Summary
Here we describe an in vitro primary culture system for C. elegans germline stem cells. This culture system was used to identify a bacterial folate as a positive regulator of germ cell proliferation. Folates are a family of B-complex vitamins that function in one-carbon metabolism to allow the de novo synthesis of amino acids and nucleosides. We show that germ cell proliferation is stimulated by the folate 10-formyl-tetrahydrofolate-Glun both in vitro and in animals. Other folates that can act as vitamins to rescue folate deficiency lack this germ cell stimulatory activity. The bacterial folate precursor dihydropteroate also promotes germ cell proliferation in vitro and in vivo, despite its inability to promote one-carbon metabolism. The folate receptor homolog FOLR-1 is required for the stimulation of germ cells by 10-formyl-tetrahydrofolate-Glun and dihydropteroate. This work defines a folate and folate-related compound as exogenous signals to modulate germ cell proliferation.
eTOC blurb
Chaudhari and Mukherjee et al. describe an in vitro culture system for C. elegans germ cells A subset of bacterial folates was identified as a stimulatory signal for germ cell proliferation. Folates stimulate germ cell proliferation through a pathway that is independent of their role as vitamins in one-carbon metabolism.
Introduction
Animal germ stem cells (GSCs) are adult stem cell populations that provide reproductive cells to allow species propagation. C. elegans hermaphrodite GSCs proliferate in adult stem cell niches located in the distal regions of the two gonad arms (Hansen and Schedl, 2013). Primary cultures of C. elegans germ cells have not been previously reported. C. elegans embryonic cells can be cultured, but not propagated, in an L-15-based culture medium (Christensen et al., 2002). In this study, we describe a primary culture system for C. elegans germ cells that utilizes a culture medium with substantially different characteristics than L-15 medium. The in vitro culture system allows the analysis of relatively pure populations of germ cells that are isolated from germline tumorous mutant strains. Two external signals, Notch and Insulin/IGF-like, are known to promote the proliferation of GSCs (Hansen and Schedl, 2013; Michaelson et al., 2010). We used the in vitro culture system to identify bacterial folate as a new signal that promotes GSC proliferation.
Folates are a group of B vitamins whose canonical role is in one carbon transfer for the de novo synthesis of: thymidine; purines; methionine, and the methyl donor S-adenosylmethionine (Selhub, 2002) (Fig. 1A). Folates comprise moieties of a pteridine ring, para-aminobenzoic acid (PABA), and one or more glutamate residues (Glun) in γ-linkages to the terminal glutamate (Fig. 1B). Folates differ from each other by: 1) the states of oxidation of the pteridine ring, i.e. dihydrofolate, DHF, or tetrahydrofolate, THF; 2) modification of the 5- and 10- position of the pteridine ring by substitution with formate (5-formyl-, 10-formyl-, and 5,10-methenyl-THF), formaldehyde (5,10-methylene-THF), or methanol (5-methyl-THF); and 3) the number of glutamate residues (Fig. 1B,C). The three forms of formylated THF are interconvertible: 5,10-methenyl-THF, which is stable at acid pH (1.5–2.6), is converted to 5-formyl-THF at pH 4.0–5.5 and to 10-formyl-THF at neutral and higher pH, and vice versa (Stover and Schirch, 1992).
Figure 1. Partial one-carbon metabolism cycle and folate structures.
(A) Diagram of partial one-carbon metabolism cycle; modified from (Zhao and Goldman, 2003).
(B) Schematic of the last stages of folate biosynthesis in bacteria. The enzymatic reactions to create dihydrofolate (DHF) only occur in organisms that are capable of de novo folate synthesis.
(C) Structures of one-carbon metabolism folates.
The bonds for PABA and Glu are shown in-line.
In mammals, there are three types of folate transporters. The reduced folate carrier, RFC, is a low-affinity, high-capacity transporter that brings folates into all cells of the body (Matherly et al., 2007). The proton-coupled folate transporter, PCFT, functions at low pH to transport folates from acidic pH environments, such as the mammalian small intestine (Desmoulin et al., 2012). Folate receptors, FRs, are high-affinity, low-capacity transporters that have been shown to function in the transcytosis of folates across polarized cell barriers (Grapp et al., 2013; Henderson et al., 1995; Selhub et al., 1987).
Folates are synthesized by bacteria, plants, fungi, and certain protozoa and archaea (Rossi et al., 2011). Folates cannot be synthesized de novo by animals, and hence are classified as vitamins that must be obtained from the animal’s diet or microbiota. As is true for other animals, C. elegans requires folates for one-carbon metabolism. Inactivation of the C. elegans RFC homolog FOLT-1/RFC results in severely reduced germ cell numbers and sterility (Austin et al., 2010).
Our work demonstrates that a specific bacterial folate and pteroate (a folate-related compound) stimulate germ cell proliferation in a manner that can be distinguished from the canonical role of folates in one-carbon metabolism.
Results
Cellularization of tumorous germ cells
In an effort to understand the regulation of GSC survival and proliferation, we sought to create a primary culture system for C. elegans germ cells. Wild-type germ cells are syncytial (Hansen and Schedl, 2013) and therefore cannot be isolated as viable cells. We found that germ cells can be isolated from the tumorous germline mutant strain glp-1(ar202); cki-2(ok2105); daf-16(mu86) (hereafter glp-1(gf); cki-2; daf-16) (Fig. 2A). Staining with the dye calcein-AM shows that the cells have intact plasma membranes, as calcein-AM is converted by cellular esterases to a fluorescent form that is unable to cross intact plasma membranes (Fig. 2A).
Figure 2. Optimization of C. elegans germ cell culture conditions.
(A) Images of glp-1(gf); cki-2; daf-16 germ cells one-day post-isolation in CeM1 medium: phase contrast; and calcein-AM live-cell stain. Scale bar, 20 μm.
(B–E) Live cell counts for glp-1(gf); cki-2; daf-16 germ cells are shown for all panels. (B) CeM1 maintains germ cell viability more effectively than L-15 medium. (C) Germ cell viability decreases when CeM1 lacks the specified components. (D) Pretreatment of FBS with Amberlite IRA 400-CL and charcoal-dextran increases germ cell viability. (E) Bacterial extract (with or without heat inactivation at 60°C for 30 min) promotes initial germ cell proliferation. The same full CeM1 control was analyzed in (B) and (D), and is shown in each panel for comparison.
For all figures, error bars reflect standard error of the mean, SEM. See also Figures S1 and S2, and Table S1.
To determine the percentage of germ cells among the isolated cells, we compared the number of germ cells isolated from glp-1(gf); cki-2; daf-16 mutants and wild-type adult hermaphrodites. Wild-type germ cells are not cellularized and therefore would not survive in culture. glp-1(gf); cki-2; daf-16 mutants have increased numbers of germ cells, but appear to have approximately the same number of somatic cells as wild type. Therefore, any significant increase in the number of isolated cells from glp-1(gf); cki-2; daf-16 mutants can be attributed to germ cells. glp-1(gf); cki-2; daf-16 mutants released an average of 6023 ± 196 live cells per adult hermaphrodite, while wild type released an average of 37 ± 10 live cells per adult hermaphrodite (n = 3 and n = 6 sets of 25 animals, respectively). These results suggest that over 99% of the glp-1(gf); cki-2; daf-16 isolated cells are germ cells.
The glp-1(gf); cki-2; daf-16 strain contains: a temperature-sensitive, gain-of-function (gf) allele of the Notch receptor gene glp-1, which promotes germ cell proliferation at the non-permissive temperature (Pepper et al., 2003); and loss-of-function mutations of cki-2 (encoding a CDK-inhibitor) and daf-16 (encoding a FOXO transcription factor), both of which normally function to inhibit GSC proliferation (Kalchhauser et al., 2011; Michaelson et al., 2010). In glp-1(gf); cki-2; daf-16 mutants, germ cell proliferation is not constrained to the distal stem cell niche but occurs throughout the gonad. This is demonstrated by the presence of cells in mitosis throughout the gonad, as shown by immunofluorescence staining with the mitotic marker anti-phosphohistone H3 (Ser10) antibody (Hendzel et al., 1997) (Fig. S1). The addition of cki-2(lf) and daf-16(lf) mutations to the glp-1(ar202) mutation produced more mitotic proliferation, as demonstrated by the significantly decreased region of the gonad in which the meiotic marker HIM-3 is present relative to glp-1(ar202) mutants alone (Hansen et al., 2004) (Fig. S1).
