Abstract
Aims
To assess feasibility of classifying skin tone using Munsell color chart values and to compare Munsell-based skin tone categories to ethnicity/race to predict pressure ulcer risk.
Background
Pressure ulcer classification uses level of visible tissue damage, including skin discoloration over bony prominences. Prevention begins with early detection of damage. Skin discoloration in those with dark skin tones can be difficult to observe, hindering early detection.
Design
Observational cohort of 417 nursing home residents from 19 nursing homes collected between 2009 – 2014, with weekly skin assessments for up to 16 weeks.
Methods
Assessment included forearm and buttocks skin tone based on Munsell values (Dark, Medium, Light) at three time points, ethnicity/race medical record documentation, and weekly skin assessment on trunk and heels.
Results
Inter-rater reliability was high for forearm and buttock values and skin tone. Mean Munsell buttocks values differed significantly by ethnicity/race. Across ethnicity/race, Munsell value ranges overlapped, with the greatest range among African-Americans. Trunk pressure ulcer incidence varied by skin tone, regardless of ethnicity/race. In multinomial regression, skin tone was more predictive of skin damage than ethnicity/race for trunk locations but ethnicity/race was more predictive for heels.
Conclusions
Given the overlap of Munsell values across ethnicity/race, color charts provide more objective measurement of skin tone than demographic categories. An objective measure of skin tone can improve pressure ulcer risk assessment among patients for whom current clinical guidelines are less effective.
Keywords: nursing skin assessment, nursing home care, pressure ulcers, nurse pressure ulcer detection, ethnicity, gerontology
INTRODUCTION
Pressure ulcers (PUs) are areas of skin and tissue damage caused by pressure and shear forces compressing tissues between bony prominences and an external surface. PUs are used as indicators of care quality across health care settings, represent a patient safety event, and reflect a multifactorial costly condition for vulnerable populations. PUs are classified according to level of visible tissue damage; where a stage 1 PU and suspected Deep Tissue Injury (DTI) are skin discoloration over bony prominences (red and maroon/purple, respectively [NPUAP/EPUAP 2014]). Stage 2 PUs are partial thickness wounds and stage 3/4 PUs are full thickness wounds. Prevention begins with early detection of skin discoloration, often the first indicator of PU damage. If recognized prevention strategies are implemented, early PU damage may be reversible. If unrecognized, damage may progress to more severe full thickness PUs.
Care providers have difficulty—even those with expertise and experience—in detecting skin color changes in persons with dark skin tones. In countries with diverse populations, this can have a profound impact on the ability to prevent pressure damage. For example, in the United States, epidemiological studies show that Hispanic and African American patients have higher prevalence of more severe full-thickness pressure ulcers compared with Caucasians (NPUAP/EPUAP 2014). Thus, there are likely problems identifying early PU development, leading to delay of effective prevention strategies. Such problems are true in other areas of health care such as treatment of child-birth related and sexual assault injuries, where skin color changes indicate injury (Everett et al. 2012). When ethnicity or race is used as a proxy for skin tone, health disparities attributed to societal or organizational sources may be confounded with imprecise detection methods. For this reason, an objective measure of skin tone may be more useful for evaluation PU risk.
Background
Ethnicity and race have often been used as a proxy for skin tone. In sexual assault injury, injury prevalence is significantly higher for Caucasian compared with African American women (Sommers et al. 2009). Related to PU development, multiple investigators describe higher prevalence of severe PUs and virtually no prevalence of early PUs among African Americans compared with Caucasian patients (Baumgarten et al. 2004, Hinds et al. 2013, Li et al. 2011, Nixon et al. 2007). However, in these circumstances, ethnicity or race is not a reliable determinant of skin tone because of the wide variation of skin tones in ethnic/racial groups (Everett et al. 2012, Sommers et al. 2009). There are few tools available for determining skin tone. The Fitzpatrick Skin Type Classification Scale, developed in Australia, has been used in dermatology to classify a person’s skin type based on the person’s sensitivity to ultraviolet rays (Fitzpatrick 1998). The tool’s utility on persons with dark skin tones, specifically for non-White ethnic/racial groups such as Asians, African Americans, and Hispanics may not be valid due to its development and application primarily for persons of European culture (Pinchon et al. 2010). The scale inherently applies the criterion of skin’s reaction to sunlight, using the words ‘tan’ and ‘burn’, which reflects and is biased toward the experience of Europeans’ or Caucasians’ experience to sun reactivity. Thus, using this tool to categorize skin tone excludes or miscategorizes the majority of persons with dark skin tones.
