Abstract
Some Lactobacillus spp. suppress Helicobacter pylori in the stomach and have potential therapeutic applications for the treatment of gastrointestinal conditions. In this study, the effects of Lactobacillus strains on functional dyspepsia associated with H. pylori infection were examined. Volunteers were screened using the 13C-urea breath test (UBT) and H. pylori stool test, and 131 participants who met the selection criteria (mean age: 48.9 years) were randomly given L. gasseri OLL2716-containing yogurt or placebo yogurt once daily for 12 weeks. Gastrointestinal symptoms (epigastric pain, bloating, postprandial fullness, nausea, and heartburn) and the levels of serum pepsinogen (PG), 13C-UBT, and H. pylori stool antigen were assessed. No significant differences were observed between the groups in UBT results, H. pylori stool antigens, or the serum PGI/II ratio. In the L. gasseri group, postprandial fullness was significantly lower at the end of the trial compared to the initial level (p < 0.05) and significantly fewer patients had a VAS score of >10 for bloating compared to the placebo group (p < 0.05). Dietary supplementation with L. gasseri OLL2716-containing yogurt may effectively suppress dyspeptic symptoms in H. pylori-infected patients. This study was registered at the University Hospital Medical Network Clinical Trial Registry (UMIN000016746).
1. Introduction
Functional dyspepsia describes a heterogeneous group of GI disorders, which may be related to atrophic gastritis. The symptoms include epigastric pain and burning and postprandial fullness, without evidence of organic disease. In Japan, functional dyspepsia has recently been recognized as a disease covered by national insurance [1]. The underlying pathophysiological mechanisms are unclear, and it has been suggested that intestinal inflammation and H. pylori infection may play a role [2]. The treatment of functional dyspepsia is challenging, and there is no effective therapy.
A recent pilot study indicated that a diet enriched in probiotics may alleviate dyspeptic symptoms [3]. Some strains of lactic acid bacteria, including Lactobacillus spp., have beneficial probiotic effects in the GI by suppressing H. pylori and reducing the associated inflammation [4–6]. The addition of Lactobacillus acidophilus to H. pylori treatment regimens (i.e., antibiotics) significantly improves H. pylori eradication rates [5, 6]. A dietary product containing L. johnsonii prevents H. pylori colonization of the GI tract in children [6], which is consistent with the results of our previous study showing that H. pylori cannot colonize L. salivarius-inoculated gnotobiotic mice [7]. Lactobacillus spp. exhibit antimicrobial activity against H. pylori both in vitro and in vivo [7, 8], and beneficial effects of Lactobacillus-fermented milk products on H. pylori infection have been documented in humans [9, 10]. In a nonrandomized controlled trial, we previously found that the twice-daily consumption of yogurt containing L. gasseri strain OLL2716 for 8 weeks effectively treated H. pylori infection [9], suggesting that H. pylori colonization may be suppressed by the continuous ingestion of Lactobacillus-fermented milk products.
Despite these previous studies, the effects of dietary probiotic supplementation on functional dyspepsia are still unclear. The aims of this multicenter, double-blind, placebo-controlled clinical trial were to clarify the relationship between H. pylori infection and functional dyspepsia and to analyze the clinical effectiveness of L. gasseri OLL2716-containing yogurt consumed once daily for 12 weeks in alleviating functional dyspepsia and H. pylori infection.
2. Patients and Methods
2.1. Participants
The study participants were recruited from individuals who visited the hospitals (Tokai University Hospital, Social Insurance Shiga Hospital, Kawasaki Medical School Hospital, and Tokyo Medical College Hospital) for annual health checks. In the Japanese population, atrophic gastritis usually develops after the age of 30 years [11]; therefore, individuals ≥30 years old who tested positive for H. pylori infection were included in the study. Exclusion criteria were as follows: organic disorders, such as gastric cancer, gastric and duodenal ulcers, and pyloric stenosis; the use of nonsteroidal anti-inflammatory drugs, acid-inhibitory drugs (proton pump inhibitors or H2 blockers), and anti-flatulence agents; antibiotic treatment, including H. pylori eradication therapy, within 6 months of the study or the intention to use antibiotics for H. pylori eradication during the study; and the consumption of yogurt or other lactic acid bacteria-fermented beverages. A total of 131 individuals diagnosed with H. pylori by both the 13C-urea breath test (UBT; cut-off value ≥ 2.5‰) and stool antigen positivity were invited to participate and were included in the study after providing written informed consent. This clinical trial was approved by the Ethics Review Committee of each participating medical facility and was registered at the University Hospital Medical Network Clinical Trial Registry (UMIN000016746).