Development of an in vitro culture medium for C. elegans germ cells
Embryonic C. elegans cells can be maintained, but not propagated, in L-15-based cell culture medium (Christensen et al., 2002). We observed that isolated germ cells die rapidly in the L-15 medium (Fig. 2B). After carrying out a systematic analysis of culture medium components, we prepared a medium optimized for germ cell culture called CeM1 (C. elegans medium 1) (Table S1). Cell survival in CeM1 was extended relative to L-15 medium by altering: the base medium (3:1 Schneider’s insect:L-15 medium); fetal bovine serum (FBS) concentration (8%); heat-inactivation of FBS at 65°C for 30 min; osmolality (390 mOsm/kg); and pH (6.5) (Fig. S2; data not shown). Additionally, the following CeM1 components contribute to germ cell survival: reduced L-glutathione; RPMI vitamins; the sugar trehalose; and cholesterol and heme, for which C. elegans are auxotrophic (Rao et al., 2005; Shim et al., 2002) (Fig. 2C).
We tested different FBS lots and observed a partial negative correlation between the levels of thyroxine (Atlanta Biologicals data sheets) and the ability of the FBS to support germ cell viability (data not shown). Steroid hormones, such as thyroxine, can be removed by exposing FBS to the anion-exchange resin Amberlite IRA 400-CL and charcoal-dextran (Leake et al., 1987; Wiedemann et al., 1972). Treatment of FBS with both reagents significantly increased germ cell survival (Fig. 2D). The full CeM1 medium can maintain the viability of a majority of isolated germ cells for a period of one month (Fig. 2).
Bacteria can differentially stimulate C. elegans germ cell proliferation
We considered the possibility that C. elegans’ major dietary component, bacteria, could regulate germ cell proliferation. To test this, we created a bacterial extract of the Escherichia coli K-12 strain HT115(DE3) (hereafter HT115), which is used for feeding RNAi (Timmons and Fire, 1998). Addition of HT115 bacterial extract to CeM1 medium increased the number of germ cells over the first three days in culture, suggesting that bacterial component(s) induce germ cell proliferation (Fig. 2E). Heat-inactivation of the bacterial extract (60°C for 30 min) did not affect its ability to induce transient proliferation, suggesting that the active compound(s) are not particularly heat-sensitive (Fig. 2E).
In laboratory settings, C. elegans is propagated on a monoxenic diet of a single bacterial species. To assess the effects of extracts from different bacteria, we created extracts from two additional bacteria: E. coli B strain OP50, which is the standard laboratory diet; and Comamonas aquatica DA1877, which accelerates C. elegans growth (MacNeil et al., 2013). Bacterial extracts from the three strains were added to germ cells isolated from the tumorous mutant strain glp-1(gf); cki-2; daf-16 (hereafter referred to as “isolated germ cells”). Incorporation of the thymidine-analog EdU was used to follow DNA replication 24 to 48 hr post-isolation. Typically, 3–10% of the isolated germ cells incorporate EdU in the absence of bacterial extract. The addition of the bacterial extracts increased the percentage of cells incorporating EdU, with HT115 and DA1877 extracts having more activity than OP50 extract (Fig. 3A).
Figure 3. Bacterial folates stimulate germ cell proliferation in vitro and in vivo.
(A) EdU incorporation in isolated germ cells increases when CeM1 is supplemented with extract from bacteria grown with 2.5 mM PABA, but not from adding PABA alone at the indicated concentrations (in μM). Dash indicates buffer control.
(B) The percentages of germline tumors in glp-1(gf); cki-2; daf-16 mutants at 18°C increase on diets of live bacteria grown with PABA compared to ethanol carrier control (EtOH). The concentrations of PABA are in μM (for panels B, C, F).
(C) Tumor frequency with a diet of heat-killed OP50 bacteria supplemented with PABA at 20°C (the higher temperature compensates for the suboptimal diet of heat-killed bacteria). Dash indicates no addition.
(D) EdU incorporation in isolated germ cells using extracts from bacteria treated with the DHFR-inhibitor trimethoprim (TRI). Dash indicates buffer control. The concentration of TRI was 2.5 μg/ml (for panels D and E).
(E) Tumor frequency at 18°C with diets of bacteria treated with TRI. Dash indicates no addition.
(F) The number of germ cell nuclei in the proliferative zone of wild-type hermaphrodite gonads (analyzed 36 hr post-adulthood) when animals were fed diets of the indicated bacteria supplemented with PABA.
(G) The number of cells expressing seam cell marker scm::GFP per lateral side in cul-1 homozygous and heterozygous mutants fed a diet of OP50 bacteria with or without PABA supplementation.
The same buffer and control bacterial extract samples were analyzed in (A) and (D), and are shown in each panel for comparison. For all figures, asterisks above bars denote statistical significance relative to the control, and asterisks above lines are for comparisons below the lines: *P<0.05; **P<0.01; ***P<0.001; ****P<0.0001; ns = not significant. Statistics are described in the Experimental Procedures section. See also Figures S3–S5.
We tested the effect of diets of the three bacteria on germ cell proliferation in vivo by analyzing the frequency of germline tumor formation in the glp-1(gf); cki-2; daf-16 mutant strain grown at a semi-permissive temperature (Fig. S3A). Hereafter, references to “tumor frequency” will imply that the assay was performed with glp-1(gf); cki-2; daf-16 mutants. Similar results were obtained whether tumor frequency was scored blinded or non-blinded (see Experimental Procedures). Consistent with the EdU incorporation data, the frequency of visible tumors at the semi-permissive temperature of 18°C was higher with a diet of DA1877 or HT115 than with OP50 (Fig. 3B). Diets of the three bacteria appear to stimulate germ cell proliferation through a common pathway, as mixed diets of the different bacteria did not synergistically increase tumor formation (Fig. S3B).
Bacterial folates stimulate germ cell proliferation
Several bacteria-derived compounds have been implicated in modulating C. elegans biological processes, including: folates, which reduce lifespan (Virk et al., 2012); tryptophan metabolite(s), which alter the expression of detoxification genes (Gracida and Eckmann, 2013); and vitamin B12, which accelerates growth (Watson et al., 2014). Supplementing bacteria with tryptophan or vitamin B12 did not stimulate germ cell proliferation (Fig. S4). In contrast, our analysis of folates found that they are linked to the stimulation of germ cell proliferation.
Most bacteria are capable of the de novo synthesis of folate through a pathway that includes the condensation of the dihydropteridine ring with PABA, followed by the addition of one or more glutamates (Glu) (Fig. 1B). PABA can be rate-limiting for folate synthesis in bacteria (Sybesma et al., 2003). To test if increasing the level of bacterial folates increases germ cell proliferation, we supplemented bacteria with PABA. Diets of the three bacteria supplemented with PABA produced an increase in tumor frequencies (Fig. 3B). Similarly, adding extracts from bacteria supplemented with PABA to isolated germ cells increased the percentage of cells incorporating EdU (Fig. 3A). As expected, based on the inability of animals to use PABA to create folates, adding PABA directly to isolated germ cells had no affect on EdU incorporation (Fig. 3A). Similarly, adding PABA to a diet of heat-killed bacteria, which cannot metabolize the PABA, had no stimulatory effect on tumor formation (Fig. 3C).
