Abstract
Purpose of review
Catastrophic antiphospholipid syndrome (CAPS) is a severe manifestation of APS. While affecting only 1% of patients with APS, the condition is frequently fatal if not recognized and treated early. Here, we will review the current approach to diagnosis and treatment of CAPS.
Recent findings
Data from the international “CAPS registry,” spearheaded by the European Forum on Antiphospholipid Antibodies, have improved our understanding of at-risk patients, typical clinical features, and associated/precipitating diagnoses. Current guidelines also continue to support a role for anticoagulants and glucocorticoids as foundation therapy in all patients. Finally, new basic science and case series suggest that novel therapies, such as rituximab and eculizumab warrant further study.
Summary
Attention to associated diagnoses such as infection and systemic lupus erythematosus (SLE) are critical at the time of diagnosis. All patients should be treated with anticoagulation, corticosteroids, and possibly plasma exchange. In patients with SLE, cyclophosphamide should also be considered. In refractory or relapsing cases, new therapies such as rituximab and possibly eculizumab may be options, but need further study.
Keywords: Antiphospholipid syndrome, Catastrophic antiphospholipid syndrome (CAPS), Lupus anticoagulant, Thrombosis, Microangiopathic hemolytic anemia (MAHA)
INTRODUCTION
Antiphospholipid syndrome (APS) is an autoimmune disease characterized by the cardinal manifestations of thrombosis and pregnancy loss [1]. At the same time, the systemic nature of APS is highlighted by other associated features include cytopenias, cognitive dysfunction, cardiac valve disease, renal impairment, and skin ulcers [2]**. Patients with APS have circulating antiphospholipid antibodies, as measured by assays for anticardiolipin IgG or IgM; anti-beta-2 glycoprotein I (β2GPI) IgG or IgM; or lupus anticoagulant [1]; at least one of these tests should be durably positive to classify a patient as having APS. The cause of APS is unknown, although it is well established that about half of diagnoses will occur in the background of systemic lupus erythematosus (SLE) [3]; the remaining cases are designated as “primary APS.” Although APS is clearly an autoimmune disorder, it has no proven immunomodulatory treatment. Rather, day-to-day therapy focuses on managing downstream morbidity with medications such as heparin and warfarin [4]*.
A rare, life-threatening presentation of APS has been coined catastrophic antiphospholipid syndrome, or CAPS. CAPS is characterized by simultaneous involvement of multiple organs, with histology demonstrating myriad small-vessel occlusions suggestive of a thrombotic storm [5]. We should point out that the majority of what we know about this condition must be credited to the impressive international “CAPS registry,” managed by the European Forum on Antiphospholipid Antibodies. This collection of clinical data for almost 500 CAPS episodes is periodically reviewed in systematic fashion [6], with the most recent endeavor published in 2014 [7]**.
For this article, published literature was systematically reviewed as described in the Supplementary Methods.
PATHOPHYSIOLOGY
As few specimens from CAPS patients are available for study, essentially all concepts regards CAPS pathophysiology are derived from investigations of classic APS [8]. In classic APS, antiphospholipid antibodies engage phospholipid surfaces via intermediary lipid-binding proteins such as β2GPI, and thereby activate endothelial cells, platelets, monocytes, trophoblast cells, and neutrophils with prothrombotic implications [9,10]*. Mechanistically (and with relevance to potential therapies), cell activation is dependent on both the intracellular transcription factor NF-κB and the extracellular complement cascade [9,11-13]. In addition to direct cell activation, antiphospholipid antibodies may also disrupt natural anti- and pro-coagulant regulators in vivo, such as annexin V, protein C, prothrombin, and tissue factor [14]. In 1998, Kitchens reported on six cases (at least three of which would meet current criteria for CAPS), and hypothesized that thrombosis can beget more thrombosis in at-risk patients via an increase in inhibitors of fibrinolysis [15,16]. While it is unclear exactly how antiphospholipid antibodies potentiate this thrombotic storm, many CAPS patients clearly improve with anticoagulation (see below), and an imbalance between fibrin generation and lysis must be at least partially responsible for CAPS. At the same time, there is clinical evidence of a cytokine cascade/systemic inflammatory response syndrome (SIRS) in many CAPS patients [17]. As will be discussed below, the approach to treatment has therefore focused on quieting not just thrombosis, but also the SIRS response.
DIAGNOSIS
The earliest description of CAPS dates to 1984 [18], followed soon thereafter by several case descriptions [19]. In 1992, Dr. Ronald Asherson formally defined CAPS as “widespread coagulopathy, strongly antiphospholipid antibody related, but totally distinct and separate from any of the other recognized inherited/acquired coagulopathies [19].” CAPS diagnostic criteria were originally proposed by Asherson in 2002 [20], subsequently endorsed by an international consensus [21], and ultimately validated in 2005 [5]. These criteria ask the clinician to look for the rapid onset of thrombosis in multiple organs, as well as circulating antiphospholipid antibodies (Table 1) [5]. As diagnosis is essentially dependent on the presence of these antibodies [5], and as almost 50% of patients will have CAPS as their first manifestation of APS [7]**, a high index of suspicion is needed.
Table 1.
Preliminary criteria for the classification of catastrophic APS
|
Definite catastrophic APS
|
Usually, clinical evidence of vessel occlusions, confirmed by imaging techniques when appropriate. Renal involvement is defined by a 50% rise in serum creatinine, severe systemic hypertension (> 180/100mmHg) and/or proteinuria (> 500mg/24hours).
For histopathological confirmation, significant evidence of thrombosis must be present, although vasculitis may coexist occasionally.