The Munsell System of Color Notation (Munsell chart) provides a more objective assessment of skin tone. This is a numerical notation system used internationally to describe and compare colors of many substances. Alphanumeric designations are assigned to color samples based on three qualities: hue, value, and chroma. Hue is the color quality that is described as red (R), yellow (Y), green (G), blue (B) and purple (P) and occurs as a range of values for a band of color with a total of 40 hues. Value is the lightness or darkness of a color, ranging from 0 for pure black - 10 for pure white. Chroma can be described as the vividness or saturation of a color (for example a low chroma value indicates a more washed out color, such as pastel colors). Sets of color tiles arranged by Munsell hue, value, and chroma are commercially available and inexpensive. By matching the perforated color tile that best matches a material, the color of that material can be described using Munsell notation. For example, the Munsell chart 5YR contains color tiles with yellow/red hues with values ranging from 2.5 to 8.0 and chroma ranging from 1 to 8. A yellow/red of medium lightness and fairly saturated would have a Munsell value of 5YR 5/6, with ‘5YR’ indicating the color in the middle of the yellow/red hue band, ‘5/’ indicating medium value (lightness), and a chroma of ‘6’ indicating moderately saturated.
Munsell charts (www.munsell.com) have been used to describe human skin tone specifically related to dermatology (Gitelson 1965, Lim & Tham 1997, Reisfeld 2000, Riordan et al. 2001, Sivarajan & Mackay 2005). Munsell charts have been used to describe lightening of capillary vascular malformations (‘port wine stains’) exposed to pulsed dye laser treatments as an objective method to determine therapy response (Sivarajan & Mackay 2005). Lim and Than (1997) used Munsell charts to evaluate effectiveness of glycolic acid peel treatments for melasma in Asian women by categorizing skin hyperpigmentation through treatments. Both patients and dermatologists were able to detect skin color change using Munsell charts.
Central to detecting early pressure-induced tissue damage, Munsell charts have been used to assess blue and red skin coloration. Reisfeld (2000) assessed blue tones in skin overlying veins using the Munsell color chart with the 5YR hue range in 2.5 unit increments (the same chart used in this study). Others have used the 5YR Munsell chart to classify skin color to study tissue reflectance spectroscopy erythema detection algorithms (Riordan et al. 2001). In this study, skin color was classified by value (2.5–8, lower indicating darker) and chroma (1–8, higher indicating redder) and, of the 20 subjects, nine Caucasians were classified with Munsell values ranging from 7–8, three Hispanic subjects were classified values ranging from 6–7, and eight African Americans with values from 3–6. Use of the Munsell charts in this study categorized subjects’ skin as ’dark’ or ‘light’ toned to identify which algorithms detected erythema with high sensitivity and specificity across all skin tones.
THE STUDY
Aims
The purpose of this study is to report the validity, reliability and feasibility of using Munsell charts to objectively classify skin tone in nursing home (NH) residents at risk for PU development, to examine the incidence of PUs across Munsell skin tone categories, and to compare ability of Munsell values to ethnicity/race category to predict PU development.
Design
The study is observational, involving research staff observation of skin color and weekly skin assessments on trunk and heel areas for up to 16 weeks among NH residents from 19 NHs.
Participants
Data for this study were obtained from a large prospective cohort study conducted in 2009–2014 to evaluate use of a handheld dermal phase meter that measures subepidermal moisture, edema or water in skin and tissues, as a method of detecting early PUs in NH residents. The primary aim of the study involved longitudinal analysis of skin damage, so residents with less than three weeks of observations are excluded from the analysis dataset. Of the 490 enrolled in the study, 417 participants had at least three weeks of data, 42 withdrew themselves from the study, 10 were withdrawn by the principal investigator, and 21 died or were discharged before completing three weeks of measurement. Study design specifically targeted the recruitment of large numbers of minority NH residents. Munsell color tiles were used to objectively classify participant skin tone. Participants were followed for 16 weeks, with Munsell skin tone assessments obtained initially (baseline), at 8 weeks, and on completion of the larger study (week 16).
