Abstract
With the aging population, there is a rising prevalence of degenerative spinal deformity and need of surgical care for these patients. Surgical treatment for adult spinal deformity (ASD) is often fraught with a high rate of complications. Minimally invasive surgery (MIS) has for the past decade been adopted by spine surgeons to treat ASD in the hopes of reducing access-related morbidity and perioperative complications. The benefits of MIS approach in general and recent development of MIS techniques to avoid long-term complications such as pseudoarthrosis or proximal junctional kyphosis are reviewed.
Keywords: Adult spinal deformity, Complications, Minimally invasive, Sagittal imbalance, Scoliosis
Introduction
The prevalence of degenerative spinal deformity has been increasing, secondary to the increase in the aging population. This increase has led to the need of surgical care for these patients [1]. Advanced spinal degeneration and subsequent deformity can lead to incapacitating pain, neurological deficits, and disability. While conservative treatment remains the mainstay of management for most patients, surgical intervention for severe deformity is chosen with the desire for patients to have a better quality of life. There are many large case series with long-term follow-up demonstrating that operative intervention can reduce visual analog scale (VAS) score and Oswestry Disability Index (ODI), and improve the patient’s quality of life [2–5].
Nevertheless, medical comorbidities, osteoporosis, and severe spinal deformity in the aging population pose challenges to spine surgeons. Open surgical treatment for adult spinal deformity (ASD) can be associated with high rates of complications [6, 7]. A multicenter prospective study conducted to assess perioperative and postoperative complication rates associated with ASD surgery with a minimum of 2-year follow-up period by Smith et al. showed that 52.2 % of patients were affected by at least one perioperative complication, and 69.8 % of patients experienced at least one complication at some point during the perioperative time or minimum 2-year follow-up. A total of 82 patients (28.2 %) required one or more reoperations [8•]. The most common complications following open surgery for ASD are approach-related (i.e., blood loss, wound infection), implant-related, radiographic (i.e., pseudoarthrosis, adjacent segment failure, inadequate correction, etc.), neurological, or medical (i.e., cardiac, pulmonary, renal, gastrointestinal, etc.).
Contemporary spine surgeons have strived to develop techniques to achieve similar outcomes while minimizing complications with the use of minimally invasive surgery (MIS). These techniques have emerged as a valuable option for managing degenerative spinal disorders and have been adopted in the spine surgeon’s armamentarium to treat ASD. The goal of MIS is to accomplish the intended surgical goals of its open counterpart while decreasing access-related and perioperative complications. Newer techniques have also been developed to minimize long-term complications such as pseudoarthrosis or proximal junctional kyphosis.
MIS to reduce approach related complications
One of the major differences between the MIS and open approaches are that MIS preserves paraspinal musculature, which are potent posterior stabilizing muscles. Stripping of the tedinous attachments, denervation, thermal injury from cautery, and crushing injury from retractors are usual causes of muscular injury during the open procedures [9, 10]. MIS minimizes injury to the paraspinal muscles through the use of specialized instruments and surgical techniques. Additionally, MIS techniques reduce blood loss, minimize postsurgical pain, and expedite patient’s recovery, while minimizing the risk of wound infections due to limited surgical exposure [11, 12].
Open spinal surgeries for ASD can pose a major risk on patients with limited cardiac or pulmonary functional reserve. Large amounts of fluids given during open procedures can increase renal functional demand and lead to electrolyte imbalances due to perioperative fluid shifting. MIS approaches typically have less blood loss and significantly reduce the need to give large amounts of fluids. Additionally, patients that undergo MIS procedures typically have earlier mobilization, which reduces the occurrence of venous thromboembolism. Lastly, MIS procedures typically do not require large incisions or extensive muscular dissection; thus, most patients require less narcotic use. This leads to a decrease in the occurrence of ileus, which is not uncommon for patients undergoing open procedures [13, 14].
Recent innovative MIS techniques and their application in ASD to avoid complications
MIS shares the same surgical goals of the open ASD surgery, i.e., decompression, instrumentation, fusion, and realignment. Several MIS techniques and their capability to reduce the complications of their open counterparts are described below.