If the patient had not been previously diagnosed as having an APS, the laboratory confirmation requires that presence of antiphospholipid antibodies must be detected on two or more occasions at least six weeks apart (not necessarily at the time of the event), according to the proposed preliminary criteria for the classification of definite APS.9
The lupus anticoagulant (a functional test for antiphospholipid antibodies) is identified by demonstrating prolongation of a phospholipid-dependent clotting assay that does not correct with mixing, but can be quenched with excess phospholipids [22]. Indeed, lupus anticoagulant is the antiphospholipid antibody test that best predicts thrombotic events in classic APS [23]*, and 82% of patients in the CAPS registry have been reported to have a positive lupus anticoagulant [6]. Clinical states that prolong clotting times, such as therapeutic anticoagulation or disseminated intravascular coagulation (DIC), introduce inherent complications (and the potential for false positives) into lupus anticoagulant testing. While experienced centers may be able to handle lupus anticoagulant detection in patients treated with vitamin K antagonists [24,25]*, there is not universal agreement on this point. In addition, our center and most others strongly discourage testing in patients receiving inhibitory anticoagulants such as the various heparin formulations or the novel oral anticoagulants [25,26]*. Further emphasizing the need to exercise caution in interpreting the lupus anticoagulant, some percentage of critical care patients (on the order of 50%) may have a positive lupus anticoagulant secondary to other issues (infection, catecholamine administration, or cancer) entirely independent of APS [27,28]. Fortunately, 83% of patients with CAPS have been reported to have anticardiolipin IgG [6], and detecting high titers of either anticardiolipin or anti-β2GPI will certainly increase the clinician’s confidence regarding the diagnosis of APS/CAPS. In summary, relying on an isolated lupus anticoagulant should be done with trepidation in critically-ill patients in whom the aforementioned confounders may exist. Our recommendation is to always consult with local hematologists and coagulation lab personnel for help in sending and interpreting these functional assays in critically-ill patients.
A key to the diagnosis of CAPS is a careful consideration and exclusion of other syndromes that can simultaneously target multiple organs, and in particular the microvasculature of these organs (Table 2). This complex topic has been reviewed in detail in an excellent, recent review [29]**. Hyperferritinemia is emerging as a biomarker that may help distinguish CAPS from classic APS (~800 ng/ml vs. ~100 ng/ml; p<0.001) [30]; however, it is not yet clear whether elevated ferritin would help segregate CAPS from other causes of critical illness. CAPS can present with microangiopathic hemolytic anemia (21.7% of patients in the CAPS registry) [7]**, and differentiating from thrombotic thrombocytopenic purpura (TTP) is among the most common questions that arises clinically. Antiphospholipid antibodies are rarely present in idiopathic TTP, and when they do occur are almost always at a level below 40 U/ml in anticardiolipin and anti-β2GPI assays [29,31]** (in cases of lupus-associated TTP, we are unaware of studies regarding levels of antiphospholipid antibodies). Also helpful in differentiating CAPS from TTP is the number of schistocytes. While these can be seen in CAPS, the levels are typically well below the standard set by TTP [29,31]**. Finally, a clinical pearl suggested by some experts is that lung involvement would favor CAPS over TTP [29]**. Of course, and as has been pointed out by others [29]**, these various conditions can present on a spectrum and with clinical overlap, requiring an adaptable and multispecialty approach to the care of these patients.
Table 2.
Differential diagnosis of catastrophic antiphospholipid syndrome
| CAPS | TTP-HUS | DIC | HELLP | SRC | HIT | |
|---|---|---|---|---|---|---|
| Microvascular thrombosis | + | + | + | + | + | − |
| Macrovascular thrombosis | + | − | − | + | − | + |
| Hemorrhage | − | − | + | +/− | − | − |
| Multiple organ failure | ++ | +/− | +/− | +/− | − | − |
| Renal failure | +/− | ++ | − | +/− | ++ | − |
| Altered mental status | +/− | +/− | − | +/− | − | − |
| Pulmonary disease† | ++/− | − | +/− | +/− | − | − |
| Cardiac disease‡ | +/− | − | − | − | − | − |
| Pregnancy | +/− | − | +/− | + | − | − |
| Infection | +/− | +/− | +/− | − | − | − |
| Malignancy | +/− | − | +/− | − | − | − |
| Hemolytic anemia | +/− | ++ | +/− | + | + | − |
| Schistocytes | +/− | ++ | +/− | + | + | − |
| Thrombocytopenia | +/− | ++ | + | + | +/− | ++ |
| Prolonged PTT | +/− | − | + | − | − | − |
| Fibrinogen | Normal | Normal | ↓ | Normal | Normal | Normal |
| Liver enzymes | ↑ | Normal | Normal | ↑ ↑ | Normal | Normal |
| Antiphospholipid abs* | ++ | − | − | − | − | − |
| ADAMTS13 | Normal | ↓ ↓ ↓ | ↓ | Normal | Normal | Normal |
| Anti-PF4 | − | − | − | − | − | ++ |
CAPS, catastrophic antiphospholipid syndrome; TTP, thrombotic thrombocytopenic purpura; HUS, hemolytic uremic syndrome; HIT, heparin-induced thrombocytopenia; HELLP, hemolysis, elevated liver enzymes, low platelets; DIC, disseminated intravascular coagulation; SRC, scleroderma renal crisis; PTT, partial thromboplastin time; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; Anti-PF4, anti-platelet factor 4
Especially acute respiratory distress syndrome
Especially myocardial infarction or new reduction in ejection fraction
High titers (>40 U/ml) are very specific for CAPS; low titers are possible in other scenarios.
ETIOLOGY AND ASSOCIATIONS
It is well accepted that for APS in general, and especially for CAPS, a second hit is needed to tip a primed system toward thrombotic events. In the most recent analysis of the CAPS registry, almost two-thirds of CAPS cases (65.4%) were attributable to a clear precipitating factor [7]**. These included infection (46.7%), malignancy (17.6%), surgery (16.8%), and sub-therapeutic anticoagulation (10.9%) [7]**. It is possible that infection is even more strongly linked to CAPS when the analysis is limited to children; for example, a query of the CAPS registry found infection in 60.9% of children, as compared to 26.8% of adults (p< 0.001) [32]*.
Infection
Infection is the most common trigger of CAPS [7]**, and administration of LPS in an animal model promotes antiphospholipid antibody-mediated microvascular thrombosis [13]. Organisms that have been commonly linked to CAPS include E. coli, Klebsiella, Salmonella, Shigella, Staphylococcus, and Streptococcus [7]**. Other viral infections, such as herpesviruses and HIV, have also been reported as precipitators of CAPS [6,33]. There is even a report of mycobacterium-induced CAPS in a patient with chronic HIV and hepatitis C infections [34]. Key sites of infection are respiratory tract, cutaneous, urinary tract, gastrointestinal tract, and systemic/sepsis [35]. Finally, beyond its role as an initiator, infection is the cause of mortality in 14.1% of CAPS cases, attributable to bacterial sepsis, candidiasis, cerebral abscess, or Pneumocystis-associated pneumonia [36].