To further address the contribution of bacterial folates or folate-related compounds to germ cell proliferation, we used the antibiotic trimethoprim (TRI), which inhibits dihydrofolate reductase (DHFR) in bacteria to block the generation of THF. TRI reduces overall THF folate levels in E. coli; however, while the levels of poly-Glu3 or higher THF folates become undetectable upon TRI exposure, the levels of mono- and di-Glu THF folates modestly increase (Kwon et al., 2008). We observed that incubating HT115 and DA1877 bacteria with 2.5 μg/ml TRI prior to creating extract reduced the extract’s ability to stimulate EdU incorporation in isolated germ cells (Fig. 3D). Pretreatment of OP50 with TRI did not have an obvious effect on the extract’s (normally lower) level of stimulating DNA replication (Fig. 3D). Similarly, feeding glp-1(gf); cki-2; daf-16 mutants a diet of HT115 or DA1877 grown on TRI reduced tumor frequency, while a diet of OP50 grown on TRI did not significantly reduce tumor frequency (Fig. 3E). The lack of effect of TRI on an OP50 diet or OP50 bacterial extract indicates that TRI by itself has no appreciable effect on germ cell proliferation, but rather mediates its effects via its action on specific bacteria.
We wanted to determine if the choice of bacterial diet and increasing bacterial folate production affects GSC proliferation in wild-type animals. Increases or decreases in mitotic proliferation of GSCs expand or contract the proliferative zone of the gonad to alter the number of germ cell nuclei in the zone (Michaelson et al., 2010). When wild-type hermaphrodites were fed a diet of HT115 or DA1877 bacteria, the number of germ cell nuclei in the proliferative zone was higher compared to an OP50 diet (Fig. 3F). Supplementing the bacteria with PABA increased the number of germ cell nuclei in the proliferative zone for OP50 and HT115 diets, with a higher mitotic index for the HT115 diet, suggesting that a diet with increased levels of folates increases proliferative germ cell numbers (Figs 3F, S5A). Incubation of the three bacteria with TRI reduced the numbers of mitotic germ cells per gonad arm in wild-type hermaphrodites fed a diet of DA1877, but had less effect on OP50 and HT115 diets (Fig. S5B). Overall, these results suggest that increased levels of bacterial folates can increase mitotic germ cell proliferation in wild-type hermaphrodites.
To confirm that folates are the active bacterial component, folates were purified from bacteria and tested for their ability to induce germ cell proliferation. Total bacterial extract, purified folates, and folate-free, flow-through extract were tested on isolated germ cells for their effect on DNA replication. Total extract and purified folates increased the number of cells incorporating EdU when added to isolated germ cells (Fig. 4A; Table S2). In contrast, the addition of the folate-free flow-through extract reduced EdU incorporation, suggesting that in the absence of folates, bacterial extract negatively impacts germ cell cultures. The addition of purified folates to a diet of heat-killed OP50 bacteria also increased tumor frequency (Fig. 4B).
Figure 4. Purified folates stimulate germ cell proliferation.
(A) Germ cell stimulatory activity segregates with purified folates during affinity purification. Equal percentages of total bacterial extract; folate-free extract (post-folate purification); or folates purified from the extract were added to isolated germ cells and EdU incorporation was assessed. The concentrations of the purified folates were: 0.063 μM (OP50); 0.038 μM (HT115); and 0.055 μM (DA1877).
(B) The effect of purified folates and the reduced folates shown on tumor frequency at 20°C when added to heat-killed bacteria. Equal volumes of purified folates were used; the concentrations of purified folates for experiments (B) and (C) were: 0.21 μM (OP50); 0.13 μM (HT115); and 0.18 μM (DA1877).
(C) The number of germ cell nuclei in the proliferative zone of wild-type hermaphrodite gonads for animals fed a diet of heat-killed OP50 supplemented with purified folates from the indicated bacteria.
(D) Purified folates from the indicated bacteria stimulate EdU incorporation at 0.06 μM, while the basic reduced folates, 5-methyl-THF (5m-THF), 5-formyl-THF (5f-THF), and THF, are not active at concentrations of 0.1 to 100 μM.
To determine if purified folates increase the number of mitotic cells in wild-type animals, we added purified folates from OP50, DA1877, or HT115 to heat-killed OP50 bacteria and allowed wild-type animals to develop from eggs on these plates. The addition of purified folates from HT115 and DA1877 increased the number of nuclei in the proliferative zone, with the mitotic index statistically higher for DA1877 folates (Figs 4C, S5C). These results indicate that bacterial folates promote GSC proliferation in wild-type hermaphrodites.
Our previous analysis used equal volumes of isolated purified folates. To compare the relative activity of the purified folates among the three bacteria, we added equal concentrations of purified folates (0.06 μM) to isolated germ cells. The purified folates from HT115 induced a higher percentage of cells incorporating EdU than purified folates from OP50 and DA1877 (Fig. 4D). Folates purified from the mouse microbiota also have potent activity in stimulating EdU incorporation in isolated germ cells, suggesting that the active folate(s) are present in diverse microbial settings (Fig. S5D).
10-formyl-THF-Glun stimulates germ cell proliferation
CeM1 medium contains the synthetic folate folic acid at 2.8 μM, which is 40-fold higher than the concentration of purified folates that stimulate increased EdU incorporation in isolated germ cells. Therefore, bacterial folates provide a signal that is not provided by folic acid.
We tested the germ cell stimulatory activity of the reduced monoglutamyl forms of folates, including racemic (S,R) THF, 5-formyl-THF (folinic acid), and 5-methyl-THF, as well as the biologically active (S) isomer for the latter two folates. We found that these basic folates, which can promote one-carbon metabolism when added to other animal cells, were unable to stimulate germ cell DNA replication even at concentrations significantly higher than the purified bacterial folates (Fig. 4D). The addition of 5-methyl-THF and THF to a diet of heat-killed bacteria also did not have a major effect on tumor frequency (Fig. 4B).
In an effort to identify the active germ cell-stimulatory folate(s), we analyzed the folates from OP50, HT115, and DA1877 bacteria grown under normal or PABA-supplemented conditions. Ion-pair high performance liquid chromatography (HPLC) was used to separate the affinity-purified folates, which were then identified by their stereotypical UV absorbance spectra (Selhub et al., 1980). Four folate species were detected in the bacterial extracts: 10-formyl-THF-Glun; THF-Glun; 5-formyl-THF-Glun; and 5-methyl-THF-Glun (Fig. S6). The folates from DA1877 consisted predominantly of folates with three Glu residues, the most abundant of which was 5-methyl-THF-Glu3 (Fig. S6C). In contrast, OP50 and HT115 had primarily formylated folates with 3–7 Glu residues (Fig. S6A,B). Notably, the only folate species that increased upon growth with PABA in all three bacterial species was 10-formyl-THF-Glun.
We isolated individual DA1877 folate fractions using HPLC (Fig. 5A). The 5-methyl-THF-Glu1,3 fractions lacked stimulatory activity, consistent with our analysis of pure folates (Fig. 5B,C). In contrast, the 10-formyl-THF-Glu3 and 5,10-methenyl-THF-Glu3 fractions stimulated EdU incorporation in isolated germ cells and increased tumor frequency when added to a diet of heat-killed bacteria (Fig. 5B,C). The activity of 5,10-methenyl-THF-Glu3 is likely to be due to its conversion to 10-formyl-THF-Glu3, which would occur because the assays were performed at neutral pH. The first peak from the chromatogram, whose molecular identity we could not determine, also exhibited stimulatory activity (data not shown). Overall, our results suggest that 10-formyl-THF-Glun can stimulate germ cell proliferation.
Figure 5. 10-formyl-THF and 5,10-methenyl-THF isolated from bacteria stimulate germ cells.
(A) Chromatogram showing UV absorbance (milli-absorbance units) of affinity-purified DA1877 folates separated by ion-pair chromatography. Folate species identified by UV spectra are labeled.
(B and C) Affinity-purified folate fractions, containing the indicated folates, were isolated from DA1877 bacteria grown with PABA supplementation and tested for their ability to induce DNA replication (EdU incorporation) in isolated germ cells (B) or increase tumor frequency at 20°C with a diet of heat-killed bacteria (C). 10-formyl-THF (10f-THF); 5,10-methenyl-THF (5,10me-THF).
See also Figure S6.
