Abstract
Influence of various structural patterns in a series of novel bi- and tricyclic N-heterocycles on the activity against Leishmania major and Leishmania panamensis has been studied and compounds that are active in the low micromolar region have been identified. Both quinolines and tetrahydrooxazinoindoles (TOI) proved to have significant antileishmanial activities, while substituted indoles were inactive. We have also showed that a chloroquine analogue induces Leishmania killing by modulating macrophage activation.
Introduction
Leishmaniasis is a tropical disease with a significant global health burden that is caused by Leishmania flagellate protozoa.1 Twenty Leishmania species that are pathogenic for humans have been identified. They are transmitted by several sand flies species, Phlebotomus in the Old World and Lutzomya in the New World.2 Leishmaniasis has a wide spectrum of clinical manifestations depending on the Leishmania species and the immunological status of the host. These include localized and diffused cutaneous leishmaniasis, mucocutaneous form and visceral disease.3
Leishmania species differ in virulence, vectors preferences and geographic distribution. However, all species have a similar life cycle involving a motile, flagellated stage in the midgut of vector (promastigote) and an intracellular non-motile stage (amastigote) in host macrophages.4 Macrophages are the most important effector cells in Leishmania infection, and their appropriate activation is required to eliminate the parasite. The destruction of the parasite by macrophages depends on the production of nitric oxide (NO), tumor necrosis factor (TNF), interleukin (IL)-1 among other mediators, and is negatively affected by a variety of factors including IL-10.5
Current treatments for leishmaniasis are based in drugs whose specific mechanisms of action are poorly understood. The most used drugs (e.g. pentavalent antimonials, pentamidine, amphotericin B and miltefosine) require lengthy treatments and have high toxicity and serious side effects.6 Furthermore, resistance to some of these drugs has been reported for a diversity of Leishmania strains.7 Consequently, the search for new drugs for the treatment of the disease that carries a multitude of health and socioeconomic problems in endemic countries is an enduring challenge.
Several families of compounds have been tested for antileishmania activity.8 Both, natural products and synthetic compounds have been recently identified as promising leads against leishmaniasis. Particularly promising scaffolds include quinolines and indoles. Antiparasitic,9 antibacterial,10 antineoplastic,11 and antiviral12 activities have been reported for quinoline derivatives. For instance, naphthylisoquinoline alkaloids showed low micromolar activities against Leishmania donovani,13 as well as against intracellular amastigote stage of Leishmania major.14 Similarly, the naturally-occurring hypocrellin A was found to be more active against L. donovani in vitro than amphotericin B and pentamidine.15 Synthetic antileishmanial 1,4-anthraquinones have also been described.16 Recently, abietane-type diterpenoids have emerged as potent antileishmanial agents.17
In this study we evaluated the effects on intracellular amastigotes and promastigotes of L. panamensis and L. major of three families of bi- and tricyclic N-heterocycles: tetrahydrooxazinoindoles (TOIs) 1, quinolines 2, and indoles 3 (Fig. 1). Since quinolines, e.g. amodiaquine, chloroquine, mefloquine, and primaquine have been successfully used as antimalarials, they may hold promise as a new class of antileishmanial agents. Indeed, amodiaquine and its basic side chain-modified analogues have been found to have a significant antileishmanial activity.18 In addition, 7-chloro-4-quinolinyl hydrazones have shown strong activity against the intracellular parasite.19 We have recently developed an efficient method of synthesis of 2-susbtituted quinolines from quinoline N-oxides that allows for a simple access to various substituted quinolines, including 2-substituted derivatives of amodiaquine and chloroquine.20 Using this method, we have prepared a series of quinolines bearing substituents in 2, 4, 5, 7 and 8 positions, including novel amodiaquine and chloroquine analogues. The 1,2-oxazine moiety in tetrahydrooxazinoindoles (TOIs) 1 resembles pyridine in amodiaquine and chloroquine. In addition to structural similarity, quinolines and 1,2-oxazines both contain a weekly basic nitrogen atom that may be important for the antileishmanial activity. Hence, it was of interest to compare these two classes of basic N-heterocycles. Our recently described method of tetrahydrooxazinoindoles (TOIs) synthesis offered a facile entry to the novel 1,2-oxazine-containing framework in racemic and enantioselective fashion.21 Since TOI framework contains an indole moiety, a series of substituted indoles were also prepared, and their antileishmanial activities have been compared with the quinolines and TOIs.
Fig. 1.
Structures of studied N-heterocycles.