Malignancy
Both cancer and chemotherapy are well-known precipitators of CAPS [37]. Not surprisingly, malignancy-associated CAPS has demonstrated a trend toward higher mortality (61% vs. 42%, p=0.07) [37]. Specific malignancies linked to CAPS include hematological (26%), lung (17%), and colon (9%) [38].
Pregnancy
In a series of 255 CAPS cases, 15 occurred during pregnancy, with notable features that include HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, placental infarctions, pelvic vein thromboses, and thrombotic microangiopathy [39]. CAPS was the first-ever manifestation of APS in 4 of the 15, underscoring the need to keep CAPS in the differential diagnosis for all patients [39]. Interestingly, mortality attributable to pregnancy-associated CAPS was very different between this study (46%) [39], and a more recent series [40], perhaps attributable to advancement in management of CAPS in recent years.
SLE
Although not a precipitating factor in the same way as infection or malignancy, identification of the presence of SLE is critical to the management of CAPS, both in terms of diagnosis (as the well-known association between SLE and APS would place CAPS higher on the differential) and therapy (as discussed below). In general, SLE-associated CAPS occurs in younger patients, associates with more cerebral involvement, and has higher mortality [41]. Specifically, reported mortality is on the order of 58% in SLE, as compared to 35% in primary APS [41]. Perhaps related to the baseline treatment of SLE patients with immunosuppressive therapy, infection was an especially common precipitator of CAPS in SLE, as compared to primary APS (p=0.006) [41].
MORBIDITY AND MORTALITY
CAPS likely complicates around 1% of APS cases. For example, a European study of 1,000 APS patients found nine new cases over 10 years of follow-up (five of whom died) [42]**. In an Italian series focusing exclusively on patients with “triple-positive” APS serology, 4/160 patients (2.5%) were diagnosed with CAPS [43]. Indeed, triple-positive APS (when a patient has positive testing for anticardiolipin, anti-β2GPI, and lupus anticoagulant) is also associated with a higher risk of thrombosis in classic APS [43].
CAPS, by definition, targets multiple organ systems simultaneously. The systems most likely to be affected include renal (73%), pulmonary (58.9%), central nervous system (55.9%), cardiac (49.7%), cutaneous (45.4%), peripheral vessels (36.2%), intestinal (24%), spleen (16.7%), adrenal glands (10.6%), pancreas (7.2%), and bone marrow (3.1%) [7]**. Other rare targets include testicles/ovaries, the prostate gland, and even acalculous cholecystitis [7]**. It is worth noting that a Japanese series found a higher percentage of CNS involvement, on the order of 87% [44].
In early case series, mortality from CAPS was estimated at around 50% [45]. In CAPS patients who die, death is most commonly attributed to cerebral involvement (19.5%). Other etiologies are cardiac (14.1%), infection (14.1%), multi-organ failure (12.4%), pulmonary (7.1%), and abdominal (4.5%) [36]. Autopsy results found evidence of microthrombosis in 84.5% of patients. Other common autopsy findings were infarction (53.4%), non-infectious endocarditis (27.6%), and large vessel thrombosis (19%) [46]. In non-lupus CAPS, mortality is predicted by age over 36, more organs involved, and need for hemodialysis [41]. In lupus-associated CAPS, adrenal involvement predicted higher mortality, while thrombocytopenia was associated with better outcomes [41]. A final point is that CAPS, not surprisingly, predicts a more severe variety of APS. Of patients who survived their first CAPS episode, 16% ultimately died as the result of a different APS manifestation [47].
THERAPY
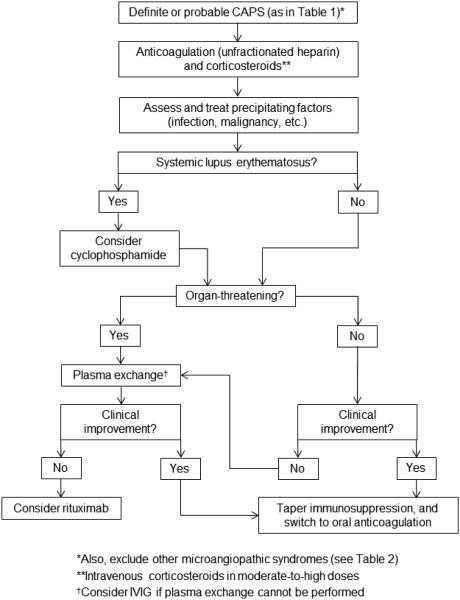
The mortality of CAPS is high [45], but has improved in recent years (survival now 63%), presumably the result of greater education about, and employment of, therapy [7]**. Currently, expert consensus recommends the use of anticoagulation and corticosteroids in essentially all patients, with a strong consideration for the further addition of plasma exchange or intravenous immunoglobulin (IVIG) (Figure 1). Addition of cyclophosphamide should also be considered in patients with SLE. It is important to note that this is a field without any prospective studies, and the best data comes from analysis of the aforementioned CAPS registry.
Figure 1.
Proposed algorithm for treatment of CAPS.
Anticoagulation
The most compelling evidence for any treatment in CAPS regards the use of anticoagulation. In an analysis of 280 patients from the CAPS registry, 244 received any type of anticoagulation, and 36 received none [6]. Survival was significantly better in the anticoagulated patients (63% vs. 22%, p<0.0001) [6]. A common question regards the specific type of anticoagulation. While there is no evidence to favor unfractionated heparin over low-molecular-weight heparin or warfarin [46], most critically ill patients should probably receive unfractionated heparin given its reversibility [7]**. Another practical point is that patients with a positive lupus anticoagulant may have an elevated PTT at baseline; therefore, following additional readouts (for example, an anti-factor Xa assay) is recommended. Fibrinolytics, such as streptokinase, have been utilized in a small number of reports [48,49], but there is no standard recommendation for their use, even in refractory cases. Similarly, there is no known role for antiplatelet agents or the novel oral anticoagulants in CAPS.
