Abstract
Preeclampsia (PE) is a form of gestational hypertension that complicates ~ 5 percent of pregnancies worldwide. Over 70 percent of the fatal cases of PE are attributed to cerebral edema, intracranial hemorrhage, and eclampsia. The etiology of PE originates from abnormal remodeling of the maternal spiral arteries, creating an ischemic placenta that releases factors that drive the pathophysiology. An initial neurological outcome of PE is the absence of the autonomically regulated cardiovascular adaptations to pregnancy. PE patients exhibit sympathetic overactivation, in comparison to both normotensive pregnant and hypertensive non-pregnant females. Moreover, PE diminishes baroreceptor reflex sensitivity (BRS) beyond that observed in healthy pregnancy. The absence of the cardiovascular adaptations to pregnancy, combined with sympathovagal imbalance and a blunted BRS leads to life-threatening neurological outcomes. Behaviorally, the increased incidences of maternal depression, anxiety, and post-traumatic stress disorder (PTSD) in PE are correlated to low fetal birth weight, intrauterine growth restriction (IUGR) and premature birth. This review addresses these neurological consequences of PE that present in the gravid female both during and after the index pregnancy.
Keywords: Pregnancy, hypertension, preeclampsia, sympathovagal imbalance, disinhibition
Introduction
Preeclampsia (PE) is a distinct form of gestational hypertension that typically presents after the 20th week of gestation and complicates ~5 percent of pregnancies worldwide (1, 2), with developing countries having an incidence almost seven times higher than that of industrialized nations (3). The current diagnostic criteria classifies PE (in the absence of proteinuria) as hypertension associated with thrombocytopenia (platelet count less than 100,00 / microliter), liver dysfunction with liver transaminase blood levels at least double the normal concentration, elevated serum creatinine in excess of 1.1 mg/dL, pulmonary edema, and /or new-onset cerebral or visual impairments (4). PE results in more than 60,000 maternal deaths per year (3, 5), with over 70 percent of these fatal cases being neurological in cause, and are attributed to cerebral edema, intracranial hemorrhage, and eclampsia (3, 5–7). The exact etiology of PE is unknown, but the disorder originates from abnormal remodeling of the spiral arteries at the maternal-placental interface, creating an ischemic placenta that releases factors that drive the pathophysiology (8, 9). An initial neurological outcome of PE is the impairment of visceral motor autonomic control of maternal hemodynamics (Table 1), such that the cardiovascular physiological adaptations characteristic of healthy pregnancy are absent (10–14). One of these critical adaptations is the biphasic alteration of autonomic firing patterns, which adjusts sympathovagal balance to meet the metabolic demands of pregnancy (15, 16). PE is distinguished by sympathetic overactivation, where sympathetic firing is three times higher than in normotensive pregnant females, and quadruple the rate of hypertensive non-pregnant females (17, 18). Moreover, PE blunts baroreceptor reflex sensitivity (BRS) beyond that observed in healthy pregnancy (14, 19–21).
Table 1.
The visceral motor autonomic influence of the cardiovascular physiological adaptations of pregnancy vs. the maternal hemodynamics of PE. In healthy pregnancy, higher brain centers direct autonomic neural circuitry to produce an expanded blood volume, increased cardiac output, decreased peripheral vascular resistance, and biphasic alterations in sympathetic firing. However, in PE, these adaptations are absent, resulting in an insufficient blood volume increase, decreased cardiac output, increased peripheral vascular resistance, and sympathetic overexcitation.
| Autonomic Influence of the Cardiovascular Physiological Adaptations of Pregnancy | ||||
|---|---|---|---|---|
| Cardiovascular Physiological Component |
Higher Brain Inputs |
Key Neural Circuitry |
Adaptive Mechanism of Healthy Pregnancy |
Maladaptive Perturbations of PE |
| Blood Volume |
Circumventricular Organs of the Lamina Terminals |
|
|
|
| Cardiac Output (CO) |
Rostral Ventro- lateral medulla (RVLM) |
|
|
|
| Peripheral Vascular Resistance (PVR) |
RVLM |
|
|
|
| Sympatho- vagal Balance |
Solitary Tract Nucleus (NTS), Caudal Ventro- lateral Medulla (CVLM), RVLM |
|
|
|
Abbreviations: PVN, paraventricular nucleus; SON, supraoptic nucleus; IUGR, intrauterine growth restriction; IMLCC, intermediolateral cell column; T1–T4, thoracic spinal cord levels 1–4; ACh, acetylcholine; NE, norepinephrine; cAMP, cyclic AMP; PKA, protein kinase A; T1–L2, thoracic spinal cord levels T1–L2; AT1R-AABs, agonistic autoantibodies to the angiotensin AT1 receptor; AT1-B2 heterodimer, AT1 receptor – Bradykinin-2 receptor heterodimer; Ang 1–7, Angiotensin 1–7; CN IX, cranial nerve X (glossopharyngeal nerve); CN X, cranial nerve X (vagus nerve); BR, baroreceptor; LF:HF, low frequency to high frequency ratio (index of sympathovagal balance).
In the absence of the cardiovascular adaptations to pregnancy (Figure 1), the combination of an impaired BRS, sympathovagal imbalance, and pathogenic factors from the ischemic placenta advance the sequelae of life-threatening white matter lesions (22–24), cerebral edema and hemorrhaging (25–27) that can lead to executive dysfunction (28–30). Furthermore, PE establishes predispositions to anxiety (31–33), depression (34–36), and PTSD (37–42) in the gravid female, which are significantly correlated to low fetal birth weight, IUGR, and premature birth (32–36, 38). This review examines the neural control of blood pressure in healthy pregnancy, and addressess the resulting neurological outcomes of PE that present both during and after the index pregnancy.
Figure 1.
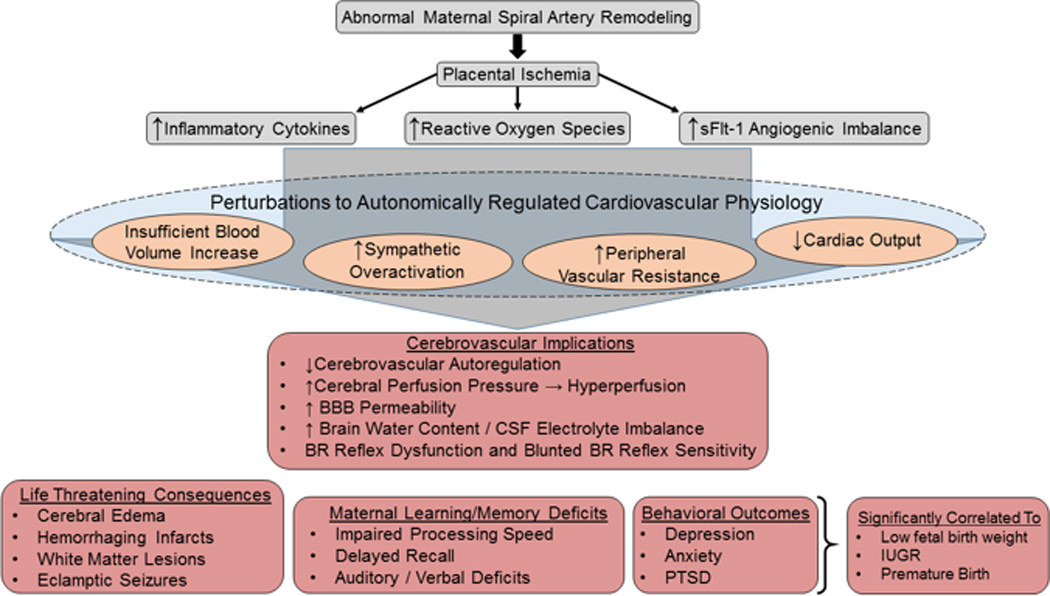
The neurological consequences and life-threatening implications of PE. The initial pathophysiology of PE (gray boxes) originates from abnormal remodeling of the spiral arteries at the maternal-placental interface that creates placental ischemia/hypoxia. The ischemic placenta releases inflammatory cytokines, reactive oxygen species, and the anti-angiogenic factor sFlt-1. Furthermore, in the absence of the autonomically regulated cardiovascular adaptations to pregnancy, perturbations in cardiovascular physiology (orange circles) result. The combination of ischemic placental pathogenic factors and the lack of cardiovascular adaptations to pregnancy, lead to cerebrovascular implications (middle red box) that include autoregulatory breakthrough, increased BBB permeability, CSF electrolyte imbalance, BRS impairment, sympathovagal imbalance. The life-threatening consequences (bottom left red box) include white matter lesions, cerebral edema, hemorrhaging infarcts, and eclamptic seizures. Executive dysfunction (bottom middle and right red boxes) include impaired processing speed that can persist for decades after the index pregnancy, delayed recall, and auditory /verbal memory impairments. The behavioral outcomes of depression, anxiety, and PTSD are all significantly associated with low fetal birth weight, IUGR, and premature birth.
The Autonomic Regulation of Cardiovascular Physiological Adaptations of Healthy Pregnancy vs. the Maternal Hemodynamics of PE
One of the initial and most dramatic changes observed in the gravid female is an expanded blood volume (43). To prepare for a typical blood loss of 500 ml (vaginal) to 1 liter (Cesarean) during delivery, osmoregulatory brain circuity (44) acts via neuroendocrine mechanisms to elicit increases in total blood volume beginning at 6 weeks gestation (45). During pregnancy, the plasma osmolality threshold for stimulating osmoreceptors is lowered from the normal 285 to 270 mOsm/kg (46). Relaxin secreted by the corpus luteum (47) acts on the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ that project to and excite the magnocellular neurons in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus (48). Axons of these PVN and SON cell bodies project into the posterior pituitary, where they release vasopressin into the general circulation (44, 49, 50). Vasopressin acts on renal V2 receptors to upregulate aquaporin-2 water channel expression on the cells of the distal convoluted tubule and collecting duct, thereby increasing the amount of water reabsorbed from urine and returning it to the blood volume (51, 52). As a result, by 32 weeks gestation 1.2 -1.6 liters has been added to the total blood volume (53), which is almost exclusively increased plasma volume (43). In stark contrast, preeclamptic women exhibit an increase of only ~ 200 mL in blood volume, which is directly correlated to the low birth weight and intrauterine growth restriction (54, 55) seen in children that are born during the index pregnancy (10).
Another critical adaptation that occurs in response in to this expanded blood volume is an increase in cardiac output (CO) (56). The metabolic demands of the fetoplacental unit require that 25% of the CO be allotted to arterial blood flow to the uterus (57). To increase the CO, the RVLM of the brain stem sends excitatory inputs to the cardiac preganglionic sympathetic nerves, residing in the intermediolateral cell column (IMLCC) of the spinal cord at thoracic levels T1–T4 (58–60). Exiting the cord via spinal nerves at the same levels, these cardiac preganglionic nerves synapse with postganglionic fibers within paravertebral ganglia (59–61). Cardiac postganglionic sympathetic fibers project to the sinoatrial and the atrioventricular nodes of the heart, and release norepinephrine that binds to β1 adrenergic receptors (60, 62). The resulting signaling pathway increases cyclic AMP and activates protein kinase A, which leads to increased cardiac output (58, 60, 63, 64).
Influenced by this autonomic input, the CO increases early in the 1st trimester of normal pregnancy so that by 37–42 weeks gestation the average output is 6 L/minute (65–67). Three factors contribute to this increase in CO, each varying in dominance over the time course of gestation. Beginning in the 1st trimester, stroke volume and preload, both associated with the expansion in total blood volume, participates in the augmentation of CO (65, 68–70). Conversely, in the 2nd and 3rd trimesters, the HR, increased by 15 to 20 beats/min, becomes the primary cause of the increased CO which enables adequate nutrient/waste exchange to the developing fetus (65, 71). In clinical cases of preeclampsia, however, cardiac output is significantly reduced with an increase in peripheral vascular resistance (72, 73). This pathophysiological reduction in CO is manifested in preeclamptic patients by asymmetrical remodeling and hypertrophy of the left ventricle (74, 75). Moreover, increased levels of atrial and brain natriuretic peptides are directly correlated to the degree of left ventricular dysfunction in preeclampsia (12).
Within the context of the increases in blood volume and CO, the adaptation of a decrease in the peripheral vascular resistance (PVR) occurs in healthy pregnancy. The visceral motor autonomic innervation of the systemic vasculature produces forceful vasoconstriction by increasing the PVR (76, 77). Postganglionic sympathetic nerves terminate in the tunica adventitia of arteries and arterioles and release NE that diffuses into the tunica media to bind to α1 or α2 adrenergic receptors expressed on vascular smooth muscle cells producing vasoconstriction (59–61). In healthy pregnancy, this vasoconstriction is attenuated, and PVR is reduced by complex neurohormonal interactions that diminish the sensitivity to angiotensisn II (AngII) (78–80), offsetting the vasoconstrictive effects of thromboxane (81), and lowering both plasma osmolarity and arterial load (82–84). Moreover, starting at the fifth week of gestation, progesterone and prostaglandin levels promote vasodilation, and as a result the PVR decreases ~10% from baseline levels (76, 85, 86). The PVR reaches its lowest level at 20 weeks’ gestation, ~ 35% decrease from baseline levels, and persists at this level through week 32 (65, 77, 87). Prostacyclin, an endothelium-derived eicosanoid, counteracts the vasoconstrictive effects of AngII during pregnancy (79, 80). Moreover, enhanced release of nitric oxide attenuates the actions of thromboxane by preventing the phosphorylation of myosin light chains in vascular smooth muscle (88, 89). In addition, production of relaxin increases during the first trimester and at term (82, 84). This peptide contributes both to the reduction in PVR and to the increase in CO (82, 84). Thus, despite the presence of increased AngII levels during normal pregnancy, a decreased sensitivity to AngII results (90–92).