Data Collection
Medical record data
At baseline, and monthly thereafter, research staff extracted medical and demographic information from all consented participants’ medical records and their most recent Resident Assessment Instrument Minimum Data Set (MDS). The MDS is a multi-domain assessment tool that is completed on admission and at quarterly intervals for residents in Medicare and Medicaid accredited NHs in the U.S. Ethnicity/racial category was determined based on medical record admission documentation, which codes ethnicity/racial categories as American Indian, Asian, African American, Hispanic, and Caucasian.
Braden Scale Risk Assessment scores
Research staff assessed participants’ risk for pressure ulcer development at baseline and each month using the Braden scale. The Braden Scale is composed of six subscales that conceptually reflect degrees of sensory perception, moisture, activity, mobility, nutrition, and friction and shear (Bergstrom et al. 1996, 1998). All subscales are rated from 1–4 (1=best, 4= worst for subscale), except for friction and shear, which is rated from 1–3. The subscales may be summed for a total score, with a range from 6–23, with lower scores indicating lower function and higher risk for developing a PU. In older patients, cutoff scores of 17 or 18 have been shown to be predictors of PU development (Bergstrom et al. 1996).
Visual skin assessment
Trained research staff assessed skin health through direct independent visual skin assessments of the sacrum, buttocks, ischium, and heels each week for 16 weeks. Visual skin assessment training, developed by the second author, an expert in wound classification, emphasized early PU damage, stage 1 PUs, and DTI because DTI and early damage may be transient and difficult to detect (NPUAP/EPUAP 2014). Erythema and skin discoloration was rated as present or absent and then coloration severity graded as minimal (pink-light red in light skin tones or slight deepening of normal color in dark skin tones), moderate (bright red in light skin, tones; purple in dark skin tones), or severe (dark red to purple in light skin tones, black to blue-grey in dark skin tones). Erythema was defined as moderate skin discoloration for all skin tones, and additionally with blanching for light skin tones (based on the finger method of palpation to elicit the blanch response). Stage 1 PUs were defined as severe skin discoloration for all skin tones, and additionally, as moderate skin discoloration with nonblanching for light skin tones. PUs were classified using the National Pressure Ulcer Advisory Panel’s (NPUAP’s) 2009 six categories. The presence or absence of urinary or fecal incontinence and ointments or skin creams were also recorded.
Skin tone assessment using Munsell color charts
Two trained raters independently assessed skin color on the ulnar aspect of the forearm midway between wrist and elbow and the upper inner quadrant of the right or left buttock using the 5YR Munsell color chart with 33 color tiles representing scores of 2.5–8.0 for value and 0.1–0.8 for chroma. Skin assessments were performed at baseline, 8 and 16 weeks. Measurements were obtained after ensuring no presence of feces or urine, ointments or skin creams and in locations without scar tissue. Use of the Munsell color chart is quick (less than one minute), straight-forward, uncomplicated, and intuitive. Research staff training in use of the Munsell chart required less than fifteen minutes; certification involved measurement with five persons and review of results with the trainer.
Ethical Considerations
The university Human Subject Protection Committee approved the protocol. Research staff obtained written informed consent to participate in the current study from residents who were able to provide informed consent or from their designated representatives for residents unable to provide consent.
Data Analysis
Demographic and medical data were analyzed using descriptive statistics with mean (standard deviation [SD]) for continuous variables and frequency and proportion for categorical variables. Munsell values were analyzed overall and by ethnicity/racial categories (African American, Asian, Caucasian, Hispanic) for stability from baseline to 8 and 16 weeks and relationship between buttock and forearm readings using Pearson’s correlation coefficient and for seasonal variation and differences in reliability of raters based on gender using Students t test.
