Abstract
Background
Asthma accounts for 0.4% of all deaths worldwide, a figure that increases annually. Ketamine induces bronchial smooth muscle relaxation, and increasing evidence suggests that its anti-inflammatory properties might protect against lung injury and ameliorate asthma. However, there is a lack of evidence of the usefulness and mechanism of ketamine in acute asthma exacerbation. This study aimed to analyze the therapeutic effects and mechanism of action of ketamine on acute ovalbumin (OVA)-induced murine asthma.
Material/Methods
In vivo, BALB/c mice with OVA-induced asthma were treated with or without ketamine (25 or 50 mg/mL). Serum, lung sections, and mononuclear cell suspensions from the lung were collected for histological, morphometric, immunofluorescence, microRNA, quantitative polymerase chain reaction, regulatory T cell identification, cytokine, and Western blotting analyses. In vitro, bronchial epithelial cells were cultured to analyze the effect and mechanism of ketamine on epithelial-mesenchymal transition (EMT) and transforming growth factor-β (TGF-β) signaling.
Results
The inhalation of ketamine 25 or 50 mg/mL markedly suppressed OVA-induced airway hyper-responsiveness and airway inflammation, significantly increased the percentage of CD4+CD25+ T cells, and significantly decreased OVA-induced up-regulation of TGF-β1 and the EMT. MiR-106a was present at higher amounts in OVA-induced lung samples and was suppressed by ketamine treatment. The in vitro results showed that TGF-β1-induced EMT was suppressed by ketamine via miR-106a level regulation.
Conclusions
Ketamine ameliorates lung fibrosis in OVA-induced asthmatic mice by suppressing EMT and regulating miR-106a level, while ketamine inhalation might be a new therapeutic approach to the treatment of allergic asthma.
MeSH Keywords: Asthma, Epithelial-Mesenchymal Transition, Ketamine, MicroRNAs, Transforming Growth Factor beta1
Background
Asthma is a chronic inflammatory disorder characterized by the infiltration of inflammatory cells such as macrophages and eosinophils, airway hyper-responsiveness (AHR), and airflow obstruction [1,2]. Asthma accounts for 0.4% of all deaths worldwide, and its incidence is increasing annually. Indeed, it has become a significant cause of morbidity and mortality in developed countries, especially in children [3,4].
A number of studies have suggested that regulatory T cells (Tregs), the immunosuppressive cytokine interleukin-10 (IL-10), and transforming growth factor-β (TGF-β) comprise the primary mechanisms by which probiotics suppress allergic inflammation [5]. TGF-β is essential to the maintenance of immunologic self-tolerance [6] and induces the conversion of naive CD4+CD25- T cells to CD4+CD25+ T cells through FOXP3 [7] induction. TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of CD4+CD25+ T cells. The immunological background describing the spontaneous remission of immunoglobulin E – dependent allergy primarily includes IL-10 and/or the CD4+CD25+ T cell pathway [8]. Dermatophagoides farinae – specific anergy of T cells is likely induced by increased IL-10 levels that are initially produced by specific T cells after exposure to relevant mite allergens during remission [9].
MicroRNAs (miRNAs) are single stranded non-coding RNAs of approximately 19–25 nucleotides in length that regulate gene expression by inhibiting protein translation and might be involved in the regulation of inflammatory processes [10]. miRNAs have also been shown to act as an important therapeutic target in allergy asthma in many reports [11–13]. It is reported that alterations of airway epithelial cell miRNA levels are a common feature of patients with asthma [14].
Ketamine, an intravenous anesthetic, is a unique drug with anxiolytic, analgesic, amnesic, and dissociative properties [15]. Ketamine has bronchodilatory properties and also has anti-inflammatory and anti-hyper-responsiveness effects; thus, it might be useful for the treatment of acute asthma exacerbation [16, 17]. Evidence of the usefulness of ketamine for the treatment of acute asthma exacerbation has been reported; however, its mechanism is not yet clear [17].
In the present study, we investigated the effects of ketamine in an ovalbumin (OVA)-induced murine model of acute asthma. We observed that ketamine ameliorates OVA-induced murine asthma by increasing the percentage of CD4+CD25+ regulatory T cells (Tregs) and the IL-10 level, as well as suppressing the epithelial-mesenchymal transition (EMT); furthermore, we analyzed microRNA and found that miR-106a regulation by ketamine may be a key mechanism in asthma therapy.
Material and Methods
Animals
The experimental protocol used in this study and animal care were approved by the institutional guidelines set by the Animal Research Committee of Southwest Medical University and adhered to the National Institutes of Health guidelines for the use of experimental animals. Specific pathogen-free 8-week-old female BALB/c mice weighing 20±2 g were provided by the Experimental Animal Center of Luzhou Medical College. The mice were individually bred and housed under pathogen-free conditions at a temperature of 24–28°C and 50–60% humidity in a 12-h light/12-h dark cycle. The mattresses in the cages were changed every other day. Both water and food (standard chow provided by the Animal Center of Luzhou Medical College) were freely accessible. We took the utmost care to alleviate any pain and suffering on the part of the mice.
Asthma model and inhalational exposure
The mice were sensitized and challenged with OVA using a previously described protocol with some modifications [18] (Figure 1A). Four groups (n=10 each) were included in this study: control, OVA, OVA+ketamine 25 mg/mL, and OVA+ketamine 50 mg/mL. Briefly, aluminum hydroxide (alum) solution (Sigma, St. Louis, MO, USA) was diluted in saline to 25% (vol/vol) and mixed with OVA overnight. Approximately 20 μg of OVA was adsorbed to alum particles in 100 μL of alum solution that was injected intraperitoneally on days 1 and 14. Ten mice in the control group were intraperitoneally injected with saline on day 1 and day 14.
Figure 1.
Ketamine inhalation ameliorated lung fibrosis and the symptoms of ovalbumin (OVA)-induced asthma in mice. (A) Schematic representation of the study design. (B–E) Hematoxylin and eosin staining in the right lung of each mouse. Bar: 50 μm. (F) Effects of ketamine on inflammatory scores in the lung tissues obtained from OVA-sensitized and -challenged mice. The lungs were sampled after measurement of airway reactivity and scored under a light microscope at ×40 magnification. The bars represent mean ±SEM (n=6–8/group). (G) Infiltration of inflammatory cells in the bronchoalveolar lavage fluid. (H) The airway hyper-responsiveness was determined as the mean response to methacholine at 24 h after the last OVA (or normal saline) challenge using the Buxco system as described in the Material and Methods section. The data are presented as mean ±SE for 10 mice in each group of three independent experiments. * p<0.05 compared with control; ** p<0.01 compared with control; # p<0.05 compared with the OVA group.
