Abstract
Objective
Adenocarcinoma of esophagogastric junction (AEG) is a lethal malignancy featured with early metastasis, poor prognosis, and few treatment options. Matrix metalloproteinase (MMP) and metalloproteinase suppressor (TIMP) have been considered to be associated with cancer invasion and metastasis. In our study, we evaluated expressions of MMP-9, MMP-2, TIMP-1, and TIMP-2 in AEG and their correlation with clinicopathological parameters and the overall survival rate.
Methods
Expressions of MMP-9, MMP-2, TIMP-1, and TIMP-2 in specimens from 120 AEGs were detected by immunohistochemistry. The correlations between expressions of these four proteins and clinicopathological characters were analyzed by chi-square test. Moreover, the prognostic value of these four biomarkers was evaluated by univariate analysis with Kaplan–Meier method and multivariate analysis with Cox regression model.
Results
The positive expression rate of MMP-9, MMP-2, TIMP-1, and TIMP-2 was 65%, 53%, 70%, and 49%, respectively, in the detected 120 AEG samples. MMP-9 was significantly associated with poorly histological differentiation (P=0.001), lymph node metastasis (P=0.007), and UICC stage (P=0.008). TIMP-1 showed significantly reversed correlations with histological differentiation (P=0.001), lymph node metastasis (P=0.007), and Union for International Cancer Control stage (P=0.008). Univariate analysis revealed that lymph node metastasis (P=0.002), depth of invasion (P=0.050), and MMP-9+/TIMP-1 phonotype (P<0.001) were significantly associated with the overall survival rate. Multivariate analyses demonstrated that MMP-9+/TIMP-1–phenotype was an independent prognostic factor in AEGs.
Conclusion
Detection of MMP-9 and TIMP-1 expression allows stratification of AEG patients into different survival categories and can be useful for precise individual evaluation and survival prediction.
Keywords: adenocarcinoma of esophagogastric junction, MMP-9, MMP-2, TIMP-1, TIMP-2, metastasis, prognosis
Introduction
Adenocarcinoma of esophagogastric junction (AEG) is a kind of malignant tumor originated from the distal esophagus and the esophagogastric junction.1,2 During the past 2 decades, the morbidity of AEG increased rapidly.3,4 AEG is characterized by poor prognosis because of extensive locoregional invasion and distant metastasis at the time of diagnosis.5,6 Tumor progression is a multistep process, in which many genes and proteins are involved.7 The function of extracellular matrix (ECM) in many kinds of cancers have been revealed, but the clinical significance in AEG is still in mist.
The matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that are involved in degrading ECM and facilitating tumor invasion.8 MMP-9 and MMP-2 are gelatinases of MMP family and degrade substrates of type IV collagen and gelatin.9,10 Previous evidence has demonstrated that MMP-2 and/or MMP-9 are associated with invasion and metastasis in esophageal carcinoma. However, these experiments were predominantly carried on esophageal squamous cell carcinoma; whether the conclusion suits for AEG are quite debatable without experiments because of different pathological types.
TIMP-1 and TIMP-2 are natural suppressors of MMP-9 and MMP-2 and are theoretically presumed to prevent tumor growth, locoregional invasion, and metastasis.11 However, paradoxical roles of TIMPs were reported to promote tumorigenesis in growth-stimulating and antiapoptotic manner.12,13 Till date, there is little evidence about the clinical significance of TIMP-1 and TIMP-2 in human AEG, and the prognostic value of their interaction with MMP-9 and MMP-2 is not investigated until now.
In our study, we investigated the expression of MMP-9, MMP-2 and their suppressors TIMP-1 and TIMP-2 in 120 cases of AEGs to explore the associations with clinical pathological features. Moreover, we estimated their prognostic value with univariate and multivariate analysis.
Methods
Our study consisted of 120 patients with AEGs (males 63; females 57) who underwent surgical resections between 2006 and 2012 in Jinan Central Hospital affiliated to Shandong University and Qilu Hospital affiliated to Shandong University. Follow-up data were available for 106 patients, ranging from 2 to 62 months. Detailed clinical and pathological profiles were collected from medical records. Tumor staging and histological classifications were assessed according to the seventh edition of American Joint Committee on Cancer TNM classification.14 The study was approved by the Human Research Review Committee of Jinan Central Hospital and Qilu Hospital. Written informed consent was obtained from all the patients.