Corticosteroids
Corticosteroids have been proposed as a means to blunt the inflammatory aspects of CAPS, and are recommended by most experts [7]**. It should, however, be noted that because corticosteroids have typically been employed in combination with anticoagulation, there is little data to independently support their use. For example, in an analysis of 242 patients from the CAPS registry, 190 were treated with any corticosteroid regimen [46]. Of the 190, 65 received an intravenous pulse (methylprednisolone 500-1000 mg daily for 1-3 days) and 64 received 1-2 mg/kg/day (methylprednisolone equivalent, oral or intravenous); the specifics were unknown in the remainder [46]. When considering only the presence or absence of corticosteroids (and ignoring concomitant therapy), there was no difference in recovery between the groups (55.8% vs. 56.9%, p=not significant) [46]. Further, in the same study, 11 patients received corticosteroids as monotherapy, and only 2 of the 11 survived [46]. Despite these negative data, it should again be noted that in the most recent analyses of CAPS therapy, corticosteroids are used in combination with anticoagulation more than 99% of the time [7]**. While no dosing regimen is evidence-based in CAPS, expert consensus favors the use of high doses, similar to what is employed in severe manifestations of SLE (i.e., methylprednisone 500 mg daily × 3 days) [50]. Also in line with the treatment of SLE, more moderate doses (~1 mg/kg/day) should be considered in patients with significant infectious complications.
Plasma exchange and IVIG
Given the known benefit of plasma exchange in patients with a different type of microangiopathy (TTP), as well as the accepted pathogenicity of antiphospholipid antibodies themselves [9], plasma exchange has been proposed as a potential therapy in CAPS [49]. Retrospective data has demonstrated a survival of 77.8% for 18 patients treated with triple therapy (anticoagulation, corticosteroids, and plasma exchange) as compared to 55.4% for the patients who did not receive this treatment (p=0.083) [46]. In another series of patients, plasma exchange was utilized in 21 patients, alongside other standard-of-care treatment; 16 had complete control of CAPS, and 3 had a partial response [51]. It should be noted that most reported patients in the literature have received plasma exchange with fresh frozen plasma (rather than albumin) as the replacement fluid [29]**. As plasma may contain beneficial antithrombotic factors [52], it is unclear whether similar outcomes would be seen with albumin as the replacement fluid (or with simple plasmapheresis).
Regarding IVIG, its addition to anticoagulation and corticosteroids seems to associate with improved mortality (although most analyses have grouped plasma exchange and IVIG together given low numbers of patients). For example, in an analysis of 342 patients, the 160 who received triple therapy with anticoagulation, corticosteroids, and IVIG and/or plasma exchange had better survival than those patients who received neither plasma exchange nor IVIG (p=0.04) [7]**. Typically used at doses of 0.4 g/kg/day × 5 days, IVIG may be particularly favored in patients with thrombocytopenia, and has the advantage of being immunomodulatory rather than immunosuppressive. Further, IVIG is a treatment that has at times been used in classic APS, for example to address growth restriction in pregnancy [53], and even to treat patients with recurrent thrombosis [54]. Some clinicians may prefer to use IVIG in combination with plasma exchange. If that is the case, then it should not be administered until plasma exchange is complete. Other practical considerations include the association of IVIG with both increased thrombotic risk and worsening renal function in patients who do not have CAPS [55]. We would therefore recommend using sucrose-reduced preparations that are less nephrotoxic, and avoiding IVIG in patients who are not being actively treated with anticoagulation.
Cyclophosphamide
An important consideration is whether immunosuppressive therapy beyond corticosteroids should be administered. When CAPS occurs in a patient with SLE, our recommendation would be to strongly consider cyclophosphamide (typically administered intravenously at doses of 500-750 mg/m2, adjusted for renal function, as has been utilized for other organ- and life-threatening manifestations of SLE). Indeed, in an analysis of 103 patients with SLE-CAPS, cyclophosphamide (administered to 47% of patients) decreased mortality [odds ratio=0.20 (0.06-0.71); p=0.013] [41]. In the same analysis, there were 126 CAPS patients without SLE, of whom 15% received cyclophosphamide. In these non-SLE patients, cyclophosphamide actually associated with increased mortality [odds ratio=8.5 (1.91-37.83); p=0.005], although with the caveat that patients who received cyclophosphamide had more severe disease based on the number of organs involved [41]. When considering the timing of cyclophosphamide, the clinician should know that the duration of its effects will be measured in weeks. As such, ensuring control of any complicating infections is important before administration.
Rituximab
Rituximab is a humanized anti-CD20 monoclonal antibody, devised to target B-cell malignancies, but now also proven efficacious in autoimmune diseases such as rheumatoid arthritis and small-vessel vasculitis [56]. Additionally, rituximab treatment (either alone or in combination with cyclophosphamide) has been reported to be successful in refractory manifestations of SLE including nephritis, neuropsychiatric disease, and autoimmune hemolytic anemia [57]. In a review utilizing the CAPS registry, 20 rituximab-treated patients were identified, all of whom were concomitantly treated with other potent modalities (100% anticoagulation, 85% corticosteroids, 80% IVIG, 65% plasma exchange, and 20% cyclophosphamide) [58]. In 8 of these 20 cases, rituximab was part of first-line therapy (due to either severity of disease or underlying lymphoma); in the remaining cases, rituximab was administered due to progressive manifestations or declining clinical status [58]. With the caveat that no comparison group was included in the study, it is notable that 15 of the 20 rituximab-treated patients (75%) recovered from their CAPS episode [58]. It should also be pointed out that a pilot study of rituximab in classic APS showed a good safety profile, and perhaps some efficacy against non-criteria manifestations of APS such as skin ulcers [59]. A practical point is that if plasma exchange is utilized, rituximab administration should not occur until that course is complete.
Eculizumab
Eculizumab is a humanized monoclonal antibody that prevents C5 complement cleavage/activation [60], and is approved as treatment for atypical hemolytic-uremic syndrome (aHUS) and paroxysmal nocturnal hemoglobinuria. In aHUS, approved dosing is 900 mg once weekly for 4 weeks, and 1200 mg every-other-week thereafter [61]. As complement activation has been considered a key factor in animal models of APS thrombosis [12,13], eculizumab has sometimes been considered in refractory cases of CAPS. For example, APS is associated with poor outcomes following renal transplant, and two cases have been reported in which a CAPS-like picture was mitigated (and renal function preserved) with administration of eculizumab [62]*. Additionally, several case reports have suggested efficacy in CAPS patients who failed to respond to triple therapy, and sometimes also rituximab [63-66]. With caution, eculizumab may therefore be considered as a medication of last resort in CAPS, when organ- and life-threatening disease persists despite treatment with triple therapy. But, the clinician must acknowledge that, at this point in time, its utilization is based almost exclusively on the promising work in animal models [13], and a case-report literature that is likely affected by publication bias. Going forward, complement inhibition via a different agent is currently being explored for non-criteria manifestations of classic APS such as thrombocytopenia, skin ulcers, and nephropathy (www.clinicaltrials.gov NCT02128269). This clinical trial may give a better sense of safety (and possibly efficacy) of the medication in APS.