Decompression
While back pain is one of the most common presenting symptoms among patients with ASD due to instability or malalignment of the spine, patients can also present with radicular pain or neurogenic claudication. It is crucial to evaluate clinically and radiographically if the patient requires direct decompression or the patient can benefit from indirect decompression that can be achieved with MIS techniques. Indirect decompression of the spinal canal, lateral recess, or neuroforamen can be accomplished by restoration of disc height or reduction of spondylolisthesis following the placement of a large foot print interbody cage through a mini-open anterior or lateral approach [15]. (Fig. 1) This approach has the benefits of avoiding prior surgical scar and obviating nerve root injury/unintentional durotomy, as ASD patients often have prior surgeries. Suitable candidates for indirect decompression are those with disc bulge, collapsed disc, lateral, retro-, or spondylolisthesis, and degenerative scoliosis with fractional curve. The technique may not adequately decompress stenosis in the setting of fused articular processes, bridging anterior osteophytes or severe stenosis from ligamentous hypertrophy. In cases with severe stenosis and less flexible segment, direct decompression is required. In these cases, a posterior laminotomy or foraminotomy can be performed through a mini-open approach or a tubular retractor [16].
Fig. 1.
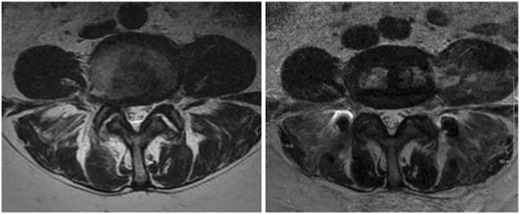
Preoperative (left) and postoperative (right) axial T2-weighted MRI through the L4/5 disc space showing a placement of an interbody cage through a mini-open left-sided retroperitoneal transpsoas approach. Noted is the widening of the central canal and neuroforamen
Instrumentation
Options for instrumentation in MIS are similar to those offered by open procedures. They include interbody spacers and pedicle screws and are used to correct malalignment and improve spinal stability.
Interbody fusion via the placement of cages restores the disc high and provides a mechanism for indirect decompression of the neural elements. These interbody cages also provide load-bearing capacity and structural stability. They assist with realignment of the coronal and sagittal deformity. Additionally, they increase fusion rates because of the wide footprint across the length of the vertebral body [17]. There are many types of MIS approaches to place interbody cages. The most common types are discussed below.
Anterior lumbar interbody fusion (ALIF) has long been considered the gold standard technique for interbody fusion. It affords a direct access for discectomy, release of anterior longitudinal ligament (ALL) to restore lordosis, and placement of a large cage for arthrodesis. The procedure can be performed through a mini-open or laparoscopic approach with minimal access-related complications [18].
Transforaminal lumbar interbody fusion (TLIF) has been the workhorse of posterior surgery for ASD. It is especially useful at the lumbosacral junction due to high nonunion rate at this segment. Some of the advantages of this approach include familiar posterior approach and anatomy and direct decompression of neural structures. Wang et al. reported his experience using MIS TLIF for ASD and demonstrated statistically significant improvement in all radiographic parameters [19]. However, TLIF requires an extensive bony removal, and the size of the implants is limited by the access corridor. These smaller footprint implants resting on the weaker central part of the vertebral endplate are more susceptible to subsidence. Recent advance of expandable interbody cage devices has overcome some of the limitations while facilitating alignment and stability [20].
Lateral lumbar interbody fusion (LLIF) has recently gained popularity. First described by Pimenta in 2001, the technique uses a retroperitoneal transpsoas corridor to perform a lumbar interbody fusion without violating the ALL, posterior longitudinal ligament (PLL), and the posterior tension band [21]. The lateral approach has been used as part of a minimally invasive anterior-only or anterior-posterior procedure in the management of ASD. It may also be combined with open posterior fusion with osteotomies to achieve sagittal and coronal balance in ASD patients. The restoration of disc height and correction of alignment can be achieved through the ligamentotaxis created with the intact ALL and PLL [21]. The large footprint cage spanning the apophyseal ring reduces the risk of cage subsidence (Fig. 2). The approach, however, has its own set of approach-related complications as it uses the transpsoas corridor, which is in proximity with the lumbar plexus and can cause femoral nerve injury especially at L4/5 level [22]. The risk can be reduced with the use of directional real time EMG. In a prospective study of 107 patients undergoing LLIF as part of correction surgeries for adult scoliosis (average 4.4 levels treated), Isaacs et al. reported a low transfusion rate (4.7 %) and short hospital stay (average 3.8 days). Thirteen (12.1 %) individuals experienced 14 major complications: 1 myocardial infarction, 1 sepsis, 1 deep venous thrombosis, 3 wound infection from posterior open surgery, 1 kidney injury, and 7 motor deficits. The 7 patients with motor deficits had residual weakness at 6-month follow-up or presented with more than two grades decrease in strength at any point. Six patients had complete resolution of weakness. One patient with 1/5 weakness of the proximal muscles of the lower extremity that was caused by lumbar plexus injury improved to 4/5 by the 6-month visit [23].