Immunohistochemistry
Specimens were deparaffinized and rehydrated by successively passing through xylene and ethanol of gradually reducing concentration. After washing in phosphate-buffered saline for three times, slides were infiltrated in 0.01 M citrate buffer (pH 6.0) and boiled for 15 minutes in microwave for antigen retrieval. Then 3% hydrogen peroxide was dropped onto the slides to quench the endogenous peroxidase activity.
The sections were incubated with rabbit anti-human monoclonal antibody against MMP-9 (1:500 dilution; ab58803; Abcam), MMP-2 (1:500 dilution; ab2462; Abcam), TIMP-1 (1:500 dilution; ab109125; Abcam), and TIMP-2 antibodies (1:500 dilution; ab79472; Abcam). The slides were incubated overnight at 4°C and then incubated with horseradish peroxidase for 30 minutes at 37°C; 3,3-diaminobenzidine tetrahydrochloride was used as chromogen for visualization.
The results of immunohistochemistry were evaluated by Rajkumar score,15 in which the staining intensity was scored as (range: 0 to 3) and the percentage of positive cells as (range: 0 to 4 [0, {0%–10%}, 1 {11%–25%}, 2 {26%–50%}, 3 {51%–75%}, and 4 {76%–100%}]). After multiplying the scores of two parameters, slides with scores of 8 or higher were classified as high expression and slides with scores less than 8 as lower expression.
Statistical analysis
The associations between expressions of the four items with clinicopathological variables were assessed by chi-square test. Kaplan–Meier survival functions were used to plot overall survival, and statistical significance was analyzed by log-rank test. Multivariate Cox proportional hazards model was constructed to analyze significant variables verified by univariate analysis. P-values were calculated with significance level <0.05. All calculations were performed with SPSS software package, standard Version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Associations between MMP-9, MMP-2, TIMP-1, TIMP-2, and clinicopathological variables
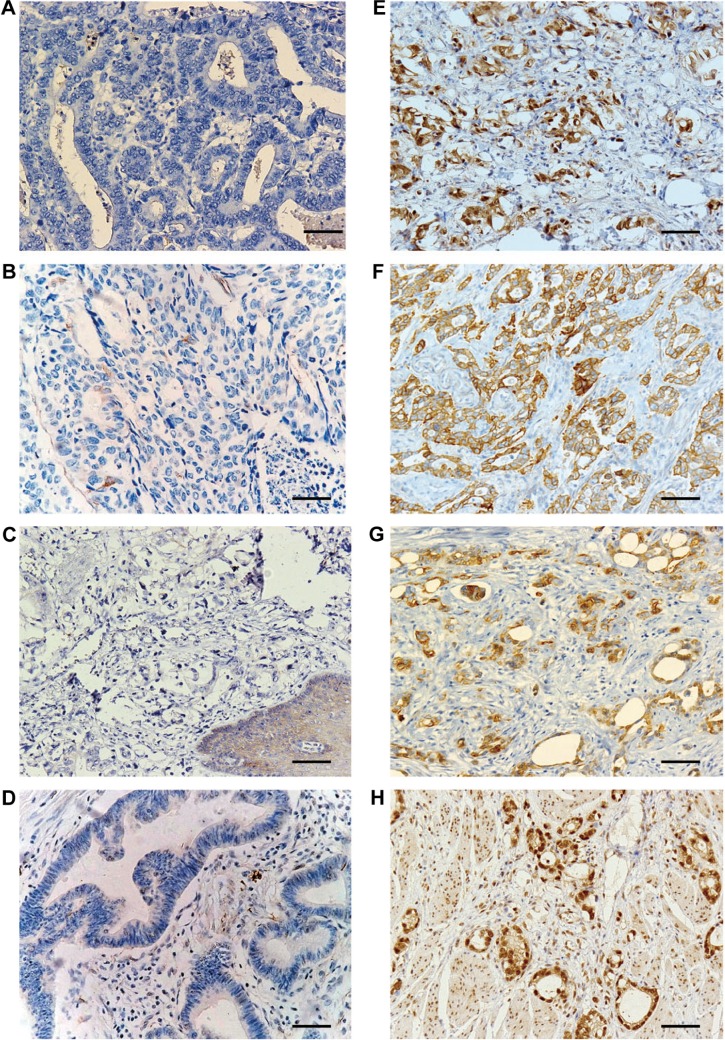
MMP-9- and MMP-2-positive immunoreactivity was localized in the cytoplasm and plasma membrane of tumor cells, while TIMP-1- and TIMP-2-positive staining was observed in the tumor cell cytoplasm and surrounding stromal cells (Figure 1). MMP-9, MMP-2, TIMP-1, and TIMP-2 overexpression were observed in 71 (59.2%), 62 (51.7%), 84 (70%), and 63 (52.5%) of 120 AEGs, respectively. In this experiment, MMP-9 was significantly associated with poor histological differentiation (P=0.002), lymph node metastasis (P=0.0.018), and UICC stage (P=0.010) but not with age (P=0.140), tumor size (P=0.218), and depth of invasion (P=0.800). TIMP-1 expression showed reversed significantly correlations with histological differentiation (P=0.031), lymph node metastasis (P=0.041) and UICC stage (P=0.010). By contrast, no significant associations were identified in MMP-2 and TIMP-2 with other clinicopathological parameters in this study (Table 1).
