lamellar bodies of rat alveolar type II cells after membrane fusion (Kittelberger et al., 2016). Interestingly, knockdown of Myo1b increases actin contraction rates, whereas loss of Myo1c decreases the rate of contraction of the lamellar body actin coat, thus suggesting that whereas Myo1c actively contracts the actin coat to expel surfactant proteins, Myo1b might act to slow contraction, thus fine-tuning the kinetics of this process. In addition to the effects of Myo1e on actin dynamics, Myo1e knockdown results in decreased early endosomal transport of transferrin toward the perinuclear region (Cheng et al., 2012). Both Myo1e and Myo1g (Dart et al., 2012; Swanson et al., 1999), but not Myo1f (Kim et al., 2006), have been found to have a role in phagocytosis and phagosome closure, again alluding to very different functions for the two closely related long-tailed myosin-I isoforms Myo1e and 1f. Although myosin-I motors have been repeatedly implicated in trafficking processes, conclusive cellular evidence of myosin-I-driven transport along actin filaments, rather than acting to sort or deform membranes, is still needed.
Myosin-I is a molecular dock or tether
Both Myo1b and Myo1c have been suggested to function as anchors or tethers between membranes and/or other proteins, as well as actin filament tracks (see poster). For example, Myo1c has been proposed to facilitate docking of GLUT4-containing vesicles at the plasma membrane prior to fusion in response to insulin stimulation (Boguslavsky et al., 2012; Bose et al., 2002, 2004; Chen et al., 2007; Huang et al., 2005; Yip et al., 2008). In vitro work suggests that Myo1c is in fact capable of halting processive microtubule-based transport at actin intersections, which not only implicates Myo1c in cargo docking, but also connects microtubule- and actin-based transport pathways (McIntosh et al., 2015). Additionally, in vitro and cellular studies have shown that cargo docking by Myo1c is regulated by the presence of non-muscle tropomyosin, which might spatially regulate the location of cargo docking to tropomyosin-free filaments just beneath the plasma membrane (Kee et al., 2015; McIntosh et al., 2015). Myo1c has also been implicated in the mechanical adaptation of signaling in inner ear hair cells (Batters et al., 2004; Gillespie et al., 1993; Holt et al., 2002; Lin et al., 2011), as well as in the regulation of a Na+ channel after antidiuretic hormone stimulation in the kidney collection ducts (Wagner et al., 2005). Myo1b has been proposed to tether amino acid transporters to the apical plasma membrane of kidney cells, thereby facilitating neutral amino acid transport across the membrane; however, more investigation is needed to differentiate this hypothesis from other potential roles, such as facilitating membrane fusion of vesicles containing amino acid transporters, and/or mediating the transport and/or sorting of these cargo to the apical plasma membrane (Komaba and Coluccio, 2015). Similarly, Myo1a is important for the retention and/or localization of sucrose isomaltase in the intestinal brush border membrane, although it is again unclear whether it is the trafficking, sorting or docking functions that are most important (Tyska and Mooseker, 2004).
Biochemical kinetic, structural and single-molecule experiments initially led to the proposal that Myo1b can act as a tension-sensitive motor (Coluccio and Geeves, 1999; Veigel et al., 1999); more recently, direct evidence has shown that when Myo1b goes through its mechanochemical cycle while experiencing low resistive load, when its ATP-dependent actin-detachment rate is slowed by nearly two orders-of-magnitude (Laakso et al., 2008, 2010). In other words, Myo1b is termed a ‘tension-sensitive tether’ because when experiencing no mechanical force, the motor detaches from actin filaments within a second after binding to actin and undergoing its powerstroke; however, under resistive loads as low as 1 pN, Myo1b remains anchored to actin filaments for nearly 100 s. These low forces are well within the range of those expected to take place during intracellular transport and mechanotransduction (Gillespie and Cyr, 2004; Hendricks et al., 2012; Soppina et al., 2009). Recent structural and biophysical studies have shown that the tension sensitivity of Myo1b is due in part to a structural element located within the N-terminus of the motor (Greenberg et al., 2015; Shuman et al., 2014). In contrast, despite similar biochemical properties and working stroke sizes, the ATP-dependent actin-detachment kinetics of Myo1c are largely independent of forces that are smaller than 2 pN. Cellular studies have yet to clarify how the remarkable tension-sensitivity of Myo1b translates into distinct functional roles from Myo1c; however, the discrete cellular functions and localizations of Myo1b and Myo1c, despite similar tissue expression patterns, suggest a physiological relevance for these mechanical-load-dependent differences.
Myosin-I powers membrane deformation
Given that myosin-I isoforms link membranes to the actin cytoskeleton, they are ideally poised to provide tension, deform the plasma membrane and participate in tubulation of organelle membranes (see poster). Indeed, Myo1a is instrumental for vesicular shedding off the tip of microvilli in the intestines, which is important for membrane turnover, microvillar health and antimicrobial hydrolase release into the intestinal lumen (McConnell and Tyska, 2007; McConnell et al., 2009). Myo1b is associated with a wide range of plasma membrane geometries, including cell protrusions (Komaba and Coluccio, 2010), lamellipodia, membrane ruffles, filopodia and the cleavage furrow of dividing cells (Lewis and Bridgman, 1996; Ruppert et al., 1995; Tang and Ostap, 2001). However, it is still unclear how exactly Myo1b affects these plasma membrane geometries. One known cellular function for Myo1b in filopodia is in ephrin receptor B2 (EphB2) signaling for cell–cell repulsion of Hek293 cells, which is important for tissue patterning and homeostasis (Prospéri et al., 2015). Nevertheless, more work is still required to elucidate the molecular functions and relevance of these Myo1b localizations.
