Abstract
Technical advancements have enabled the spinal deformity surgeon to correct severe spinal mal-alignment. However, proximal adjacent segment pathology (ASP) remains a significant issue. Examples include proximal junctional kyphosis (PJK) and proximal junctional failure (PJF). Agreement on the definition, classification, and pathophysiology of PJK and PJF remains incomplete, and an understanding of the risk factors, means of prevention, and treatment of this problem remains to be elucidated. In general, PJK is a relatively asymptomatic radiographic diagnosis managed with patient reassurance and monitoring. On the other hand, PJF is characterized by mechanical instability, pain, and more severe kyphosis, with potential for neurologic compromise. Patients who develop PJF more often require revision surgery than those with PJK. This chapter will review the current understanding of PJK and PJF.
Keywords: Adjacent segment degeneration, Proximal junctional kyphosis, Proximal junctional failure, Acute proximal junctional failure, Topping off syndrome, Acute proximal junctional collapse
Introduction
Pedicle screw instrumentation constructs have become the cornerstone in the treatment of adult spinal deformity and instability. They are known to provide greater rigidity and enhanced ability to correct and maintain spinal alignment. Biomechanical data, however, demonstrate that increased construct stiffness is associated with increased loading within adjacent segments [1–9]. Increasingly stiff constructs can create vulnerability at the proximal segments and, in some cases, lead to proximal junctional pathology with radiographic and clinical manifestations [10–12]. Adjacent segment degeneration (ASD) is a well-documented phenomenon that can occur after thoracolumbar or lumbar spinal fusion [5, 6, 13–24]. Proximal junctional kyphosis (PJK) is a relatively more benign form of junctional pathology, manifesting primarily as a minimally symptomatic radiographic diagnosis [16, 19, 25, 26]. On the other hand, proximal junctional failure (PJF) represents a more severe form of junctional pathology associated with mechanical failure and increased risk of neurologic injury, deformity, pain, and the need for revision surgery [27, 28, 29•, 30]. PJF has important clinical implications especially for elderly patients with poor bone density. In this population, increased loads in the setting of decreased bone strength can lead to adjacent segment failure [5, 15, 31, 32]. When proximal junctional failure manifests with clinical symptoms, treatment can be complex, typically requiring osteotomy and extension of instrumentation and fusion. Recently, an increased amount of information describing the incidence, classification, prevention, and treatment of this problem has been developed.
Definition, epidemiology, and clinical significance
Proximal junctional kyphosis
PJK manifests as the development of minimally symptomatic kyphosis immediately above a spinal fusion construct [16, 19, 25, 26]. There is no consensus regarding a precise radiographic definition of PJK. Glattes et al. originally defined PJK as a sagittal Cobb angle between the uppermost instrumented vertebra (UIV) and the two levels above the UIV (UIV+2) of 10° or greater and at least 10° greater than the pre-operative measurement [16]. Bridwell et al. [33] and O’Shaughnessey et al. [34] used 20° as the cutoff for defining PJK. More recently, Helgeson et al. [35] described PJK as a postoperative increase of 15° or more between the UIV and UIV+1 (instead of UIV+2) [35]. To date, Glatte’s definition of PJK appears to be the most commonly utilized in the literature.
Sacramento-Dominguez et al. [36] evaluated the reproducibility of using the UIV+1 and UIV+2 to measure PJK. Although they demonstrated moderate to very high intra- and inter-rater reliability, the authors could not conclude which of the two vertebrae is the better landmark to use for measuring PJK [36]. Further work has recently shown that radiographic measurement of kyphosis from UIV to UIV+2 is highly repeatable, with or without presence of PJF and at either upper thoracic or thoracolumbar junction [37].
Proximal junctional failure
PJF is more severe than PJK and is becoming increasingly recognized as one of the most frequent reasons for reoperation after adult spinal deformity surgery. It may result in a higher need for revision surgery, a greater risk of neurologic injury, increased deformity, and pain [27, 28, 29•, 30]. Other terms used to describe this phenomenon have included “topping off syndrome,” “proximal junctional fracture,” and “proximal junctional acute collapse.” These terms highlight the associated structural failure and mechanical instability that distinguish this more severe form of proximal junctional pathology from its more common and more benign PJK counterpart. The estimated cost of revision surgery after PJF is $77,432, indicating a greater clinical and economic burden of this condition [18].
The structural failure that occurs with PJF can present as vertebral body fracture, implant pullout or breakage, and/or disruption of the posterior osseo-ligamentous complex [27, 29•]. Development of a single definition and classification system for PJF remains a challenge. Yagi and colleagues defined PJF as a symptomatic PJK requiring any type of revision surgery [38]. Hostin et al. [29•] and Smith et al. [30] defined acute PJF as 15° or more of PJK along with fracture of the UIV or UIV+1, failure of UIV fixation, or need for extension of instrumentation within 6 months of the index surgery. Hart and colleagues [28] described PJF on the basis of 10° or greater postoperative increase in kyphosis between the UIV and UIV+2, along with one or more of the following features: fracture of the vertebral body of the UIV or UIV+1, posterior osseo-ligamentous disruption, or pullout of instrumentation at the UIV (Fig. 1).
Fig. 1.