Figure 1.
Representative cases of IHC staining for MMP-2, MMP-9, TIMP-1, and TIMP-2 of AEG.
Notes: (A and B) Case negative for MMP-2 and MMP-9 for IHC staining; (C and D) case negative for TIMP-1 and TIMP-2 for IHC staining; (E and F) strong membrane and cytoplasm staining of MMP-2 and MMP-9; (G and H) strong expression of TIMP-1 and TIMP-2 in the cytoplasm. Magnification ×200; Scale bar =50 μm.
Abbreviations: IHC, immunohistochemistry; MMP, matrix metalloproteinase; AEG, adenocarcinoma of esophagogastric junction.
Table 1.
Association of expression of MMP-9, MMP-2, TIMP-1, and TIMP-2 with clinicopathological parameters in AEG
| Parameters | N | MMP-9 positive
|
MMP-2 positive
|
TIMP-1 positive
|
TIMP-2 positive
|
||||
|---|---|---|---|---|---|---|---|---|---|
| (%) | P-value | (%) | P-value | (%) | P-value | (%) | P-value | ||
| Age (years) | |||||||||
| <60 | 50 | 34 (68.0) | 0.140 | 26 (52.0) | 0.951 | 32 (64.0) | 0.243 | 41 (58.6) | 0.470 |
| ≥60 | 70 | 37 (52.9) | 36 (51.4) | 52 (74.3) | 32 (56.1) | ||||
| Sex | |||||||||
| Male | 63 | 38 (60.3) | 0.787 | 33 (52.4) | 0.822 | 40 (63.5) | 0.115 | 32 (55.2) | 0.588 |
| Female | 57 | 33 (57.9) | 29 (50.9) | 44 (77.2) | 24 (49.0) | ||||
| Tumor size (cm) | |||||||||
| <5 | 62 | 40 (64.5) | 0.218 | 30 (48.4) | 0.457 | 42 (67.7) | 0.691 | 0.800 | |
| ≥5 | 58 | 21 (53.4) | 32 (55.2) | 42 (72.4) | |||||
| Well | 23 | 7 (30.4) | 10 (43.5) | 17 (73.9) | |||||
| Histological differentiation | |||||||||
| Depth of invasion | |||||||||
| Poor | 49 | 36 | 0.800 | 25 (51.0) | 0.457 | 28 (57.1) | 0.011* | 13 (39.4) | 0.143 |
| T1–T2 | 58 | 35 (60.3) | 32 (55.2) | 47 (81.0) | |||||
| T3–T4 | 62 | 36 (58.1) | 30 (48.4) | 37 (59.7) | 37 (59.7) | ||||
| Lymph node metastasis | |||||||||
| Positive | 74 | 50 (67.6) | 0.018* | 36 (48.6) | 0.401 | 57 (77.0) | 0.041* | 39 (52.7) | 0.955 |
| Negative | 46 | 21 (45.7) | 26 (56.5) | 27 (58.7) | 24 (52.2) | ||||
| UICC stage | |||||||||
| I+II | 59 | 28 (47.5) | 0.010* | 29 (49.2) | 0.588 | 48 (81.4) | 0.010* | 26 (44.1) | |
| III+IV | 61 | 43 (70.5) | 33 (54.1) | 36 (59.0) | 37 (60.7) | ||||
| Adjuvant chemotherapy | |||||||||
| Yes | 89 | 55 (61.8) | 0.320 | 50 (56.2) | 0.142 | 65 (73.0) | 0.258 | 46 (51.7) | 0.836 |
| No | 31 | 16 (51.6) | 12 (38.7) | 19 (61.3) | 17 (54.8) | ||||
Note:
Chi-square test, difference with P<0.05.