Our results show that most of the tested compounds are more active against intracellular amastigotes than on promastigotes of both Leishmania species assayed. L. panamensis amastigotes appear to be more sensitive to our active compounds than L. major amastigotes. Interestingly we also found that one of the compounds inhibited the production of IL-10 by macrophages infected with either L. panamensis or L. major.
Further, we describe herein that some TOIs are competent antileishmanial agents, and their activity is related to the presence of the 1,2-oxazine moiety. This result, along with the data on the antileishmanial activity of several substituted quinolines, provide important insights into the antileishmanial activity of N-heterocycles and point to the potential of 1,2-oxazines22 as new structural frameworks for biomolecular applications.23
Results and Discussion
Preparation of the compounds used in the antileishmanial tests
The tetrahydrooxazinoindole compounds 4 bearing substituents in positions 3, 4, 4a, 5, 6, and 9 were accessed using the inverse electron demand [4+2] cycloaddition reaction of indoles 5 with transient nitrosoalkenes that were generated in situ from α-chlorooximes 6 (Scheme 1).21 The indoles 5 were prepared by means of N-alkylation and a reductive C3-alkylation (See Supporting Information for details).
Scheme 1.
Structure and preparation of tetrahydrooxazinoindoles (TOIs).
The enantiomerically enriched TOIs were prepared using Cu(DM-Binap)OTf as a catalyst, while racemic TOIs 4 were prepared using Cu(rac-Binap)OTf as a catalyst, in the presence of silver carbonate as a chloride scavenger and a base. The TOI products were obtained in good to excellent yields. The 4-chloro-substituted TOIs were isolated as single diastereomers, in line with the previously observed results.21
X-ray crystal structures were elucidated for compounds 7–11 (Fig. 2), aiding in the confirmation of the structure of the TOI products and their precursors. Interestingly, although TOI compounds 9 and 10 were prepared with >90% ee, only a small amount of racemic crystals was obtained, indicating that the racemate is less soluble than both enantiomers, as previously observed for other scalemic mixtures.24 The indoles, quinolines and TOIs were selected based on the combination of ready synthetic availability and structural diversity.
Fig. 2.
Single crystal X-ray crystallographic structures of compounds 7–11.
Screening of compounds against L. major and L. panamensis promastigotes
Libraries of TOIs, indoles and quinolines were tested against L. major and L. panamensis extracellular promastigotes. To evaluate the effect of compounds on promastigotes of both Leishmania species we performed a first screening at a fixed concentration of 10 μM. Any compound inducing a growth inhibition of 50% or more, was further tested using a four concentration points including 1, 3, 10 and 30 μM. Viability of promastigotes was assessed by using an ATP-bioluminescence assay after 24 hours of incubation with the compound. 25 Our results showed that none of the tested compounds were active for L. major promastigotes (Tables 1 and 2), whereas four compounds from the TOI library (10% of the total number of the tested compounds) were effective in the killing of L. panamensis promastigotes, with IC50 values ranging from 8 to 12 μM (Table 2). Several compounds, e.g. 11–14, showed some toxicity to uninfected macrophages.
Table 1.
Antileishmanial activity of select quinoline derivatives.a
| Compound | Structure | IC50 (μM) ± SD
|
Macrophage cytotoxicity CC50 (μM) | SI (L. major/L. panamensis)b | |||
|---|---|---|---|---|---|---|---|
| Intracellular L. major amastigotes | Intracellular L. panamensis amastigotes | L. major promastigotes | L. panamensis promastigotes | ||||
| 12 |

|
1.65±0.3 | 1.07±0.51 | NA | NA | 14.03±2.65 | 8.5/13.1 |
| 15 |

|
5.19±2.33 | 3.29±2.72 | NA | NA | >30 | 9.7/15.3 |
NA, non-active. IC50 and CC50 values are mean ± standard deviation (SD) of two independent experiments. The control drug was amphotericin B, with an IC50 value for intracellular amastigotes of 0.103 μM for L. panamensis and 0.157 μM for L. major. The IC50 of amphotericin B for promastigotes was 0.1 μM for L. panamensis and 0.243 μM for L. major.
The selectivity index (SI) is calculated as the ratio between CC50 on peritoneal macrophages and IC50 on intracellular L. major or L. panamensis amastigotes. SI for amphotericin B is 1723.3 for L. panamensis and 1130.5 for L. major.
Table 2.