However, in PE the PVR is elevated due to a heightened sensitivity to AngII (93), contributing to the hypertensive state. Several studies suggest that agonistic autoantibodies against the AngII AT1 receptor (AT1R-AABs) are associated with enhanced AngII sensitivity (94–98). AT1R-AABs have been observed to increase production of the NF-κB / NADPH oxidase-mediated formation of reactive oxygen species (ROS) (99), plasminogen activator inhibitor, (100, 101), and soluble fms-related tyrosine kinase-1 (sFlt-1) (102). However, this significant correlation between AT1R-AAB titer and elevated PVR is observed mostly in severe cases of PE (103, 104). In addition, levels of the vasodilatory heptapeptide angiotensin 1–7 (Ang 1–7), which attenuates the vasoconstrictive properties of AngII, are significantly diminished in PE (91). Further amplifying AngII sensitivity in PE is the increased expression of the angiotensin 1 receptor - bradykinin 2 receptor (AT1-B2) heterodimer on blood cells and omental vessels (105–107), that increase the PVR by accentuating AngII-mediated vasoconstriction. The pathological elevations in PVR observed in PE are manifested by increased arterial stiffness that not only reveals endothelial dysfunction, but also indicates the vulnerability to left ventricular hypertrophy and cardiac irregularities (74, 108, 109), all of which contribute to the hypertension associated with PE.
Critical to understanding the characteristic enhanced AngII sensitivity of PE are the changes that occur to the renin-angiotensin-aldosterone systems (RAAS) in both the kidney (110–113) and more importantly the placenta (114–131). Mistry et al., (119) observed that the placental RAAS exhibits the same degree of autonomy as the renal RAAS, but whose components are derived from both maternal and fetal sources. Rising estrogen plasma levels during pregnancy activate the placental RAAS, with concurrent increases in angiotensinogen (AGT) and renin levels (110, 114, 120, 132). Compared to the chorionic villi, the maternal decidua produces greater amounts of both total and active renin (114, 123). The hallmark placental ischemia observed in PE triggers increased expression of prorenin receptors (PRRs), which promote the generation of angiotensin I from AGT, and the subsequent cleavage of AngII from Ang I by angiotensin converting enzyme (ACE) (119). Morover, the binding of prorennin to PRRs exerts nearly a four-fold increase in the catalytic efficiency of PRR (117, 133).
The placental RAAS demonstrates high sensitivity to tissue levels of reactive oxygen species (ROS), which serve as a signal for both angiogenesis (116) and placental development (117). Overactivation of the placental RAAS, such as in cases of placental ischemia, results in excess local production of AngII, which acting on AT1Rs expressed by placental trophoblasts (124), can lead to the deleterious effects of low birthweight and intrauterine growth restriction (119). Recently, Zhou et al., (130) have elucidated the mechanism between placental RAAS overactivation and the enhanced pressor response observed in PE. The AGT protein maintains a state of equilibrium between its oxidized and reduced forms (130). Excessive ROS production by the ischemic placenta catalyzes a disulfide linkage in the angiotensin portion of AGT, and this oxidized form of AGT results in the four-fold incease in Ang I production (117, 130), subsequent cleavage to AngII, and then enhanced pressor response brought about by the vasoconstrictive properties of AngII (119, 134). Thus, maternally derived AngII produced from the uterine placental bed acts in an endocrine manner to produce vasoconstriction of the uterine spiral arteries, further exacerbating placental ischemia (114, 115).
As observed by Nartita et al. (120), overactivation of the placental RAAS by placental ischemia leads to increased AngII-mediated pressor response and sympathetic nerve activity. If left untreated, the resulting hypertension can increase the risk of the loss of CBF autoregulation, increase in cerebral perfusion pressure, and eventual damage to the blood brain barrier (135, 136). These initial neurological outcomes resulting from overactivation of the placental RAAS can lead to life threatening neurological consequences, which will be addressed later in the text.
The above described cardiovascular hemodynamic alterations of pregnancy are mediated by the sympathetic and parasympathetic divisions of the autonomic nervous system via sympathovagal balance (137, 138). Sympathovagal balance has been assessed in healthy pregnant and preeclamptic women by spectral analysis of heart rate variability (HRV) by comparing the ratio of the low frequency (0.04–0.15 Hz) to high frequency (0.15–0.40 Hz) domains of the HRV, termed the LF:HF ratio (139–142). During the first trimester of normal healthy pregnancy, a dominant HF exists (0.15–0.40 Hz), which is consistent with a robust vagal control of heart rate (16). By the third trimester, however, a gradual increase in the LF:HF ratio indicates that a biphasic change in autonomic inputs occurs, characterized by a vagal withdrawal over heart rate and a dominant sympathetic tone (15, 16, 143). Kuo et al., observed that this biphasic shift towards augmented sympathetic tone in late pregnancy is in part attributed to aortocaval compression by the gravid uterus (16, 144, 145). In these cases, the gravid uterus compresses upon the maternal abdominal aorta and inferior vena cava, resulting in both a decrease in cardiac output and venous return, and eliciting compensatory changes in sympathetic tone (146).
In PE, however, the abnormal uterine perfusion adversely affects autonomic control of cardiovascular function (14, 147), such that the LF:HF ratio is elevated beyond that seen in normotensive pregnant females (140, 142, 148), indicative of sympathetic overactivation and sympathovagal imbalance (11, 17, 149–151). Thus, the impaired regulation of heart rate and blood pressure by sympathetic overactivation (141, 152) further exacerbates the abnormal placental perfusion and renal blood flow in preeclamptic women (153). This sympathetic overactivation may be the result of placental pathogenic factors (e.g., inflammatory cytokines) acting primarily on brainstem nuclei that govern sympathovagal balance (154–156) of cardiovascular physiology. In other forms of hypertension, the mechanism is disinhibition of the RVLM by upregulating the expression of GABAB receptors in the regions of the nucleus of the solitary tract that receive baroreceptor afferents (Figure 2) (157–163). Whether this mechanism is active in PE is an area ripe for new research.
Figure 2.
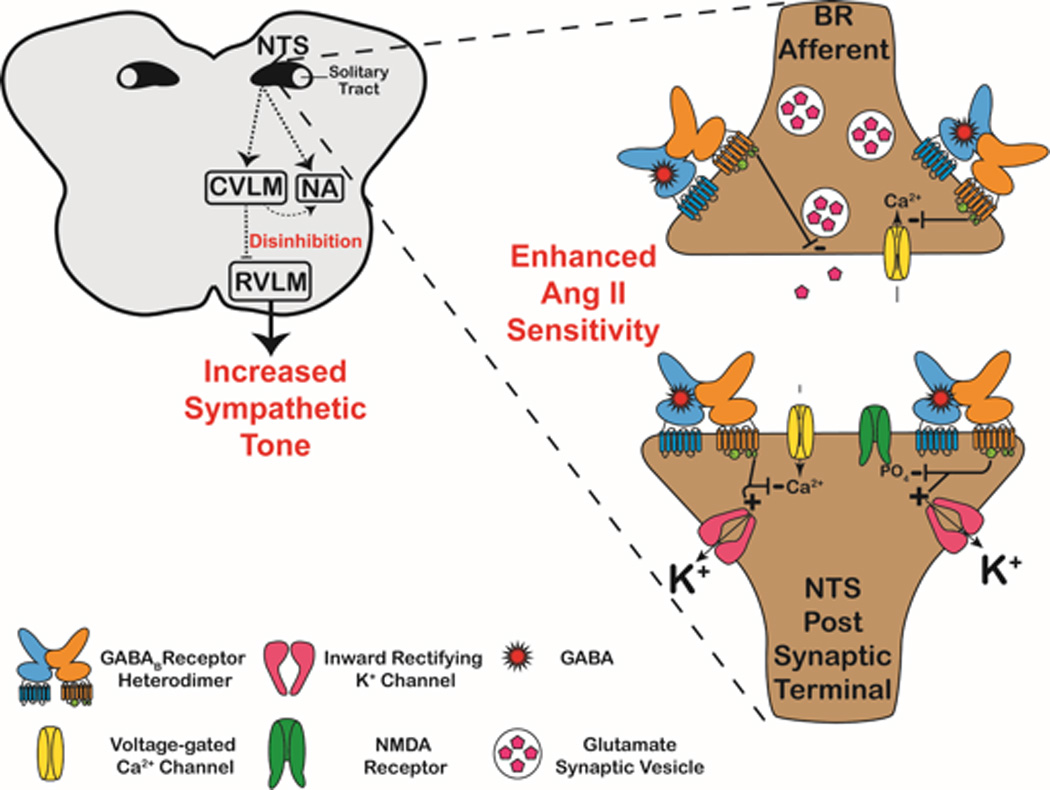
GABABR-mediated disinhibition of the RVLM as a proposed mechanistic target for future PE studies. Neurons in the dorsomedial regions of the caudal NTS receive and integrate BR afferent inputs. Normally, BR inputs excite these NTS neurons, which in turn, excites the caudal ventrolateral medulla (CVLM). The CVLM reduces sympathetic tone using dual circuitries. First, the CVLM excites preganglionic parasympathetic neurons in the nucleus ambiguus (NA) which projects (via the vagus nerve) to the heart to reduce the heart rate and stroke volume. Secondly, the CVLM projects inhibitory (GABAergic) projections to the “vasomotor center” RVLM. Inhibition of the RVLM reduces the sympathetic tone to the blood vessels, heart, and kidneys. However, in several hypertensive studies, GABABR-mediated inhibition of these NTS neurons occurs (expanded synapse in figure). In the presence of AngII, upregulated expression of GABABR occurs on NTS neurons. Presynaptically, GABABRs inhibit N-type (CaV2.2) & P/Q-type (CaV2.1) Ca2+ channels, and thus reduce the probability of glutamate release into the synaptic cleft. Postsynaptically, GABABRs hyperpolarize NTS neurons by activating inward-rectifying K+ channels, inhibiting L-type Ca2+ channels, and producing a voltage-sensitive Mg2+ block of NMDA receptors, as well as preventing their phosphosphorylation by protein kinase A (PKA). Hyperpolarization of NTS neurons by GABABRs result in insufficient excitatory input (dashed arrows) to the NA and CVLM. Decreased parasympathetic input to the heart via the NA results in increases in heart rate and stroke volume. More importantly, the hyperpolarized NTS neurons cannot excite the CVLM, resulting in a disinhibition of the RVLM. No longer governed by the GABAergic tone from the CVLM, the RVLM increases sympathetic tone to the vasculature, heart, and kidneys. Given that PE is characterized by enhanced AngII sensitivity, an impaired BR reflex, and sympathetic overexcitation, this GABABR-mediated disinhibition of the RVLM is proposed as a mechanistic target for future PE studies.
Despite the routine clinical use of the LF:HF ratio in assessing sympathovagal balance, caution should be taken when interpreting data due to confounding non-neural factors and mathematical influences (164). For instance, the HF peak, typically associated with parasympathetic activity, may exhibit up to a 10% change in frequency due to augmented sympathetic nerve activation (164–166). Conversely, the upper limits of sympathetic activation may be offset by cardiac parasympathetic input, such as that observed during the “diving reflex” (167), where marked bradycardia occurs despite a robust increase in sympathetic nerve output (164, 168). Mathematical confounding variables (169) can occur when the heart rate variability (HRV) is not divided by the average R-R interval (164, 170). Moreover, respiratory influences on the LF:HF ratio, as is observed in heart transplant patients that lack cardiac innervation (164), exhibit an atrial stretch contributioin to the LF:HF ratio during the respiratory cycle (164, 171). Given these limitations of the LF:HF ratio, microneurography of skeletal muscle sympathetic nerve activity (MSNA) may be an alternative method of accurately evaluating sympathovagal balance.
Microneurography clinically assesses multiunit sympathetic activity of the peripheral vasculature by performing percutaneous recordings of action potentials conducted by peripheral nerves (172, 173). Sex-dependent differences are observed in MSNA recordings, such that women exhibit β-adrenergic vasodilation that counteracts the vasoconstrictive properties associated with increased MSNA (174). This effect has been observed in normotensive pregnant women, who demonstrated enhanced MSNA in spite of no significant change in blood pressure (141, 152, 172). Microneurographic studies of preeclamptic women reported amplified MSNA when compared to normotensive pregnant women, but were not significantly different from women diagnosed with pregnancy-induced hypertension (PIH), suggesting that sympathetic hyperactivity is not solely responsible for the renal dysfunction presented in cases of PE (172, 175). These data emphasize that it is unclear whether sympathetic hyperactivity actually causes gestational hypertension, or if it is a mechanistic consequence of angiogenic imbalance and endothelial dysfunction (172, 176).
The Baroreceptor Reflex in Healthy Pregnancy and Preeclampsia
Though it was once regarded as a short-term regulator of abrupt changes in blood pressure, the BR reflex is now considered to play an integral role in chronic hypertensive states (177).
Basic Circuitry of the Baroreceptor Reflex
Located within the arterial walls of the aortic arch and immediately rostral to the bifurcation of the common carotid artery within the carotid sinus (178) stretch-sensitive baroreceptors (BRs) detect beat-to-beat fluctuations in arterial pressure (62, 179, 180). Aortic BRs transmit impulses via the vagus nerve (CN X), while the carotid BRs send afferent signals via the glossopharyngeal nerve (CN IX). The cell bodies of the aortic BRs and carotid BRs reside in the nodose and petrosal ganglia, respectively, and they both make excitatory (glutamatergic) synapses on neurons in the dorsomedial region of the caudal NTS (62, 181, 182). The NTS receives and integrates these BR afferent inputs, and projects excitatory terminals to the caudal ventrolateral medulla (CVLM) (62, 181, 182). In turn, the CVLM excites preganglionic parasympathetic neurons in the nucleus ambiguus (NA), which also receives excitatory input from the NTS, and projects (via the vagus nerve) to the heart to reduce the heart rate and stroke volume (62, 181). More importantly, the CVLM projects inhibitory (GABAergic) projections to the “vasomotor center” of cardiovascular system, the RVLM (59, 181, 183). The RVLM projects to and synapses on preganglionic sympathetic neurons located in the IMLCC of the spinal cord (59, 179, 182). Inhibition of the RVLM reduces the sympathetic tone to IMLCC, and it is this decrease in sympathetic nerve activity to the blood vessels, heart, and kidneys, that produces a corresponding decrease in mean arterial pressure (180, 181, 183).
Alterations of the BR Reflex in Healthy and Preeclamptic Pregnancies
The CNS nuclei of the BR reflex circuitry express receptors for sex hormones (184). Estradiol has been observed to affect BRS, while progesterone modulates the sympathetic output of the RVLM (184). The neurosteroid 3-α-hydroxydihydroprogesterone (3-α-OH-DHP), a metabolite of progesterone, augments inhibitory GABAergic tone of the RVLM by binding to GABAA receptors expressed by RVLM neurons, and increasing Cl− conductance (185, 186). Through this hormonal modulation of neural excitability, normal pregnancy is characterized by a reduction in BRS (185, 187–190).