Abbreviations: MMP, matrix metalloproteinase; AEG, adenocarcinoma of esophagogastric junction; T, tumor (TNM staging system); TIMP, tissue inhibitor of metalloproteinase; UICC, Union for International Cancer Control.
Univariate and multivariate analyses in AEG
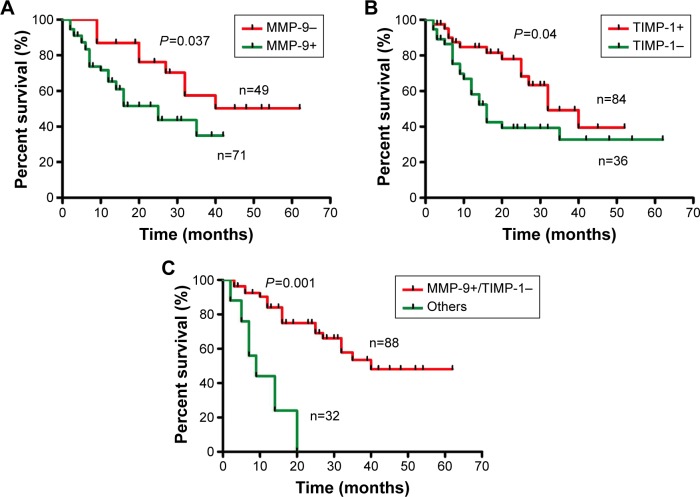
Univariate analysis revealed that lymph node metastasis (P=0.001), depth of invasion (P=0.024), and adjuvant chemotherapy (P=0.050) were significantly associated with overall survival (Table 2). Likewise, MMP-9 and TIMP-1 were associated with overall survival with statistical significance P=0.037 and P=0.040, respectively. In the subgroup of MMP-9+/TIMP-, the median survival was 15.0 months compared to 35 months of other phenotypes as the control group. Moreover, MMP-9+/TIMP- was demonstrated to correlate with poorer prognosis (P=0.001) (Figure 2). Subsequently, multivariate analysis was performed with Cox regression model. The six parameters which were proved to have prognostic significance in univariate analysis were included: depth of invasion, lymph node metastasis, TIMP-1, MMP-9, MMP-9+/TIMP-1-, and adjuvant chemotherapy. All the independent prognostic factors confirmed by multivariate analysis were displayed, and the factors without clinical significance were excluded from Table 3. Notably, MMP-9+/TIMP- was included in the prognostic panel (hazard ratio [95% confidence interval]: 3.014 [2.432–3.237], P=0.002) in multivariate analysis (Table 3).
Table 2.
