Abstract
Background
Annuloplasty ring dehiscence is a well described mechanism of mitral valve repair failure. Defining the mechanisms underlying dehiscence may facilitate its prevention.
Methods
Factors governing suture dehiscence were examined using an ovine model. Following undersized ring annuloplasty in live animals (N=5), Cyclic Force (FC) acting on sutures during cardiac contraction were measured using custom transducers. FC was measured at 10 suture positions, throughout cardiac cycles with peak left ventricular pressure (LVPmax) of 100, 125, and 150mmHg. Suture pullout testing was conducted on explanted mitral annuli (N=12) to determine suture holding strength at each position. Finally, relative collagen density differences at suture sites around the annulus were assessed by two-photon excitation fluoroscopy.
Results
Anterior FC exceeded posterior at each LVPmax (e.g. 2.8±1.3 vs. 1.8±1.2N at LVPmax=125mmHg, p<0.01). Anterior holding strength exceeded posterior (6.4±3.6 vs. 3.9±1.6N, p<0.0001). Based on FC at LVPmax=150mmHg, margin of safety before suture pullout was vastly higher between the trigones (exclusive) versus elsewhere (4.8±0.9 versus 1.9±0.5N, p<0.001). Margin of safety exhibited strong correlation to collagen density (R2=0.947).
Conclusions
Despite lower cyclic loading on posterior sutures, the weaker posterior mitral annular tissue creates higher risk of dehiscence, apparently due to reduced collagen content. Sutures placed atop the trigones are less secure than predicted, due to a combination of reduced collagen and higher overall rigidity in this region. These findings highlight the inter-trigonal tissue as the superior anchor, and have implications on the design and implantation techniques for next-generation mitral prostheses.
Keywords: mitral valve repair, myocardial mechanics, heart valve prosthesis (annuloplasty ring), reoperation (mitral valve), surgery techniques
The spectrum of corrective devices for mitral valve (MV) regurgitation (MR) is diverse, with heavy emphasis on reconstructive annuloplasty rings and prosthetic valve replacements. One increasingly acknowledged mode of postoperative device failure is dehiscence, in which the sutures anchoring device to mitral annulus pullout from the tissue. Partial or complete dehiscence has been demonstrated with varying devices and patient groups. Various studies of reoperation for failed annuloplasty-based MV repairs have reported the proportion of failures attributable to annular suture dehiscence to fall between 13–42%.[1–3] Looking specifically at degenerative MR, Dumont et al reported an overall suture dehiscence rate below 1%,[3] while Chitwood et al reported a rate of 2.3% among one center’s first 300 robotic repairs.[4] Kronzon et al reported 18 additional dehiscence cases across etiologies, encompassing 8 rings and 10 prosthetic valves.[5] Demonstrating that this risk extends to functional MR repair, a large number of case studies have additionally been reported.[6–10] While these data collectively highlight the potential for dehiscence even at high-volume centers, its incidence may be greater at low-volume centers. Bolling et al reported that, among 1088 surgeons at 639 hospitals in the Society of Thoracic Surgeons Adult Cardiac Surgery Database, the median number of isolated MV operations per year was 5.[11] Among all surgeons, devices, and patient etiologies, the overall incidence of suture dehiscence remains uncertain.
The consequences of dehiscence may be severe and include acute recurrent MR, endocarditis, device embolization, device migration and excessive patient morbidity.[9, 10, 12] Yet, the factors influencing its occurrence remain unclear. One clue comes from Spratt et al, who measured the total force necessary for complete ring dehiscence ex vivo, and observed increased strength using a flexible ring with a running suture.[13] Still, no data have identified why sutures more commonly dehisce from the posterior aspect of the annulus.[7–9, 12] Further uncertainty surrounds the contributions of suture technique, device size and shape, hemodynamic conditions, annular microstructure, or combinations thereof.[14–16] Developing a comprehensive understanding of these potential factors is an important step toward reducing the risk of device dehiscence, establishing optimal pre-clinical regulatory standards for future MV devices, and advancing implantation procedures.
