Abstract
Intestinal hormone-producing cells represent the largest endocrine system in the body, but remarkably little is known about enteroendocrine cell type specification in the embryo and adult. We analyzed stage- and cell type-specific deletions of Nkx2.2 and its functional domains in order to characterize its role in the development and maintenance of enteroendocrine cell lineages in the mouse duodenum and colon. Although Nkx2.2 regulates enteroendocrine cell specification in the duodenum at all stages examined, it controls the differentiation of progressively fewer enteroendocrine cell populations when deleted from Ngn3+ progenitor cells or in the adult duodenum. During embryonic development Nkx2.2 regulates all enteroendocrine cell types, except gastrin and preproglucagon. In developing Ngn3+ enteroendocrine progenitor cells, Nkx2.2 is not required for the specification of neuropeptide Y and vasoactive intestinal polypeptide, indicating that a subset of these cell populations derive from an Nkx2.2-independent lineage. In adult duodenum, Nkx2.2 becomes dispensable for cholecystokinin and secretin production. In all stages and Nkx2.2 mutant conditions, serotonin-producing enterochromaffin cells were the most severely reduced enteroendocrine lineage in the duodenum and colon. We determined that the transcription factor Lmx1a is expressed in enterochromaffin cells and functions downstream of Nkx2.2. Lmx1a-deficient mice have reduced expression of Tph1, the rate-limiting enzyme for serotonin biosynthesis. These data clarify the function of Nkx2.2 in the specification and homeostatic maintenance of enteroendocrine populations, and identify Lmx1a as a novel enterochromaffin cell marker that is also essential for the production of the serotonin biosynthetic enzyme Tph1.
KEY WORDS: Nkx2.2, Lmx1a, Enteroendocrine cells, Serotonin, Intestine, Enterochromaffin
Summary: Conditional deletions of Nkx2.2 in the mouse intestine reveal that Lmx1a functions downstream of Nkx2.2 in serotonin-expressing enterochromaffin cells and regulates Tph1 – a key serotonin synthesis enzyme.
INTRODUCTION
The intestinal epithelium comprises five terminally differentiated cell types: the absorptive enterocytes and the secretory Paneth cells, goblet cells, tuft cells and enteroendocrine cells. Enterocytes are the major cell population in the intestine and are important for nutrient absorption. Paneth cells produce antimicrobial peptides and lysozyme, and possibly provide the stem cell niche (Porter et al., 2002; Sato et al., 2011). Goblet cells secrete mucins and thereby establish and maintain the protective mucus layer (Kim and Ho, 2010). Tuft cells comprise a rare cell population marked by doublecortin-like kinase 1 (Dclk1) expression (Gerbe et al., 2011) and are implicated in chemoreception (Gerbe et al., 2012; Sato, 2007). Enteroendocrine cells are the hormone-producing cells in the intestine. Although they represent only 1% of the cells in the intestinal epithelium, they secrete at least fifteen different types of hormones (May and Kaestner, 2010; Rindi et al., 2004) and represent the largest endocrine system in the body. Enteroendocrine cells are found in the small and large intestine and are classified by their location and principal hormone and peptide product. However, most enteroendocrine cells express more than one hormone (Arnes et al., 2012a; Egerod et al., 2012; Habib et al., 2012; Sykaras et al., 2014) and can be identified by chromogranin A (Chga) expression. Since enteroendocrine cells secrete many different hormones, they control a variety of physiological functions in the intestine and body, including gut motility, glucose homeostasis, appetite and food intake.
The serotonin [5-hydroxytryptamine (5-HT)]-producing enterochromaffin cells are the largest enteroendocrine cell population in the intestine. Approximately 90% of the 5-HT in the body is synthesized in the gut, but it is also produced in the CNS. Biosynthesis of 5-HT is a two-step process. The first step involves the conversion of the essential amino acid tryptophan to 5-hydroxytryptophan by the rate-limiting enzyme tryptophan hydroxylase (Tph). Two Tph enzymes have been found to mediate this conversion; Tph1 is expressed in the enterochromaffin cells in the intestine, whereas Tph2 is only found in the brain (Walther et al., 2003). Subsequently, 5-hydroxytryptophan becomes decarboxylated by the enzyme 5-hydroxytryptophan decarboxylase to 5-HT (Manocha and Khan, 2012).
Several transcription factors are known to regulate the enteroendocrine cell lineages. The basic helix-loop-helix (bHLH) protein neurogenin 3 (Ngn3, or Neurog3) is expressed in enteroendocrine progenitor cells and is required for induction of the enteroendocrine cell lineage (Jenny et al., 2002; Lopez-Diaz et al., 2007; Schonhoff et al., 2004). Ngn3−/− mice do not develop enteroendocrine cells in the intestinal epithelium (Jenny et al., 2002). In addition, a number of transcription factors specify subpopulations of enteroendocrine cells downstream of Ngn3, including Arx (Beucher et al., 2012; Du et al., 2012), Foxa1/2 (Ye and Kaestner, 2009), Isl1 (Terry et al., 2014), Insm1 (Gierl et al., 2006), Neurod1 (Mutoh et al., 1997; Naya et al., 1997), Pax4 (Beucher et al., 2012; Larsson et al., 1998) and Pax6 (Larsson et al., 1998). The NK2 homeobox 2 (Nkx2.2) transcription factor also regulates cell fate decisions within the enteroendocrine cell lineage in the embryo (Desai et al., 2008; Wang et al., 2009); however, postnatal lethality of Nkx2.2−/− mice (Briscoe et al., 1999; Sussel et al., 1998) precludes functional analysis of Nkx2.2 in the adult intestine. Since the intestinal epithelium undergoes constant turnover in the adult, we sought to investigate whether Nkx2.2 is required for enteroendocrine cell subtype specification in the adult as well.
In this study, we demonstrate that deletion of Nkx2.2 specifically in the intestinal epithelium in the embryo and the adult, and deletion of Nkx2.2 in Ngn3+ enteroendocrine progenitor cells, results in loss of most enteroendocrine cell types and an increase in the ghrelin (Ghrl) + cell population within the duodenum. Deletion of Nkx2.2 from the large intestine affects only a small number of enteroendocrine cell populations. Interestingly, Ghrl- and 5HT-producing cells are the most affected populations in the duodenum and colon. Overall, the intestine-specific Nkx2.2 deletion displays a developmental phenotype that is similar to that of global Nkx2.2 null mice (Desai et al., 2008; Wang et al., 2009), indicating that the misspecification of enteroendocrine cells is due to intestinal cell-intrinsic functions of Nkx2.2. Deletion of Nkx2.2 from the adult intestinal epithelium did not affect the duodenal expression of cholecystokinin (Cck), gastrin (Gast), neuropeptide Y (Npy), secretin (Sct) and vasoactive intestinal polypeptide (Vip), indicating that Nkx2.2 is dispensable for the homeostatic maintenance of these enteroendocrine lineages in the adult. Additional analysis of the intestinal epithelium in Nkx2.2 mutant mouse models carrying deletions of either the tinman (TN) domain or the NK2-specific domain (SD) revealed discrete functions of these Nkx2.2 regulatory domains in enteroendocrine cell specification. By determining gene changes that were common to the small and large intestine of all Nkx2.2 mutant mice evaluated, we identified Tph1 and the LIM homeobox transcription factor 1 alpha (Lmx1a) to be coordinately downregulated. Gene expression and deletion analyses also revealed Lmx1a to be a novel marker of 5-HT+ enterochromaffin cells, which is essential for 5-HT biosynthesis in the intestine.
RESULTS
Characterization of Nkx2.2Δint mice
Expression of the homeodomain transcription factor Nkx2.2 in the murine intestine begins at embryonic day (E) 15.5 and persists into adulthood (Desai et al., 2008; Wang et al., 2009). To analyze the function of Nkx2.2 in the adult intestine, we specifically deleted Nkx2.2 in the intestinal epithelium using a conditional Nkx2.2 allele (Mastracci et al., 2013) and the VillinCre/+ transgene (Madison et al., 2002). Intestine-specific deletion of Nkx2.2 circumvents the early postnatal lethality of Nkx2.2−/− mice caused by the pancreatic defect (Sussel et al., 1998). Nkx2.2flox/flox;VillinCre/+ or Nkx2.2flox/lacZ;VillinCre/+ mice are referred to hereafter as Nkx2.2Δint mice. To verify that deletion of Nkx2.2 is restricted to the intestine and does not occur in other organs, we performed PCR for the recombined Nkx2.2 allele in several representative tissues. As expected, a recombined product was only detected in intestinal tissues (Fig. S1A). Furthermore, qPCR analysis of the duodenum and colon of 6-week-old adult Nkx2.2Δint mice showed significant ablation of Nkx2.2 in the intestine (Fig. S1B).
Nkx2.2Δint mice at all ages were viable and indistinguishable from their littermate controls, with no significant change in body weight (Fig. S2A). Interestingly, we observed a small but significant increase in the length of the small but not large intestine of 6-week-old Nkx2.2Δint mice (Fig. S2B,C,J). Increases in both the villus and crypt lengths in the small intestine appeared to contribute to the overall change in length (Fig. S2D-I). Interestingly, the change in intestinal length occurred gradually and was transient: there were no length differences in neonatal animals and intestine length had normalized by 19-20 weeks (Fig. S2K,L). The transient manifestation of this phenotype at the post-weaning stage suggests that the change in length might be due to an adaptive effect.
