Abstract
Purpose
To investigate changes in limbal basal epithelial cell density in eyes with limbal stem cell deficiency (LSCD) using in vivo confocal laser scanning microscopy
Design
retrospective observational comparative study
Methods
A total of 43 eyes of 30 patients diagnosed with LSCD were included in the study. Ten eyes from normal subjects were included as control. Confocal imaging of the central cornea, and the superior, nasal, inferior and temporal limbus were collected using the Heidelberg Retina Tomograph III Rostock Corneal Module. Basal cell density in all locations was measured by two independent observers.
Results
The mean basal cell density of the normal group was 9264 ±598 cells/mm2 in the cornea and 7120 ±362 cells/mm2 in the limbus. In the LSCD group, the mean basal cell density in the cornea decreased 31.0% (6389 ±1820 cells/mm2, p<0.001) and in the limbus decreased 23.6% (5440 ±1123 cells/mm2, p<0.001) compared to that in the control. There was a trend of basal cell density decline in more advanced stage of LSCD. The basal cell density declined in the unaffected regions at a similar degree as that in the affected region in sectoral LSCD (p>0.05). The basal cell diameter increased by 24.6% in the cornea (14.7 μm) and by 15.7% in the limbus (15.5 μm) compared to the control.
Conclusions
Basal cell density in both central cornea and limbus decreases in LSCD. LSCs are affected globally and basal cell density could be used as a parameter to measure LSC function at the early stages of the disease process.
INTRODUCTION
Limbal stem cells (LSC) are responsible for the normal homeostasis and wound repair of the corneal epithelium. When there is an insufficient number of functional stem cells, a normal transparent corneal epithelial surface cannot be maintained and the eventual result is limbal stem cell deficiency (LSCD).1,2 There are multiple etiologies of LSCD, and the hallmark is invasion of the conjunctival epithelium on the cornea. Etiologies of LSCD include multiple surgeries, Stevens-Johnson syndrome, chemical injury, chronic contact lens wear, aniridia, and chronic keratitis. Common signs of LSCD are opacity and irregularity of epithelium, recurrent epithelial defects, and neovascularization. Common symptoms include photophobia, pain, redness, tearing, and decreased vision.3,4
The limbus harbors LSCs, and the palisades of Vogt are thought to be a site of the LSC niche.5,6 Damage to the stem cell niche and its population impairs long-term regeneration of corneal epithelial cells, although acute injury to the cornea epithelium may be healed in the short term by existing corneal transient amplifying cells.7,8 Damage to the limbus also leads to the loss of the barrier that prevents invasion of the conjunctiva onto the ocular surface.9 Conjunctivalization of the corneal surface leads to significant visual loss and eventually blindness.
The diagnosis of LSCD is mostly based on clinical presentation. Persistent epithelial defect due to impaired epithelial wound healing that resulted from an insufficient number of functional LSCs is one common presentation. Stippled fluorescein staining in a vortex pattern and late fluorescein staining are indicative of abnormal corneal epithelium or conjunctival epithelium, which are typical early signs of LSCD. However, these clinical signs are often subtle and are not pathognomonic. Impression cytology can detect the presence of conjunctival goblet cells on the corneal surface and thus confirms the diagnosis of LSCD.10 However, goblet cells might be absent from some patients with chemical burns, Stevens-Johnson syndrome, or topical medication toxicity from long-term glaucoma medications.11–13 Sensitivity of impression cytology is also low. A negative result does not necessary rule out LSCD.
In vivo laser scanning confocal microscopy has been used to understand the microstructural changes in normal and pathologic conditions including various epithelial diseases, and to investigate quantifiable parameters, such as density and size.14 On the basis of previous work, corneal basal cell density and subbasal nerve density were identified as potential parameters for the diagnosis of LSCD and for the characterization of the severity of LSCD.15 In the current study, we investigated whether limbal basal cell density is another parameter in assessing LSCD.