One systematic approach to evaluating the roles of these mechanistic factors in device dehiscence is to (i)quantify the cyclic suture forces on an implanted device in vivo at varying hemodynamic conditions, (ii)quantify margins of safety for suture dehiscence by comparing measured in vivo forces to corresponding suture holding strengths around the annular circumference, and (iii)relate these margins of safety to the variation in cellular microstructure around the annular circumference. An undersized, flat, rigid ring, which constricts the annulus both in-plane and out-of-plane, provides the most effective case study for cyclic suture forces in vivo. The present study sought to accomplish these objectives through the innovative application of suture force transducers, pullout testing, and histological techniques in healthy ovine animals and explanted hearts.
Material and Methods
Suture Force Transducers and In Vivo Experimental Protocol
To measure tension in individual annuloplasty ring sutures, novel transducers with data acquisition system were previously developed and validated (Figure 1A).[17] Transducers are designed to report suture tension as a positive force tracing. Before use in this study, the transducers were calibrated with known forces exceeding those anticipated in vivo, as previously described. Sets of transducers (N=10) were fixed to size 24 Physio rings (Edwards Lifesciences, Irvine, CA). Transducers were fixed to the annuloplasty rings at the left trigone (LT), right trigone (RT), and at evenly spaced locations around the remaining circumference. This amounted to four transducers on the anterior aspect and six on the posterior (1B); effective ring size was 26. Transducer signals were zeroed prior to implantation in the flaccid, cardioplegic heart. To implant the instrumented ring, one mattress suture was passed through and tied to each transducer (i.e. ten total sutures), by the same method used for tying sutures to a ring’s suture cuff.
Figure 1.
Design and implementation of custom suture force transducers, first reported by Siefert et al.[17] (A) Schematic representation of transducer, highlighting suture holes for ring mounting (a), annuloplasty mattress suture passages (b), strain gauge for force measurement (c), and wiring (d). (B) Each instrumented ring contains 4 anterior (orange) and 6 posterior (purple) transducers. (C) Device implantation. (D) Device (arrow) imaged by fluoroscopy post-implantation.
Suture forces were measured in vivo in 5 healthy Dorsett hybrid sheep. Animals received care in compliance with protocols approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania in accordance with the guidelines for humane care (National Institutes of Health Publication 85–23, revised 1996). All in vivo experimental procedures for ring implantation (1C–D) and measurement were performed as we previously described.[17]
Ex Vivo Suture Pullout Testing
Ovine hearts were acquired from a local market (N=12). These were previously frozen; prior studies have established previously frozen MVs and annuli as suitable structural models for living tissue.[18, 19] MVs (annulus and leaflets only) were excised and mounted to a custom fixture with the atrial face oriented upwards (Figure 2A–C). Ten Y-31 2-0 Ti·Cron sutures were passed through the same ten positions studied in vivo. Placement adhered strictly to published annuloplasty guidelines (1–2mm above hinge line, 10mm bite width, minimum 10° between needle and leaflet hinge).[20]
Figure 2.
Ex vivo suture Holding Strength testing. (A–B) MV before and after explantation from ovine heart. (C) Tie-down to custom fixture using two continuous suture loops. Test sutures harnessed until pullout trial (red arrows). (D) Each suture was pulled out independently, at a perpendicular angle to the test plate.
Suture pullouts were conducted using an ElectroForce3200 uniaxial tester (Bose Corporation, Eden Prairie, MN). The fixture was attached to the lower testing arm in line with a 25N load cell (SMT1-22; Interface, Scottsdale, AZ). Each suture was sequentially tied to the upper arm and steadily pulled (2D) until it tore through the tissue or until the upper arm reached its maximum displacement (13mm). The fixture featured a biaxial traverse, enabling each suture to be positioned directly beneath the testing arm and pulled normal to the annulus. In terms of native MV geometry, this pulling is approximately radially inward, i.e. the approximate direction of suture tension following undersized annuloplasty in vivo. Throughout testing, MVs were kept topically hydrated with 0.75% saline spray.
In Vitro Annulus Microstructure Analysis
MV annulus tissue was excised from ovine hearts (N=5), at each of four positions: LT, RT, and the central regions of the anterior (Ant) and posterior (Post) circumference. Tissue samples were paraffinized and sectioned to 30μm. The final 30μm slices were roughly 5x3mm in the apical-basal and radial directions, respectively, such that the endocardial surface ran along one long edge of the sample.