Loss of most enteroendocrine cell populations in the duodenum of Nkx2.2Δint mice
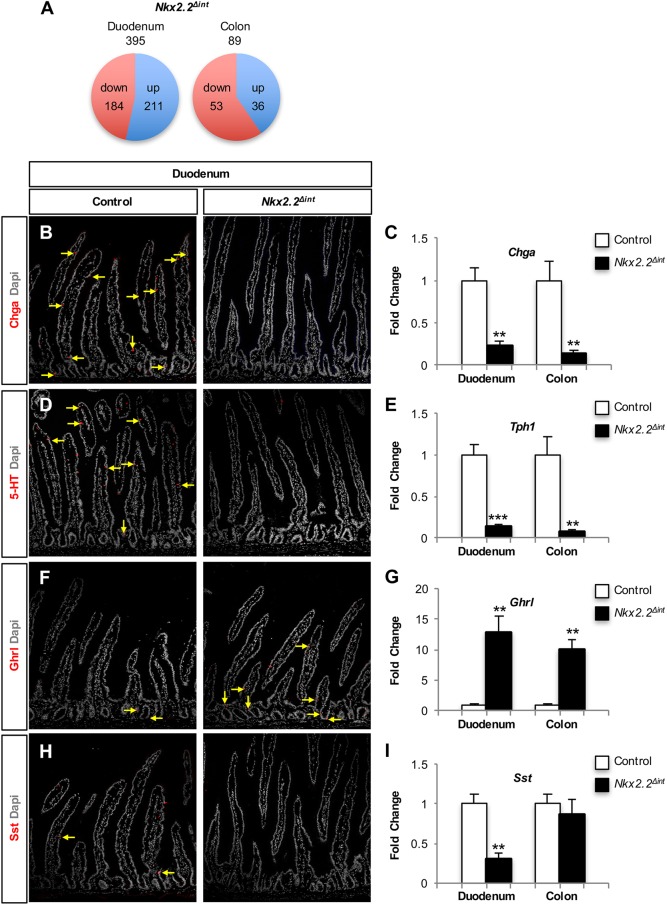
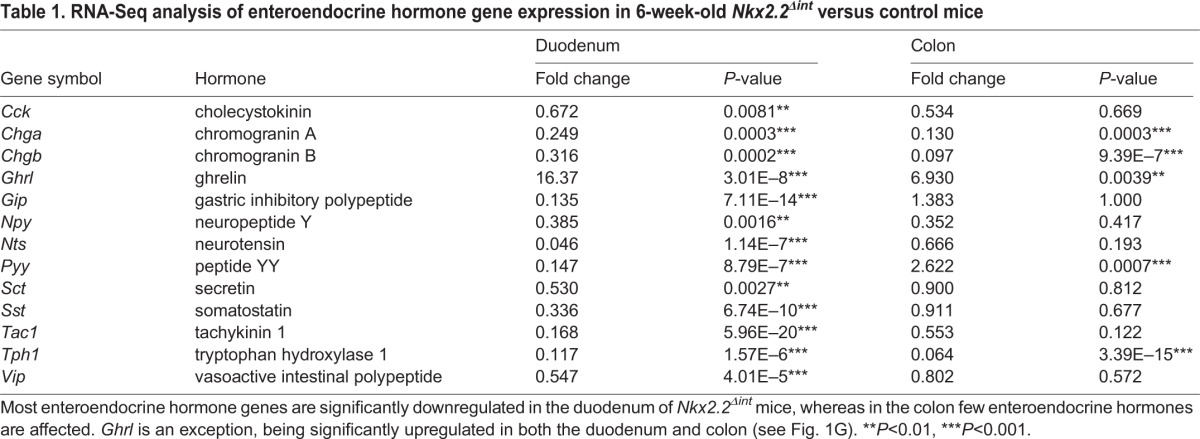
Since the enteroendocrine lineages within the duodenum are well characterized and Nkx2.2 is expressed at the highest levels within this region of the intestine, we chose to focus on the duodenum to analyze the precise molecular changes in the intestinal epithelium of Nkx2.2Δint mice. RNA-Seq analysis of the duodenum of 6-week-old Nkx2.2Δint mice demonstrated that expression of 395 genes was significantly altered compared with controls, with a slightly larger proportion of genes upregulated than downregulated (Fig. 1A). Similar to E18.5 mice carrying a null mutation of Nkx2.2 (Nkx2.2−/−) (Desai et al., 2008; Wang et al., 2009), Nkx2.2Δint mice displayed altered enteroendocrine cell lineages. In the Nkx2.2Δint duodenum, the enteroendocrine cell marker Chga, as well as the hormones Cck, gastric inhibitory polypeptide (Gip), Npy, neurotensin (Nts), Sct, somatostatin (Sst), tachykinin 1 (Tac1), Tph1 and Vip showed significantly decreased expression. The only hormone that demonstrated a higher expression level in the duodenum of Nkx2.2Δint mice was Ghrl (Table 1). Interestingly, the expression of Gast and ppGcg is altered in the small intestine of Nkx2.2−/− mice (Desai et al., 2008), but did not appear changed in the duodenum of Nkx2.2Δint mice (Gast, fold change 0.22, P=0.38; ppGcg, fold change 1.15, P=0.68), suggesting that these changes could be secondary to loss of Nkx2.2 in the CNS or pancreas. In the intestinal epithelium, the ppGcg product is processed to glucagon-like peptide 1 (GLP-1), an incretin hormone that is important for glucose homeostasis. Consistent with unchanged ppGcg expression in the duodenum of Nkx2.2Δint mice, we could not detect a change in blood glucose levels in fed mice or after glucose challenge in an intraperitoneal glucose tolerance test (ipGTT) (Fig. S3).
Fig. 1.
Expression analysis of the duodenum and colon of Nkx2.2Δint mice. (A) RNA-Seq analysis of the duodenum and colon of 6-week-old Nkx2.2Δint mice revealed 211 significantly upregulated and 184 significantly downregulated genes in the duodenum and 36 significantly upregulated and 53 significantly downregulated genes in the colon (P<0.05). Among the significantly downregulated genes in the duodenum are most of the enteroendocrine cell hormones, except Ghrl, which is significantly upregulated (see Table 1). In the colon, few enteroendocrine hormones are changed. (B-I) Immunofluorescence of the duodenum (B,D,F,H) and qPCR analysis of the duodenum and colon (C,E,G,I; n=5) of 6-week-old Nkx2.2Δint mice, showing significant reduction in expression of the enteroendocrine marker Chga (B,C) and the rate-limiting enzyme for 5-HT biosynthesis Tph1 (D,E). The expression of the hormone Ghrl is significantly higher in the duodenum, as well as in the colon, than in controls (F,G). Expression of the hormone Sst is significantly reduced in the duodenum of Nkx2.2Δint mice, but is unchanged in the colon (H,I). Arrows indicate hormone-positive cells. **P<0.01, ***P<0.001.
Table 1.
RNA-Seq analysis of enteroendocrine hormone gene expression in 6-week-old Nkx2.2Δint versus control mice
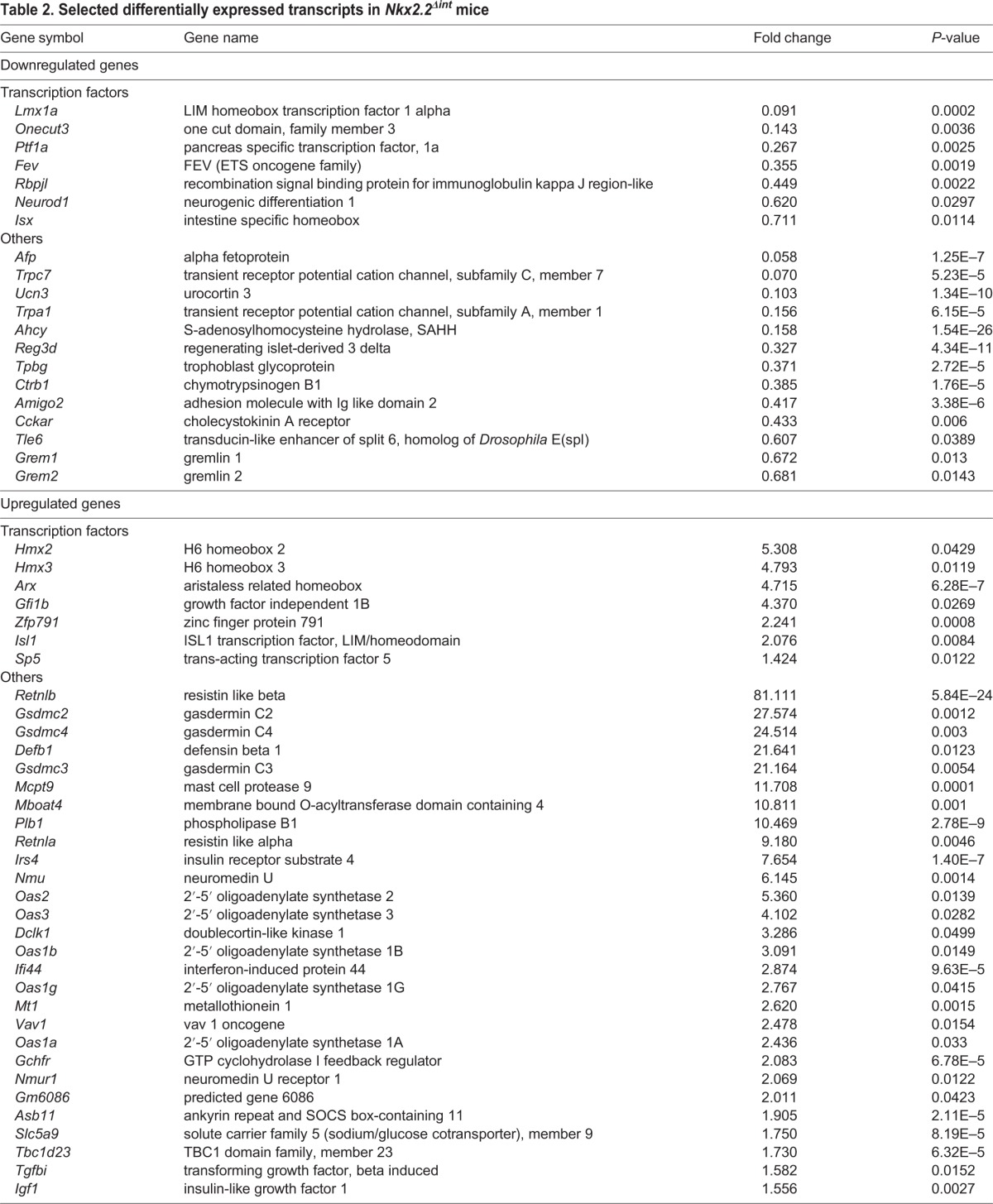
We confirmed the transcriptome results by qPCR analysis of gene expression in the duodenum and immunofluorescence analysis of the small intestine. The R26RTomato reporter (Madisen et al., 2010) was used to identify recombined areas of the small intestine in Nkx2.2Δint mice (data not shown). Representative images of immunofluorescent stainings of the small intestine from Nkx2.2Δint and control mice were consistent with the changes in transcript levels. In particular, both analyses demonstrated the absence and/or decrease of Chga+ enteroendocrine cells (Fig. 1B,C), including the 5-HT+ and Sst+ subpopulations (Fig. 1D,E,H,I), whereas the Ghrl+ cell number was increased (Fig. 1F,G). With the exception of Sst, the hormonal gene expression changes observed in the duodenum of 6-week-old Nkx2.2Δint mice were also observed in the colon (Fig. 1C,E,G,I). Consistent with the change in Sst+ cells in the duodenum of 6-week-old Nkx2.2Δint mice (Fig. 1H,I), urocortin 3 (Ucn3) was also decreased (Table 2). A recent study demonstrated a reduction in the Sst+ cell number in the pancreas of Ucn3-deficient mice (van der Meulen et al., 2015), suggesting that there is a similar relationship between Ucn3+ and Sst+ cells in both the pancreas and intestine.