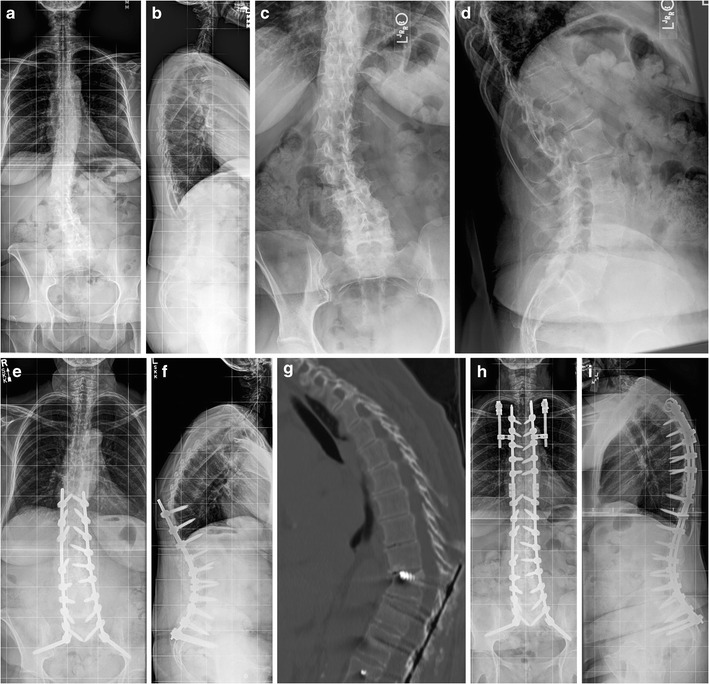
a, b Preoperative AP and lateral full-length radiographs demonstrating lumbar degenerative scoliosis with coronal imbalance. c, d Preoperative lumbosacral radiographs re-demonstrating the lumbar curve and L4-5 spondylolisthesis. e, f Postoperative radiographs illustrating L4-5 and L5-S1 laminectomy, decompression, and transforaminal lumbar interbody fusion, and T10-pelvis fusion and instrumentation with proximal junctional failure occurring within 2 months after surgery. g Postoperative CT scan illustrating T10 vertebral body fracture, screw cut-out, and kyphotic deformity, characterizing proximal junctional failure. Revision surgery was indicated for progressive kyphosis and worsening pain. The patient did not have neurologic compromise. h, i Postoperative radiographs demonstrating extension of fusion and instrumentation to T4, Smith-Peterson osteotomy at T9 and T10, and prophylactic bilateral rib fixation to T3 using VEPTR
Epidemiology and clinical significance
It is difficult to determine the prevalence of these conditions in the adult population due to the varied definitions of PJK and PJF. Different authors report the prevalence of PJK ranging from 20 to 39 % after spinal deformity fusion surgeries [16, 19, 39–42]. While the prevalence of PJF has been reported to range between 1.4 and 35 % [29•, 30, 38].
Experts continue to debate whether PJK is simply a radiographic diagnosis or has potential clinical implications for patient outcomes. Most studies have failed to demonstrate that PJK significantly diminishes clinical outcomes [16, 19, 39, 40, 42]. Only when using 20° as the threshold for defining PJK did Bridwell et al. report a significant difference in self-image subscale scores of the SRS-22 [33]. In a large retrospective study, Kim et al. also demonstrated higher rates of pain in patients with PJK (29.4 %) compared to those without PJK (0.9 %), and that the presence of upper back pain had an odds ratio of 12.5 for prediction of PJK [43]. There is also evidence that PJK can be progressive and that increased absolute PJK angles (in some cases likely an indication of structural failure) are directly correlated with pain and inversely correlated with function [43, 44].
Current literature suggests that separating PJK and PJF as two separate conditions may be overly simplistic. PJK and PJF are different clinical entities residing on the proximal junctional pathology spectrum. With worsening degrees of PJK, patients can develop the structural failures that characterize PJF. This may be accompanied by subsequent pain, neurologic deficit, gait difficulties, sagittal imbalance, and social isolation. While patients with PJK may be initially asymptomatic, Hart et al. [27] report that nearly half (47.4 %) of patients who developed acute PJF required revision surgery within 6 months of their index procedure.
Risk factors
The etiologies of PJK and PJF are likely multifactorial as no study has elucidated a single variable that strongly and consistently predicts their development. However, several major risk factors for PJK and PJF have been described. The potentially modifiable risk factors include greater curvature correction [30, 33, 45–49], combined anterior-posterior spinal fusion [19, 33, 42, 44, 50, 51], fusion to the sacro-pelvis [30, 34, 40–42, 44, 52], and residual sagittal imbalance [53]. Non-modifiable factors with clear correlation to PJK development include the following: older age (>55 years) [19, 22, 33, 45] and severe pre-operative sagittal imbalance [30, 42, 44, 46, 49, 52, 54–56]. Other less well-established but likely risk factors include low bone density [44], presence of a comorbidity [33], and high body mass index [22, 33].
There remains conflicting evidence regarding whether the type of instrumentation used at the UIV, the number of levels fused, or the location of the UIV influence the risk of PJK development. The use of hooks, wires, or pedicle screws at the proximal level has not been consistently shown to significantly affect the risk of PJK across studies [35, 40, 46, 50, 52, 55]. There are studies demonstrating that both a greater and lesser number of levels fused [33, 55] are both risk factors for PJK. Similarly, both a UIV at the upper and lower thoracic level have been associated with the development of PJK [26, 33, 51].
Modes of failure and classification
Modes of failure
Given that the prevalence of elevated thoracic kyphosis ranges between 20 and 40 % in the general population, and that is more common in geriatric patients, some authors have suggested that PJK represents a recurrence of deformity and/or natural history of aging rather than a postoperative complication. This assertion is supported by the fact that many of the radiographic features associated with the development of PJK mimic the natural history of kyphosis with normal aging: osteopenia, facet joint degeneration, disc height loss and wedging, and compression deformities of vertebrae [16, 57]. The true etiology may be multifactorial, involving iatrogenic effects of altered mechanics and adjacent segment surgical injury, along with deformity progression and the processes of natural aging. Indeed, several authors have submitted evidence suggesting that surgical disruption of the posterior soft tissue tension band, construct stiffness, and correction forces may all play an important role in the pathogenesis of PJK [24, 26, 35, 42, 57–59].
Unlike PJK, the underlying pathology for PJF appears to be an acute structural event, most typically early in the postoperative period, although it may also include progressive deformity occurring over months to years [18, 22, 24, 28, 29•]. Hostin and colleagues [29•] reported that fracture was the most common mechanism of failure (47 %), followed by soft tissue disruption (44 %). They found that 9 % of their patient cohort experienced PJF as a result of trauma, and screw pullout accounted for approximately 9 % of failures. This variety in failure mechanisms accounts for the spectrum of severity in clinical presentations of PJF. Fracture subluxation and dislocation of the adjacent segment(s) have also been reported [22, 24, 29•, 57, 60]. Hostin and colleagues [29•] found that failure resulted more frequently from vertebral body fractures when the UIV ended in the thoracolumbar region, while when the UIV ended in the upper thoracic spine, soft tissue disruption and subluxation without fracture or instrumentation failure were the more common modes of failure [29•].