Each sample was imaged under a 10X objective (1.66x1.66μm resolution) by two-photon excitation fluoroscopy using a laser scanning microscope (LSM 710 NLO; Zeiss, Oberkochen, Germany). Using an 800nm excitation wavelength, collagen was detected in one channel at 390–420nm via second harmonic generation from the fibers,[21] while non-specific extracellular structures were detected in another channel at 485–700nm. To maximize sensitivity and accuracy in assessing relative differences in microstructure, identical microscope acquisition settings (gain and offset) were used for each sample from a given MV. Among the four samples from a given MV, the sample with the strongest signal from either channel was identified. Settings were optimized for this sample, and held constant for the remaining three samples.
Mean pixel intensity (MPI) in each channel was quantified over the sample area using a custom MATLAB code. Before averaging across samples, each MPI was normalized by the Ant MPI from the same valve. That is, for the normalized MPI (nMPI) from valve i, position j:
| (1) |
Data Analysis
In vivo, the cyclic force (FC) acting on a suture was defined as the difference between the maximum and minimum force recorded for a given cardiac cycle. All reported FC values are averages from ten consecutive cycles with LVPmax of 100, 125, or 150mmHg, as indicated. All data was processed using a custom MATLAB code (MathWorks, Natick, MA). Following ex vivo suture pullouts, Holding Strength (HS) was defined as the peak recorded force. If the testing arm reached maximum displacement before complete pullout occurred, the final (peak) load was used to conservatively approximate tearing force. To estimate suture dehiscence risk, a margin of safety, termed Residual Strength (RS), was computed at each suture position as the difference between mean HS and mean FC at LVPmax=150mmHg. This elevated LVPmax was chosen as a worst-case.
For FC and HS, significance of pairwise differences among positions was assessed by Bayesian ANOVA, using p<0.05 as the critical threshold (OpenBUGS 3.2.3; OpenBUGS, Helsinki, Finland). All other statistical analysis (including pairwise comparison of both pooled forces and regional microstructural composition) was performed by Student’s t-test; critical p-values are as indicated (Minitab16; Minitab Inc., State Collage, PA).
Results
Animal Characteristics
A description of the animal population used, including heart rates during acquisition of the reported forces, is presented in Table 1. In each case, the animal’s mitral annulus was measured as size 30, and was successfully downsized by two full sizes using an instrumented ring with effective size 26. Under anesthesia, the Doppler-derived mean transmitral pressure gradient increased by 3±1mmHg following ring implantation. Representative force recordings from a three second interval are shown in Figure 3. At each position, real-time forces exhibited strong coupling to LVP and low inter-cycle variability.
Table 1.
Dataset Summary
|
In vivo- cyclic forces
| ||
| Sample size | 5 | |
| Positions tested | 10 | |
| Animal Weight (kg) | 58.2±12.4 | |
| Annulus size | 30±0 | |
| Ring size | 26 | |
| HR at LVPmax 100mmHg (bpm) | 111±14 | |
| HR at LVPmax 125mmHg (bpm) | 122±13 | |
| HR at LVPmax 150mmHg (bpm) | 134±15 | |
|
Ex vivo- pullout forces
| ||
| Sample size | 12 | |
| Positions tested | 10 | |
| Successful pullouts | 112/120 | |
|
In vitro- microstructural analysis
| ||
| Sample size | 5 | |
| Positions tested | 4 | |
HR, heart rate;LVPmax,maximum left ventricular pressure
Figure 3.

Representative coupled in vivo recordings of LVP and annuloplasty suture forces. Each trace corresponds to one mattress suture. The primary contributors to the observed increases in suture tension during systole are (i) constraint of the native annular shape to that of a flat, undersized ring, (ii) radial expansion of contracting myocardial fibers, and (iii) pressure acting on the valve leaflets, which may further pull the annulus and sutures.[22] Note, baseline pre-tension (i.e. the minimum force in each trace) has been zeroed, to highlight the amplitude differences among the ten sutures. This baseline force is nonzero and positive, and is a subject of ongoing investigation.
In Vivo Suture Force Measurement
For cardiac cycles with LVPmax of 100, 125, and 150mmHg, grand mean FC across all positions were 1.9±1.2, 2.2±1.4, and 2.4±1.5N, respectively. Each successive 25mmHg increase in LVPmax caused a change in FC of 14±16%. Among all 50 sutures, the absolute minimum and maximum FC at 125mmHg were 0.3 and 6.6N (4 o’clock and 3 o’clock, respectively). Averaged over 5 subjects, the minimum and maximum FC at 125mmHg were 1.0±0.5 and 3.7±1.6N (7 o’clock and RT, respectively).