Table 2.
Selected differentially expressed transcripts in Nkx2.2Δint mice
Although Nkx2.2 is expressed at lower levels in the colon, we sought to determine whether Nkx2.2 also regulates the enteroendocrine lineages in the large intestine. Comparative transcriptome analysis of the Nkx2.2Δint colon revealed a smaller number of gene changes but, similar to the duodenum, comprising both upregulated and downregulated genes (Fig. 1A). There was a severe reduction in expression of the pan-endocrine genes Chga and Chgb, indicating that the absence of Nkx2.2 also affects enteroendocrine lineages of the colon (Table 1, Fig. 1C). The expression of Tph1 and Ghrl was significantly altered (Table 1, Fig. 1E,G). However, the function of Nkx2.2 appears to be more limited in the colon as many of the hormones that were regulated by Nkx2.2 in the duodenum were not affected by the deletion of Nkx2.2 in the colon (Table 1, Fig. 1I). This suggests that the regulation of these lineages in the colon is independent of Nkx2.2 or, as in the case of Gip and Cck, these hormones are not highly expressed in the colon.
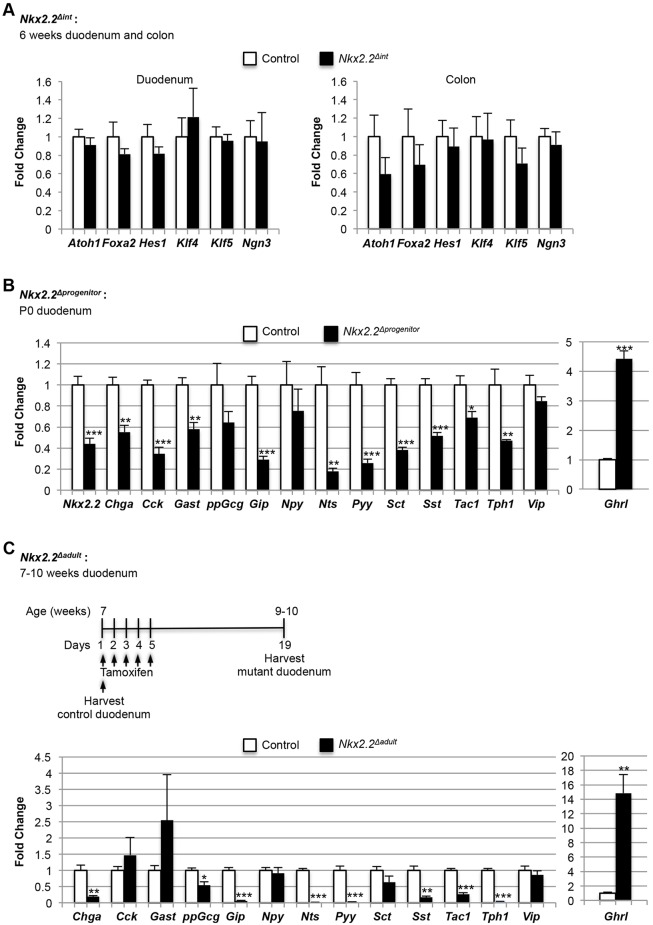
Since the expression of the enteroendocrine progenitor marker Ngn3 was not changed in the duodenum or colon of Nkx2.2Δint mice (Fig. 2A), it is unlikely that the change in enteroendocrine hormone expression is due to a loss of enteroendocrine progenitor cells or of the upstream progenitor populations that contribute to enteroendocrine cell lineages. For example, there is no change in atonal homolog 1 (Atoh1), which is essential for the production of all secretory cells, including enteroendocrine cells (Shroyer et al., 2007; Yang et al., 2001). Furthermore, hairy and enhancer of split 1 (Hes1), which functions upstream of Atoh1 and Ngn3 (Jensen et al., 2000; Kopinke et al., 2011), is also unchanged (Fig. 2A). Expression of even earlier genes, such as Kruppel-like factor 5 (Klf5), which marks early proliferative populations that contribute to proper cellular differentiation (Bell and Shroyer, 2015), and forkhead box A2 (Foxa2) and Klf4, which are expressed in most cells of the upper crypt and villus (Katz et al., 2002; Ye and Kaestner, 2009), is also unchanged (Fig. 2A). The lack of expression changes in these early markers of intestinal progenitor populations suggests that the defect in enteroendocrine cell specification in Nkx2.2Δint mice is likely to be specific to the enteroendocrine lineage and downstream of Ngn3+ progenitor formation.
Fig. 2.
qPCR expression analysis of the duodenum or colon of Nkx2.2Δint, Nkx2.2Δprogenitor and Nkx2.2Δadult mice. (A) Analysis of the duodenum and colon of 6-week-old Nkx2.2Δint mice (n=5) shows no changes in Atoh1, Foxa2, Hes1, Klf4, Klf5 and Ngn3. (B) The P0 Nkx2.2Δprogenitor duodenum (n=5) revealed significantly decreased expression of most hormones analyzed, except for higher expression of Ghrl and no change in expression for ppGcg, Npy and Vip. (C) Nkx2.2Δadult mice were tamoxifen injected at 7 weeks of age for 5 consecutive days (days 1-5) and the duodenum harvested 14 days after the last injection (day 19; n=4). The duodenum of 7-week-old control mice was harvested at day 1 (n=3). qPCR analysis showed a significant reduction in expression of Chga, ppGcg, Gip, Nts, Pyy, Sst, Tac1 and Tph1, but an increase in Ghrl, in the duodenum of the 7- to 10-week-old Nkx2.2Δadult mice. Expression of the hormones Cck, Gast, Npy, Sct and Vip was unchanged. *P<0.05, **P<0.01, ***P<0.001.
In addition to the changes in enteroendocrine hormone expression in the duodenum, we detected differences in several transcription factors necessary for the development of specific enteroendocrine cell subtypes. Neurod1 was significantly downregulated in the duodenum of 6-week-old Nkx2.2Δint mice, consistent with the decrease in Cck and Sct expression, the two cell populations regulated by Neurod1 (Naya et al., 1997), whereas aristaless related homeobox (Arx) and Isl1 were highly upregulated (Table 2). Consistent with the increase in Ghrl+ cells, there was elevated expression of membrane bound O-acyltransferase domain containing 4 (Mboat4), the enzyme that is co-expressed with Ghrl and converts the Ghrl peptide to its biologically active, acylated form (Gutierrez et al., 2008, 2012; Kang et al., 2012) (Table 2).
Intriguingly, there was a striking upregulation of several duodenal genes that have antimicrobial and antiviral activity, and that have been implicated in the host immune response and/or inflammation in the intestine. Among these were resistin like alpha and beta (Retnla and Retnlb) (Artis et al., 2004; Munitz et al., 2009; Wang et al., 2005), defensin beta 1 (Defb1) (Morrison et al., 2002), mast cell protease 9 (Mcpt9) (Friend et al., 1998), interferon-induced protein 44 (Ifi44) (Hallen et al., 2007), vav 1 oncogene (Vav1) (Spurrell et al., 2009), TBC1 domain family, member 23 (Tbc1d23) (De Arras et al., 2012) and several genes encoding 2′-5′ oligoadenylate synthetases (Oas1a, Oas1b, Oas1g, Oas2, Oas3) (Mashimo et al., 2003). Given that expression of Nkx2.2 is restricted to the intestinal epithelium, these gene expression changes are likely to be secondary to the dysregulation of enteroendocrine populations, such as cells expressing 5-HT, Cck and Ghrl, and support the emerging concept that enteroendocrine hormones can play immunomodulatory roles in the gut (Worthington, 2015).
It is also interesting to note that the entire gasdermin C cluster (Gsdmc, Gsdmc2, Gsdmc3, Gsdmc4) was significantly upregulated in the duodenum and colon of 6-week-old Nkx2.2Δint mice (Table 2). Although it has been demonstrated that these genes are expressed in the intestinal epithelium, the function of this subfamily is relatively uncharacterized (Tamura et al., 2007).
Deletion of Nkx2.2 in Ngn3-expressing enteroendocrine progenitor cells and in the adult intestine
To determine whether Nkx2.2 functions in enteroendocrine progenitor cells to regulate subsequent lineage decisions in the duodenum, we deleted Nkx2.2 from Ngn3+ cells using the Ngn3Cre/+ allele (Schonhoff et al., 2004). Since Nkx2.2flox/flox;Ngn3Cre/+ mice (referred to hereafter as Nkx2.2Δprogenitor) die shortly after birth with severe hyperglycemia due to the absence of insulin-producing cells in the pancreas (Sussel, et al., 1998; A. J. Churchill and L.S., unpublished), we examined the duodenum at postnatal day (P) 0 by qPCR. Although expression of Nkx2.2 was only decreased by 50% in the duodenum of Nkx2.2Δprogenitor mice, we observed reduced expression of Chga, Cck, Gast, Gip, Nts, peptide YY (Pyy), Sct, Sst, Tac1 and Tph1. The expression of ppGcg, Npy and Vip was unchanged (Fig. 2B). Similar to Nkx2.2Δint mice, Ghrl expression was significantly upregulated, although to a lesser extent (Fig. 1G, Fig. 2B). Compared with Nkx2.2Δint mice, the significantly downregulated hormones were less drastically affected in Nkx2.2Δprogenitor mice. However, this could be a consequence of lower Ngn3-Cre recombination efficiency; alternatively, it could be due to the fact that Nkx2.2 is expressed in only ∼80% of Ngn3+ cells (Wang et al., 2009).