Classification
Several studies have proposed a classification system for PJK and PJF [27, 38, 42, 61]. Yagi and colleagues initially presented their PJK classification in 2011 and subsequently modified it in 2014 [38, 42]. While their modified classification is simple and easy to use, it lacks prognostic information and does not guide management. The ideal classification should both guide treatment and provide information regarding severity of the pathology. Recently Hart et al. [61] and the International Spine Study Group (ISSG) proposed a Proximal Junctional Kyphosis Severity Scale (PJKSS) that assigns points to six different components thought to be important in the evaluation and management of PJK/PKF (Table 1). Points are summed to give a total severity score. The PJKSS has been shown to strongly correlate with HRQOL outcome scores and indication for revision surgery [62•]. Its inter- and intra-observer reproducibility and reliability has also been demonstrated [63].
Table 1.
Proximal Junctional Kyphosis Severity Scale (PJKSS). PJKSS assigns points to six different parameters thought to play important roles in the evaluation and management of PJK/PKF. Points are summed to give a total severity score. The PJFSS has been shown to strongly correlate with HRQOL outcome scores and indication for revision surgery [61]
| Hart-International Spine Study Group (ISSG) Proximal Junctional Kyphosis Severity Scale | ||
|---|---|---|
| Parameter | Qualifier | Severity Score |
| Neurologic deficit | None | 0 |
| Radicular pain | 2 | |
| Myelopathy/motor deficit | 4 | |
| Focal pain | None | 0 |
| VAS ≤4 | 1 | |
| VAS ≥5 | 3 | |
| Instrumentation problem | None | 0 |
| Partial fixation loss | 1 | |
| Prominence | 1 | |
| Complete fixation loss | 2 | |
| Change in kyphosis/PLC integrity | 0–10° | 0 |
| 10–20° | 1 | |
| >20° | 2 | |
| PLC failure | 2 | |
| UIV/UIV+1 fracture | None | 0 |
| Compression fracture | 1 | |
| Burst/chance fracture | 2 | |
| Translation | 3 | |
| Level of the UIV | Thoracolumbar junction | 0 |
| Upper thoracic spine | 1 | |
VAS visual analogue scale, PLC posterior ligamentous complex, UIV upper instrumented vertebra
Evaluation and preoperative planning
Evaluation
Failure to recognize and differentiate PJF from PJK and initiate the proper workup and treatment can put patients at risk of neurologic compromise. Unlike patients with PJK, patients with PJF can experience loss of neurologic function. Although pain can be substantial, some patients may have limited new complaints [18, 22, 24, 27, 29•]. On physical examination, the patient’s gait and posture should be noted and compared to previous findings. Kyphotic deformity, tenderness to palpation at the proximal junction of instrumentation, and implant prominence and skin tenting should be assessed. If there are concerns, infection should be considered in the differential diagnosis and the appropriate blood work should be ordered (CBC with differential, erythrocyte sedimentation rate, C-reactive protein). A thorough neurologic examination should be performed to evaluate for evidence of spasticity and myelopathy. Upright 36-in.-long cassette AP and lateral X-rays, and if indicated, advanced imaging such as computed tomography (CT) and magnetic resonance imaging (MRI) are essential in the complete assessment of symptomatic patients.
Preoperative planning
When revision surgery is planned, performing a thorough history and physical exam and obtaining a complete imaging workup are mandatory. Full-length 36-in. standing anteroposterior and lateral radiographs allow for accurate assessment of segmental and global spinal alignment parameters. Inclusion of the femoral heads within the field of view is required for spino-pelvic parameter measurements. In addition, supine hyperextension lateral radiograph over a bolster can provide information regarding the flexibility of the kyphotic deformity. Preoperative CT with sagittal and coronal reconstructions is helpful in identifying anterior ankylosis, as well as delineating vertebral fractures and instrumentation fracture or pullout. CT can also be valuable in evaluating prior fusions and planning osteotomies. MRI or CT myelogram should be obtained if there is suspicion for neural element compression. Bone mineral density should be measured if it has not been done within the previous 6 months. Osteoporosis or osteopenia should be treated with teriparatide if possible prior to consideration for elective revision surgery in order to reduce the chance of a second recurrence. If the procedure is more urgent, then it can be started post-operatively.
Prevention strategies
Soft tissue considerations
Failure to respect the soft tissues around the UIV is considered a risk factor for PJK [50]. As such, measures taken to preserve the interspinous ligaments, the supraspinous ligaments, the supra-adjacent facets, and their capsules at the upper end of the instrumentation construct are thought to mitigate the risk of PJK and PJF [50, 57]. Fluoroscopic localization of the UIV is a useful strategy to avoid inadvertent overexposure and unnecessary soft tissue damage. Unfortunately, in cases of elderly patients, especially those with a history of multiple previous spinal surgeries, even the most meticulous surgical dissection and instrumentation techniques cannot always offset pre-existing atrophic and degenerated soft tissues.
Avoidance of implant pre-loading at the top of the fusion construct
Introduction of a preload by forcing under-contoured rods into the top screws of a long fusion construct may predispose the patient to developing proximal junctional degeneration. The authors recommend careful and meticulous in situ bending of the rods such that they lay fully seated within the screw heads at the proximal two levels. One can be sure that the rods are not preloaded when locking caps can be placed without additional force required to reduce the rods inside the screw heads.