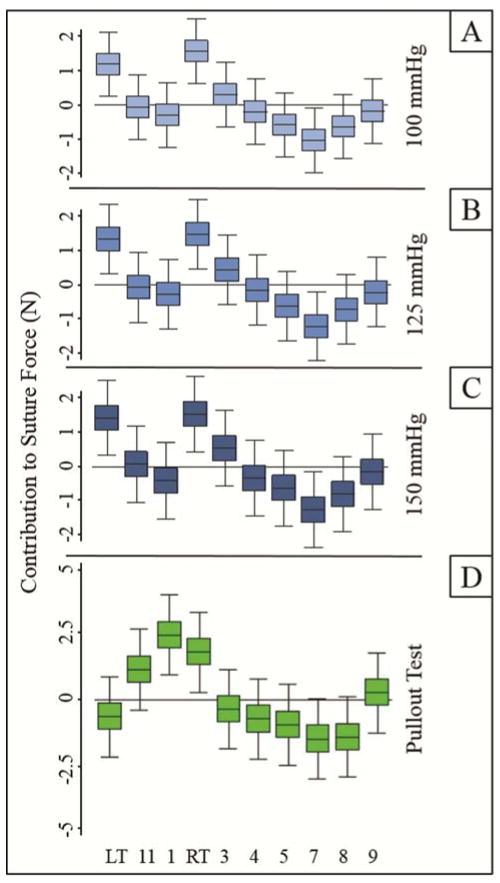
FC at each position and LVPmax is plotted in Figure 4. ANOVA revealed a series of pairwise differences between positions (Figure 5A–C). Notably, all significant differences were observed to exist between either LT or RT and a posterior position. FC was also pooled and analyzed by region. Anterior sutures experienced higher FC than posterior sutures (p<0.01 at each LVPmax). At 125mmHg, the disparity between anterior and posterior mean FC was 1.0N (2.8±1.3 vs. 1.8±1.2N).
Figure 4.
Cyclic Forces (FC) on each suture, recorded in vivo post-cardiopulmonary bypass (mean±SE).FC was computed from ten consecutive cycles with LVPmax of 100, 125, or 150mmHg. Anterior FC(orange) exceeded posterior FC (purple) by an average of 1.0–1.1N depending on LVPmax (p<0.01 at each LVPmax).
Figure 5.
ANOVA plots, reporting 95% Credible Intervals for the influence of each suture position on (A–C) Cyclic Forces or (D) Holding Strength. Positive or negative contribution to suture force at a given position indicates that position experienced force that was either greater or less than the grand mean force, respectively. Position pairs whose whiskers do not overlap are significantly different (p<0.05).
Ex Vivo Holding Strength (HS) and Residual Strength (RS)
The suture successfully pulled from the annulus in 93.3% of trials (Table 1). Each of the 8 unsuccessful trials were along the anterior aspect. The global mean suture force was 4.9±2.8N. The absolute minimum and maximum HS were 1.3 and 17.6N (8 o’clock and RT, respectively).
Mean HS at each position is given in Figure 6A. The minimum and maximum mean HS were 3.1±1.3 and 7.4±3.7N (7 o’clock and 1 o’clock, respectively). The HS profile roughly mirrors the FC profile. However, whereas the two largest FC magnitudes were located at either trigone, the two largest HS were between the trigones (with RT and LT the third- and fourth-strongest locations). ANOVA revealed a series of pairwise differences between HS (Figure 5D). The significant differences (p<0.05) were all between either 1 o’clock or RT and a posterior position. Pooled by region, anterior HS exceeded posterior by 2.5N (6.4±3.6 vs. 3.9±1.6N, p<0.0001).
Figure 6.
(A) Suture Holding Strength (HS) at each position, determined by ex vivo suture pullout testing. All anterior (orange) HS exceeded all posterior (purple) HS, including an average differential of 2.5N (p<0.0001). (B) Residual Strength (RS) at each position, i.e. the difference between mean HS and mean Cyclic Force with LVPmax of 150mmHg (Figure 3). Anterior RS exceeded posterior RS by 1.4N on average (p=0.10).