Intensive care unit (ICU) recommendations
Experts have recommended minimizing arterial instrumentation when possible (given risk of new clots), and also utilizing lung-protective ventilation, glycemic control, and gastric ulcer preventative measures (given high doses of corticosteroids and anticoagulation) [67]. These are recommendations with which we agree.
SUMMARY
Establishing the diagnosis of CAPS requires a high index of suspicion, as outcomes are poor without timely diagnosis and targeted therapy. CAPS is not the only systemic syndrome to present with microangiopathy, and so high titers of antiphospholipid antibodies should be sought as confirmation of the diagnosis. Common associations include infection, malignancy, and SLE, and all should be considered as early as possible in the patient’s course.
We would strongly recommend anticoagulation initially with unfractionated heparin in all patients, and consultation with a hematologist regarding dosing/monitoring. At the same time, administration of corticosteroids is recommended by experts, and has been employed in the majority of patients who survive (although the data to support their use is less compelling than for anticoagulation). Patients with SLE should additionally receive cyclophosphamide (500-750 mg/m2), adjusted for renal function, unless there is a contraindication such as serious infection. In patients with organ-threatening manifestations, we would recommend the early initiation of plasma exchange (or possibly IVIG in some circumstances). Indeed, the concept of “triple therapy” with anticoagulation, corticosteroids, and plasma exchange/IVIG, is marginally supported by retrospective data, and is recommended in most expert reviews.
Rituximab (possibly in combination with cyclophosphamide) is also a consideration in patients who have relapsed previously, or who are not responding to the above therapy. Animal data has suggested a role for the complement cascade in both obstetric and thrombotic APS, and eculizumab can be considered in refractory cases, as long as the highly experimental nature of this therapy is recognized.
Supplementary Material
KEY POINTS.
❖ A high index of suspicion is needed to make the diagnosis of CAPS, as many patients (up to 50%) will have CAPS as the first manifestation of APS.
❖ While there are no prospective studies regarding treatment of CAPS, experts in the field recommend anticoagulation and corticosteroids for essentially all patients.
❖ Based on retrospective data, patients treated with triple therapy (anticoagulation, corticosteroids, and plasma exchange/IVIG) may have better outcomes than patients treated with anticoagulation and corticosteroids alone.
❖ Cyclophosphamide seems to improve survival when CAPS occurs in patients with SLE; this is in contrast to patients without SLE, who have worse outcomes with cyclophosphamide.
ACKNOWLEDGEMENTS
We would like to acknowledge Whitney Townsend, MLIS, Informationist at the University of Michigan Taubman Health Sciences Library, for her assistance with the literature search.
FINANCIAL SUPPORT AND SPONSORHIP
NMK was supported by Security Forces Hospital Program, Ministry of Interior, Riyadh, Saudi Arabia. WJM was supported by the Mary Piazza Lupus Research Fund and the Michael and Marcia Klein Lupus Research Fund. JSK received funding from NIH K08AR066569 and a career development award from the Burroughs Wellcome Fund.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts to disclose.
REFERENCES
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, Koike T, Meroni PL, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. PG DEG. [DOI] [PubMed] [Google Scholar]
- 2**.Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, Fleming N, Domingues V, Sciascia S, Lyra JO, et al. The relevance of "non-criteria" clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev. 2015;14:401–414. doi: 10.1016/j.autrev.2015.01.002. This study analyzed nine potential manifestations of APS that are not part of current classification criteria. The critical appraisal found that current literature best supports an association between APS and three noncriteria manifestations: APS nephropathy, heart valve lesions, and livedo reticularis. Efforts like this help frame a discussion about possible broadening of current classification criteria. [DOI] [PubMed] [Google Scholar]
- 3.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 4*.Erkan D, Aguiar CL, Andrade D, Cohen H, Cuadrado MJ, Danowski A, Levy RA, Ortel TL, Rahman A, Salmon JE, et al. 14th International Congress on Antiphospholipid Antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev. 2014;13:685–696. doi: 10.1016/j.autrev.2014.01.053. This task force review describes classic APS management, inclusive of traditional, adjuvant, and experimental therapies. A detailed analysis of the evidence to support each treatment is also included. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Font J, Gomez-Puerta JA, Espinosa G, Cucho M, Bucciarelli S, Ramos-Casals M, Ingelmo M, Piette JC, Shoenfeld Y, et al. Validation of the preliminary criteria for the classification of catastrophic antiphospholipid syndrome. Ann Rheum Dis. 2005;64:1205–1209. doi: 10.1136/ard.2004.025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cervera R, Bucciarelli S, Plasin MA, Gomez-Puerta JA, Plaza J, Pons-Estel G, Shoenfeld Y, Ingelmo M. Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the "CAPS Registry". J Autoimmun. 2009;32:240–245. doi: 10.1016/j.jaut.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 7**.Cervera R, Rodriguez-Pinto I, Colafrancesco S, Conti F, Valesini G, Rosario C, Agmon-Levin N, Shoenfeld Y, Ferrao C, Faria R, et al. 14th International Congress on Antiphospholipid Antibodies Task Force Report on Catastrophic Antiphospholipid Syndrome. Autoimmun Rev. 2014;13:699–707. doi: 10.1016/j.autrev.2014.03.002. This task force analysis focuses on CAPS, reviewing available evidence on pathogenesis, laboratory and clinical features, diagnosis, management, and direction of future research. Importantly, the most recent analysis of the CAPS registry is included here. [DOI] [PubMed] [Google Scholar]