Vertebral cement augmentation
Recently, Kebaish et al. [64] provided biomechanical data from a cadaveric study that suggested a possible role of prophylactic vertebral cement augmentation (vertebroplasty of both the UIV and UIV+1) in reducing the risk of junctional fractures in patients with osteoporosis. The authors specifically noted that augmentation of only the UIV level provided no statistically significant benefit in preventing proximal junctional fractures. In a clinical study, Hart et al. [18] reported that prophylactic vertebroplasty of the UIV and UIV+1 levels not only reduced the risk of PJF but was also cost effective when compared to the cost of a revision procedure. Currently, there is little guidance for surgeons in determining how many levels on which to perform prophylactic cement augmentation. Kayanja et al. [65] performed a study comparing the biomechanics of augmenting different numbers of vertebral levels. They reported that bone mineral density rather than the number of augmented levels was the most important determinant of construct strength and stiffness, and concluded that cement augmentation should be performed at the most at risk levels and not arbitrarily at the UIV and/or UIV+1 [65]. Cement augmentation, however, is not without its own risks. There is evidence suggesting that cement augmentation reduces intervertebral disc nutrition and alters loading mechanics, which can accelerate adjacent segment degeneration and stenosis [24, 66–68]. The added stiffness anteriorly may also put the posterior column at greater risk of catastrophic failure. We have witnessed this sequelae in our practice and have largely stopped performing routine prophylactic vertebroplasty, although some surgeons do continue to support this approach.
Selection of the appropriate level and instrumentation for the UIV
As demonstrated by the literature reviewed in this chapter thus far, while proper selection of the UIV level is important, there does not appear to be any level immune to the risk of ASP. However, the presence of thoracic hyperkyphosis has important implications for surgical planning as it is a well-known risk factor for the development of PJK and PJF [30, 42, 44, 46, 47, 49, 52, 55, 56, 69]. Therefore, in a patient with thoracic hyperkyphosis, extending the fusion and instrumentation to the upper thoracic levels is considered desirable to minimize the risk of PJK and PJF and to achieve appropriate sagittal realignment.
While there is more consistent data in the adolescent scoliosis literature demonstrating the beneficial effect of hook and hybrid instrumentation at the proximal construct in decreasing the risk of PJK [35, 46, 55], the evidence in the adult population is inconclusive at best. In their biomechanical investigation of six adult spine models, Cammarata and colleagues [50] reported that the use of hooks and transition rods with reduced proximal diameter at the UIV was effective at reducing biomechanical effects thought to play important roles in the pathogenesis of PJK and PJF. Unfortunately, this biomechanical finding has not translated to clear clinical benefits. The currently available clinical evidence is contradictory, with some studies [70] supporting and other studies [16, 33, 34, 40] failing to find a consistent statistically significant association between proximal instrumentation type and PJK/PJF.
Matching age-appropriate spino-pelvic alignment goals
Schwab and colleagues demonstrated that lumbar lordosis (LL) should be within 9° of pelvic incidence (PI = LL ± 9°) [71]. Restoring this spino-pelvic relationship has since become a central tenet of adult deformity surgery. However, there are at least two described circumstances in which the association between PI and LL deviates from this linear equation. The first is in patients with extremely high or low PI measurements. Patients with extremely high PI (>70°) actually require slightly less lumbar lordosis than the PI = LL ± 9 o equation would suggest, while those with a low PI (<40°) require slightly more. The second situation where deviation from the typical PI–LL mismatch goals is recommended is in the elderly. Recently, the International Spine Study Group has demonstrated that the spino-pelvic alignment values that correspond with HRQOL scores (PT, PI–LL mismatch, SVA) are substantially greater at baseline in older patients compared to younger patients [72•]. The authors therefore advocate for incorporating the patient’s age into the determination of their operative spino-pelvic alignment goals. Adjusting for age-appropriate alignment goals and avoiding overly strict adherence to PI–LL relationship rules at the extremes of anatomic variability may reduce the risk of over correction and subsequent development of PJK and PJF.
Prophylactic rib fixation
Hart et al. [28, 73] introduced the concept of prophylactic rib fixation without fusion at the level of the UIV+1. Early in the development of this technique, we utilized vertical expandable prosthetic titanium rib (VEPTR) hooks inserted at the medioposterior portion the UIV+1 ribs. Two separate longitudinal incisions (approximately 3 cm long) are made over the medioposterior portion of the UIV+1 ribs. The ribs are exposed in a subperiosteal manner circumferentially, with care taken to avoid inadvertent violation of the pleural cavity. The VEPTR hooks are then placed around the exposed ribs, which are then connected to titanium rods that are tunneled subcutaneously and connected to the midline rods bilaterally via connectors (Fig. 1). The second iteration of this technique involved the use of sublaminar bands instead of VEPTR hooks. More recently, sublaminar hooks have been used in a manner similar to the VEPTR. Preliminary analysis supports the efficacy of this technique in reducing the risk of PJF [74].
Spinous process augmentation
As disruption of the posterior ligamentous complex is thought to play an important role in the pathogenesis of PJK and PJF, technical measures aimed at reinforcing the posterior tension band may be effective in reducing the risk for developing proximal junctional problems. The authors have developed a technique of looping and a Mersilene tape around the spinous processes of the UIV and UIV+1 in order to recreate a functional posterior tension band. Most recently, the authors have begun using a technique developed by Christopher Ames, MD utilizing two sublaminar bands. This is achieved by looping the bands in a weave configuration in opposite directions through drilled holes at the spino-lamina junction of the UIV+1, UIV, and UIV−1. This effectively creates a tension band loop encompassing the involved levels. The bands are tensioned and the clamps are securely locked to each of the main rods.
Treatment concepts
Currently, there is no standardized consensus to guide the surgeon in determining which patients with PJK would benefit most from revision surgery. In general, patients who are asymptomatic are managed with reassurance, education, and close monitoring. On the other hand, those with significant symptoms and higher severity of deformity or instability can be considered for revision surgery. Some authors suggest that treatment may be essential even in the absence of symptoms in patients with disruption of the posterior column, due to risk of deformity progression and neurologic injury [19, 44].
Hart et al. [27] reported that about 47 % of patients with PJF underwent revision surgery within 6 months of their index procedure. The authors also determined several factors that may influence the surgeon to recommend revision surgery for PJF: traumatic etiology, severity of kyphosis, combined anterior/posterior approaches at the index surgery, female sex, and higher SVA at the time of revision [27]. Interestingly, mode of failure (soft tissue vs. bony), age and BMI, number of levels fused, and location of UIV did not statistically correlate with the decision to revise [27]. Smith et al. [30] also identified other factors affecting the decision to perform revision surgery, including the presence of instrumentation failure, uncontrolled pain, neurologic deficits, and myelopathy. Of note, they also reported that the revision rates differed by the location of the UIV. In their patient cohort, the revision rate was much higher when the UIV was located in the lumbar or lower thoracic spine, compared to the upper thoracic spine [30].