RS at each position is given in Figure 6B. Mean RS was 2.5N; minimum and maximum RS were 1.1 and 5.4N (LT and 1 o’clock, respectively). Despite the low LT value, the anterior aspect still exhibited greater mean RS than the posterior by 1.4N (3.3±1.8 vs. 1.9±0.3N, p=0.10). Grouped differently, anterior sutures placed between the trigones exhibited mean RS of 4.8±0.9N, versus 1.9±0.5N everywhere else (p<0.001).
Annulus Microstructure Analysis
Annulus tissue microstructure was clearly visible by autofluorescence, without histological staining. A sharply delineated collagenous band was typically located toward the endocardial surface (Figure 7). The thickness and density of this band varied among samples. nMPI was fixed to a value of 1 for the Ant position. For collagen, nMPI was approximately 2-fold higher at Ant than at any other position.
Figure 7.
(A) Normalized mean pixel intensity (nMPI), averaged over 5 MVs. (B) Collagen density at a given suture position, indicated by nMPI, correlated to Residual Strength at that position. (C) Representative MV annulus section at site of suture passage, visualized by autofluorescence; non-specific fibers (red) vs. collagen (green). Endocardial surface along right edge; leaflet hinge at lower-right corner. LT, left trigone; Ant, central anterior; RT, right trigone; Post, central posterior. *p<0.05; 3p<0.005.
Excluding Ant, collagen nMPI ranged from 0.44±0.09 (LT) through 0.54±0.13 (RT) (each p<0.05 versus Ant). In contrast, for non-specific structures, nMPI outside of Ant ranged from 1.01±0.25 (LT) to 1.21±0.27 (RT). No significant differences existed among non-specific structure nMPIs. Collagen density correlated strongly to RS (R2=0.947; 7B). Note, to estimate Ant or Post RS, the two closest positions were averaged (11–1 o’clock or 5–7 o’clock, respectively).
Comment
A significant number of implantable repair and replacement prosthetic MV devices are commercially available. Many devices rely on suture-based anchoring to the annulus, and the consequent risk for suture dehiscence is only beginning to be understood. The present study utilized clinically relevant models to explore the in vivo cyclic suture forces, ex vivo suture pullout forces, and regional variation in microstructure within the mitral annulus. The study successfully demonstrated how regional variations in collagen content impact the magnitude of in vivo suture forces and the force necessary to tear sutures from the annular tissue.
Extending from our recent pilot experiments,[17] this study has provided measurements of cyclic suture forces for an implanted MV device. At all positions, suture forces rose in systole, and fell in diastole (Figure 3). Three primary aspects of cardiac contraction likely contribute to these force dynamics. First, the native shape of the annulus is constrained to that of the flat, undersized ring. During systole, aortic root filling causes the central anterior annulus to rise, while ventricular contraction pulls the trigones apically. This accentuation of the annulus’ out-of-plane saddle shape is opposed by the annuloplasty ring, with the sutures absorbing the resulting tension. Second, when contracting myocardial fibers shorten along their length during systole, they also expand radially. This thickening will further expand the loops of suture, which are already tied taut around the fibers. Finally, systolic pressure on the leaflets has been shown to pull the annulus radially inward;[22] this tension, which is greatest on the anterior aspect, likely propagates through the tissue to the sutures. As a net effect, FC was observed to trend positively with LVPmax, but to vary significantly with suture position. When considered in isolation, the greater observed anterior FC appears to contradict the high proportion of posterior suture dehiscence observed clinically. Yet, ex vivo suture pullout force was also found to vary by position, with significantly higher anterior suture HS. Taken together, the FC and HS data provided a margin of safety at each position which mirrored clinical experience: after peak contractile loading, anterior sutures placed between the trigones exhibited a RS approximately 2.5 fold higher than those placed elsewhere (Figure 6B).
Suture breakage and pullout are recognized modes of failure in ISO 5840-3 and FDA guidance for heart valve replacements and annuloplasty rings.[23–25] Failures by breakage and pullout recognizably extend to other device-annulus anchors (e.g. hooks, clips, pledgets, or coils) that are common design features of minimally invasive or percutaneous MV repair devices. An anchoring mechanism’s loading and holding strength dynamics may be impacted by numerous aspects, including but not limited to its size, position in the annulus (height, depth, or circumferential position), and material stiffness. When the potential for breakage or pullout is identified, pullout testing is recommended to determine the strength of annular anchoring. As current devices and anchoring methods vary widely,[26] the justification of in vitro test methods and reference values becomes critical for anchor design verification and risk assessment.