Conversely, the BR reflex is impaired in PE beyond that observed in healthy pregnancy (14, 19), and presents with beat-to-beat variations in blood pressure, heart rate, and BRS that are utilized clinically to predict cases of PE (191, 192). When combined with Doppler sonography (for detection of reduced uterine perfusion), analysis of these beat-to-beat variations in blood pressure, heart rate, and BR reflex sensitivity, PE is predicted with a positive predictive accuracy of over 71% (21).
These clinical observations of BR reflex pathophysiology associated with PE provide the rationale for future studies on the regions of the NTS that receive and integrate BR afferents inputs. Indeed, a variety of hypertensive models have demonstrated mechanistic alterations occurring on NTS neurons (160–163, 193–201). While none of these studies modeled PE, the molecular pathways implicated in the increased pressor response warrant investigation in an animal model of PE (202). The electrophysological alterations that were observed in NTS neurons in these animal models are summarized below.
Diverse Models of Hypertension Demonstrate Electrophysiological Alterations Occuring in the NTS
GABAB receptors (GABABRs) are metabotropic G-protein coupled receptors (GPCRs) (203, 204) that hyperpolarize the neuron (Figure 2) presynaptically (205, 206) and postsynaptically (203–206). NTS neurons hyperpolarized by increased GABABR activity cannot excite the CVLM and NA, nor can the CVLM sufficiently excite the preganglionic parasympathetic neurons of the NA (158, 180, 195, 207). As a result, the NA provides insufficient parasympathetic input to the heart, and the CVLM no longer inhibits the RVLM, creating a sympathovagal imbalance that leads to vasoconstriction, increased heart rate, and enhanced renal sympathetic nerve activity (158, 180, 195, 207). Pharmacologic studies in spontaneously hypertensive rats (SHRs) (162, 208–210), and hypertensive Sprague-Dawley rats induced with a one-kidney figure-8 renal wrap (163, 195, 198, 201, 211) have demonstrated that the enhanced expression and activation of GABABRs in the regions of the NTS that receive and integrate BR afferent inputs results in hypertension. Additionally, transfer of the GABABR gene into the NTS of normotensive rats resulted in rapid onset of hypertension, increased heart rate, and increased plasma norepinephrine levels, all of which remained elevated for the duration of the 14-day study (157). This GABABR-mediated pressor response is potentiated by the actions of AngII on NTS neurons (161).
NTS neurons express angiotensin II Type 1 receptors (AT1Rs) with one of the highest receptor densities within the BBB (212, 213), and AngII acts at these receptors to dampen the BR afferent inputs in both hypertensive and normotensive animals (214, 215). Moreover, AngII increases the expression of GABABRs on NTS neurons (161) and synergistically enhances the pressor response (160). Thus, AngII blunts BR afferent inputs by augmenting the GABABR-mediated inhibitory currents on NTS neurons, leading to disinhibition of the RVLM and increased sympathetic tone to the heart, vasculature, and kidneys (160, 163, 180, 207). Therefore, given the heightened AngII sensitivity observed in PE (216), and that the NTS neurons that receive BR afferent inputs exhibit one of the highest AT1R expression levels within the BBB (212, 213), these GABABRs on NTS neurons would serve as prime mechanistic targets for future studies employing an animal model of PE (202).
In addition to GABABRs, alpha2-adrenoreceptors (α2-adrenoreceptors) represent an alternative mechanistic target for blood pressure control in PE. The activation of alpha2-adrenoreceptors (α2-adrenoreceptors) expressed by astrocytes residing in the NTS elicits a decrease in blood pressure (197, 217, 218). However, when these astrocytic α2-adrenoreceptors engage in cross-talk with neuronal adenosine-1 receptors (A1Rs) (196), or form heterodimers with µ-opoid receptors (197), hypertension can occur. Synaptic release of norepinephrine activates astrocytic α2-adrenoreceptors, which triggers the extracellular release of ATP, that is then hydrolyzed to adenosine (196, 219, 220). The adenosine binds to presynaptic A1Rs and induces hyperpolarization by inactivating Ca2+ inward channels and activating K+ channels (196, 221). Studies employing SHRs observed increased A1R expression in the NTS (194, 222), with enhanced sensitivity to adenosine (222) that blunted BRS resulting in a hypertensive state (193, 196, 223, 224). A hypertensive response in the NTS can also occur when µ-opioid receptors form heterodimers specifically with the α2A class of α2-adrenoreceptors (α2A-ARs) (197, 225). Pharmacologic studies of SHRs demonstrated this hypertensive effect by microinjecting a µ-opioid agonist into the NTS, in which the µ-opioid-α2A-AR heterodimers blocked nitric oxide-mediated vasodilation (197). Conversely, administration of a µ-opioid antagonist into the NTS prohibits the formation of µ-opioid-α2A-AR heterodimers, resulting in a decrease in blood pressure in SHRs but not in WKY rats (197, 226). These results in rat models of primary hypertension implicate GABABRs and alpha2-adrenoreceptors as prime candidates for mechanistics studies examining the neurogenic control of blood pressure in PE.
Cerebrovascular and Higher Function Neurological Consequences of PE
In addition to the neural control of cardiovascular maladaptations that occur in PE, PE also induces deleterious neurological outcomes to affected mothers. Next, we examine the effects PE has on brain volume, cerebral hemodynamics, white matter lesions, electrophysiological profile, (Table 2) and cognitive and behavioral impairments.
Table 2.
Alterations in brain volume, cerebral hemodynamics, white matter integrity, and electrophysiological profile observed in maternal brains after a preeclamptic index pregnancy. Normal pregnancy is associated with a reduction in both gray and matter volumes, and an enlargement of the ventricular spaces, with these changes reversing postpartum. However, in preeclampsia this atrophy in brain volume and expansion of the ventricles can persist for decades. In moderate to severe case of PE, cerebrovascular autoregulation is lost, resulting in increased BBB permeability, brain water content, and an exacerbated risk of vasogenic edema. The pattern and distribution pattern of white matter lesions correlate to neurological outcome. The occipital lobe, a region of interest in visual disturbances, exhibits electrophysiological changes as a result of preeclampsia.
| CNS Component | Healthy Pregnancy | Preeclampsia | |
|---|---|---|---|
| Brain Volume | Gray Matter | Atrophy reverses postpartum | Atrophy persists decades after index pregnancy |
| White Matter | |||
| Ventricular Spaces | Expansion reverses postpartum |
Enlarged ventricles persist decades after index pregnancy |
|
| Cerebral Hemodynamics |
Cerebrovascular Autoregulation | --- | Decreased |
| Cerebral Perfusion Pressure | --- | Increased | |
| BBB Permeability | --- | ||
| Brain Water Content | --- | ||
| Risk of Vasogenic Edema | --- | ||
| White Matter Lesions (WMLs) |
WML Distribution Pattern | --- | Strong correlation to neurological outcome |
| Sympathetic Innervation | --- | Regions with meager innervation (e.g., occipital lobe) are more susceptible to WMLs |
|
| Electrophysiology | EEG | --- | Diffuse and focal slowing of delta and theta waves in occipital lobe |
Volume Changes in the Brains of Preeclamptic Women During the Peripartum Period
Observed in humans (227), and in rodents (228), healthy gravid females undergo a reduction in both gray and white matter volumes of the brain, while the volume of the lateral ventricles are increased. These changes in volume in the brain and ventricular spaces begin at the moment of placental implantation, peak at term, and slowly reverse months after delivery. Moreover, human studies have shown that while the brain decreased in volume, there were also concomitant volume increases in the heart, kidneys, thyroid gland, and extracellular fluid, with all changes reversing within six months postpartum (227, 228). However, these volumetric changes were even more pronounced in human PE patients (227, 228). Women in the PE group had significantly smaller brain volumes, with corresponding increases in lateral ventricular volumes. A recent study involving a large multiethnic and racially diverse sample observed that women with a history of hypertensive pregnancy had smaller brain volumes and larger degrees of atrophy decades after the index pregnancy (229). While not a sole indicator of neurological dysfunction, these alterations in brain size and volume are accompanied by changes in cerebral hemodynamics, electrophysiology, cognition, and behavior in human PE patients.
Cerebral Hemodynamics in Healthy vs. Preeclamptic Gravid Females
In normotensive individuals, cerebral blood flow (CBF) is maintained at ~50 mL per 100 g of brain tissue per minute, given that the cerebral perfusion pressure (CPP) and intracranial pressure is in the range of 60 to 150 mm Hg (61, 135, 136). When the CPP exceeds 150 mm Hg, autoregulation can no longer be maintained and “breakthrough” occurs, such that the decrease in cerebrovascular resistance (CVR) results in hyperperfusion, blood brain barrier (BBB) disruption, and vasogenic edema (61, 135, 136), which can contribute to neurological complications associated with hypertensive encephalopathy and eclampsia (61, 135, 136). Significant changes in cerebral hemodynamics have been observed in both clinical (230–232) and animal model (233) studies of PE. Compared to normotensive pregnant women, PE patients exhibit augmented CPP in the middle (231), anterior, and posterior (230) cerebral arteries, with accompanying changes in cerebral artery resistance indices (230). An animal model PE study confirmed that placental ischemia was the driving force of the CBF pathology, and that the increased brain water content was the result of increased BBB permeability and smaller diameter cerebral vessels being burdened with increased pressure (233). Thus, it is possible that the PE-decreased CVR and hyperperfusion causes the brain to be susceptible to vasogenic edema by creating an unfavorable hydrostatic pressure gradient when pressure is elevated (61, 135, 136).
Anatomical Distribution and Volume of White Matter Lesions in PE
White matter lesions (WMLs) are a common neurological corollary that result from the altered hemodynamics of PE (5, 234, 235). Diagnostic imaging reveals that these WMLs can persist for years after the index pregnancy (22–24, 229). Counterintuitively, the pattern of distribution of WMLs associated with preeclampsia differs from that of Posterior Reversible Encephalopathy Syndrome (PRES). PRES is more often associated with eclampsia (7, 234). The WMLs resulting from PRES tend to predominate in the occipital, parietal, and frontal lobes, are hemispheric, and bilaterally symmetric (25). The WMLs seen in PE patients are distributed in the frontal lobes (24), and tend to dominate in cases of early-onset PE (23). Interestingly, a relationship exists between WML distribution patterns and the degree of sympathetic innervation supplied to the brain regions most at risk for sustaining WMLs (236). Myogenic and neurogenic elements comprise cerebral autoregulation, in which proper neurogenic function is dependent upon sympathetic innervation (236). In PRES, elevated blood pressure weakens myogenic homeostatic mechanisms via vascular endothelial dysfunction, causing cerebral autoregulation to rely more upon its neurogenic component (236–238). As a result, brain regions with robust sympathetic innervation (e.g. frontal lobe) are relatively safeguarded against serum extravasation through vasoconstriction. This contrasts with regions such as the occipital lobe, which receives meager sympathetic innervation, that are more susceptible to developing WMLs after being exposed to acute oscillations in cerebral blood pressure (236, 239).
There is a strong correlation between the presence and distribution of WMLs and poor neurological outcome in PE, particularly when accompanied by cerebral edema, intracranial hemorrhage, and eclampsia (6, 7). Moreover, during the peripartum period 47% of ischemic strokes are the result of severe PE, and these strokes account for 12% of the annual maternal death rate globally (240–243). One quarter of PE patients that suffer an ischemic stroke will incur permanent brain damage (5). Diagnostic imaging studies of PE patients that sustained a cerebrovascular accident demonstrate white matter lesions in the frontal, parietal, insular, and temporal lobes in women 10–26 years after being diagnosed with PE or eclampsia (23, 24).
Electrophysiological Changes Exhibited by Healthy, Preeclamptic, and Eclamptic Gravid Females
The electroencephalogram (EEG) is sensitive enough to distinguish if an intra-partum seizure has resulted from eclampsia or if the mother suffered an epileptic seizure during labor (244, 245). Furthermore, hypertensive PE and eclamptic women exhibit changes in EEG recordings compared to normotensive pregnant and non-pregnant females (244). PE and eclamptic women demonstrate both diffuse and focal slowing of delta and theta waves, typically localized to the occipital lobe (234, 244–247). Eclamptic women exhibit a significantly greater number of spike discharges than PE patients (234, 244–247). Collectively, these anatomical, hemodynamic, histopathological, and electrophysiological changes that result from PE are manifested in cognitive and behavioral impairments.
Cognitive and Behavioral Impairments in PE: Associations with Increased Risk of Low Fetal Birth Weight, Intrauterine Growth Restriction, and Premature Birth
Maternal cognitive dysfunction is associated with PE (Figure 3) (28–30), where impairment severity correlates to the total number of eclamptic seizures (248). A pilot study suggested that these self-reported deficits from formerly PE women exhibited auditory-verbal memory deficits, impaired learning, and delayed recall, all of which were independent of depression or anxiety (29). However, a long-term follow up study found no evidence of neurocognitive dysfunction despite the self-reporting of impairments, but the investigators did conclude that this increase in self-reported deficits is an indicator for cognitive impairment and/or dementia later in life (28). A more recent study concluded that hypertensive pregnancy disorders may be independent risk factors of cognitive decline, after adjusting for cardiovascular disease and known cardiovascular disease risk factors (229). Human subjects with a mean age of ~61 years, from a large multiracial sample, demonstrated significant deficits in processing speed decades after the index pregnancy, even when the results were adjusted for abnormal estimated glomerular filtration rate (eGFR) (229).
Figure 3.
