Abstract
Heart failure is common and results in substantial morbidity and mortality. Current guideline-based therapies for heart failure with reduced ejection fraction, including beta-blockers, angiotensin converting enzyme (ACE) inhibitors, and aldosterone antagonists aim to interrupt deleterious neurohormonal pathways and have shown significant success in reducing morbidity and mortality associated with heart failure. Continued efforts to further improve outcomes in patients with heart failure with reduced ejection fraction have led to the first new-in-class medications approved for heart failure since 2005, ivabradine and sacubitril/valsartan. Ivabradine targets the If channels in the sinoatrial node of the heart, decreasing heart rate. Sacubitril/valsartan combines a neprilysin inhibitor that increases levels of beneficial vasodilatory peptides with an angiotensin receptor antagonist. On a background of previously approved, guideline-directed medical therapies for heart failure, these medications have shown improved clinical outcomes ranging from decreased hospitalizations in a select group of patients to a reduction in all-cause mortality across all pre-specified subgroups. In this review, we will discuss the previously established guideline-directed medical therapies for heart failure with reduced ejection fraction, the translational research that led to the development of these new therapies, and the results from the major clinical trials of ivabradine and sacubitril/valsartan.
Introduction
Heart failure is a source of significant morbidity and mortality in the United States1 and is responsible for billions of dollars spent in direct medical expenditures and lost revenue due to reduced productivity2. In the past three decades, dramatic advances have been made in the understanding of the pathophysiology of heart failure and the development of pharmacologic therapies that improve functional status and reduce hospitalizations and mortality for patients with heart failure with reduced ejection fraction3–7. These advances have led to guideline recommendations for the use of certain beta-blockers, angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers, and aldosterone antagonists in patients with symptomatic heart failure with reduced ejection fraction. However, despite these guideline-directed medical treatments, aimed at blockade of the neurohormonal mechanisms of heart failure, heart failure remains the cause of one in nine deaths in the United States1 and is the number one cause of hospitalization. Recognizing this, effort has continued to identify new pathways in heart failure for modification in patients already receiving the benefit of these proven medications. Secondary analysis of major beta-blocker trials and data from large heart failure registries revealed that heart failure patients with lower heart rates have improved outcomes. This led to the prospective trials that have shown the sinoatrial “funny” current (If) inhibitor, ivabradine, improves outcomes in selected patients with heart failure8. Additionally, while blockade of the renin-angiotensin-aldosterone (RAA) system has been a cornerstone of heart failure therapy, more recent research has noted the important effects of the body’s own mechanisms to counter the volume expansion and vasoconstriction seen in heart failure. Efforts to augment these natural systems resulted in the approval of sacubitril, a neprilysin-inhibitor, given in combination with the angiotensin receptor blocker (ARB) valsartan in the treatment of heart failure with reduced ejection fraction9. In this review, we will summarize the current knowledge of the pharmacologic treatment of chronic heart failure and then explore the first new-in-class medications to be approved by the FDA for the treatment of heart failure since 2005, ivabradine and sacubitril/valsartan (LCZ696).
Guideline-Directed Medical Therapy
Heart failure is the inability of the heart to maintain enough cardiac output to distal organs to meet metabolic demand and is heralded by symptoms that include dyspnea, edema, and fatigue. The decreased perfusion and arterial pressure activate regulatory systems in the body’s neural and hormonal pathways designed to compensate for the weakened heart. The most important of these is the RAA system, in which decreased perfusion to the juxtaglomerular cells in the kidney result in an increase in renin levels. Renin is responsible for the conversion of angiotensinogen to angiotensin I (AT I) which is, in turn, converted to angiotensin II (AT II). AT II has a host of effects, including vasoconstriction, promotion of anti-diuretic hormone (ADH) and aldosterone secretion, and an increase in sympathetic tone10. Baroreceptor feedback in the neural axis further increases the adrenergic drive through direct nerve innervation on the heart and adrenal glands, increasing circulating catecholamines that increase heart rate and cardiac contractility11. The physiologic end goal of the neurohormonal cascade is a compensatory attempt to restore organ perfusion through increased systemic vascular resistance, plasma volume, and cardiac output.