Box-whisker plots were used to illustrate Munsell value overlap across ethnicity/racial groups. Inter-rater reliability was assessed two ways: with intraclass correlation coefficient (ICC) for continuous variables for the first digit of the actual Munsell value and with Kappa coefficient for defined skin tone groups. Analyses were conducted with SPSS Statistics for Windows, Version 21.0. (IBM Corp., Armonk, NY). Statistical significance was set at p = 0.05.
To compare PU incidence between Munsell categories and ethnicity/racial groups ANOVA was used (conducted with SAS, Version 9.2 for Windows). Using generalized multinominal logistic regression analyses with covariates of age, gender, Braden Scale for PU risk (for sacral location), diabetes mellitus (for heels location), and controlling for clustering we compared use of Munsell categories to ethnicity/racial groups as a predictor for PU development using Wald statistic and likelihood ratio chi-square for the models and Bayesian information criterion (BIC) to compare models (conducted with STATA, version 13.1). Statistical significance was set at p = 0.05.
Validity and Reliability
Two observers obtained duplicate measurements at randomly selected observation visits. Paired observers were blinded to each other’s ratings. Spearman correlation coefficient was used to evaluate reliability of the Braden Scale because of the ordinal nature of the measurement and the small range of observed values (60% fall within a range of 13–18). Of the randomly selected reliability visits, 464 were during visits when Braden Scale data were collected; the correlation was 0.81 at baseline visits and 0.77 at monthly visits. Agreement of the visual skin assessment was calculated on 2,124 pairs of observations with 96% agreement overall (Kappa=0.72).
RESULTS
Table 1 presents demographic and functional characteristics of the participants on the variables most relevant to this study. Similar to typical nursing home residents in the United States, participants were on average 77 years old and represented major ethnic groups in the United States, with about one-third Caucasian (non-Hispanic white). Slightly more than half were women. On average, NH residents were at risk for developing a PU (mean Braden Scale= 15.6 (SD 3.2); scores below 18 indicate at risk). Participants were functionally dependent with 25% totally dependent for bed mobility and 43% required extensive assistance with bed mobility.
Table 1.
Participant Demographic and Functional Characteristics
| Characteristics (N=417) | Mean (SD) or Number (%) |
|---|---|
| Age | 76.5 (14.8) |
| Female (N (%)) | 243 (58%) |
| Ethnicity/Race (N (%)) | |
| African American | 122 (29%) |
| Asian American | 50 (12%) |
| Caucasian | 156 (37%) |
| Hispanic | 89 (21%) |
| MDS Bed Mobility score | 2.7 (1.2) |
| MDS Transfer score | 3.1 (1.0) |
| MDS Urinary Incontinence | 2.4 (1.5) |
| MDS Bowel Incontinence | 2.5 (1.5) |
| Braden Scale score | 15.6 (3.2) |
Note. MDS=Minimum Data Set; Bed Mobility, Transfer scored as 1= independent, 4=dependent; Urinary and Bowel Incontinence scored as 1=continent, 4=incontinent all the time; Braden Scale for Predicting Pressure Sores Scale scored as 6=high risk; 23=no risk.
Over time, mean Munsell scores were consistent for baseline-8 week and baseline-16 week for both arm (intraclass correlation (ICC) r=0.86 and r=0.85, respectively, both p<0.001) and buttock (r=0.90 and r=0.91, respectively, both p<0.001) across all ethnicity/racial groups. We examined seasonal variation in Munsell values by comparing participants who engaged in the study during summer months when arm values might be expected to change with sun exposure (June through September) with those who participated during winter months (October through January). The mean Munsell value of the arm for all ethnicity/racial groups remained approximately the same from June to September (6.7–6.9) and from October to January (6.7–6.7). As would be expected, seasonal changes also did not apply to Munsell values obtained from buttocks.
The Munsell values obtained from the arm and buttock were similar (ICC r=0.88, p<0.001). The correlation was lower among Asian and Caucasian participants (ICC r=0.53 and r=0.55, respectively, both p<0.001), indicating greater difference in arm Munsell values compared with African Americans participants (ICC r=0.83, p<0.001) and Hispanic participants (ICC r=0.64, p<0.001).