Antileishmanial activity of select tetrahydrooxazinoindoles (TOIs).a

| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | R4 | R5 | IC50 (μM) ± SD
|
|||||
| Intracellular L. major amastigotes | Intracellular L. panamensis amastigotes | L. major promastigotes | L. panamensis promastigotes | Macrophage cytotoxicity CC50 (μM) | SI (L. major/L. panamensis)b | ||||||
| 9 | H |

|
Me | Ph | H | 12.2±0.19 | 0.8±0.49 | NA | NA | >30 | 5.96/90.9 |
| 11 | H | All | All | Ph | H | NA | 1.95±0.99 | NA | 11.60±0.20 | 28.48±2.19 | NA/14.6 |
| 13 | H | Bn | iPr | Ph | H | 3.84±2.5 | 0.87±0.14 | NA | NA | 9.15±1.24 | 2.38/10.5 |
| 14 | H |

|
iPr | Ph | H | NA | 0.35±0.18 | NA | 8.28±0.83 | 30 | NA/85.7 |
| 16 | H | All | cPent | Ph | H | 4.30±1.73 | 1.22±0.63 | NA | 12.53±0.54 | >30 | 9.57/33.7 |
| 17 | H | All | All | Ph | Cl | 1.92±1.81 | NA | NA | NA | >30 | 29.3/NA |
| 18 | H | All | All | 2-Naphth | H | 6.13±0.05 | NA | NA | NA | >30 | 12.2/NA |
| 19 | H | All | All | p-MeOPh | Cl | 5.57±0.53 | NA | NA | NA | >30 | 13.3/NA |
| 20 | H |

|
iPr | Ph | H | NA | 1.13±0.21 | NA | 11.51±0.32 | >30 | NA/47.0 |
NA, non-active. IC50 and CC50 values are mean ± standard deviation (SD) of two independent experiments. The control drug was amphotericin B, with an IC50 value for intracellular amastigotes of 0.103 μM for L. panamensis and 0.157 μM for L. major. The IC50 of amphotericin B for promastigotes was 0.1 μM for L. panamensis and 0.243 μM for L. major.
The selectivity index (SI) is calculated by the ratio between CC50 on peritoneal macrophages and IC50 on intracellular L. major or L. panamensis amastigotes. SI for amphotericin B is 1723.3 for L. panamensis and 1130.5 for L. major.
Antileishmanial activity of compounds on intracellular amastigotes
The evaluation of the effect of compounds against L. major and L. panamensis intracellular amastigotes was performed by using the Giemsa staining method.26 As described above for promastigotes, a first screening was performed at a compound concentration of 10 μM. Accordingly, active compounds were subjected to a four-point dose response evaluation. Active compounds and their IC50 values are presented in Table 1 (quinolines 12 and 15) and Table 2 (TOI compounds 9, 11, 13, 14, 16–20).
Compounds from the quinoline family showed similar effect on both Leishmania species. Only two quinoline derivatives (10 % of the compounds tested) were active and exhibited similar IC50 values for L. major and L. panamensis (Table 1 and Fig S2). Compound 12 exhibited cytotoxic effects on macrophages with a 50% cytotoxic concentration (CC50) of 14.03 μM. However, that cytotoxic concentration is still tenfold higher than the IC50 calculated for L. panamensis and L. major (1.07±0.51 and 1.65±0.3 respectively). Both quinoline derivatives, however, have similar values of selectivity index (Table 1).
Compounds from TOIs family showed differential activity against both Leishmania species. Compounds 17, 18 and 19 were exclusively active against intracellular L. major whereas compounds 11, 14, and 20 showed effect only for L. panamensis, – both promastigotes and amastigotes (Table 2). These differences in sensitivity to some compounds were previously described for Leishmania species.27 Compounds 9, 13 and 16 were active for amastigotes of both species but the effect on L. panamensis was higher (IC50= 0.8, 1.22, 0.87 μM respectively) than on L. major (IC50= 12.27, 4.30, 3.84 μM) (Table 2). It is important to note that SI values are consistently higher for L. panamensis than for L. major (Tables 1 and 2), suggesting that L. panamensis is more sensitive to both families of compounds than L. major. Compounds of the indole series showed no activity against any Leishmania species or stage tested (Table S1).
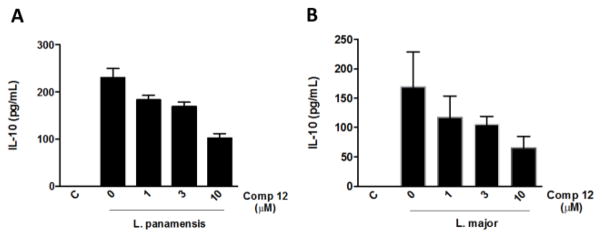
Considering the specificity of the most active compounds for the amastigote form, we evaluated the possible immunomodulatory effect of compounds 9, 12, 13, 15, and 17–19. Our results showed that compound 12 inhibited production of IL-10 by macrophages infected with L. panamensis and L. major (Fig. 3) in a dose dependent manner. These results suggest that the compound-induced parasite killing mechanism may include a regulation of the macrophage activation.