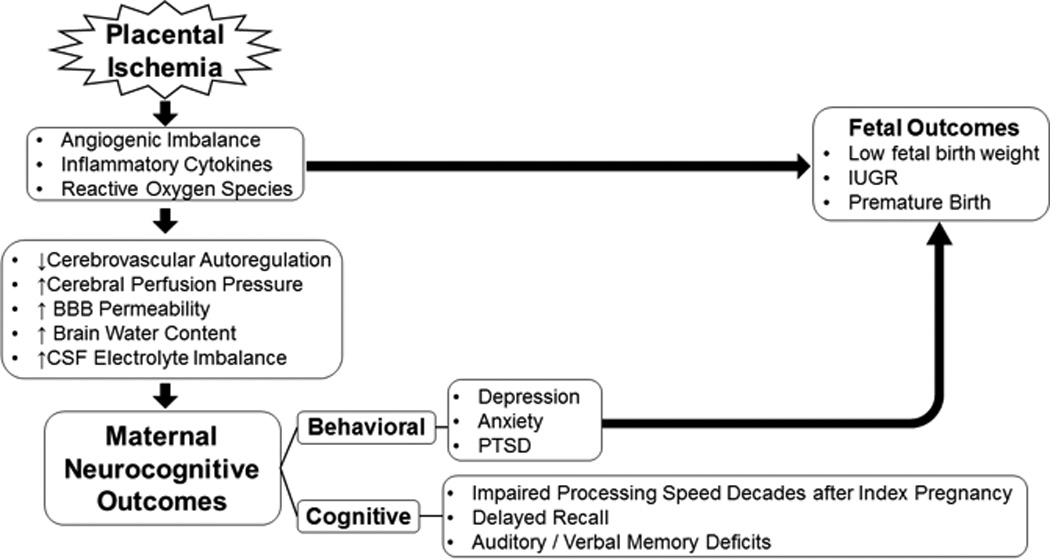
Cognitive and behavioral deficits associated with preeclampsia, and their relationship to fetal outcomes. The ischemic placenta releases a myriad of pathogenic factors that lead to endothelial dysfunction. The resulting pathophysiology, if left untreated, can develop into cerebrovascular abnormalities which include a loss of autoregulation, increase in BBB permeability, with a resulting imbalance in CSF electrolyte composition. Collectively, these insults to the CNS can manifest into learning and memory deficits that can persist for decades after the index pregnancy. Moreover, behavioral outcomes of depression, anxiety, and PTSD are significantly associated with low fetal birth weight, IUGR, and preterm birth.
The indices for depression (31, 249, 250) and anxiety (31, 38, 249) are elevated in PE, while the frequency of PTSD is increased for several years after the index pregnancy (37–42). One clinical study observed that the risk for postpartum depression was associated not to the severity of PE, but rather to its consequences (e.g., perinatal death), even after adjusting for the confounding variables of age, ethnicity, and educational level of the mother (250). While resilience shielding against psychological stress (251) and psychotherapeutic treatment (252) can attenuate the duration of the episode, a previous history of depression coupled with experiencing a preeclamptic index pregnancy can significantly contribute to the onset of PTSD and exacerbate the anxiety of planning future pregnancies (39). The deleterious effects of prenatal maternal psychosocial stressors on fetal development are well documented, where increased incidences of maternal depression (34–36) and anxiety (31–33) are significantly correlated to low fetal birth weight, IUGR, and premature birth (32–36, 38).
Conclusion
First reported over a century ago (253), the ischemic placenta was identified as the source of pathogenic factors that generate the clinical presentations of PE. Moreover, experiments performed in 1940 confirmed that delivery/removal of the ischemic placenta results in full regression of the maternal syndrome (9, 254). Current research continues to focus on the ischemic placenta by targeting the pathogenic factors that drive angiogenic imbalance (255), increases in reactive oxygen species (ROS) (256), and peripheral inflammation (257). These approaches are based upon the recommendations of the 2013 Task Force on Hypertension in Pregnancy, which concluded that current FDA-approved antihypertensive therapies have no effect on the progression of PE, may further exacerbate placental ischemia, and expose both the expectant mother and developing fetus to possible deleterious side effects (4).
To be sure, while PE is initiated at the maternal-placental interface, the poor patient outcomes are predominantly neurological and occur in the brain. As illustrated in Figure 1, the absence of the autonomically-regulated adaptations to pregnancy contributes to the development of potential life-threatening neuropathology, including increased BBB permeability and brain water content, the appearance of white matter lesions, and the loss of cerebrovascular regulation. Finally, the poor outcomes of PE are manifested as executive dysfunction, cognitive impairment, depression, anxiety, and PTSD. These behavioral outcomes are significantly associated with low fetal birth weight, IUGR, and premature birth.
In parallel with the current ischemic placental studies of PE pathophysiology, we have proposed that future studies of PE should address the neural control of blood pressure in animal models, and how known circulating factors (for example, anti-angiogenic proteins, angII, and inflammatory cytokines) influence activity of brainstem nuclei controlling blood pressure. The intent of these proposed studies is to identify mechanistic targets, and to direct therapeutic agents against these targets so as to reduce both the neurological outcomes and the number of fatal cases of PE.
References
- 1.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ (Clinical research ed) 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. European journal of obstetrics, gynecology, and reproductive biology. 2013;170(1):1–7. doi: 10.1016/j.ejogrb.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Geneva: World Health Organization; 2011. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. [PubMed] [Google Scholar]
- 4.American College of O, Gynecologists, Task Force on Hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstetrics and gynecology. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 5.Zeeman GG. Neurologic complications of pre-eclampsia. Semin Perinatol. 2009;33(3):166–172. doi: 10.1053/j.semperi.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Isler CM, Rinehart BK, Terrone DA, Martin RW, Magann EF, Martin JN., Jr Maternal mortality associated with HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome. American journal of obstetrics and gynecology. 1999;181(4):924–928. doi: 10.1016/s0002-9378(99)70343-1. [DOI] [PubMed] [Google Scholar]
- 7.Okanloma KA, Moodley J. Neurological complications associated with the pre-eclampsia/eclampsia syndrome. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2000;71(3):223–225. doi: 10.1016/s0020-7292(00)00295-2. [DOI] [PubMed] [Google Scholar]
- 8.Al-Jameil N, Aziz Khan F, Fareed Khan M, Tabassum H. A brief overview of preeclampsia. Journal of Clinical Medicine Research. 2014;6(1):1–7. doi: 10.4021/jocmr1682w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nature reviews Nephrology. 2014;10(8):466–480. doi: 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown MA, Gallery ED. Volume homeostasis in normal pregnancy and pre-eclampsia: physiology and clinical implications. Bailliere's clinical obstetrics and gynaecology. 1994;8(2):287–310. doi: 10.1016/s0950-3552(05)80322-0. [DOI] [PubMed] [Google Scholar]
- 11.Eneroth-Grimfors E, Westgren M, Ericson M, Ihrman-Sandahl C, Lindblad LE. Autonomic cardiovascular control in normal and pre-eclamptic pregnancy. Acta Obstet Gynecol Scand. 1994;73(9):680–684. doi: 10.3109/00016349409029402. [DOI] [PubMed] [Google Scholar]
- 12.Melchiorre K, Sharma R, Thilaganathan B. Cardiovascular implications in preeclampsia: an overview. Circulation. 2014;130(8):703–714. doi: 10.1161/CIRCULATIONAHA.113.003664. [DOI] [PubMed] [Google Scholar]
- 13.Melchiorre K, Thilaganathan B. Maternal cardiac function in preeclampsia. Current opinion in obstetrics & gynecology. 2011;23(6):440–447. doi: 10.1097/GCO.0b013e32834cb7a4. [DOI] [PubMed] [Google Scholar]
- 14.Voss A, Baumert M, Baier V, Stepan H, Walther T, Faber R. Autonomic cardiovascular control in pregnancies with abnormal uterine perfusion. American journal of hypertension. 2006;19(3):306–312. doi: 10.1016/j.amjhyper.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Matsuo H, Inoue K, Hapsari ED, Kitano K, Shiotani H. Change of autonomic nervous activity during pregnancy and its modulation of labor assessed by spectral heart rate variability analysis. Clinical and experimental obstetrics & gynecology. 2007;34(2):73–79. [PubMed] [Google Scholar]
- 16.Kuo CD, Chen GY, Yang MJ, Lo HM, Tsai YS. Biphasic changes in autonomic nervous activity during pregnancy. British journal of anaesthesia. 2000;84(3):323–329. doi: 10.1093/oxfordjournals.bja.a013433. [DOI] [PubMed] [Google Scholar]
- 17.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia -- a state of sympathetic overactivity. The New England journal of medicine. 1996;335(20):1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 18.Tanrikulu L, Naraghi R, Ernst V, Voigt F, Hastreiter P, Doerfler A, et al. Neurovascular compression of medulla oblongata – Association for gestation-induced hypertension. Medical Hypotheses. 2015;84(6):605–610. [Google Scholar]
- 19.Hines T, Beauchamp D, Rice C. Baroreflex control of sympathetic nerve activity in hypertensive pregnant rats with reduced uterine perfusion. Hypertension in pregnancy. 2007;26(3):303–314. doi: 10.1080/10641950701415598. [DOI] [PubMed] [Google Scholar]
- 20.Silver HM, Tahvanainen KU, Kuusela TA, Eckberg DL. Comparison of vagal baroreflex function in nonpregnant women and in women with normal pregnancy, preeclampsia, or gestational hypertension. American journal of obstetrics and gynecology. 2001;184(6):1189–1195. doi: 10.1067/mob.2001.112871. [DOI] [PubMed] [Google Scholar]
- 21.Walther T, Wessel N, Malberg H, Voss A, Stepan H, Faber R. A combined technique for predicting pre-eclampsia: concurrent measurement of uterine perfusion and analysis of heart rate and blood pressure variability. Journal of hypertension. 2006;24(4):747–750. doi: 10.1097/01.hjh.0000217858.27864.50. [DOI] [PubMed] [Google Scholar]
- 22.Aukes AM, de Groot JC, Aarnoudse JG, Zeeman GG. Brain lesions several years after eclampsia. American journal of obstetrics and gynecology. 2009;200(5):504, e1–e5. doi: 10.1016/j.ajog.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Aukes AM, De Groot JC, Wiegman MJ, Aarnoudse JG, Sanwikarja GS, Zeeman GG. Long-term cerebral imaging after pre-eclampsia. BJOG : an international journal of obstetrics and gynaecology. 2012;119(9):1117–1122. doi: 10.1111/j.1471-0528.2012.03406.x. [DOI] [PubMed] [Google Scholar]
- 24.Wiegman MJ, Zeeman GG, Aukes AM, Bolte AC, Faas MM, Aarnoudse JG, et al. Regional distribution of cerebral white matter lesions years after preeclampsia and eclampsia. Obstetrics and gynecology. 2014;123(4):790–795. doi: 10.1097/AOG.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 25.Junewar V, Verma R, Sankhwar PL, Garg RK, Singh MK, Malhotra HS, et al. Neuroimaging features and predictors of outcome in eclamptic encephalopathy: a prospective observational study. AJNR American journal of neuroradiology. 2014;35(9):1728–1734. doi: 10.3174/ajnr.A3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. American journal of obstetrics and gynecology. 2004;190(3):714–720. doi: 10.1016/j.ajog.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Zeeman GG, Hatab MR, Twickler DM. Increased cerebral blood flow in preeclampsia with magnetic resonance imaging. American journal of obstetrics and gynecology. 2004;191(4):1425–1429. doi: 10.1016/j.ajog.2004.05.069. [DOI] [PubMed] [Google Scholar]
- 28.Postma IR, Bouma A, Ankersmit IF, Zeeman GG. Neurocognitive functioning following preeclampsia and eclampsia: a long-term follow-up study. American journal of obstetrics and gynecology. 2014;211(1):37, e1–e9. doi: 10.1016/j.ajog.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 29.Brussé I, Duvekot J, Jongerling J, Steegers E, De Koning I. Impaired maternal cognitive functioning after pregnancies complicated by severe pre-eclampsia: a pilot case-control study. Acta Obstetricia et Gynecologica Scandinavica. 2008;87(4):408–412. doi: 10.1080/00016340801915127. [DOI] [PubMed] [Google Scholar]
- 30.Postma IR, Groen H, Easterling TR, Tsigas EZ, Wilson ML, Porcel J, et al. The brain study: Cognition, quality of life and social functioning following preeclampsia; An observational study. Pregnancy Hypertension: An International Journal of Women's Cardiovascular Health. 2013;3(4):227–234. doi: 10.1016/j.preghy.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Abedian Z, Soltani N, Mokhber N, Esmaily H. Depression and anxiety in pregnancy and postpartum in women with mild and severe preeclampsia. Iranian journal of nursing and midwifery research. 2015;20(4):454–459. doi: 10.4103/1735-9066.161013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Heinen AG, van Geijn HP. Psychosocial predictors of low birthweight: a prospective study. British journal of obstetrics and gynaecology. 1999;106(8):834–841. doi: 10.1111/j.1471-0528.1999.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 33.Wainstock T, Anteby E, Glasser S, Shoham-Vardi I, Lerner-Geva L. The association between prenatal maternal objective stress, perceived stress, preterm birth and low birthweight. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26(10):973–977. doi: 10.3109/14767058.2013.766696. [DOI] [PubMed] [Google Scholar]
- 34.Ciesielski TH, Marsit CJ, Williams SM. Maternal psychiatric disease and epigenetic evidence suggest a common biology for poor fetal growth. BMC pregnancy and childbirth. 2015;15:192. doi: 10.1186/s12884-015-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang H, Coleman S, Bridge JA, Yonkers K, Katon W. A meta-analysis of the relationship between antidepressant use in pregnancy and the risk of preterm birth and low birth weight. General hospital psychiatry. 2014;36(1):13–18. doi: 10.1016/j.genhosppsych.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uguz F, Gezginc K, Yazici F. Are major depression and generalized anxiety disorder associated with intrauterine growth restriction in pregnant women? A case-control study. General hospital psychiatry. 2011;33(6):640, e7–e9. doi: 10.1016/j.genhosppsych.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Tan P, Evsen MS, Soydinc HE, Sak ME, Ozler A, Turgut A, et al. Increased psychological trauma and decreased desire to have children after a complicated pregnancy. Journal of the Turkish German Gynecological Association. 2013;14(1):11–14. doi: 10.5152/jtgga.2013.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaugler-Senden IP, Duivenvoorden HJ, Filius A, De Groot CJ, Steegers EA, Passchier J. Maternal psychosocial outcome after early onset preeclampsia and preterm birth. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(3):272–276. doi: 10.3109/14767058.2011.573829. [DOI] [PubMed] [Google Scholar]