To determine whether Nkx2.2 is also required for maintenance of the enteroendocrine cell lineages during normal cellular turnover in the adult, we deleted Nkx2.2 from the duodenum of 9- to 10-week-old tamoxifen-injected Nkx2.2flox/flox;VillinCreERT2/+ and Nkx2.2flox/lacZ;VillinCreERT2/+ mice (referred to hereafter as Nkx2.2Δadult), and compared them with 7-week-old Nkx2.2Δadult mice that were not tamoxifen injected. Analysis of the intestine from Nkx2.2floxflox;VillinCreERT2/+ mice showed that Nkx2.2 was efficiently deleted after tamoxifen injection (Fig. S4A). In addition, PCR analysis exclusively for the recombined Nkx2.2flox/+ allele, but not the Nkx2.2lacZ/+ allele, confirmed successful tamoxifen-induced deletion of exon 2 of Nkx2.2 specifically in the duodenum (Fig. S4B). Chga, Ghrl, Gip, Nts, Pyy, Sst, Tac1 and Tph1 displayed a similar expression change as in the developing duodenum of Nkx2.2Δint mice (Table 1, Fig. 2C). Ghrl was similarly upregulated, regardless of the timing of Nkx2.2 deletion from the intestine (Fig. 1G, Fig. 2C; Fig. S5A,B). Interestingly, the expression of Cck, Gast, Npy, Sct and Vip was unchanged in tamoxifen-injected Nkx2.2Δadult mice, suggesting that Nkx2.2 might not be required for the continued production of these enteroendocrine subtypes in the adult. Furthermore, ppGcg was expressed at significantly lower levels in tamoxifen-injected Nkx2.2Δadult mice (Fig. 2C). We conclude that Nkx2.2 is necessary for the maintenance of only a subset of enteroendocrine cell populations in the adult.
Changes in hormone expression after mutating the SD or TN domain of Nkx2.2
Previous studies in the ventral neural tube and pancreas have shown that the TN domain of Nkx2.2 is important for interaction with the transducin-like enhancer of split (Tle) proteins (Muhr et al., 2001; Papizan et al., 2011) to regulate gene expression. The function of the SD domain of Nkx2.2 is unknown but appears to be important for endocrine cell differentiation in the pancreas (J. Levine and L.S., unpublished). To determine whether the TN or SD domains contribute to the distinct functional activities of Nkx2.2 in regulating the various enteroendocrine lineages, we generated VillinCre/+;Nkx2.2flox/TN or Nkx2.2flox/SD mice. These mice, hereafter referred to as TNint or SDint mice, express the respective mutant allele of Nkx2.2 in the intestine and are wild type for Nkx2.2 in other tissues.
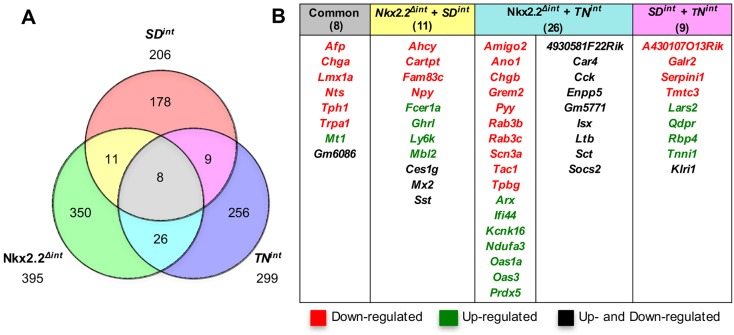
Transcriptome analysis of the duodenum of 6-week-old TNint or SDint mice revealed significant gene expression changes: 299 genes were significantly altered in expression in the TNint duodenum versus 206 genes in the duodenum of SDint mice. By comparing the datasets from Nkx2.2Δint, TNint and SDint mice, we found that only eight genes were significantly changed in the duodenum of all three mutant mouse strains (Fig. 3A). Six genes were significantly downregulated (Fig. 3B), including the enteroendocrine cell marker Chga, the enteroendocrine hormone Nts, Tph1, alpha fetoprotein (Afp) and transient receptor potential cation channel, subfamily A, member 1 (Trpa1). All of these genes have been shown to be expressed in enteroendocrine cells (Cho et al., 2014; Rindi et al., 2004; Tyner et al., 1990), suggesting that the TN and SD domains in Nkx2.2 are important for enteroendocrine cell specification, especially for the Nts+ and 5-HT+ cell subtypes (Fig. 3B; Fig. S6D-G). In addition, Ghrl+ cell numbers were also increased in both mutants (Fig. S6A-C,G). However, Ghrl expression was significantly increased in the SDint mice (Fig. 3B) but only trended up in the TNint mutant (fold change 1.78, P=0.08). Interestingly, metallothionein 1 (Mt1) is the only gene that was highly upregulated in all three mutants. Since Mt1 functions as an antioxidant, its upregulation might be due to a secondary response to altered gut hormone ratios. Comparison of Nkx2.2Δint with the TNint or SDint mice revealed that the TN domain alone is important for Pyy, Sct and Tac1 expression, whereas the SD domain alone is necessary for Npy and Sst expression (Fig. 3B).
Fig. 3.
Comparison of gene expression changes in the duodenum of Nkx2.2Δint, SDint and TNint mice. (A) Venn diagram summarizing gene expression changes in the duodenum of Nkx2.2Δint, SDint and TNint mice identified by RNA-Seq. (B) List of the eight genes with significantly changed expression in the duodenum of Nkx2.2Δint, SDint and TNint mice; the 11 genes significantly changed in Nkx2.2Δint and SDint mice; the 26 genes that are changed in both Nkx2.2Δint and TNint mice; and the nine genes significantly differentially expressed in SDint and TNint.
Lmx1a is expressed downstream of Nkx2.2 and regulates 5-HT production
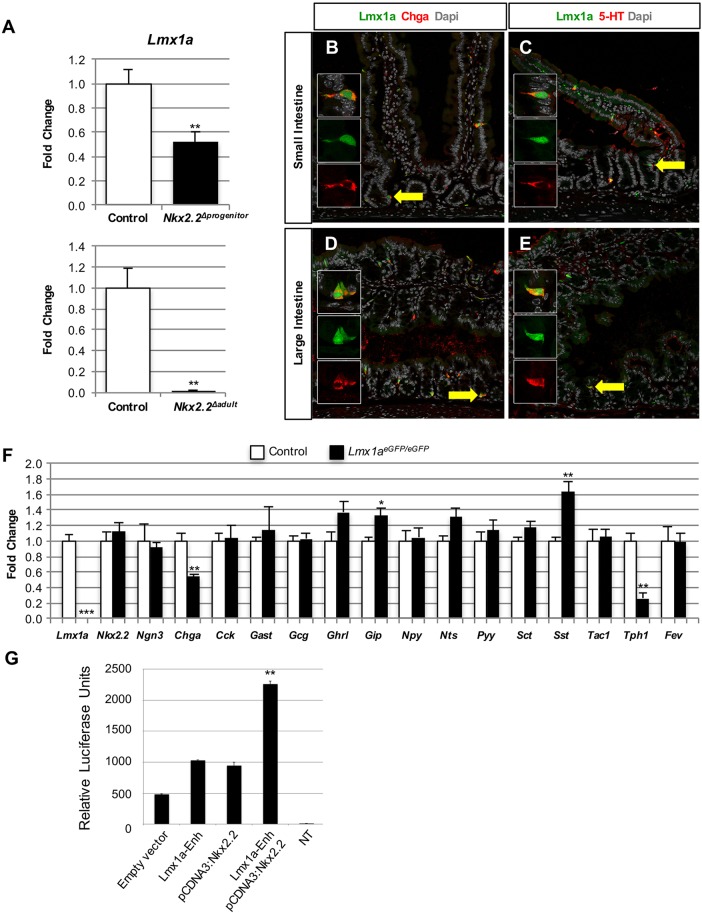
A notably downregulated gene in the duodenum of Nkx2.2Δint, SDint and TNint mice encoded the transcription factor Lmx1a. Since expression of Tph1, the rate-limiting enzyme for 5-HT biosynthesis, was decreased to a similar degree in mice carrying each of the three mutant Nkx2.2 alleles (Fig. 3B), we hypothesized that Lmx1a might be expressed in 5-HT+ cells in the intestine. Furthermore, Lmx1a expression was significantly downregulated in the duodenum of Nkx2.2Δprogenitor and Nkx2.2Δadult mice, corresponding to the observed decrease in Tph1 expression (Fig. 2B,C, Fig. 4A). Interestingly, Lmx1b, a paralog of Lmx1a, regulates serotonergic neuron development in the brain downstream of Nkx2.2 (Cheng et al., 2003; Ding et al., 2003), suggesting that Lmx1a could be important for the specification of 5-HT+ cells. We performed immunofluorescence staining on the duodenum of 6-week-old Lmx1aeGFP/+ mice and confirmed that Lmx1a is expressed in Chga+ enteroendocrine cells in the epithelium of both the small and large intestine, and is specifically expressed in 5-HT+ cells (Fig. 4B-E). To investigate whether Lmx1a is important for the differentiation of 5-HT+ cells, we assessed the intestinal phenotype of homozygous Lmx1aeGFP/eGFP null mice (Deng et al., 2011). Since these mice die shortly after birth, we analyzed the small intestine of newborn Lmx1aeGFP/eGFP mice. Chga and Tph1 expression was significantly reduced (Fig. 4F). It has been demonstrated that Fev (Pet1) + cells can lineage label 5-HT+ cells; however, deletion of Fev does not affect the formation of the enterochromaffin population (Wang et al., 2010). Interestingly, Fev expression, as well as that of Nkx2.2, was unchanged in the small intestine of newborn Lmx1aeGFP/eGFP mice (Fig. 4F), suggesting that Lmx1a functions independently of Fev and downstream of Nkx2.2 to regulate Tph1 and 5-HT biosynthesis in the gut.