Descriptions of in vitro test methods and reference values for annular anchor pullout testing are limited in the literature. In a study evaluating the safety of valvular prostheses for magnetic resonance imaging, annular suture pullouts were conducted. Among patients with varying valvular disease, single sutures were shown to tear from the annular tissue at 4.9±3.6N.[27] These forces agree strongly with the single suture pullout forces reported herein (4.9±2.8N). This similarity between differing test setups, species, and tissue properties (diseased vs. healthy) further supports the use of healthy, previously frozen ovine tissue in pullout experiments.
The asymmetry in RS observed herein is likely explained by asymmetry in collagen along the annular circumference. Two-photon excitation autofluorescence revealed significantly denser collagen between the trigones (Ant) as at the LT, RT, or Post. This was tightly correlated to RS (R2=0.947); a 50% reduction in collagen from the Ant position was associated with a 3N drop in RS. (Figure 7). Recent work by Gunning et al showed annular collagen is the principal driver of porcine annular mechanical strength under circumferential loading.[14] Further, they observed higher collagen content on the anterior quadrant of the annulus. However, this colorimetric analysis used an atrial view of the entire quadrant, at relatively low spatial resolution. Thus, the critical zone of transition from high to low collagen density remained to be defined. Our data complement this work, suggesting the average location of this transition zone must be interior from either trigone. These findings provide motivation to consider annular heterogeneity in the development of sutureless devices, as well. Transcatheter MV Replacements (TMVRs), for example, anchor and seal in part through radial expansion against the annulus. We predict the tissue’s resistance to conforming to the device will vary in a spatial pattern mirroring that observed in this study; this may significantly influence risk of paravalvular leakage or TMVR migration.
The heightened FC at the trigones may be explained on a biomechanical basis. Both collagen and myocardium are viscoelastic;[28, 29] under contractile loading, suture tension would induce these materials to strain in a time-dependent fashion, counteracting additional gains in tension. In contrast, the cardiac skeleton does not possess such compliance. Sutures placed directly atop the trigones may therefore be expected to reach greater tensions more quickly, as systolic contraction tends to pull them radially inward and apically. Yet, the lower collagen density atop the trigones also adversely affects that region’s HS. In this way, the trigonal combination of high rigidity/moderate failure strength presents similar risk to the posterior aspect’s low rigidity/low failure strength. In contrast, the high-collagen inter-trigonal region (exclusive of the trigones themselves) features high failure strength and only moderate rigidity. It is thus uniquely established as an optimal suture anchoring zone, as evidenced by its superior margin of safety. These findings may explain the more common clinical observation of posterior device dehiscence.
RS in this study was as low as 1.1N, computed from mean HS and mean FC using Physio rings. This equates to a safety factor of only 30%. Given such a low safety factor, in combination with numerous potential sources of variability that may exist clinically, the prevalence of dehiscence is understandable. The complex interaction between patient anatomy and etiology would make predicting the risk for a specific patient inherently uncertain. More extreme undersizing to correct severe dilatation, for example, may exacerbate FC, while collagen degeneration may further reduce HS. Compounding this uncertainty is the interaction of these factors with the shape and material properties of the selected device.
Ultimately, these findings should inform advanced device design as well as surgical decision-making and technique, such that dehiscence risk can be minimized on a patient-specific basis. However, the present study was limited to a single ring design and healthy tissue. Ongoing studies are exploring the influence of ring shape, size, and stiffness on FC, as well as the added risk of suture dehiscence in the setting of heart failure/ischemic MR.[16] The data reported in the present study will provide a baseline for interpreting the impact of left ventricular and/or MV dysfunction on annuloplasty sutures. A few additional limitations in the present study must be noted. Though an ovine model was used, various data suggest comparable annular mechanics between sheep and humans.[30] One key difference which may favor higher FC in humans is degree of systolic annular area reduction, which has been shown to be 10% in sheep vs. 26% in humans.[20, 31] The extension of our findings to human annuloplasty suture forces therefore requires further attention. Also, although suture tie-down force (i.e. pre-tension) is not expected to vary significantly by position, device, or etiology, it has not yet adequately been quantified. Tie-down force may add to suture dehiscence risk and is also the subject of an ongoing study. Finally, use of previously frozen, post-rigor mortis hearts, while expected not to affect collagen quantification, may slightly compromise collagen strength. We expect living tissue to be slightly more durable with respect to suture pullout.
