Abstract
Based on data obtained from oral, pancreatic and lung cancers, glioblastoma, and melanoma, we have established that natural killer (NK) cells target cancer stem-like cells (CSCs). CSCs displaying low MHC class I, CD54, and PD-L1 are killed by cytotoxic NK cells and are differentiated by split anergized NK cells through both membrane bound and secreted forms of TNF-α and IFN-γ. NK cells select and differentiate both healthy and transformed stem-like cells, resulting in target cell maturation and shaping of their microenvironment. In our recent studies, we have observed that oral, pancreatic, and melanoma CSCs were capable of forming large tumors in humanized bone marrow, liver, thymus (hu-BLT) mice with fully reconstituted human immune system. In addition, major human immune subsets including NK cells, T cells, B cells, and monocytes were present in the spleen, bone marrow, peripheral blood, and tumor microenvironment. Similar to our previously published in vitro data, CSCs differentiated with split anergized NK cells prior to implantation in mice formed smaller tumors. Intravenous injection of functionally potent osteoclast-expanded NK cells inhibited tumor growth through differentiation of CSCs in humanized mice. In this review, we present current approaches, advances, and existing limitations in studying interactions of the immune system with the tumor, in particular NK cells with CSCs, using in vivo preclinical hu-BLT mouse model. In addition, we discuss the use of osteoclast-expanded NK cells in targeting cancer stem-like tumors in humanized mice—a strategy that provides a much-needed platform to develop effective cancer immunotherapies.
Keywords: Osteoclast-expanded NK cells, Cancer immunotherapy, BLT humanized mice, CSCs, CITIM 2015
Natural killer cells select and differentiate healthy and transformed stem cells
There is increasing evidence that many human tumors contain a stem-like population, known as cancer stem-like cells (CSCs), which are capable of self-renewal and sustain tumor growth. Our laboratory, as well as others, has previously shown that natural killer (NK) cells target both normal hematopoietic stem cells (HSCs) and malignant stem-like cells [1–3]. NK cells lose the ability to mediate cytotoxicity and down-modulate CD16 receptor expression upon interaction with CSCs, while they maintain high levels of interferon-γ (IFN-γ) secretion, a functional outcome that we have previously coined as split anergy [4–8]. In contrast, differentiated tumor cells neither induce down-modulation of CD16 nor induce split anergy in NK cells [5, 9].
Based on our data obtained from a number of tumors including oral squamous cancer cells, glioblastoma, melanoma, pancreatic and lung cancer, we have established that CSCs with low major histocompatibility complex (MHC) class I, CD54, and PD-L1 (also known as programmed death-ligand 1, B7H1) expression are killed by cytotoxic NK cells, while the remaining CSCs can be differentiated by split anergized NK cells through cell–cell contact and secretion of membrane bound and secreted forms of tumor necrosis factor-α (TNF-α) and IFN-γ, respectively [10]. We have also shown that differentiation by split anergized NK cells leads to growth inhibition of CSCs and upregulation of MHC class I, CD54, and PD-L1 expression, resulting in resistance of differentiated tumor cells to NK cell-mediated cytotoxicity [10, 11].
Studies conducted using mice have provided valuable insights into the tumor and immune cell interactions. However, despite overall structural similarity, human and murine immune systems are significantly different. The major immune cells in human blood are neutrophils (50–70 %), while lymphocytes constitute only 30–50 %. The proportions in murine blood are quite distinct: the majority of cells are lymphocytes (75–90 %), with B cells being the most abundant [12]. Major differences have also been noted between human and mouse innate immune systems (reviewed in [12]). Although proportions of NK cells in murine and human blood are similar, their phenotypes and activation dynamics are quite distinct [12, 13]. CD3−/CD16+CD56dim phenotype is typical for human cytotoxic NK cells, whereas the regulatory CD3−/CD16low/negCD56bright NK cells mediate differentiation of stem cells in humans [2, 14]. Murine NK cells can be characterized by DX5 expression and CD11b+/CD27− and CD11b−/CD27+ phenotypes, respectively [13, 15, 16]. We have recently shown that primary non-activated human NK cells are able to mediate cytotoxicity, and upon interleukin-2 (IL-2) activation, significantly increase their cytotoxic potential against a number of human stem-like tumors after a short period of activation. In contrast, naïve murine NK cells do not mediate significant cytotoxicity and require an extended period of IL-2 activation to become cytotoxic against the majority of tumor targets [17]. Human NK cells use killer-cell immunoglobulin-like receptor (KIR) family members as inhibitory receptors for MHC class I molecules, whereas murine NK cells rely on Ly49 protein family members with highly divergent structures. In addition, human NKG2D receptors bind to MICA, MICB and/or ULBP 1–6 ligands, whereas mouse ligands for NKG2D include HAE60 and Rae1β [12, 13]. Therefore, investigators with an interest in studying the role of NK cells in tumor immunity should consider these differences when using mice in pre-clinical models of human cancers.
In this review, we discuss current strategies, advances and existing limitations in studying interactions of the immune system, in particular NK cells, with cancer cells in humanized mice—the best available preclinical model to test novel cancer immunotherapies. In addition, we present our recent studies that demonstrate the potential use of human NK cells in in vivo selection and differentiation of cancer stem-like tumors in hu-BLT mice.
NK cells in xenogeneic implantation of human cells
The use of athymic nude (Foxn1) mice that are T cell deficient, and severe combined immunodeficiency (scid) mice initiated an era of immunodeficient mice for xenotransplantation [18–20]. Nude mice, the oldest mouse model, have an intact humoral adaptive system and innate immune system characterized by high NK cell activity, which limits engraftment with most primary solid human tumors and virtually disqualifies engraftment of normal or malignant human hematopoietic cells ([21], Table 1). The discovery of the spontaneous “scid” mutation in the C.B17 strain [18], allowed for engraftment of a broader range of human solid tumors that could not persist in nude mice ([22], Table 1).
Table 1.