Two weeks after the last injection, the mice received aerosol exposure to phosphate-buffered saline (PBS) or ketamine 25 or 50 mg/mL (ketamine hydrochloride 100 mg/2 mL injection diluted with sterile PBS in a nebulizer) for 30 min. Then, they were subsequently challenged with 1% OVA in saline through the nebulizer for 20 min daily for 3 days. The control group followed by challenge with saline for 3 days. The mice were sacrificed 48 hours after the last challenge, and the plasma and lung tissues were harvested for the analysis.
Bronchoalveolar lavage fluid leukocyte count
The lungs were flushed twice with cold 0.5% fetal bovine serum in 1 mL of PBS. Bronchoalveolar lavage fluid (BALF) was obtained after lavage and centrifuged at 2200×g for 5 min at 4°C. The pellets were resuspended in 50 μL of PBS, and the total number of cells was counted with a hemocytometer.
Isolation of immune cells from the lungs
The murine lung tissue was digested in Hank’s medium (containing collagenase I 150 μg/mL). After red blood cell lysis, the dissociated cells were underlaid with 5 mL of lymphocyte separation solution (Mediatech, Montronge, France) and centrifuged at 2200 rpm for 20 min. The mononuclear cells in the middle layer were collected for flow cytometric analysis. The neutrophils were collected from the bottom of the tube.
AHR measurements
The mice were anesthetized at 24 h after the last OVA challenge, and a tracheotomy was performed as previously described [19]. Briefly, the mice were placed in a whole-body chamber (Biaera Technologies, Hagerstown, Maryland, USA), and basal readings were obtained for 3 min and then averaged. Aerosolized saline combined with methacholine (MeCh) 5–50 mg/mL was inhaled for 3 min after each MeCh inhalation. The lung resistance was calculated after the administration of each dose.
Histological and morphometric analyses
The right lung of each mouse was fixed in 10% (vol/vol) formalin overnight and processed according to standard histological protocols. The tissues were ultimately embedded in paraffin (Paraplast® Plus; Leica Biosystems, Ayer Rajah, Singapore) and cut into 3-μm sections using a rotary microtome (Accu-Cut® SRM™ 200; Sakura, California, USA). The sections were stained with hematoxylin and eosin (H&E) (Sigma) to examine the histology of the airways and cellular infiltration into the peribronchial connective tissues [20]. The inflammatory scores were based on the presence of congestion, hemorrhage, edema (alveolar and interstitial), and/or inflammation (airway lumen, airway wall, alveolar, interstitial, and perivascular) [21].
Treg cell identification and cytokine analysis
Anti-mouse CD4+ (fluorescein isothiocyanate [FITC]) (Bioscience, San Diego, California, USA) and anti-mouse CD25+ (phycoerythrin [PE]) (Milteny Biotech, Bergisch Gladbach, Germany) antibodies were added to the whole blood samples. A flow cytometric analysis was performed to gate CD4+CD25+ cells using a fluorescence-activated cell sorter (FACS) Aria Flow Cytometry System (BD Biosciences, San Jose, California, USA). FITC- and PE-labeled immunoglobulin G (BD Pharmingen, San Diego, California, USA) served as the isotype control. The serum IL-10 and TGF-β1 levels were analyzed using an enzyme-linked immunosorbent assay (Abcam, Cambridge, UK). Staining with isotype control antibodies was performed in all experiments.
Cell culture
Bronchial epithelial cells (BEAS-2B; ATCC, Manassas, Virginia, USA) were seeded on six-well culture dishes and cultured in Clonetics™ Bronchial Epithelial Cell Growth Medium (Lonza, Alpharetta, Georgia, USA) supplemented with 10% fetal bovine serum. When the cells reached 70% confluence, the medium was changed and the cells were cultured in serum-free media for 24 hours. The cells were then subjected to stimulation with ketamine (1 mg/mL) for 1 hour. TGF-β1 5 ng/mL (PeproTech, Rocky Hill, New Jersey, USA) was then added to the cells for an additional 48 hours.
Wound healing assay
Using a pipette tip at an approximately 30° angle, each well was given a straight scratch to simulate a wound. After 48 h had passed, the number of cells that migrated into the wounded area was counted under a light microscope. Six different areas were evaluated in each group, and the experiment was repeated twice with similar results.
Immunofluorescence
Frozen lung tissue sections (5-μm thickness) were used for double-positive labeling with E-cadherin and α-smooth muscle actin (α-SMA) by the protocol in a previous report [22]. Briefly, the frozen sections were fixed in acetone for 10 min at −30°C, blocked in 2% bovine serum albumin (BSA)/PBS for 30 min at room temperature (RT), and then subsequently incubated in primary antibody (1:100) for 1 hour at RT and washed three times for 5 min each with PBS. After that, the slides were incubated with the secondary antibodies (1:200) for 30 min and washed with PBS three times, and then were mounted with mounting medium containing DAPI (Sigma, St Louis, Missouri, USA). The immunolabeled sections were analyzed through fluorescence microscopy (Axio Vert.A1, Carl Zeiss Microscopy GmbH, Jena, Germany). For each mouse, ×300 magnification pictures were obtained of six different areas.
MicroRNA array analysis
Total RNA was isolated using a miRNeasy Kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instructions. Quality-confirmed total RNA samples were determined with the Quant-iT™ RNA Assay kit (Invitrogen, Carlsbad, California, USA). The samples were labeled with cyanine 3-pCp and subsequently hybridized to the Agilent mouse microRNA microarray release version 15 (1881 mouse miRNAs represented) using a microRNA Complete Labeling and Hyb Kit (Agilent Technologies) for 20 h. After washing, the slides were scanned with a G2565BA scanner, and the data were analyzed and monitored with Agilent Feature Extraction Software version 9.5.1 and GeneSpring GX software version 12.5 (Agilent Technologies).
Real-time quantitative reverse transcription–polymerase chain reaction
Total RNA was extracted from lung tissue using Trizol reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions. Real-time quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed in 25-μL aliquots containing 10 pmol of primers, while the expression levels of E-cadherin and α-SMA were detected using an ABI PRISM 7300 sequence detection system (Applied Biosystems Inc., New York, New York, USA). The following PCR primers were used: E-cadherin forward, 5′-ACA GCC CCG CCT TAT GATT-3′; E-cadherin reverse, 5′-TCG GAA CCG CTT CCT TCA-3′; α-SMA forward, 5′-GTC CAC CGC AAA TGC TTC TAA-3′; and α-SMA reverse, 5′-AAA ACA CAT TAA CGA GTC AG-3′. Three independent experiments were performed in triplicate. The relative mRNA expression levels of E-cadherin and α-SMA were calculated using 18s (forward 5′-TGT GCC GCT AGA GGT GAA ATT-3′, reverse 5′-TGG CAA ATG CTT TCG CTTT-3′) as a reference gene. The miRNA expression profile for each sample was analyzed using a miRNA TaqMan panel of individual miRNAs (Applied Biosystems Inc.). The ΔCT method was used to calculate the relative expression (compared with the mean expression of all miRNAs measured) of each miRNA, and each sample was normalized to the small nucleolar RNA (snoRNA) RNU44.