Fig. 3.
Compound 12 inhibits the production of IL-10 from Leishmania-infected macrophages. Peritoneal macrophages from Balb/c mice were infected with L. panamensis (A) or L. major (B) and treated with compound 12. Supernatants were collected after 24 hours of the stimulus and levels of IL-10 were measured by Elisa. Data represent mean ± SEM from stimuli performed in duplicates and are representative of two independent experiments.
Discussion
Leishmaniasis is recognized as one of the most neglected diseases, and the development of new drugs against leishmaniasis is an important therapeutic goal.28
There is evidence of recent appearance of Leishmania resistance to antimonials – the current first line of treatment.7b,d Molecular and biochemical differences among species influence the sensitivity of Leishmania species to different chemical agents, complicating the search for antileishmanial drugs with broad activity profile. Here, we studied synthetic compounds of three classes and identified positive hits that inhibit the intracellular amastigotes and promastigotes of L. panamensis and L. major. We also showed that L. panamensis is more sensitive than L. major to these compounds. Quinoline compounds were previously identified as efficient antimalarial and antileishmanial agents.18 Several quinoline derivatives showed inhibitory capacity against different Leishmania species that is comparable to reference drugs.8 In view of the lack of antileishmanial activity of substituted indoles, we further focused on the study of the influence of substituents in the oxazine ring of the TOI compounds. In the TOI series, presence of the bulky aromatic rings in the N9 and C4a positions generally led to the loss of antileishmanial activity. Allyl groups in N9 and C4a resulted in higher activities than both larger benzylic and smaller (methyl) groups. On the other hand, both aromatic substituents and ester groups in C3 were well tolerated. Displacement of the O1 with TsN (S14, see Table S2) led to the loss of activity, further highlighting the importance of substitution in the C ring of the TOI system.
The C4 position in 1,2-oxazines is generally difficult to access synthetically. Hence, influence of the substituents in C4 position was tested with TOI compounds bearing a chlorine atom anti to the C4a substituent. Interestingly, compounds 17 and 19 were found to be active only against intracellular L. major amastigotes, indicating that significant selectivity can be achieved through modulation of the 1,2-oxazine moiety.
In general, TOI framework has provided more hits than quinolines. In the quinoline series, only two compounds (12 and 15) that are structurally related to amodiaquine and chloroquine exhibited significant activity against intracellular L. major and L. panamensis amastigotes, with no activity against promastigotes (Table 1).
It has been known that there are important differences in the sensitivity towards chemical agents between both parasite stages, and between different Leishmania species.25,29 We showed herein that, of the 39 assayed compounds, none was active against L. major promastigotes, while 20% of the compounds were active against L. major intracellular amastigotes. Promastigotes differ biologically from amastigotes in metabolism, morphology and surface composition and these differences have an impact on the sensitivity of parasites to chemical agents.30 Moreover, to be active against amastigotes, compounds must cross the cellular membrane and maintain its stability in the intracellular environment. Additionally, some compounds may be toxic to the parasite only when metabolized inside the macrophage, then showing the behavior of being inactive in promastigotes and active in the amastigote. Another interesting possibility may be that some of the compounds, instead of being toxic directly to the parasite, may be able to activate the macrophage to fully develop their antiparasitic activity. We also showed here that compound 12 inhibits the production of IL-10 by macrophages infected with L. panamensis and L. major. It is well-known that IL-10 is an antiinflammatory mediator that inhibits a variety of macrophage functions including phagocytosis, expression of co-stimulatory molecules and production of pro-inflammatory cytokines, with important consequences in macrophage activation.31 Interleukin 10 has been implicated as a key factor in the survival of Leishmania infection both in vitro and in vivo. High levels of IL-10 have been linked to leishmaniasis progression and parasite persistence.32 The addition of IL-10 to L. major-infected macrophages results in uncontrolled parasite replication.32a Our results suggest that the antileishmanial activity of compound 12 might be, at least in part, mediated by the modulation of the macrophage activation. The selectivity index of this chloroquine analogue was 3 to 4 times higher (8.5/13.1 L. major/L. panamensis) than the SI of chloroquine (3.1) previously reported under similar experimental conditions33 Since chloroquine is an approved drug, these SI values suggest that compound 12 is a promising candidate for further therapeutic investigation.