2.2. Study Protocol
The primary end-point was a decrease in H. pylori load assessed by the UBT and H. pylori antigen levels in stool samples. The secondary end-points were improvements in gastric mucosal inflammation/atrophic gastritis assessed by serum levels of pepsinogen (PG) I and pepsinogen II and changes in dyspeptic symptoms. The participants were randomly divided into two groups using a double-blind method. The placebo group (n = 67) received 90 g of yogurt containing milk, sugar, and stevia and fermented with L. delbrueckii and Streptococcus thermophilus (approximately 1010 CFU). The experimental group (n = 64) received the same yogurt supplemented with L. gasseri OLL2716 (≥109 CFU). The participants were requested to consume yogurt once daily between meals for 12 weeks; the two yogurt types were identical in appearance and taste.
2.3. Evaluation Parameters
To assess the effects of yogurt consumption, all study parameters were measured before and after the experimental period. The participants were requested to fast on the day of the UBT. They were orally given a 100 mg UBIT tablet (Otsuka Pharmaceutical Co., Tokyo, Japan) and 13CO2 levels in the breath were measured by mass spectrometry. H. pylori stool antigens were detected using enzyme immunoassays (Testmate pylori antigen test; Wakamoto Pharmaceutical Co., Tokyo, Japan) and an OD value of ≥0.1 was considered positive. Serum PG levels were determined using a radioimmunoassay.
Functional dyspepsia was evaluated according to Japanese guidelines [1] using a visual analogue scale (VAS). During the study, the participants kept a diary in which they recorded compliance and scored the severity of GI symptoms (upper abdominal pain, bloating, indigestion, nausea, vomiting, and heartburn) from 1 (none) to 10 (severe pain, never experienced before). Laboratory tests (hematology, clinical chemistry, and urinalysis) were also performed before and after the completion of the study.
During the product consumption phase, 3 and 4 participants from the experimental and placebo groups, respectively, used prohibited concomitant drugs and were excluded from the analysis. Therefore, data were compared between 61 L. gasseri OLL2716 yogurt consumers and 63 placebo yogurt consumers (n = 124 in total).
2.4. Statistical Analysis
The number of patients required to detect a difference of 0.5% between the groups was calculated. Based on a decrease in the expected mean of 0.5%, a significance level of 5%, and a power of 80%, at least 102 subjects in total were required. Participants' characteristics were compared between the groups using Pearson's χ 2 test. Stool antigen, UBT, PG, and VAS results before and after product consumption were compared using the Wilcoxon signed rank sum test. The Mann-Whitney test was used for comparisons between groups. The data are expressed as medians (range), and the level of statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS 11.5J Windows software (SPSS Japan Inc., Tokyo, Japan).
3. Results and Discussion
There were no significant differences in the demographic and initial clinical characteristics (Table 1) or compliance between the L. gasseri and placebo groups, and none of the participants experienced any adverse events during the study.
Table 1.
Participant characteristics.
| Lactobacillus gasseri group (n = 61) | Placebo group (n = 63) | Group comparison∗ | |||
|---|---|---|---|---|---|
| Median | 25–75 percentile | Median | 25–75 percentile | p value | |
| Age | 51.0 | 44.0–56.0 | 48 | 41.0–54.0 | 0.214 |
| Gender (male/female) | 27/34 | 17/46 | 0.06 | ||
| Stool Hp antigens | 1.45 | 0.38–2.38 | 1.217 | 0.38–2.83 | 0.622 |
| UBT | 19.4 | 10.4–35.0 | 19.6 | 10.3–36.7 | 0.924 |
| PGI/PGII | 2.6 | 2.1–3.5 | 2.7 | 2.0–3.9 | 0.468 |
| PGI | 61.0 | 45.2–76.5 | 59.2 | 42.0–74.5 | 0.589 |
| PGII | 21.4 | 16.4–32.5 | 20.6 | 16.9–27.5 | 0.635 |
∗Mann-Whitney test.