METHODS
This observational, cross-sectional comparative study was approved by the Institutional Review Board at the University of California, Los Angles. Each patient underwent a comprehensive eye examination, slit lamp microscopy and in vivo laser scanning confocal microscopy. Twenty-five eyes also underwent impression cytology after in vivo confocal microscopy. Ten eyes of normal subjects without ocular pathology by slit lamp examination and without previous history of ocular disease were selected as the control group.
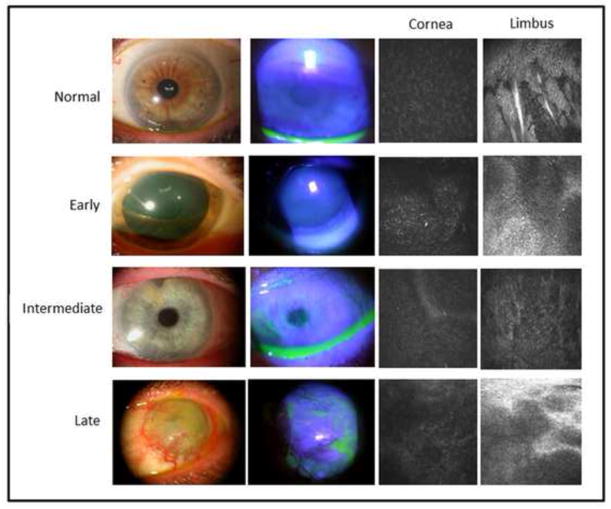
Based on the results of the slit lamp examination and fluorescein staining, we characterized LSCD in the 43 affected eyes as early, intermediate, or late stage according to the criteria previously reported.15 Briefly, the early stage was characterized by stippling or late fluorescein staining; the intermediate stage was characterized by persistent, late fluorescein staining in a vortex pattern; and the late stage was characterized by the same vortex pattern of staining and a history of cornea epithelial defect or persistent epithelial defect. Representative slit lamp photos are shown in Figure 1. Affected limbal and corneal areas were identified by the location of fluorescein staining observed during slit lamp examination. Affected areas were then stratified into superior, nasal, inferior, and temporal limbal sections. Unaffected areas were determined as the limbal sections outside of the affected regions. Each patient’s chart was also reviewed to determine any underlying etiology and predisposing factors that led to LSCD.
Figure 1.
Representative slit lamp photos (left column) and fluorescein staining patterns (middle left column) of normal controls and of patients with early, intermediate, and late stage limbal stem cell deficiency are shown. Representative in vivo confocal images of the corneal (middle right column) and limbal (right column) basal epithelium in normal controls and in patients with limbal stem cell deficiency are shown.
Confocal Microscopy Analysis
Confocal images were taken by the Heidelberg Retina Tomograph III Rostock Corneal Module Confocal Microscope (Heidelberg Engineering GmBH, Dossenheim, Germany). A minimum of three scans of the central cornea and of the superior, nasal, inferior, and temporal limbus were collected. A minimum of three image frames of the corneal or limbal basal cell layer at each location were selected if the images clearly showed cellular morphology. Each image frame was 400 × 400 μm. Two independent, masked observers then proceeded to measure basal cell density in all three images as recommended by the manufacturer and as previously reported.15 Cell size was calculated by dividing the size of the area by the number of cells within this area. The average cell diameter was then derived from the calculated cell area.
Statistical Analysis
Statistical analyses were performed using SAS software version 9.4 (SAS, Inc, Cary, North Carolina, USA). Intraclass correlation coefficients were used to assess the reliability of density measurements obtained by two independent observers. Kruskal-Wallis tests and two-tailed t-tests were used for analysis when appropriate. Any p value less than 0.05 was considered to indicate statistical significance.
RESULTS
Forty-three eyes of 30 patients diagnosed with LSCD from 2010 to 2014 were included in this study. Of those 30 patients with LSCD (Supplemental Table 1), the mean age was 59.3 years (range, 24–94 years) for the LSCD group and 46.3 years (range, 27–88 years) for the control group (p=0.14). Twenty-two eyes in the LSCD group were of female patients and 21 were of male patients (p=0.38). The diagnosis of LSCD was confirmed in 11 eyes by impression cytology. The overall leading etiologies of LSCD were contact lens wear (32.6%) and multiple surgeries (27.9%).