- 39.Stramrood CA, Wessel I, Doornbos B, Aarnoudse JG, van den Berg PP, Schultz WC, et al. Posttraumatic stress disorder following preeclampsia and PPROM: a prospective study with 15 months follow-up. Reproductive sciences (Thousand Oaks, Calif) 2011;18(7):645–653. doi: 10.1177/1933719110395402. [DOI] [PubMed] [Google Scholar]
- 40.Hoedjes M, Berks D, Vogel I, Franx A, Visser W, Duvekot JJ, et al. Symptoms of post-traumatic stress after preeclampsia. Journal of psychosomatic obstetrics and gynaecology. 2011;32(3):126–134. doi: 10.3109/0167482X.2011.599460. [DOI] [PubMed] [Google Scholar]
- 41.van Pampus MG, Wolf H, Weijmar Schultz WC, Neeleman J, Aarnoudse JG. Posttraumatic stress disorder following preeclampsia and HELLP syndrome. Journal of psychosomatic obstetrics and gynaecology. 2004;25(3–4):183–187. doi: 10.1080/01674820400017863. [DOI] [PubMed] [Google Scholar]
- 42.Engelhard IM, van Rij M, Boullart I, Ekhart TH, Spaanderman ME, van den Hout MA, et al. Posttraumatic stress disorder after pre-eclampsia: an exploratory study. General hospital psychiatry. 2002;24(4):260–264. doi: 10.1016/s0163-8343(02)00189-5. [DOI] [PubMed] [Google Scholar]
- 43.Chesley LC. Plasma and red cell volumes during pregnancy. American journal of obstetrics and gynecology. 1972;112(3):440–450. doi: 10.1016/0002-9378(72)90493-0. [DOI] [PubMed] [Google Scholar]
- 44.Brunton PJ, Arunachalam S, Russel JA. Control of neurohypophysial hormone secretion, blood osmolality and volume in pregnancy. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2008;59(Suppl 8):27–45. [PubMed] [Google Scholar]
- 45.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesthesia and analgesia. 2010;110(5):1368–1373. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 46.Cheung KL, Lafayette RA. Renal physiology of pregnancy. Advances in chronic kidney disease. 2013;20(3):209–214. doi: 10.1053/j.ackd.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sunn N, Egli M, Burazin TC, Burns P, Colvill L, Davern P, et al. Circulating relaxin acts on subfornical organ neurons to stimulate water drinking in the rat. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(3):1701–1706. doi: 10.1073/pnas.022647699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKinley MJ, Mathai ML, McAllen RM, McClear RC, Miselis RR, Pennington GL, et al. Vasopressin secretion: osmotic and hormonal regulation by the lamina terminalis. Journal of neuroendocrinology. 2004;16(4):340–347. doi: 10.1111/j.0953-8194.2004.01184.x. [DOI] [PubMed] [Google Scholar]
- 49.Atherton JC, Dark JM, Garland HO, Morgan MR, Pidgeon J, Soni S. Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol. 1982;330:81–93. doi: 10.1113/jphysiol.1982.sp014330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barrett KE, Barman SM, Boitano S, Brooks HL. Hypothalamic Regulation of Hormonal Functions. In: Barrett KE, Barman SM, Boitano S, Brooks HL, editors. Ganong’s Review of Medical Physiology. 25th. New York, NY: McGraw-Hill; 2016. [Google Scholar]
- 51.Durr JA, Stamoutsos B, Lindheimer MD. Osmoregulation during pregnancy in the rat. Evidence for resetting of the threshold for vasopressin secretion during gestation. The Journal of clinical investigation. 1981;68(2):337–346. doi: 10.1172/JCI110261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Progress in brain research. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- 53.Pritchard JA. CHANGES IN THE BLOOD VOLUME DURING PREGNANCY AND DELIVERY. Anesthesiology. 1965;26:393–399. doi: 10.1097/00000542-196507000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Rosso P, Donoso E, Braun S, Espinoza R, Fernandez C, Salas SP. Maternal hemodynamic adjustments in idiopathic fetal growth retardation. Gynecologic and obstetric investigation. 1993;35(3):162–165. doi: 10.1159/000292690. [DOI] [PubMed] [Google Scholar]
- 55.Salas SP, Rosso P, Espinoza R, Robert JA, Valdes G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstetrics and gynecology. 1993;81(6):1029–1033. [PubMed] [Google Scholar]
- 56.Ouzounian JG, Elkayam U. Physiologic changes during normal pregnancy and delivery. Cardiology clinics. 2012;30(3):317–329. doi: 10.1016/j.ccl.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Tan EK, Tan EL. Alterations in physiology and anatomy during pregnancy. Best practice & research Clinical obstetrics & gynaecology. 2013;27(6):791–802. doi: 10.1016/j.bpobgyn.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Gordan R, Gwathmey JK, Xie LH. Autonomic and endocrine control of cardiovascular function. World journal of cardiology. 2015;7(4):204–214. doi: 10.4330/wjc.v7.i4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haines DE. In: Fundamental Neuroscience for Basic and Clinical Applications. 4th. Haines DE, editor. Philadelphia, PA: Saunders An Imprint of Elsevier; 2013. p. 504. Fourth ed. [Google Scholar]
- 60.Thomas GD. Neural control of the circulation. Advances in physiology education. 2011;35(1):28–32. doi: 10.1152/advan.00114.2010. [DOI] [PubMed] [Google Scholar]
- 61.Hall JE, Guyton AC. Guyton and Hall Textbook of Medical Physiology. 12th. Philadelphia, PA: Saunders; 2011. p. 1120. [Google Scholar]
- 62.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7(5):335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 63.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351(6322):145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 64.Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacological reviews. 2001;53(2):319–356. [PubMed] [Google Scholar]
- 65.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. The American journal of physiology. 1989;256(4 Pt 2):H1060–H1065. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 66.Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- 67.Bamfo JE, Kametas NA, Chambers JB, Nicolaides KH. Maternal cardiac function in normotensive and pre-eclamptic intrauterine growth restriction. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2008;32(5):682–686. doi: 10.1002/uog.5311. [DOI] [PubMed] [Google Scholar]
- 68.Capeless EL, Clapp JF. Cardiovascular changes in early phase of pregnancy. American journal of obstetrics and gynecology. 1989;161(6 Pt 1):1449–1453. doi: 10.1016/0002-9378(89)90902-2. [DOI] [PubMed] [Google Scholar]
- 69.Easterling TR, Carlson KL, Schmucker BC, Brateng DA, Benedetti TJ. Measurement of cardiac output in pregnancy by Doppler technique. American journal of perinatology. 1990;7(3):220–222. doi: 10.1055/s-2007-999486. [DOI] [PubMed] [Google Scholar]
- 70.Mabie WC, DiSessa TG, Crocker LG, Sibai BM, Arheart KL. A longitudinal study of cardiac output in normal human pregnancy. American journal of obstetrics and gynecology. 1994;170(3):849–856. doi: 10.1016/s0002-9378(94)70297-7. [DOI] [PubMed] [Google Scholar]
- 71.Kazerooni T, Khosropananh S. Second trimester cardiac output and its predictive value for preeclampsia. Saudi medical journal. 2006;27(10):1526–1529. [PubMed] [Google Scholar]
- 72.Bosio PM, McKenna PJ, Conroy R, O'Herlihy C. Maternal central hemodynamics in hypertensive disorders of pregnancy. Obstetrics and gynecology. 1999;94(6):978–984. doi: 10.1016/s0029-7844(99)00430-5. [DOI] [PubMed] [Google Scholar]
- 73.De Paco C, Kametas N, Rencoret G, Strobl I, Nicolaides KH. Maternal cardiac output between 11 and 13 weeks of gestation in the prediction of preeclampsia and small for gestational age. Obstetrics and gynecology. 2008;111(2 Pt 1):292–300. doi: 10.1097/01.AOG.0000298622.22494.0c. [DOI] [PubMed] [Google Scholar]
- 74.Melchiorre K, Sutherland GR, Baltabaeva A, Liberati M, Thilaganathan B. Maternal cardiac dysfunction and remodeling in women with preeclampsia at term. Hypertension. 2011;57(1):85–93. doi: 10.1161/HYPERTENSIONAHA.110.162321. [DOI] [PubMed] [Google Scholar]
- 75.Melchiorre K, Sutherland GR, Watt-Coote I, Liberati M, Thilaganathan B. Severe myocardial impairment and chamber dysfunction in preterm preeclampsia. Hypertension in pregnancy. 2012;31(4):454–471. doi: 10.3109/10641955.2012.697951. [DOI] [PubMed] [Google Scholar]
- 76.Carbillon L, Uzan M, Uzan S. Pregnancy, vascular tone, and maternal hemodynamics: a crucial adaptation. Obstetrical & gynecological survey. 2000;55(9):574–581. doi: 10.1097/00006254-200009000-00023. [DOI] [PubMed] [Google Scholar]
- 77.Easterling TR, Benedetti TJ, Schmucker BC, Millard SP. Maternal hemodynamics in normal and preeclamptic pregnancies: a longitudinal study. Obstetrics and gynecology. 1990;76(6):1061–1069. [PubMed] [Google Scholar]
- 78.Allen R, Castro L, Arora C, Krakow D, Huang S, Platt L. Endothelium-derived relaxing factor inhibition and the pressor response to norepinephrine in the pregnant rat. Obstetrics and gynecology. 1994;83(1):92–96. [PubMed] [Google Scholar]
- 79.Brown GP, Venuto RC. Angiotensin II receptor alterations during pregnancy in rabbits. The American journal of physiology. 1986;251(1 Pt 1):E58–E64. doi: 10.1152/ajpendo.1986.251.1.E58. [DOI] [PubMed] [Google Scholar]
- 80.Paller MS. Mechanism of decreased pressor responsiveness to ANG II, NE, and vasopressin in pregnant rats. The American journal of physiology. 1984;247(1 Pt 2):H100–H108. doi: 10.1152/ajpheart.1984.247.1.H100. [DOI] [PubMed] [Google Scholar]
- 81.Ylikorkala O, Makila UM. Prostacyclin and thromboxane in gynecology and obstetrics. American journal of obstetrics and gynecology. 1985;152(3):318–329. doi: 10.1016/s0002-9378(85)80221-0. [DOI] [PubMed] [Google Scholar]
- 82.Dschietzig T, Bartsch C, Richter C, Laule M, Baumann G, Stangl K. Relaxin, a pregnancy hormone, is a functional endothelin-1 antagonist: attenuation of endothelin-1-mediated vasoconstriction by stimulation of endothelin type-B receptor expression via ERK-1/2 and nuclear factor-kappaB. Circulation research. 2003;92(1):32–40. doi: 10.1161/01.res.0000051884.27117.7e. [DOI] [PubMed] [Google Scholar]
- 83.Lanni SM, Tillinghast J, Silver HM. Hemodynamic changes and baroreflex gain in the supine hypotensive syndrome. American journal of obstetrics and gynecology. 2002;187(6):1636–1641. doi: 10.1067/mob.2002.127304. [DOI] [PubMed] [Google Scholar]
- 84.McGuane JT, Debrah JE, Debrah DO, Rubin JP, Segal M, Shroff SG, et al. Role of relaxin in maternal systemic and renal vascular adaptations during gestation. Annals of the New York Academy of Sciences. 2009;1160:304–312. doi: 10.1111/j.1749-6632.2009.03829.x. [DOI] [PubMed] [Google Scholar]
- 85.Duvekot JJ, Cheriex EC, Pieters FA, Menheere PP, Peeters LH. Early pregnancy changes in hemodynamics and volume homeostasis are consecutive adjustments triggered by a primary fall in systemic vascular tone. American journal of obstetrics and gynecology. 1993;169(6):1382–1392. doi: 10.1016/0002-9378(93)90405-8. [DOI] [PubMed] [Google Scholar]
- 86.Gilson GJ, Samaan S, Crawford MH, Qualls CR, Curet LB. Changes in hemodynamics, ventricular remodeling, and ventricular contractility during normal pregnancy: a longitudinal study. Obstetrics and gynecology. 1997;89(6):957–962. doi: 10.1016/s0029-7844(97)85765-1. [DOI] [PubMed] [Google Scholar]
- 87.Clark SL, Cotton DB, Lee W, Bishop C, Hill T, Southwick J, et al. Central hemodynamic assessment of normal term pregnancy. American journal of obstetrics and gynecology. 1989;161(6 Pt 1):1439–1442. doi: 10.1016/0002-9378(89)90900-9. [DOI] [PubMed] [Google Scholar]
- 88.Anggard E. Nitric oxide: mediator, murderer, and medicine. Lancet. 1994;343(8907):1199–1206. doi: 10.1016/s0140-6736(94)92405-8. [DOI] [PubMed] [Google Scholar]
- 89.Podjarny E, Mandelbaum A, Bernheim J. Does nitric oxide play a role in normal pregnancy and pregnancy-induced hypertension? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1994;9(11):1527–1529. [PubMed] [Google Scholar]
- 90.Holden DP, Fickling SA, Whitley GS, Nussey SS. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. American journal of obstetrics and gynecology. 1998;178(3):551–556. doi: 10.1016/s0002-9378(98)70437-5. [DOI] [PubMed] [Google Scholar]
- 91.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine. 2002;18(3):239–245. doi: 10.1385/ENDO:18:3:239. [DOI] [PubMed] [Google Scholar]
- 92.Novak J, Reckelhoff J, Bumgarner L, Cockrell K, Kassab S, Granger JP. Reduced sensitivity of the renal circulation to angiotensin II in pregnant rats. Hypertension. 1997;30(3 Pt 2):580–584. doi: 10.1161/01.hyp.30.3.580. [DOI] [PubMed] [Google Scholar]
- 93.Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(3):543–549. doi: 10.2215/CJN.03761106. [DOI] [PubMed] [Google Scholar]
- 94.LaMarca B, Parrish M, Ray LF, Murphy SR, Roberts L, Glover P, et al. Hypertension in response to autoantibodies to the angiotensin II type I receptor (AT1-AA) in pregnant rats: role of endothelin-1. Hypertension. 2009;54(4):905–909. doi: 10.1161/HYPERTENSIONAHA.109.137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Herse F, Verlohren S, Wenzel K, Pape J, Muller DN, Modrow S, et al. Prevalence of agonistic autoantibodies against the angiotensin II type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension. 2009;53(2):393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 96.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. American journal of hypertension. 2005;18(3):330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, et al. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110(12):1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 98.Wallukat G, Neichel D, Nissen E, Homuth V, Luft FC. Agonistic autoantibodies directed against the angiotensin II AT1 receptor in patients with preeclampsia. Canadian journal of physiology and pharmacology. 2003;81(2):79–83. doi: 10.1139/y02-160. [DOI] [PubMed] [Google Scholar]