Reliability of Munsell ratings for arm and buttock among the 30 raters is shown in Table 2. For all ethnicity/racial groups, inter-rater reliability for buttocks at baseline was high (ICC r = 0.97 (p<0.001) and reproducible (Kappa coefficient=0.84). Findings were similar for other study time points (data not shown) and for arm location. ICCs were highest when rating African Americans (r=0.93, p<0.001) and lowest for Caucasians (r=0.91, p<0.001). We also examined inter-rater reliability by rater gender and found no significant difference for female pairs compared with other pairs (female pairs: r=0.99, N=102 paired observations vs other pairs: r=0.97, N=303 paired observations, p=0.84). Raters included African Americans (N=4), Asians (N=4), Caucasians (N=15) and Hispanics (N=7).
Table 2.
Inter-Rater Reliability for Buttock Munsell Score (ICC) and Skin tone Categories (Kappa) by Ethnicity/Race and Overall
| Ethnicity/Race | N | Intraclass Correlation Coefficient* | Kappa Coefficient* |
|---|---|---|---|
| African American | 122 | .927 | .712 |
| Asian American | 50 | .925 | .874 |
| Caucasian | 156 | .907 | .776 |
| Hispanic | 89 | .924 | .731 |
| Total | 417 | .972 | .836 |
All p<.001
As shown in Figure 1, a large overlap was observed in the Munsell values across ethnicity/racial groups (Munsell values 5.3–8.3). The range was greatest for African Americans 3.1–8.3, followed by Hispanics (4.2–8.4). Caucasians and Asian Americans had the narrowest range of Munsell values (5.2–8.4 and 5.3–8.4, respectively).
Figure 1.
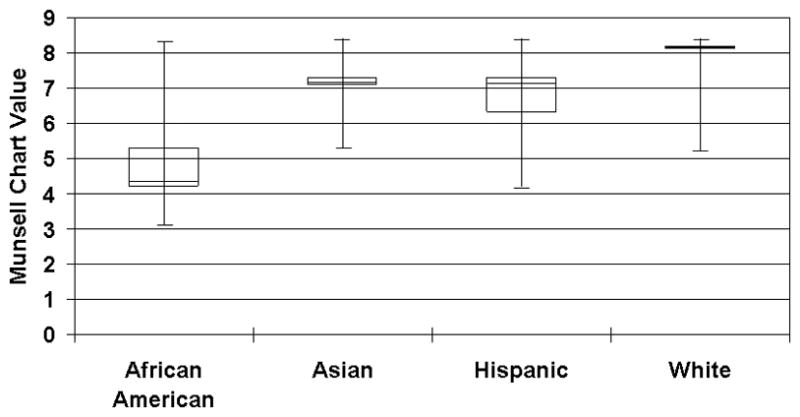
Box and whisker plot of Munsell chart values by ethnicity group
Munsell values were used to group participants into one of three skin tone groups: light skin tones (values 7–8); medium skin tones (values 5–6) and dark skin tones (values 2.5–4). Table 3 shows the proportion of participants in each skin tone category by ethnicity/race. Only African Americans are represented in each of the skin tone groups, indicating a broad range of skin color and thus a broad range of PU risk based on skin tone.
Table 3.