Beyond facilitating plasma membrane deformations, Myo1b has been shown to participate in the formation of post-Golgi carriers from the trans-Golgi in conjunction with processive microtubule-based motors (Almeida et al., 2011). Additionally, a recent study has demonstrated that Myo1b alone can tubulate giant unilamellar vesicles along fascin-bundled actin in an in vitro reconstitution assay (Yamada et al., 2014). Similarly, Myo1c has been found to participate in the tubulation and recycling of glycosylphosphatidylinositol-anchored lipid-raft-rich membrane components toward the plasma membrane (Brandstaetter et al., 2012), thereby influencing cholesterol-dependent lysosome and autophagosome fusion (Brandstaetter et al., 2014). Myo1d is involved in the fusion of organelle membranes, in particular, the fusion of early endosomes from the apical or basolateral membrane with recycling endosomes (Huber et al., 2000). Finally, Myo1c has been found to promote ER sheet stabilization rather than reticular patterning, likely by coupling actin dynamics to membrane geometry (Joensuu et al., 2014). In all of these intracellular membrane deformations, however, it is unclear whether myosin-I affects membrane deformation directly, by producing the force required to deform the membrane, or provides the resistive anchoring that is necessary for other motors to power the tubulation event. Further investigation is therefore needed to clarify how myosin-I motors interact with processive actin- and microtubule-based motors, the local cytoskeleton, and other factors involved in membrane deformation such as Bin, Amphiphysin and Rvs (BAR) domain proteins to induce membrane deformation within the cell.
Conclusions
The myosin-I family of molecular motors consists of eight different isoforms that participate in a wide range of cell biological processes that require generation or regulation of membrane tension, formation of cell adhesions and changes in the actin architecture. Additionally, myosin-I motors affect intracellular trafficking, function as tension-sensitive docks or tethers and power membrane deformation. More work is needed to understand how these myosin-I motors are targeted to their site of action (based on tail domain and motor domain preferences) and function to accomplish these distinct and varied cellular tasks. In order to fully understand the underlying physiology, a continued interdisciplinary approach is required to integrate the cell biological, biochemical, biophysical and structural features of myosin-I molecular motors.
Acknowledgements
The authors would like to thank Michael Woody, Michael Greenberg, and the rest of the Ostap Laboratory for helpful discussions during preparation of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
Our research was supported by the National Institutes of Health [grant numbers T32 AR053461-08 to B.B.M., P01 GM087253 to E.M.O., R01 GM057247 to E.M.O.]. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster and individual poster panels are available for downloading at http://jcs.biologists.org/lookup/doi/10.1242/jcs.186403.supplemental
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/doi/10.1242/jcs.186403.supplemental
References
- Adams R. J. and Pollard T. D. (1989). Binding of myosin I to membrane lipids. Nature 340, 565-568. 10.1038/340565a0 [DOI] [PubMed] [Google Scholar]
- Almeida C. G., Yamada A., Tenza D., Louvard D., Raposo G. and Coudrier E. (2011). Myosin 1b promotes the formation of post-Golgi carriers by regulating actin assembly and membrane remodelling at the trans-Golgi network. Nat. Cell Biol. 13, 779-789. 10.1038/ncb2262 [DOI] [PubMed] [Google Scholar]
- Arif E., Wagner M. C., Johnstone D. B., Wong H. N., George B., Pruthi P. A., Lazzara M. J. and Nihalani D. (2011). Motor protein Myo1c is a podocyte protein that facilitates the transport of slit diaphragm protein Neph1 to the podocyte membrane. Mol. Cell. Biol. 31, 2134-2150. 10.1128/MCB.05051-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J.-I., Oh S.-K., Kim D.-B., Choi S.-Y., Kim U.-K., Lee K.-Y. and Lee S.-H. (2012). Targeted massive parallel sequencing: the effective detection of novel causative mutations associated with hearing loss in small families. Orphanet J. Rare Dis. 7, 60 10.1186/1750-1172-7-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler M., Kroschewski R., Stöffler H. E. and Behrmann T. (1994). Rat myr 4 defines a novel subclass of myosin I: identification, distribution, localization, and mapping of calmodulin-binding sites with differential calcium sensitivity. J. Cell Biol. 126, 375-389. 10.1083/jcb.126.2.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batters C., Arthur C. P., Lin A., Porter J., Geeves M. A., Milligan R. A., Molloy J. E. and Coluccio L. M. (2004). Myo1c is designed for the adaptation response in the inner ear. EMBO J. 23, 1433-1440. 10.1038/sj.emboj.7600169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesh A. E., Nambiar R., McConnell R. E., Mao S., Tabb D. L. and Tyska M. J. (2010). Differential localization and dynamics of class I myosins in the enterocyte microvillus. Mol. Biol. Cell 21, 970-978. 