- 99.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107(12):1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 100.Xia Y, Kellems RE. Angiotensin receptor agonistic autoantibodies and hypertension: preeclampsia and beyond. Circulation research. 2013;113(1):78–87. doi: 10.1161/CIRCRESAHA.113.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. Journal of the Society for Gynecologic Investigation. 2003;10(2):82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi Y, Yamamoto T, Chishima F, Takahashi H, Suzuki M. Autoantibodies isolated from patients with preeclampsia induce soluble endoglin production from trophoblast cells via interactions with angiotensin II type 1 receptor. American journal of reproductive immunology (New York, NY : 1989) 2015;73(4):285–291. doi: 10.1111/aji.12340. [DOI] [PubMed] [Google Scholar]
- 103.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension. 2010;55(2):386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Velloso EP, Pimentel RL, Braga JF, Cabral AC, Reis ZS, Bader M, et al. Identification of a Novel Agonist-Like Autoantibody in Preeclamptic Patients. American journal of hypertension. 2015 doi: 10.1093/ajh/hpv099. [DOI] [PubMed] [Google Scholar]
- 105.AbdAlla S, Abdel-Baset A, Lother H, el Massiery A, Quitterer U. Mesangial AT1/B2 receptor heterodimers contribute to angiotensin II hyperresponsiveness in experimental hypertension. Journal of molecular neuroscience : MN. 2005;26(2–3):185–192. doi: 10.1385/JMN:26:2-3:185. [DOI] [PubMed] [Google Scholar]
- 106.AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nature medicine. 2001;7(9):1003–1009. doi: 10.1038/nm0901-1003. [DOI] [PubMed] [Google Scholar]
- 107.Quitterer U, AbdAlla S. Vasopressor meets vasodepressor: The AT1-B2 receptor heterodimer. Biochemical pharmacology. 2014;88(3):284–290. doi: 10.1016/j.bcp.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 108.Dennis AT. Transthoracic echocardiography in women with preeclampsia. Current opinion in anaesthesiology. 2015;28(3):254–260. doi: 10.1097/ACO.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 109.Mahendru AA, Everett TR, Wilkinson IB, Lees CC, McEniery CM. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. Journal of hypertension. 2014;32(4):849–856. doi: 10.1097/HJH.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 110.August P, Lenz T, Ales KL, Druzin ML, Edersheim TG, Hutson JM, et al. Longitudinal study of the renin-angiotensin-aldosterone system in hypertensive pregnant women: deviations related to the development of superimposed preeclampsia. American journal of obstetrics and gynecology. 1990;163(5 Pt 1):1612–1621. doi: 10.1016/0002-9378(90)90639-o. [DOI] [PubMed] [Google Scholar]
- 111.Chesley LC, Talledo E, Bohler CS, Zuspan FP. VASCULAR REACTIVITY TO ANGIOTENSIN II AND NOREPINEPHRINE IN PREGNANT WOMEN. American journal of obstetrics and gynecology. 1965;91:837–842. doi: 10.1016/0002-9378(65)90462-x. [DOI] [PubMed] [Google Scholar]
- 112.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacological reviews. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 113.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003;41(1):42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Anton L, Merrill DC, Neves LA, Diz DI, Corthorn J, Valdes G, et al. The uterine placental bed Renin-Angiotensin system in normal and preeclamptic pregnancy. Endocrinology. 2009;150(9):4316–4325. doi: 10.1210/en.2009-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Anton L, Merrill DC, Neves LA, Stovall K, Gallagher PE, Diz DI, et al. Activation of local chorionic villi angiotensin II levels but not angiotensin (1–7) in preeclampsia. Hypertension. 2008;51(4):1066–1072. doi: 10.1161/HYPERTENSIONAHA.107.103861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Delforce SJ, Wang Y, Van-Aalst ME, Corbisier de Meaultsart C, Morris BJ, Broughton-Pipkin F, et al. Effect of oxygen on the expression of renin-angiotensin system components in a human trophoblast cell line. Placenta. 2016;37:1–6. doi: 10.1016/j.placenta.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 117.Kurlak LO, Mistry HD, Cindrova-Davies T, Burton GJ, Broughton Pipkin F. Human placental renin-angiotensin system in normotensive and pre-eclamptic pregnancies at high altitude and after acute hypoxia-reoxygenation insult. J Physiol. 2016;594(5):1327–1340. doi: 10.1113/JP271045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marques FZ, Pringle KG, Conquest A, Hirst JJ, Markus MA, Sarris M, et al. Molecular characterization of renin-angiotensin system components in human intrauterine tissues and fetal membranes from vaginal delivery and cesarean section. Placenta. 2011;32(3):214–221. doi: 10.1016/j.placenta.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 119.Mistry HD, Kurlak LO, Broughton Pipkin F. The placental renin-angiotensin system and oxidative stress in pre-eclampsia. Placenta. 2013;34(2):182–186. doi: 10.1016/j.placenta.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 120.Nartita T, Ichihara A, Matsuoka K, Takai Y, Bokuda K, Morimoto S, et al. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta. 2016;37:72–78. doi: 10.1016/j.placenta.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 121.Pringle KG, Tadros MA, Callister RJ, Lumbers ER. The expression and localization of the human placental prorenin/renin-angiotensin system throughout pregnancy: roles in trophoblast invasion and angiogenesis? Placenta. 2011;32(12):956–962. doi: 10.1016/j.placenta.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 122.Pringle KG, Zakar T, Yates D, Mitchell CM, Hirst JJ, Lumbers ER. Molecular evidence of a (pro)renin/ (pro)renin receptor system in human intrauterine tissues in pregnancy and its association with PGHS-2. Journal of the renin-angiotensin-aldosterone system : JRAAS. 2011;12(3):304–310. doi: 10.1177/1470320310376554. [DOI] [PubMed] [Google Scholar]
- 123.Shaw KJ, Do YS, Kjos S, Anderson PW, Shinagawa T, Dubeau L, et al. Human decidua is a major source of renin. The Journal of clinical investigation. 1989;83(6):2085–2092. doi: 10.1172/JCI114121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shibata E, Powers RW, Rajakumar A, von Versen-Hoynck F, Gallaher MJ, Lykins DL, et al. Angiotensin II decreases system A amino acid transporter activity in human placental villous fragments through AT1 receptor activation. American journal of physiology Endocrinology and metabolism. 2006;291(5):E1009–E1016. doi: 10.1152/ajpendo.00134.2006. [DOI] [PubMed] [Google Scholar]
- 125.Sykes SD, Mitchell C, Pringle KG, Wang Y, Zakar T, Lumbers ER. Methylation of promoter regions of genes of the human intrauterine Renin Angiotensin system and their expression. International journal of endocrinology. 2015;2015:459818. doi: 10.1155/2015/459818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y, Lumbers ER, Sykes SD, Pringle KG. Regulation of the Renin-Angiotensin System Pathways in the Human Decidua. Reproductive sciences (Thousand Oaks, Calif) 2015;22(7):865–872. doi: 10.1177/1933719114565029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang Y, Pringle KG, Lumbers ER. The effects of cyclic AMP, sex steroids and global hypomethylation on the expression of genes controlling the activity of the renin-angiotensin system in placental cell lines. Placenta. 2013;34(3):275–280. doi: 10.1016/j.placenta.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 128.Wang Y, Pringle KG, Sykes SD, Marques FZ, Morris BJ, Zakar T, et al. Fetal sex affects expression of renin-angiotensin system components in term human decidua. Endocrinology. 2012;153(1):462–468. doi: 10.1210/en.2011-1316. [DOI] [PubMed] [Google Scholar]
- 129.Yilmaz Z, Yildirim T, Yilmaz R, Aybal-Kutlugun A, Altun B, Kucukozkan T, et al. Association between urinary angiotensinogen, hypertension and proteinuria in pregnant women with preeclampsia. Journal of the renin-angiotensin-aldosterone system : JRAAS. 2015;16(3):514–520. doi: 10.1177/1470320313510585. [DOI] [PubMed] [Google Scholar]
- 130.Zhou A, Carrell RW, Murphy MP, Wei Z, Yan Y, Stanley PL, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468(7320):108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhou A, Dekker GA, Lumbers ER, Lee SY, Thompson SD, McCowan LM, et al. The association of AGTR2 polymorphisms with preeclampsia and uterine artery bilateral notching is modulated by maternal BMI. Placenta. 2013;34(1):75–81. doi: 10.1016/j.placenta.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 132.Hsueh WA, Luetscher JA, Carlson EJ, Grislis G, Fraze E, McHargue A. Changes in active and inactive renin throughout pregnancy. The Journal of clinical endocrinology and metabolism. 1982;54(5):1010–1016. doi: 10.1210/jcem-54-5-1010. [DOI] [PubMed] [Google Scholar]
- 133.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. The Journal of clinical investigation. 2002;109(11):1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Higuchi S, Ohtsu H, Suzuki H, Shirai H, Frank GD, Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clinical science (London, England : 1979) 2007;112(8):417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 135.Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50(1):14–24. doi: 10.1161/HYPERTENSIONAHA.106.079442. [DOI] [PubMed] [Google Scholar]
- 136.Cipolla MJ, Bishop N, Chan SL. Effect of pregnancy on autoregulation of cerebral blood flow in anterior versus posterior cerebrum. Hypertension. 2012;60(3):705–711. doi: 10.1161/HYPERTENSIONAHA.112.198952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ekholm EM, Erkkola RU. Autonomic cardiovascular control in pregnancy. European journal of obstetrics, gynecology, and reproductive biology. 1996;64(1):29–36. doi: 10.1016/0301-2115(95)02255-4. [DOI] [PubMed] [Google Scholar]
- 138.Ekholm EM, Piha SJ, Erkkola RU, Antila KJ. Autonomic cardiovascular reflexes in pregnancy. A longitudinal study. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 1994;4(4):161–165. doi: 10.1007/BF01826181. [DOI] [PubMed] [Google Scholar]
- 139.Ekholm EM, Tahvanainen KU, Metsala T. Heart rate and blood pressure variabilities are increased in pregnancy-induced hypertension. American journal of obstetrics and gynecology. 1997;177(5):1208–1212. doi: 10.1016/s0002-9378(97)70041-3. [DOI] [PubMed] [Google Scholar]
- 140.Flood P, McKinley P, Monk C, Muntner P, Colantonio LD, Goetzl L, et al. Beat-to-Beat Heart Rate and Blood Pressure Variability and Hypertensive Disease in Pregnancy. American journal of perinatology. 2015 doi: 10.1055/s-0035-1548542. [DOI] [PubMed] [Google Scholar]
- 141.Greenwood JP, Stoker JB, Walker JJ, Mary DA. Sympathetic nerve discharge in normal pregnancy and pregnancy-induced hypertension. Journal of hypertension. 1998;16(5):617–624. doi: 10.1097/00004872-199816050-00009. [DOI] [PubMed] [Google Scholar]
- 142.Yang CC, Chao TC, Kuo TB, Yin CS, Chen HI. Preeclamptic pregnancy is associated with increased sympathetic and decreased parasympathetic control of HR. American journal of physiology Heart and circulatory physiology. 2000;278(4):H1269–H1273. doi: 10.1152/ajpheart.2000.278.4.H1269. [DOI] [PubMed] [Google Scholar]
- 143.Pal GK, Shyma P, Habeebullah S, Shyjus P, Pal P. Spectral analysis of heart rate variability for early prediction of pregnancy-induced hypertension. Clinical and experimental hypertension (New York, NY : 1993) 2009;31(4):330–341. doi: 10.1080/10641960802621333. [DOI] [PubMed] [Google Scholar]
- 144.Chen GY, Kuo CD. The effect of the lateral decubitus position on vagal tone. Anaesthesia. 1997;52(7):653–657. doi: 10.1111/j.1365-2044.1997.114-az0106.x. [DOI] [PubMed] [Google Scholar]
- 145.Kuo CD, Chen GY, Yang MJ, Tsai YS. The effect of position on autonomic nervous activity in late pregnancy. Anaesthesia. 1997;52(12):1161–1165. doi: 10.1111/j.1365-2044.1997.254-az0387.x. [DOI] [PubMed] [Google Scholar]
- 146.Lee SW, Khaw KS, Ngan Kee WD, Leung TY, Critchley LA. Haemodynamic effects from aortocaval compression at different angles of lateral tilt in non-labouring term pregnant women. British journal of anaesthesia. 2012;109(6):950–956. doi: 10.1093/bja/aes349. [DOI] [PubMed] [Google Scholar]
- 147.Rang S, Wolf H, Montfrans GA, Karemaker JM. Non-invasive assessment of autonomic cardiovascular control in normal human pregnancy and pregnancy- associated hypertensive disorders: a review. Journal of hypertension. 2002;20(11):2111–2119. doi: 10.1097/00004872-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 148.Lucini D, Strappazzon P, Dalla Vecchia L, Maggioni C, Pagani M. Cardiac autonomic adjustments to normal human pregnancy: insight from spectral analysis of R-R interval and systolic arterial pressure variability. Journal of hypertension. 1999;17(12 Pt 2):1899–1904. doi: 10.1097/00004872-199917121-00019. [DOI] [PubMed] [Google Scholar]
- 149.Cunningham FG, Lindheimer MD. Hypertension in pregnancy. The New England journal of medicine. 1992;326(14):927–932. doi: 10.1056/NEJM199204023261405. [DOI] [PubMed] [Google Scholar]
- 150.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet. 1993;341(8858):1447–1451. doi: 10.1016/0140-6736(93)90889-o. [DOI] [PubMed] [Google Scholar]