Fig. 2.
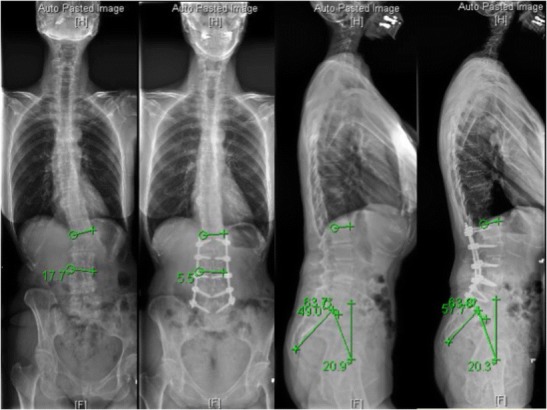
Preoperative and postoperative 36-in.-long cassette radiographs obtained in a patient who underwent a minimally invasive correction of ASD including L1/2, L2/3, L3/4, and L4/5 lateral lumbar interbody fusion and posterior L1–L5 percutaneous pedicle screws. The coronal Cobb angle reduced from 17.7° to 5.5° and lumbar lordosis increased from 49° to 57.7°
Percutaneous pedicle screws allow spine surgeons to apply spinal fixation [24•]. In patients with ASD, percutaneous pedicle screw instrumentation is an effective means of providing posterior support and optimizing correction of spinopelvic parameters as well as sagittal and coronal imbalance. Placement of screws through multiple small paramedian incisions reduces the severity of paraspinal muscle injury. Percutaneous iliac screws or sacral alar iliac screw have also been described recently to further stabilize long fusion constructs [25, 26].
Fusion
Pseudoarthrosis remains a major concern after surgical correction of ASD. Most MIS techniques rely on interbody fusion and large foot print implants through a non-violated healthy environment for robust fusion. However, minimal posterior exposure limits the fusion potential in the posterolateral aspect of the spine.
Realignment
Studies have demonstrated that correction of the sagittal imbalance to a sagittal vertical axis (SVA) of less than 5 cm, lumbar lordosis (LL) − pelvic incidence (PI) mismatch within 9°, and pelvic tilt (PT) <20° leads to improved outcomes [27]. Inadequate correction not only correlates with poor outcomes but also increases the risks of proximal junction kyphosis and pseudoarthrosis. The realignment for ASD can be performed through a circumferential MIS (cMIS) or a hybrid technique (Fig. 3). The cMIS involves anterior column support by placing interbody grafts (ALIF, TLIF, LLIF) and percutaneous posterior segmental instrumentation through an entirely MIS approach. Historically, the cMIS approach has been satisfactory at correcting coronal but deficient at correcting sagittal imbalance [28]. Dakwar et al. reviewed the clinical outcome of 25 patients undergoing cMIS lateral and posterior approach for ASD. The mean blood loss was 53 ml per level and the mean length of stay was 6.2 days. Postoperative VAS scores and ODI improved significantly. However, one third of the cases failed to achieve adequate restoration of sagittal balance [29]. Wang and Mummaneni, on the other hand, reported an improvement of coronal Cobb angles from 31.4 to 11.5° and lumbar lordosis from 37.4 to 47.5° in a group of 23 ASD patients treated with cMIS approach [30].
Fig. 3.