Advantages and limitations of common immunodeficient and humanized mouse models used in studies of NK cells in tumor immunity
| Mouse strain/model | Murine NK cells | Human NK cells | Engraftment of solid tumor cell lines, Engraftment of primary tumors | Engraftment of hematologic malignancies, Engraftment of normal hematopoietic cells | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|---|
| Athymic nude | +++ | − | +, ± | −, − |
Well-characterized, hairless, s.c. tumors are easy to assess |
Intact humoral adaptive system and innate immune system Very limited engraftment mainly due to high NK cell activity |
[21, 64] |
| C.B17-scid | ++ | − | +, + | ±, ± | Allows for engraftment of a broader range of human solid tumors compared to nude mice |
Intact innate immune system: normal antigen presentation, myeloid cell development, and NK cell functions Limited engraftment due to remaining NK cell activity “Leakiness” in older mice –production of small numbers of mature T and B cells |
[18, 22, 65] |
| NOD-scid | + (Defective) | − | ++, ++ | +, + | Allows for engraftment of large numbers of solid tumors and hematological malignancies. |
Some tumors fail to engraft or grow efficiently, mainly due to remaining NK cell activity “Leakiness” of residual T and B cells Mice develop thymic lymphomas by 8–9 months, not suitable for long-term studies Sensitivity to radiation |
[23–26] |
| NSG | − | − | +++, +++ | ++, +++ |
Profoundly immunodeficient Allows for engraftment of a variety of solid tumors and blood cancers Allows for engraftment and differentiation of human hematopoietic stem cells into multi-lineage subsets Best strain for humanization |
Human cell engraftment is better in newborns compared to adults. Sensitivity to radiation |
[27–29, 66] |
| CD34+ hu-NSG | − | + (not fully functional, decreased NK cell receptors) | +++, +++ (PDX) | ++, +++ |
Permits differentiation of all major human blood cell types including NK cells Stable, long-term engraftment Allows for growth of non-HLA-matched CSC-like tumors Can be used for testing cancer immunotherapeutics |
Human NK cells are present in low numbers and they require IL-15/IL-15Rα to increase function T cells are selected in the context of mouse MHC Sensitivity to radiation Limited mucosal immunity |
([28, 29, 38, 43–46, 54], data not published) |
| Hu-BLT hu-NSG | − | + (not fully functional, decreased NK cell receptors) | +++, not determined | Not determined, +++ |
Permits differentiation of all major human blood cell types including NK cells T cells are HLA-restricted Mucosal and adaptive immune functions Allows for growth of non-HLA-matched CSC-like tumors Best available model for testing cancer immunotherapeutics in the context of fully reconstituted human immune system |
Human NK cells are detected in low numbers Fetal tissue accessibility Surgical procedure required More prone to GvHD than CD34+ humanized model Sensitivity to radiation |
([28, 33, 34, 39], data not published) |
The rejection of tumor and hematopoietic xenografts was partially overcome with the development of the non-obese diabetic (NOD)-scid strain via introduction of the Prdkcscid mutated gene from C.B17-scid mice into a NOD inbred strain with several impairments in innate immunity [23]. The resulting NOD-scid mice were deficient in NK cells [24, 25]. Although NOD-scid mice support the growth of a large number of solid tumors and hematological malignancies, still a portion of tumors fails to engraft or grow efficiently, mainly due to the remaining NK cell activity and other residual innate immune functions ([24, 26], Table 1).
The most recent introduction of a genetically engineered complete null mutation of the interleukin 2 receptor subunit gamma (IL2Rγ) into the NOD-scid mice gave rise to one of the most immunodeficient strains known to date—NOD-scid IL2Rγnull (NSG) [27]. NSG, as well as NOD-Rag1nullIL2Rγnull (NRG) strains, are profoundly immunodeficient, which is the key feature that supports growth of various malignancies, and most importantly, differentiation of human HSCs into multi-lineage subsets ([28, 29], Table 1).
Humanized mice as a platform for studying human NK cells in the context of the reconstituted human immune system
Various strategies have been implemented to create humanized mice with modifications in the source and type of donor cells, injection route, recipient age, type of transplanted cells, irradiation source, and surgical implantation of human tissues to support immune cell reconstitution. One of the simplest approaches involves injection of immunodeficient mice with human peripheral blood mononuclear cells (PBMCs) obtained from healthy donors or patients [30, 31]. Peripheral blood mononuclear cells circulate in the blood and either die or migrate to other tissues, but the utility of such mice is limited to short-term experiments as mature immune cells circulating in the body initiate graft versus host (GvHD) disease against murine recipients [32]. CD34+ cells injected into newly born or adult NSG mice stably engraft bone marrow and are capable of differentiating into all the hematopoietic lineages of the human immune system. The major limitation of the CD34+ humanization model is the lack of human thymus ([28], Table 1). The hu-BLT represents the most advanced and complete humanization model created to date [28]. The engraftment protocol includes surgical implantation of human fetal liver and thymus under the renal capsule of NSG mice followed by the intravenous injection of CD34+ hematopoietic cells from the same donor to support full reconstitution of human bone marrow [33, 34]. Positive and negative selection of developing T cells in such mice occurs in the presence of human thymus. Hu-BLT is the only known humanized mouse model that displays mucosal immunity [35]. HSCs develop, at least to some extent, into T cells, B cells, NK cells, monocytes, myeloid-derived suppressor cells (MDSCs), macrophages, dendritic cells (DCs), erythrocytes, and platelets in tissues of hu-BLT ([29, 36–38], Table 1). Long-term peripheral reconstitution of human CD45+ immune cells is usually within the 30–80 % range as detected in the peripheral blood, spleen, and bone marrow (Fig. 1, manuscript in preparation). Human immune cells are detected in the reproductive tract of females, intestines and rectum [39, 40], and gingiva (manuscript in preparation). As demonstrated by Stoddart et al. [35], hu-NSG-BLT mice have substantially higher levels of human leukocyte reconstitution in peripheral blood than hu-NOD-scid-BLT mice. However, the immune system suffers from several weaknesses, including the poor development of antigen-specific antibody response [41]. The above features make NSG-based hu-BLT mice arguably the best available model for studying human immunity, thus far.