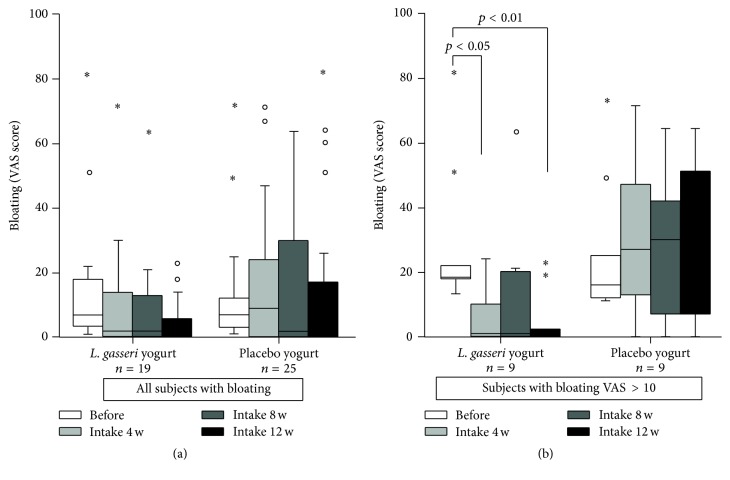
There were no significant differences in the UBT or stool H. pylori antigen values between the start and end of the study in either group (Table 2). Serum PGI and PGII levels in both groups were significantly higher at the end of the study period compared to the initial levels (p < 0.05), and the PGI/II ratios remained unchanged (Table 2). Based on the VAS results, the incidences of gastrointestinal symptoms before the test products were consumed were as follows: epigastric pain, 23.4%; bloating, 35.5%; postprandial fullness, 39.5%; nausea, 28.2%; and heartburn, 29.8% (Table 3). At least one of these symptoms was reported by 58% of the participants (72 out of 124), and these patients were considered dyspeptic. The VAS score for postprandial fullness in the L. gasseri group was significantly higher before than after the consumption of the test yogurt (p < 0.05; Figure 1). Although no significant differences were observed in bloating according to the VAS analysis, the number of subjects with a VAS bloating score of >10 was significantly lower in the L. gasseri group than in the placebo group (p < 0.05; Figure 2). Overall, these results indicate that the consumption of L. gasseri OLL2716-containing yogurt alleviated some dyspeptic symptoms in individuals infected with H. pylori.
Table 2.
Effects of Lactobacillus gasseri consumption on Helicobacter pylori infection and serum pepsinogen levels.
| Parameters | Group | Before | After | Before versus after | Group comparison | ||
|---|---|---|---|---|---|---|---|
| Median | 25–75 percentile | Median | 25–75 percentile | p value∗ | p value∗∗ | ||
| Stool Hp antigen | L. gasseri group | 1.45 | 0.38–2.38 | 1.17 | 0.48–2.13 | 0.588 | 0.958 |
| Placebo group | 1.22 | 0.38–2.83 | 1.24 | 0.44–2.50 | 0.584 | ||
|
| |||||||
| UBT | L. gasseri group | 19.4 | 10.4–35.0 | 21.9 | 11.1–31.2 | 0.395 | 0.516 |
| Placebo group | 19.6 | 10.3–36.7 | 19.9 | 10.3–32.8 | 0.958 | ||
|
| |||||||
| PGI/PGII | L. gasseri group | 2.6 | 2.1–3.5 | 2.7 | 2.1–3.6 | 0.537 | 0.414 |
| Placebo group | 2.7 | 2.0–3.9 | 2.7 | 2.1–3.7 | 0.604 | ||
|
| |||||||
| PGI | L. gasseri group | 61.0 | 45.2–76.5 | 63.3 | 48.5–79.6 | 0.006 | 0.497 |
| Placebo group | 59.2 | 42.0–74.5 | 62.3 | 43.9–77.0 | 0.018 | ||
|
| |||||||
| PGII | L. gasseri group | 21.4 | 16.4–32.5 | 22.5 | 16.1–35.1 | 0.012 | 0.725 |
| Placebo group | 20.6 | 16.9–27.5 | 24.3 | 16.4–29.8 | 0.003 | ||
L. gasseri group, n = 61; placebo group, n = 63.
∗Wilcoxon signed rank sum test.
∗∗Mann-Whitney test.
Table 3.
Dyspeptic symptoms.
| Symptom | Lactobacillus gasseri group (n = 61) | Placebo group (n = 63) | ||||
|---|---|---|---|---|---|---|
| Before, n (%) | After, n (%) | p | Before, n (%) | After, n (%) | p | |
| Postprandial fullness | 18/61 (29.5%)∗ | 20/61 (32.8%) | 0.696 | 31/63 (49.2%) | 24/61 (38.1%) | 0.209 |
| Epigastric pain | 8/61 (13.1%)∗∗ | 13/61 (21.3%) | 0.230 | 21/63 (33.3%) | 27/63 (42.9%) | 0.271 |
| Heartburn | 17/61 (27.9%) | 17/61 (27.9%) | 1.000 | 20/63 (31.7%) | 20/63 (31.7%) | 1.000 |
| Nausea | 16/61 (26.2%) | 15/61 (24.6%) | 0.835 | 19/63 (30.1%) | 17/63 (27.0%) | 0.693 |
| Bloating | 19/61 (31.1%) | 21/61 (34.4%) | 0.700 | 25/63 (39.7%) | 29/63 (23.8%) | 0.471 |
∗ p = 0.025 and ∗∗ p = 0.008 versus the placebo group.