Univariate analysis of overall survival in AEG
| Parameters | Category | No of events (%) | Median survival time (months) | 95% confidence interval | P-value |
|---|---|---|---|---|---|
| Depth of invasion | T1+T2 | 19 (32.8) | 28 | 24.3–36.5 | 0.024 |
| T3+T4 | 33 (53.2) | 20 | 19.5–36.1 | ||
| Lymph node metastasis | Negative | 17 (36.9) | 40 | 21.0–58.6 | 0.001 |
| Positive | 35 (47.3) | 30 | 17.0–34.3 | ||
| TIMP-1 | Negative | 20 (55.6) | 29 | 25.6–40.1 | 0.040 |
| Positive | 32 (38.1) | 33 | 30.8–40.4 | ||
| MMP-9 | Negative | 36 (50.7) | 41 | 30.6–43.3 | 0.037 |
| Positive | 16 (32.7) | 22 | 13.4–31.7 | ||
| MMP-9+/TIMP-1- | Yes | 21 (65.6) | 18 | 14.2–30.5 | 0.001 |
| No | 31 (35.2) | 35 | 28.1–47.6 | ||
| Adjuvant chemotherapy | No | 15 (48.4) | 26 | 22.8–34.5 | 0.050 |
| Yes | 37 (41.5) | 31 | 29.6–40.2 |
Abbreviations: AEG, adenocarcinoma of esophagogastric junction; MMP-9, matrix metalloproteinase 9; T, tumor (TNM staging system); TIMP, tissue inhibitor of metalloproteinase.
Figure 2.
Survival curves stratified by MMP-9 and TIMP-1 in AEG (Kaplan–Meier method).
Notes: Univariate analysis of MMP-9 and TIMP-1 in AEG is shown in (A and B), respectively. (C) The outcome of MMP-9-positive and TIMP-1-negative phenotype was significantly worse than other combinations of MMP-9 and TIMP-1.
Abbreviations: MMP-9, matrix metalloproteinase 9; AEG, adenocarcinoma of esophagogastric junction.
Table 3.
Multivariate analysis of overall survival in AEG
| Parameters | Category | HR | 95% CI | P-value |
|---|---|---|---|---|
| N stage | Negative | 1 | ||
| Positive | 2.337 | 2.024–4.166 | 0.008 | |
| T stage | I+II | 1 | ||
| III+IV | 1.987 | 1.651–2.304 | 0.035 | |
| MMP-9+/TIMP-1- | Others | 1 | ||
| Positive | 3.014 | 2.432–3.237 | 0.002 |
Abbreviations: HR, hazard ratio; CI, confidence interval; MMP-9, matrix metalloproteinase 9.
Discussion
In this study, we evaluated the expressions of MMP-2, MMP-9 and their natural suppressors TIMP-1 and TIMP-2 in AEG based on immunohistochemistry. Among all the MMPs, MMP-2 and MMP-9 are well identified because of the ability to degrade basement membrane type IV collagen, which would facilitate tumor cells invasion. The expression of MMP-9 was correlated significantly with tumor differentiation (P=0.020) and lymph node metastasis (P=0.018) but not with the T stage, age, and sex. In survival analysis, the expression of MMP-9 was proved to correlate with poorer survival, but the evidence is not sufficient to consider MMP-9 as an independent prognostic marker in multivariate analysis. Our results correspond to the majority of the previous studies on AEGs and expand the understanding of AEG biomarker study.16–18 As far as we know, we investigated the expression of MMP-2, MMP-9, TIMP-1, and TIMP-2 in AEG and furthermore evaluated their clinical significance including prognostic value for the first time. As a result, we demonstrated that MMP-9+/TIMP-1- phenotype could predict more unfavorable prognosis.
Recent studies on the functions of TIMPs in cancer progression were quite controversial. Elevated TIMP-1 expression was reported to promote cancer cell proliferation and invasion and proved to correlate with cancer progression and unfavorable prognosis in certain tumor types.12,19,20 In esophageal cancers, Mori et al21 demonstrated that high TIMP-1 mRNA in esophageal tumor was associated with advanced stage and poor prognosis, but this study only focused on esophageal cancer and the main pathological type of esophageal cancer is squamous carcinoma. Unlike previous studies, our study focused on AEG, which is totally different with esophageal squamous cancer in the aspect of tumor site and pathologic type. In our study, TIMP-1 was demonstrated to be expressed more frequently in well-differentiated AEG with significant difference (P=0.031) and to be negatively correlated with lymph node metastasis (P=0.041). Our observation was in concordance with the report by Vegh et al, who found that TIMP-1 expression was lower in positive lymph nodes cases in contrast to those with negative nodes (P<0.0005) in a cohort of 23 adenocarcinoma samples.22 In tumor tissues, the source of TIMP-1 is not only carcinoma cells per se but also macrophages or fibroblasts around tumor nests; the diversity of methodology in detection of TIMP-1 and the heterogeneity of cancers among these experiments ought to be taken into account. One possible explanation is that in AEG, elevated TIMP-1 is more likely to have a host response to regulate MMP activity and maintain ECM integrity, which control tumor invasion. TIMP-2 inhibits the activity of MMP-2, thus suppressing carcinoma invasion and metastasis,23,24 which of course needs to be demonstrated by further experiments.