Western blot analysis
The lung sections were prepared in a lysis buffer containing 0.5 M NaF, 0.1 M Na3VO4, and protease inhibitor cocktail. The protein lysates were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The separated protein lysates were blotted onto SP PVDF membranes (Invitrogen, Carlsbad, California, USA) using a semidry transfer method. After blocking in 5% BSA/Tris-buffered saline with Tween 20 (TBST), the membranes were incubated with E-cadherin or α-SMA antibodies (1:1000 for primary E-cadherin or α-SMA antibodies; 1:10,000 for β-actin) and shaking at 4°C overnight. The membranes were washed three times with PBS and incubated with 1:2000 secondary antibodies for 1 h at RT. The bands were incubated in an enhanced chemiluminescence detection system (Pierce Biotechnology, Rockford, Illinois, USA) for 5 min, and the pictures were taken with an ImageQuant LAS 400 software (GE Healthcare Life Sciences, Uppsala, Sweden).
Statistical analysis
The data are presented as means ±SEM. Statistical comparisons among the treatment groups were performed using randomized-design two-way analysis of variance followed by the Newman-Keuls post hoc test for more than two groups or unpaired Student’s t-test for two groups using Prism software (GraphPad Inc., La Jolla, California, USA) as appropriate. Statistical significance was defined as P<0.05. Descriptive and analytical analyses were performed by the Pearson correlation index in cases of normal distributions, while the inter-group comparisons were made using Student’s t-test or the chi-square test as appropriate.
Results
Ketamine inhalation ameliorated lung fibrosis and OVA-induced asthma symptoms in mice
After challenge, some asthma symptoms were observed in OVA-induced mice, such as shortness of breath, an arched back, cyanotic lips, and scratching or irritability. These symptoms were significantly ameliorated in the ketamine inhalation group, whereas some mice showed slight irritability in the negative group. We performed H&E staining to evaluate fibrosis in the lungs and observed significant infiltration of inflammatory cells around the bronchioles and vascular tissues in the lungs of OVA-induced mice compared with the lungs of the control mice (Figure 1B–1F). BALF cell counts revealed that most of the invading cells were macrophages and eosinophils with some lymphocytes and neutrophils, and their levels were decreased after ketamine treatment (Figure 1G). The inflammation scores showed that OVA-induced inflammation was ameliorated after ketamine inhalation (Figure 1E). The AHR was significantly increased in OVA-induced asthmatic mice compared with control mice, whereas the mean Penh value in ketamine-treated mice was decreased compared with that of OVA-induced mice (Figure 1F).
Ketamine increased CD4+CD25+ T cell counts in OVA-induced asthmatic mice
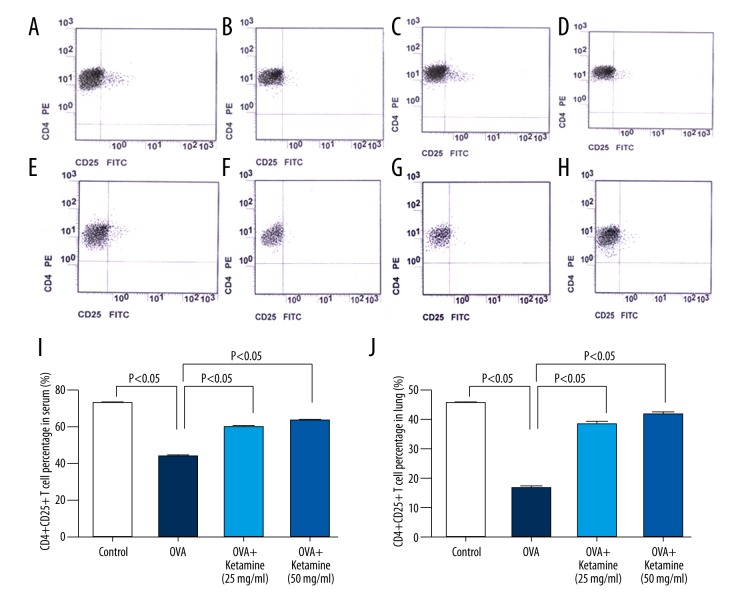
To evaluate the effects of ketamine on CD4+CD25+ T cells, the expression of CD4+CD25+ T cells in the serum and a mononuclear cell suspension obtained from the lung tissue was analyzed through flow cytometry. The serum or mononuclear cell suspension was immunofluorescently stained with anti-CD4 PE and anti-CD25 FITC-conjugated antibodies, and the percentage of CD4+CD25+ T cells was analyzed. The results showed that, compared with the control group, the percentage of CD4+CD25+ T cells in the serum was significantly decreased in the OVA-induced asthma group; however, after ketamine treatment, this percentage increased (Figure 2A–2D, 2I).
Figure 2.
Ketamine increased CD4+CD25+ T cells in mice with ovalbumin-induced asthma. (A–G) Flow cytometric analysis was performed to gate CD4+CD25+ cells using a fluorescence activated cell sorter Aria Flow Cytometric System. Fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-labeled immunoglobulin G served as the isotype control. The x-axis shows CD25-FITC, the y-axis shows CD4-PE, and the right superior quadrant represents the percentage of CD4+CD25+ regulatory T cells (Treg). (A–D) Flow cytometric analysis of CD4 and CD25 expression on the cell surface of peripheral blood lymphocytes. (E–G) Flow cytometric analysis of CD4 and CD25 expression on the cell surface of lung lymphocytes. (H) Quantification of the percentage of CD4+CD25+ Treg in the serum. (I) Quantification of the percentage of CD4+CD25+ Treg in the lungs.