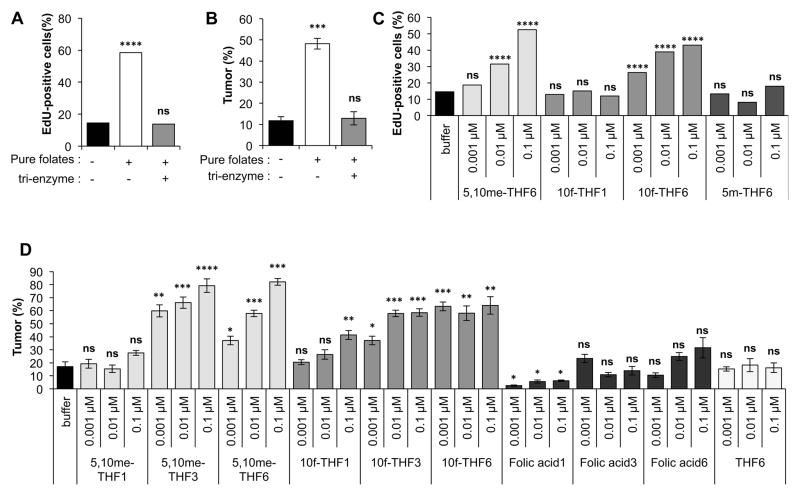
To clarify the importance of the number of glutamate residues, we converted purified OP50 bacterial folates from poly-Glu3–7 to mono-Glu by treatment with tri-enzyme (a mixture of chicken pancreas conjugase, alpha amylase, and pronase) (Martin et al., 1990). The conversion to mono-Glu folates abolished the stimulatory activity in EdU incorporation assays with isolated germ cells and tumor frequency assays (Fig. 6A,B). The stimulation of tumor frequency was also abolished when purified DA1877 bacterial folates were converted to mono-Glu using conjugase enzyme alone (Fig. S5E).
Figure 6. Poly-glutamate increases 10-formyl-THF-Glun germ cell stimulatory activity.
(A and B) Purified folates from OP50 were treated with tri-enzyme to convert poly-Glu folates to mono-Glu folates. Germ cell stimulatory activity was assessed by analyzing EdU incorporation in isolated germ cells (A) and tumor frequency at 20°C with a diet of heat-killed bacteria (B). The concentration of purified folates was 0.06 μM for (A) and 0.12 μM for (B).
(C and D) Comparison of the activity of synthetic folates with 1, 3, 6 Glu residues in the EdU incorporation assay with isolated germ cells (C) and tumor frequency assay at 18°C (D).
See also Figure S5.
To further confirm that poly-Glu contributes to germ cell stimulatory activity, we synthesized folates with 1, 3, or 6 Glu from folic acid-Glu1,3,6: THF-Glu1,3,6; 5-methyl-THF-Glu1,3,6; 5,10-methenyl-THF-Glu1,3,6; and 10-formyl-THF-Glu1,3,6. We observed that folic acid, 5-methyl-THF, and THF were not active in stimulating increased tumor frequencies irrespective of the number of Glu residues (Figs 6D; S5F). In contrast, 10-formyl-THF and 5,10-methenyl-THF increased tumor frequency in vivo and EdU incorporation in isolated germ cells in vitro, with greater activity with higher numbers of poly-glutamates (Figs 6C,D; S5F). Significantly, 10-formyl-THF-Glu3,6 and 5,10-methenyl-THF-Glu3,6 had activity even at the lowest concentration tested: 1 nM (Fig. 6C,D). The addition of 5,10-methenyl-THF-Glu6 stimulated the transient proliferation of germ cells in vitro, similar to what we had observed with HT115 bacterial extract (Fig. S5G). The ability of the synthetic folates 10-formyl-THF-Glun and 5,10-methenyl-THF-Glun to match the stimulatory activity of the folates isolated from bacteria confirms the identity of the bacterial stimulatory folates.
Folates stimulate germ cell proliferation independently of one-carbon metabolism
In other animals, multiple folates can act as single vitamin sources to reconstitute all of the folates required for one-carbon metabolism (Zhao et al., 2009). One potential model to explain the specificity of germ cell stimulation is that perhaps, unlike other animals, C. elegans can only utilize a single folate as a vitamin source. To address the role of folates as vitamins, we used the folt-1/RFC mutant as a means to deplete folate levels.
In mammals, the ubiquitously-expressed RFC transports the bulk of systemic folates into tissues (Zhao et al., 2009). In C. elegans, FOLT-1/RFC is required for ~80% of folate uptake into animals (Balamurugan et al., 2007). folt-1(ok1467) deletion mutants, when grown on a diet of OP50 bacteria, have severe defects in germ cell proliferation, with few germ cells per gonad arm (Austin et al., 2010). Strikingly, we found that providing folt-1/RFC mutants a diet of OP50 supplemented with PABA rescued the germ cell number defect, and allowed 100% of the folt-1/RFC mutant adult hermaphrodites to become gravid (Fig. 7A). Therefore, the folt-1/RFC mutant is responsive to increased folate levels.
Figure 7. Folates and pteroates stimulate germ cell proliferation independently of a role as vitamins.
(A) folt-1(ok1467) mutant sterility is rescued by growth on OP50 supplemented with PABA. The percentage of gravid animals is shown (n = 20 each).
(B) Stimulatory and non-stimulatory folates can rescue folate deficiency, but dihydropteroate cannot. folt-1(ok1467) mutants were fed heat-killed, folate-depleted pabC mutant bacteria with the indicated folates or folate-related compounds (10 μM). Germ cell numbers per gonad arm from mid/late L4-stage larvae were scored blindly.
(C and D) Dihydropteroate stimulates EdU incorporation in isolated germ cells (C) and tumor frequency at 18°C (D). For (C), the experiment was performed at the same time as Fig. 6C, and the same control is shown for comparison.
(E) folr-1/FR RNAi blocks the stimulatory effect of dihydropteroate, 5,10-methenyl-THF6, and 5,10-methenyl-THF1 on tumor frequency at 18°C. The concentrations are in μM.
(F) folr-1/FR RNAi blocks the stimulatory effect of dihydropteroate and 5,10-methenyl-THF-Glu6 on EdU incorporation in isolated germ cells.
See also Figure S7.
To further reduce the levels of folates, folt-1/RFC mutants were fed a diet of heat-killed, folate-depleted pabC mutant bacteria, which are unable to produce PABA (Roux and Walsh, 1993). To deplete folates in pabC mutants, the bacteria were incubated 24 hr in PABA-free minimal media. The resulting folate-depleted pabC bacteria had only 2.3% of the folate level of the parental E. coli K-12 strain (data not shown).
To test the ability of folates to rescue folate deficiency, heat-killed, folate-depleted pabC bacteria were supplemented with 10 μM of either the non-stimulatory folate S-5-formyl-THF-Glu1 or the stimulatory folate 5,10-methenyl-THF-Glu1. Both folates were able to rescue germ cell proliferation in the folate-depleted folt-1/RFC mutants, with the non-stimulatory S-5-formyl-THF-Glu1 exhibiting more activity (Fig. 7B). Racemic 5-formyl-THF-Glu1 (folinic acid) also rescues C. elegans sterility due to folate deficiency (Virk et al., 2016). These results suggest that the effectiveness of a folate to function as a vitamin does not correlate with its ability to stimulate germ cell proliferation under normal growth conditions.
To directly test if a folate-related compound can stimulate germ cell proliferation independently of one-carbon metabolism, we analyzed dihydropteroate. Pteroates are comprised of a pteridine ring and PABA moieties, but lack glutamates (Fig. 1B). Dihydropteroate is a precursor to all folate synthesis in bacteria. Animals are unable to convert pteroates to folates because they lack the enzyme (dihydrofolate synthase) that is required to add glutamate to pteroates (Fig. 1B). Animals therefore cannot utilize pteroates for one-carbon metabolism. As expected for a compound that cannot support one-carbon metabolism, dihydropteroate was unable to rescue the folate deficiency of folt-1/RFC mutants grown on folate-depleted bacteria (Fig. 7B).
Significantly, dihydropteroate stimulated increased EdU incorporation in isolated germ cells and tumor frequency, although it was less active than 5,10-methenyl-THF-Glu6 in inducing the proliferation of isolated germ cells (Figs 7C,D; S5G). The ability of dihydropteroate to stimulate germ cell proliferation implies that the stimulation occurs independently of one-carbon metabolism.