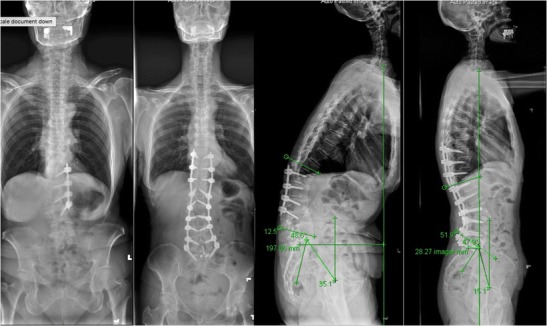
Preoperative and postoperative 36-in.-long cassette radiographs obtained in a patient who had prior L2–5 laminectomy, fusion, and left-sided instrumentation. She underwent a hybrid minimally invasive correction of ASD including removal of old hardware, posterior column osteotomy, placement of L1–4 pedicle screws in an open fashion, T10–12, L5–S1 percutaneous screws followed by L5/S1 ALIF, L1/2, 2/3, 3/4, 4/5 lateral lumbar interbody fusion, L3/4 anterior longitudinal ligament release, and placement of T10–S1 rods. The sagittal vertical axis reduced from 19.7 to 2.8 cm, the lumbar lordosis increased from 12.5° to 51.9°, and the pelvic tilt decreased from 35.1° to 15.1°
The hybrid MIS technique is an alternative, which involves the incorporation of the aforementioned cMIS techniques with traditional open posterior surgery that includes segmental osteotomies and instrumentation. Not surprisingly, the influence of anterior column reconstruction on sagittal balance and spinopelvic parameters is significantly enhanced when combined with an open posterior approach. In a consecutive case series, Park et al. reported similar clinical outcomes following cMIS and hybrid surgeries; however, the degree of sagittal and coronal plane correction was greater in the hybrid group at the expense of a higher complication rate [31]. The major and minor complication rate in the hybrid group was 55 % compared to only 33 % for the cMIS group.
Uribe et al. examined the incidence of complications associated with surgical approaches (cMIS, hybrid, or open) in three cohorts of patients propensity matched for age, SVA, number of levels fused posteriorly, and lumbar coronal Cobb angle [32•]. No significant differences existed among the groups with regard to preoperative demographics (sex, age, ASA score, number of comorbidities, and previous spine surgery). There was statistically less estimated blood loss in the cMIS group (669 ml) than in the open group (2322 ml, p < 0.001). The open procedures were on average shorter than the hybrid procedures (367 vs. 665 min, p < 0.001) but not significantly different from the cMIS procedures (507 min). All three groups had a statistically significant decrease in their VAS and ODI scores. Postoperatively, the open group had a smaller PI-LL mismatch than the cMIS group (6 vs. 17°, p < 0.03). The open group also had a greater change in the PI-LL mismatch than the cMIS group (−14 vs. −3°, p = 0.04). On average, there were 1.06 complications per patient for the open group, 0.84 per patient in the hybrid group, and only 0.30 per patient in the c MIS group (p = 0.04) The comparison of intraoperative complications revealed that the cMIS group had significantly fewer complications than the hybrid group, which in turn had fewer complications than the open group. Open surgical approaches may be more powerful in obtaining sagittal alignment, but do so with an increase in complications.
Proximal junctional kyphosis (PJK) above the upper instrumented vertebra is a common complication of ASD surgery and may be related to disruption of the facet joints and posterior ligamentous complex. The incidence of PJK in patients treated for adult deformity may exceed 25 % [33]. In two groups of patients (68 each) undergoing cMIS or hybrid procedures for ASD, that were propensity matched for preoperative PI-LL mismatch and change in LL, Mummaneni et al. reported that 30.9 % of patients developed radiographic PJK in the cMIS group compared to 52.9 % in the hybrid group (p = 0.01) [34]. Three patients (4.4 %) in the cMIS group required revision surgery for PJK compared with seven (10.3 %) in the hybrid group (p = 0.2).
Three-column osteotomies such as pedicle subtraction osteotomy (PSO) and vertebral column resection (VCR) are often required in patients with severe sagittal imbalance or rigid focal deformity. These procedures have been associated with a higher risk of complications [35]. Although a mini-open PSO is feasible, the technique remains challenging [36]. Anterior column reconstruction involving the combination of lateral lumbar discectomy, ALL release, and placement of hyperlordotic grafts through a mini-open lateral retroperitoneal approach has recently been implemented to avoid PSO or VCR. Early studies demonstrated a gain of 10–15° sagittal corrections per level of ALL release with low blood loss, much shorter operative times, and reduced morbidity [37, 38]. A shorter fusion construct may also be feasible using ALL release.