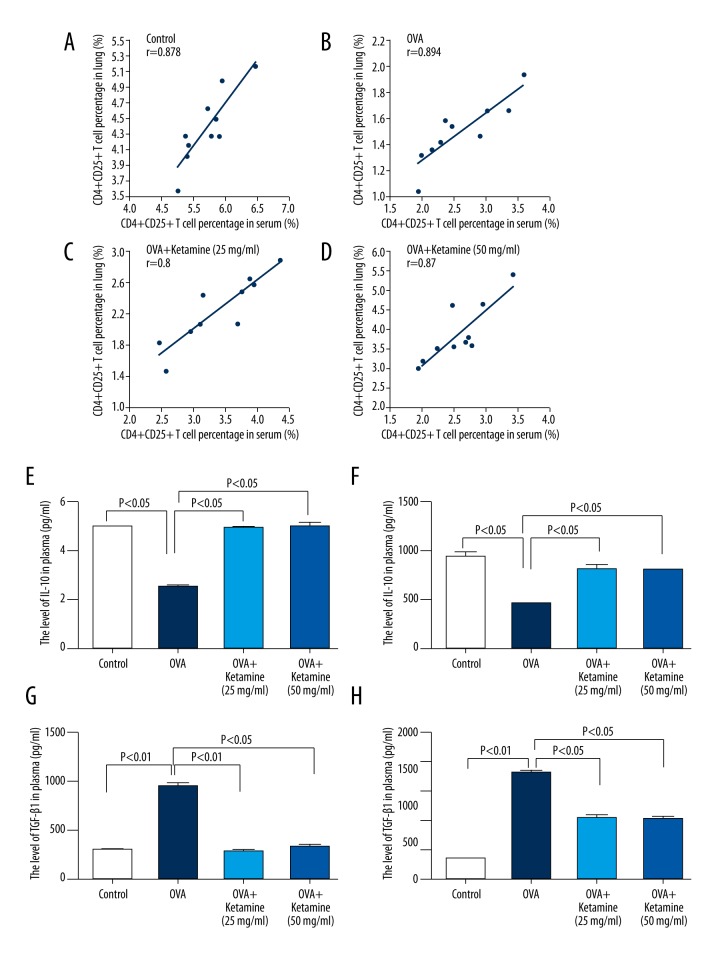
We observed similar results in the mononuclear cell suspension obtained from the lung tissue (Figure 2E–2H, 2J). We used Pearson’s correlation to determine the correlation between the serum and mononuclear cell suspension. The results showed a positive correlation between the changes in CD4+CD25+ T cell counts in the serum and the mononuclear cell suspension obtained from the lung tissue in each group (Figure 3A–3D; control group, r=0.878, p<0.01; OVA group, r=0.894, p<0.01; ketamine 25 mg/mL, r=0.8, p<0.01; ketamine 50 mg/mL, r=0.87, p<0.01).
Figure 3.
Positive correlation between the changes in CD4+CD25+ T cells in the serum and the mononuclear cell suspension obtained from lung tissue and the levels of interleukin (IL)-10 and transforming growth factor (TGF)-β1 in the serum of acute asthmatic mice. (A–D) Pearson’s correlation was used to determine the correlation between the changes in CD4+CD25+ in the serum and the mononuclear cell suspension from the lung tissues of mice in each group. (A) Control group; (B) ovalbumin (OVA) group; (C) OVA+ketamine 25 mg/mL group; (D) OVA+ketamine 50 mg/mL group. The r value is shown in each figure. (E–F) The levels of IL-10 in the serum and lung tissue were analyzed using enzyme-linked immunosorbent assay (ELISA). Staining with isotype control antibodies was performed in all experiments. E, IL-10 level in the serum. F, IL-10 level in the lung tissue. (G–H) TGF-β1 levels in the serum and lung tissue were analyzed using ELISA. (G) TGF-β1 levels in the serum. (H) TGF-β1 levels in the lung tissue.
Ketamine increased IL-10 levels and decreased TGF-β1 levels in the serum and lung tissue of asthmatic mice
Next we analyzed the mechanism underlying the effect of ketamine on asthma. Several studies have reported that Tregs regulate the immune system by suppressing Th1/Th2 responses [23,24]. As crucial suppressor T cells, Tregs release the immunosuppressive cytokines IL-10 and TGF-β1, which mediate cytotoxic lymphocyte associated antigen-4 (CTLA-4) expression in CD4+CD25+ Tregs [25]. We also analyzed the IL-10 and TGF-β1 levels in the serum and lung tissue. Compared with the control group, the IL-10 levels were significantly decreased in the serum and lungs of OVA-induced mice; however, after ketamine treatment, these levels were increased (Figure 3E, 3F). TGF-β1 is a cytokine involved in airway pathology that is primarily released from the submucosa by eosinophils, the most important cells in airway inflammation. We observed that TGF-β1 levels were significantly decreased in the plasma and lung tissue of OVA-induced acute asthmatic mice and that ketamine treatment increased the levels (Figure 3G, 3H).
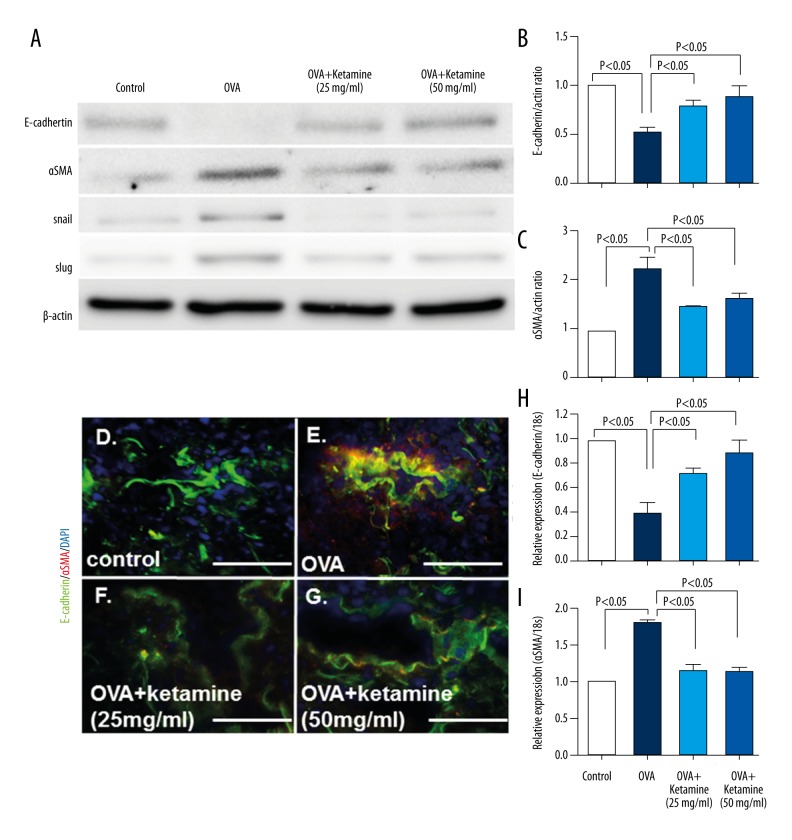
Ketamine suppressed EMT in the lungs of asthmatic mice
TGF-β1–induced EMT was recently demonstrated in human bronchial epithelial cells [26–28]. Our Western blot analysis showed that epithelial marker E-cadherin levels were decreased and mesenchymal cell marker α-SMA, Snail, and Slug levels were increased in OVA-induced asthmatic mice, and these effects were reversed after ketamine treatment (Figure 4A–4C), suggesting that OVA-induced EMT was suppressed after ketamine inhalation. The analysis of EMT through co-expression of the epithelial marker E-cadherin and the mesenchymal marker α-SMA showed that OVA-induced mice exhibited a significantly increased number of cells during EMT compared with control mice (Figure 4D–4G, yellow). Ketamine-treated mice exhibited significantly fewer cells during EMT compared with untreated asthmatic mice. The qRT-PCR also showed that E-cadherin levels were decreased and α-SMA levels were increased in OVA-induced asthmatic mice, whereas ketamine reversed these effects (Figure 4H, 4I).