The folate receptor homolog FOLR-1 is required for the stimulation of germ cell proliferation
The mammalian FRs, α, β, and γ, transport folates, but have more restricted tissue expression than RFC (Zhao et al., 2009). Notably, mammalian FR can bind both folates and pteroates (Sodji et al., 2015). C. elegans contains an apparent ortholog of FR, folr-1 (C17G1.1). Although FOLR-1/FR and human FRγ proteins only share 12% identity and 25.5% similarity, they are the top scores in the two respective species using reciprocal psi-BLAST searches (Altschul et al., 1997). FOLR-1/FR has a predicted signal peptide and transmembrane domain that is compatible with cell surface localization (Cserzo et al., 2002; Petersen et al., 2011).
In contrast to folt-1/RFC mutants, RNAi depletion of folr-1/FR does not appear to affect the basal number of germ cells, and folr-1(RNAi) hermaphrodites lay the same number of eggs as wild type (Fig. S7A). This suggests that unlike FOLT-1/RFC, FOLR-1/FR is not essential for the uptake of folates to function as vitamins. Strikingly, RNAi depletion of folr-1/FR abolishes the stimulatory effect of purified bacterial folates, 10-formyl-THF-Glu6, 5,10-methenyl-THF-Glu1,6, and dihydropteroate both in vivo (for tumor frequency) and in vitro (for EdU incorporation) (Figs 7E,F, S7B). At the fully non-permissive temperature of 25°C, folr-1/FR RNAi only modestly suppresses tumor formation in glp-1(gf); cki-2; daf-16 mutants, but blocks the response to PABA supplementation, indicating that it still blocks the stimulatory effect of folates (Fig. S7C). These results suggest that both 10-formyl-THF-Glun and dihydropteroate stimulate germ cell proliferation through a FOLR-1-dependent pathway.
A folate-enriched diet increases cell number in somatic hyperplasia
We wanted to determine if bacterial folates could stimulate somatic cell division using the cul-1 mutant, which exhibits hyperplasia of larval somatic cell lineages (Kipreos et al., 1996). cul-1(e1756) mutants expressing a hypodermal seam cell GFP marker were fed diets of OP50 or OP50 supplemented with 10 μM PABA. Both cul-1 homozygous and heterozygous mutants exhibited increased seam cell numbers when on the diet supplemented with PABA (Fig. 3G). Notably, the hyperplasia in cul-1 heterozygotes is a synthetic phenotype, as cul-1 heterozygotes do not exhibit hyperplasia under normal growth conditions (Kipreos et al., 1996) and wild-type animals do not exhibit seam cell hyperplasia on a diet of OP50 supplemented with PABA (Fig. 3G).
We were unable to use heat-killed bacteria to assess the effect of pure folates or pteroates because cul-1 homozygotes arrest development on heat-killed bacteria, and cul-1 heterozygotes do not exhibit hyperplasia on heat-killed bacteria with added folates, potentially due to the suboptimal diet (data not shown). Nevertheless, these results suggest that a diet with increased bacterial folates can stimulate the proliferation of a somatic cell lineage.
Discussion
In this study, we describe the first primary culture system for C. elegans germ cells. The CeM1 medium that we created differs markedly from the L-15-based medium used for C. elegans embryonic and larval cell cultures. CeM1 medium can maintain the viability of germ cells for up to one month, thereby providing an experimental platform for the study of nearly homogeneous populations of germ cells.
10-formyl-THF-Glun is a germ cell-stimulatory folate
Our results show that bacterial folates act as an exogenous signal to stimulate an adult stem cell population. Surprisingly, many folate species (folic acid, THF, 5-formyl-THF, and 5-methyl-THF) are unable to stimulate germ cells under normal growth conditions, despite the fact that these folates are readily taken up by animals, including C. elegans (Balamurugan et al., 2007). Instead, we observed germ cell stimulatory activity only with the folates 10-formyl-THF-Glun and 5,10-methenyl-THF-Glun, with increasing activity with larger numbers of poly-Glu. The ability of 5,10-methenyl-THF-Glun to stimulate germ cells is unlikely to reflect its own activity, as it converts to 10-formyl-THF-Glun within minutes at the neutral pH used in our experiments.
Bacterial folates and related compounds can stimulate C. elegans germ cells independently of one-carbon metabolism
We observed that the non-stimulatory folate S-5-formyl-THF-Glu1 rescued the folate deficiency of folate-depleted folt-1/RFC mutant germ cells more effectively than the stimulatory folate 5,10-methenyl-THF-Glu1. This suggests that the ability of a folate to act as a vitamin does not correlate with its ability to stimulate germ cell proliferation under normal growth conditions. Additionally, 10-formyl-THF-Glun can stimulate C. elegans germ cell proliferation at a concentration of 1 nM, which is lower than the levels required for one-carbon metabolism in mammals (Geng et al., 2015; Neuhouser et al., 2011).
Dihydropteroate can also stimulate germ cell proliferation. Dihydropteroate is unable to function in one-carbon metabolism in animal cells, and consistently, its addition was unable to rescue folate deficiency in C. elegans. Significantly, the stimulation of germ cell proliferation by both dihydropteroate and 10-formyl-THF-Glun requires the presence of FOLR-1/FR. The mammalian homolog of FOLR-1/FR can bind both folates and pteroates (Sodji et al., 2015). These results suggest that dihydropteroate and 10-formyl-THF-Glun stimulate germ cell proliferation through a FOLR-1/FR-dependent pathway that is independent of one-carbon metabolism.
Dihydropteroate is present in all bacteria that are capable of de novo folate biosynthesis. However, we did not observe detectable levels of dihydropteroate by chromatography in extracts from the three bacteria (data not shown). This suggests that in these three bacteria, dihydropteroate is not the predominant germ cell stimulatory signal, with 10-formyl-THF-Glun and 5,10-methenyl-THF-Glun present at much higher levels. It is possible that in the wild, other bacteria produce higher levels of dihydropteroate that contribute to the stimulation of C. elegans germ cell proliferation.
One obvious question is why 10-formyl-THF and dihydropteroate, but not other folates, were selected during evolution to regulate germ cell proliferation. In this regard, it is notable that 10-formyl-THF and dihydropteroate are particularly unstable relative to other folates and folate-related compounds (Blakley, 1969; Schircks Laboratories data sheets). Potentially, the labile nature of these folate and folate-related compounds allows a tighter linkage between the presence of live bacteria and germ cell proliferation.
The folate receptor and signaling
The finding that FOLR-1/FR is required for the stimulation of C. elegans germ cell proliferation is interesting in light of recent mammalian cancer research. In many cancers, FRs are overexpressed, and this is associated with neoplastic progression and poor prognosis (Kelemen, 2006). FRα promotes proliferation, migration, and invasiveness of SKOV-3 ovarian cancer cells, which have high-level FRα expression; while surprisingly, RFC acts oppositely to reduce cell proliferation, migration, and invasiveness (Siu et al., 2012). Notably, FRα only contributes 20–30% of the uptake of folate in SKOV-3 and four other ovarian cancer cell lines, while RFC is responsible for ~70% of their folate uptake (Corona et al., 1998). Similarly, in C. elegans, FOLT-1/RFC is required for the majority of folate uptake to allow basal germ cell proliferation and fertility, while FOLR-1/FR is required for stimulatory folate signaling but is not essential for providing folates for basal germ cell proliferation.
Recent emerging evidence suggests the potential for human FR to function in cell signaling independently of one-carbon metabolism. The addition of folates to cells activates intracellular signaling pathways in a FR-dependent manner in time periods that are shorter than would be expected from changes in one-carbon metabolism. The addition of folic acid to mammalian cells has been reported to induce the phospho-activation of c-src tyrosine kinase, ERK kinase, and STAT transcription factor in a FRα-dependent manner within 2–5 minutes of stimulation (Hansen et al., 2015; Lin et al., 2012; Zhang et al., 2009). Additionally, FRα itself has been reported to translocate to the nucleus and function as a transcription factor in human tissue culture cells after stimulation with 453 μM of folic acid (Boshnjaku et al., 2012). These studies used folic acid, a non-natural, synthetic folate, at elevated, non-physiological levels of 10 to 600 μM. These concentrations are orders of magnitude higher than the concentration of folates in human serum, which are 8.6 to 29.7 nM for the 5th–95th percentiles of an unsupplemented population (Hustad et al., 2000). This raises the question of the physiological relevance of the observations. In contrast, our results show biological effects with 1 nM of a specific, naturally-available folate.