Conclusion
The MIS approach has its inherent benefits of lower intraoperative blood loss, less postoperative pain, reduced surgical morbidities, and faster return of function among others. Early results are promising, however, ASD patients with severe fixed sagittal imbalance and spinopelvic malalignment are not ideal candidates for MIS surgery alone [39, 40]. The MIS approach remains in its early stage and work remains to produce more robust data with longer follow-up to make definitive claims regarding its efficacy and safety. Proper selection of MIS as the sole approach or as an adjunct to open techniques will maximize the benefits of this approach while minimizing the risk of complications [41].
Compliance with ethical standards
Conflict of interest
Chun-Po Yen and Yusef I. Mosley declare that they have no conflict of interest.
Juan S. Uribe reports grants and personal fees from NUVASIVE outside of the submitted work.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Complications in Spine Surgery
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Schwab F, Dubey A, Gamez L, et al. Adult scoliosis: prevalence, SF-36, and nutritional parameters in an elderly volunteer population. Spine (Phila Pa 1976) 2005;30(9):1082–5. doi: 10.1097/01.brs.0000160842.43482.cd. [DOI] [PubMed] [Google Scholar]
- 2.Best NM, Sasso RC. Outpatient lumbar spine decompression in 233 patients 65 years of age or older. Spine (Phila Pa 1976) 2007;32(10):1135–9. doi: 10.1097/01.brs.0000261486.51019.4a. [DOI] [PubMed] [Google Scholar]
- 3.Bridwell KH, Glassman S, Horton W, et al. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine (Phila Pa 1976) 2009;34(20):2171–8. doi: 10.1097/BRS.0b013e3181a8fdc8. [DOI] [PubMed] [Google Scholar]
- 4.Li G, Passias P, Kozanek M, et al. Adult scoliosis in patients over sixty-five years of age: outcomes of operative versus nonoperative treatment at a minimum two-year follow-up. Spine (Phila Pa 1976) 2009;34(20):2165–70. doi: 10.1097/BRS.0b013e3181b3ff0c. [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Klineberg E, Schwab F, et al. Change in classification grade by the SRS-Schwab adult spinal deformity classification predicts impact on health-related quality of life measures: prospective analysis of operative and nonoperative treatment. Spine (Phila Pa 1976) 2013;38(19):1663–71. doi: 10.1097/BRS.0b013e31829ec563. [DOI] [PubMed] [Google Scholar]
- 6.Glassman SD, Hamill CL, Bridwell KH, Schwab FJ, Dimar JR, Lowe TG. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976) 2007;32(24):2764–70. doi: 10.1097/BRS.0b013e31815a7644. [DOI] [PubMed] [Google Scholar]
- 7.Pateder DB, Gonzales RA, Kebaish KM, Cohen DB, Chang JY, Kostuik JP. Short-term mortality and its association with independent risk factors in adult spinal deformity surgery. Spine (Phila Pa 1976) 2008;33(11):1224–8. doi: 10.1097/BRS.0b013e3181714a66. [DOI] [PubMed] [Google Scholar]
- 8.•.Smith JS, Klineberg E, Lafage V, Shaffrey CI, Schwab F, Lafage R, et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J Neurosurg Spine. 2016;6:1–14. A prospective multicenter study detailing complications associated with open surgery for adult spinal deformities. [DOI] [PubMed]
- 9.Kim CW. Scientific basis of minimally invasive spine surgery: prevention of multifidus muscle injury during posterior lumbar surgery. Spine (Phila Pa 1976) 2010;35(26 Suppl):S281–6. doi: 10.1097/BRS.0b013e3182022d32. [DOI] [PubMed] [Google Scholar]
- 10.Fan SW, Hu ZJ, Fang XQ, Zhao FD, Huang Y, Yu HJ. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg. 2010;2(3):194–200. doi: 10.1111/j.1757-7861.2010.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker SL, Adogwa O, Witham TF, Aaronson OS, Cheng J, McGirt MJ. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): literature review and cost analysis. Minim Invasive Neurosurg. 2011;54(1):33–7. doi: 10.1055/s-0030-1269904. [DOI] [PubMed] [Google Scholar]