Fig. 4.
Lmx1a is expressed in 5-HT-expressing cells. (A) qPCR analysis of the duodenum of Nkx2.2Δprogenitor (n=5) and Nkx2.2Δadult (control, n=3; mutant, n=4) mice reveals a significant reduction in Lmx1a in Nkx2.2Δprogenitor mice and absence of Lmx1a expression in Nkx2.2Δadult mice compared with controls. (B-E) Immunofluorescence analysis of the duodenum of 6-week-old Lmx1aeGFP/+ mice shows that Lmx1a is expressed in Chga+ enteroendocrine cells (B,D) and in 5-HT+ cells (C,E) in the small (B,C) and large (D,E) intestine. Arrows indicate co-expressing cells that are shown at higher magnification in the insets. (F) qPCR analysis of the small intestine of P0 Lmx1aeGFP/EGFP mice shows that Chga and Tph1 expression is significantly reduced, whereas Gip and Sst are upregulated (n=4). (G) Luciferase reporter assays in MIN6 cells. The pGL4.27:Lmx1a enhancer element (Lmx1a-Enh) and pcDNA3:myc-Nkx2.2 expression plasmid were co-transfected into MIN6 cells. Luciferase values were normalized to Renilla activity to account for transfection efficiencies (n=3). *P<0.05, **P<0.01, ***P<0.001.
To determine whether Nkx2.2 directly activates Lmx1a, we identified an active Lmx1a enhancer element (mm9; Chr1:169730978-169733204) in the ENCODE dataset that contained two Nkx2.2 consensus binding sites. Since an enterochromaffin cell line is not available, we tested the ability of Nkx2.2 to activate the Lmx1a enhancer in the closely related pancreatic MIN6 cell line (Ishihara et al., 1993). In this cellular context, Nkx2.2 was able to activate the Lmx1a enhancer in a luciferase assay (Fig. 4G). Future studies in a more relevant cellular context will be necessary to confirm whether endogenous Lmx1a is a direct target of Nkx2.2 in the intestinal enterochromaffin cells.
DISCUSSION
Previous studies have analyzed the function of the homeodomain transcription factor Nkx2.2 in the brain and pancreas (Briscoe et al., 1999; Sussel et al., 1998). Its role in the intestine has only been analyzed in a global knockout during embryonic development (Desai et al., 2008; Wang et al., 2009). In this study, we analyzed the intrinsic function of Nkx2.2 in the duodenum by deleting Nkx2.2 specifically in the intestinal epithelium using VillinCre/+ mice (Madison et al., 2002). Nkx2.2Δint mice are viable, but display a transiently elongated small intestine and a reduction in most enteroendocrine hormones. Despite the dramatic changes in gut hormones, glucose homeostasis remains normal. Interestingly, mice with an intestine-specific deletion of the enteroendocrine progenitor marker Ngn3, which display a loss of all enteroendocrine cells, have a different metabolic phenotype. Ngn3Δint mice are severely growth retarded, frequently die in the first week after birth, have a smaller intestine and have improved glucose clearance (Mellitzer et al., 2010). We postulate that the differences in metabolic phenotypes between these two mouse models might be attributed to the hormone Ghrl, which is highly upregulated in the Nkx2.2Δint mice (this study), but downregulated in the Ngn3Δint mutant (Mellitzer et al., 2010).
The transient increase in length of the small intestine of 6-week-old Nkx2.2Δint mice, as opposed to the decrease seen in the Ngn3Δint mice (Mellitzer et al., 2010), is also of interest. The increased length could be due to compensatory intestinal growth as an adaptive response to the alteration of gut hormone expression. For example, the altered ratios of hormones that either stimulate or inhibit proliferation, such as Gast and Sst (Thomas et al., 2003), could favor excess growth. Alternatively, we observed increased expression of insulin-like growth factor 1 (Igf1) in the Nkx2.2Δint mice at 6 weeks of age (Table 2). Igf1 is positively regulated by luminal nutrients and is able to promote growth of the epithelium of the small intestine. Furthermore, transgenic mice expressing human IGF1 exhibit a longer small intestine as well as increased villus length and crypt depth similar to Nkx2.2Δint mice (Ohneda et al., 1997). A study in rats also showed that Ghrl administration increases serum levels of IGF1, thereby stimulating duodenal growth (Warzecha et al., 2006), suggesting that the increase in Ghrl contributes to the increase in intestinal length in Nkx2.2Δint mice. It is possible that these compensatory responses are not triggered in the Ngn3Δint mice because they have a more uniform lack of all hormones.
Similar to the Nkx2.2 null mice, we demonstrated that most enteroendocrine hormones are significantly reduced in 6-week-old Nkx2.2Δint mice, with the exception of Ghrl, which is highly increased, and Gast and ppGcg, which are unchanged (Fig. S7A). However, in contrast to deletion of Nkx2.2 throughout the intestinal epithelium, Npy and Vip expression was unchanged in the Nkx2.2Δprogenitor mice, suggesting that the differentiation of these two subtypes is independent of Nkx2.2 function in the Ngn3+ progenitor cells (Fig. S7B). Deletion of Nkx2.2 in the adult intestine (Nkx2.2Δadult) also showed that Nkx2.2 plays an essential role postnatally in maintaining enteroendocrine specification during the normal turnover of enteroendocrine cells. However, during normal turnover, Nkx2.2 does not appear to be required for maintaining the Cck+, Gast+, Npy+, Sct+ and Vip+ enteroendocrine cell populations, suggesting that although these cell lineages are specified by Nkx2.2 in the embryo they are maintained in the adult by alternative mechanisms. Alternatively, ppGcg was specifically downregulated in Nkx2.2Δadult mice (Fig. S7C), suggesting there might be distinct regulatory programs for GLP-1+ cells in the adult versus the developing duodenum. Currently, it is not well understood why some enteroendocrine cell populations should be differentially regulated.
Our studies also begin to clarify the position of Nkx2.2 within the known enteroendocrine regulatory pathways. For example, the expression of Neurod1, a transcription factor that is essential for Cck+ and Sct+ cell development (Naya et al., 1997), was severely reduced in Nkx2.2Δint mice, suggesting that Nkx2.2 regulates the Cck+ and Sct+ cell lineages through the regulation of Neurod1. Furthermore, there is a correlation between Nkx2.2 and Arx regulation of Sst, in that Sst is upregulated in Arx-deficient intestine (Beucher et al., 2012; Du et al., 2012) and downregulated when Arx expression is increased in Nkx2.2Δint mice, suggesting that Sst might be regulated by Arx downstream of Nkx2.2.
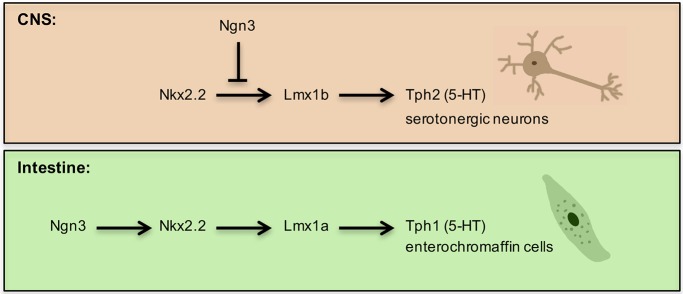
In addition to elucidating the relationship between known intestinal regulatory proteins, our studies have identified Lmx1a as a novel regulator of the 5-HT signaling pathway in the gut. Enterochromaffin cells are the major source of 5-HT in the body, regulating a variety of processes, including gut motility (Gershon, 2013). Although Lmx1a RNA expression has been reported in the intestine, the function of Lmx1a was not investigated (Makarev and Gorivodsky, 2014). We have demonstrated that Lmx1a is co-expressed with Chga and 5-HT in enterochromaffin cells and is essential for the expression of Tph1, the gut-specific 5HT-synthesizing gene. Interestingly, the Lmx1a paralog Lmx1b regulates Tph2 (Song et al., 2011) and serotonergic neuron development downstream of Nkx2.2 in the brain (Cheng et al., 2003; Song et al., 2011). Since Lmx1b is only expressed at extremely low levels in the intestine (Makarev and Gorivodsky, 2014), it is likely that Lmx1a performs analogous functions downstream of Nkx2.2 for intestinal 5-HT+ cell development. Interestingly, other regulators of 5-HT+ cell development do not appear to be conserved between the intestine and CNS. For example, Fev functions downstream of Nkx2.2 in the brain to specify 5-HT neurons (Cheng et al., 2003; Hendricks et al., 2003). In the intestine, however, expression of Fev was unchanged in Nkx2.2Δint mice. Furthermore, although Fev+ cells lineage-label 5-HT+ cells, Fev-deficient mice do not show a change in Tph1 or Chga expression, or in 5-HT+ cell numbers (Wang et al., 2010). In addition, Ascl1 – another key regulator of hindbrain 5-HT+ cells (Tsarovina et al., 2004) – is not expressed in the intestine. Furthermore, in the brain Ngn3 represses the serotonergic neuron fate (Carcagno et al., 2014), whereas in the intestine Ngn3 is expressed in enteroendocrine progenitor cells and is required for all enteroendocrine lineages, including 5-HT+ enterochromaffin cells. These findings suggest that, although Nkx2.2 and Lmx1a/b may represent conserved essential components of the transcriptional pathway regulating 5-HT+ lineages, other constituents of these pathways in the intestine and CNS have diverged to provide important tissue-specific 5-HT+ cell identities (Fig. 5).
Fig. 5.
Regulation of 5-HT biosynthesis in the CNS and intestine by Nkx2.2. In the CNS, Lmx1b is downstream of Nkx2.2 and is required for 5-HT biosynthesis by regulating Tph2 expression. Ngn3 represses the serotonergic fate. In the intestine, Nkx2.2 is downstream of the enteroendocrine progenitor marker Ngn3. We identified Lmx1a, a paralog of Lmx1b, downstream of Nkx2.2 as a regulator of Tph1 expression and thereby controls 5-HT biosynthesis in enterochromaffin cells.