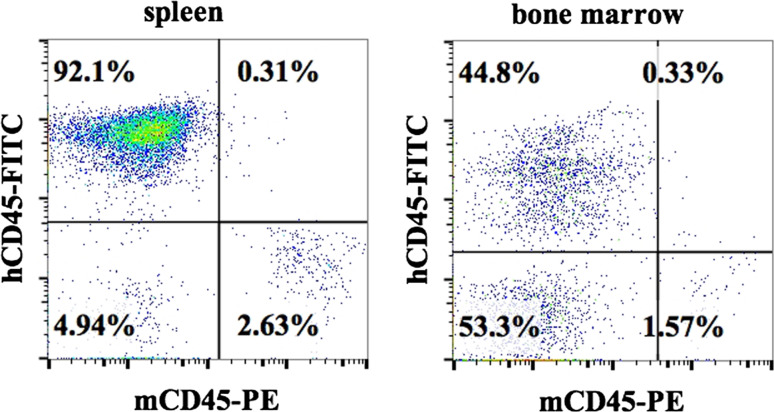
Fig. 1.
Reconstituted human immune cells in hu-BLT mice represent the majority of immune effectors. Hu-BLT mice were prepared on NSG background by surgical implantation of pieces of human fetal liver and thymus under the renal capsule followed by the intravenous injection of CD34+ hematopoietic cells from the same donor to support full reconstitution of human bone marrow. Percentages of human and murine immune cells were analyzed in cells isolated from spleen (left) and bone marrow (right) 12 weeks after reconstitution by staining with anti-human-CD45 and anti-mouse-CD45 antibodies followed by flow cytometry
Based on our previous data, we selected human oral squamous cancer stem cells (OSCSCs) as a candidate to test tumor initiation and growth in the presence of the non-histocompatibility leukocyte antigen (HLA)-matched mature immune system in hu-BLT mice [1, 10]. We found that non-HLA-matched OSCSCs were not rejected when injected 8–12 weeks after reconstitution of the human immune system and they could form visible tumors in the oral cavity of humanized animals. We also observed infiltration of major human immune subsets including NK cells, T cells, B cells and monocytes in the tumor microenvironment. Interestingly, OSCSCs that were differentiated with split anergized NK cell supernatants containing TNF-α and IFN-γ prior to injection grew much slower in humanized mice. Similarly, we demonstrated the growth of cancer stem-like pancreatic tumors and melanoma in hu-BLT mice, whereas in vitro differentiation of pancreatic CSCs before injection led to upregulation of surface receptors including MHC class I and inhibited growth of tumor cells, both in culture systems and formation of tumors in vivo (manuscript in preparation). Our data are consistent with JAX laboratories, which demonstrated growth of patient-derived xenografts (PDXs) of different origins in CD34+ hu-mice without prior matching (unpublished data). However, because differentiation stages of those PDXs were not known, it is likely that those tumors contained CSC populations, capable of giving rise to primary tumors in the presence of competent T cells.
In a study by Vatakis [42], two different melanoma cell lines derived from patients were successfully implanted in hu-BLT mice and treated with T cell based immunotherapy. Interestingly, in our studies, NSG immunodeficient mice developed relatively larger tumors with faster kinetics when compared to hu-BLT mice. Similarly, mice bearing K562 erythroleukemia tumors and injected with CD34+ HSC progenitors survived significantly longer in comparison with their K562 bearing immunodeficient Balb/c Rag2−/− γc −/− counterparts suggesting growth inhibition of malignant cells by reconstituted human immune cells [43].
Our laboratory has demonstrated that aggressive stem-like tumors, including melanoma, oral and pancreatic CSCs, are characterized by low classical MHC class I expression and therefore, may be resistant to recognition and lysis by engrafted allogeneic T cells, which could, in part, explain the lack of rejection of cancer cells in mice by non-HLA-matched immune cells ([1, 10, 11], manuscript in preparation). However, cancer cells lacking MHC class I expression should be susceptible to lysis by NK cells. Collectively, the data that we, and others, have obtained suggest that autologous NK cells reconstituted in humanized mice might be lower in number and function to effectively prevent CSCs from establishing and growing.
Little is known about the phenotype and function of NK cells in humanized mice. Accumulated evidence suggests that NK cell numbers and functions in both CD34+ and hu-BLT mouse models do not precisely reflect those observed in humans [41, 44–47]. Along with others, we have also observed low frequencies and decreased cytotoxicity of NK cells in peripheral blood, bone marrow, spleen and liver of humanized animals in comparison with data obtained from human peripheral blood. Implantation of stem-like tumors further decreased cytotoxicity and cytokine secretion of reconstituted NK cells (manuscript in preparation).
NK cell development and maintenance is regulated by a variety of factors, IL-15 being one of the most important [48, 49]. Deficiency in IL-15 has been shown to result in the absence of mature NK cells in both mice and humans [50, 51]. It has been demonstrated that NK cells were developed in CD34+ Balb/c Rag2−/− IL2Rγ−/− humanized mice in the absence of IL-15; however, they were detected in much lower numbers, mainly in the thymus and lymph nodes [44]. These NK cells displayed CD56bright/CD16− phenotype similar to NK cells isolated from human lymph nodes. Following 24-h activation with IL-2, CD56bright/CD16− NK cells secreted IFN-γ but did not mediate cytotoxicity [52]. A decrease in cytotoxicity accompanied by significant IFN-γ production and CD56bright phenotype is also the hallmark of split anergized NK cells treated with IL-2+ CD16mAb [11]. Chen et al. also showed that human NK cells were detected at very low percentages in peripheral blood, bone marrow, lung, spleen, and liver of CD34+ NSG hu-mice.