Two independent observers performed the basal cell density measurements in a masked fashion. There was high agreement between the observers, with an intraclass correlation coefficient of 0.977 (Supplemental Figure). The mean basal cell density of the normal control group was 9264 ± 598 cells/mm2 in the cornea and 7120 ± 362 cells/mm2 in the limbus. The highest density was in the superior limbus (7458 ± 355 cells/mm2), and lower densities were observed in the temporal limbus (7434 ± 653 cells/mm2), the inferior limbus (7042 ± 775 cells/mm2), and the nasal limbus (6782 ± 582 cells/mm2). The mean basal cell density of the nasal limbus differed significantly from that of the superior limbus (p=0.031) and the temporal limbus (p=0.039). All other pairwise comparisons between limbal regions did not reveal significant differences (Supplemental Table 2).
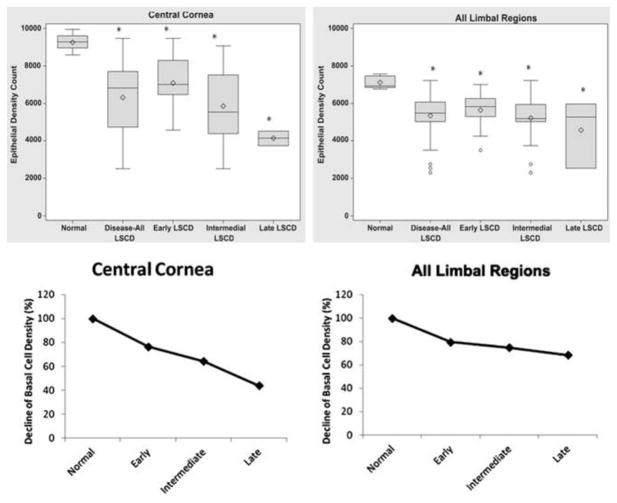
The mean basal cell density of the cornea (6389 ± 1820 cells/mm2) was 31.0% lower in the LSCD group than in the control group (p<0.001; Figure 2). In addition, the mean basal cell density of all four limbal regions (5440 ± 1123 cells/mm2) was 23.6% lower in the LSCD group than in the control (p<0.001; Figure 2). Because contact lens wear was the leading cause of LSCD in our series, we compared basal cell density in contact lens wearers and non-contact lens wearers. There was no significant difference between the two groups in corneal basal cell density (6818 ± 1668 cells/mm2 and 6068 ± 1845 cells/mm2, respectively; p=0.22) or limbal basal cell density (5386 ± 690 cells/mm2 and 5350 ± 1299 cells/mm2, respectively; p=0.69). This result indicates that the decrease in basal cell density is a general phenomenon in LSCD and not limited to the contact lens etiology.
Figure 2.
Box-whisker plots of the basal cell density of the central cornea (top left) and the average of all four limbal regions (top right) are shown. The relative basal cell density (%; limbal stem cell deficiency versus normal control) in patients with limbal stem cell deficiency in relation to that of normal control subjects is shown for the cornea (bottom left) and all limbal regions (bottom right). Abbreviation: LSCD, limbal stem cell deficiency. Each asterisk denotes p<0.05.
When the mean basal cell density of different stages of LSCD were compared, there was a trend toward a decrease in basal cell density in the cornea and limbal regions in more advanced disease. The mean basal cell density of the cornea was lower by 23.5% in the early stage, 35.6% in the intermediate stage, and 56.2% in the late stage. The differences in corneal basal cell density between respective stages were significant (for each comparison, p<0.05). Compared with the mean basal cell density of all limbal regions in control subjects, the mean basal cell density of all four limbal regions of patients with LSCD was 20.5% lower in the early stage, 25.3% lower in the intermediate stage, and 31.7% lower in the late stage (Figure 1). No significant differences in the mean limbal basal cell density were found among different stages.