10.1091/mbc.E09-07-0638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benesh A. E., Fleming J. T., Chiang C., Carter B. D. and Tyska M. J. (2012). Expression and localization of myosin-1d in the developing nervous system. Brain Res. 1440, 9-22. 10.1016/j.brainres.2011.12.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi J., Chase S. E., Pellenz C. D., Kurihara H., Fanning A. S. and Krendel M. (2013). Myosin 1e is a component of the glomerular slit diaphragm complex that regulates actin reorganization during cell-cell contact formation in podocytes. Am. J. Physiol. Ren. Physiol. 305, F532-F544. 10.1152/ajprenal.00223.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguslavsky S., Chiu T., Foley K. P., Osorio-Fuentealba C., Antonescu C. N., Bayer K. U., Bilan P. J. and Klip A. (2012). Myo1c binding to submembrane actin mediates insulin-induced tethering of GLUT4 vesicles. Mol. Biol. Cell 23, 4065-4078. 10.1091/mbc.E12-04-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A., Guilherme A., Robida S. I., Nicoloro S. M. C., Zhou Q. L., Jiang Z. Y., Pomerleau D. P. and Czech M. P. (2002). Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420, 821-824. 10.1038/nature01246 [DOI] [PubMed] [Google Scholar]
- Bose A., Robida S., Furcinitti P. S., Chawla A., Fogarty K., Corvera S. and Czech M. P. (2004). Unconventional myosin myo1c promotes membrane fusion in a regulated exocytic pathway. Mol. Cell. Biol. 24, 5447-5458. 10.1128/MCB.24.12.5447-5458.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstaetter H., Kendrick-Jones J. and Buss F. (2012). Myo1c regulates lipid raft recycling to control cell spreading, migration and Salmonella invasion. J. Cell Sci. 125, 1991-2003. 10.1242/jcs.097212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandstaetter H., Kishi-Itakura C., Tumbarello D. A., Manstein D. J. and Buss F. (2014). Loss of functional MYO1C/myosin 1c, a motor protein involved in lipid raft trafficking, disrupts autophagosome-lysosome fusion. Autophagy 10, 2310-2323. 10.4161/15548627.2014.984272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy J. D., Emery B., Kaushal A., Foo L. C., Zamanian J. L., Christopherson K. S., Xing Y., Lubischer J. L., Krieg P. A., Krupenko S. A. et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J. Neurosci. 28, 264-278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. H., Stephan D. A., Hasson T., Fukushima K., Nelissen C. M., Chen A. F., Jun A. I., Ramesh A., Van Camp G. and Smith R. J. H. (2001). Myo1f as a candidate gene for nonsyndromic deafness, dfnb15. Arch. Otolaryngol. Neck Surg. 127, 921-925. 10.1001/archotol.127.8.921 [DOI] [PubMed] [Google Scholar]
- Chen X.-W., Leto D., Chiang S.-H., Wang Q. and Saltiel A. R. (2007). Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell 13, 391-404. 10.1016/j.devcel.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Cheng J., Grassart A. and Drubin D. G. (2012). Myosin 1E coordinates actin assembly and cargo trafficking during clathrin-mediated endocytosis. Mol. Biol. Cell 23, 2891-2904. 10.1091/mbc.E11-04-0383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang C.-H., Carpenter A. E., Fuchsova B., Johnson T., de Lanerolle P. and Belmont A. S. (2006). Long-range directional movement of an interphase chromosome site. Curr. Biol. 16, 825-831. 10.1016/j.cub.2006.03.059 [DOI] [PubMed] [Google Scholar]
- Collins K., Sellers J. R. and Matsudaira P. (1990). Calmodulin dissociation regulates brush border myosin I (110-kD-calmodulin) mechanochemical activity in vitro. J. Cell Biol. 110, 1137-1147. 10.1083/jcb.110.4.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccio L. M. and Geeves M. A. (1999). Transient kinetic analysis of the 130-kDa myosin I (MYR-1 Gene Product) from rat liver A MYOSIN I DESIGNED FOR MAINTENANCE OF TENSION? J. Biol. Chem. 274, 21575-21580. 10.1074/jbc.274.31.21575 [DOI] [PubMed] [Google Scholar]
- Cordonnier M.-N., Dauzonne D., Louvard D. and Coudrier E. (2001). Actin filaments and myosin I Alpha cooperate with microtubules for the movement of lysosomes. Mol. Biol. Cell 12, 4013-4029. 10.1091/mbc.12.12.4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J., Ting-Beall H. P., Hochmuth R. M., Sheetz M. P. and Titus M. A. (1999). Myosin I contributes to the generation of resting cortical tension. Biophys. J. 77, 1168-1176. 10.1016/S0006-3495(99)76968-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart A. E., Tollis S., Bright M. D., Frankel G. and Endres R. G. (2012). The motor protein myosin 1G functions in FcγR-mediated phagocytosis. J. Cell Sci. 125, 6020-6029. 10.1242/jcs.109561 [DOI] [PubMed] [Google Scholar]