In conclusion, our data show that Nkx2.2 is required for the specification of enteroendocrine cells during development and that it is necessary for the maintenance of most enteroendocrine lineages in the adult (Fig. S7). In addition, we have identified Lmx1a as a novel marker for 5-HT+ cells that is expressed downstream of Nkx2.2 to regulate Tph1 expression, which is analogous to the role of Lmx1b in CNS 5-HT+ cells (Fig. 5). Further investigation of the shared and distinct regulatory pathways of 5-HT+ cells in the intestine and CNS will help elucidate the important regulatory mechanisms that regulate superficially similar cell types in two different tissues.
MATERIALS AND METHODS
Animals
Mice were housed and treated in accordance with the animal care protocol (AAAG3206) approved by Columbia University's Institutional Animal Care and Use Committee (IACUC). Mice were maintained on a C57BL/6J background (The Jackson Laboratory). VillinCre/+ [B6.SJL-Tg(Vil-cre)997Gum/J] (Madison et al., 2002) and R26RTomato [B6.Cg-Gt(ROSA)26-Sortm14(CAG-tdTomato)Hze/J] (Madisen et al., 2010) mice were obtained from The Jackson Laboratory. Nkx2.2flox/+, Nkx2.2lacZ/+, Nkx2.2TN/+, Ngn3Cre/+, VillinCreERT2/+ and Lmx1aeGFP/+ mice were described previously (Arnes et al., 2012b; Deng et al., 2011; el Marjou et al., 2004; Mastracci et al., 2013; Papizan et al., 2011; Schonhoff et al., 2004). Nkx2.2SD/+ mice (J. Levine and L.S., unpublished) were genotyped with the following PCR primers: 5′-GCGGCAGCACCGGCAGCCGCA-3′ and 5′-GACAACGTTAACGTTGGGATG-3′. To analyze recombination of the Nkx2.2flox/+ allele in different tissues, the following primers were used, resulting in a 464 bp PCR product: 5′-TCCTTTTAAAAATCTGCCCACGTCT-3′ and 5′-GAGGTCAACTAGGCCTCAACTTGGT-3′. Unless otherwise indicated, adult mice were analyzed at 6 weeks of age. In all experiments, Nkx2.2flox/+, Nkx2.2lacZ/+, Nkx2.2flox/flox or wild-type mice were used as controls.
To delete Nkx2.2 in adult mice, 7-week-old Nkx2.2flox/flox;VillinCreERT2/+ and Nkx2.2flox/lacZ;VillinCreERT2/+ mice were injected intraperitoneally with 100 μl tamoxifen (100 mg/ml; Sigma, T5648) for 5 consecutive days (days 1-5) and analyzed 2 weeks after the last injection (day 19; Fig. 2C). These mice are referred to as Nkx2.2Δadult mice. Seven-week-old Nkx2.2Δadult mice that were not injected with tamoxifen served as controls for the qPCR analysis and were dissected at day 1 (Fig. 2C). Tamoxifen was prepared in corn oil (Sigma, C8267).
Metabolic analysis
To analyze blood glucose levels in the fed state, measurements were obtained at the same time of day while mice were kept on a regular chow diet. Intraperitoneal glucose tolerance tests (ipGTTs) were performed after a 16 h overnight fast, followed by an intraperitoneal glucose injection (2 g/kg body weight). Blood glucose was measured at 0, 15, 30, 45, 60, 90, 120 and 150 min after the glucose injection. Blood glucose measurements were taken with an Accu-Check Compact Plus glucose monitor (Model GT; Roche).
Histology and immunofluorescence
Intestines were cut longitudinally, washed with cold PBS and rolled into ‘swiss rolls' (Moolenbeek and Ruitenberg, 1981). After overnight fixation in 4% paraformaldehyde at 4°C, samples were cryopreserved with 30% sucrose and cryo-embedded in Tissue-Tek O.C.T. (Fisher Scientific, 14-373-65). Sections were cut to 5 μm thickness.
For immunofluorescence staining, sections were incubated for 15 min in 0.3% H2O2, washed in PBS and blocked for 30 min at room temperature with 10% donkey serum (Fisher Scientific, NC9624464) in PBT (PBS with 0.3% Triton X-100). Primary antibodies were diluted in 5% donkey serum in PBT and incubated on the sections overnight at 4°C. The following primary antibodies were used: rabbit anti-chromogranin A (1:500-1000; ImmunoStar, 20085), goat anti-ghrelin (1:200; Santa Cruz Biotechnology, sc-10368), rabbit anti-5-HT (1:200; ImmunoStar, 20079), rabbit anti-Sst (1:200; Phoenix Pharmaceuticals, H-060-03) and rat anti-Sst (1:500; Abcam, ab30788). The GFP signal was detected by direct fluorescence of the protein. After washing with PBT, sections were incubated with appropriate secondary antibodies diluted in 5% donkey serum in PBT for 2 h at room temperature. Secondary antibodies were conjugated with Alexa 488 or Alexa 647 (1:200; Jackson ImmunoResearch). Nuclei were stained with DAPI (1:1000; Invitrogen) for 15 min at room temperature. Sections were mounted with fluorescence mounting medium (Dako, S3023). Images were acquired with either a Zeiss LSM710 confocal microscope (Zen 2012 software) or a Leica DM5500B upright microscope (LAS AF version 2.6.0.7266 software).
To analyze tissue morphology, sections were stained with Alcian Blue (pH 2.5; Sigma, A-3157) to visualize goblet cells and counterstained with Nuclear Fast Red (Vector Laboratories, NC9483816).
Intestine measurements
To analyze the length of villi and the depth of crypts, 35 well-sectioned villi or crypts in the outermost layer of the ‘swiss roll' of the adult small intestine (Moolenbeek and Ruitenberg, 1981) were measured using ImageJ v1.48 (http://imagej.nih.gov/ij/).
Gene expression analysis
The duodenum or colon of adult mice (2 cm, measured from the stomach or caecum), 1 cm of the duodenum or the whole small intestine of newborn mice was dissected and stored at −20°C in RNAlater (Ambion, AM7021) until total RNA was extracted using the RNeasy Mini or Midi Kit (Qiagen, 74106 or 75144). cDNA was prepared with random hexamer primers and the SuperScript III First-Strand Synthesis System (Invitrogen, 18080-051). Quantitative real-time PCR (qPCR) was performed with TaqMan assays (Applied Biosystems; Table S1) and qPCR MasterMix (AnaSpec, RT-QP2X-03-15+) on a CFX96 Touch Real-Time PCR Detection System (Bio-Rad). A standard two-step real-time PCR program was used with an annealing temperature of 61°C and 40 cycles of amplification. All gene expression values were normalized to cyclophilin B (Ppib) and the fold change between wild-type and mutant samples calculated. All samples were analyzed in triplicate.
RNA sequencing was performed by the Columbia Genome Center (Columbia University) on the duodenum (n=3) and colon (n=2) of 6-week-old mice. Libraries were prepared from total RNA [RNA integrity number (RIN)>8] with the TruSeq RNA Preparation Kit (Illumina). Libraries were then sequenced using the HiSeq2000 instrument (Illumina). More than 20 million reads were mapped to the mouse genome (UCSC/mm9) using TopHat (Trapnell et al., 2009) (v2.0.4) with four mismatches and ten maximum multiple hits. Significantly differentially expressed genes were calculated using DEseq (Anders and Huber, 2010). RNA-Seq data have been deposited at GEO under series accession numbers GSE72761, GSE72762, GSE72764 and GSE78902.
Luciferase assays
A 2.226 kb fragment containing an active Lmx1a enhancer element (mm9; Chr1:169730978-169733204) was cloned into the pGL4.27 luciferase vector (Promega). 1 µg of the experimental vector pGL4.27:Lmx1a enhancer region (Lmx1a-Enh) was co-transfected with 0.1 µg Renilla luciferase vector pRL into MIN6 cells (Ishihara et al., 1993) in triplicate. The MIN6 cells were recently validated in our laboratory by RNA-Seq and tested for contamination. Luciferase activity was measured after 48 h using the Dual Luciferase Assay System (Promega). pcDNA3:myc-Nkx2.2 (pcDNA3:Nkx2.2) has been described previously (Anderson et al., 2009; Raum et al., 2006). Luciferase values were normalized to Renilla activity to account for transfection efficiencies and expressed as fold increase over the empty vector.
Data analysis
Results are expressed as mean±s.e.m. Statistical analysis on qPCR data and measurements was performed using a two-tailed unpaired Student's t-test. P<0.05 was considered significant. Fig. 3A was made with the help of Venn diagram generator (http://www.bioinformatics.lu/venn.php).
Acknowledgements
We thank S. Robine (Institut Curie, Paris, France) for the VillinCreERT2 mouse line; Giselle Dominguez and Shouhong Xuan for providing technical assistance; the Columbia University Genome Center for performing RNA-Seq; and members of the L.S. lab for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
S.G. designed and performed experiments, analyzed data and wrote the paper. D.C.G. assisted with the immunostaining and cell counting experiments. D.A.B. performed the colon RNA-Seq studies and the luciferase assays. J.M.D. provided Lmx1a mutant mice and edited the manuscript. T.L.M. assisted with generation of the Nkx2.2 floxed allele and edited the manuscript. T.P. and J.E. made the Lmx1aeGFP mice and edited the manuscript. L.S. oversaw the entire project, designed experiments, analyzed data and wrote the paper.
Funding
Funding was provided by the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases [R01 DK082590] and American Diabetes Association [7-11-MN-61]. Deposited in PMC for release after 12 months.
Data availability
The RNA-Seq data discussed in this publication have been deposited in NCBI Gene Expression Omnibus and are accessible through GEO series accession numbers GSE72761 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72761), GSE72762 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72762), GSE72764 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE72764) and GSE78902 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE78902).