Skin Tone Categories and Incidence of Trunk Skin Damage by Ethnicity/Race (N (%))
| Ethnicity/Skin Tone | N (% of ethnicity) | Erythema/Stage 1 | Stage 2/3/4 |
|---|---|---|---|
| African American | 122 | 17 (14%) | 21 (17%) |
| Dark skin tone | 66 (54%) | 3 (4%) | 8 (12%) |
| Medium skin tone | 45 (37%) | 10 (22%) | 10 (22%) |
| Light skin tone | 11 (9%) | 4 (36%) | 3 (27%) |
| Asian American | 50 | 32 (64%) | 12 (24%) |
| Dark skin tone | 0 | -- | -- |
| Medium skin tone | 11 (22%) | 7 (64%) | 4 (36%) |
| Light skin tone | 39 (78%) | 25 (64%) | 8 (21%) |
| Caucasian | 156 | 44 (28%) | 37 (24%) |
| Dark skin tone | 0 | -- | -- |
| Medium skin tone | 8 (5%) | 3 (37%) | 1 (12%) |
| Light skin tone | 148 (95%) | 41 (28%) | 36 (24%) |
| Hispanic | 89 | 39 (44%) | 16 (18%) |
| Dark skin tone | 2 (2%) | 1 (50%) | 0 |
| Medium skin tone | 29 (33%) | 11 (38%) | 7 (24%) |
| Light skin tone | 58 (65%) | 27 (47%) | 9 (15%) |
| Total | 417 | 132 (32%) | 86 (21%) |
| Dark skin tone | 68 (16%) | 4 (6%) | 8 (12%) |
| Medium skin tone | 93 (22%) | 31 (33%) | 22 (24%) |
| Light skin tone | 256 (61%) | 97 (38%) | 56 (22%) |
Note. Logistic regression models predicting incidence for erythema/Stage 1 and for Stage 2/3/4 were run separately for skin ton and ethnicity, with gender, age, and Braden score as covariates. Skin tone was a significant predictor of erythema/Stage 1 incidence (p=0.003), but not Stage 2/3/4 incidence. Ethnicity was not a significant predictor for either level of skin damage.
Incident skin damage for erythema/stage 1 PU and stage 2/3/4 PU for trunk locations (sacrum, buttocks, ischium, trochanter) is presented in Table 3 by Munsell skin tone category, both within ethnicity/race and overall. Notably, erythema/stage 1 PU are less often observed among residents with dark and medium skin tones, regardless of ethnicity/race.
The multinominal logistic regression models comparing use of Munsell skin tone categories with ethnicity/race along with covariates of Braden Scale for Risk of PUs, gender, and age demonstrated significant findings for Munsell skin tone as a predictor of erythema (RRR=7.66, p<0.001, 95% confidence interval [CI] 4.32—13.56) and stage 1 PU (RRR=5.87, p<0.001, 95% CI 2.55—13.55) at the sacral site. Ethnicity/race was a significant predictor of erythema (RRR=1.71, p<0.001, 95% CI 1.47—1.99) and stage 2 and greater PU (RRR=0.78, p<0.001, 95% CI 0.69—0.87) at the sacral site (LR chi-square=581.05, p<0.0001; Wald chi-square=92.07, p<0.001 for Munsell models; LR chi-square=521.35, p<0.001; Wald chi-square=53.61, p<0.001 for ethnicity/race models). Bayesian information criterion (BIC) showed a difference of 4.269 between the Munsell skin tone model and the ethnicity/race model and provides positive support for the Munsell skin tone model with covariates of age and Braden Score for PU risk. In models conducted for the heel location with diabetes and age as covariates, the opposite was true—the BIC showed a difference of 57.87 between models providing very strong support for the ethnicity/race model.
DISCUSSION
In this study, a Munsell color chart was used to classify the skin tone of NH residents from a variety of ethnicity/race groups. Values were reliably and easily collected over time and from skin on two anatomic locations, arm and buttocks. As expected, patterns of early levels of skin damage, as defined by visual skin assessment, were difficult to detect for residents with dark skin tone, regardless of ethnicity/race.
In a previous cross-sectional study, Everett, Budescu, and Sommers (2012) defined the variation of skin tone across ethnic/racial groups and how this variation can be used to guide clinical practice. The study included 237 female participants: Asian (N=13), Black/African American (N=101), Caucasian (N=118), and Biracial (N=5). Skin color measurements of the volar forearm and upper inner arm were obtained with a hand-held ColorTec spectrophotometer that was calibrated to CIELAB color space standards. For both anatomic locations, African American participants were more likely to have lower values on the light to dark spectrum and a wider range of on the red-green spectrum and the yellow-blue spectrum than any other ethnicity/race group.