- Diakonova M., Bokoch G. and Swanson J. A. (2002). Dynamics of cytoskeletal proteins during Fcγ receptor-mediated phagocytosis in macrophages. Mol. Biol. Cell 13, 402-411. 10.1091/mbc.01-05-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diz-Muñoz A., Krieg M., Bergert M., Ibarlucea-Benitez I., Muller D. J., Paluch E. and Heisenberg C.-P. (2010). Control of directed cell migration in vivo by membrane-to-cortex attachment. PLoS Biol. 8, e1000544 10.1371/journal.pbio.1000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doberstein S. K. and Pollard T. D. (1992). Localization and specificity of the phospholipid and actin binding sites on the tail of Acanthamoeba myosin IC. J. Cell Biol. 117, 1241-1249. 10.1083/jcb.117.6.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaudy F., Ferrara A., Esposito L., Hertzano R., Ben-David O., Bell R. E., Melchionda S., Zelante L., Avraham K. B. and Gasparini P. (2003). Multiple mutations of MYO1A, a cochlear-expressed gene, in sensorineural hearing loss. Am. J. Hum. Genet. 72, 1571-1577. 10.1086/375654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Eswarappa S. M., Hitomi M. and Fox P. L. (2012). Myo1c facilitates G-actin transport to the leading edge of migrating endothelial cells. J. Cell Biol. 198, 47-55. 10.1083/jcb.201111088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath K. R. and Burgess D. R. (1993). Golgi-derived vesicles from developing epithelial cells bind actin filaments and possess myosin-I as a cytoplasmically oriented peripheral membrane protein. J. Cell Biol. 120, 117-127. 10.1083/jcb.120.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath K. R., Trimbur G. M. and Burgess D. R. (1994). Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J. Cell Biol. 126, 661-675. 10.1083/jcb.126.3.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeser E. A., Ignacio C. M. G., Krendel M. and Ostap E. M. (2010). Myo1e binds anionic phospholipids with high affinity. Biochemistry 49, 9353-9360. 10.1021/bi1012657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishilevich S., Zimmerman S., Kohn A., Stein T. I., Kolker E., Olender, T., Safran M. and Lancet D. (2016). Genic insights from integrated human proteomics in GeneCards. Database 2016, baw030 10.1093/database/baw030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier N. C., Masters T. A. and Sheetz M. P. (2012). Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 22, 527-535. 10.1016/j.tcb.2012.07.005 [DOI] [PubMed] [Google Scholar]
- Gérard A., Patino-Lopez G., Beemiller P., Nambiar R., Ben-Aissa K., Liu Y., Totah F. J., Tyska M. J., Shaw S. and Krummel M. F. (2014). Detection of rare antigen-presenting cells through t cell-intrinsic meandering motility, mediated by myo1g. Cell 158, 492-505. 10.1016/j.cell.2014.05.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G. and Cyr J. L. (2004). Myosin-1c, the hair cell's adaptation motor. Annu. Rev. Physiol. 66, 521-545. 10.1146/annurev.physiol.66.032102.112842 [DOI] [PubMed] [Google Scholar]
- Gillespie P. G., Wagner M. C. and Hudspeth A. J. (1993). Identification of a 120 kd hair-bundle myosin located near stereociliary tips. Neuron 11, 581-594. 10.1016/0896-6273(93)90071-X [DOI] [PubMed] [Google Scholar]
- Gillespie P. G., Albanesi J. P., Bähler M., Bement W. M., Berg J. S., Burgess D. R., Burnside B., Cheney R. E., Corey D. P., Coudrier E. et al. (2001). Myosin-I nomenclature. J. Cell Biol. 155, 703-704. 10.1083/jcb.200110032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. J., Lin T., Shuman H. and Ostap E. M. (2015). Mechanochemical tuning of myosin-I by the N-terminal region. Proc. Natl. Acad. Sci. USA 112, E3337-E3344. 10.1073/pnas.1506633112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Gauthier N. C., Cheng-Han Y., Zuanning Y., Pontes B., Ohmstede M., Martin R., Knölker H.-J., Döbereiner H.-G., Krendel M. et al. (2013). Myosin 1E localizes to actin polymerization sites in lamellipodia, affecting actin dynamics and adhesion formation. Biol. Open 2, 1288-1299. 10.1242/bio.20135827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J.-J., Wang G., Pisitkun T., Patino-Lopez G., Nagashima K., Knepper M. A., Shen R.-F. and Shaw S. (2008). Enrichment of distinct microfilament-associated and GTP-binding-proteins in membrane/microvilli fractions from lymphoid cells. J. Proteome Res. 7, 2911-2927. 10.1021/pr800016a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden S. M., Wolenski J. S. and Mooseker M. S. (1990). Binding of brush border myosin I to phospholipid vesicles. J. Cell Biol. 111, 443-451. 10.1083/jcb.111.2.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegan P. S., Ostertag E., Geurts A. M. and Mooseker M. S. (2015). Myosin Id is required for planar cell polarity in ciliated tracheal and ependymal epithelial cells. Cytoskeleton 72, 503-516. 10.1002/cm.21259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks A. G., Holzbaur E. L. F. and Goldman Y. E. (2012). Force measurements on cargoes in living cells reveal collective dynamics of microtubule motors. Proc. Natl. Acad. Sci. USA 109, 18447-18452. 10.1073/pnas.1215462109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokanson D. E. and Ostap E. M. (2006). Myo1c binds tightly and specifically to phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate. Proc. Natl. Acad. Sci. USA 103, 3118-3123. 10.1073/pnas.0505685103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokanson D. E., Laakso J. M., Lin T., Sept D. and Ostap E. M. (2006). Myo1c binds phosphoinositides through a putative pleckstrin homology domain. Mol. Biol. Cell 17, 4856-4865. 10.1091/mbc.E06-05-0449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. R., Gillespie S. K. H., Provance D. W. Jr., Shah K., Shokat K. M., Corey D. P., Mercer J. A. and Gillespie P. G. (2002). A Chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371-381. 10.1016/S0092-8674(02)00629-3 [DOI] [PubMed] [Google Scholar]