We have shown here that novel synthetic derivatives of several families of compounds are promising antileishmanial hits, opening new possibilities for further development of 1,2-oxazine-based antileishmanial agents as a way towards new and effective drugs against this neglected disease. Due to the differences in Leishmania species sensitivity to drugs, the search of species-specific antileishmanial drugs has been encouraged. The higher values of SI for L. panamensis than for L. major for the active compounds described herein indicate that they are good leads in the development of L. panamensis-specific drugs.
Conclusions
In conclusion, a targeted library of tetrahydrooxazinoindoles (TOIs) was synthesized, and antileishmanial activities were discovered for a number of the TOI compounds. The activity was compared with indoles that were found to be inactive, and with quinolines. For quinolines, only amodiaquine and chloroquine analogues were found to be active. We have identified that the antileishmanial activity of the chloroquine analogue 12 may also be due to a modulation of macrophage activation. The activity of TOI compounds opens an avenue for the search of structurally novel antileishmanial agents and for further elucidation of their mechanism of action.
Experimental
Materials and methods
Dichloromethane was dried and purified under an argon atmosphere using an LC technology Solutions’ SP-1 Solvent Purifier All oximes were synthesized according to the literature procedure.21 All heterocyclic N-oxides were synthesized according to reported procedures.20b N′-(2-chloro-1-phenylethylidene)-4-methylbenzenesulfonohydrazide was synthesized according to literature procedure.34 All other reagents were purchased and used without further purification. Column chromatography was performed using CombiFlash Rf-200 (Teledyne-Isco) automated flash chromatography system. 1H, 13C, 19F NMR spectra were recorded at 500 (1H), 125 (13C), and 282 MHz (19F) on Varian Mercury VX 300 and Agilent Inova 500 instruments in CDCl3 solutions. Chemical shifts (δ) are reported in parts per million (ppm) from the residual solvent peak and coupling constants (J) in Hz. Proton multiplicity is assigned using the following abbreviations: singlet (s), doublet (d), triplet (t), quartet (quart.), quintet (quint.), septet (sept.), multiplet (m), broad (br). Infrared measurements were carried out neat on a Brüker Vector 22 FT-IR spectrometer fitted with a Specac diamond attenuated total reflectance (ATR) module.
General procedure for the synthesis of 1,2-oxazines
To an oven dried flask was added 3Å MS (5 scoops), CuOTf ½ PhMe (10–20 mol%), rac-BINAP or (S)-DM-BINAP (10–20 mol%) and dichloromethane (0.1–0.2M). The reaction was stirred for 15 min and then cooled to –78 °C under argon. Indole (1 equiv.), oxime (1 equiv.) and silver carbonate (3 equiv.) were added sequentially. The reaction was allowed to warm to either −20 or −15 °C and was stirred for the specified time. The reaction mixtures were then filtered, concentrated under reduced pressure, and purified by column chromatography [hexanes/EtOAc, silica gel] to yield the desired products.
Mice
Female and male Balb/c mice, 8 weeks of age, were provided by INDICASAT’s animal facility. Animals were maintained with 12 hours light/dark cycle, at a constant temperature of 24 °C with free access to food and water. All experimental procedures were approved by the Institutional Animal Care and Use Committee of INDICASAT (IACUC-14-002) and were based in the strict observance of the ethic guidelines related to the handling of lab animals in accordance with international regulations and those established by INDICASAT.
Parasites
Promastigotes of L. panamensis (MHOM/PA/94/PSCI-1) and L. major (Restrepo et al., 2013) were cultured at 25°C, in Schneider medium (Sigma) supplemented with 20% FBS (Gibco). Parasite virulence for both strains was maintained by inoculating them previously in hamster.
Promastigote Inhibition Assay
L. panamensis and L. major parasites from stationary phase culture were washed with PBS 1X and centrifuged at 1700xg for 10 minutes. Parasites were diluted in Schneider media supplemented with 20 % FBS and seeded in 96 well white opaque plate (Thermo Scientific, Nunc) at a density of 2×106 parasites per well in a volume of 99 μL. Each well was treated with 1 μL of compound at a concentration of 10 μM in screening assays and later at 1, 3, 10 and 30 μM in dose-response assays. The parasites were incubated at 25°C during 24 hours. After incubation period, 50 μL of CellTiter-Glo® reagent (Promega) was added to each well for lysing the parasites and the plate was incubated at room temperature for 10 minutes to stabilize the luminescent signal. The resulting ATP was recorded in relative-light units (RLU) in a multi-detection microplate reader (Synergy HT-Biotek).