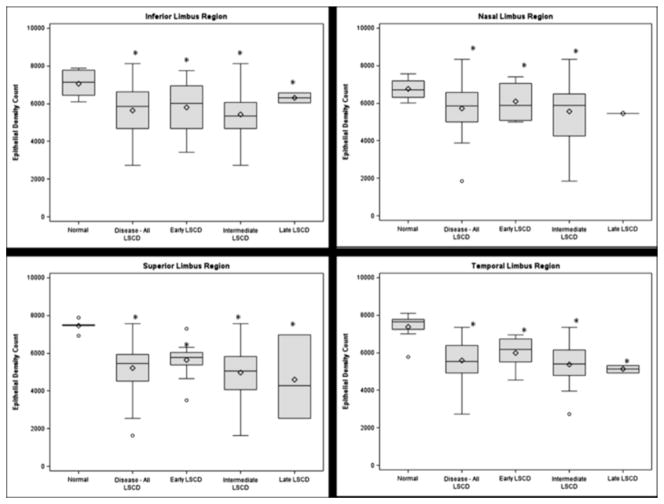
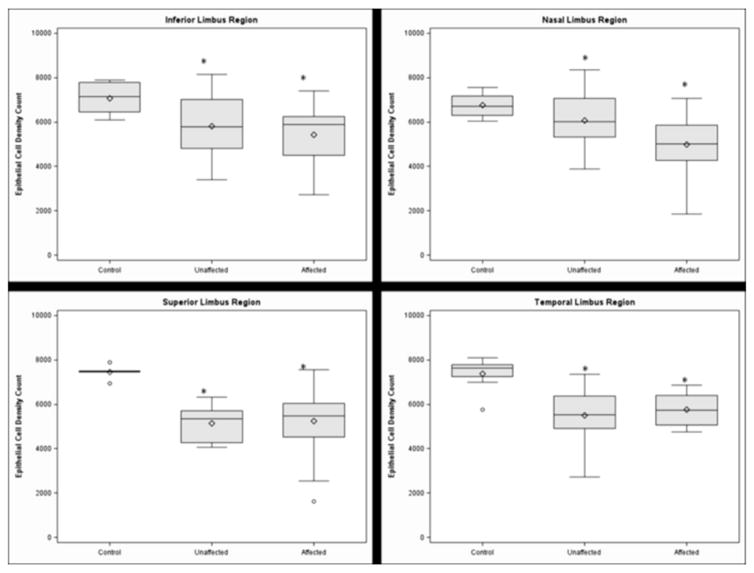
Significant decreases in densities were observed in all four limbal regions with LSCD When compared with those in the respective region in normal eyes (all p<0.05, Figure 3). Additionally, we examined the basal cell density in limbal regions that appeared clinically unaffected by LSCD. The mean basal cell density was also significantly decreased in the unaffected regions at a similar degree as in the affected regions (for each comparison, p<0.05; Figure 4). The cell borders became indistinguishable in many of the eyes with late-stage LSCD; therefore, it was not feasible to reliably determine the cell density.
Figure 3.
Box-whisker plots of the limbal basal cell density in each region are shown: inferior (top left), nasal (top right), superior (bottom left), and temporal limbus (bottom right). Each asterisk denotes p<0.05.
Figure 4.
Box-whisker plots of the limbal basal cell density of the clinically affected and unaffected regions in the inferior (top left), nasal (top right), superior (bottom left), and temporal limbus (bottom right). Each asterisk denotes p<0.05.
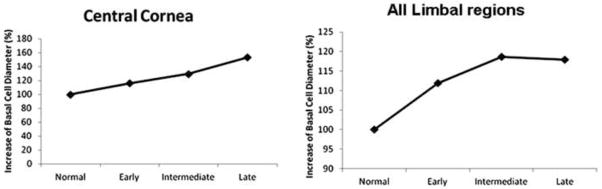
When cell density was converted to cell size, the mean cell diameters of the normal control group were 11.8 μm in the cornea and 13.4 μm in the limbus (Supplemental Table 4). The mean cell diameters of the LSCD group were 14.7 μm in the cornea and 15.5 μm in the limbus (Figure 4).