- Huang J., Imamura T., Babendure J. L., Lu J.-C. and Olefsky J. M. (2005). Disruption of microtubules ablates the specificity of insulin signaling to glut4 translocation in 3t3-l1 adipocytes. J. Biol. Chem. 280, 42300-42306. 10.1074/jbc.M510920200 [DOI] [PubMed] [Google Scholar]
- Huber L. A., Fialka I., Paiha K., Hunziker W., Sacks D. B., Bähler M., Way M., Gagescu R. and Gruenberg J. (2000). Both calmodulin and the unconventional myosin myr4 regulate membrane trafficking along the recycling pathway of mdck cells. Traffic 1, 494-503. 10.1034/j.1600-0854.2000.010607.x [DOI] [PubMed] [Google Scholar]
- Inouye M., Ripatti S., Kettunen J., Lyytikäinen L.-P., Oksala N., Laurila P.-P., Kangas A. J., Soininen P., Savolainen M. J., Viikari J. et al. (2012). Novel loci for metabolic networks and multi-tissue expression studies reveal genes for atherosclerosis. PLoS Genet. 8, e1002907 10.1371/journal.pgen.1002907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joensuu M., Belevich I., Rämö O., Nevzorov I., Vihinen H., Puhka M., Witkos T. M., Lowe M., Vartiainen M. K. and Jokitalo E. (2014). ER sheet persistence is coupled to myosin 1c–regulated dynamic actin filament arrays. Mol. Biol. Cell 25, 1111-1126. 10.1091/mbc.E13-12-0712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Taylor W. R. and Thornton J. M. (1992). The rapid generation of mutation data matricies from protein sequences. Comput. Appl. Biosci. 8, 275-282. [DOI] [PubMed] [Google Scholar]
- Jung G., Wu X. and Hammer J. A. (1996). Dictyostelium mutants lacking multiple classic myosin I isoforms reveal combinations of shared and distinct functions. J. Cell Biol. 133, 305-323. 10.1083/jcb.133.2.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee A. J., Yang L., Lucas C. A., Greenberg M. J., Martel N., Leong G. M., Hughes W. E., Cooney G. J., James D. E., Michael Ostap E. et al. (2015). An actin filament population defined by the tropomyosin Tpm3.1 regulates glucose uptake. Traffic 16, 691-711. 10.1111/tra.12282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. V., Mehal W. Z., Dong X., Heinrich V., Pypaert M., Mellman I., Dembo M., Mooseker M. S., Wu D. and Flavell R. A. (2006). Modulation of cell adhesion and motility in the immune system by myo1f. Science 314, 136-139. 10.1126/science.1131920 [DOI] [PubMed] [Google Scholar]
- Kittelberger N., Breunig M., Martin R., Knölker H.-J. and Miklavc P. (2016). The role of myosin 1c and myosin 1b in surfactant exocytosis. J. Cell Sci. 129, 1685-1696. 10.1242/jcs.181313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler D., Struchholz S. and Bähler M. (2005). The two IQ-motifs and Ca2+/calmodulin regulate the rat myosin 1d ATPase activity. FEBS J. 272, 2189-2197. 10.1111/j.1742-4658.2005.04642.x [DOI] [PubMed] [Google Scholar]
- Komaba S. and Coluccio L. M. (2010). Localization of myosin 1b to actin protrusions requires phosphoinositide binding. J. Biol. Chem. 285, 27686-27693. 10.1074/jbc.M109.087270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaba S. and Coluccio L. M. (2015). Myosin 1b regulates amino acid transport by associating transporters with the apical plasma membrane of kidney cells. PLoS ONE 10, e0138012 10.1371/journal.pone.0138012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravtsov D. V., Caputo C., Collaco A., Hoekstra N., Egan M. E., Mooseker M. S. and Ameen N. A. (2012). Myosin ia is required for cftr brush border membrane trafficking and ion transport in the mouse small intestine. Traffic 13, 1072-1082. 10.1111/j.1600-0854.2012.01368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M., Osterweil E. K. and Mooseker M. S. (2007). Myosin 1E interacts with synaptojanin-1 and dynamin and is involved in endocytosis. FEBS Lett. 581, 644-650. 10.1016/j.febslet.2007.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M., Kim S. V., Willinger T., Wang T., Kashgarian M., Flavell R. A. and Mooseker M. S. (2009). Disruption of myosin 1e promotes podocyte injury. J. Am. Soc. Nephrol. 20, 86-94. 10.1681/ASN.2007111172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyselá K., Philimonenko A. A., Philimonenko V. V., Janáček J., Kahle M. and Hozák P. (2005). Nuclear distribution of actin and myosin I depends on transcriptional activity of the cell. Histochem. Cell Biol. 124, 347-358. 10.1007/s00418-005-0042-8 [DOI] [PubMed] [Google Scholar]
- Laakso J. M., Lewis J. H., Shuman H. and Ostap E. M. (2008). Myosin I can act as a molecular force sensor. Science 321, 133-136. 10.1126/science.1159419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso J. M., Lewis J. H., Shuman H. and Ostap E. M. (2010). Control of myosin-I force sensing by alternative splicing. Proc. Natl. Acad. Sci. USA 107, 698-702. 10.1073/pnas.0911426107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A. K. and Bridgman P. C. (1996). Mammalian myosin Iα is concentrated near the plasma membrane in nerve growth cones. Cell Motil. Cytoskeleton 33, 130-150. [DOI] [PubMed] [Google Scholar]
- Lin T., Tang N. and Ostap E. M. (2005). Biochemical and motile properties of myo1b splice isoforms. J. Biol. Chem. 280, 41562-41567. 10.1074/jbc.M508653200 [DOI] [PubMed] [Google Scholar]