Amastigote Inhibition Assay
Peritoneal resident macrophages from Balb/c mice were collected by peritoneal lavage with cold PBS 1X (AppliChem). Cells were seeded in RPMI (Gibco) with 10% FBS (Gibco) at a density of 1×106 cells per well in 24 well plates with a round glass coverslip in each well and cultured for 2 h at 37°C in an atmosphere of 5% CO2. Non-adherent cells were removed by washing and adherent macrophages were infected with late stationary phase promastigotes at 1:30 ratio (cell:parasite) for L. panamensis and 1:10 for L. major during 1 hour at 37°C, 5% CO2. Non-internalized promastigotes were removed by washing with RPMI media. Infected macrophages were treated with the compounds at a final concentration of 10 μM. Dose-response curves were produced for active compounds using concentrations of 1, 3, 10 and 30 μM. Amphotericin B (Sigma) was used as positive control. Infected macrophages were incubated for 24 hours at 37°C in 5% CO2. All negative controls and stimulus were performed in the presence of 0.1% DMSO (Sigma) since compounds are solubilized in this solvent. After incubation, supernatants were collected for evaluation of the presence of IL-10 and coverslips were washed once with PBS, fixed with Methanol (Merck), and stained with Giemsa (Sigma). The infection rate was calculated by counting the number of amastigotes per cell in a total of 250 cells. The percentage of parasite inhibition was calculated as
Macrophage cytotoxicity assay
Peritoneal resident macrophages from Balb/c mice were cultured at 37°C in 5% CO2 in the presence of 3, 10, 30 and 100 μM of active compounds. Twenty-four hours later incubation supernatants were removed, then 100 μl of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma) (0.5 mg/mL) dissolved in RPMI were added to each well and cells were incubated by 4 hours at 37°C. The MTT is reduced in living cells mitochondria to purple formazan crystals. The supernatants were discarded and formazan crystals were dissolved in 100 μl of 0.04 M HCl in isopropanol. The optical density was analyzed at 570 nm using an ELISA plate reader. The percentage of viable cells was calculated as % viability = (OD sample/OD control) × 100%. All experimental cells were cultured in the presence of medium plus 10% FCS and 0.1% DMSO.
Statistical Analysis
Results were analyzed using the GraphPad Prism 5 statistical software package (GraphPad software, La Jolla). Half maximal inhibitory concentrations (IC50 and CC50) were calculated adjusting a sigmoidal dose-response curve following GraphPad Prism 5 procedure.
Supplementary Material
Acknowledgments
Financial support by the Welch Foundation (AX-1788), National Institute of the General Medical Sciences (SC3GM105579), the University of Texas at San Antonio, the National Science Foundation (CHE-1455061), and the Sistema Nacional de Investigacion, Republic of Panama (SNI-34-2014) is gratefully acknowledged. Mass spectroscopic analysis was supported by a grant from the National Institute on Minority Health and Health Disparities (G12MD007591). We thank Hector Cruz and Kissy de Gracias (INDICASAT) for their help with the intracellular amastigotes experiments.
Footnotes
Electronic Supplementary Information (ESI) available: Experimental procedures and spectroscopic data. See DOI: 10.1039/b000000x/
Contributor Information
Oleg V. Larionov, Email: oleg.larionov@utsa.edu.
Patricia L. Fernández, Email: pllanes@indicasat.org.pa.