- 151.Schobel HP, Frank H, Naraghi R, Geiger H, Titz E, Heusser K. Hypertension in patients with neurovascular compression is associated with increased central sympathetic outflow. Journal of the American Society of Nephrology : JASN. 2002;13(1):35–41. doi: 10.1681/ASN.V13135. [DOI] [PubMed] [Google Scholar]
- 152.Greenwood JP, Scott EM, Stoker JB, Walker JJ, Mary DA. Sympathetic neural mechanisms in normal and hypertensive pregnancy in humans. Circulation. 2001;104(18):2200–2204. doi: 10.1161/hc4301.098253. [DOI] [PubMed] [Google Scholar]
- 153.Airaksinen KE, Kirkinen P, Takkunen JT. Autonomic nervous dysfunction in severe pre-eclampsia. European journal of obstetrics, gynecology, and reproductive biology. 1985;19(5):269–276. doi: 10.1016/0028-2243(85)90040-1. [DOI] [PubMed] [Google Scholar]
- 154.Khan GAN, Ishrat N, Zulquarnain Analysis of heart rate variability in pre-eclamptic pregnancy: a study employing frequency domain analysis. Int J Reprod Contracept Obstet Gynecol. 2014;3(4):1037–1042. [Google Scholar]
- 155.Pal GK, Shyma P, Habeebullah S, Pal P, Nanda N, Shyjus P. Vagal withdrawal and sympathetic overactivity contribute to the genesis of early-onset pregnancy-induced hypertension. International Journal of Hypertension. 2011;2011:361417. doi: 10.4061/2011/361417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Poulet R, Gentile MT, Vecchione C, Distaso M, Aretini A, Fratta L, et al. Acute hypertension induces oxidative stress in brain tissues. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26(2):253–262. doi: 10.1038/sj.jcbfm.9600188. [DOI] [PubMed] [Google Scholar]
- 157.Li B, Liu Q, Xuan C, Guo L, Shi R, Zhang Q, et al. GABAB receptor gene transfer into the nucleus tractus solitarii induces chronic blood pressure elevation in normotensive rats. Circulation journal : official journal of the Japanese Circulation Society. 2013;77(10):2558–2566. doi: 10.1253/circj.cj-13-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zubcevic J, Potts JT. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Experimental physiology. 2010;95(9):909–918. doi: 10.1113/expphysiol.2010.054007. [DOI] [PubMed] [Google Scholar]
- 159.Li DP, Pan HL. Role of GABAB receptors in autonomic control of systemic blood pressure. Advances in pharmacology (San Diego, Calif) 2010;58:257–286. doi: 10.1016/S1054-3589(10)58011-6. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Q, Yao F, O'Rourke ST, Qian SY, Sun C. Angiotensin II enhances GABA(B) receptor-mediated responses and expression in nucleus tractus solitarii of rats. American journal of physiology Heart and circulatory physiology. 2009;297(5):H1837–H1844. doi: 10.1152/ajpheart.00354.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yao F, Sumners C, O'Rourke ST, Sun C. Angiotensin II increases GABAB receptor expression in nucleus tractus solitarii of rats. American journal of physiology Heart and circulatory physiology. 2008;294(6):H2712–H2720. doi: 10.1152/ajpheart.00729.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Spary EJ, Maqbool A, Saha S, Batten TF. Increased GABA B receptor subtype expression in the nucleus of the solitary tract of the spontaneously hypertensive rat. Journal of molecular neuroscience : MN. 2008;35(2):211–224. doi: 10.1007/s12031-008-9055-9. [DOI] [PubMed] [Google Scholar]
- 163.Zhang W, Herrera-Rosales M, Mifflin S. Chronic hypertension enhances the postsynaptic effect of baclofen in the nucleus tractus solitarius. Hypertension. 2007;49(3):659–663. doi: 10.1161/01.HYP.0000253091.82501.c0. [DOI] [PubMed] [Google Scholar]
- 164.Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Frontiers in physiology. 2013;4:26. doi: 10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cohen MA, Taylor JA. Short-term cardiovascular oscillations in man: measuring and modelling the physiologies. J Physiol. 2002;542(Pt 3):669–683. doi: 10.1113/jphysiol.2002.017483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Taylor JA, Myers CW, Halliwill JR, Seidel H, Eckberg DL. Sympathetic restraint of respiratory sinus arrhythmia: implications for vagal-cardiac tone assessment in humans. American journal of physiology Heart and circulatory physiology. 2001;280(6):H2804–H2814. doi: 10.1152/ajpheart.2001.280.6.H2804. [DOI] [PubMed] [Google Scholar]
- 167.Fagius J, Sundlof G. The diving response in man: effects on sympathetic activity in muscle and skin nerve fascicles. J Physiol. 1986;377:429–443. doi: 10.1113/jphysiol.1986.sp016196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eckberg DL, Mohanty SK, Raczkowska M. Trigeminal-baroreceptor reflex interactions modulate human cardiac vagal efferent activity. J Physiol. 1984;347:75–83. doi: 10.1113/jphysiol.1984.sp015054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sacha J, Pluta W. Different methods of heart rate variability analysis reveal different correlations of heart rate variability spectrum with average heart rate. Journal of electrocardiology. 2005;38(1):47–53. doi: 10.1016/j.jelectrocard.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 170.Sacha J, Pluta W. Alterations of an average heart rate change heart rate variability due to mathematical reasons. International journal of cardiology. 2008;128(3):444–447. doi: 10.1016/j.ijcard.2007.06.047. [DOI] [PubMed] [Google Scholar]
- 171.Bernardi L, Keller F, Sanders M, Reddy PS, Griffith B, Meno F, et al. Respiratory sinus arrhythmia in the denervated human heart. Journal of applied physiology (Bethesda, Md : 1985) 1989;67(4):1447–1455. doi: 10.1152/jappl.1989.67.4.1447. [DOI] [PubMed] [Google Scholar]
- 172.Fu Q. Microneurographic research in women. Frontiers in physiology. 2012;3:278. doi: 10.3389/fphys.2012.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wallin BG. Sympathetic Microneurography. In: Robertson D, editor. Primer on the Autonomic Nervous System. San Diego, CA: Elsevier; 2012. pp. 389–392. [Google Scholar]
- 174.Charkoudian N, Wallin BG. Sympathetic neural activity to the cardiovascular system: integrator of systemic physiology and interindividual characteristics. Comprehensive Physiology. 2014;4(2):825–850. doi: 10.1002/cphy.c130038. [DOI] [PubMed] [Google Scholar]
- 175.Greenwood JP, Scott EM, Walker JJ, Stoker JB, Mary DA. The magnitude of sympathetic hyperactivity in pregnancy-induced hypertension and preeclampsia. American journal of hypertension. 2003;16(3):194–199. doi: 10.1016/s0895-7061(02)03256-9. [DOI] [PubMed] [Google Scholar]
- 176.Fu Q, Levine BD. Autonomic circulatory control during pregnancy in humans. Seminars in reproductive medicine. 2009;27(4):330–337. doi: 10.1055/s-0029-1225261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lohmeier TE, Warren S, Cunningham JT. Sustained activation of the central baroreceptor pathway in obesity hypertension. Hypertension. 2003;42(1):96–102. doi: 10.1161/01.HYP.0000076092.10923.FD. [DOI] [PubMed] [Google Scholar]
- 178.Barman SM, Barrett KE, Boitano S, Brooks HL. Cardiovascular Regulatory Mechanisms. In: Barman SM, Barrett KE, Boitano S, Brooks HL, editors. Ganong's Review of Medical Physiology. 23rd. New York: Lange Medical, McGraw-Hill; 2010. pp. 555–568. [Google Scholar]
- 179.Lohmeier TE, Drummond HA. The Baroreflex in the Pathogenesis of Hypertension. In: Lip GYH, Hall JE, editors. Comprehensive Hypertension. Philadelphia, PA: Elsevier; 2007. pp. 265–279. [Google Scholar]
- 180.Mifflin SW. What does the brain know about blood pressure? News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society. 2001;16:266–271. doi: 10.1152/physiologyonline.2001.16.6.266. [DOI] [PubMed] [Google Scholar]
- 181.Andresen MC, Doyle MW, Jin YH, Bailey TW. Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Annals of the New York Academy of Sciences. 2001;940:132–141. doi: 10.1111/j.1749-6632.2001.tb03672.x. [DOI] [PubMed] [Google Scholar]
- 182.Lohmeier TE, Iliescu R. The Baroreflex as a Long-Term Controller of Arterial Pressure. Physiology (Bethesda, Md) 2015;30(2):148–158. doi: 10.1152/physiol.00035.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Accorsi-Mendonca D, Machado BH. Synaptic transmission of baro- and chemoreceptors afferents in the NTS second order neurons. Autonomic neuroscience : basic & clinical. 2013;175(1–2):3–8. doi: 10.1016/j.autneu.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 184.Brunt VE, Miner JA, Kaplan PF, Halliwill JR, Strycker LA, Minson CT. Short-term administration of progesterone and estradiol independently alter carotid-vasomotor, but not carotid-cardiac, baroreflex function in young women. American journal of physiology Heart and circulatory physiology. 2013;305(7):H1041–H1049. doi: 10.1152/ajpheart.00194.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Brooks VL, Cassaglia PA, Zhao D, Goldman RK. Baroreflex function in females: changes with the reproductive cycle and pregnancy. Gender medicine. 2012;9(2):61–67. doi: 10.1016/j.genm.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Brooks VL, Dampney RA, Heesch CM. Pregnancy and the endocrine regulation of the baroreceptor reflex. American journal of physiology Regulatory, integrative and comparative physiology. 2010;299(2):R439–R451. doi: 10.1152/ajpregu.00059.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Azar AS, Brooks VL. Impaired baroreflex gain during pregnancy in conscious rats: role of brain insulin. Hypertension. 2011;57(2):283–288. doi: 10.1161/HYPERTENSIONAHA.110.162354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hines T. Baroreceptor afferent discharge in the pregnant rat. American journal of physiology Regulatory, integrative and comparative physiology. 2000;278(6):R1433–R1440. doi: 10.1152/ajpregu.2000.278.6.R1433. [DOI] [PubMed] [Google Scholar]
- 189.Laiprasert JD, Hamlin RL, Heesch CM. Afferent baroreceptor discharge in pregnant rats. American journal of physiology Heart and circulatory physiology. 2001;281(6):H2456–H2462. doi: 10.1152/ajpheart.2001.281.6.H2456. [DOI] [PubMed] [Google Scholar]
- 190.Voss A, Malberg H, Schumann A, Wessel N, Walther T, Stepan H, et al. Baroreflex sensitivity, heart rate, and blood pressure variability in normal pregnancy. American journal of hypertension. 2000;13(11):1218–1225. doi: 10.1016/s0895-7061(00)01199-7. [DOI] [PubMed] [Google Scholar]
- 191.Fischer C, Voss A. Three-Dimensional Segmented Poincare Plot Analyses SPPA3 Investigates Cardiovascular and Cardiorespiratory Couplings in Hypertensive Pregnancy Disorders. Frontiers in bioengineering and biotechnology. 2014;2:51. doi: 10.3389/fbioe.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hermida RC, Ayala DE, Mojon A, Fernandez JR, Silva I, Ucieda R, et al. High sensitivity test for the early diagnosis of gestational hypertension and preeclampsia. I. Predictable variability of cardiovascular characteristics during gestation in healthy and hypertensive pregnant women. Journal of perinatal medicine. 1997;25(1):101–109. doi: 10.1515/jpme.1997.25.1.101. [DOI] [PubMed] [Google Scholar]
- 193.Carrettiero DC, Ferrari MF, Fior-Chadi DR. alpha2-Adrenergic receptor distribution and density within the nucleus tractus solitarii of normotensive and hypertensive rats during development. Autonomic neuroscience : basic & clinical. 2012;166(1–2):39–46. doi: 10.1016/j.autneu.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 194.Carrettiero DC, Fior-Chadi DR. Adenosine A1 receptor distribution in the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. Journal of neural transmission (Vienna, Austria : 1996) 2004;111(4):465–473. doi: 10.1007/s00702-003-0104-9. [DOI] [PubMed] [Google Scholar]
- 195.Mei L, Zhang J, Mifflin S. Hypertension alters GABA receptor-mediated inhibition of neurons in the nucleus of the solitary tract. American journal of physiology Regulatory, integrative and comparative physiology. 2003;285(6):R1276–R1286. doi: 10.1152/ajpregu.00255.2003. [DOI] [PubMed] [Google Scholar]
- 196.Santiago FE, Fior-Chadi DR, Carrettiero DC. Alpha2-adrenoceptor and adenosine A1 receptor within the nucleus tractus solitarii in hypertension development. Autonomic neuroscience : basic & clinical. 2015;187:36–44. doi: 10.1016/j.autneu.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 197.Sun GC, Ho WY, Chen BR, Cheng PW, Cheng WH, Hsu MC, et al. GPCR dimerization in brainstem nuclei contributes to the development of hypertension. British journal of pharmacology. 2015;172(10):2507–2518. doi: 10.1111/bph.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vitela M, Mifflin SW. gamma-Aminobutyric acid(B) receptor-mediated responses in the nucleus tractus solitarius are altered in acute and chronic hypertension. Hypertension. 2001;37(2 Pt 2):619–622. doi: 10.1161/01.hyp.37.2.619. [DOI] [PubMed] [Google Scholar]
- 199.Yao ST. Alpha-adrenergic receptors in the nucleus tractus solitarii: fitting a new piece to a complex puzzle. Experimental physiology. 2009;94(7):771–772. doi: 10.1113/expphysiol.2009.048009. [DOI] [PubMed] [Google Scholar]
- 200.Zhang W, Mifflin S. Plasticity of GABAergic mechanisms within the nucleus of the solitary tract in hypertension. Hypertension. 2010;55(2):201–206. doi: 10.1161/HYPERTENSIONAHA.109.146407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang W, Mifflin S. Chronic hypertension enhances presynaptic inhibition by baclofen in the nucleus of the solitary tract. Hypertension. 2010;55(2):481–486. doi: 10.1161/HYPERTENSIONAHA.109.145151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. American journal of physiology Heart and circulatory physiology. 2012;303(1):H1–H8. doi: 10.1152/ajpheart.00117.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Benarroch EE. GABAB receptors: structure, functions, and clinical implications. Neurology. 2012;78(8):578–584. doi: 10.1212/WNL.0b013e318247cd03. [DOI] [PubMed] [Google Scholar]