DISCUSSION
Use of in vivo laser scanning microscopy has increased in the diagnosis of infectious keratitis, corneal dystrophies, and recently LSCD. Previous reports have described cellular changes in different stages of LSCD.15–17 In the current study, we have expanded the investigation of basal cell density in limbal regions. All measurements were obtained in a masked fashion by two independent observers. A very high interclass correlation (0.977) of the measurements indicates that the potential for variation is low. In addition, the average cell diameter in the control obtained in our study, 11.8 μm, is within the reported range of 10–12 μm,18–21 and the basal cell density of the limbus in our normal control is also comparable to those previously published. 21 These findings further validate our method used in the current study.
This larger study confirms our previous findings that basal cell density in the central cornea decreases significantly in eyes with LSCD and that the degree of reduction positively correlates with the severity of the disease. Basal cell density was also found to decrease significantly in the limbus in eyes with LSCD. A significant decrease (8.9%) in limbal basal cell density in an older age group (age >45 years) has been reported.21 The decrease in our LSCD group was more than 20.5% and the average ages of subjects in our control group and LSCD group were similar. Therefore, we believe that this large reduction of basal cell density in the limbus of patients with LSCD was not due to age but due to the pathology of LSCD. This reduction was not limited to contact lens–wearing subjects, but rather a general phenomenon seen in all other etiologies of LSCD.
Congenital aniridia–induced LSCD has been studied with in vivo confocal microscopy, and findings of such studies have typically been similar to those seen in LSCD in general.16,17 In particular, basal cell density in patients with severe aniridia-related keratopathy was significantly reduced and comparable in value to the basal cell density in patients with late-stage disease in our study. Based on basal cell density alone, it appears that in later stages of aniridia-related keratopathy, the damage to the limbus may be as much or more than that due to other etiologies of LSCD. It is also possible that due to loss of cell borders in the late stage of LSCD, we were unable to obtain a reliable cell count. This has led to a sampling bias in the advanced stage of LSCD towards a higher basal cell density.
Although in vivo confocal microscopy has yet to definitely distinguish LSCs, cellular characteristics such as a high nuclear:cytoplasic ratio and small cell size have been suggested. Limbal crypts within the regions of Palisades of Vogt and deep limbal stroma are regions where LSCs are proposed to locate.6,22–24 These limbal crypt structures are found to be predominantly in the superior and inferior limbus. Injury to these regions may exert a more detrimental effect on the LSCs. In addition, a decrease in basal cell density corresponds to a general increase in basal cell size in eyes with LSCD (Supplement Table 3). This result suggests that there was a loss of the smaller cell population in the limbus that are presumed to be LSCs given their expression pattern and have been found to have greater proliferative potential in vitro.25 When there are insufficient LSCs, there might be more stress on the remaining stem cells to proliferate more frequently than they are capable of. This stress in turn could further deplete the remaining stem cell population. These larger cells could represent differentiated cells, which express different cell surface markers and possibly influence cell-cell interactions.26 Additionally, these larger cells have limited regenerative potential.
A significant reduction in basal cell density was already seen in the early stage of LSCD in both the cornea (23.5%) and limbus (20.5%), and basal cell density was further decreased in intermediate stage. Therefore, basal cell density reduction in the cornea and limbus is likely to be an early sign of LSCD. These findings also suggest LSCD to be a much more insidious with widespread progression that cannot be observed clinically at the early stage. In addition, the cellular changes may occur as a global phenomenon and precede the clinical presentation in LSCD. Invasion of conjunctival epithelial cells onto the cornea is likely a late-stage finding, and this explains the low yield of positive impression cytology results in the early stage of LSC dysfunction/deficiency. This hypothesis is also supported by a surprising finding from the current study that the degree of basal cell density reduction was similar in the clinically affected and unaffected limbal regions in eyes with sectoral LSCD. Therefore, basal cell density could be one parameter to assess the population and function of LSCs. Identification of such early dysfunction of LSCs can be used to guide treatment and prevent further damage. The cornea has the ability to repair itself in the short term by using its remaining transient amplifying cells.7 Signs of dysfunction of the limbus may be best detected by in vivo laser scanning confocal microscopy.