The delivery of human IL-15/IL-15Rα but not IL-15 alone drastically improved NK cell development in bone marrow of humanized mice [46]. Strowig et al. [38] demonstrated that NK cells reconstituted from human HSCs in NSG mice were present in all blood-perfused organs including spleen, liver, bone marrow and lungs. However, about 50 % of those reconstituted NK cells expressed NKp46+ but not CD56, and thus resembled immature NKp46+/CD56− NK cells found in human cord blood (hCB). Consistently, both reconstituted NK cell populations and hCB NK cells are reported to have decreased cytotoxicity and significantly lower cytokine production when compared to NK cells from human peripheral blood, but those functions can be restored with either IL-15 or polyinosinic/polycytidylic acid (poly(I:C)) treatment. Interestingly, several groups reported the presence of NKp46+/CD56− “resting” or “immature” NK cell phenotype in humanized models that could explain differential function and activation of reconstituted NK cells in comparison with human peripheral blood NK cells [46, 53, 54]. These findings collectively suggest the pre-activation requirement for reconstituted NK cells in humanized mice in order to obtain fully functional and potent NK cells that can mimic human adult peripheral blood NK cells. Whether NKp46+/CD56− subsets are immature NK populations or those that have interacted in vivo with the targets and subsequently down-modulated CD56 surface receptors requires further investigation.
NK cell receptor downregulation as the potential mechanism for the detection of low NK cell frequencies in hu-BLT
Human NK cells are detected and further divided into cytotoxic and regulatory phenotypes using common NK cell surface receptors such as CD16 and CD56. Among four stages of NK cell maturation, CD16+CD56dimCD69− NK cells are found to select and kill stem cells, whereas the regulatory CD16−CD56brightCD69+ NK cells mediate differentiation [2, 55]. In addition, expression of activating receptors such as NKp30, NKp44 and NKp46 is used to determine frequency and activation status of NK cells [55, 56].
It is known that the cytotoxic function of NK cells is suppressed in the tumor microenvironment by a number of distinct factors and decreased peripheral blood NK cell cytotoxicity has also been reported in cancer patients. In addition, NK cell cytotoxicity is suppressed after their interaction with undifferentiated cells, such as CSCs or immature immune cells including monocytes, which leads to a gain in NK cell regulatory function. As a result of interaction of NK cells with CSCs, downregulation of CD16 receptor, as well as other activating receptors, has been reported in a variety of tumors, which correlates with disease progression [57–60]. In this regard, we have also analyzed the frequencies of circulating CD3−/CD14−/CD19−/CD16−/CD56dim/neg NK cells in stage III and IV progressing melanoma patients and found higher percentages of CD16 and CD56 receptor-low/negative NK cells in these patients in comparison with stage II melanoma patients responding to therapy, as well as healthy individuals (manuscript in preparation). The increased frequencies of such receptor-low/negative NK cells in patients with advanced stages of cancer may be the result of prior receptor triggering, potent signaling and eventual down-modulation of these NK cell receptors by CSCs, making such NK cells virtually undetectable if these receptors are used for their identification.
Similar receptor down-modulation may also occur for NK cells in the xenogeneic environment of humanized mice. It has been reported that reconstituted NK cells display “immature” or “anergized” phenotypes that can be characterized by downregulation of several receptors including CD16 [38, 45, 47]. NKp46+/CD56neg NK cells of similar phenotype to hCB NK cells have also been detected in CD34+ humanized mice [61].
The downregulation of receptors commonly used for NK cell detection and phenotyping raises questions regarding the true frequencies of NK cells in cancer patients as well as in humanized mice. In our recent in vivo studies, we have observed that autologous human NK cells isolated from hu-BLT mice with or without tumors expressed very low levels of common NK cell receptors and were also poorly cytotoxic. Similar receptor down-modulation to those of autologous NK cells was also observed when osteoclast-expanded NK cells were injected into hu-BLT humanized mice (manuscript in preparation). Interestingly, even though we could see very low numbers of NK cells using common NK cell markers at the initiation of ex vivo cultures, we could detect significant expansion and numbers of NK cells after a week of culture with IL-2. Further ex vivo expansion and IL-2 activation of such cells not only led to restoration of NK cell receptor expression but also augmented cytotoxicity.
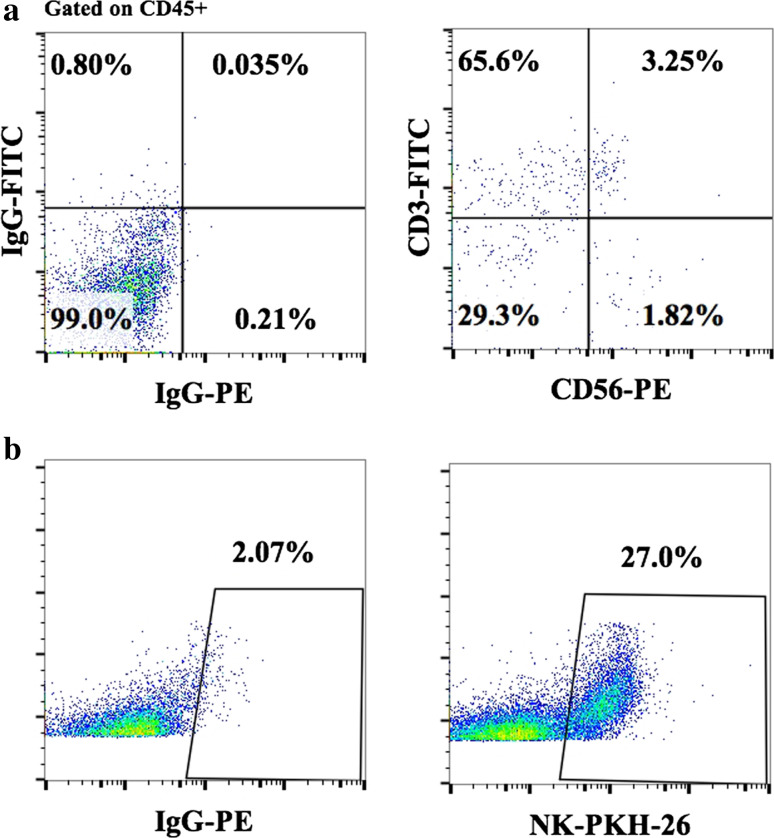
To track distribution of osteoclast-expanded NK cells in various tissue compartments, NK cells were labeled with Paul Karl Horan fluorescent dye (PKH-26) prior to intravenous injection into tumor-bearing hu-BLT animals. Indeed, PKH-26-stained NK cells could be detected in various tissue compartments including those in the tumor microenvironment, even though no or much lower frequencies of NK cells were identified using NK cell receptor surface expression (Fig. 2, manuscript in preparation). Thus, such observations underscore the need for novel or improved detection methods to determine the true frequencies of receptor-low NK cells in vivo.