Our work provides a direct link between microbial factors and the regulation of an adult stem cell population. The importance of the microbiota for animal health has recently been recognized (Clemente et al., 2012). Microbiota-derived folates are readily absorbed by the human host (Camilo et al., 1996). In diverse human populations, the initial colonization of the gut is enriched for microbes capable of de novo folate synthesis, indicating that humans harbor folate-synthesizing bacteria throughout their lifespan (Yatsunenko et al., 2012). We observed that folates isolated from mouse microbiota are potent stimulators of C. elegans germ cells, indicating that the active folates are present at high levels in the mammalian microbiota. Our work therefore suggests the possibility that microbiota-derived folates can act as signaling molecules, potentially to the host or to other organisms that may reside within the host, such as parasitic nematodes.
Experimental Procedures
CeM1 medium preparation
CeM1 medium was prepared with the ingredients listed in Table S1. CeM1 was sterile filtered through 0.22 μm 150 ml filter units (Millipore). Three sequential treatments were performed on the FBS prior to its inclusion in CeM1: heat inactivation; and treatments with Amberlite IRA 400-CL and charcoal-dextran (see Supplemental Experimental Procedures).
Germ cell isolation and primary culture
To obtain synchronous adult germline tumorous mutants, eggs were isolated by sodium hypochlorite treatment (Sulston and Hodgkin, 1988). The eggs were transferred to a 3xNGM plate (an NGM agar plate (Sulston and Hodgkin, 1988) with 3x peptone concentration) with a lawn of OP50 bacteria, and grown at 25°C for four days to ensure that all animals became adults with germline tumors. The animals were washed four times with M9 salt solution (Sulston and Hodgkin, 1988) in 15 ml polystyrene tubes (Falcon) to remove live bacteria and transferred to a 12.5 cm2 cell culture flask (Corning) containing 2.5 ml of M9 solution supplemented with: heat-killed OP50 bacteria; 200 units penicillin and 0.2 mg streptomycin per ml); 25 μg/ml tetracycline (Sigma-Aldrich, 87128); 34 μg/ml chloramphenicol (Research Products International); 50 μg/ml kanamycin; 0.02% normocin; and 5 μg/ml cholesterol. Animals were incubated overnight in the antibiotic-supplemented M9 solution. The next day, animals were washed four times with phosphate buffered saline (PBS) in 15 ml polystyrene tubes, washed one time with CeM1, and then resuspended in 2 ml of CeM1 in a 35 mm tissue culture dish (Falcon). Animals were transferred with a platinum wire to a 120 μl spot of CeM1 in another 35 mm culture dish. Germ cells were released by cutting animals into quarters using 31 gauge needles (Becton Dickinson). Cells were collected with three sequential washes with 1 ml of CeM1, collected into a 15 ml polypropylene tube and spun at 300–1000 rpm for 1 min to pellet body parts and large cell aggregates. The supernatant was transferred to a new 15 ml tube and spun at 2000 rpm for 5 min to pellet individual cells. The cells were resuspended in full CeM1 (unless otherwise stated) and transferred to a tissue culture dish. If multi-well tissue culture dishes were used, the outer wells were filled with PBS to keep the inner wells humidified, then sealed with parafilm to prevent loss of moisture, and incubated at 25°C.
Bacterial Extract
Bacteria were grown overnight in 2xYT medium that was either unsupplemented for OP50, or supplemented with 25 μg/ml tetracycline for HT115, or 100 μg/ml streptomycin for DA1877. The bacteria were collected by centrifugation at 4000 rpm for 30 min at 4°C, washed two times with sterile 0.9% NaCl, and the bacterial pellet was frozen at −80°C. Bacterial pellets were lyophilized under vacuum at room temperature. Crushed bacterial pellet was added at 0.08 g/ml to folate-extraction buffer (1% Na ascorbate, 20 mM phosphate buffer, pH 6.5), vortexed, and rotated at room temperature in the dark for 1 hr. The bacteria were spun out in a microcentrifuge at 13,300 rpm for 15 min. The supernatant was transferred to a new microcentrifuge tube and extracted once with 1:1 phenol:choloroform and three times with chloroform to remove proteins; spun out and transferred to a new tube to remove any residual chloroform, and then sterile filtered using a 0.2 μm syringe filter. The bacterial extract used in Fig. 2E was prepared with water instead of folate-extraction buffer and was used immediately after preparation.
Tumor frequency assay
Eggs isolated by sodium hypochlorite treatment were placed on 1x NGM plates seeded with live bacteria or heat-killed OP50 bacteria with the indicated experimental additives. Assays with live bacteria were performed at the semi-permissive temperature of 18°C; assays with heat-killed bacteria were performed at 20°C. L4-stage animals were transferred onto fresh plates, and the percentages of adult animals with tumors were scored two days later by observation with a dissecting microscope. Tumor frequency assays were performed in triplicate with ~100 animals per replicate. Several tumor frequency assays were performed blind, including Figs 6B, 7D, S5E, and S5F. The supplements trimethoprim (cat. no. 92131) and PABA (100536) were from Sigma-Aldrich; vitamin B12 (103278) was from MP Biomedicals.
Counts of live isolated germ cells
Counts of live isolated germ cells were performed with the live-cell stain calcein-AM and dead-cell stain ethidium homodimer (Zhang et al., 2011). The numbers of live cells were obtained by counting cells stained with 1 μM calcein-AM (Sigma-Aldrich, C1359), 0.1 μM Ethidium homodimer (Sigma-Aldrich, E1903), and with or without 2 μg/ml Hoechst 33342 (Sigma-Aldrich, B2261). A minimum of three counts were made for each sample using a cellometer counting grid (CP2, Nexcelom Bioscience LLC) analyzed with an inverted fluorescence microscope (Zeiss Axio Observer.A1); cell count variation is presented as SEM. Typically, germ cells were isolated from 25 adult hermaphrodites for 0.5 ml/well of a 24-well plate.
Counts of germ cells in mid-L4-stage larvae
Counts of germ cells in mid-L4-stage larvae were performed with animals fixed with 95% ethanol for 10 min as described (Killian and Hubbard, 2005), and then stained with 2 μg/ml Hoechst 33342 in PBS. Germ cell counts were performed blind, with the identity of the treatment masked. The germ cells in one gonad arm per animal were counted. Mid-L4-stage larvae were identified based on vulva morphology. Larvae selected for germ cell counts had vulva morphologies categorized as L4.1 to L4.3 on the L4.0–L4.9 vulval morphology scale that has been previously defined (Mok et al., 2015). For Fig. 7B, between 9 and 17 animals were analyzed per condition.
EdU-incorporation assay
Isolated germ cells from glp-1(gf); cki-2; daf-16 mutant adults were incubated in 80 μl of CeM1 in a 96-well plate, with the germ cells from approximately eight adults per well. 24 hr post-isolation, EdU was added to a concentration of 20 μM. At 48 hr post-isolation, cells were harvested and processed with the Click-iT Alexa Fluor 488 Imaging kit (Life Technologies), according to the manufacturer’s instructions. Cells were subsequently stained with 2 μg/ml Hoechst 33342 DNA stain and analyzed by fluorescence microscopy for EdU staining of DNA, with images of EdU Alexa Fluor 488 staining taken initially, and then images of Hoechst staining taken subsequently. Typically, 150–200 cells were counted for each condition.