References
- 1.Read A, Hurwitz I, Durvasula R. In: Dynamic Models of Infectious Diseases, Vol. 1: Vector-Borne Diseases. Rao Vadrevu Sree Hari, Durvasula Ravi., editors. [Google Scholar]
- 2.(a) Killick-Kendrick R. Med Vet Entomol. 1990;4:1. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]; (b) Miranda A, Carrasco R, Paz H, Pascale JM, Samudio F, Saldaña A, Santamaria G, Mendoza Y, Calzada JE. Am J Trop Med Hyg. 2009;81:565. doi: 10.4269/ajtmh.2009.08-0265. [DOI] [PubMed] [Google Scholar]
- 3.(a) Silveira FT, Lainson R, Corbett CE. Mem Inst Oswaldo Cruz. 2004;99:239. doi: 10.1590/s0074-02762004000300001. [DOI] [PubMed] [Google Scholar]; (b) Gelanew T, Hurissa Z, Diro E, Kassahun A, Kuhls K, Schönian G, Hailu A. Am J Trop Med Hyg. 2011;84:906. doi: 10.4269/ajtmh.2011.11-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volf P, Hostomska J, Rohousova I. Parasite. 2008;15:237. doi: 10.1051/parasite/2008153237. [DOI] [PubMed] [Google Scholar]
- 5.(a) Santos JL, Andrade AA, Dias AAM, Bonjardim CA, Reis LFL, Teixeira SMR, Horta MF. J Interferon Cytokine Res. 2006;26:682. doi: 10.1089/jir.2006.26.682. [DOI] [PubMed] [Google Scholar]; (b) Liu D, Uzonna JE. Frontiers Cel Infect Microbiol. 2012;2:682. doi: 10.3389/fcimb.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochia MC, Andrade CA. Acta Tropica. 2011;118:87. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 7.(a) Sundar S. Trp Med Int Health. 2001;6:849. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]; (b) Romero GA, Guerra MV, Paes MG, Macedo VO. Am J Trop Med Hyg. 2001;65:456. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]; (c) Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verástequi C, Lazo M, Loayza-Muro R, De Doncker S, Maurer A, Chappuis F, Dujardin JC, Llanos-Cuentas A. Infect Dis. 2007;195:1846. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]; (d) Palacios R, Osorio LE, Grajalew LF, Ochoa MT. Am J Trop Med Hyg. 2001;64:187. doi: 10.4269/ajtmh.2001.64.187. [DOI] [PubMed] [Google Scholar]; (e) Oliveira LF, Schubach AO, Martins MM, Passos SL, Oliveira RV, Marzochia MC, Andrade CA. Acta Tropica. 2011;118:87. doi: 10.1016/j.actatropica.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Liñares GE, Ravaschino EL, Rodriguez JB. Curr Med Chem. 2006;13:335. doi: 10.2174/092986706775476043. [DOI] [PubMed] [Google Scholar]
- 9.(a) Achan J, Talisuna AO, Erhart A, Yeka A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D’Alessandro U. Malar J. 2011;10:144. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bompart D, Núñez-Durán J, Rodríguez D, Kouznetsov VV, Meléndez Gómez CM, Sojo F, Arvelo F, Visbal G, Alvarez A, Serrano-Martín X, García-Marchán Y. Bioorg Med Chem. 2013;21:4426. doi: 10.1016/j.bmc.2013.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Metwally KA, Abdel-Aziz LM, Lashine e-SM, Husseiny MI, Badawy RH. Bioorg Med Chem. 2006;14:8675. doi: 10.1016/j.bmc.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Sissi C, Palumbo M. Curr Med Chem Anticancer Agents. 2003;3:439. doi: 10.2174/1568011033482279. [DOI] [PubMed] [Google Scholar]
- 12.Bedoya LM, Abad MJ, Calonge E, Saavedra LA, Gutierrez CM, Kouznetsov VV, Alcami J, Bermejo P. Antiviral Res. 2010;87:338. doi: 10.1016/j.antiviral.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Bringmann G, Dreyer M, Faber JH, Dalsgaard PW, Stærk D, Jaroszewski J, Ndangalasi H, Mbago F, Brun P, Reichert M, Maksimenka K, Christensen SB. J Nat Prod. 2003;66:1159. doi: 10.1021/np030077b. [DOI] [PubMed] [Google Scholar]
- 14.Ponte-Sucre A, Faber JH, Gulder T, Kajahn I, Pedersen SEH, Schultheis M, Bringmann G, Moll H. Antimicrob Agents Chemother. 2007;51:188. doi: 10.1128/AAC.00936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrob Agents Chemother. 2004;8:4450. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolognesi ML, Lizzi F, Perozzo R, Brun R, Cavalli A. Bioorg Med Chem Lett. 2008;18:2272. doi: 10.1016/j.bmcl.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Pirttimaa M, Nasereddin A, Kopelyanskiy D, Kaiser M, Yli-Kauhaluoma J, Oksman-Caldentey K-M, Brun R, Jaffe CL, Moreira VM, Alakurtti S. J Nat Prod. 2016;79:362. doi: 10.1021/acs.jnatprod.5b00990. [DOI] [PubMed] [Google Scholar]
- 18.Guglielmo S, Bertinaria M, Rolando B, Crosetti M, Fruttero R, Yardley V, Croft SL, Gasco A. Eur J Med Chem. 2009;44:5071. doi: 10.1016/j.ejmech.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Coimbra ES, Antinarelli LMR, da Silva AD, Bispo MLF, Kaiser CR, de Souza MVN. Chem Biol Drug Des. 2013;81:658. doi: 10.1111/cbdd.12112. [DOI] [PubMed] [Google Scholar]
- 20.Larionov OV, Stephens D, Mfuh A, Chavez G. Org Lett. 2014;16:864. doi: 10.1021/ol403631k.For preparation of N-oxides, see: Larionov OV, Stephens D, Chavez G, Mfuh A, Arman H, Naumova A, Skenderi B. Org Biomol Chem. 2014;12:3026. doi: 10.1039/c4ob00115j.