In our study, we demonstrated that tumors with enhanced MMP-9 and decreased TIMP-1 expression could predict a more unfavorable prognosis of patients with AEGs. Nowadays, more detailed stratification of cancers by genes and proteins tends to be a promising field for individual treatment relying on developing high-throughput techniques such as mass spectrum and microarray. Unfortunately, similar studies on AEG were not performed partially because of tumor specificity of AEG. Our finding proved that patients with MMP-9+/TIMP-1- phenotype had worse prognosis, indicating that those patients are high-risk group for tumor recurrence and progression. Meanwhile, these patients had more urgent need for chemotherapy. AEG is a special kind of tumor that exhibits different biological behaviors in both esophageal and gastric cancers. However, studies on AEG biomarkers need more attention compared with studies on esophageal and gastric cancers. We hope our study could expand the understanding of AEG and may help find more underlying mechanism of AEG progression.
Conclusion
Our finding demonstrated that MMP-9+/TIMP-1- phenotype is associated with poorer prognosis and could be identified as an independent prognostic factor in AEG. Detection of MMP-9 and TIMP-1 expression status is necessary for the stratification of AEG patients into different survival categories, and it can be useful to provide more precise individual evaluation and survival prediction.
Acknowledgments
The authors thank Dr Wang Pingan, Dr Yang Hui, Dr Shi Weichen, Dr Liu Hongda, Dr Song Xie, and Dr Chen Hongqiang for their professional advice. This study was supported by Independent Innovation Foundation of Shandong University (Grant No 2014TB026).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97(2):142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Rudiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232(3):353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crew KD, Neugut AI. Epidemiology of upper gastrointestinal malignancies. Semin Oncol. 2004;31(4):450–464. doi: 10.1053/j.seminoncol.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa S, Yoshikawa T. Adenocarcinoma of the esophagogastric junction: incidence, characteristics, and treatment strategies. Gastric Cancer. 2010;13(2):63–73. doi: 10.1007/s10120-010-0555-2. [DOI] [PubMed] [Google Scholar]
- 5.Siewert JR, Stein HJ, Feith M. Adenocarcinoma of the esophago-gastric junction. Scand J Surg. 2006;95(4):260–269. doi: 10.1177/145749690609500409. [DOI] [PubMed] [Google Scholar]
- 6.Feith M, Stein HJ, Siewert JR. Adenocarcinoma of the esophagogastric junction: surgical therapy based on 1602 consecutive resected patients. Surg Oncol Clin N Am. 2006;15(4):751–764. doi: 10.1016/j.soc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Gu ZD, Li JY, Li M, et al. Matrix metalloproteinases expression correlates with survival in patients with esophageal squamous cell carcinoma. Am J Gastroenterol. 2005;100(8):1835–1843. doi: 10.1111/j.1572-0241.2005.50018.x. [DOI] [PubMed] [Google Scholar]
- 8.Ii M, Yamamoto H, Adachi Y, Maruyama Y, Shinomura Y. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med. 2006;231(1):20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 9.Thornton KJ, Kamange-Sollo E, White ME, Dayton WR. Role of G protein-coupled receptors (GPCR), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), heparin-binding epidermal growth factor-like growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (IGF-1R) in trenbolone acetate-stimulated bovine satellite cell proliferation. J Anim Sci. 2015;93(9):4291–4301. doi: 10.2527/jas.2015-9191. [DOI] [PubMed] [Google Scholar]