Figure 1.
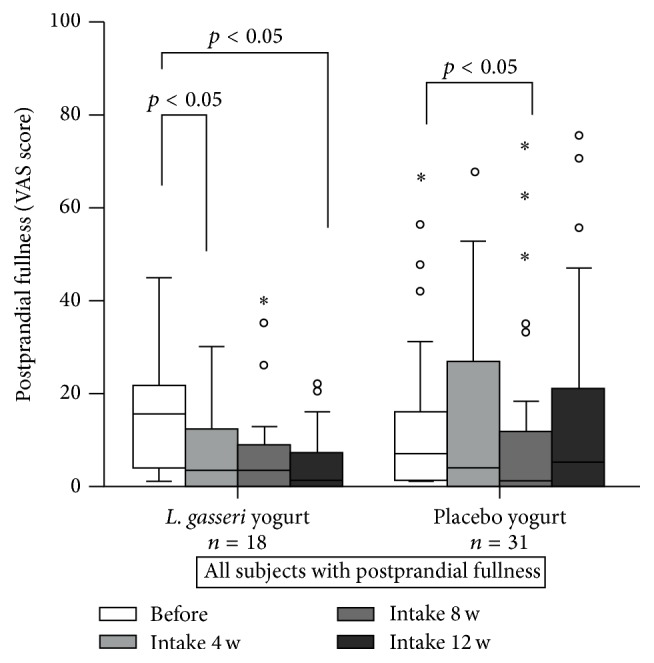
Changes in the severity score for postprandial fullness. Participants were asked to take Lactobacillus gasseri OLL2716-containing yogurt or placebo yogurt (control) once daily for 12 weeks, and the severity of dyspeptic symptoms was analyzed according to a visual analogue scale (VAS) as follows: 1, no symptoms; 10, severe pain, never experienced before. ∘: less than 3 times the height of the box. ∗: more than 1.5 times the height of the box.
Figure 2.
Changes in the bloating severity score. Participants were asked to take Lactobacillus gasseri OLL2716-containing yogurt or placebo yogurt (control) once daily for 12 weeks and the severity of dyspeptic symptoms was analyzed according to a visual analogue scale (VAS) as follows: 1, no symptoms; 10, severe pain, never experienced before. (a) The total number of participants with bloating; (b) the number of participants with a bloating severity score of >10. ∘: less than 3 times the height of the box. ∗: more than 1.5 times the height of the box.
In our previous study, we found that the consumption of L. gasseri OLL2716-containing yogurt twice daily for 8 weeks reduced the density of H. pylori and ameliorated gastritis in 31 patients, as evidenced by a significant decrease in UBT values and increase in the PGI/II ratio [9]. Furthermore, it improved H. pylori eradication rates when it was included as a first-line triple therapy with antibiotics [5]. In this study, we tested once-daily yogurt intake to promote compliance to the study protocol over a longer experimental period (12 weeks). This intake schedule did not produce significant changes in the UBT and stool antigen results or the PGI/II ratio. In other words, the reduced dose used in this study was not sufficient to eradicate H. pylori.
PG levels are associated with gastric mucosa functional activity, and a PGI/PGII ratio of <3 is a marker of atrophic gastritis [12]. In patients with H. pylori infection, increases in serum PGII concentrations to greater than 12 ng/mL and decreases in the PGI/PGII ratio to below 4.0 were used as the cut-offs for the diagnosis of H. pylori infection, with sensitivity and specificity of 90.0% and 93.5%, respectively [13]. However, the effects of probiotics on serum PG levels are unclear. Igarashi et al. [14] have shown that the ingestion of L. gasseri increases PGI levels in proton pump inhibitor users, whereas Miki et al. [15] have reported that fermented milk containing Bifidobacterium bifidum decreases PGI and the PGI/II ratio in patients with mild mucosal atrophy. The mechanisms by which L. gasseri and other probiotic bacteria influence PG levels remain unknown and require further investigation.