- 21.Zhang Y, Stephens D, Hernandez G, Mendoza R, Larionov OV. Chem Eur J. 2012;52:16612. doi: 10.1002/chem.201203435. [DOI] [PubMed] [Google Scholar]
- 22.For a review on the applications of other nitroxy compounds, e.g. N-oxides in medicinal and biomolecular chemistry, see: Mfuh AM, Larionov OV. Curr Med Chem. 2015;22:2819. doi: 10.2174/0929867322666150619104007.
- 23.For naturally occurring bioactive 1,2-oxazines, see: Stipanovic RD, Howell CR. J Antibiot. 1982;35:1326. doi: 10.7164/antibiotics.35.1326.Garo E, Starks CM, Jensen PR, Fenical W, Lobkovsky E, Clardy J. J Nat Prod. 2003;66:423. doi: 10.1021/np0204390.Li DL, Chen YC, Tao MH, Li HH, Zhang WM. Helv Chim Acta. 2012;95:805.Mfuh AM, Zhang Y, Stephens DE, Arman HD, Larionov OV. J Am Chem Soc. 2015;137:8050. doi: 10.1021/jacs.5b05205.
- 24.Belokon YN, Bespalova NB, Churkina TD, Cisarova I, Ezernitskaya MG, Harutyunyan SR, Hrdina R, Kagan HB, Kocovsky P, Kochetkov KA, Larionov OV, Lyssenko KA, North M, Polasek M, Peregudov AS, Prisyazhnyuk VV, Vyskocil S. J Am Chem Soc. 2003;125:12860–12871. doi: 10.1021/ja035465e. [DOI] [PubMed] [Google Scholar]
- 25.De Muylder G, Ang KK, Chen S, Arkin MR, Engel JS, McKerrow JH. PloS Negl Trop Dis. 2011;5:1253. doi: 10.1371/journal.pntd.0001253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neal RA, Croft SL. J Antimicrob Chemother. 1984;14:463. doi: 10.1093/jac/14.5.463. [DOI] [PubMed] [Google Scholar]
- 27.Croft SL, Yardley V, Kendrick H. Trans R Soc Trop Med Hyg. 2002;96(Suppl 1):S127. doi: 10.1016/s0035-9203(02)90063-5. [DOI] [PubMed] [Google Scholar]
- 28.Matlashewski G, Arana B, Kroeger A, Be-Nazir A, Mondal D, Nabi S, Banjara M, Das M, Marasini B, Das P, Medley G, Satoskar A, Nakhasi H, Argaw D, Reeder J, Olliaro P. Lancet Global Health. 2014;2:683. doi: 10.1016/S2214-109X(14)70318-3. [DOI] [PubMed] [Google Scholar]
- 29.Siqueira-Neto JL, Song O-R, Oh H, Sohn J-H, Yang G, Nam J, Jan J, Cechetto J, Lee CB, Moon S, Genovesio A, Chatelain E, Christophe T, Freitas-Junior LH. PloS Negl Trop Dis. 2010;4:675. doi: 10.1371/journal.pntd.0000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeersch M, da Luz RI, Tote K, Timmermans JP, Cos P, Maes L. Antimicrob Agents Chemother. 2009;53:3855. doi: 10.1128/AAC.00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore KW, de R, Malefyt W, Coffman RL, O’Garra A. Annu Rev Immunol. 2001;19:683. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 32.(a) Kane MM, Mosser DM. J Immunol. 2001;166:1141. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]; (b) Schwarz T, Remer KA, Nahrendorf W, Masic A, Siewe L, Müller W, Roers A, Moll H. PLoS Pathog. 2013;9:e1003476. doi: 10.1371/journal.ppat.1003476. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. J Exp Med. 2001;194:1497. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vale-Costa S, Costa-Gouveia J, Pérez B, Silva T, Teixeira C, Gomes P, Gomes MS. Antimicrob Agents Chemother. 2013;57:5112. doi: 10.1128/AAC.00557-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hatcher JM, Coltart DM. J Am Chem Soc. 2010;132:4546. doi: 10.1021/ja100932q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.