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.130682.supplemental
References
- Anders S. and Huber W. (2010). Differential expression analysis for sequence count data. Genome Biol. 11, R106 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. R., Torres C. A., Solomon K., Becker T. C., Newgard C. B., Wright C. V., Hagman J. and Sussel L. (2009). Cooperative transcriptional regulation of the essential pancreatic islet gene NeuroD1 (beta2) by Nkx2.2 and neurogenin 3. J. Biol. Chem. 284, 31236-31248. 10.1074/jbc.M109.048694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnes L., Hill J. T., Gross S., Magnuson M. A. and Sussel L. (2012a). Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS ONE 7, e52026 10.1371/journal.pone.0052026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnes L., Leclerc K., Friel J. M., Hipkens S. B., Magnuson M. A. and Sussel L. (2012b). Generation of Nkx2.2:lacZ mice using recombination-mediated cassette exchange technology. Genesis 50, 612-624. 10.1002/dvg.22037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D., Wang M. L., Keilbaugh S. A., He W., Brenes M., Swain G. P., Knight P. A., Donaldson D. D., Lazar M. A., Miller H. R. P. et al. (2004). RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. USA 101, 13596-13600. 10.1073/pnas.0404034101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell K. N. and Shroyer N. F. (2015). Krupple-like factor 5 is required for proper maintenance of adult intestinal crypt cellular proliferation. Dig. Dis. Sci. 60, 86-100. 10.1007/s10620-014-3307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucher A., Gjernes E., Collin C., Courtney M., Meunier A., Collombat P. and Gradwohl G. (2012). The homeodomain-containing transcription factors Arx and Pax4 control enteroendocrine subtype specification in mice. PLoS ONE 7, e36449 10.1371/journal.pone.0036449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J., Sussel L., Serup P., Hartigan-O'Connor D., Jessell T. M., Rubenstein J. L. and Ericson J. (1999). Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature 398, 622-627. 10.1038/19315 [DOI] [PubMed] [Google Scholar]
- Carcagno A. L., Di Bella D. J., Goulding M., Guillemot F. and Lanuza G. M. (2014). Neurogenin3 restricts serotonergic neuron differentiation to the hindbrain. J. Neurosci. 34, 15223-15233. 10.1523/JNEUROSCI.3403-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Chen C. L., Luo P., Tan M., Qiu M., Johnson R. and Ma Q. (2003). Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J. Neurosci. 23, 9961-9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.-J., Callaghan B., Bron R., Bravo D. M. and Furness J. B. (2014). Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tissue Res. 356, 77-82. 10.1007/s00441-013-1780-x [DOI] [PubMed] [Google Scholar]
- De Arras L., Yang I. V., Lackford B., Riches D. W. H., Prekeris R., Freedman J. H., Schwartz D. A. and Alper S. (2012). Spatiotemporal inhibition of innate immunity signaling by the Tbc1d23 RAB-GAP. J. Immunol. 188, 2905-2913. 10.4049/jimmunol.1102595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Andersson E., Hedlund E., Alekseenko Z., Coppola E., Panman L., Millonig J. H., Brunet J.-F., Ericson J. and Perlmann T. (2011). Specific and integrated roles of Lmx1a, Lmx1b and Phox2a in ventral midbrain development. Development 138, 3399-3408. 10.1242/dev.065482 [DOI] [PubMed] [Google Scholar]
- Desai S., Loomis Z., Pugh-Bernard A., Schrunk J., Doyle M. J., Minic A., McCoy E. and Sussel L. (2008). Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev. Biol. 313, 58-66. 10.1016/j.ydbio.2007.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y.-Q., Marklund U., Yuan W., Yin J., Wegman L., Ericson J., Deneris E., Johnson R. L. and Chen Z.-F. (2003). Lmx1b is essential for the development of serotonergic neurons. Nat. Neurosci. 6, 933-938. 10.1038/nn1104 [DOI] [PubMed] [Google Scholar]
- Du A., McCracken K. W., Walp E. R., Terry N. A., Klein T. J., Han A., Wells J. M. and May C. L. (2012). Arx is required for normal enteroendocrine cell development in mice and humans. Dev. Biol. 365, 175-188. 10.1016/j.ydbio.2012.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerod K. L., Engelstoft M. S., Grunddal K. V., Nøhr M. K., Secher A., Sakata I., Pedersen J., Windeløv J. A., Füchtbauer E.-M., Olsen J. et al. (2012). A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology 153, 5782-5795. 10.1210/en.2012-1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Marjou F., Janssen K.-P., Chang B. H.-J., Li M., Hindie V., Chan L., Louvard D., Chambon P., Metzger D. and Robine S. (2004). Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186-193. 10.1002/gene.20042 [DOI] [PubMed] [Google Scholar]
- Friend D. S., Ghildyal N., Gurish M. F., Hunt J., Hu X., Austen K. F. and Stevens R. L. (1998). Reversible expression of tryptases and chymases in the jejunal mast cells of mice infected with Trichinella spiralis. J. Immunol. 160, 5537-5545. [PubMed] [Google Scholar]
- Gerbe F., van Es J. H., Makrini L., Brulin B., Mellitzer G., Robine S., Romagnolo B., Shroyer N. F., Bourgaux J.-F., Pignodel C. et al. (2011). Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J. Cell Biol. 192, 767-780. 10.1083/jcb.201010127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbe F., Legraverend C. and Jay P. (2012). The intestinal epithelium tuft cells: specification and function. Cell. Mol. Life Sci. 69, 2907-2917. 10.1007/s00018-012-0984-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D. (2013). 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr. Opin. Endocrinol. Diabetes Obes. 20, 14-21. 10.1097/MED.0b013e32835bc703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierl M. S., Karoulias N., Wende H., Strehle M. and Birchmeier C. (2006). The zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 20, 2465-2478. 10.1101/gad.381806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J. A., Solenberg P. J., Perkins D. R., Willency J. A., Knierman M. D., Jin Z., Witcher D. R., Luo S., Onyia J. E. and Hale J. E. (2008). Ghrelin octanoylation mediated by an orphan lipid transferase. Proc. Natl. Acad. Sci. USA 105, 6320-6325. 10.1073/pnas.0800708105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J. A., Willency J. A., Knierman M. D., Coskun T., Solenberg P. J., Perkins D. R., Higgs R. E. and Hale J. E. (2012). From ghrelin to ghrelin's O-acyl transferase. Methods Enzymol. 514, 129-146. 10.1016/B978-0-12-381272-8.00009-X [DOI] [PubMed] [Google Scholar]
- Habib A. M., Richards P., Cairns L. S., Rogers G. J., Bannon C. A. M., Parker H. E., Morley T. C. E., Yeo G. S. H., Reimann F. and Gribble F. M. (2012). Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 153, 3054-3065. 10.1210/en.2011-2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallen L. C., Burki Y., Ebeling M., Broger C., Siegrist F., Oroszlan-Szovik K., Bohrmann B., Certa U. and Foser S. (2007). Antiproliferative activity of the human IFN-alpha-inducible protein IFI44. J. Interferon Cytokine Res. 27, 675-680. 10.1089/jir.2007.0021 [DOI] [PubMed] [Google Scholar]
- Hendricks T. J., Fyodorov D. V., Wegman L. J., Lelutiu N. B., Pehek E. A., Yamamoto B., Silver J., Weeber E. J., Sweatt J. D. and Deneris E. S. (2003). Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron 37, 233-247. 10.1016/S0896-6273(02)01167-4 [DOI] [PubMed] [Google Scholar]
- Ishihara H., Asano T., Tsukuda K., Katagiri H., Inukai K., Anai M., Kikuchi M., Yazaki Y., Miyazaki J.-I. and Oka Y. (1993). Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 36, 1139-1145. 10.1007/BF00401058 [DOI] [PubMed] [Google Scholar]
- Jenny M., Uhl C., Roche C., Duluc I., Guillermin V., Guillemot F., Jensen J., Kedinger M. and Gradwohl G. (2002). Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21, 6338-6347. 10.1093/emboj/cdf649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J., Pedersen E. E., Galante P., Hald J., Heller R. S., Ishibashi M., Kageyama R., Guillemot F., Serup P. and Madsen O. D. (2000). Control of endodermal endocrine development by Hes-1. Nat. Genet. 24, 36-44. 10.1038/71657 [DOI] [PubMed] [Google Scholar]
- Kang K., Schmahl J., Lee J.-M., Garcia K., Patil K., Chen A., Keene M., Murphy A. and Sleeman M. W. (2012). Mouse ghrelin-O-acyltransferase (GOAT) plays a critical role in bile acid reabsorption. FASEB J. 26, 259-271. 10.1096/fj.11-191460 [DOI] [PubMed] [Google Scholar]
- Katz J. P., Perreault N., Goldstein B. G., Lee C. S., Labosky P. A., Yang V. W. and Kaestner K. H. (2002). The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 129, 2619-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S. and Ho S. B. (2010). Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12, 319-330. 10.1007/s11894-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopinke D., Brailsford M., Shea J. E., Leavitt R., Scaife C. L. and Murtaugh L. C. (2011). Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development 138, 431-441. 10.1242/dev.053843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L.-I., St-Onge L., Hougaard D. M., Sosa-Pineda B. and Gruss P. (1998). Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech. Dev. 79, 153-159. 10.1016/S0925-4773(98)00182-8 [DOI] [PubMed] [Google Scholar]
- Lopez-Diaz L., Jain R. N., Keeley T. M., VanDussen K. L., Brunkan C. S., Gumucio D. L. and Samuelson L. C. (2007). Intestinal Neurogenin 3 directs differentiation of a bipotential secretory progenitor to endocrine cell rather than goblet cell fate. Dev. Biol. 309, 298-305. 10.1016/j.ydbio.2007.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T. A., Sunkin S. M., Oh S. W., Zariwala H. A., Gu H., Ng L. L., Palmiter R. D., Hawrylycz M. J., Jones A. R. et al. (2010). A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 13, 133-140. 10.1038/nn.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison B. B., Dunbar L., Qiao X. T., Braunstein K., Braunstein E. and Gumucio D. L. (2002). Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275-33283. 10.1074/jbc.M204935200 [DOI] [PubMed] [Google Scholar]