A separate study by Swiatoniowski et al. (2013) compared the use of the von Luschan skin color tiles (a set of 36 standardized, opaque, colored glass tiles) to spectrophotometry. Measurements of the forehead, inner upper arms and hands were collected with African Americans (N=66), Asians (N=14), Caucasian (N=150), Hispanics (N=8), and ’Other’ race (N=8). Consecutive tiles are not consistent in chromatic order or degree of color difference, so matching tiles to the skin pigmentation was often difficult and resulted in many tiles at the ends of the chromatic scale left unused. In addition, because glass has different physical properties from skin, the indices obtained from measuring the tiles directly with a reflectometer cannot be compared with those obtained from human skin.
The Munsell color chart provides a way to objectively rate skin tone and the reliability in this study did not vary by skin tone. In contrast, the Fitzpatrick Skin Type Classification Scale, used by Pichon et al. (2010) to evaluate the risk of skin cancer among African Americans, had limited utility among persons with dark skin tones. Differences in Munsell values by anatomic site were explained through greater exposure of arm skin to pigment-altering light. Similarly, differences over time were explained by seasonal differences in exposure to sunlight.
The Munsell color chart required minimal staff training for use and negligible time for assessment making it a practical tool for potential use in clinical practice as a method of refining PU risk assessment. Current practice focuses on attention to specific ethnicity/racial groups as more or less at risk for PU development however use of a an objective measure of skin tone such as a Munsell color chart may increase sensitivity of risk assessment and further target aggressive prevention strategies to those persons in whom early signs of pressure damage are masked by skin color.
Limitations
One limitation of the current study is that Munsell values were not collected for the heel. It is possible, and even likely for those with dark skin tones, that skin on the trunk may be classified in a different category than skin on the heel. For clinical usefulness, it may be necessary to directly assess the skin tone for the anatomic location being monitored.
While there were high levels of participation in this study by residents of ethnic minorities, there are still groups that were not included. We did not recruit significant numbers of Pacific Islanders, Native Americans, or those with ancestry from the Indian sub-continent nor did we account for persons identifying with more than one ethnicity/race group. Further work that includes NH residents from these ethnic groups would greatly enhance the generalizability of the findings.
CONCLUSION
This study demonstrates the inaccuracy of describing skin color by ethnicity/race and the value of using the Munsell Color Chart to assess skin color. Further, as the population becomes increasingly multiracial, tools such as the Munsell color chart become essential in assessing skin tone. More importantly, accurate skin color baseline measurements are imperative assessments for detection of Stage 1 pressure ulcer development in patients with darker skin tones. Use of a Munsell color chart provides a practical objective method of increasing sensitivity of PU risk assessment for clinical practice and research.
SUMMARY STATEMENT.
Why is this research or review needed?
More effective tools for determining risk for early skin damage among persons of various ethnicities or racial groups is needed to eliminate to health care disparities in pressure ulcer prevention.
Evidence about pressure ulcer prevalence and incidence among persons with dark skin tones is important for the discussion of improved pressure ulcer prevention and identifying those at increased risk.
What are the key findings?
Skin tone ranges, measured reliabily over time and across raters for all nursing home residents using Munsell color charts, overlapped across ethnicity/race categories, with the greatest range among African-Americans.
Skin tone was more predictive of skin damage on trunk locations than ethnicity/race, such that those with darker skin tones had more severe levels of skin damage.
How should the findings be used to influence policy/practice/research/education?
Clinical tools that use other measures of tissue damage need to be identified to enable early high-quality pressure ulcer risk assessment among all patients, regardless of skin tone.
Studies of ethnic/racial disparities regarding pressure ulcer incidence and prevalence should also include information about skin tone assessment techniques to determine if differences in detection accuracy are confounding results.
In clinical practice, use of ethnicity/race measures to identify pressure ulcer risk may be insufficient without a measure of skin tone such as that obtained with Munsell color charts.
Acknowledgments
Funding
This project was supported by a grant from the National Institute of Nursing Research to BBJ (5R01NR010736). Data collection and analyses was supported in part by the UCLA Claude Pepper Older Americans Independence Center funded by the National Institute on Aging (5P30AG028748).
Footnotes
No conflict of interest has been declared by the authors.
Author Contributions:
- substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
-
drafting the article or revising it critically for important intellectual content.