While these mechanisms may help in an acute setting, over time the chronic, continuous feedback becomes deleterious, leading to pathologic ventricular remodeling, worsening heart failure, and perpetuating a downward spiral. Extended beta-receptor activation increases myocardial metabolic demands, contributes to adverse ventricular remodeling, predisposes to dangerous arrhythmias, and speeds myocyte death11. The continuous activation of the RAA system leads to remodeling of the ventricle, volume overload, and increased ventricular fibrosis10. In light of this, current guideline therapy in chronic heart failure aims to interrupt this process. The Studies of Left Ventricular Dysfunction (SOLVD) and Vasodilator-Heart Failure Trial II (V-HeFT II) trials showed that ACE inhibitors reduced the risk of death by 17% and death or hospitalization by up to 30% compared to placebo, and they were superior to the nonspecific vasodilators hydralazine and isosorbide dinitrate6,12. Other trials showed that angiotensin receptor antagonists could improve outcomes in patients intolerant of ACE inhibitors but did not reduce mortality when added on to an ACE inhibitor. Studies testing beta-blockade in heart failure with reduced ejection fraction, including the Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) and Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trials, showed an additional mortality decrease of up to 35% when added to background ACE inhibitor therapy3,4. The Randomized Aldactone Evaluation Study (RALES) trial showed a remarkable, additional 30% all-cause mortality reduction with the aldosterone antagonist spironolactone when added to ACE inhibitor and beta-blocker therapy7, and subsequent trials showed benefit in mild heart failure with reduced ejection fraction. Taken together, pharmacologic therapy aimed at interrupting the neurohormonal feedback system can decrease two year mortality by 50% and the risk of hospitalization by 64% in patients with heart failure with reduced ejection fraction13. It is on this background of guideline-directed medical therapies that new classes of heart failure therapies were evaluated.
Ivabradine
Resting heart rate has long been shown to have prognostic significance. Follow-up from the Framingham Heart Study demonstrated in an adult population without previous myocardial infarction or heart failure that a higher resting heart rate was associated with increased risk of cardiovascular and all-cause mortality. Each standard deviation of increase in baseline heart rate was associated with a 17% increase in the risk of all-cause mortality over a median follow-up of 19 years, even when adjusted for co-morbidities and activity level14. Multiple studies have demonstrated heart rate to be a predictive and modifiable marker in patients with heart failure in sinus rhythm. Although there has been some question as to whether there is a mechanistic relationship between elevated heart rate and worse cardiac function15 or whether heart rate is secondary in importance to dose of beta-blockers16, most analyses suggest that heart rate reduction is associated with lower risk.
Given the relationship between heart rate and mortality in heart failure it may be expected that beta-blockers with higher chronotropic suppression would have a larger benefit in heart failure patients. The was essentially only one large, randomized, and double-blind study, the Carvedilol or Metoprolol European Trial (COMET)17, in which there were similar long-term heart rate reductions. Outside of head-to-head trials, there are limitations in comparing differences in heart rate reduction and mortality reduction relative to placebo among different trials of various beta-blockers under varying conditions. However, it appears that the beta-blocker trials that showed the largest heart rate reduction, even with similar agents, had the largest impact on mortality. In a meta-analysis of 23 beta-blocker trials, there were greater mortality reductions as a function of the magnitude of heart rate reduction achieved within the trial18. For every heart rate reduction of 5 bpm with beta-blocker treatment, a commensurate 18% reduction (confidence interval [CI], 6% to 29%) in the risk for death occurred. If heart rate reduction is independent of the other protective effects of beta-blockers, then non-beta-blocker medications that lower the heart rate may be of benefit.