- 8.Ortega-Hernandez OD, Agmon-Levin N, Blank M, Asherson RA, Shoenfeld Y. The physiopathology of the catastrophic antiphospholipid (Asherson's) syndrome: Compelling evidence. Journal of Autoimmunity. 2009;32:1–6. doi: 10.1016/j.jaut.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 10*.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, Hernandez-Ramirez D, Bockenstedt PL, Liaw PC, Cabral AR, et al. Release of Neutrophil Extracellular Traps by Neutrophils Stimulated With Antiphospholipid Antibodies: A Newly Identified Mechanism of Thrombosis in the Antiphospholipid Syndrome. Arthritis Rheumatol. 2015;67:2990–3003. doi: 10.1002/art.39247. This paper found evidence of exuberant neutrophil extracellular trap (NET) formation in patients with primary APS. Given the emerging association between NETs and thrombosis, novel immunomodulatory approaches to treatment may eventually arise from this work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meroni PL, Raschi E, Testoni C, Tincani A, Balestrieri G, Molteni R, Khamashta MA, Tremoli E, Camera M. Statins prevent endothelial cell activation induced by antiphospholipid (anti-beta(2)-glycoprotein I) antibodies - Effect on the proadhesive and proinflammatory phenotype. Arthritis and Rheumatism. 2001;44:2870–2878. doi: 10.1002/1529-0131(200112)44:12<2870::aid-art475>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52:2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 13.Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, Bossi F, Ziller F, Sblattero D, Meroni P, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood. 2005;106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 14.Meroni PL, Ronda N, De Angelis V, Grossi C, Raschi E, Borghi MO. Role of anti-beta2 glycoprotein I antibodies in antiphospholipid syndrome: in vitro and in vivo studies. Clin Rev Allergy Immunol. 2007;32:67–74. doi: 10.1007/BF02686083. [DOI] [PubMed] [Google Scholar]
- 15.Kitchens CS. Thrombotic storm: When thrombosis begets thrombosis. American Journal of Medicine. 1998;104:381–385. doi: 10.1016/s0002-9343(98)00061-8. [DOI] [PubMed] [Google Scholar]
- 16.D'Angelo A, Kluft C, Verheijen JH, Rijken DC, Mozzi E, Mannucci PM. Fibrinolytic shut-down after surgery: impairment of the balance between tissue-type plasminogen activator and its specific inhibitor. Eur J Clin Invest. 1985;15:308–312. doi: 10.1111/j.1365-2362.1985.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa G, Bucciarelli S, Cervera R, Gomez-Puerta JA, Font J. Laboratory studies on pathophysiology of the catastrophic antiphospholipid syndrome. Autoimmun Rev. 2006;6:68–71. doi: 10.1016/j.autrev.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Dosekun AK, Pollak VE, Glas-Greenwalt P, Kant KS, Penovich P, Lebron-Berges A, Weiss MA, Levinson JE. Ancrod in systemic lupus erythematosus with thrombosis. Clinical and fibrinolysis effects. Arch Intern Med. 1984;144:37–42. [PubMed] [Google Scholar]
- 19.Asherson RA. The catastrophic antiphospholipid syndrome. J Rheumatol. 1992;19:508–512. [PubMed] [Google Scholar]
- 20.Asherson RA, Espinosa G, Cervera R, Font J, Reverter JC. Catastrophic antiphospholipid syndrome: proposed guidelines for diagnosis and treatment. J Clin Rheumatol. 2002;8:157–165. [PubMed] [Google Scholar]
- 21.Asherson RA, Cervera R, de Groot PG, Erkan D, Boffa MC, Piette JC, Khamashta MA, Shoenfeld Y. Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus. 2003;12:530–534. doi: 10.1191/0961203303lu394oa. [DOI] [PubMed] [Google Scholar]
- 22.Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, De Groot PG, Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the S, Standardisation Committee of the International Society on T, Haemostasis Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7:1737–1740. doi: 10.1111/j.1538-7836.2009.03555.x. [DOI] [PubMed] [Google Scholar]
- 23*.Gebhart J, Posch F, Koder S, Perkmann T, Quehenberger P, Zoghlami C, Ay C, Pabinger I. Increased mortality in patients with the lupus anticoagulant: the Vienna Lupus Anticoagulant and Thrombosis Study (LATS) Blood. 2015;125:3477–3483. doi: 10.1182/blood-2014-11-611129. This work highlights the importance of lupus anticoagulant as a predictor of mortality in APS patients. [DOI] [PubMed] [Google Scholar]
- 24*.Isert M, Miesbach W, Stoever G, Lindhoff-Last E, Linnemann B. Screening for lupus anticoagulants in patients treated with vitamin K antagonists. Int J Lab Hematol. 2015 doi: 10.1111/ijlh.12409. This laboratory study presents a methodology utilized at a single-center for detecting the lupus anticoagulant in patients receiving vitamin K antagonists. [DOI] [PubMed] [Google Scholar]
- 25*.Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, de Groot P, Lakos G, Lambert M, Meroni P, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev. 2014;13:917–930. doi: 10.1016/j.autrev.2014.05.001. Bertolaccini et al: This task force focused on lab testing as it pertains to APS diagnosis. In addition to current “criteria” lab tests, the literature is also appraised as it pertains to non-criteria antibodies such as anti-phosphatidylserine/prothrombin and anti-β2GPI IgA. [DOI] [PubMed] [Google Scholar]