- Lin T., Greenberg M. J., Moore J. R. and Ostap E. M. (2011). A hearing loss-associated myo1c mutation (r156w) decreases the myosin duty ratio and force sensitivity. Biochemistry 50, 1831-1838. 10.1021/bi1016777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Ortega O., Ovalle-García E., Ortega-Blake I., Antillón A., Chávez-Munguía B., Patiño-López G., Fragoso-Soriano R. and Santos-Argumedo L. (2016). Myo1g is an active player in maintaining cell stiffness in B-lymphocytes. Cytoskeleton 73, 258-268. 10.1002/cm.21299 [DOI] [PubMed] [Google Scholar]
- Manceva S., Lin T., Pham H., Lewis J. H., Goldman Y. E. and Ostap E. M. (2007). Calcium regulation of calmodulin binding to and dissociation from the myo1c regulatory domain. Biochemistry 46, 11718-11726. 10.1021/bi700894h [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravillas-Montero J. L., Gillespie P. G., Patiño-López G., Shaw S. and Santos-Argumedo L. (2011). Myosin 1c participates in b cell cytoskeleton rearrangements, is recruited to the immunologic synapse, and contributes to antigen presentation. J. Immunol. 187, 3053-3063. 10.4049/jimmunol.1004018 [DOI] [PubMed] [Google Scholar]
- Mazzolini R., Dopeso H., Mateo-Lozano S., Chang W., Rodrigues P., Bazzocco S., Alazzouzi H., Landolfi S., Hernández-Losa J., Andretta E. et al. (2012). Brush border Myosin Ia has tumor suppressor activity in the intestine. Proc. Natl. Acad. Sci. USA 109, 1530-1535. 10.1073/pnas.1108411109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini R., Rodrigues P., Bazzocco S., Dopeso H., Ferreira A. M., Mateo-Lozano S., Andretta E., Woerner S. M., Alazzouzi H., Landolfi S. et al. (2013). Brush border myosin Ia inactivation in gastric but not endometrial tumors. Int. J. Cancer 132, 1790-1799. 10.1002/ijc.27856 [DOI] [PubMed] [Google Scholar]
- McConnell R. E. and Tyska M. J. (2007). Myosin-1a powers the sliding of apical membrane along microvillar actin bundles. J. Cell Biol. 177, 671-681. 10.1083/jcb.200701144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R. E. and Tyska M. J. (2010). Leveraging the membrane-cytoskeleton interface with myosin-1. Trends Cell Biol. 20, 418-426. 10.1016/j.tcb.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell R. E., Higginbotham J. N., Shifrin D. A., Tabb D. L., Coffey R. J. and Tyska M. J. (2009). The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 185, 1285-1298. 10.1083/jcb.200902147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh B. B., Holzbaur E. L. F. and Ostap E. M. (2015). Control of the Initiation and Termination of Kinesin-1-Driven Transport by Myosin-Ic and Nonmuscle Tropomyosin. Curr. Biol. 25, 523-529. 10.1016/j.cub.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna J. M. D. and Ostap E. M. (2009). Kinetics of the Interaction of myo1c with Phosphoinositides. J. Biol. Chem. 284, 28650-28659. 10.1074/jbc.M109.049791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele C., Iatropoulos P., Donadelli R., Calabria A., Maranta R., Cassis P., Buelli S., Tomasoni S., Piras R., Krendel M. et al. (2011). MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N. Engl. J. Med. 365, 295-306. 10.1056/NEJMoa1101273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H., Bowers B. and Korn E. D. (1989). Plasma membrane association of Acanthamoeba myosin I. J. Cell Biol. 109, 1519-1528. 10.1083/jcb.109.4.1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamori Y., Emoto M., Fukuda N., Taguchi A., Okuya S., Tajiri M., Miyagishi M., Taira K., Wada Y. and Tanizawa Y. (2006). Myosin motor Myo1c and its receptor NEMO/IKK-γ promote TNF-α–induced serine307 phosphorylation of IRS-1. J. Cell Biol. 173, 665-671. 10.1083/jcb.200601065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambiar R., McConnell R. E. and Tyska M. J. (2009). Control of cell membrane tension by myosin-I. Proc. Natl. Acad. Sci. USA 106, 11972-11977. 10.1073/pnas.0901641106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebl T., Pestonjamasp K. N., Leszyk J. D., Crowley J. L., Oh S. W. and Luna E. J. (2002). Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 277, 43399-43409. 10.1074/jbc.M205386200 [DOI] [PubMed] [Google Scholar]
- Nielsen J. A., Maric D., Lau P., Barker J. L. and Hudson L. D. (2006). Identification of a novel oligodendrocyte cell adhesion protein using gene expression profiling. J. Neurosci. 26, 9881-9891. 10.1523/JNEUROSCI.2246-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak K. D., Peterson M. D., Reedy M. C. and Titus M. A. (1995). Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J. Cell Biol. 131, 1205-1221. 10.1083/jcb.131.5.1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Kim H., Shin B., Lee K. H., Yeo M. G. and Song W. K. (2013). Interaction of Crk with myosin-1c participates in fibronectin-induced cell spreading. Int. J. Biol. Sci. 9, 778-791. 10.7150/ijbs.6459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olety B., Wälte M., Honnert U., Schillers H. and Bähler M. (2010). Myosin 1G (Myo1G) is a haematopoietic specific myosin that localises to the plasma membrane and regulates cell elasticity. FEBS Lett. 584, 493-499. 10.1016/j.febslet.2009.11.096 [DOI] [PubMed] [Google Scholar]