Figure 4.
Ketamine reduced epithelial-mesenchymal transition (EMT) via transforming growth factor (TGF)-β1 in the lungs of asthmatic mice. (A) Western blot analysis of the lung tissue samples. The protein lysate (15 μg) was separated on polyacrylamide gels and transferred onto a polyvinylidene fluoride membrane. The immunoreactive bands were analyzed using the enhanced chemiluminescence method. Representative data from four mice in each group are shown. (B, C) Densitometric analysis results of the E-cadherin (B) and α-smooth muscle actin (α-SMA) (C) levels normalized to actin (n=4 in each group). The data are expressed as mean ±SEM on the graph. (D–G) Immunofluorescence microscopy analysis of E-cadherin and α-SMA expression in asthmatic mice. Bar: 50 μm. (H–I) mRNA expression. The expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase expression (n=4–5 per group). (H) E-cadherin. (I) α-SMA.
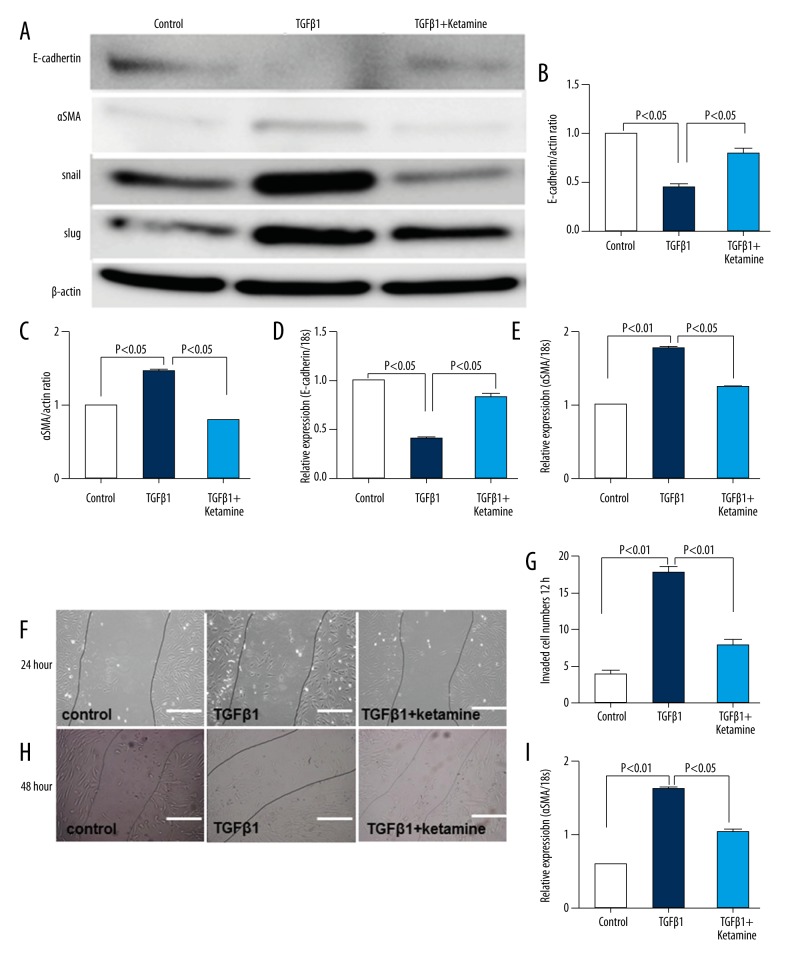
TGF-β1–induced EMT was suppressed by ketamine in BEAS-2B cells
In cultured BEAS-2B cells, we found that TGF-β1–induced EMT was suppressed by ketamine through the induction of E-cadherin protein level and the suppression of α-SMA, Snail, and Slug protein levels (Figure 5A–5C). The qRT-PCR showed same results for E-cadherin and α-SMA levels (Figure 5D, 5E). In a cell wound healing model, the invading cell numbers were increased by TGF-β1 but suppressed by ketamine at 24 and 48 hours (Figure 5F–5I).
Figure 5.
Ketamine suppressed the epithelial-mesenchymal transition in BEAS-2B cells. (A) Western blot analysis of the cell lysis. The protein lysate (10 μg) was separated on polyacrylamide gels and transferred onto a polyvinylidene fluorescence membrane. The immunoreactive bands were analyzed using an enhanced chemiluminescence method. Representative data from four wells in each group are shown. (B, C) Densitometric analysis of E-cadherin (B) and α-smooth muscle actin (α-SMA) (C) normalized to actin (n=4 in each group). The data are expressed as mean ± SEM on the graph. (D, E) Quantitative reverse transcription-polymerase chain reaction analysis of E-cadherin and α-SMA expression in cells. (F, H) Wound healing experiment for control and ketamine with or without TGF-β1 treatment. Bar: 50 μm. (G) Quantification of invaded cell number in wound healing after 24 hours. (I) Quantification of invaded cell number in wound healing after 48 hours.