- 26*.Martinuzzo ME, Barrera LH, Da MA, Otaso JC, Gimenez MI, Oyhamburu J. Frequent false-positive results of lupus anticoagulant tests in plasmas of patients receiving the new oral anticoagulants and enoxaparin. Int J Lab Hematol. 2014;36:144–150. doi: 10.1111/ijlh.12138. This is a consideration of how new oral direct thrombin inhibitors and factor Xa inhibitors may impact lupus anticoagulant testing. Based on their findings, the authors discourage assaying lupus anticoagulant in these patients. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel C, Stoiser B, Locker GJ, Laczika K, Quehenberger P, Kapiotis S, Frass M, Pabinger I, Knobl P. Frequent development of lupus anticoagulants in critically ill patients treated under intensive care conditions. Critical Care Medicine. 2002;30:763–770. doi: 10.1097/00003246-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Vassalo J, Spector N, de Meis E, Rabello LS, Rosolem MM, do Brasil PE, Salluh JI, Soares M. Antiphospholipid antibodies in critically ill patients with cancer: a prospective cohort study. J Crit Care. 2014;29:533–538. doi: 10.1016/j.jcrc.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 29**.Rodriguez-Pinto I, Espinosa G, Cervera R. Catastrophic APS in the context of other thrombotic microangiopathies. Curr Rheumatol Rep. 2015;17:482. doi: 10.1007/s11926-014-0482-z. This excellent and thorough review article explores the hematological differential diagnosis of CAPS. Included are clinical pearls and nuances that will help the reader distinguish CAPS from alternatives (TTP-HUS, DIC, etc.) [DOI] [PubMed] [Google Scholar]
- 30.Agmon-Levin N, Rosario C, Katz BSP, Zandman-Goddard G, Meroni P, Cervera R, Stojanovich L, Blank M, Pierangeli SS, Praprotnik S, et al. Ferritin in the antiphospholipid syndrome and its catastrophic variant (cAPS) Lupus. 2013;22:1327–1335. doi: 10.1177/0961203313504633. [DOI] [PubMed] [Google Scholar]
- 31.Montecucco C, Di Lauro M, Bobbio-Pallavicini E, Longhi M, Caporali R, De Gennaro F, Ascari E. Anti-phospholipid antibodies and thrombotic thrombocytopenic purpura. Clin Exp Rheumatol. 1987;5:355–358. [PubMed] [Google Scholar]
- 32*.Berman H, Rodriguez-Pinto I, Cervera R, Gregory S, de Meis E, Rodrigues CE, Aikawa NE, de Carvalho JF, Springer J, Niedzwiecki M, et al. Pediatric catastrophic antiphospholipid syndrome: descriptive analysis of 45 patients from the "CAPS Registry". Autoimmun Rev. 2014;13:157–162. doi: 10.1016/j.autrev.2013.10.004. This is the first detailed description of CAPS in a pediatric population. A relevant clinical finding is that CAPS is the first manifestation of APS more frequently in pediatric patients. [DOI] [PubMed] [Google Scholar]
- 33.Damian L, Rednic S, Cristea A, Felea I, Nicola M, Nicoara I, Fodor D, Albu A, Bolosiu HD. Viral-induced catastrophic antiphospholipid syndrome: Two cases with favourable outcome. Annals of the Rheumatic Diseases. 2004;63:330–330. [Google Scholar]
- 34.Ku EW, Mizrachi A, Cohn J. Catastrophic Antiphospholipid Syndrome secondary to mycobacterium tuberculosis infection: A case report. Blood. 2003;102:107B–107B. [Google Scholar]
- 35.Asherson RA, Cervera R, Piette JC, Shoenfeld Y, Espinosa G, Petri MA, Lim E, Lau TC, Gurjal A, Jedryka-Goral A, et al. Catastrophic antiphospholipid syndrome: clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore) 2001;80:355–377. doi: 10.1097/00005792-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Bucciarelli S, Espinosa G, Cervera R. The CAPS Registry: morbidity and mortality of the catastrophic antiphospholipid syndrome. Lupus. 2009;18:905–912. doi: 10.1177/0961203309106833. [DOI] [PubMed] [Google Scholar]
- 37.Miesbach W, Asherson RA, Cervera R, Shoenfeld Y, Gomez Puerta J, Espinosa G, Bucciarelli S. The role of malignancies in patients with catastrophic anti-phospholipid (Asherson's) syndrome. Clin Rheumatol. 2007;26:2109–2114. doi: 10.1007/s10067-007-0634-x. [DOI] [PubMed] [Google Scholar]
- 38.Miesbach W, Asherson RA, Cervera R, Shoenfeld Y, Gomez Puerta J, Bucciarelli S, Espinoza G, Font J. The catastrophic antiphospholipid (Asherson's) syndrome and malignancies. Autoimmun Rev. 2006;6:94–97. doi: 10.1016/j.autrev.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Puerta JA, Cervera R, Espinosa G, Asherson RA, Garcia-Carrasco M, da Costa IP, Andrade DC, Borba EF, Makatsaria A, Bucciarelli S, et al. Catastrophic antiphospholipid syndrome during pregnancy and puerperium: maternal and fetal characteristics of 15 cases. Ann Rheum Dis. 2007;66:740–746. doi: 10.1136/ard.2006.061671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanouna G, Morel N, Le Thi Huong D, Josselin L, Vauthier-Brouzes D, Saadoun D, Kettaneh A, Levesque K, Le Guern V, Goffinet F, et al. Catastrophic antiphospholipid syndrome and pregnancy: an experience of 13 cases. Rheumatology (Oxford) 2013;52:1635–1641. doi: 10.1093/rheumatology/ket167. [DOI] [PubMed] [Google Scholar]
- 41.Bayraktar UD, Erkan D, Bucciarelli S, Espinosa G, Asherson R. The clinical spectrum of catastrophic antiphospholipid syndrome in the absence and presence of lupus. J Rheumatol. 2007;34:346–352. [PubMed] [Google Scholar]
- 42**.Cervera R, Serrano R, Pons-Estel GJ, Ceberio-Hualde L, Shoenfeld Y, de Ramon E, Buonaiuto V, Jacobsen S, Zeher MM, Tarr T, et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann Rheum Dis. 2015;74:1011–1018. doi: 10.1136/annrheumdis-2013-204838. A cohort of 1,000 APS patients was followed prospectively, giving us some sense of the incidence of CAPS in patients with an established diagnosis of APS. [DOI] [PubMed] [Google Scholar]
- 43.Pengo V, Ruffatti A, Legnani C, Gresele P, Barcellona D, Erba N, Testa S, Marongiu F, Bison E, Denas G, et al. Clinical course of high-risk patients diagnosed with antiphospholipid syndrome. J Thromb Haemost. 2010;8:237–242. doi: 10.1111/j.1538-7836.2009.03674.x. [DOI] [PubMed] [Google Scholar]
- 44.Ichikawa K, Koike T. Catastrophic antiphospholipid syndrome in Japanese population. Arthritis and Rheumatism. 2003;48:S361–S361. [Google Scholar]
- 45.Asherson RA, Cervera R, Piette JC, Font J, Lie JT, Burcoglu A, Lim K, Munoz-Rodriguez FJ, Levy RA, Boue F, et al. Catastrophic antiphospholipid syndrome - Clinical and laboratory features of 50 patients. Medicine. 1998;77:195–207. doi: 10.1097/00005792-199805000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Bucciarelli S, Espinosa G, Cervera R, Erkan D, Gomez-Puerta JA, Ramos-Casals M, Font J, Asherson RA. Mortality in the catastrophic antiphospholipid syndrome: causes of death and prognostic factors in a series of 250 patients. Arthritis Rheum. 2006;54:2568–2576. doi: 10.1002/art.22018. [DOI] [PubMed] [Google Scholar]