Folate analysis
Folates for chromatography analysis were prepared as follows. Lyophilized bacteria were resuspended at a concentration of 0.09–0.1 mg/ml in folate-extraction buffer (2% sodium ascorbate, 0.05 M 2-mercaptoethanol), boiled for 15 min, then spun in a centrifuge at 30,000xg for 30 min to remove insoluble components. Aliquots (2 ml) of the supernatants were mixed with 18 ml of potassium phosphate buffer containing 1% sodium ascorbate. Purified folates were isolated by passage through affinity columns (2.4 ml bed volume) containing purified milk folate-binding protein which was immobilized to a Sepharose matrix (Selhub et al., 1980).
For HPLC detection of folate species, 250 μl of the purified folate was mixed with 1% sodium ascorbate, 0.01 M potassium phosphate pH 7.5. A 0.9 ml aliquot was injected into a 4.6×250 mm Betasil Phenyl analytic column and eluted under acid conditions using acetonitrile gradient and detection by UV, fluorescence, and electrochemical signals (Bagley and Selhub, 2000) (data not shown). The use of multisignaling allowed better identification of the various peaks that were eluted from the column.
The microbial assay was used to determine folate concentration. Purified extract was treated with conjugase using the tri-enzyme system, as described (Poo-Prieto et al., 2006), and folate was then analyzed using 96 well plates, as described (Tamura et al., 1990).
Statistical analysis
Two-tailed Student’s t-test was used to analyze the three replicates of tumor frequencies, the data for mitotic index, numbers of germ cell nuclei per proliferative zone, egg numbers per animal, and the number of germ cells per gonad arm. The chi-squares test was used to analyze the percentages of EdU positive cells. The nonparametric Mann-Whitney test was used to analyze the number of phosphohistone H3 positive cells per gonad arm. All error bars reflect standard error of the mean (SEM).
Additional methods are provided in the Supplemental Information.
Supplementary Material
Highlights.
A tissue culture system maintains primary cultures of C. elegans germ cells
Bacteria-derived folates promote germ cell proliferation both in vitro and in vivo
Specific folates stimulate proliferation independently of their roles as vitamins
The folate germ cell-stimulatory pathway requires the folate receptor homolog FOLR-1
Acknowledgments
We thank M.J. McEachern and members of the Kipreos lab for critical comments on the manuscript, M.C. Zetka, D.B. Hausman, and S. Dougan for reagents or resources. Some C. elegans strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). This work was supported by grants from NSF (MCB-1138454) and NIH/NIGMS (R01 GM074212) (ETK), and support from United States Department of Agriculture cooperative agreement 51520-008-04S (JS). Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the United States Department of Agriculture.
Footnotes
Author Contributions
Conceptualization, E.T.K. and J.S.; Methodology, E.T.K. and J.S.; Investigation, S.N.C., M.M., A.S.V., G.B., M.M.R., C.Q.N., and E.T.K.; Resources, J.S.; Writing – Original Draft, E.T.K. and J.S.; Writing – Review and Editing, S.N.C., M.M., E.T.K., J.S., M.M.R., and L.P.; Visualization, M.M. and S.N.C.; Supervision, E.T.K., J.S., and L.P.; Funding Acquisition, E.T.K. and J.S.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MU, Liau WS, Balamurugan K, Ashokkumar B, Said HM, LaMunyon CW. Knockout of the folate transporter folt-1 causes germline and somatic defects in C. elegans. BMC developmental biology. 2010;10:46. doi: 10.1186/1471-213X-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley PJ, Selhub J. Analysis of folate form distribution by affinity followed by reversed-phase chromatography with electrical detection. Clinical chemistry. 2000;46:404–411. [PubMed] [Google Scholar]
- Balamurugan K, Ashokkumar B, Moussaif M, Sze JY, Said HM. Cloning and functional characterization of a folate transporter from the nematode Caenorhabditis elegans. American journal of physiology. 2007;293:C670–681. doi: 10.1152/ajpcell.00516.2006. [DOI] [PubMed] [Google Scholar]
- Blakley RL. The Biochemistry of Folic Acid and Related Pteridines. Amsterdam, Netherlands: North-Holland Publishing Company; 1969. [Google Scholar]
- Boshnjaku V, Shim KW, Tsurubuchi T, Ichi S, Szany EV, Xi G, Mania-Farnell B, McLone DG, Tomita T, Mayanil CS. Nuclear localization of folate receptor alpha: a new role as a transcription factor. Sci Rep. 2012;2:980. doi: 10.1038/srep00980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilo E, Zimmerman J, Mason JB, Golner B, Russell R, Selhub J, Rosenberg IH. Folate synthesized by bacteria in the human upper small intestine is assimilated by the host. Gastroenterology. 1996;110:991–998. doi: 10.1053/gast.1996.v110.pm8613033. [DOI] [PubMed] [Google Scholar]
- Christensen M, Estevez A, Yin X, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM, 3rd, Strange K. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33:503–514. doi: 10.1016/s0896-6273(02)00591-3. [DOI] [PubMed] [Google Scholar]
- Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona G, Giannini F, Fabris M, Toffoli G, Boiocchi M. Role of folate receptor and reduced folate carrier in the transport of 5-methyltetrahydrofolic acid in human ovarian carcinoma cells. International journal of cancer. 1998;75:125–133. doi: 10.1002/(sici)1097-0215(19980105)75:1<125::aid-ijc19>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Cserzo M, Eisenhaber F, Eisenhaber B, Simon I. On filtering false positive transmembrane protein predictions. Protein Eng. 2002;15:745–752. doi: 10.1093/protein/15.9.745. [DOI] [PubMed] [Google Scholar]
- Desmoulin SK, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer biology & therapy. 2012;13:1355–1373. doi: 10.4161/cbt.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Gao R, Chen X, Liu X, Liao X, Li Y, Liu S, Ding Y, Wang Y, He J. Folate deficiency impairs decidualization and alters methylation patterns of the genome in mice. Mol Hum Reprod. 2015;21:844–856. doi: 10.1093/molehr/gav045. [DOI] [PubMed] [Google Scholar]
- Gracida X, Eckmann CR. Fertility and germline stem cell maintenance under different diets requires nhr-114/HNF4 in C. elegans. Curr Biol. 2013;23:607–613. doi: 10.1016/j.cub.2013.02.034. [DOI] [PubMed] [Google Scholar]
- Grapp M, Wrede A, Schweizer M, Huwel S, Galla HJ, Snaidero N, Simons M, Buckers J, Low PS, Urlaub H, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun. 2013;4:2123. doi: 10.1038/ncomms3123. [DOI] [PubMed] [Google Scholar]
- Hansen D, Hubbard EJ, Schedl T. Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev Biol. 2004;268:342–357. doi: 10.1016/j.ydbio.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Hansen D, Schedl T. Stem cell proliferation versus meiotic fate decision in Caenorhabditis elegans. Advances in experimental medicine and biology. 2013;757:71–99. doi: 10.1007/978-1-4614-4015-4_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen MF, Greibe E, Skovbjerg S, Rohde S, Kristensen AC, Jensen TR, Stentoft C, Kjaer KH, Kronborg CS, Martensen PM. Folic acid mediates activation of the pro-oncogene STAT3 via the Folate Receptor alpha. Cell Signal. 2015;27:1356–1368. doi: 10.1016/j.cellsig.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Henderson GI, Perez T, Schenker S, Mackins J, Antony AC. Maternal-to-fetal transfer of 5-methyltetrahydrofolate by the perfused human placental cotyledon: evidence for a concentrative role by placental folate receptors in fetal folate delivery. J Lab Clin Med. 1995;126:184–203. [PubMed] [Google Scholar]
- Hendzel MJ, Wei Y, Mancini MA, Van Hooser A, Ranalli T, Brinkley BR, Bazett-Jones DP, Allis CD. Mitosis-specific phosphorylation of histone H3 initiates primarily within pericentromeric heterochromatin during G2 and spreads in an ordered fashion coincident with mitotic chromosome condensation. Chromosoma. 1997;106:348–360. doi: 10.1007/s004120050256. [DOI] [PubMed] [Google Scholar]
- Hustad S, Ueland PM, Vollset SE, Zhang Y, Bjorke-Monsen AL, Schneede J. Riboflavin as a determinant of plasma total homocysteine: effect modification by the methylenetetrahydrofolate reductase C677T polymorphism. Clinical chemistry. 2000;46:1065–1071. [PubMed] [Google Scholar]