Ketamine-induced EMT suppression was regulated through miRNA-106a
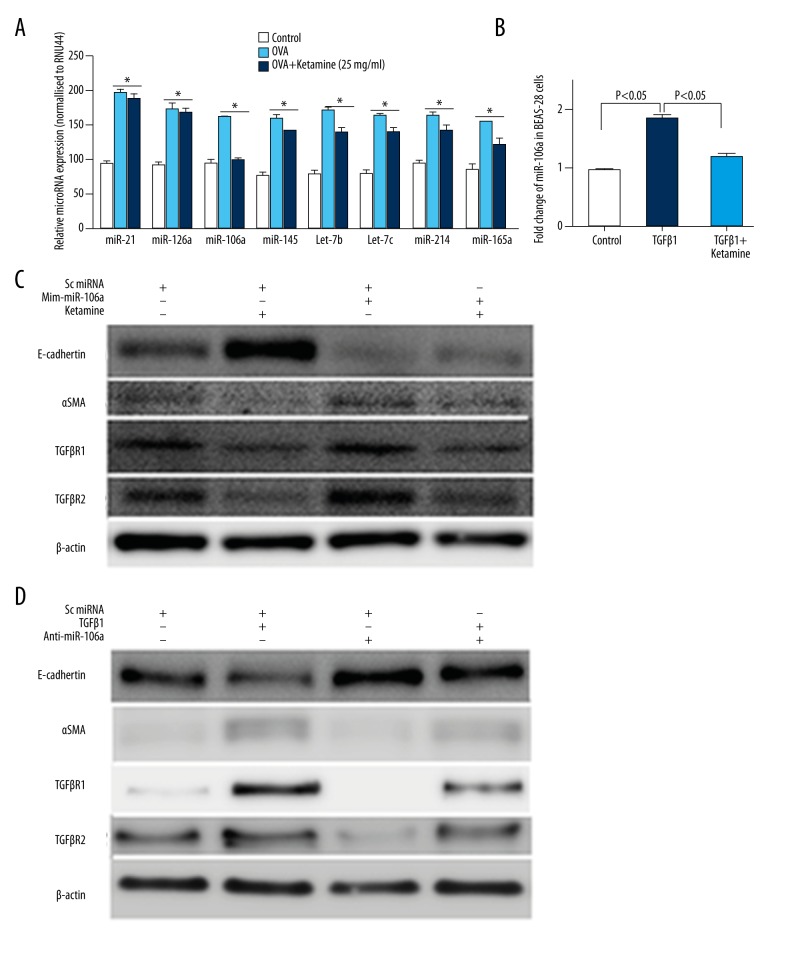
Finally, to identify the underlying mechanisms of how ketamine ameliorates asthma, we analyzed the animals’ microRNA profiles. As shown in previous reports [13,29–32], several miRNAs, including miR-126a, miR-145, miR-106a, miR-21, let-7b, let-7c, miR-214, and miR146a, were highly enriched in the mouse lung, giving strong hybridization signals on the miRNA arrays (data not shown). Among the miRNAs, we found that the miRNA-106a level was most suppressed in ketamine-treated compared with OVA mice (Figure 6A). Also, in BEAS-2B cells, qRT-PCR revealed that TGF-β1 induced a high miR-106a level that was suppressed by ketamine treatment.
Figure 6.
Ketamine-induced suppression of the epithelial-mesenchymal transition was associated with miR-106a regulation in BEAS-2B cells. (A) Individual microRNAs were measured in lung tissue using semi-quantitative real-time reverse transcription–polymerase chain reaction. The ΔCT method was used to calculate the relative expression (compared with the mean expression of all miRNAs measured) of each miRNA, and each sample was normalized to the small nucleolar RNA (snoRNA) RNU44. (B) MiR-106a analyzed in the control and ketamine with or without transforming growth factor-β1 (TGF-β1)-treated BEAS-2B cells. (C) Western blot analysis of the cell lysis. BEAS-2B cells were transfected with mimic-miR-106a with or without ketamine. E-cadherin, α-SMA, and TGF-βR1/2 were analyzed. (D) Western blot analysis of the cell lysis. BEAS-2B cells were transfected with antagomir-miR-106a with or without TGF-β1. E-cadherin, α-SMA, and TGF-βR1/2 were analyzed. * p<0.05 compared with control.
To analyze the further mechanism of miR-106a on EMT and ketamine, we also treated BEAS-2B cells with mimic-miR106a or antagomir-miR106a, and our Western blot results revealed that the E-cadherin protein level was suppressed by mimic-106a transfection, while the -SMA level was increased, which exhibited induced EMT compared with control miRNA treatment, while TGF-βR1/2 was increased by mimic-106a and ketamine treatment revised the results (Figure 6C). Furthermore, TGF-β1-induced EMT and TGF-βR1/2 protein levels were suppressed by antagomir-miR-106a (Figure 6D). Taken together, those data revealed that ketamine suppressed EMT and TGF-β signaling via regulating miR-106a levels.
Discussion
In the present study, we analyzed OVA-induced asthmatic mice in an acute asthma model and showed that ketamine inhalation ameliorated the symptoms and lung fibrosis of OVA-induced asthmatic mice and that these changes were associated with increased CD4+CD25+T cell counts and IL-10 levels. Ketamine significantly suppressed EMT and TGF-β signaling by regulating miR-106a levels in vivo and in vitro. Thus, ketamine might be an effective therapy for acute asthma in children.
Asthma is the most frequently occurring disease in children [33]. Treg numbers are associated with airway inflammation degree in asthma [34]. Strong evidence implicates CD4+CD25+ T cells in the regulation of allergic disease. The transfer of CD4+CD25+ T cells to sensitized mice reduced AHR and eosinophilic inflammation [35,36]. We also observed that, in OVA-induced asthmatic mice, the mean percentage of CD4+CD25+ cells was significantly decreased compared with that of control mice. Mice treated with ketamine inhalation showed significantly improved asthma symptoms, a phenomenon that was likely associated with decreased AHR and inflammatory cell infiltration, thereby increasing the percentage of CD4+CD25+ cells in the serum and mononuclear cell suspensions obtained from the murine lung tissues.
The development of inflammation in asthma involves an intricate network of cytokines that recruit and activate numerous immune cells [37]. Among these immune cells, eosinophils are the hallmark of asthma and the primary source of TGF-β1 in asthmatic airways [38]. In the present study, there was significant infiltration of macrophages and eosinophils around the bronchioles and vascular tissues in the lungs of OVA-induced asthmatic mice compared with the lungs of control mice, and these effects were reduced after ketamine treatment. TGF-β1 levels were also increased in OVA-induced asthmatic mice compared with control mice and suppressed after ketamine inhalation. These data also showed that levels of IL-10, the most important suppressor in asthma, were significantly decreased in the serum of OVA-induced asthmatic mice and increased after ketamine treatment. Taken together, these results suggest that ketamine ameliorates asthma fibrosis by regulating the percentage of CD4+CD25+ cells and the levels of IL-10 and TGF-β1 in the serum and lung tissue.