- Ostap E. M. and Pollard T. D. (1996). Overlapping functions of myosin-I isoforms? J. Cell Biol. 133, 221-224. 10.1083/jcb.133.2.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouderkirk J. L. and Krendel M. (2014). Myosin 1e is a component of the invadosome core that contributes to regulation of invadosome dynamics. Exp. Cell Res. 322, 265-276. 10.1016/j.yexcr.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino-Lopez G., Aravind L., Dong X., Kruhlak M. J., Ostap E. M. and Shaw S. (2010). Myosin 1G is an abundant class I myosin in lymphocytes whose localization at the plasma membrane depends on its ancient divergent pleckstrin homology (PH) domain (Myo1PH). J. Biol. Chem. 285, 8675-8686. 10.1074/jbc.M109.086959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percipalle P., Fomproix N., Cavellán E., Voit R., Reimer G., Krüger T., Thyberg J., Scheer U., Grummt I. and Farrants A.-K. Ö. (2006). The chromatin remodelling complex WSTF–SNF2h interacts with nuclear myosin 1 and has a role in RNA polymerase I transcription. EMBO Rep. 7, 525-530. 10.1038/sj.embor.7400657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestic-Dragovich L., Stojiljkovic L., Philimonenko A. A., Nowak G., Ke Y., Settlage R. E., Shabanowitz J., Hunt D. F., Hozak P. and Lanerolle P. de. (2000). A myosin I isoform in the nucleus. Science 290, 337-341. 10.1126/science.290.5490.337 [DOI] [PubMed] [Google Scholar]
- Petzoldt A. G., Coutelis J.-B., Géminard C., Spéder P., Suzanne M., Cerezo D. and Noselli S. (2012). DE-Cadherin regulates unconventional Myosin ID and Myosin IC in Drosophila left-right asymmetry establishment. Development 139, 1874-1884. 10.1242/dev.047589 [DOI] [PubMed] [Google Scholar]
- Philimonenko V. V., Zhao J., Iben S., Dingová H., Kyselá K., Kahle M., Zentgraf H., Hofmann W. A., de Lanerolle P., Hozák P. et al. (2004). Nuclear actin and myosin I are required for RNA polymerase I transcription. Nat. Cell Biol. 6, 1165-1172. 10.1038/ncb1190 [DOI] [PubMed] [Google Scholar]
- Pollard T. D. and Korn E. D. (1973). Acanthamoeba Myosin I. ISOLATION FROM ACANTHAMOEBA CASTELLANII OF AN ENZYME SIMILAR TO MUSCLE MYOSIN. J. Biol. Chem. 248, 4682-4690. [PubMed] [Google Scholar]
- Prospéri M.-T., Lépine P., Dingli F., Paul-Gilloteaux P., Martin R., Loew D., Knölker H.-J. and Coudrier E. (2015). Myosin 1b functions as an effector of EphB signaling to control cell repulsion. J. Cell Biol. 210, 347-361. 10.1083/jcb.201501018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrpassopoulos S., Feeser E. A., Mazerik J. N., Tyska M. J. and Ostap E. M. (2012). Membrane-bound Myo1c powers asymmetric motility of actin filaments. Curr. Biol. 22, 1688-1692. 10.1016/j.cub.2012.06.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G., Cordonnier M.-N., Tenza D., Menichi B., Dürrbach A., Louvard D. and Coudrier E. (1999). Association of Myosin I alpha with endosomes and lysosomes in mammalian cells. Mol. Biol. Cell 10, 1477-1494. 10.1091/mbc.10.5.1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert C., Kroschewski R. and Bähler M. (1993). Identification, characterization and cloning of myr 1, a mammalian myosin-I. J. Cell Biol. 120, 1393-1403. 10.1083/jcb.120.6.1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert C., Godel J., Muller R. T., Kroschewski R., Reinhard J. and Bähler M. (1995). Localization of the rat myosin I molecules myr 1 and myr 2 and in vivo targeting of their tail domains. J. Cell Sci. 108, 3775-3786. [DOI] [PubMed] [Google Scholar]
- Salas-Cortes L., Ye F., Tenza D., Wilhelm C., Theos A., Louvard D., Raposo G. and Coudrier E. (2005). Myosin Ib modulates the morphology and the protein transport within multi-vesicular sorting endosomes. J. Cell Sci. 118, 4823-4832. 10.1242/jcs.02607 [DOI] [PubMed] [Google Scholar]
- Schietroma C., Yu H.-Y., Wagner M. C., Umbach J. A., Bement W. M. and Gundersen C. B. (2007). A role for Myosin 1e in cortical granule exocytosis in xenopus oocytes. J. Biol. Chem. 282, 29504-29513. 10.1074/jbc.M705825200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr E. H., Joyce M. P. and Greene L. A. (1993). Mammalian myosin I alpha, I beta, and I gamma: new widely expressed genes of the myosin I family. J. Cell Biol. 120, 1405-1416. 10.1083/jcb.120.6.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H., Greenberg M. J., Zwolak A., Lin T., Sindelar C. V., Dominguez R. and Ostap E. M. (2014). A vertebrate myosin-I structure reveals unique insights into myosin mechanochemical tuning. Proc. Natl. Acad. Sci. USA 111, 2116-2121. 10.1073/pnas.1321022111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sielski N. L., Ihnatovych I., Hagen J. J. and Hofmann W. A. (2014). Tissue specific expression of Myosin IC Isoforms. BMC Cell Biol. 15, 8 10.1186/1471-2121-15-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowron J. F., Bement W. M. and Mooseker M. S. (1998). Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil. Cytoskeleton 41, 308-324. [DOI] [PubMed] [Google Scholar]
- Sokac A. M., Schietroma C., Gundersen C. B. and Bement W. M. (2006). Myosin-1c couples assembling actin to membranes to drive compensatory endocytosis. Dev. Cell 11, 629-640. 10.1016/j.devcel.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soppina V., Rai A. K., Ramaiya A. J., Barak P. and Mallik R. (2009). Tug-of-war between dissimilar teams of microtubule motors regulates transport and fission of endosomes. Proc. Natl. Acad. Sci. USA 106, 19381-19386. 10.1073/pnas.0906524106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spéder P., Ádám G. and Noselli S. (2006). Type ID unconventional myosin controls left–right asymmetry in Drosophila. Nature 440, 803-807. 10.1038/nature04623 [DOI] [PubMed] [Google Scholar]