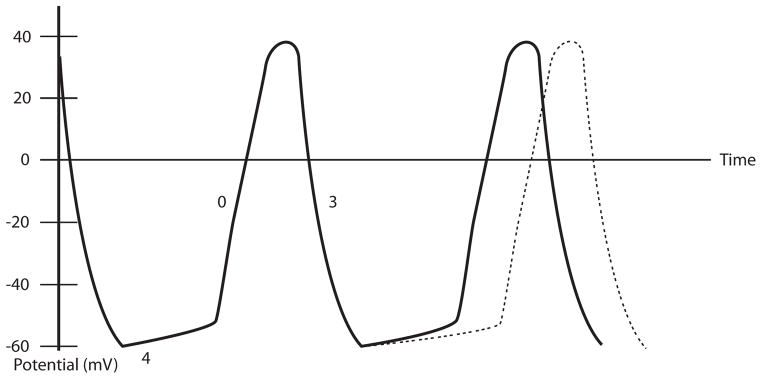
While the intrinsic control of heart rate is a complex balance of various cellular processes, the “funny” (If) current is felt to play a significant role in the continuous, rhythmic depolarization of the heart and in modulation of the heart rate. The time between firing events of the sinoatrial node (SAN), and thus the heart rate, is determined by the slope of phase 4 in the SAN action potential, which is determined by the If19. Antagonism of beta 1-receptors in the SAN reduces levels of intracellular cyclic adenosine monophosphate (cAMP), which in turn slows the If and decreases firing of the SAN, and this is the mechanism by which beta-blockers reduce heart rate. However, beta-receptors are found throughout the heart and antagonism provides other beneficial effects, including preventing catecholamine-driven myocyte death and reducing ventricular arrhythmias20. Ivabradine is a drug that blocks the hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels that are responsible for the If, resulting in a shallower phase 4 slope of the SAN action potential21 (Figure 1). In this manner, ivabradine slows heart rate without the other cardiovascular effects, both positive and negative, of beta-blockers.
Figure 1.
Diagram of SAN Action Potential and Effect of Ivabradine
Legend: Phase 0 – depolarization, phase 3 – repolarization, phase 4 – spontaneous depolarization, solid line – normal SAN action potential, dashed line – SAN action potential under the effect of ivabradine. Adapted from DiFrancesco D21.
The Morbidity-mortality Evaluation of the If Inhibitor Ivabradine in Patients with Coronary Disease and Left Ventricular Dysfunction (BEAUTIFUL) trial was a multinational, randomized, placebo-controlled trial involving 10,917 patients with proven coronary artery disease, an ejection fraction of 40% or lower, and a resting heart rate greater than 60 bpm22. Ivabradine, either 5mg or 7.5mg twice daily, was titrated to a target of 50 to 60 bpm without symptoms related to bradycardia. At entry, 84% of patients were on beta-blocker therapy, 83% had New York Heart Association (NYHA) class II or III heart failure symptoms, and the average ejection fraction was 32.4%. At the end of the trial, with a median follow-up of 19 months and a mean difference in heart rate of 6.4 bpm at one year, there was no difference in all-cause mortality (10.4% v. 10.1%), hospital admission for heart failure (7.8% v. 7.9%), or admission to the hospital for myocardial infarction or unstable angina (5.8% v. 5.5%). Serious adverse events were similar in both groups, but there was a higher rate of study drug discontinuation with ivabradine (28% v. 16%), owing mainly to bradycardia. Per the reported analysis, there was no significant interaction in the outcome based on the use of beta-blockers at study entry, although the types and dosages were not specified.
Based on prior evidence suggesting that heart rates greater than 70–75bpm were associated with worse outcomes, the authors had a pre-specified subgroup in which the entry resting heart rates were greater than 70bpm. In final analysis, there was a statistically significant treatment effect for this subgroup, and ivabradine was associated with decrease in hospital admissions for unstable angina or myocardial infarction (3.1% v. 4.9%) and coronary revascularizations (2.8% v. 4.0%). Heart failure admissions, all-cause mortality, and cardiovascular mortality were still not significantly different.