- Makarev E. and Gorivodsky M. (2014). Islet1 and its co-factor Ldb1 are expressed in quiescent cells of mouse intestinal epithelium. PLoS ONE 9, e95256 10.1371/journal.pone.0095256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manocha M. and Khan W. I. (2012). Serotonin and GI disorders: an update on clinical and experimental studies. Clin. Transl. Gastroenterol. 3, e13 10.1038/ctg.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashimo T., Glaser P., Lucas M., Simon-Chazottes D., Ceccaldi P. E., Montagutelli X., Despres P. and Guenet J.-L. (2003). Structural and functional genomics and evolutionary relationships in the cluster of genes encoding murine 2′,5′-oligoadenylate synthetases. Genomics 82, 537-552. 10.1016/S0888-7543(03)00176-9 [DOI] [PubMed] [Google Scholar]
- Mastracci T. L., Lin C.-S. and Sussel L. (2013). Generation of mice encoding a conditional allele of Nkx2.2. Transgenic Res. 22, 965-972. 10.1007/s11248-013-9700-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May C. L. and Kaestner K. H. (2010). Gut endocrine cell development. Mol. Cell. Endocrinol. 323, 70-75. 10.1016/j.mce.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G., Beucher A., Lobstein V., Michel P., Robine S., Kedinger M. and Gradwohl G. (2010). Loss of enteroendocrine cells in mice alters lipid absorption and glucose homeostasis and impairs postnatal survival. J. Clin. Invest. 120, 1708-1721. 10.1172/JCI40794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenbeek C. and Ruitenberg E. J. (1981). The “Swiss roll”: a simple technique for histological studies of the rodent intestine. Lab. Anim. 15, 57-59. 10.1258/002367781780958577 [DOI] [PubMed] [Google Scholar]
- Morrison G., Kilanowski F., Davidson D. and Dorin J. (2002). Characterization of the mouse beta defensin 1, Defb1, mutant mouse model. Infect. Immun. 70, 3053-3060. 10.1128/IAI.70.6.3053-3060.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhr J., Andersson E., Persson M., Jessell T. M. and Ericson J. (2001). Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell 104, 861-873. 10.1016/S0092-8674(01)00283-5 [DOI] [PubMed] [Google Scholar]
- Munitz A., Seidu L., Cole E. T., Ahrens R., Hogan S. P. and Rothenberg M. E. (2009). Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J. Immunol. 182, 2357-2363. 10.4049/jimmunol.0803130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H., Fung B. P., Naya F. J., Tsai M.-J., Nishitani J. and Leiter A. B. (1997). The basic helix-loop-helix transcription factor BETA2/NeuroD is expressed in mammalian enteroendocrine cells and activates secretin gene expression. Proc. Natl. Acad. Sci. USA 94, 3560-3564. 10.1073/pnas.94.8.3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya F. J., Huang H.-P., Qiu Y., Mutoh H., DeMayo F. J., Leiter A. B. and Tsai M.-J. (1997). Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/neuroD-deficient mice. Genes Dev. 11, 2323-2334. 10.1101/gad.11.18.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda K., Ulshen M. H., Fuller C. R., D'Ercole A. J. and Lund P. K. (1997). Enhanced growth of small bowel in transgenic mice expressing human insulin-like growth factor I. Gastroenterology 112, 444-454. 10.1053/gast.1997.v112.pm9024298 [DOI] [PubMed] [Google Scholar]
- Papizan J. B., Singer R. A., Tschen S.-I., Dhawan S., Friel J. M., Hipkens S. B., Magnuson M. A., Bhushan A. and Sussel L. (2011). Nkx2.2 repressor complex regulates islet beta-cell specification and prevents beta-to-alpha-cell reprogramming. Genes Dev. 25, 2291-2305. 10.1101/gad.173039.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E. M., Bevins C. L., Ghosh D. and Ganz T. (2002). The multifaceted Paneth cell. Cell. Mol. Life Sci. 59, 156-170. 10.1007/s00018-002-8412-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raum J. C., Gerrish K., Artner I., Henderson E., Guo M., Sussel L., Schisler J. C., Newgard C. B. and Stein R. (2006). FoxA2, Nkx2.2, and PDX-1 regulate islet beta-cell-specific mafA expression through conserved sequences located between base pairs -8118 and -7750 upstream from the transcription start site. Mol. Cell. Biol. 26, 5735-5743. 10.1128/MCB.00249-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi G., Leiter A. B., Kopin A. S., Bordi C. and Solcia E. (2004). The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann. N. Y. Acad. Sci. 1014, 1-12. 10.1196/annals.1294.001 [DOI] [PubMed] [Google Scholar]
- Sato A. (2007). Tuft cells. Anat. Sci. Int. 82, 187-199. 10.1111/j.1447-073X.2007.00188.x [DOI] [PubMed] [Google Scholar]
- Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M. and Clevers H. (2011). Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415-418. 10.1038/nature09637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonhoff S. E., Giel-Moloney M. and Leiter A. B. (2004). Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 270, 443-454. 10.1016/j.ydbio.2004.03.013 [DOI] [PubMed] [Google Scholar]
- Shroyer N. F., Helmrath M. A., Wang V. Y.-C., Antalffy B., Henning S. J. and Zoghbi H. Y. (2007). Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology 132, 2478-2488. 10.1053/j.gastro.2007.03.047 [DOI] [PubMed] [Google Scholar]
- Song N.-N., Xiu J.-B., Huang Y., Chen J.-Y., Zhang L., Gutknecht L., Lesch K. P., Li H. and Ding Y.-Q. (2011). Adult raphe-specific deletion of Lmx1b leads to central serotonin deficiency. PLoS ONE 6, e15998 10.1371/journal.pone.0015998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurrell D. R., Luckashenak N. A., Minney D. C., Chaplin A., Penninger J. M., Liwski R. S., Clements J. L. and West K. A. (2009). Vav1 regulates the migration and adhesion of dendritic cells. J. Immunol. 183, 310-318. 10.4049/jimmunol.0802096 [DOI] [PubMed] [Google Scholar]
- Sussel L., Kalamaras J., Hartigan-O'Connor D. J., Meneses J. J., Pedersen R. A., Rubenstein J. L. and German M. S. (1998). Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development 125, 2213-2221. [DOI] [PubMed] [Google Scholar]
- Sykaras A. G., Demenis C., Cheng L., Pisitkun T., McLaughlin J. T., Fenton R. A. and Smith C. P. (2014). Duodenal CCK cells from male mice express multiple hormones including ghrelin. Endocrinology 155, 3339-3351. 10.1210/en.2013-2165 [DOI] [PubMed] [Google Scholar]
- Tamura M., Tanaka S., Fujii T., Aoki A., Komiyama H., Ezawa K., Sumiyama K., Sagai T. and Shiroishi T. (2007). Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics 89, 618-629. 10.1016/j.ygeno.2007.01.003 [DOI] [PubMed] [Google Scholar]
- Terry N. A., Walp E. R., Lee R. A., Kaestner K. H. and May C. L. (2014). Impaired enteroendocrine development in intestinal-specific Islet1 mouse mutants causes impaired glucose homeostasis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G979-G991. 10.1152/ajpgi.00390.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. P., Hellmich M. R., Townsend C. M. Jr and Evers B. M. (2003). Role of gastrointestinal hormones in the proliferation of normal and neoplastic tissues. Endocr. Rev. 24, 571-599. 10.1210/er.2002-0028 [DOI] [PubMed] [Google Scholar]
- Trapnell C., Pachter L. and Salzberg S. L. (2009). TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105-1111. 10.1093/bioinformatics/btp120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarovina K., Pattyn A., Stubbusch J., Muller F., van der Wees J., Schneider C., Brunet J.-F. and Rohrer H. (2004). Essential role of Gata transcription factors in sympathetic neuron development. Development 131, 4775-4786. 10.1242/dev.01370 [DOI] [PubMed] [Google Scholar]
- Tyner A. L., Godbout R., Compton R. S. and Tilghman S. M. (1990). The ontogeny of alpha-fetoprotein gene expression in the mouse gastrointestinal tract. J. Cell Biol. 110, 915-927. 10.1083/jcb.110.4.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen T., Donaldson C. J., Caceres E., Hunter A. E., Cowing-Zitron C., Pound L. D., Adams M. W., Zembrzycki A., Grove K. L. and Huising M. O. (2015). Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 21, 769-776. 10.1038/nm.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther D. J., Peter J.-U., Bashammakh S., Hortnagl H., Voits M., Fink H. and Bader M. (2003). Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299, 76 10.1126/science.1078197 [DOI] [PubMed] [Google Scholar]
- Wang M.-L., Shin M. E., Knight P. A., Artis D., Silberg D. G., Suh E. and Wu G. D. (2005). Regulation of RELM/FIZZ isoform expression by Cdx2 in response to innate and adaptive immune stimulation in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G1074-G1083. 10.1152/ajpgi.00442.2004 [DOI] [PubMed] [Google Scholar]
- Wang Y.-C., Gallego-Arteche E., Iezza G., Yuan X., Matli M. R., Choo S.-P., Zuraek M. B., Gogia R., Lynn F. C., German M. S. et al. (2009). Homeodomain transcription factor NKX2.2 functions in immature cells to control enteroendocrine differentiation and is expressed in gastrointestinal neuroendocrine tumors. Endocr. Relat. Cancer 16, 267-279. 10.1677/ERC-08-0127 [DOI] [PubMed] [Google Scholar]
- Wang Y.-C., Zuraek M. B., Kosaka Y., Ota Y., German M. S., Deneris E. S., Bergsland E. K., Donner D. B., Warren R. S. and Nakakura E. K. (2010). The ETS oncogene family transcription factor FEV identifies serotonin-producing cells in normal and neoplastic small intestine. Endocr. Relat. Cancer 17, 283-291. 10.1677/ERC-09-0243 [DOI] [PubMed] [Google Scholar]
- Warzecha Z., Dembinski A., Ceranowicz P., Dembinski M., Cieszkowski J., Konturek S. J., Polus A., Pawlik W. W., Kuwahara A., Kato I. et al. (2006). Influence of ghrelin on gastric and duodenal growth and expression of digestive enzymes in young mature rats. J. Physiol. Pharmacol. 57, 425-437. [PubMed] [Google Scholar]
- Worthington J. J. (2015). The intestinal immunoendocrine axis: novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem. Soc. Trans. 43, 727-733. 10.1042/BST20150090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Bermingham N. A., Finegold M. J. and Zoghbi H. Y. (2001). Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155-2158. 10.1126/science.1065718 [DOI] [PubMed] [Google Scholar]
- Ye D. Z. and Kaestner K. H. (2009). Foxa1 and Foxa2 control the differentiation of goblet and enteroendocrine L- and D-cells in mice. Gastroenterology 137, 2052-2062. 10.1053/j.gastro.2009.08.059 [DOI] [PMC free article] [PubMed] [Google Scholar]