In general, extension of the fusion (with or without decompression) may be all that is required if the spine is flexible and global balance can be achieved without the use of osteotomies. However, if the spine above the fusion construct is rigid and the kyphotic deformity is severe, the use of osteotomies may be indicated. Smith-Peterson or Ponte osteotomies are sometimes adequate to restore sagittal alignment if the intervertebral discs are supple and there is no anterior column ankylosis (Fig. 1). Three column osteotomies, such as pedicle subtraction osteotomy or vertebral column resection, are reserved for cases of severe, rigid deformity or when neurological compromise due to anterior spinal cord compression is present. The use of anterior interbody support should be considered when significant anterior column defect (>50 % bone loss) exists or to aid in obtaining greater sagittal alignment correction and improving fusion rates. Yagi et al. [38] recently reported a 48 % recurrence rate of PJK/PJF at the new UIV after revision surgery. Of those patients with a recurrence PJK/PJF, 82 % required repeat revision surgeries, highlighting the importance of prophylactic procedures at the time of revision to reduce the risk of recurrence.
Conclusion
Advances in surgical techniques and technology have revolutionized the treatment of adult spinal deformity. The ability to perform aggressive global realignment of spinal deformities has also led to the discovery of new complications such as PJK and PJF. Spine surgeons are beginning to reach consensus on the definition, classification, and pathophysiology of these entities. However, the risk factors, means of prevention, and treatment strategies for this problem remain incompletely described. While PJK is generally an asymptomatic radiographic diagnosis, PJF is a more serious condition on the pathology spectrum with significant clinical, psychosocial, and economic ramifications that often requires revision surgery and proximal extension of the fusion construct. Continued research on PJK and PJF will be needed in order to reduce the incidence and impact of this challenging complication.
Compliance with ethical standards
Conflict of interest
Ngoc-Lam M. Nguyen and Christopher Y. Kong declare that they have no conflict of interest.
Robert A. Hart is a board member for CSRS and ISSLS. He reports personal fees from DePuySynthes, Globus, Medtronic, and Seaspine. Dr. Hart also reports grants from Medtronic and ISSGF, and stock from Spine Connect.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Complications in Spine Surgery
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Bastian L, Lange U, Knop C, Tusch G, Blauth M. Evaluation of the mobility of adjacent segments after posterior thoracolumbar fixation: a biomechanical study. Eur Spine J. 2001;10(4):295–300. doi: 10.1007/s005860100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chow DH, Luk KD, Evans JH, Leong JC. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine. 1996;21(5):549–55. doi: 10.1097/00007632-199603010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ha KY, Schendel MJ, Lewis JL, Ogilvie JW. Effect of immobilization and configuration on lumbar adjacent-segment biomechanics. J Spinal Disord. 1993;6(2):99–105. doi: 10.1097/00002517-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Lee CK, Langrana NA. Lumbosacral spinal fusion. A biomechanical study. Spine. 1984;9(6):574–81. doi: 10.1097/00007632-198409000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Park P, Garton HJ, Gala VC, Hoff JT, McGillicuddy JE. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29(17):1938–44. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 6.Shono Y, Kaneda K, Abumi K, McAfee PC, Cunningham BW. Stability of posterior spinal instrumentation and its effects on adjacent motion segments in the lumbosacral spine. Spine. 1998;23(14):1550–8. doi: 10.1097/00007632-199807150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Untch C, Liu Q, Hart R. Segmental motion adjacent to an instrumented lumbar fusion: the effect of extension of fusion to the sacrum. Spine. 2004;29(21):2376–81. doi: 10.1097/01.brs.0000143667.55696.bd. [DOI] [PubMed] [Google Scholar]
- 8.Yang SW, Langrana NA, Lee CK. Biomechanics of lumbosacral spinal fusion in combined compression-torsion loads. Spine. 1986;11(9):937–41. doi: 10.1097/00007632-198611000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Yoganandan N, Pintar F, Maiman DJ, Reinartz J, Sances A, Jr, Larson SJ, et al. Kinematics of the lumbar spine following pedicle screw plate fixation. Spine. 1993;18(4):504–12. doi: 10.1097/00007632-199318040-00015. [DOI] [PubMed] [Google Scholar]
- 10.Javedan SP, Dickman CA. Cause of adjacent-segment disease after spinal fusion. Lancet. 1999;354(9178):530–1. doi: 10.1016/S0140-6736(99)00201-9. [DOI] [PubMed] [Google Scholar]
- 11.Kasliwal MK, Shaffrey CI, Lenke LG, Dettori JR, Ely CG, Smith JS. Frequency, risk factors, and treatment of distal adjacent segment pathology after long thoracolumbar fusion: a systematic review. Spine. 2012;37(22 Suppl):S165–79. doi: 10.1097/BRS.0b013e31826d62c9. [DOI] [PubMed] [Google Scholar]
- 12.Kraemer P, Fehlings MG, Hashimoto R, Lee MJ, Anderson PA, Chapman JR, et al. A systematic review of definitions and classification systems of adjacent segment pathology. Spine. 2012;37(22 Suppl):S31–9. doi: 10.1097/BRS.0b013e31826d7dd6. [DOI] [PubMed] [Google Scholar]
- 13.Aota Y, Kumano K, Hirabayashi S. Postfusion instability at the adjacent segments after rigid pedicle screw fixation for degenerative lumbar spinal disorders. J Spinal Disord. 1995;8(6):464–73. doi: 10.1097/00002517-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 14.DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine. 2006;31(19 Suppl):S144–51. doi: 10.1097/01.brs.0000236893.65878.39. [DOI] [PubMed] [Google Scholar]