Interestingly, we also observed that EMT was induced by OVA and that ketamine suppressed EMT in OVA-induced mice compared with non-treated OVA mice. Levels of the epithelial marker E-cadherin were decreased, while those of the mesenchymal cell marker α-SMA, Snail, and Slug were increased in OVA-induced asthmatic mice. These effects were reversed after ketamine treatment. Thus, these data showed that ketamine suppressed OVA-induced EMT. Indeed, EMT plays an important role in the progression of airway epithelial fibrosis and the down-regulation of tight junctions [39]. Growth factors, inflammatory mediators, and matricellular proteins induce the down-regulation of epithelial cell–cell adhesion and promote mesenchymal gene expression of vimentin and α-SMA [40].
The physiological relevance of miR-106a–mediated post-transcriptional regulation of IL-10 was identified in an OVA-induced asthma model [41]. MiR-106a knockdown alleviates AHR, inflammation, increased Th2 responses, goblet cell metaplasia, and subepithelial fibrosis [42]. In the present study, the OVA-induced miR-106a level was suppressed in the lung of ketamine-treated OVA-induced asthmatic mice. In vitro experiments also showed that EMT and TGF-βR1/2 levels were mediated by ketamine via miR-106a regulation. We further found that TGF-βR2 was mediated through mimic-miR106a or antagomir-miR106a, which confirmed that TGF-βR2 is a target of miR-106a [43].
Conclusions
Taken together, the results of the present study showed that ketamine inhalation inhibits the inflammatory cascade response in an experimental asthma model in vivo. The inhalation of ketamine 25 or 50 mg/mL markedly suppressed OVA-induced AHR, airway inflammation, and airway inflammatory cell infiltration, and significantly increased the percentage of CD4+CD25+ T cells. Those effects were associated with the suppression of EMT and TGF-β signaling via regulating miR-106a levels in vivo and in vitro (Figure 7). These findings collectively indicate that ketamine ameliorates OVA-induced asthma in mice and may provide a new therapeutic approach for the treatment of allergic asthma.
Figure 7.
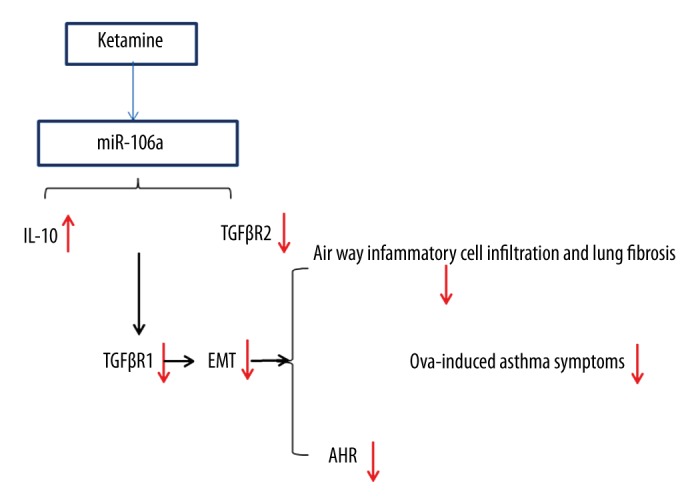
Possible mechanism of ketamine on ovalbumin (OVA)-induced asthma. Ketamine regulated miR-106a, which targeted interleukin-10 and transforming growth factor (TGF)-βR2, leading to suppression of the epithelial-mesenchymal transition and TGF-β1 levels, finally suppressing OVA-induced airway hyper-responsiveness, airway inflammation, and airway inflammatory cell infiltration.
Acknowledgments
The authors thank Yanzheng He for his critical insights and suggestions. Dr. Li Song, the guarantor of this work, had full access to the data obtained in the present study and accepts responsibility for its integrity and accuracy. Dr. Shi Sen performed the morphometric analysis and contributed to manuscript editing. Yuhong Sun contributed to some of the animal experiments and discussion. Liqun Mo contributed to some of the animal care and manuscript editing and discussion.
Footnotes
Competing interest statement
All authors: no conflicts.
Source of support: Departmental sources
References
- 1.Balaha MF, Tanaka H, Yamashita H, et al. Oral Nigella sativa oil ameliorates ovalbumin-induced bronchial asthma in mice. Int Immunopharmacol. 2012;14(2):224–31. doi: 10.1016/j.intimp.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Park JS, Park CS. The search for genetic variants and epigenetics related to asthma. Allergy Asthma Immunol Res. 2011;3(4):236–44. doi: 10.4168/aair.2011.3.4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4s–12s. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 4.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;(381):1–24. [PubMed] [Google Scholar]
- 5.Kwon HK, Lee CG, So JS, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci USA. 2010;107(5):2159–64. doi: 10.1073/pnas.0904055107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198(12):1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber S, Schramm C, Lehr HA, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173(11):6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 8.Karlsson MR, Rugtveit J, Brandtzaeg P. Allergen-responsive CD4+CD25+ regulatory T cells in children who have outgrown cow’s milk allergy. J Exp Med. 2004;199(12):1679–88. doi: 10.1084/jem.20032121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noma T, Sugawara Y, Ogawa N, et al. Dermatophagoides-induced interleukin-10 production by peripheral blood lymphocytes from patients with asthma in remission. Pediatr Allergy Immunol. 2004;15(5):459–68. doi: 10.1111/j.1399-3038.2004.00176.x. [DOI] [PubMed] [Google Scholar]
- 10.Chen XM, Splinter PL, O’Hara SP, LaRusso NF. A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J Biol Chem. 2007;282(39):28929–38. doi: 10.1074/jbc.M702633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neilson JR, Zheng GX, Burge CB, Sharp PA. Dynamic regulation of miRNA expression in ordered stages of cellular development. Genes Dev. 2007;21(5):578–89. doi: 10.1101/gad.1522907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez A, Vigorito E, Clare S, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316(5824):608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattes J, Collison A, Plank M, et al. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci USA. 2009;106(44):18704–9. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solberg OD, Ostrin EJ, Love MI, et al. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186(10):965–74. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jat KR, Chawla D. Ketamine for management of acute exacerbations of asthma in children. Cochrane Database Syst Rev. 2012;11:CD009293. doi: 10.1002/14651858.CD009293.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reich DL, Silvay G. Ketamine: An update on the first twenty-five years of clinical experience. Can J Anaesth. 1989;36(2):186–97. doi: 10.1007/BF03011442. [DOI] [PubMed] [Google Scholar]