Conclusions
Significantly denser collagen in the region of the anterior mitral annulus between the trigones creates an optimal zone for device anchoring. The posterior annulus, despite imparting low cyclic loads to sutures, is prone to suture dehiscence on account of its low HS. The trigonal regions themselves are poorer anchors than originally thought, due to the combined effect of high cyclic loading and relatively low collagen content. Further studies evaluating different devices and diseased animal models are underway.
Acknowledgments
Partially supported by a fellowship from the National Science Foundation (DGE-1148903: ELP) and by the National Heart, Lung and Blood Institute (HL113216).
This work was partially supported by a fellowship from the National Science Foundation (DGE-1148903: ELP) and by the National Heart, Lung and Blood Institute (HL113216).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cerfolio RJ, et al. Reoperation after valve repair for mitral regurgitation: Early and intermediate results. The Journal of thoracic and cardiovascular surgery. 1996;111(6):1177–1184. doi: 10.1016/s0022-5223(96)70219-2. [DOI] [PubMed] [Google Scholar]
- 2.Gillinov AM, et al. Reoperation for failure of mitral valve repair. The Journal of thoracic and cardiovascular surgery. 1997;113(3):467–475. doi: 10.1016/S0022-5223(97)70359-3. [DOI] [PubMed] [Google Scholar]
- 3.Dumont E, et al. Reoperation after mitral valve repair for degenerative disease. The Annals of thoracic surgery. 2007;84(2):444–450. doi: 10.1016/j.athoracsur.2007.03.078. [DOI] [PubMed] [Google Scholar]
- 4.Chitwood WR, et al. Robotic mitral valve repairs in 300 patients: A single-center experience. The Journal of thoracic and cardiovascular surgery. 2008;136(2):436–441. doi: 10.1016/j.jtcvs.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 5.Kronzon I, et al. Real-time 3-dimensional transesophageal echocardiography in the evaluation of post-operative mitral annuloplasty ring and prosthetic valve dehiscence. Journal of the American College of Cardiology. 2009;53(17):1543–1547. doi: 10.1016/j.jacc.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishna H. Incidental toe finding-carpentier mitral annuloplasty ring dehiscence during heart transplantation. Annals of cardiac anaesthesia. 2008;11(1):49. doi: 10.4103/0971-9784.38451. [DOI] [PubMed] [Google Scholar]
- 7.Shapira AR, et al. Dehiscence of mitral annuloplasty ring. Circulation. 2005;112(18):e305–e305. doi: 10.1161/01.CIRCULATIONAHA.104.509570. [DOI] [PubMed] [Google Scholar]
- 8.Levack MM, et al. Annuloplasty ring dehiscence in ischemic mitral regurgitation. The Annals of thoracic surgery. 2012;94(6):2132. doi: 10.1016/j.athoracsur.2012.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones-Haywood M-M, et al. Percutaneous closure of mitral paravalvular leak. Journal of cardiothoracic and vascular anesthesia. 2013;27(1):168–177. doi: 10.1053/j.jvca.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 10.Tsang W, et al. Early mitral annuloplasty ring dehiscence with migration to the descending aorta. Journal of the American College of Cardiology. 2009;54(17):1629–1629. doi: 10.1016/j.jacc.2009.03.090. [DOI] [PubMed] [Google Scholar]
- 11.Bolling SF, et al. Predictors of mitral valve repair: Clinical and surgeon factors. The Annals of thoracic surgery. 2010;90(6):1904–1912. doi: 10.1016/j.athoracsur.2010.07.062. [DOI] [PubMed] [Google Scholar]
- 12.Ciobanu AO, et al. Catastrophic mitral prosthesis dehiscence diagnosed by three-dimensional transesophageal echocardiography. Journal of Clinical Ultrasound. 2014;42(4):249–251. doi: 10.1002/jcu.22079. [DOI] [PubMed] [Google Scholar]
- 13.Spratt JR, et al. Strength comparison of mitral annuloplasty ring and suturing combinations: An in-vitro study. The Journal of heart valve disease. 2012;21(3):286–292. [PubMed] [Google Scholar]