- 47.Erkan D, Asherson RA, Espinosa G, Cervera R, Font J, Piette JC, Lockshin MD, Catastrophic Antiphospholipid s Long term outcome of catastrophic antiphospholipid syndrome survivors. Annals of the Rheumatic Diseases. 2003;62:530–533. doi: 10.1136/ard.62.6.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bucciarelli S, Espinosa G, Asherson RA, Cervera R, Claver G, Gomez-Puerta JA, Ramos-Casals M, Ingelmo M, Font J, Catastrophic Antiphospholipid S The acute respiratory distress syndrome in catastrophic antiphospholipid syndrome: analysis of a series of 47 patients. Annals of the Rheumatic Diseases. 2006;65:81–86. doi: 10.1136/ard.2005.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asherson RA. The catastrophic antiphospholipid syndrome, 1998. A review of the clinical features, possible pathogenesis and treatment. Lupus. 1998;7(Suppl 2):S55–62. doi: 10.1177/096120339800700214. [DOI] [PubMed] [Google Scholar]
- 50.Isenberg DA, Morrow WJ, Snaith ML. Methyl prednisolone pulse therapy in the treatment of systemic lupus erythematosus. Ann Rheum Dis. 1982;41:347–351. doi: 10.1136/ard.41.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uthman I, Shamseddine A, Taher A. The role of therapeutic plasma exchange in the catastrophic antiphospholipid syndrome. Transfus Apher Sci. 2005;33:11–17. doi: 10.1016/j.transci.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 52.Cervera R, Espinosa G. Update on the catastrophic antiphospholipid syndrome and the "CAPS Registry". Semin Thromb Hemost. 2012;38:333–338. doi: 10.1055/s-0032-1304718. [DOI] [PubMed] [Google Scholar]
- 53.Branch DW, Peaceman AM, Druzin M, Silver RK, El-Sayed Y, Silver RM, Esplin MS, Spinnato J, Harger J, Grp PLS A multicenter, placebo-controlled pilot study of intravenous immune globulin treatment of antiphospholipid syndrome during pregnancy. American Journal of Obstetrics and Gynecology. 2000;182:122–127. doi: 10.1016/s0002-9378(00)70500-x. [DOI] [PubMed] [Google Scholar]
- 54.Sciascia S, Giachino O, Roccatello D. Prevention of thrombosis relapse in antiphospholipid syndrome patients refractory to conventional therapy using intravenous immunoglobulin. Clinical and Experimental Rheumatology. 2012;30:409–413. [PubMed] [Google Scholar]
- 55.Shoenfeld Y, Katz U. IVIg therapy in autoimmunity and related disorders: our experience with a large cohort of patients. Autoimmunity. 2005;38:123–137. doi: 10.1080/08916930500059633. [DOI] [PubMed] [Google Scholar]
- 56.Weiner GJ. Rituximab: mechanism of action. Semin Hematol. 2010;47:115–123. doi: 10.1053/j.seminhematol.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murray E, Perry M. Off-label use of rituximab in systemic lupus erythematosus: a systematic review. Clinical Rheumatology. 2010;29:707–716. doi: 10.1007/s10067-010-1387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berman H, Rodriguez-Pinto I, Cervera R, Morel N, Costedoat-Chalumeau N, Erkan D, Shoenfeld Y, Espinosa G. Rituximab use in the catastrophic antiphospholipid syndrome: descriptive analysis of the CAPS registry patients receiving rituximab. Autoimmun Rev. 2013;12:1085–1090. doi: 10.1016/j.autrev.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 59.Erkan D, Vega J, Ramon G, Kozora E, Lockshin MD. A Pilot Open-Label Phase II Trial of Rituximab for Non-Criteria Manifestations of Antiphospholipid Syndrome. Arthritis and Rheumatism. 2013;65:464–471. doi: 10.1002/art.37759. [DOI] [PubMed] [Google Scholar]
- 60.Chighizola CB, Favalli EG, Meroni PL. Novel mechanisms of action of the biologicals in rheumatic diseases. Clin Rev Allergy Immunol. 2014;47:6–16. doi: 10.1007/s12016-013-8359-x. [DOI] [PubMed] [Google Scholar]
- 61.Cofiell R, Kukreja A, Bedard K, Yan Y, Mickle AP, Ogawa M, Bedrosian CL, Faas SJ. Eculizumab reduces complement activation, inflammation, endothelial damage, thrombosis, and renal injury markers in aHUS. Blood. 2015;125:3253–3262. doi: 10.1182/blood-2014-09-600411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62*.Lonze BE, Zachary AA, Magro CM, Desai NM, Orandi BJ, Dagher NN, Singer AL, Carter-Monroe N, Nazarian SM, Segev DL, et al. Eculizumab prevents recurrent antiphospholipid antibody syndrome and enables successful renal transplantation. Am J Transplant. 2014;14:459–465. doi: 10.1111/ajt.12540. This small series reports on three APS patients in whom eculizumab was utilized in the context of renal transplantation. [DOI] [PubMed] [Google Scholar]
- 63.Kronbichler A, Frank R, Kirschfink M, Szilagyi A, Csuka D, Prohaszka Z, Schratzberger P, Lhotta K, Mayer G. Efficacy of eculizumab in a patient with immunoadsorption-dependent catastrophic antiphospholipid syndrome: a case report. Medicine (Baltimore) 2014;93:e143. doi: 10.1097/MD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barratt-Due A, Floysand Y, Orrem HL, Kvam AK, Holme PA, Bergseth G, Tjonnfjord GE, Mollnes TE. Complement inhibition may be lifesaving in catastrophic antiphospholipid syndrome. Molecular Immunology. 2015;67:122–122. [Google Scholar]
- 65.Shapira I, Andrade D, Allen SL, Salmon JE. Brief report: induction of sustained remission in recurrent catastrophic antiphospholipid syndrome via inhibition of terminal complement with eculizumab. Arthritis Rheum. 2012;64:2719–2723. doi: 10.1002/art.34440. [DOI] [PubMed] [Google Scholar]
- 66.Zikos TA, Sokolove J, Ahuja N, Berube C. Eculizumab Induces Sustained Remission in a Patient With Refractory Primary Catastrophic Antiphospholipid Syndrome. J Clin Rheumatol. 2015;21:311–313. doi: 10.1097/RHU.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 67.Erkan D. Therapeutic and prognostic considerations in catastrophic antiphospholipid syndrome. Autoimmun Rev. 2006;6:98–103. doi: 10.1016/j.autrev.2006.06.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.