- Stöffler H.-E. and Bähler M. (1998). The ATPase activity of Myr3, a rat Myosin I, is allosterically inhibited by its own tail domain and by Ca2+ binding to its light chain calmodulin. J. Biol. Chem. 273, 14605-14611. 10.1074/jbc.273.23.14605 [DOI] [PubMed] [Google Scholar]
- Stöffler H. E., Ruppert C., Reinhard J. and Bähler M. (1995). A novel mammalian myosin I from rat with an SH3 domain localizes to Con A-inducible, F-actin-rich structures at cell-cell contacts. J. Cell Biol. 129, 819-830. 10.1083/jcb.129.3.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J. L., Merriman B., Cantor R. M., Geschwind D. H. and Nelson S. F. (2007). High density SNP association study of a major autism linkage region on chromosome 17. Hum. Mol. Genet. 16, 704-715. 10.1093/hmg/ddm015 [DOI] [PubMed] [Google Scholar]
- Swanljung-Collins H. and Collins J. H. (1991). Ca2+ stimulates the Mg2(+)-ATPase activity of brush border myosin I with three or four calmodulin light chains but inhibits with less than two bound. J. Biol. Chem. 266, 1312-1319. [PubMed] [Google Scholar]
- Swanson J. A., Johnson M. T., Beningo K., Post P., Mooseker M. and Araki N. (1999). A contractile activity that closes phagosomes in macrophages. J. Cell Sci. 112, 307-316. [DOI] [PubMed] [Google Scholar]
- Taki T., Akiyama M., Saito S., Ono R., Taniwaki M., Kato Y., Yuza Y., Eto Y. and Hayashi Y. (2005). The MYO1F, unconventional myosin type 1F, gene is fused to MLL in infant acute monocytic leukemia with a complex translocation involving chromosomes 7, 11, 19 and 22. Oncogene 24, 5191-5197. 10.1038/sj.onc.1208711 [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A. and Kumar S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725-2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N. and Ostap E. M. (2001). Motor domain-dependent localization of myo1b (myr-1). Curr. Biol. 11, 1131-1135. 10.1016/S0960-9822(01)00320-7 [DOI] [PubMed] [Google Scholar]
- Tang N., Lin T., Yang J., Foskett J. K. and Ostap E. M. (2007). CIB1 and CaBP1 bind to the myo1c regulatory domain. J. Muscle Res. Cell Motil. 28, 285-291. 10.1007/s10974-007-9124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassopoulou-Fishell M., Deeley K., Harvey E. M., Sciote J. and Vieira A. R. (2012). Genetic variation in Myosin 1H contributes to mandibular prognathism. Am. J. Orthod. Dentofacial Orthop. 141, 51-59. 10.1016/j.ajodo.2011.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Jung J.-J., Inamdar S. M., Nihalani D. and Choudhury A. (2013). The myosin motor Myo1c is required for VEGFR2 delivery to the cell surface and for angiogenic signaling. Am. J. Physiol. Heart Circ. Physiol. 304, H687-H696. 10.1152/ajpheart.00744.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuo H. and Coluccio L. M. (2013). Myosin-1c regulates the dynamic stability of E-cadherin–based cell–cell contacts in polarized Madin–Darby canine kidney cells. Mol. Biol. Cell 24, 2820-2833. 10.1091/mbc.E12-12-0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska M. J. and Mooseker M. S. (2004). A role for myosin-1A in the localization of a brush border disaccharidase. J. Cell Biol. 165, 395-405. 10.1083/jcb.200310031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyska M. J., Mackey A. T., Huang J.-D., Copeland N. G., Jenkins N. A. and Mooseker M. S. (2005). Myosin-1a is critical for normal brush border structure and composition. Mol. Biol. Cell 16, 2443-2457. 10.1091/mbc.E04-12-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veigel C., Coluccio L. M., Jontes J. D., Sparrow J. C., Milligan R. A. and Molloy J. E. (1999). The motor protein myosin-I produces its working stroke in two steps. Nature 398, 530-533. 10.1038/19104 [DOI] [PubMed] [Google Scholar]
- Wagner M. C., Blazer-Yost B. L., Boyd-White J., Srirangam A., Pennington J. and Bennett S. (2005). Expression of the unconventional myosin Myo1c alters sodium transport in M1 collecting duct cells. Am. J. Physiol. Cell Physiol. 289, C120-C129. 10.1152/ajpcell.00569.2003 [DOI] [PubMed] [Google Scholar]
- Wang F.-S., Liu C.-W., Diefenbach T. J. and Jay D. G. (2003). Modeling the role of Myosin 1c in neuronal growth cone turning. Biophys. J. 85, 3319-3328. 10.1016/S0006-3495(03)74751-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J., Ouderkirk J. L., Krendel M. and Lang R. (2015). Class I myosin Myo1e regulates TLR4-triggered macrophage spreading, chemokine release, and antigen presentation via MHC class II. Eur. J. Immunol. 45, 225-237. 10.1002/eji.201444698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A., Mamane A., Lee-Tin-Wah J., Di Cicco A., Prévost C., Lévy D., Joanny J.-F., Coudrier E. and Bassereau P. (2014). Catch-bond behaviour facilitates membrane tubulation by non-processive myosin 1b. Nat. Commun. 5, 3624 10.1038/ncomms4624 [DOI] [PubMed] [Google Scholar]
- Yip M. F., Ramm G., Larance M., Hoehn K. L., Wagner M. C., Guilhaus M. and James D. E. (2008). CaMKII-mediated phosphorylation of the myosin motor myo1c is required for insulin-stimulated glut4 translocation in adipocytes. Cell Metab. 8, 384-398. 10.1016/j.cmet.2008.09.011 [DOI] [PubMed] [Google Scholar]