- 12.McGirt MJ, Parker SL, Lerner J, Engelhart L, Knight T, Wang MY. Comparative analysis of perioperative surgical site infection after minimally invasive versus open posterior/transforaminal lumbar interbody fusion: analysis of hospital billing and discharge data from 5170 patients. J Neurosurg Spine. 2011;14(6):771–8. doi: 10.3171/2011.1.SPINE10571. [DOI] [PubMed] [Google Scholar]
- 13.Bach K, Ahmadian A, Deukmedjian A, Uribe JS. Minimally invasive surgical techniques in adult degenerative spinal deformity: a systematic review. Clin Orthop Relat Res. 2014;472(6):1749–61. doi: 10.1007/s11999-013-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mundis GM, Akbarnia BA, Phillips FM. Adult deformity correction through minimally invasive lateral approach techniques. Spine (Phila Pa 1976) 2010;35(26 Suppl):S312–21. doi: 10.1097/BRS.0b013e318202495f. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira L, Marchi L, Coutinho E, Pimenta L. A radiographic assessment of the ability of the extreme lateral interbody fusion procedure to indirectly decompress the neural elements. Spine (Phila Pa 1976) 2010;35(26 Suppl):S331–7. doi: 10.1097/BRS.0b013e3182022db0. [DOI] [PubMed] [Google Scholar]
- 16.Kelleher MO, Timlin M, Persaud O, et al. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine. 2010;35:E981–7. doi: 10.1097/BRS.0b013e3181c46fb4. [DOI] [PubMed] [Google Scholar]
- 17.Castro C, Oliveira L, Amaral R, Marchi L, Pimenta L. Is the lateral transpsoas approach feasible for the treatment of adult degenerative scoliosis? Clin Orthop Relat Res. 2014;472(6):1776–83. doi: 10.1007/s11999-013-3263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung SK, Lee SH, Lim SR, et al. Comparative study of laparoscopic L5-S1 fusion versus open mini-ALIF, with a minimum 2-year follow-up. Eur Spine J. 2003;12(6):613–7. doi: 10.1007/s00586-003-0526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12(6):694–9. doi: 10.3171/2009.12.SPINE09621. [DOI] [PubMed] [Google Scholar]
- 20.Cannestra AF, Peterson MD, Parker SR, Roush TF, Bundy JV, Turner AW. MIS expandable interbody spacers: a literature review and biomechanical comparison of an expandable MIS TLIF with conventional TLIF and ALIF. Spine (Phila Pa 1976) 2016;41(Suppl 8):S44–9. doi: 10.1097/BRS.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 21.Ozgur BM, Aryan HE, Pimenta L, Taylor WR. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J. 2006;6(4):435–43. doi: 10.1016/j.spinee.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Uribe JS, Arredondo N, Dakwar E, Vale FL. Defining the safe working zones using the minimally invasive lateral retroperitoneal transpsoas approach: an anatomical study. J Neurosurg Spine. 2010;13(2):260–6. doi: 10.3171/2010.3.SPINE09766. [DOI] [PubMed] [Google Scholar]
- 23.Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 2010;35(26 Suppl):S322–30. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 24.•.Beckman JM, Murray G, Bach K, Deukmedjian A, Uribe JS. Percutaneous Minimally Invasive (MIS) Guide Wire-less Self-Tapping Pedicle Screw Placement in the Thoracic and Lumbar Spine: Safety and Initial Clinical Experience: Technical Note. Neurosurgery. 2015. A novel technique of placing percutaneous pedicle screws. [DOI] [PubMed]
- 25.Wang MY, Ludwig SC, Anderson DG, Mummaneni PV. Percutaneous iliac screw placement: description of a new minimally invasive technique. Neurosurg Focus. 2008;25(2) doi: 10.3171/FOC/2008/25/8/E17. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien JR, Matteini L, Yu WD, Kebaish KM. Feasibility of minimally invasive sacropelvic fixation: percutaneous S2 alar iliac fixation. Spine (Phila Pa 1976) 2010;35(4):460–4. doi: 10.1097/BRS.0b013e3181b95dca. [DOI] [PubMed] [Google Scholar]