Based on these findings, another trial of ivabradine focusing solely on patients with heart failure with reduced ejection fraction was conducted, the Systolic Heart Failure Treatment with the If Inhibitor Ivabradine Trial (SHIFT). Trial designs were somewhat similar, although in SHIFT, patients needed to have NYHA class II-IV heart failure with an ejection fraction of 35% of lower and a resting heart rate greater than 70 bpm to be enrolled8. While the target heart rate range was the same, investigators allowed doses of ivabradine as low as 2.5mg twice daily in addition to 5mg or 7.5mg twice daily. During the trial, there was a better separation in heart rates, with a mean difference of 9.1 bpm at one year. After a median follow-up of 22.9 months, ivabradine was associated with an 18% relative reduction in the primary end-point of cardiovascular death or hospital admission for heart failure (24% v. 29%), although this was driven primarily by the reduction in hospital admissions (16% v. 21%, p<0.0001). All-cause and cardiovascular mortality were not significantly different between ivabradine and placebo, however heart failure mortality was reduced with ivabradine. In terms of adverse events, the ivabradine arm had higher rates of symptomatic (5% v. 1%) bradycardia, rings or spots of lights in vision named phosphenes (3% v. 1%), and atrial fibrillation (9% v. 8%). Only symptomatic and asymptomatic bradycardias were more likely to lead to study drug discontinuation with ivabradine.
At study entry, 89% of patients in SHIFT reported being on beta-blocker therapy. However, 14% of these patients were on beta-blockers that are not part of the American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the management of chronic systolic heart failure8,23. Of those on therapy, only 56% of patients were on at least half the recommended dose with just over half of those patients at target recommended dose. A comprehensive secondary analysis was performed examining the reasons for below-target therapies and the effects of varying beta-blocker doses and baseline heart rates on outcomes24. A history of COPD or asthma, lower blood pressure, increasing age, and concurrent treatment with amiodarone or digoxin were all associated with a lack of beta-blocker therapy or sub-target dosing. While there was a trend towards a decreased effect of ivabradine on the primary outcome with increased beta-blocker doses, after controlling for the impact of baseline heart rate, there was no significant difference—beta-blocker therapy did not significantly alter the effect of ivabradine in isolation. Analysis also showed a significant interaction of the treatment effect of ivabradine with baseline heart rate, with a greater treatment effect of ivabradine when the baseline heart rate was higher and the subsequent heart rate reduction was greater25. The increased magnitude of heart rate reduction with a higher starting heart rate is consistent with the use-dependent molecular model of ivabradine.21
Pre-specified subgroup analysis showed a near significant (p=0.059) interaction between the treatment group and etiology of cardiomyopathy, suggesting a more pronounced benefit of ivabradine in patients with non-ischemic cardiomyopathies as compared to those with an ischemic etiology. This result is interesting in the context of other trials of ivabradine. The previously described BEAUTIFUL trial was negative in its primary end-point, and although these patients had higher ejection fractions and lower heart rates than in the SHIFT trial, they all had an ischemic cardiomyopathy. A large, randomized trial, the Study Assessing the Morbidity Mortality Benefits of the If Inhibitor Ivabradine in Patients with Coronary Artery Disease (SIGNIFY) trial, found no benefit of ivabradine in addition to guideline-directed medical therapy, including beta-blockers, for stable coronary artery disease in patients without heart failure. Pre-specified sub-group analysis of the SIGNIIFY trial revealed that patients with limiting angina, compared to non-limiting angina, actually had higher rates of the primary endpoint, death from cardiovascular causes or nonfatal myocardial infarction, when on ivabradine compared to placebo40. While not conclusive, these results suggest that further study may potentially be warranted to delineate a patient population that more clearly benefits from ivabradine.
In the SHIFT trial, Ivabradine therapy was also associated with improved quality of life (QOL) and echocardiographic indices. In a substudy of just under 10% of the total study population, patients treated with ivabradine had greater reductions in left ventricular end systolic volume index (−7 mL/m2 v. −0.9 mL/m2) and left ventricular end diastolic volume index (−7.9 mL/m2 v. −1.8 mL/m2), and a greater increase in ejection fraction (+2.4% v. −0.1%)26. This reversal of ventricular remodeling was seen across all subgroups including varying beta-blocker dose, etiology of heart failure, and baseline ejection fraction. Patients on ivabradine also experienced greater increases in their Kansas City Cardiomyopathy Questionnaires (KCCQ), a previously validated tool to assess the various limitations of heart failure. Patients taking ivabradine had a larger increase (6.7 v. 4.3) in their overall summary score (OSS), in which a higher number represents less limitation in the physical, social, and psychological areas27.