- 10.Antunes LA, Antunes LS, Kuchler EC, et al. Analysis of the association between polymorphisms in MMP2, MMP3, MMP9, MMP20, TIMP1, and TIMP2 genes with white spot lesions and early childhood caries. Int J Paediatr Dent. 2015 doi: 10.1111/ipd.12202. [DOI] [PubMed] [Google Scholar]
- 11.Khokha R, Waterhouse P, Yagel S, et al. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989;243(4893):947–950. doi: 10.1126/science.2465572. [DOI] [PubMed] [Google Scholar]
- 12.Zeng ZS, Cohen AM, Zhang ZF, Stetler-Stevenson W, Guillem JG. Elevated tissue inhibitor of metalloproteinase 1 RNA in colorectal cancer stroma correlates with lymph node and distant metastases. Clin Cancer Res. 1995;1(8):899–906. [PubMed] [Google Scholar]
- 13.Nomura H, Fujimoto N, Seiki M, Mai M, Okada Y. Enhanced production of matrix metalloproteinases and activation of matrix metalloproteinase 2 (gelatinase A) in human gastric carcinomas. Int J Cancer. 1996;69(1):9–16. doi: 10.1002/(SICI)1097-0215(19960220)69:1<9::AID-IJC3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar T, Stamp GW, Pandha HS, Waxman J, Gullick WJ. Expression of the type 1 tyrosine kinase growth factor receptors EGF receptor, c-erbB2 and c-erbB3 in bladder cancer. J Pathol. 1996;179(4):381–385. doi: 10.1002/(SICI)1096-9896(199608)179:4<381::AID-PATH603>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Bashash M, Shah A, Hislop G, et al. Genetic polymorphisms at TIMP3 are associated with survival of adenocarcinoma of the gastroesophageal junction. PloS One. 2013;8(3):e59157. doi: 10.1371/journal.pone.0059157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allott EH, Lysaght J, Cathcart MC, et al. MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation. Mol Carcinog. 2013;52(2):144–154. doi: 10.1002/mc.21840. [DOI] [PubMed] [Google Scholar]
- 18.Murray GI, Duncan ME, O’Neil P, McKay JA, Melvin WT, Fothergill JE. Matrix metalloproteinase-1 is associated with poor prognosis in oesophageal cancer. J Pathol. 1998;185(3):256–261. doi: 10.1002/(SICI)1096-9896(199807)185:3<256::AID-PATH115>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Urbanski SJ, Edwards DR, Hershfield N, et al. Expression pattern of metalloproteinases and their inhibitors changes with the progression of human sporadic colorectal neoplasia. Diagn Mol Pathol. 1993;2(2):81–89. [PubMed] [Google Scholar]
- 20.Birgisson H, Nielsen HJ, Christensen IJ, Glimelius B, Brunner N. Preoperative plasma TIMP-1 is an independent prognostic indicator in patients with primary colorectal cancer: a prospective validation study. Eur J Cancer. 2010;46(18):3323–3331. doi: 10.1016/j.ejca.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Mori M, Mimori K, Sadanaga NI, et al. Prognostic impact of tissue inhibitor of matrix metalloproteinase-1 in esophageal carcinoma. Int J Cancer. 2000;88(4):575–578. doi: 10.1002/1097-0215(20001115)88:4<575::aid-ijc9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Vegh I, Santiuste AD, Colina F, et al. Relationship between biomarker expression and allelic alteration in esophageal carcinoma. J Gastroenterol Hepatol. 2007;22(12):2303–2309. doi: 10.1111/j.1440-1746.2006.04374.x. [DOI] [PubMed] [Google Scholar]
- 23.DeClerck YA, Imren S. Protease inhibitors: role and potential therapeutic use in human cancer. Eur J Cancer. 1994;30A(14):2170–2180. doi: 10.1016/0959-8049(94)00460-m. [DOI] [PubMed] [Google Scholar]
- 24.Imren S, Kohn DB, Shimada H, Blavier L, DeClerck YA. Overexpression of tissue inhibitor of metalloproteinases-2 retroviral-mediated gene transfer in vivo inhibits tumor growth and invasion. Cancer Res. 1996;56(13):2891–2895. [PubMed] [Google Scholar]