Accurate staging of LSCD is important in monitoring disease progression and treatment outcome. Transplantation of autologous stem cells has been shown to cure LSCD.27 However, quantitative criteria for identification of candidates for transplantation are lacking. Parameters such as corneal and limbal basal cell density, and subbasal nerve density could be used to classify the degree of LSCD. Seeing firsthand the health of the epithelial tissue at a cellular level can provide a better evaluation of the viability of the graft and the recovery of normal corneal epithelium.28
There are limitations to this study. Assessment of epithelial cell density requires discernible cells visualized on image frames. Loss of clear cellular borders, cell clumping, and fibrosis in advanced disease limited the measurement of cell density and perhaps led to some bias toward the selection of only those regions that were less severely affected to obtain cell counts in the intermediate and late stages. Patients with late stage LSCD were commonly found to have corneal and limbal epithelium replaced by conjunctival cells accompanied by stromal scarring and infiltration of inflammatory cells. Thus, limbal basal cell density often could not be obtained in late stage disease because very few basal cells could be identified. Because of such selection bias in the more advanced stage of LSCD, the actual basal cell density in more severe LSCD is probably much lower than the values we obtained. The assessment of basal cell density in such late stage might be better relied on the characteristics of cell morphology.29
In summary, basal cell density in the limbus significantly decreases in LSCD, and basal cell density could serve as one of the parameters to measure LSC function, particularly in the early stage of the disease process.
Supplementary Material
Figure 5.
Relative basal cell diameter (%; limbal stem cell deficiency versus normal control) is shown for the central cornea (left) and total limbus (right).
Acknowledgments
Funding/Support: This work was funded in part by an unrestricted grant from Research to Prevent Blindness. LC was supported by the National Nature Science Foundation of China (Youth project, No.81201643), China Postdoctoral Science Foundation (No.128357).
Footnotes
Financial Disclosures: SXD received grant support from the National Eye Institute (R01 EY021797) and California Institute for Regenerative Medicine (TR2 TR2-01768) to study limbal stem cells. All other authors have no financial disclosures.
References
- 1.Notara M, Alatza A, Gilfillan J, et al. In sickness and in health: Corneal epithelial stem cell biology, pathology and therapy. Experimental eye research. 2010;90(2):188–195. doi: 10.1016/j.exer.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Tseng SC. Concept and application of limbal stem cells. Eye. 1989;3(Pt 2):141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 3.Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency: concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2000;48(2):83–92. [PubMed] [Google Scholar]
- 4.Sejpal K, Bakhtiari P, Deng SX. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr J Ophthalmol. 2013;20(1):5–10. doi: 10.4103/0974-9233.106381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlotzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Experimental eye research. 2005;81(3):247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 6.Dziasko MA, Armer HE, Levis HJ, Shortt AJ, Tuft S, Daniels JT. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal crypt. PLoS One. 2014;9(4):e94283. doi: 10.1371/journal.pone.0094283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang CY, Green CR, McGhee CN, Sherwin T. Acute wound healing in the human central corneal epithelium appears to be independent of limbal stem cell influence. Investigative ophthalmology & visual science. 2008;49(12):5279–5286. doi: 10.1167/iovs.07-1260. [DOI] [PubMed] [Google Scholar]
- 8.Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. The British journal of ophthalmology. 1994;78(5):401–408. doi: 10.1136/bjo.78.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dua HS. The conjunctiva in corneal epithelial wound healing. The British journal of ophthalmology. 1998;82(12):1407–1411. doi: 10.1136/bjo.82.12.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102(10):1476–1485. doi: 10.1016/s0161-6420(95)30842-1. [DOI] [PubMed] [Google Scholar]