On a background of guideline-directed medical therapy for chronic heart failure with reduced ejection fraction including ACE inhibitors, beta-blockers, and aldosterone antagonists, ivabradine may provide an additional reduction in hospitalizations in a carefully selected population in sinus rhythm and resting heart failure of 70 bpm8,22 with the most benefit likely in those with persistently elevated heart rates (>77 bpm).22,24,25 While ivabradine did reduce hospitalizations, it did not reduce all-cause or cardiovascular mortality, a proven benefit of beta-blockers and other guideline-based therapies and it remains critical to achieve target doses of these medications in patients prior to consideration of additional therapies.
Neprilysin Inhibition
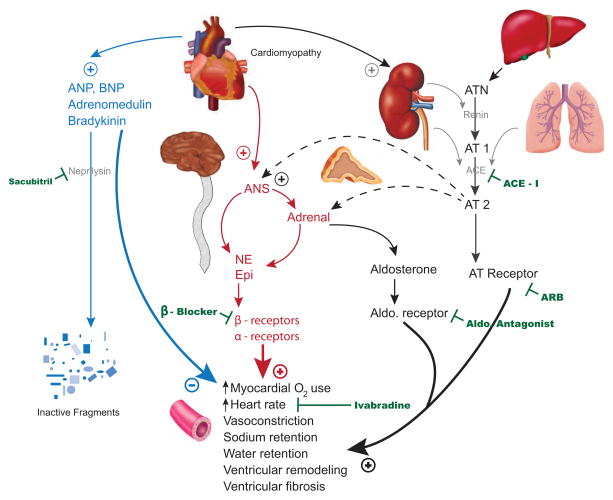
In addition to beta-blocker therapy, interruption of the RAA system activation via ACE inhibitors or angiotensin receptor antagonists and aldosterone antagonists has been a cornerstone in therapy for heart failure with reduced ejection fraction10. However, while maladaptive RAA system activation leads to increased levels of angiotensin II, ADH, and aldosterone that are harmful, heart failure is associated with a rise in counter regulatory hormones and molecules as well (Figure 2). Adrenomedullin is a peptide that has been noted to be elevated in patients with heart failure and which can cause significant vasodilation and increased glomerular filtration28. Natriuretic peptides, including atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), are produced in response to volume overload, cardiac dysfunction, and atrial stretch, and exert effects including vasodilation, diuresis, natriuresis, and prevention of cardiac hypertrophy29. Similar beneficial effects are seen from bradykinin, a product of the kallikrein-kinins system (KKS) that becomes up regulated in heart failure in parallel to the RAA system activation30, although it is also associated with angioedema in high levels.
Figure 2.
Neurohormonal Pathways in Heart Failure and Targets for Medical Therapy
Legend: ANP = atrial natriuretic peptide, BNP = brain natriuretic peptide, ANS = autonomic nervous system, NE = norepinephrine, Epi = epinephrine, ARB = angiotensin receptor blocker, ACE = angiotensin converting enzyme, ACE-I = ACE inhibitor, ATN = angiotensinogen, AT = angiotensin. Graphic credit: Margaux Reynolds.