Fig. 2.
Detection with common NK cell surface markers does not reflect the true frequencies of NK cells in humanized mice. Hu-BLT mice were prepared on NSG background, as described in Fig. 1, then subcutaneously injected with human melanoma cells, followed by therapeutic injection of osteoclast-expanded NK cells. Percentages of NK cells stained with CD45, CD3 and CD56 anti-human antibodies (a) or PKH-26-labeled expanded NKs (b) were analyzed in isolates from spleen 4 days after intravenous injection of 2 × 106 osteoclast-expanded NK cells by flow cytometric analysis
Adoptive transfer of ex vivo osteoclast-expanded NK cells eliminated cancer stem-like tumors in hu-BLT mice
As of today, very few reports have shown functional NK cell responses against tumors in humanized mice. Chen and colleagues demonstrated that IL-15 cytokine-induced NK cells isolated from CD34+ hu-mice mediated cytotoxicity against K562 cells and their treatment with poly(I:C) or culture with DC-triggered secretion of IFN-γ [45]. Consistently, NK cells that were not activated by IL-15 did not produce IFN-γ, even after poly(I:C) treatment. In addition, CD34+ hu-mice injected systemically with K562 erythroleukemia tumors were able to clear tumors over a long period of time, whereas CD56-depleted animals and immunodeficient mice were not protected by human CD56+ cells and had detectable K562 cells in all major body compartments. Moreover, splenocytes isolated from tumor-bearing animals produced more IFN-γ when re-stimulated with K562 cells in comparison with naïve mice [43].
Wege and colleagues found increased frequencies and activation of NK cells in humanized tumor mice (HTM) that were generated by simultaneous co-injection of HSCs and tumor cells [54]. NK cell numbers and activation were higher in HTM in comparison with naïve tumor-free humanized mice and IL-15/IL-15Rα further increased frequencies of mature NKp46+/CD56+ phenotype, as well as IFN-γ production by these cells in HTM. Collectively, cytokine-activated NK cells have the capacity to interact with NK cell sensitive human tumors; however, the precise mechanisms underlying elimination of these tumors by NK cells are not known.
In order to overcome the requirement for IL-15 and compensate for the lower frequencies of NK cells observed in humanized mice, we generated efficient ex vivo osteoclast-expanded human NK cells for adoptive NK cell transfer therapy of human CSCs, using osteoclasts as feeder cells. We have previously shown that this myeloid subset is a potent activator of NK cells, and their effect on the induction of cytotoxicity and secretion of cytokines and chemokines by NK cells is much stronger than when using monocytes or DCs [62]. Human osteoclasts produce IL-15, IL-12, IL-18 and IFN-α, but not IFN-γ; they express low levels of MHC class I and II, CD14, CD11b and CD54, and they do not upregulate MHC class I surface expression when treated with either the combination of TNF-α and IFN-γ or with activated NK supernatants known to increase MHC class I expression [62]. Low expression of MHC class I together with increased release of IL-15, IL-12, IL-18 and IFN-α may be the main mechanisms by which osteoclasts are able to expand functionally potent NK cells ([62], manuscript in preparation). We have found that such osteoclast-expanded NK cells proliferated to high numbers, displayed high cytotoxicity and much stronger IFN-γ secretion in comparison with IL-2 and/or split anergized (IL-2+ anti-CD16mAb activated) NK cells freshly purified from the same donors, or even NK cells expanded with dendritic cells. Indeed, with osteoclasts as feeder cells, we were able to expand and recover from 60 to 300 million highly potent NK cells from each primary NK cell (manuscript in preparation). We have also shown that a single intravenous injection of one million osteoclast-expanded NK cells into fully reconstituted tumor-bearing hu-BLT mice mediated significant reduction of oral and pancreatic tumor burden (manuscript in preparation). Osteoclast-expanded NK cell injections also mediated significant therapeutic effect in combination with whole cell cancer vaccine in hu-BLT model of advanced melanoma (manuscript in preparation). Importantly, no side effects were observed after delivery of allogeneic NK cells within xenogeneic host microenvironment, and the mice remained active without showing any signs of morbidity. We observed increased cytotoxicity and proliferation of NK cells isolated from hu-BLT mice that received osteoclast-expanded NK cell adoptive transfer, with or without tumors in comparison with autologous/intrinsic NK cells that were originally reconstituted in hu-BLT mice. Moreover, tumor cells isolated from mice receiving adoptive transfer of osteoclast-expanded NK cells demonstrated more differentiated phenotype, grew much slower, and had reduced capacity to attach to the plate and proliferate in comparison with non-NK-injected hu-BLT mice injected with the same tumors. Reduced growth rate of cancer cells correlated with increased immune cell infiltration observed in tumors of expanded NK-injected animals.
Enhanced function of osteoclast-expanded NK cells after in vitro re-stimulation from mice is likely due to activation of NK cells by cytokines and receptor binding and signaling by osteoclasts. It has been suggested that NK cells which undergo prior cytokine activation display the capacity to respond more robustly after reactivation with cytokines or via engagement of activating NK receptors [63].
It is believed that based on prior exposure, NK cells can significantly change the manner by which they respond to later activation. It is well-established that freshly isolated “naïve” NK cells are poorly cytotoxic and do not spontaneously produce cytokines without activation with IL-2, IL-15, or IL-18 cytokines and/or via CD16 receptor triggering [63]. It has been demonstrated that cytokine pre-activated NK cells produce IFN-γ upon transfer into naïve mice but have reduced regulatory capacity after 7 days. However, these cells could easily be re-stimulated, and their secretory capacity can be restored if stimulated with cytokines and/or by the engagement of their NK cell receptors with specific ligands, demonstrating memory-like phenotype [63].