- 15.Etebar S, Cahill DW. Risk factors for adjacent-segment failure following lumbar fixation with rigid instrumentation for degenerative instability. J Neurosurg. 1999;90(2 Suppl):163–9. doi: 10.3171/spi.1999.90.2.0163. [DOI] [PubMed] [Google Scholar]
- 16.Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C., 2nd Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine. 2005;30(14):1643–9. doi: 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 17.Hambly MF, Wiltse LL, Raghavan N, Schneiderman G, Koenig C. The transition zone above a lumbosacral fusion. Spine. 1998;23(16):1785–92. doi: 10.1097/00007632-199808150-00012. [DOI] [PubMed] [Google Scholar]
- 18.Hart RA, Prendergast MA, Roberts WG, Nesbit GM, Barnwell SL. Proximal junctional acute collapse cranial to multi-level lumbar fusion: a cost analysis of prophylactic vertebral augmentation. Spine J. 2008;8(6):875–81. doi: 10.1016/j.spinee.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 19.Kim YJ, Bridwell KH, Lenke LG, Glattes CR, Rhim S, Cheh G. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up. Spine. 2008;33(20):2179–84. doi: 10.1097/BRS.0b013e31817c0428. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Sagittal thoracic decompensation following long adult lumbar spinal instrumentation and fusion to L5 or S1: causes, prevalence, and risk factor analysis. Spine. 2006;31(20):2359–66. doi: 10.1097/01.brs.0000238969.59928.73. [DOI] [PubMed] [Google Scholar]
- 21.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13(3):375–7. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 22.O’Leary PT, Bridwell KH, Lenke LG, Good CR, Pichelmann MA, Buchowski JM, et al. Risk factors and outcomes for catastrophic failures at the top of long pedicle screw constructs: a matched cohort analysis performed at a single center. Spine. 2009;34(20):2134–9. doi: 10.1097/BRS.0b013e3181b2e17e. [DOI] [PubMed] [Google Scholar]
- 23.Schlegel JD, Smith JA, Schleusener RL. Lumbar motion segment pathology adjacent to thoracolumbar, lumbar, and lumbosacral fusions. Spine. 1996;21(8):970–81. doi: 10.1097/00007632-199604150-00013. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Lenke LG, Bridwell KH, Kim YJ, Koester L, Hensley M. Proximal junctional vertebral fracture in adults after spinal deformity surgery using pedicle screw constructs: analysis of morphological features. Spine. 2010;35(2):138–45. doi: 10.1097/BRS.0b013e3181c8f35d. [DOI] [PubMed] [Google Scholar]
- 25.Cho SK, Shin JI, Kim YJ. Proximal junctional kyphosis following adult spinal deformity surgery. Eur Spine J. 2014;23(12):2726–36. doi: 10.1007/s00586-014-3531-4. [DOI] [PubMed] [Google Scholar]
- 26.Denis F, Sun EC, Winter RB. Incidence and risk factors for proximal and distal junctional kyphosis following surgical treatment for Scheuermann kyphosis: minimum five-year follow-up. Spine. 2009;34(20):E729–34. doi: 10.1097/BRS.0b013e3181ae2ab2. [DOI] [PubMed] [Google Scholar]
- 27.Hart R, McCarthy I, O’Brien M, Bess S, Line B, Adjei OB, et al. Identification of decision criteria for revision surgery among patients with proximal junctional failure after surgical treatment of spinal deformity. Spine. 2013;38(19):E1223–7. doi: 10.1097/BRS.0b013e31829fedde. [DOI] [PubMed] [Google Scholar]
- 28.Hart RA, McCarthy I, Ames CP, Shaffrey CI, Hamilton DK, Hostin R. Proximal junctional kyphosis and proximal junctional failure. Neurosurg Clin N Am. 2013;24(2):213–8. doi: 10.1016/j.nec.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 29.•.Hostin R, McCarthy I, O’Brien M, Bess S, Line B, Boachie-Adjei O, et al. Incidence, mode, and location of acute proximal junctional failures after surgical treatment of adult spinal deformity. Spine. 2013;38(12):1008–15. doi: 10.1097/BRS.0b013e318271319c. [DOI] [PubMed] [Google Scholar]
- 30.Smith MW, Annis P, Lawrence BD, Daubs MD, Brodke DS. Acute proximal junctional failure in patients with preoperative sagittal imbalance. Spine J. 2015;15(10):2142–8. doi: 10.1016/j.spinee.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Simmons ED, Huckell CB, Zheng Y. Proximal kyphosis “topping off syndrome” and retrolisthesis secondary to multilevel lumbar fusion in the elderly patients. Spine J. 2004; 4(5):S114.
- 32.Toyone T, Ozawa T, Kamikawa K, Watanabe A, Matsuki K, Yamashita T, et al. Subsequent vertebral fractures following spinal fusion surgery for degenerative lumbar disease: a mean ten-year follow-up. Spine. 2010;35(21):1915–8. doi: 10.1097/BRS.0b013e3181dc846c. [DOI] [PubMed] [Google Scholar]
- 33.Bridwell KH, Lenke LG, Cho SK, Pahys JM, Zebala LP, Dorward IG, et al. Proximal junctional kyphosis in primary adult deformity surgery: evaluation of 20 degrees as a critical angle. Neurosurgery. 2013;72(6):899–906. doi: 10.1227/NEU.0b013e31828bacd8. [DOI] [PubMed] [Google Scholar]
- 34.O’Shaughnessy BA, Bridwell KH, Lenke LG, Cho W, Baldus C, Chang MS, et al. Does a long-fusion “T3-sacrum” portend a worse outcome than a short-fusion “T10-sacrum” in primary surgery for adult scoliosis? Spine. 2012;37(10):884–90. doi: 10.1097/BRS.0b013e3182376414. [DOI] [PubMed] [Google Scholar]
- 35.Helgeson MD, Shah SA, Newton PO, Clements DH, 3rd, Betz RR, Marks MC, et al. Evaluation of proximal junctional kyphosis in adolescent idiopathic scoliosis following pedicle screw, hook, or hybrid instrumentation. Spine. 2010;35(2):177–81. doi: 10.1097/BRS.0b013e3181c77f8c. [DOI] [PubMed] [Google Scholar]
- 36.Sacramento-Dominguez C, Vayas-Diez R, Coll-Mesa L, Parrilla AP, Machado-Calvo M, Pinilla JA, et al. Reproducibility measuring the angle of proximal junctional kyphosis using the first or the second vertebra above the upper instrumented vertebrae in patients surgically treated for scoliosis. Spine. 2009;34(25):2787–91. doi: 10.1097/BRS.0b013e3181b61955. [DOI] [PubMed] [Google Scholar]
- 37.Rastegar F, Contag A, Daniels A, Hiratzka J, Lin C, Chang J, Than K, Raslan A, Kong C, Nguyen N, Hostin R, Hart R. Proximal junctional kyphosis: inter- and intra-observer reliability in adult spinal deformity. Presented at the Lumbar Spine Research Society, Chicago, IL April 14–15, 2016.