Attempts have been made to potentiate the levels of these counter regulatory molecules through inhibition of the neutral endopeptidases (NEP), which include neprilysin, that are responsible for the degradation of the counter regulatory molecules into inactive components. The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) trial was a comparison of omapatrilat, a combined NEP and ACE inhibitor, against enalapril, an ACE inhibitor, in 5,770 patients with NYHA class II-IV heart failure and an ejection fraction less than or equal to 30%31. After a mean duration of 14.5 months, omapatrilat was found to be non-inferior, but not superior to enalapril in the primary endpoint of hospitalization for heart failure treated with intravenous diuretics. A modified primary endpoint—to try and more closely model that used in the SOLVD trial6—did suggest omapatrilat may be superior in reducing admission for all heart failure hospitalizations, including those treated only with oral medications, with an 11% reduction in such admissions. Omapatrilat was more potent at lowering blood pressure at peak effect, but was less effective at trough levels, causing the enalapril group to have lower recorded blood pressures at each check while those taking omapatrilat more often reported hypotension and dizziness. In the OVERTURE trial, there was a slight increase in the number of cases of angioedema (0.8% v. 0.5%), but in the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial, in which omapatrilat was compared with enalapril for hypertension management, the difference in angioedema was more concerning (2.17% for omapatrilat versus 0.68% for enalapril) including several life-threatening events32. The increase in angioedema is felt to be due to greater elevations in bradykinin levels, as bradykinin is degraded both by ACE and NEP30 and this concern was the barrier to approval of omapatrilat for use in clinical practice.
With the goal of maximizing the potential benefits of these counter regulatory hormones and minimizing the deleterious effects seen with combined NEP and ACE inhibition, LCZ696 (sacubitril/valsartan), a dual angiotensin receptor-neprilysin inhibitor (ARNi) was developed33. The Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial studied the effects of sacubitril/valsartan versus enalapril in patients with NYHA class II-IV heart failure, an ejection fraction less than 35–40%, and a heightened risk of heart failure events as evidenced by history of recent hospitalization or elevated biomarkers9. Although sacubitril/valsartan has an angiotensin receptor blocker (ARB) moiety, the comparison was made to enalapril given the more consistent data of the benefits of ACE inhibitors in heart failure as compared to ARBs34. Patients were randomized to receive either a target dose of 200mg of LCZ696 twice daily, which has an ARB equivalency to 320mg daily of valsartan, or enalapril 10mg twice daily, which were based on the target doses of the Valsartan Heart Failure Trial35 and SOLVD trial6.
The trial employed a run-in period in which patients received, in a single-blind fashion, enalapril for two weeks and then sacubitril/valsartan for four to six weeks while being assessed for adverse effects and compliance issues. Of all patients that began the run-in, 12% withdrew for an adverse event, with a higher rate in the enalapril arm, when adjusted for the differing length of run-in periods. While this run-in period, an often utilized technique for maximizing the efficiency of a trial, is unlikely to alter the validity of the outcomes from the trial, it does somewhat reduce the generalizability of the safety and tolerability findings36.
During the study, 8,399 patients were randomized and included in the analysis. After completion of enrollment and a median follow-up of 27 months, the study was halted early based on the finding of overwhelming benefit during the third interim analysis. The primary end-point, death from cardiovascular cause or first hospitalization for worsening heart failure, was reduced by 20% in the sacubitril/valsartan group, with similar reductions seen in the individual components of the composite end-point. The secondary end-points were also favorable for sacubitril/valsartan, with a 16% reduction in overall mortality and a slower decline in QOL as assessed by the KCCQ. Sacubitril/valsartan was also superior to enalapril in terms of various clinical outcomes. Patients in the sacubitril/valsartan arm required new medications, intravenous therapy, or intensified diuretic doses less often than the enalapril arm (12.4% v. 14.3%) and had a 34% reduction in emergency room visits that did not lead to hospitalization. For patients that were hospitalized, those receiving, sacubitril/valsartan needed inotropes less often (3.9% v. 5.4%) and there was a 13% reduction in the number of patients needing intensive care37. The benefits of sacubitril/valsartan were observed across all pre-specified clinically relevant subgroups with similar benefits across age, sex, race-ethnicity, comorbidities, severity of heart failure, and background therapies included in the trial.