Functional dyspepsia presents a major economic burden in modern society. Despite the high cost of investigating and treating functional dyspepsia, few therapeutic options are currently available. Systematic reviews have suggested that prokinetic therapy and the suppression of acid release via proton pump inhibitors may alleviate dyspeptic symptoms [16, 17]. However, an effective treatment for functional dyspepsia has yet to be established.
Although H. pylori infection induces changes in gastric emptying, gastrointestinal motility, gastric acid secretion, and the perception of gastric characteristics related to functional dyspepsia, the role of H. pylori in the pathogenesis of dyspepsia remains controversial [2, 18]. Thus, it is still unclear whether the eradication of H. pylori is beneficial for functional dyspepsia, and clinical trials have not revealed a clear association between H. pylori eradication and the relief of dyspeptic symptoms [2, 19].
Probiotics have been suggested to treat functional dyspepsia, but studies examining their effects are inconclusive. For example, a previous study of the effects of probiotic-enriched olive oil on dyspeptic symptoms in 44 individuals revealed a significant amelioration of nausea, pain/discomfort in the abdomen, and postprandial fullness compared to the control group [3]. However, it was a proof-of-concept study and had a very short duration (i.e., 1 week).
The current multicenter double-blind, randomized, placebo-controlled study had a duration of 12 weeks. We showed that the VAS scores for postprandial fullness and the number of patients with a bloating score of >10 decreased significantly after the consumption of L. gasseri-containing yogurt (p < 0.05), indicating long-term benefits of the probiotic for the relief of dyspeptic symptoms. L. gasseri OLL2716 has multiple beneficial properties, including acid resistance and adhesion to gastric epithelial cells in vitro (unpublished data), as well as successful competition with H. pylori for colonization of the GI tract [9]. Our data further suggest that dietary supplementation with this Lactobacillus strain has potential applications for the treatment of functional dyspepsia.
Our study had some limitations. First, the sample size of H. pylori-infected individuals with functional dyspepsia was small because the participants were recruited from people admitted to the hospitals for an annual health check. However, a power analysis indicated that this sample size was sufficient to detect differences at the 5% level. Second, we did not account for life-style habits, such as smoking and alcohol consumption, which can have confounding effects. In our future studies, we plan to address this issue. Finally, we did not strictly adhere to the Rome III criteria for functional dyspepsia [20]. Almost all Japanese citizens are covered by health insurance and can seek medical help within a month of the onset of dyspeptic symptoms; thus, these patients do not meet the Rome III criterion of a symptom duration of 6 months. We used our own questionnaire, rather than a standard questionnaire, as recommended by the Japanese guidelines for functional dyspepsia, such as the Gastrointestinal Symptom Rating Scale (GSRS). Further studies on functional dyspepsia according to the Rome III criteria are necessary to elucidate the role of probiotics, including L. gasseri OLL2716, on dyspeptic symptoms.
4. Conclusions
We demonstrated that a low dose of the L. gasseri strain OLL2716 improves dyspeptic symptoms, without inhibiting H. pylori infection. Further studies that examine higher probiotic doses and larger patient cohorts using standard diagnostic criteria for functional dyspepsia are needed to evaluate the effects of L. gasseri OLL2716 on the relief of dyspeptic symptoms.
Acknowledgments
This study was fully funded by Meiji Co., Ltd.
Competing Interests
Toshihiro Ohtsu is an employee of Meiji Co., Ltd. (Tokyo, Japan). All other authors have no potential competing interests to disclose.