Future approaches in NK cell-mediated immunotherapy
We conclude that the hu-BLT is the best available preclinical mouse model to study novel therapeutic approaches in the context of a fully reconstituted immune repertoire. Decreased NK cell function in humanized mice can be overcome by the adoptive transfer of ex vivo derived, osteoclast-expanded human NK cells and/or by the delivery of differentiation-promoting human cytokines or stable transduction of cytokine genes.
Our previous data [10, 11] and current in vivo studies indicate that NK cells are the main immune effectors that select and differentiate CSCs, resulting in tumor growth inhibition and cessation of chronic inflammation [1, 11]. CSCs differentiated by NK cells become sensitive targets for chemotherapeutic and radio therapeutic strategies [10]. Our recent findings show great promise for the use of NK cells in cancer immunotherapy since both autologous and allogeneic NK cells can be expanded ex vivo using osteoclast-based strategy, in order to substantially increase the numbers and the functional potency of NK cells before their therapeutic transfer into cancer patients. Moreover, our data also demonstrates that the adoptive transfer of osteoclast-expanded NK cells into hu-BLT mice initiates and enhances recruitment of CD8+ T cells to the tumor microenvironment. Significant upregulation of MHC class I and PD-L1 on NK-differentiated stem-like tumor cells in vivo should provide the means for effective T cell based immunotherapies and susceptibility of tumor cells to immune checkpoint inhibitors. Therefore, future directions for adoptive NK cell-mediated therapies in cancer patients should include combined regimens with conventional chemo- and radiotherapies, as well as immune checkpoint inhibitors and peptides or cancer vaccine-based therapies.
Acknowledgments
Anna K. Kozlowska was supported by the Polish Ministry of Sciences and Higher Education and Mobility Plus award.
Abbreviations
- CD
Cluster of differentiation
- CSC
Cancer stem-like cell
- DC
Dendritic cell
- hCB
Human cord blood
- HLA
Human histocompatibility leukocyte antigen
- HSC
Hematopoietic stem cell
- HTM
Humanized tumor mice
- hu-BLT
Bone marrow, liver, thymus humanized mice
- IFN
Interferon
- IL
Interleukin
- IL2Rγ
Interleukin 2 receptor subunit gamma
- K562
Mouse erythroleukemia cell line
- MHC
Major histocompatibility complex
- NK cell
Natural killer cell
- NOD
Non-obese diabetic
- NSG
NOD-scid IL2RGnull
- OSCSC
Oral squamous cancer stem cell
- PD-L1
Programmed death-ligand 1 (also known as B7H1)
- PDX
Patient-derived xenografts
- PKH
Paul Karl Horan fluorescent dye
- Poly(I:C)
Polyinosinic/polycytidylic acid
- scid
Severe combined immunodeficiency
- TNF
Tumor necrosis factor
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Fourth International Conference on Cancer Immunotherapy and Immunomonitoring (CITIM 2015), held in Ljubljana, Slovenia, 27th–30th April 2015. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Tseng H-C, Arasteh A, Paranjpe A, Teruel A, Yang W, Behel A, Alva JA, Walter G, Head C, Ishikawa T, Herschman HR, Cacalano N, Pyle AD, Park N-H, Jewett A. Increased lysis of stem cells but not their differentiated cells by Natural Killer cells; de-differentiation or reprogramming activates NK cells. PLoS ONE. 2010 doi: 10.1371/journal.pone.0011590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jewett A, Man Y-G, Tseng H-C. Dual functions of Natural Killer cells in selection and differentiation of stem cells; role in regulation of inflammation and regeneration of tissues. J Cancer. 2013;4(1):12–24. doi: 10.7150/jca.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames E, Canter RJ, Grossenbacher SK, Mac S, Chen M, Smith RC, Hagino T, Perez-Cunningham J, Sckisel GD, Urayama S, Monjazeb AM, Fragoso RC, Sayers TJ, Murphy WJ. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J Immunol. 2015;195(8):4010–4019. doi: 10.4049/jimmunol.1500447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jewett A, Cavalcanti M, Bonavida B. Pivotal role of endogenous TNF-alpha in the induction of functional inactivation and apoptosis in NK cells. J Immunol. 1997;159(10):4815–4822. [PubMed] [Google Scholar]
- 5.Jewett A, Bonavida B. Target-induced anergy of Natural Killer cytotoxic function is restricted to the NK-target conjugate subset. Cell Immunol. 1995;160(1):91–97. doi: 10.1016/0008-8749(95)80013-9. [DOI] [PubMed] [Google Scholar]
- 6.Jewett A, Bonavida B. MHC-Class I antigens regulate both the function and the survival of human peripheral blood NK cells: role of endogenously secreted TNF-alpha. Clin Immunol. 2000;96(1):19–28. doi: 10.1006/clim.2000.4871. [DOI] [PubMed] [Google Scholar]
- 7.Jewett A, Cacalano NA, Head C, Teruel A. Coengagement of CD16 and CD94 receptors mediates secretion of chemokines and induces apoptotic death of naive Natural Killer cells. Clin Cancer Res. 2006;12(7 Pt 1):1994–2003. doi: 10.1158/1078-0432.CCR-05-2306. [DOI] [PubMed] [Google Scholar]
- 8.Jewett A, Teruel A, Romero M, Head C, Cacalano N. Rapid and potent induction of cell death and loss of NK cell cytotoxicity against oral tumors by F(ab’)2 fragment of anti-CD16 antibody. Cancer Immunol Immunother: CII. 2008;57(7):1053–1066. doi: 10.1007/s00262-007-0437-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jewett A, Bonavida B. Target-induced inactivation and cell death by apoptosis in a subset of human NK cells. J Immunol. 1996;156(3):907–915. [PubMed] [Google Scholar]