References
- Baumgarten M, Margolis D, van Doorn C, Gruber-Baldini AL, Hebel JR, Zimmerman S, Magaziner J. Black/white differences in pressure ulcer incidence in nursing home residents. Journal of the American Geriatrics Society. 2004;52(8):1293–1298. doi: 10.1111/j.1532-5415.2004.52358.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Multi-site study of incidence of pressure ulcers and the relationship between risk level, demographic characteristics, diagnoses, and prescription of preventive interventions. Journal of the American Geriatrics Society. 1996;44(1):22–30. doi: 10.1111/j.1532-5415.1996.tb05633.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom N, Braden B, Kemp M, Champagne M, Ruby E. Predicting pressure ulcer risk: A multisite study of the predictive validity of the Braden scale. Nursing Research. 1998;47(5):261–269. doi: 10.1097/00006199-199809000-00005. [DOI] [PubMed] [Google Scholar]
- Everett JS, Budescu M, Sommers MS. Making sense of skin color in clinical care. Clinical Nursing Research. 2012;21(4):495–516. doi: 10.1177/1054773812446510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Archives of Dermatology. 1998;124(6):869–871. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- Gitelson S. Determination of the skin color with the use of perforated Munsell color standards. Israel Journal of Medical Sciences. 1965;1(5):994–1003. [PubMed] [Google Scholar]
- Hinds GA, Alhariri J, Klein RQ, Wilson LD. Treatment of mycosis fungoides with total skin electron beam: Response and relapse by ethnicity and sex. American Journal of Clinical Oncology. 2013;36(5):481–485. doi: 10.1097/COC.0b013e31825494d3. [DOI] [PubMed] [Google Scholar]
- Li Y, Yin J, Cai X, Temkin-Greener J, Mukamel DB. Association of race and sites of care with pressure ulcers in high-risk nursing home residents. Journal of the American Medical Association. 2011;306(2):179–186. doi: 10.1001/jama.2011.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JT, Tham SN. Glycolic acid peels in the treatment of melasma among Asian women. Dermatologic Surgery. 1997;23(3):177–9. doi: 10.1111/j.1524-4725.1997.tb00016.x. [DOI] [PubMed] [Google Scholar]
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guidelines. Washington DC: National Pressure Ulcer Advisory Panel; 2009. [Google Scholar]
- Haesler E, editor. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Clinical Practice Guidelines. Cambridge Media; Perth, Australia: 2014. [Google Scholar]
- Nixon J, Cranny G, Bond S. Skin alterations of intact skin and risk factors associated with pressure ulcer development in surgical patients: A cohort study. International Journal of Nursing Studies. 2007;44(5):655–663. doi: 10.1016/j.ijnurstu.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Pichon LC, Landrine H, Corral I, Mayer JA, Hoerster KD. Measuring skin cancer risk in African Americans: Is the Fitzpatrick skin type classification scale culturally sensitive? ’ Ethnicity & Disease. 2010;20(2):174–179. [PubMed] [Google Scholar]
- Reisfeld PL. Blue in the skin. Journal of the American Academy of Dermatology. 2000;42(4):597–605. [PubMed] [Google Scholar]
- Riordan B, Sprigle S, Linden M. Testing the validity of erythema detection algorithms. Journal of Rehabilitation Research & Development. 2001;38(1):13–22. [PubMed] [Google Scholar]
- Sivarajan V, Mackay IR. Noninvasive in vivo assessment of vessel characteristics in capillary vascular malformations exposed to five pulsed dye laser treatments. Plastic & Reconstructive Surgery. 2005;115(5):1245–1252. doi: 10.1097/01.prs.0000156776.03772.fb. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Fargo JD, Baker RB, Fisher BS, Buschur C, Zink TM. Health disparities in the forensic sexual assault examination related to skin color. Journal of Forensic Nursing. 2009;5(4):191–200. doi: 10.1111/j.1939-3938.2009.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatoniowski AK, Quillen EE, Shriver MD, Jablonski NG. Technical note: Comparing von Luschan skin color tiles and modern spectrophotometry for measuring human skin pigmentation. American Journal of Physical Anthropology. 2013;151(2):325–330. doi: 10.1002/ajpa.22274. [DOI] [PubMed] [Google Scholar]