- 14.Gunning GM, Murphy BP. Determination of the tensile mechanical properties of the segmented mitral valve annulus. Journal of biomechanics. 2014;47(2):334–340. doi: 10.1016/j.jbiomech.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Jensen MO, et al. Saddle-shaped mitral valve annuloplasty rings experience lower forces compared with flat rings. Circulation. 2008;118(14 suppl 1):S250–S255. doi: 10.1161/CIRCULATIONAHA.107.746776. [DOI] [PubMed] [Google Scholar]
- 16.Siefert AW, et al. Contractile mitral annular forces are reduced with ischemic mitral regurgitation. The Journal of thoracic and cardiovascular surgery. 2013;146(2):422–428. doi: 10.1016/j.jtcvs.2012.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siefert AW, et al. Suture forces in undersized mitral annuloplasty: Novel device and measurements. The Annals of thoracic surgery. 2014;98(1):305–309. doi: 10.1016/j.athoracsur.2014.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siefert AW, et al. In vitro mitral valve simulator mimics systolic valvular function of chronic ischemic mitral regurgitation ovine model. The Annals of thoracic surgery. 2013;95(3):825–830. doi: 10.1016/j.athoracsur.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya S, et al. Tension to passively cinch the mitral annulus through coronary sinus access: An ex vivo study in ovine model. Journal of biomechanics. 2014;47(6):1382–1388. doi: 10.1016/j.jbiomech.2014.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpentier A, et al. Carpentier's reconstructive valve surgery. Maryland Heights, Missouri: Elsevier Health Sciences; 2011. [Google Scholar]
- 21.Kwon GP, et al. Contribution of macromolecular structure to the retention of low-density lipoprotein at arterial branch points. Circulation. 2008;117(22):2919–2927. doi: 10.1161/CIRCULATIONAHA.107.754614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Z, Bhattacharya S. Papillary muscle and annulus size effect on anterior and posterior annulus tension of the mitral valve: An insight into annulus dilatation. Journal of biomechanics. 2008;41(11):2524–2532. doi: 10.1016/j.jbiomech.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Standardization IOf. Cardiovascular implants - cardiac valve prostheses. Part 3: Heart valve substitutes implanted by transcatheter techniques. 2013 [Google Scholar]
- 24.Services USDoHaH. Guidance for annuloplasty rings 510(k) submissions; final guidance for industry and fda staff. Maryland: Food and Drug Administration; 2001. [Google Scholar]
- 25.Wu Y, et al. Clinical evaluation of new heart valve prostheses: Update of objective performance criteria. The Annals of thoracic surgery. 2014;98(5):1865–1874. doi: 10.1016/j.athoracsur.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Maisano F, et al. The future of transcatheter mitral valve interventions: Competitive or complementary role of repair vs. Replacement? European heart journal. 2015:ehv123. doi: 10.1093/eurheartj/ehv123. [DOI] [PubMed] [Google Scholar]
- 27.Edwards M-B, et al. Mechanical testing of human cardiac tissue: Some implications for mri safety. Journal of Cardiovascular Magnetic Resonance. 2005;7(5):835–840. doi: 10.1080/10976640500288149. [DOI] [PubMed] [Google Scholar]
- 28.Sanjeevi R, et al. A viscoelastic model for collagen fibres. Journal of biomechanics. 1982;15(3):181–183. doi: 10.1016/0021-9290(82)90250-0. [DOI] [PubMed] [Google Scholar]
- 29.Rankin JS, et al. Viscoelastic properties of the diastolic left ventricle in the conscious dog. Circulation research. 1977;41(1):37–45. doi: 10.1161/01.res.41.1.37. [DOI] [PubMed] [Google Scholar]
- 30.Siefert AW, et al. Dynamic assessment of mitral annular force profile in an ovine model. The Annals of thoracic surgery. 2012;94(1):59–65. doi: 10.1016/j.athoracsur.2012.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rausch MK, et al. Characterization of mitral valve annular dynamics in the beating heart. Annals of biomedical engineering. 2011;39(6):1690–1702. doi: 10.1007/s10439-011-0272-y. [DOI] [PubMed] [Google Scholar]