- 38.Yagi M, Rahm M, Gaines R, Maziad A, Ross T, Kim HJ, et al. Characterization and surgical outcomes of proximal junctional failure in surgically treated patients with adult spinal deformity. Spine. 2014;39(10):E607–14. doi: 10.1097/BRS.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 39.Hyun SJ, Rhim SC. Clinical outcomes and complications after pedicle subtraction osteotomy for fixed sagittal imbalance patients : a long-term follow-up data. J Korean Neurosurg Soc. 2010;47(2):95–101. doi: 10.3340/jkns.2010.47.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim HJ, Lenke LG, Shaffrey CI, Van Alstyne EM, Skelly AC. Proximal junctional kyphosis as a distinct form of adjacent segment pathology after spinal deformity surgery: a systematic review. Spine. 2012;37(22 Suppl):S144–64. doi: 10.1097/BRS.0b013e31826d611b. [DOI] [PubMed] [Google Scholar]
- 41.Mendoza-Lattes S, Ries Z, Gao Y, Weinstein SL. Proximal junctional kyphosis in adult reconstructive spine surgery results from incomplete restoration of the lumbar lordosis relative to the magnitude of the thoracic kyphosis. Iowa Orthop J. 2011;31:199–206. [PMC free article] [PubMed] [Google Scholar]
- 42.Yagi M, Akilah KB, Boachie-Adjei O. Incidence, risk factors and classification of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Spine. 2011;36(1):E60–8. doi: 10.1097/BRS.0b013e3181eeaee2. [DOI] [PubMed] [Google Scholar]
- 43.Kim HJ, Bridwell KH, Lenke LG, Park MS, Ahmad A, Song KS, et al. Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients. Spine. 2013;38(11):896–901. doi: 10.1097/BRS.0b013e3182815b42. [DOI] [PubMed] [Google Scholar]
- 44.Yagi M, King AB, Boachie-Adjei O. Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up. Spine. 2012;37(17):1479–89. doi: 10.1097/BRS.0b013e31824e4888. [DOI] [PubMed] [Google Scholar]
- 45.Kim HJ, Bridwell KH, Lenke LG, Park MS, Song KS, Piyaskulkaew C, et al. Patients with proximal junctional kyphosis requiring revision surgery have higher postoperative lumbar lordosis and larger sagittal balance corrections. Spine. 2014;39(9):E576–80. doi: 10.1097/BRS.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 46.Kim YJ, Lenke LG, Bridwell KH, Kim J, Cho SK, Cheh G, et al. Proximal junctional kyphosis in adolescent idiopathic scoliosis after 3 different types of posterior segmental spinal instrumentation and fusions: incidence and risk factor analysis of 410 cases. Spine. 2007;32(24):2731–8. doi: 10.1097/BRS.0b013e31815a7ead. [DOI] [PubMed] [Google Scholar]
- 47.Lonner BS, Newton P, Betz R, Scharf C, O’Brien M, Sponseller P, et al. Operative management of Scheuermann’s kyphosis in 78 patients: radiographic outcomes, complications, and technique. Spine. 2007;32(24):2644–52. doi: 10.1097/BRS.0b013e31815a5238. [DOI] [PubMed] [Google Scholar]
- 48.Lowe TG, Kasten MD. An analysis of sagittal curves and balance after Cotrel-Dubousset instrumentation for kyphosis secondary to Scheuermann’s disease. A review of 32 patients. Spine. 1994;19(15):1680–5. doi: 10.1097/00007632-199408000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Maruo K, Ha Y, Inoue S, Samuel S, Okada E, Hu SS, et al. Predictive factors for proximal junctional kyphosis in long fusions to the sacrum in adult spinal deformity. Spine. 2013;38(23):E1469–76. doi: 10.1097/BRS.0b013e3182a51d43. [DOI] [PubMed] [Google Scholar]
- 50.Cammarata M, Aubin CE, Wang X, Mac-Thiong JM. Biomechanical risk factors for proximal junctional kyphosis: a detailed numerical analysis of surgical instrumentation variables. Spine. 2014;39(8):E500–7. doi: 10.1097/BRS.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 51.Kim HJ, Yagi M, Nyugen J, Cunningham ME, Boachie-Adjei O. Combined anterior-posterior surgery is the most important risk factor for developing proximal junctional kyphosis in idiopathic scoliosis. Clin Orthop Relat Res. 2012;470(6):1633–9. doi: 10.1007/s11999-011-2179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Zhao Y, Shen B, Wang C, Li M. Risk factor analysis of proximal junctional kyphosis after posterior fusion in patients with idiopathic scoliosis. Injury. 2010;41(4):415–20. doi: 10.1016/j.injury.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 53.Bjerke-Kroll B, Saiyed R, Cheung Z, Shifflett G, Sheha E, Cunningam M. Postsurgical predictors of proximal junctional kyphosis in adolescent idiopathic scoliosis. Spine J. 2015;15(10):S148. doi: 10.1016/j.spinee.2015.07.157. [DOI] [Google Scholar]
- 54.Blondel B, Schwab F, Ungar B, Smith J, Bridwell K, Glassman S, et al. Impact of magnitude and percentage of global sagittal plane correction on health-related quality of life at 2-years follow-up. Neurosurgery. 2012;71(2):341–8. doi: 10.1227/NEU.0b013e31825d20c0. [DOI] [PubMed] [Google Scholar]
- 55.Kim YJ, Bridwell KH, Lenke LG, Kim J, Cho SK. Proximal junctional kyphosis in adolescent idiopathic scoliosis following segmental posterior spinal instrumentation and fusion: minimum 5-year follow-up. Spine. 2005;30(18):2045–50. doi: 10.1097/01.brs.0000179084.45839.ad. [DOI] [PubMed] [Google Scholar]