- Kalchhauser I, Farley BM, Pauli S, Ryder SP, Ciosk R. FBF represses the Cip/Kip cell-cycle inhibitor CKI-2 to promote self-renewal of germline stem cells in C. elegans. EMBO J. 2011;30:3823–3829. doi: 10.1038/emboj.2011.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? International journal of cancer. 2006;119:243–250. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- Killian DJ, Hubbard EJ. Caenorhabditis elegans germline patterning requires coordinated development of the somatic gonadal sheath and the germ line. Dev Biol. 2005;279:322–335. doi: 10.1016/j.ydbio.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Kipreos ET, Lander LE, Wing JP, He WW, Hedgecock EM. cul-1 is required for cell cycle exit in C. elegans and identifies a novel gene family. Cell. 1996;85:829–839. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- Kwon YK, Lu W, Melamud E, Khanam N, Bognar A, Rabinowitz JD. A domino effect in antifolate drug action in Escherichia coli. Nat Chem Biol. 2008;4:602–608. doi: 10.1038/nchembio.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake RE, Freshney RI, Munir I. Steroid response in vivo and in vitro. In: Green B, Leake RE, editors. Steroid hormones: a practical approach. Oxford, England: IRL Press Limited; 1987. pp. 205–218. [Google Scholar]
- Lin SY, Lee WR, Su YF, Hsu SP, Lin HC, Ho PY, Hou TC, Chou YP, Kuo CT, Lee WS. Folic acid inhibits endothelial cell proliferation through activating the cSrc/ERK 2/NF-kappaB/p53 pathway mediated by folic acid receptor. Angiogenesis. 2012;15:671–683. doi: 10.1007/s10456-012-9289-6. [DOI] [PubMed] [Google Scholar]
- MacNeil LT, Watson E, Arda HE, Zhu LJ, Walhout AJ. Diet-induced developmental acceleration independent of TOR and insulin in C. elegans. Cell. 2013;153:240–252. doi: 10.1016/j.cell.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JI, Landen WO, Jr, Soliman AG, Eitenmiller RR. Application of a tri-enzyme extraction for total folate determination in foods. Journal - Association of Official Analytical Chemists. 1990;73:805–808. [PubMed] [Google Scholar]
- Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: translation of basic biology to cancer etiology and therapy. Cancer Metastasis Rev. 2007;26:111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- Michaelson D, Korta DZ, Capua Y, Hubbard EJ. Insulin signaling promotes germline proliferation in C. elegans. Development. 2010;137:671–680. doi: 10.1242/dev.042523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DZ, Sternberg PW, Inoue T. Morphologically defined sub-stages of C. elegans vulval development in the fourth larval stage. BMC developmental biology. 2015;15:26. doi: 10.1186/s12861-015-0076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhouser ML, Nijhout HF, Gregory JF, 3rd, Reed MC, James SJ, Liu A, Shane B, Ulrich CM. Mathematical modeling predicts the effect of folate deficiency and excess on cancer-related biomarkers. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20:1912–1917. doi: 10.1158/1055-9965.EPI-10-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper AS, Killian DJ, Hubbard EJ. Genetic analysis of Caenorhabditis elegans glp-1 mutants suggests receptor interaction or competition. Genetics. 2003;163:115–132. doi: 10.1093/genetics/163.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Poo-Prieto R, Haytowitz DB, Holden JM, Rogers G, Choumenkovitch SF, Jacques PF, Selhub J. Use of the affinity/HPLC method for quantitative estimation of folic acid in enriched cereal-grain products. The Journal of nutrition. 2006;136:3079–3083. doi: 10.1093/jn/136.12.3079. [DOI] [PubMed] [Google Scholar]
- Rao AU, Carta LK, Lesuisse E, Hamza I. Lack of heme synthesis in a free-living eukaryote. Proc Natl Acad Sci U S A. 2005;102:4270–4275. doi: 10.1073/pnas.0500877102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Amaretti A, Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011;3:118–134. doi: 10.3390/nu3010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux B, Walsh CT. p-Aminobenzoate synthesis in Escherichia coli: mutational analysis of three conserved amino acid residues of the amidotransferase PabA. Biochemistry. 1993;32:3763–3768. doi: 10.1021/bi00065a031. [DOI] [PubMed] [Google Scholar]
- Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. The journal of nutrition, health & aging. 2002;6:39–42. [PubMed] [Google Scholar]
- Selhub J, Ahmad O, Rosenberg IH. Preparation and use of affinity columns with bovine milk folate-binding protein (FBP) covalently linked to Sepharose 4B. Methods in enzymology. 1980;66:686–690. doi: 10.1016/0076-6879(80)66528-8. [DOI] [PubMed] [Google Scholar]
- Selhub J, Emmanouel D, Stavropoulos T, Arnold R. Renal folate absorption and the kidney folate binding protein. I. Urinary clearance studies. The American journal of physiology. 1987;252:F750–756. doi: 10.1152/ajprenal.1987.252.4.F750. [DOI] [PubMed] [Google Scholar]
- Shim YH, Chun JH, Lee EY, Paik YK. Role of cholesterol in germ-line development of Caenorhabditis elegans. Molecular reproduction and development. 2002;61:358–366. doi: 10.1002/mrd.10099. [DOI] [PubMed] [Google Scholar]
- Siu MK, Kong DS, Chan HY, Wong ES, Ip PP, Jiang L, Ngan HY, Le XF, Cheung AN. Paradoxical impact of two folate receptors, FRalpha and RFC, in ovarian cancer: effect on cell proliferation, invasion and clinical outcome. PloS one. 2012;7:e47201. doi: 10.1371/journal.pone.0047201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodji QH, Kornacki JR, McDonald JF, Mrksich M, Oyelere AK. Design and structure activity relationship of tumor-homing histone deacetylase inhibitors conjugated to folic and pteroic acids. Eur J Med Chem. 2015;96:340–359. doi: 10.1016/j.ejmech.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover P, Schirch V. Synthesis of (6S)-5-formyltetrahydropteroyl-polyglutamates and interconversion to other reduced pteroylpolyglutamate derivatives. Analytical biochemistry. 1992;202:82–88. doi: 10.1016/0003-2697(92)90210-x. [DOI] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 1988. pp. 587–606. [Google Scholar]
- Sybesma W, Starrenburg M, Tijsseling L, Hoefnagel MH, Hugenholtz J. Effects of cultivation conditions on folate production by lactic acid bacteria. Applied and environmental microbiology. 2003;69:4542–4548. doi: 10.1128/AEM.69.8.4542-4548.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Freeberg LE, Cornwell PE. Inhibition of EDTA of growth of Lactobacillus casei in the folate microbiological assay and its reversal by added manganese or iron. Clinical chemistry. 1990;36:1993. [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Virk B, Correia G, Dixon DP, Feyst I, Jia J, Oberleitner N, Briggs Z, Hodge E, Edwards R, Ward J, et al. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC biology. 2012;10:67. doi: 10.1186/1741-7007-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk B, Jia J, Maynard CA, Raimundo A, Lefebvre J, Richards SA, Chetina N, Liang Y, Helliwell N, Cipinska M, et al. Folate Acts in E. coli to Accelerate C. elegans Aging Independently of Bacterial Biosynthesis. Cell Rep. 2016;14:1611–1620. doi: 10.1016/j.celrep.2016.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, Caudy AA, Walhout AJ. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014;156:759–770. doi: 10.1016/j.cell.2014.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann M, Raith L, Wirtz A, Karl HJ. Trennung von freien und proteingebundenen Steroiden mit Amberlite. Z Anal Chem. 1972;261:382–385. [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Banerjee D, Kuhn JR. Isolation and culture of larval cells from C. elegans. PloS one. 2011;6:e19505. doi: 10.1371/journal.pone.0019505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Huang GW, Tian ZH, Ren DL, Wilson JX. Folate stimulates ERK1/2 phosphorylation and cell proliferation in fetal neural stem cells. Nutritional neuroscience. 2009;12:226–232. doi: 10.1179/147683009X423418. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert reviews in molecular medicine. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.