- 17.Zhu MM, Zhou QH, Zhu MH, et al. Effects of nebulized ketamine on allergen-induced airway hyperresponsiveness and inflammation in actively sensitized Brown-Norway rats. J Inflamm (Lond) 2007;4:10. doi: 10.1186/1476-9255-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q, Martin RJ, Lafasto S, et al. Toll-like receptor 2 down-regulation in established mouse allergic lungs contributes to decreased mycoplasma clearance. Am J Respir Crit Care Med. 2008;177(7):720–29. doi: 10.1164/rccm.200709-1387OC. [DOI] [PubMed] [Google Scholar]
- 19.Bao Z, Lim S, Liao W, et al. Glycogen synthase kinase-3beta inhibition attenuates asthma in mice. Am J Respir Crit Care Med. 2007;176(5):431–38. doi: 10.1164/rccm.200609-1292OC. [DOI] [PubMed] [Google Scholar]
- 20.Yang EJ, Lee JS, Yun CY, et al. Inhibitory effects of Duchesnea chrysantha extract on ovalbumin-induced lung inflammation in a mouse model of asthma. J Ethnopharmacol. 2008;118(1):102–7. doi: 10.1016/j.jep.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Cho JY, Miller M, Baek KJ, et al. Immunostimulatory DNA sequences inhibit respiratory syncytial viral load, airway inflammation, and mucus secretion. J Allergy Clin Immunol. 2001;108(5):697–702. doi: 10.1067/mai.2001.119918. [DOI] [PubMed] [Google Scholar]
- 22.Kanasaki K, Shi S, Kanasaki M, et al. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63(6):2120–31. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 23.Yang YL, Pan YQ, He BS, Zhong TY. [Changes of regulatory T cells and T helper cells in peripheral blood and their roles in the severity evaluation in children with asthma]. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13(6):482–86. [in Chinese] [PubMed] [Google Scholar]
- 24.Fu Y, Lou H, Wang C, et al. T cell subsets in cord blood are influenced by maternal allergy and associated with atopic dermatitis. Pediatr Allergy Immunol. 2013;24(2):178–86. doi: 10.1111/pai.12050. [DOI] [PubMed] [Google Scholar]
- 25.Bianchini R, Bistoni O, Alunno A, et al. CD4(+) CD25(low) GITR(+) cells: A novel human CD4(+) T-cell population with regulatory activity. Eur J Immunol. 2011;41(8):2269–78. doi: 10.1002/eji.201040943. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JR, Nishioka M, Chakir J, et al. IL-22 contributes to TGF-beta1-mediated epithelial-mesenchymal transition in asthmatic bronchial epithelial cells. Respir Res. 2013;14:118. doi: 10.1186/1465-9921-14-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kamitani S, Yamauchi Y, Kawasaki S, et al. Simultaneous stimulation with TGF-beta1 and TNF-alpha induces epithelial mesenchymal transition in bronchial epithelial cells. Int Arch Allergy Immunol. 2011;155(2):119–28. doi: 10.1159/000318854. [DOI] [PubMed] [Google Scholar]
- 28.Heijink IH, Postma DS, Noordhoek JA, et al. House dust mite-promoted epithelial-to-mesenchymal transition in human bronchial epithelium. Am J Respir Cell Mol Biol. 2010;42(1):69–79. doi: 10.1165/rcmb.2008-0449OC. [DOI] [PubMed] [Google Scholar]
- 29.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182(8):4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar M, Ahmad T, Sharma A, et al. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol. 2011;128(5):1077–85e1–10. doi: 10.1016/j.jaci.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 31.Collison A, Mattes J, Plank M, Foster PS. Inhibition of house dust mite-induced allergic airways disease by antagonism of microRNA-145 is comparable to glucocorticoid treatment. J Allergy Clin Immunol. 2011;128(1):160–7e4. doi: 10.1016/j.jaci.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Williams AE, Larner-Svensson H, Perry MM, et al. MicroRNA expression profiling in mild asthmatic human airways and effect of corticosteroid therapy. PLoS One. 2009;4(6):e5889. doi: 10.1371/journal.pone.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Dimeloe S, Richards DF, et al. Defective IL-10 expression and in vitro steroid-induced IL-17A in paediatric severe therapy-resistant asthma. Thorax. 2014;69(6):508–15. doi: 10.1136/thoraxjnl-2013-203421. [DOI] [PubMed] [Google Scholar]
- 34.Thunberg S, Gafvelin G, Nord M, et al. Allergen provocation increases TH2-cytokines and FOXP3 expression in the asthmatic lung. Allergy. 2010;65(3):311–18. doi: 10.1111/j.1398-9995.2009.02218.x. [DOI] [PubMed] [Google Scholar]
- 35.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202(11):1539–47. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol. 2008;122(3):617–24.e6. doi: 10.1016/j.jaci.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raeiszadeh Jahromi S, Mahesh PA, Jayaraj BS, et al. Serum levels of IL-10, IL-17F and IL-33 in patients with asthma: A case-control study. J Asthma. 2014;51(10):1004–13. doi: 10.3109/02770903.2014.938353. [DOI] [PubMed] [Google Scholar]
- 38.Scherf W, Burdach S, Hansen G. Reduced expression of transforming growth factor beta 1 exacerbates pathology in an experimental asthma model. Eur J Immunol. 2005;35(1):198–206. doi: 10.1002/eji.200425209. [DOI] [PubMed] [Google Scholar]
- 39.Gong JH, Cho IH, Shin D, et al. Inhibition of airway epithelial-to-mesenchymal transition and fibrosis by kaempferol in endotoxin-induced epithelial cells and ovalbumin-sensitized mice. Lab Invest. 2014;94(3):297–308. doi: 10.1038/labinvest.2013.137. [DOI] [PubMed] [Google Scholar]
- 40.Hackett TL. Epithelial-mesenchymal transition in the pathophysiology of airway remodelling in asthma. Curr Opin Allergy Clin Immunol. 2012;12(1):53–59. doi: 10.1097/ACI.0b013e32834ec6eb. [DOI] [PubMed] [Google Scholar]
- 41.Sharma A, Kumar M, Ahmad T, et al. Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J Appl Physiol (1985) 2012;113(3):459–64. doi: 10.1152/japplphysiol.00001.2012. [DOI] [PubMed] [Google Scholar]
- 42.Kai W, Qian XU, Qun WU. MicroRNAs and asthma regulation. Iran J Allergy Asthma Immunol. 2015;14(2):120–25. [PubMed] [Google Scholar]
- 43.Li Z, Yang CS, Nakashima K, Rana TM. Small RNA-mediated regulation of iPS cell generation. EMBO J. 2011;30(5):823–34. doi: 10.1038/emboj.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]