- 204.Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiological reviews. 2004;84(3):835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- 205.Emson PC. GABA(B) receptors: structure and function. Progress in brain research. 2007;160:43–57. doi: 10.1016/S0079-6123(06)60004-6. [DOI] [PubMed] [Google Scholar]
- 206.Ulrich D, Bettler B. GABA(B) receptors: synaptic functions and mechanisms of diversity. Current opinion in neurobiology. 2007;17(3):298–303. doi: 10.1016/j.conb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 207.Yin M, Sved AF. Role of gamma-aminobutyric acid B receptors in baroreceptor reflexes in hypertensive rats. Hypertension. 1996;27(6):1291–1298. doi: 10.1161/01.hyp.27.6.1291. [DOI] [PubMed] [Google Scholar]
- 208.Catelli JM, Sved AF. Enhanced pressor response to GABA in the nucleus tractus solitarii of the spontaneously hypertensive rat. Eur J Pharmacol. 1988;151(2):243–248. doi: 10.1016/0014-2999(88)90804-7. [DOI] [PubMed] [Google Scholar]
- 209.Singh R, Ticku MK. Comparison of [3H]baclofen binding to GABAB receptors in spontaneously hypertensive and normotensive rats. Brain Res. 1985;358(1–2):1–9. doi: 10.1016/0006-8993(85)90941-2. [DOI] [PubMed] [Google Scholar]
- 210.Tsukamoto K, Sved AF. Enhanced gamma-aminobutyric acid-mediated responses in nucleus tractus solitarius of hypertensive rats. Hypertension. 1993;22(6):819–825. doi: 10.1161/01.hyp.22.6.819. [DOI] [PubMed] [Google Scholar]
- 211.Durgam VR, Vitela M, Mifflin SW. Enhanced gamma-aminobutyric acid-B receptor agonist responses and mRNA within the nucleus of the solitary tract in hypertension. Hypertension. 1999;33(1 Pt 2):530–536. doi: 10.1161/01.hyp.33.1.530. [DOI] [PubMed] [Google Scholar]
- 212.Allen AM, Zhuo J, Mendelsohn FAO. Localization and function of angiotensin AT1 receptors. American journal of hypertension. 2000;13(1, Supplement 1):S31–S38. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- 213.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Expression of angiotensin type-1 (AT1) and type-2 (AT2) receptor mRNAs in the adult rat brain: a functional neuroanatomical review. Frontiers in neuroendocrinology. 1997;18(4):383–439. doi: 10.1006/frne.1997.0155. [DOI] [PubMed] [Google Scholar]
- 214.Schreihofer AM, Guyenet PG. The baroreflex and beyond: control of sympathetic vasomotor tone by GABAergic neurons in the ventrolateral medulla. Clinical and experimental pharmacology & physiology. 2002;29(5–6):514–521. doi: 10.1046/j.1440-1681.2002.03665.x. [DOI] [PubMed] [Google Scholar]
- 215.Averill DB, Diz DI. Angiotensin peptides and baroreflex control of sympathetic outflow: pathways and mechanisms of the medulla oblongata. Brain Res Bull. 2000;51(2):119–128. doi: 10.1016/s0361-9230(99)00237-3. [DOI] [PubMed] [Google Scholar]
- 216.Saxena AR, Karumanchi SA, Brown NJ, Royle CM, McElrath TF, Seely EW. Increased sensitivity to angiotensin II is present postpartum in women with a history of hypertensive pregnancy. Hypertension. 2010;55(5):1239–1245. doi: 10.1161/HYPERTENSIONAHA.109.147595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, et al. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Molecular pharmacology. 1999;56(1):154–161. doi: 10.1124/mol.56.1.154. [DOI] [PubMed] [Google Scholar]
- 218.Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, et al. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106(19):2491–2496. doi: 10.1161/01.cir.0000036600.39600.66. [DOI] [PubMed] [Google Scholar]
- 219.Accorsi-Mendonca D, Zoccal DB, Bonagamba LG, Machado BH. Glial cells modulate the synaptic transmission of NTS neurons sending projections to ventral medulla of Wistar rats. Physiol Rep. 2013;1(4):e00080. doi: 10.1002/phy2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bruno AN, Diniz GP, Ricachenevsky FK, Pochmann D, Bonan CD, Barreto-Chaves ML, et al. Hypo- and hyperthyroidism affect the ATP, ADP and AMP hydrolysis in rat hippocampal and cortical slices. Neurosci Res. 2005;52(1):61–68. doi: 10.1016/j.neures.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 221.Landolt HP, Retey JV, Adam M. Reduced neurobehavioral impairment from sleep deprivation in older adults: contribution of adenosinergic mechanisms. Frontiers in neurology. 2012;3:62. doi: 10.3389/fneur.2012.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Carrettiero DC, Fior-Chadi DR. Age-dependent changes in adenosine A1 receptor distribution and density within the nucleus tractus solitarii of normotensive and hypertensive rats. Journal of neural transmission (Vienna, Austria : 1996) 2008;115(8):1109–1118. doi: 10.1007/s00702-008-0055-2. [DOI] [PubMed] [Google Scholar]
- 223.Carrettiero DC, Almeida RS, Fior-Chadi DR. Adenosine modulates alpha2-adrenergic receptors within specific subnuclei of the nucleus tractus solitarius in normotensive and spontaneously hypertensive rats. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31(12):2177–2186. doi: 10.1291/hypres.31.2177. [DOI] [PubMed] [Google Scholar]
- 224.Carrettiero DC, da Silva SM, Fior-Chadi DR. Adenosine modulates alpha2-adrenergic receptors through a phospholipase C pathway in brainstem cell culture of rats. Autonomic neuroscience : basic & clinical. 2009;151(2):174–177. doi: 10.1016/j.autneu.2009.05.251. [DOI] [PubMed] [Google Scholar]
- 225.Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nature chemical biology. 2008;4(2):126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 226.Delbarre B, Casset-Senon D, Delbarre G, Sestillange P, Christin O. Naloxone effects on blood pressure, analgesia and diuresis in spontaneous hypertensive and normotensive rats. Neurosci Lett. 1982;30(2):167–172. doi: 10.1016/0304-3940(82)90291-9. [DOI] [PubMed] [Google Scholar]
- 227.Oatridge A, Holdcroft A, Saeed N, Hajnal JV, Puri BK, Fusi L, et al. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. AJNR American journal of neuroradiology. 2002;23(1):19–26. [PMC free article] [PubMed] [Google Scholar]
- 228.Hillerer KM, Jacobs VR, Fischer T, Aigner L. The maternal brain: an organ with peripartal plasticity. Neural Plasticity. 2014;2014:574159. doi: 10.1155/2014/574159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mielke MM, Milic NM, Weissgerber TL, White WM, Kantarci K, Mosley TH, et al. Impaired Cognition and Brain Atrophy Decades After Hypertensive Pregnancy Disorders. Circulation Cardiovascular quality and outcomes. 2016;9(1):S70–s76. doi: 10.1161/CIRCOUTCOMES.115.002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Riskin-Mashiah S, Belfort MA. Preeclampsia is associated with global cerebral hemodynamic changes. Journal of the Society for Gynecologic Investigation. 2005;12(4):253–256. doi: 10.1016/j.jsgi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 231.Riskin-Mashiah S, Belfort MA, Saade GR, Herd JA. Cerebrovascular reactivity in normal pregnancy and preeclampsia. Obstetrics and gynecology. 2001;98(5 Pt 1):827–832. [PubMed] [Google Scholar]
- 232.Williams KP, Wilson S. Variation in cerebral perfusion pressure with different hypertensive states in pregnancy. American journal of obstetrics and gynecology. 1998;179(5):1200–1203. doi: 10.1016/s0002-9378(98)70131-0. [DOI] [PubMed] [Google Scholar]
- 233.Warrington JP, Fan F, Murphy SR, Roman RJ, Drummond HA, Granger JP, et al. Placental ischemia in pregnant rats impairs cerebral blood flow autoregulation and increases blood-brain barrier permeability. Physiol Rep. 2014;2(8) doi: 10.14814/phy2.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Osmanagaoglu MA, Dinc G, Osmanagaoglu S, Dinc H, Bozkaya H. Comparison of cerebral magnetic resonance and electroencephalogram findings in pre-eclamptic and eclamptic women. The Australian & New Zealand journal of obstetrics & gynaecology. 2005;45(5):384–390. doi: 10.1111/j.1479-828X.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 235.Wiebers DO. Ischemic cerebrovascular complications of pregnancy. Archives of neurology. 1985;42(11):1106–1113. doi: 10.1001/archneur.1985.04060100092030. [DOI] [PubMed] [Google Scholar]
- 236.Demirtas O, Gelal F, Vidinli BD, Demirtas LO, Uluc E, Baloglu A. Cranial MR imaging with clinical correlation in preeclampsia and eclampsia. Diagnostic and interventional radiology (Ankara, Turkey) 2005;11(4):189–194. [PubMed] [Google Scholar]
- 237.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217(2):371–376. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 238.Schwartz RB, Mulkern RV, Gudbjartsson H, Jolesz F. Diffusion-weighted MR imaging in hypertensive encephalopathy: clues to pathogenesis. AJNR American journal of neuroradiology. 1998;19(5):859–862. [PMC free article] [PubMed] [Google Scholar]
- 239.Sakaharova AV. Regional characteristics of the noradrenergic and cholinergic innervation of the vessels of the brain surface. Biulleten' eksperimental'noi biologii i meditsiny. 1980;89(2):141–143. [PubMed] [Google Scholar]
- 240.Housni B, Bayad R, Cherkab R, Salmi S, Miguil M. Brainstem ischemia and preeclampsia. Hypertension in pregnancy. 2004;23(3):269–273. doi: 10.1081/PRG-200030312. [DOI] [PubMed] [Google Scholar]
- 241.Kittner SJ, Stern BJ, Feeser BR, Hebel R, Nagey DA, Buchholz DW, et al. Pregnancy and the risk of stroke. The New England journal of medicine. 1996;335(11):768–774. doi: 10.1056/NEJM199609123351102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium. A study in public hospitals of Ile de France. Stroke in Pregnancy Study Group. Stroke; a journal of cerebral circulation. 1995;26(6):930–936. doi: 10.1161/01.str.26.6.930. [DOI] [PubMed] [Google Scholar]
- 243.Wiebers DO. Subarachnoid hemorrhage in pregnancy. Seminars in neurology. 1988;8(3):226–229. doi: 10.1055/s-2008-1041382. [DOI] [PubMed] [Google Scholar]
- 244.Brusse IA, Peters NC, Steegers EA, Duvekot JJ, Visser GH. Electroencephalography during normotensive and hypertensive pregnancy: a systematic review. Obstetrical & gynecological survey. 2011;65(12):794–803. doi: 10.1097/OGX.0b013e31821286f1. [DOI] [PubMed] [Google Scholar]
- 245.Janzen R, Schroeder C, Heckel H. Eclampsia in the light of electroencephalographic investigations. Klinische Wochenschrift. 1952;30(45–6):1073–1079. doi: 10.1007/BF01471891. [DOI] [PubMed] [Google Scholar]
- 246.Kolstad P. The practical value of electro-encephalography in pre-eclampsia and eclampsia. Acta Obstet Gynecol Scand. 1961;40:127–138. doi: 10.3109/00016346109157854. [DOI] [PubMed] [Google Scholar]
- 247.Thomas SV, Somanathan N, Radhakumari R. Interictal EEG changes in eclampsia. Electroencephalography and clinical neurophysiology. 1995;94(4):271–275. doi: 10.1016/0013-4694(95)98478-q. [DOI] [PubMed] [Google Scholar]
- 248.Aukes AM, Wessel I, Dubois AM, Aarnoudse JG, Zeeman GG. Self-reported cognitive functioning in formerly eclamptic women. American journal of obstetrics and gynecology. 2007;197(4):365, e1–e6. doi: 10.1016/j.ajog.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 249.Delahaije DH, Dirksen CD, Peeters LL, Smits LJ. Anxiety and depression following preeclampsia or hemolysis, elevated liver enzymes, and low platelets syndrome. A systematic review. Acta Obstet Gynecol Scand. 2013;92(7):746–761. doi: 10.1111/aogs.12175. [DOI] [PubMed] [Google Scholar]
- 250.Hoedjes M, Berks D, Vogel I, Franx A, Bangma M, Darlington AS, et al. Postpartum depression after mild and severe preeclampsia. Journal of women's health (2002) 2011;20(10):1535–1542. doi: 10.1089/jwh.2010.2584. [DOI] [PubMed] [Google Scholar]
- 251.Mautner E, Stern C, Deutsch M, Nagele E, Greimel E, Lang U, et al. The impact of resilience on psychological outcomes in women after preeclampsia: an observational cohort study. Health and quality of life outcomes. 2013;11:194. doi: 10.1186/1477-7525-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Poel YH, Swinkels P, de Vries JI. Psychological treatment of women with psychological complaints after pre-eclampsia. Journal of psychosomatic obstetrics and gynaecology. 2009;30(1):65–72. doi: 10.1080/01674820802545990. [DOI] [PubMed] [Google Scholar]
- 253.Young J. The AEtiology of Eclampsia and Albuminuria and their Relation to Accidental Haemorrhage: (An Anatomical and Experimental Investigation.) Proceedings of the Royal Society of Medicine. 1914;7(Obstet Gynaecol Sect):307–348. [PMC free article] [PubMed] [Google Scholar]
- 254.Ogden E, Hildebrand GJ, Page EW. Rise of blood pressure during ischemia of gravid uterus. Proc Soc Exp Bio Med. 1940;43:49–51. [Google Scholar]
- 255.Thadhani R, Hagmann H, Schaarschmidt W, Roth B, Cingoez T, Karumanchi SA, et al. Removal of Soluble Fms-Like Tyrosine Kinase-1 by Dextran Sulfate Apheresis in Preeclampsia. Journal of the American Society of Nephrology : JASN. 2015 doi: 10.1681/ASN.2015020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Parrish MR, Wallace K, Tam Tam KB, Herse F, Weimer A, Wenzel K, et al. Hypertension in response to AT1-AA: role of reactive oxygen species in pregnancy-induced hypertension. American journal of hypertension. 2011;24(7):835–840. doi: 10.1038/ajh.2011.62. [DOI] [PubMed] [Google Scholar]
- 257.George EM, Stout JM, Stec DE, Granger JP. Heme oxygenase induction attenuates TNF-alpha-induced hypertension in pregnant rodents. Frontiers in pharmacology. 2015;6:165. doi: 10.3389/fphar.2015.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]