References
- 1.Miwa H., Kusano M., Arisawa T., et al. Evidence-based clinical practice guidelines for functional dyspepsia. Journal of Gastroenterology. 2015;50(2):125–139. doi: 10.1007/s00535-014-1022-3. [DOI] [PubMed] [Google Scholar]
- 2.Zala A. V., Walker M. M., Talley N. J. Emerging drugs for functional dyspepsia. Expert Opinion on Emerging Drugs. 2015;20(2):221–233. doi: 10.1517/14728214.2015.1009827. [DOI] [PubMed] [Google Scholar]
- 3.Ianiro G., Pizzoferrato M., Franceschi F., Tarullo A., Luisi T., Gasbarrini G. Effect of an extra-virgin olive oil enriched with probiotics or antioxidants on functional dyspepsia: a pilot study. European Review for Medical and Pharmacological Sciences. 2013;17(15):2085–2090. [PubMed] [Google Scholar]
- 4.Cruchet S., Obregon M. C., Salazar G., Diaz E., Gotteland M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition. 2003;19(9):716–721. doi: 10.1016/s0899-9007(03)00109-6. [DOI] [PubMed] [Google Scholar]
- 5.Deguchi R., Nakaminami H., Rimbara E., et al. Effect of pretreatment with Lactobacillus gasseri OLL2716 on first-line Helicobacter pylori eradication therapy. Journal of Gastroenterology and Hepatology. 2012;27(5):888–892. doi: 10.1111/j.1440-1746.2011.06985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dang Y., Reinhardt J. D., Zhou X., Zhang G. The effect of probiotics supplementation on Helicobacter pylori eradication rates and side effects during eradication therapy: a meta-analysis. PLoS ONE. 2014;9(11, article e111030) doi: 10.1371/journal.pone.0111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabir A. M. A., Aiba Y., Takagi A., Kamiya S., Miwa T., Koga Y. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut. 1997;41(1):49–55. doi: 10.1136/gut.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiba Y., Suzuki N., Kabir A. M. A., Takagi A., Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. The American Journal of Gastroenterology. 1998;93(11):2097–2101. doi: 10.1111/j.1572-0241.1998.00600.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto I., Igarashi M., Kimura K., Takagi A., Miwa T., Koga Y. Suppressive effect of Lactobacillus gasseri OLL 2716 (LG21) on Helicobacter pylori infection in humans. Journal of Antimicrobial Chemotherapy. 2001;47(5):709–710. doi: 10.1093/jac/47.5.709. [DOI] [PubMed] [Google Scholar]
- 10.Pantoflickova D., Corthésy-Theulaz I., Dorta G., et al. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Alimentary Pharmacology & Therapeutics. 2003;18(8):805–813. doi: 10.1046/j.1365-2036.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T., Yamamoto K., Fukuzawa M., et al. Helicobacter pylori infection and reflux esophagitis in young and middle-aged Japanese subjects. Journal of Gastroenterology and Hepatology. 2010;25(1):S80–S85. doi: 10.1111/j.1440-1746.2010.06228.x. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y.-K., Yu J.-C., Kang W.-M., et al. Significance of serum pepsinogens as a biomarker for gastric cancer and atrophic gastritis screening: a systematic review and meta-analysis. PLoS ONE. 2015;10(11) doi: 10.1371/journal.pone.0142080.e142080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitamura Y., Yoshihara M., Ito M., et al. Diagnosis of Helicobacter pylori-induced gastritis by serum pepsinogen levels. Journal of Gastroenterology and Hepatology. 2015;30(10):1473–1477. doi: 10.1111/jgh.12987. [DOI] [PubMed] [Google Scholar]
- 14.Igarashi M., Nagano J., Tsuda A., et al. Correlation between the serum pepsinogen I level and the symptom degree in proton pump inhibitor-users administered with a probiotic. Pharmaceuticals. 2014;7(7):754–764. doi: 10.3390/ph7070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miki K., Urita Y., Ishikawa F., et al. Effect of Bifidobacterium bifidum fermented milk on Helicobacter pylori and serum pepsinogen levels in humans. Journal of Dairy Science. 2007;90(6):2630–2640. doi: 10.3168/jds.2006-803. [DOI] [PubMed] [Google Scholar]
- 16.Mahadeva S., Ford A. C. Clinical and epidemiological differences in functional dyspepsia between the East and the West. Neurogastroenterology and Motility. 2016;28(2):167–174. doi: 10.1111/nmo.12657. [DOI] [PubMed] [Google Scholar]
- 17.Hajaghamohammadi A., Safiabadi Tali S. H., Samimi R., Oveisi S., Kazemifar A. M. Low dose furazolidone for eradication of H. pylori instead of clarithromycin: a clinical trial. Global Journal of Health Science. 2014;31:235–239. doi: 10.5539/gjhs.v7n1p235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zullo A., Hassan C., De Francesco V., et al. Helicobacter pylori and functional dyspepsia: an unsolved issue? World Journal of Gastroenterology. 2014;20(27):8957–8963. doi: 10.3748/wjg.v20.i27.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moayyedi P. Helicobacter pylori eradication for functional dyspepsia: what are we treating? Archives of Internal Medicine. 2011;171(21):1936–1937. doi: 10.1001/archinternmed.2011.541. [DOI] [PubMed] [Google Scholar]
- 20.Drossman D. A. The functional gastrointestinal disorders and the rome III process. Gastroenterology. 2006;130(5):1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]