- 27.Schwab F, Patel A, Ungar B, Farcy JP, Lafage V. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010;35(25):2224–31. doi: 10.1097/BRS.0b013e3181ee6bd4. [DOI] [PubMed] [Google Scholar]
- 28.Acosta FL, Liu J, Slimack N, Moller D, Fessler R, Koski T. Changes in coronal and sagittal plane alignment following minimally invasive direct lateral interbody fusion for the treatment of degenerative lumbar disease in adults: a radiographic study. J Neurosurg Spine. 2011;15(1):92–6. doi: 10.3171/2011.3.SPINE10425. [DOI] [PubMed] [Google Scholar]
- 29.Dakwar E, Cardona RF, Smith DA, Uribe JS. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28(3) doi: 10.3171/2010.1.FOCUS09282. [DOI] [PubMed] [Google Scholar]
- 30.Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28 doi: 10.3171/2010.1.FOCUS09286. [DOI] [PubMed] [Google Scholar]
- 31.Park P, Wang MY, Lafage V, et al. Comparison of two minimally invasive surgery strategies to treat adult spinal deformity. J Neurosurg Spine. 2015;22:374–80. doi: 10.3171/2014.9.SPINE131004. [DOI] [PubMed] [Google Scholar]
- 32.•.Uribe JS, Deukmedjian AR, Mummaneni PV, et al. Complications in adult spinal deformity surgery: an analysis of minimally invasive, hybrid, and open surgical techniques. Neurosurg Focus. 2014;36(5) doi: 10.3171/2014.3.FOCUS13534. [DOI] [PubMed] [Google Scholar]
- 33.Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C., 3rd Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine (Phila Pa 1976) 2005;30(14):1643–9. doi: 10.1097/01.brs.0000169451.76359.49. [DOI] [PubMed] [Google Scholar]
- 34.Mummaneni PV, Park P, Fu K-M, et al. Does minimally invasive percutaneous posterior instrumentation reduce risk of proximal junctional kyphosis in adult spinal deformity surgery? A propensity-matched cohort analysis. Neurosurgery. 2016;78:101–8. doi: 10.1227/NEU.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 35.Charosky S, Guigui P, Blamoutier A, Roussouly P, Chopin D, Study Group on Scoliosis Complications and risk factors of primary adult scoliosis surgery: a multicenter study of 306 patients. Spine (Phila Pa 1976) 2012;37(8):693–700. doi: 10.1097/BRS.0b013e31822ff5c1. [DOI] [PubMed] [Google Scholar]
- 36.Wang MY, Bordon G. Mini-open pedicle subtraction osteotomy as a treatment for severe adult spinal deformities: case series with initial clinical and radiographic outcomes. J Neurosurg Spine. 2016;24(5):769–76. doi: 10.3171/2015.7.SPINE15188. [DOI] [PubMed] [Google Scholar]
- 37.Deukmedjian AR, Dakwar E, Ahmadian A, Smith DA, Uribe JS. Early outcomes minimally invasive anterior longitudinal ligament release for correction of sagittal imbalance in patients with adult spinal deformity. Sci World J. 2012;2012:789698. doi: 10.1100/2012/789698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deukmedjian AR, Le TV, Baaj AA, Dakwar E, Smith DA, Uribe JS. Anterior longitudinal ligament release using the minimally invasive lateral retroperitoneal transpsoas approach: a cadaveric feasibility study and report of 4 clinical cases. J Neurosurg Spine. 2012;17(6):530–9. doi: 10.3171/2012.8.SPINE12432. [DOI] [PubMed] [Google Scholar]
- 39.Wang MY, Mummaneni PV, Fu KM, et al. Less invasive surgery for treating adult spinal deformities: ceiling effects for deformity correction with 3 different techniques. Neurosurg Focus. 2014;36 doi: 10.3171/2014.3.FOCUS1423. [DOI] [PubMed] [Google Scholar]
- 40.Kanter AS, Tempel ZJ, Ozpinar A, Okonkwo DO. A review of minimally invasive procedures for the treatment of adult spinal deformity. Spine (Phila Pa 1976) 2016;41(Suppl 8):S59–65. doi: 10.1097/BRS.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 41.Mummaneni PV, Shaffrey CI, Lenke LG, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus. 2014;36(5) doi: 10.3171/2014.3.FOCUS1413. [DOI] [PubMed] [Google Scholar]