- 56.Lee GA, Betz RR, Clements DH, 3rd, Huss GK. Proximal kyphosis after posterior spinal fusion in patients with idiopathic scoliosis. Spine. 1999;24(8):795–9. doi: 10.1097/00007632-199904150-00011. [DOI] [PubMed] [Google Scholar]
- 57.Arlet V, Aebi M. Junctional spinal disorders in operated adult spinal deformities: present understanding and future perspectives. Eur Spine J. 2013;22(Suppl 2):S276–95. doi: 10.1007/s00586-013-2676-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hollenbeck SM, Glattes RC, Asher MA, Lai SM, Burton DC. The prevalence of increased proximal junctional flexion following posterior instrumentation and arthrodesis for adolescent idiopathic scoliosis. Spine. 2008;33(15):1675–81. doi: 10.1097/BRS.0b013e31817b5bea. [DOI] [PubMed] [Google Scholar]
- 59.Rhee JM, Bridwell KH, Won DS, Lenke LG, Chotigavanichaya C, Hanson DS. Sagittal plane analysis of adolescent idiopathic scoliosis: the effect of anterior versus posterior instrumentation. Spine. 2002;27(21):2350–6. doi: 10.1097/00007632-200211010-00008. [DOI] [PubMed] [Google Scholar]
- 60.Ha Y, Maruo K, Racine L, Schairer WW, Hu SS, Deviren V, et al. Proximal junctional kyphosis and clinical outcomes in adult spinal deformity surgery with fusion from the thoracic spine to the sacrum: a comparison of proximal and distal upper instrumented vertebrae. J Neurosurg Spine. 2013;19(3):360–9. doi: 10.3171/2013.5.SPINE12737. [DOI] [PubMed] [Google Scholar]
- 61.Lau D, Clark AJ, Scheer JK, Daubs MD, Coe JD, Paonessa KJ, et al. Proximal junctional kyphosis and failure after spinal deformity surgery: a systematic review of the literature as a background to classification development. Spine. 2014;39(25):2093–102. doi: 10.1097/BRS.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 62.•.Lau D, Funao H, Clark AJ, Nicholls F, Smith J, Bess S, et al. The clinical correlation of the Hart-ISSG Proximal Junctional Kyphosis Severity Scale with health-related quality-of-life outcomes and need for revision surgery. Spine. 2016;41(3):213–23. doi: 10.1097/BRS.0000000000001326. [DOI] [PubMed] [Google Scholar]
- 63.Rastegar F, Contag A, Daniels A, Klineberg E, Eastlack R, Smith J, Hostin R, Hamilton K, Burton D, Gum J, Burton D, Hart R. Proximal Junctional Failure (PJF) severity scale inter- and intra-observer reliability. Presented at the Lumbar Spine Research Society, Chicago, IL April 14–15, 2016.
- 64.Kebaish KM, Martin CT, O’Brien JR, LaMotta IE, Voros GD, Belkoff SM. Use of vertebroplasty to prevent proximal junctional fractures in adult deformity surgery: a biomechanical cadaveric study. Spine J. 2013;13(12):1897–903. doi: 10.1016/j.spinee.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 65.Kayanja MM, Schlenk R, Togawa D, Ferrara L, Lieberman I. The biomechanics of 1, 2, and 3 levels of vertebral augmentation with polymethylmethacrylate in multilevel spinal segments. Spine. 2006;31(7):769–74. doi: 10.1097/01.brs.0000207466.40955.31. [DOI] [PubMed] [Google Scholar]
- 66.Fernandez-Baillo N, Sanchez Marquez JM, Sanchez Perez-Grueso FJ, Garcia FA. Proximal junctional vertebral fracture-subluxation after adult spine deformity surgery. Does vertebral augmentation avoid this complication? A case report. Scoliosis. 2012;7(1):16. doi: 10.1186/1748-7161-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol. 2006;27(1):217–23. [PMC free article] [PubMed] [Google Scholar]
- 68.Verlaan JJ, Oner FC, Slootweg PJ, Verbout AJ, Dhert WJ. Histologic changes after vertebroplasty. J Bone Joint Surg Am. 2004;86-A(6):1230–8. doi: 10.2106/00004623-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 69.Hart R HR, McCarthy I, et al. International Spine Study Group. Age, sagittal deformity and operative correction are risk factors for proximal junctional failure following adult spinal deformity surgery. AAOS Spine, in press.
- 70.Hassanzadeh H, Gupta S, Jain A, El Dafrawy MH, Skolasky RL, Kebaish KM. Type of anchor at the proximal fusion level has a significant effect on the incidence of proximal junctional kyphosis and outcome in adults after long posterior spinal fusion. Spine Deformity. 2013; 1(4):299–305. [DOI] [PubMed]
- 71.Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine. 2009;34(17):1828–33. doi: 10.1097/BRS.0b013e3181a13c08. [DOI] [PubMed] [Google Scholar]
- 72.•.Lafage R, Schwab F, Challier V, Henry JK, Gum J, Smith J, et al. Defining spino-pelvic alignment thresholds: should operative goals in adult spinal deformity surgery account for age? Spine. 2016;41(1):62–8. doi: 10.1097/BRS.0000000000001171. [DOI] [PubMed] [Google Scholar]
- 73.Kawaguchi S, Hart R. Evaluation, prevention, and treatment of proximal junctional failure. In: Haid RW, Schwab FJ, Shaffrey CI, Youssef JA, editors. Global spinal alignment: principles, pathologies, and procedures. St. Louis: Quality Medical Publishing, Inc; 2015. [Google Scholar]
- 74.Mohamed A, Coburn E, Hamilton K, Hiratzka J, Hart R. Prophylactic rib fixation to prevent proximal junctional failure following instrumented posterior spinal fusion in adult spinal deformity. Presented at the American Academy of Orthopaedic Surgeons Annual Meeting, March 24–28, Las Vegas, Nevada, 2015.