- 11.Arici MK, Arici DS, Topalkara A, Guler C. Adverse effects of topical antiglaucoma drugs on the ocular surface. Clinical & experimental ophthalmology. 2000;28(2):113–117. doi: 10.1046/j.1442-9071.2000.00237.x. [DOI] [PubMed] [Google Scholar]
- 12.Herreras JM, Pastor JC, Calonge M, Asensio VM. Ocular surface alteration after long-term treatment with an antiglaucomatous drug. Ophthalmology. 1992;99(7):1082–1088. doi: 10.1016/s0161-6420(92)31847-0. [DOI] [PubMed] [Google Scholar]
- 13.Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Archives of ophthalmology. 1984;102(7):1049–1051. doi: 10.1001/archopht.1984.01040030851031. [DOI] [PubMed] [Google Scholar]
- 14.Villani E, Baudouin C, Efron N, et al. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr Eye Res. 2014;39(3):213–231. doi: 10.3109/02713683.2013.842592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng SX, Sejpal KD, Tang Q, Aldave AJ, Lee OL, Yu F. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Archives of ophthalmology. 2012;130(4):440–445. doi: 10.1001/archophthalmol.2011.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagali N, Eden U, Utheim TP, et al. In vivo morphology of the limbal palisades of vogt correlates with progressive stem cell deficiency in aniridia-related keratopathy. Investigative ophthalmology & visual science. 2013;54(8):5333–5342. doi: 10.1167/iovs.13-11780. [DOI] [PubMed] [Google Scholar]
- 17.Le Q, Deng SX, Xu J. In vivo confocal microscopy of congenital aniridia-associated keratopathy. Eye. 2013;27(6):763–766. doi: 10.1038/eye.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miri A, Al-Aqaba M, Otri AM, et al. In vivo confocal microscopic features of normal limbus. The British journal of ophthalmology. 2012;96(4):530–536. doi: 10.1136/bjophthalmol-2011-300550. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi A, Sugiyama K. In vivo corneal confocal microscopic findings of palisades of Vogt and its underlying limbal stroma. Cornea. 2005;24(4):435–437. doi: 10.1097/01.ico.0000151542.15736.da. [DOI] [PubMed] [Google Scholar]
- 20.Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, Tseng SC. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Investigative ophthalmology & visual science. 2003;44(12):5125–5129. doi: 10.1167/iovs.03-0628. [DOI] [PubMed] [Google Scholar]
- 21.Patel DV, Sherwin T, McGhee CN. Laser scanning in vivo confocal microscopy of the normal human corneoscleral limbus. Investigative ophthalmology & visual science. 2006;47(7):2823–2827. doi: 10.1167/iovs.05-1492. [DOI] [PubMed] [Google Scholar]
- 22.Shortt AJ, Secker GA, Munro PM, Khaw PT, Tuft SJ, Daniels JT. Characterization of the limbal epithelial stem cell niche: novel imaging techniques permit in vivo observation and targeted biopsy of limbal epithelial stem cells. Stem cells. 2007;25(6):1402–1409. doi: 10.1634/stemcells.2006-0580. [DOI] [PubMed] [Google Scholar]
- 23.Notara M, Daniels JT. Biological principals and clinical potentials of limbal epithelial stem cells. Cell and tissue research. 2008;331(1):135–143. doi: 10.1007/s00441-007-0458-7. [DOI] [PubMed] [Google Scholar]
- 24.Dua HS, Shanmuganathan VA, Powell-Richards AO, Tighe PJ, Joseph A. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89(5):529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Paiva CS, Pflugfelder SC, Li DQ. Cell size correlates with phenotype and proliferative capacity in human corneal epithelial cells. Stem cells. 2006;24(2):368–375. doi: 10.1634/stemcells.2005-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HS, Jun Song X, de Paiva CS, Chen Z, Pflugfelder SC, Li DQ. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004;79(1):41–49. doi: 10.1016/j.exer.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rama P, Matuska S, Paganoni G, Spinelli A, De Luca M, Pellegrini G. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363(2):147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 28.Hong J, Zheng T, Xu J, et al. Assessment of limbus and central cornea in patients with keratolimbal allograft transplantation using in vivo laser scanning confocal microscopy: an observational study. Graefes Arch Clin Exp Ophthalmol. 2011;249(5):701–708. doi: 10.1007/s00417-011-1616-x. [DOI] [PubMed] [Google Scholar]
- 29.Nubile M, Lanzini M, Miri A, et al. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. American journal of ophthalmology. 2013;155(2):220–232. doi: 10.1016/j.ajo.2012.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.