- 10.Tseng H-C, Bui V, Man Y-G, Cacalano N, Jewett A. Induction of split anergy conditions Natural Killer cells to promote differentiation of stem cells through cell–cell contact and secreted factors. Front Immunol. 2014;5:269. doi: 10.3389/fimmu.2014.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng HC, Cacalano N, Jewett A. Split anergized Natural Killer cells halt inflammation by inducing stem cell differentiation, resistance to NK cell cytotoxicity and prevention of cytokine and chemokine secretion. Oncotarget. 2015;6(11):8947–8959. doi: 10.18632/oncotarget.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 13.Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA, Manz MG. Human hemato-lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol. 2013;31:635–674. doi: 10.1146/annurev-immunol-032712-095921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human Natural Killer-cell subsets. Trends Immunol. 2001;22(11):633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama WM, Kim S, French AR. The dynamic life of Natural Killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 16.Yoshizawa K, Nakajima S, Notake T, Miyagawa S, Hida S, Taki S. IL-15-high-responder developing NK cells bearing Ly49 receptors in IL-15 −/− mice. J Immunol. 2011;187(10):5162–5169. doi: 10.4049/jimmunol.1101561. [DOI] [PubMed] [Google Scholar]
- 17.Tseng HC, Arasteh A, Kaur K, Kozlowska A, Topchyan P, Jewett A. Differential cytotoxicity but augmented IFN-gamma secretion by NK cells after interaction with monocytes from humans, and those from wild type and myeloid-specific COX-2 knockout mice. Front Immunol. 2015;6:259. doi: 10.3389/fimmu.2015.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 19.Isaacson JH, Cattanach BM (1962) [Report]. Mouse News Lett 27:31
- 20.Levy EM, Yonkosky D, Schmid K, Cooperband SR. Enrichment of the murine Natural Killer (NK) and mitogen induced cellular cytotoxicity (MICC) cells using preparative free-flow high voltage electrophoresis. Prep Biochem. 1977;7(6):467–478. doi: 10.1080/00327487708065514. [DOI] [PubMed] [Google Scholar]
- 21.Nomura T, Watanabe T, Habu S. Humanized mice. Preface. Curr Top Microbiol Immunol. 2008;324:v–vi. [PubMed] [Google Scholar]
- 22.Phillips RA, Jewett MA, Gallie BL. Growth of human tumors in immune-deficient scid mice and nude mice. Curr Top Microbiol Immunol. 1989;152:259–263. doi: 10.1007/978-3-642-74974-2_31. [DOI] [PubMed] [Google Scholar]
- 23.Kataoka S, Satoh J, Fujiya H, Toyota T, Suzuki R, Itoh K, Kumagai K. Immunologic aspects of the nonobese diabetic (NOD) mouse. Abnormalities of cellular immunity. Diabetes. 1983;32(3):247–253. doi: 10.2337/diab.32.3.247. [DOI] [PubMed] [Google Scholar]
- 24.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–191. [PubMed] [Google Scholar]
- 25.Baxter AG, Cooke A. Complement lytic activity has no role in the pathogenesis of autoimmune diabetes in NOD mice. Diabetes. 1993;42(11):1574–1578. doi: 10.2337/diab.42.11.1574. [DOI] [PubMed] [Google Scholar]
- 26.Greiner DL, Hesselton RA, Shultz LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16(3):166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 27.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 28.Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012;12(11):786–798. doi: 10.1038/nri3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood. 2005;106(5):1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 31.Ito A, Ishida T, Yano H, Inagaki A, Suzuki S, Sato F, Takino H, Mori F, Ri M, Kusumoto S, Komatsu H, Iida S, Inagaki H, Ueda R. Defucosylated anti-CCR4 monoclonal antibody exercises potent ADCC-mediated antitumor effect in the novel tumor-bearing humanized NOD/Shi-scid, IL-2Rgamma(null) mouse model. Cancer Immunol Immunother. 2009;58(8):1195–1206. doi: 10.1007/s00262-008-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.King MA, Covassin L, Brehm MA, Racki W, Pearson T, Leif J, Laning J, Fodor W, Foreman O, Burzenski L, Chase TH, Gott B, Rossini AA, Bortell R, Shultz LD, Greiner DL. Human peripheral blood leucocyte non-obese diabetic-severe combined immunodeficiency interleukin-2 receptor gamma chain gene mouse model of xenogeneic graft-versus-host-like disease and the role of host major histocompatibility complex. Clin Exp Immunol. 2009;157(1):104–118. doi: 10.1111/j.1365-2249.2009.03933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimizu S, Hong P, Arumugam B, Pokomo L, Boyer J, Koizumi N, Kittipongdaja P, Chen A, Bristol G, Galic Z, Zack JA, Yang O, Chen IS, Lee B, An DS. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115(8):1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vatakis DN, Bristol GC, Kim SG, Levin B, Liu W, Radu CG, Kitchen SG, Zack JA. Using the BLT humanized mouse as a stem cell based gene therapy tumor model. J Vis Exp. 2012;70:e4181. doi: 10.3791/4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoddart CA, Maidji E, Galkina SA, Kosikova G, Rivera JM, Moreno ME, Sloan B, Joshi P, Long BR. Superior human leukocyte reconstitution and susceptibility to vaginal HIV transmission in humanized NOD-scid IL-2Rgamma(−/−) (NSG) BLT mice. Virology. 2011;417(1):154–160. doi: 10.1016/j.virol.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 37.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100(9):3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 38.Strowig T, Chijioke O, Carrega P, Arrey F, Meixlsperger S, Rämer PC, Ferlazzo G, Münz C. Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood. 2010;116(20):4158–4167. doi: 10.1182/blood-2010-02-270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olesen R, Wahl A, Denton PW, Garcia JV. Immune reconstitution of the female reproductive tract of humanized BLT mice and their susceptibility to human immunodeficiency virus infection. J Reprod Immunol. 2011;88(2):195–203. doi: 10.1016/j.jri.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, Chateau M, Nochi T, Krisko JF, Spagnuolo RA, Margolis DM, Garcia JV. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86(1):630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26(5):537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Vatakis DN, Koya RC, Nixon CC, Wei L, Kim SG, Avancena P, Bristol G, Baltimore D, Kohn DB, Ribas A, Radu CG, Galic Z, Zack JA. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci USA. 2011;108(51):E1408–E1416. doi: 10.1073/pnas.1115050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kwant-Mitchell A, Pek EA, Rosenthal KL, Ashkar AA. Development of functional human NK cells in an immunodeficient mouse model with the ability to provide protection against tumor challenge. PLoS ONE. 2009;4(12):e8379. doi: 10.1371/journal.pone.0008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pek EA, Chan T, Reid S, Ashkar AA. Characterization and IL-15 dependence of NK cells in humanized mice. Immunobiology. 2011;216(1–2):218–224. doi: 10.1016/j.imbio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci USA. 2009;106(51):21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, Corcuff E, Mortier E, Jacques Y, Spits H, Di Santo JP. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206(1):25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andre MC, Erbacher A, Gille C, Schmauke V, Goecke B, Hohberger A, Mang P, Wilhelm A, Mueller I, Herr W, Lang P, Handgretinger R, Hartwig UF. Long-term human CD34+ stem cell-engrafted nonobese diabetic/SCID/IL-2R gamma(null) mice show impaired CD8+ T cell maintenance and a functional arrest of immature NK cells. J Immunol. 2010;185(5):2710–2720. doi: 10.4049/jimmunol.1000583. [DOI] [PubMed] [Google Scholar]
- 48.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ Natural Killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87(7):2632–2640. [PubMed] [Google Scholar]
- 49.Sato T, Laver JH, Aiba Y, Ogawa M. NK cell colony formation from human fetal thymocytes. Exp Hematol. 1999;27(4):726–733. doi: 10.1016/S0301-472X(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 50.Vosshenrich CA, Ranson T, Samson SI, Corcuff E, Colucci F, Rosmaraki EE, Di Santo JP. Roles for common cytokine receptor gamma-chain-dependent cytokines in the generation, differentiation, and maturation of NK cell precursors and peripheral NK cells in vivo. J Immunol. 2005;174(3):1213–1221. doi: 10.4049/jimmunol.174.3.1213. [DOI] [PubMed] [Google Scholar]
- 51.Fischer A, Le Deist F, Hacein-Bey-Abina S, Andre-Schmutz I, Basile Gde S, de Villartay JP, Cavazzana-Calvo M. Severe combined immunodeficiency. A model disease for molecular immunology and therapy. Immunol Rev. 2005;203:98–109. doi: 10.1111/j.0105-2896.2005.00223.x. [DOI] [PubMed] [Google Scholar]
- 52.Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. Distinct roles of IL-12 and IL-15 in human Natural Killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101(47):16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strowig T, Chijioke O, Carrega P, Arrey F, Meixlsperger S, Ramer PC, Ferlazzo G, Munz C. Human NK cells of mice with reconstituted human immune system components require preactivation to acquire functional competence. Blood. 2010;116(20):4158–4167. doi: 10.1182/blood-2010-02-270678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wege AK, Ernst W, Eckl J, Frankenberger B, Vollmann-Zwerenz A, Männel DN, Ortmann O, Kroemer A, Brockhoff G. Humanized tumor mice—a new model to study and manipulate the immune response in advanced cancer therapy. Int J Cancer. 2011;129(9):2194–2206. doi: 10.1002/ijc.26159. [DOI] [PubMed] [Google Scholar]
- 55.Shurin MR, Umansky V, Malyguine A, Hurwitz AA, Apte RN, Whiteside T, Jewett A, Thanavala Y, Murphy WJ. Cellular and molecular pathways in the tumor immunoenvironment: 3rd Cancer Immunotherapy and Immunomonitoring (CITIM) meeting, 22–25 April 2013, Krakow, Poland. Cancer Immunol Immunother. 2014;63(1):73–80. doi: 10.1007/s00262-013-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of Natural Killer cell receptors. Nat Rev Immunol. 2009;9(8):568–580. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S, Solari N, Gualco M, Queirolo P, Moretta L, Mingari MC. Melanoma cells inhibit Natural Killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72(6):1407–1415. doi: 10.1158/0008-5472.CAN-11-2544. [DOI] [PubMed] [Google Scholar]
- 58.Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG, Balderas-Pena LM, Bravo-Cuellar A, Ortiz-Lazareno PC, Daneri-Navarro A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng YP, Zhang JJ, Liang WB, Tu M, Lu ZP, Wei JS, Jiang KR, Gao WT, Wu JL, Xu ZK, Miao Y, Zhu Y. Elevation of MMP-9 and IDO induced by pancreatic cancer cells mediates Natural Killer cell dysfunction. BMC Cancer. 2014;14:738. doi: 10.1186/1471-2407-14-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mamessier E, Sylvain A, Bertucci F, Castellano R, Finetti P, Houvenaeghel G, Charaffe-Jaufret E, Birnbaum D, Moretta A, Olive D. Human breast tumor cells induce self-tolerance mechanisms to avoid NKG2D-mediated and DNAM-mediated NK cell recognition. Cancer Res. 2011;71(21):6621–6632. doi: 10.1158/0008-5472.CAN-11-0792. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, McCullagh L, Mousa S, Quezado M, Herscher LL, Van Waes C. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5(6):1369–1379. [PubMed] [Google Scholar]
- 62.Tseng HC, Kanayama K, Kaur K, Park SH, Park S, Kozlowska A, Sun S, McKenna CE, Nishimura I, Jewett A. Bisphosphonate-induced differential modulation of immune cell function in gingiva and bone marrow in vivo: role in osteoclast-mediated NK cell activation. Oncotarget. 2015;6(24):20002–20025. doi: 10.18632/oncotarget.4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like Natural Killer cells. Proc Natl Acad Sci USA. 2009;106(6):1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelland LR. Of mice and men: values and liabilities of the athymic nude mouse model in anticancer drug development. Eur J Cancer. 2004;40(6):827–836. doi: 10.1016/j.ejca.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 65.Nonoyama S, Smith FO, Bernstein ID, Ochs HD. Strain-dependent leakiness of mice with severe combined immune deficiency. J Immunol. 1993;150(9):3817–3824. [PubMed] [Google Scholar]
- 66.Shultz LD, Goodwin N, Ishikawa F, Hosur V, Lyons BL, Greiner DL. Human cancer growth and therapy in immunodeficient mouse models. Cold Spring Harb Protoc. 2014;7:694–708. doi: 10.1101/pdb.top073585. [DOI] [PMC free article] [PubMed] [Google Scholar]