The PARADIGM-HF trial also demonstrated that sacubitril/valsartan had significant benefits for reducing heart failure progression. In comparison with enalapril-treated patients, fewer sacubitril/valsartan-treated patients required intensification of medical treatment for heart failure (hazard ratio [HR], 0.84; 95% CI, 0.74–0.94; p=0.003) or an emergency department visit for worsening heart failure (HR, 0.66; 95% CI, 0.52–0.85; p=0.001). The patients in the sacubitril/valsartan arm also experienced 23% fewer hospitalizations for worsening heart failure and were less likely to need implantation of a heart failure device or cardiac transplantation. The benefits with sacubitril/valsartan compared to ACE inhibitor therapy were observed early, with the reduction in heart failure hospitalization evident within the first 30 days after randomization. Based on actuarial estimates from the PARADIGM-HF trial and assuming consistent benefits with long-term use, it has been estimated that treatment with sacubitril/valsartan would result in benefits of 1 to 2 years of increased life expectancy and survival free from heart failure.
Analysis of serum biomarkers of heart failure revealed that NT-proBNP and troponin T levels were lower at 4 weeks and 8 months of therapy with sacubitril/valsartan compared to enalapril, whereas BNP was higher, which is expected given that NEP inhibition inherently increases BNP37. Therefore, BNP, while usually portending worse heart failure outcomes at higher levels38, cannot be used as such in patients on sacubitril/valsartan and may actually have reverse implications. However, the PARADIGM-HF investigators have not yet published any outcomes stratified by changes in BNP from baseline to see whether such a change could predict the subsequent clinical outcomes. While a rise in BNP would be an interesting way to demonstrate that the drug is exerting its effect, longitudinal changes may be difficult to interpret as improvements in heart failure and reduced ventricular stress may reduce proBNP, and therefore BNP, production. Therefore an eventual fall in BNP values could represent decreased production as opposed to loss of neprilysin inhibition.
Sacubitril/valsartan was generally well tolerated with fewer increases in serum creatinine > 2.5 mg/dl (3.3% v. 4.5%) or serum potassium > 6.0 mmol/liter (4.3% v. 5.6%) and fewer episodes of cough (11.3% v. 14.3%). Sacubitril/valsartan was more often associated with symptomatic hypotension (14.0% v. 9.2%) although there were fewer study medication discontinuations (17.8% v. 19.8%) in the sacubitril/valsartan arm. Given the previous concerns of angioedema seen in the OVERTURE and OCTAVE trials, PARADIGM-HF included it as blinded, adjudicated outcome. While there were more episodes of angioedema with sacubitril/valsartan compared with enalapril (19 v. 10), it was not statistically significant and there were no episodes of airway compromise in either group. There are theoretical reasons that neprilysin inhibition could interrupt a pathway involved with the breakdown of beta-amyloid. However, there were no increases in neurocognitive or ocular adverse effects detected in PARADIGM-HF.
There is the potential for dual angiotensin and NEP inhibition in patients with heart failure with preserved ejection fraction. The Prospective Comparison of ARNI with ARB on Management of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) trial was a phase II, double-blind study that examined the effect of sacubitril/valsartan in these patients. Three hundred and one patients with NYHA class II-IV heart failure, an ejection fraction 45% or higher and an elevated NT-proBNP were randomized to a goal of LCZ696 200mg twice daily or valsartan 160mg twice daily. After 12 weeks of therapy, the primary endpoint of NT-proBNP level showed a 23% greater reduction with sacubitril/valsartan as opposed to valsartan, although by 36 weeks, while the difference still favored sacubitril/valsartan it was no longer statistically significant39. Subgroup analysis suggested a greater benefit with sacubitril/valsartan in patients with diabetes or systolic blood pressures greater than 140mmHg. While this phase II trial used an intermediary end-point of NT-proBNP, a large, phase III trial with clinical endpoints is currently planned, and if positive would represent the first direct pharmacologic therapy for heart failure with preserved ejection fraction.
Dual angiotensin and NEP blockade with sacubitril/valsartan appears to well tolerated and provides significant benefit in terms of mortality, hospitalizations, and multiple symptomatic and quality of life indicators in comparison to ACE inhibitor alone9,37. On the basis of this compelling evidence, sacubitril/valsartan could replace ACE inhibitors or angiotensin receptor blocker as the cornerstone of therapy for heart failure with reduced ejection fraction.
Conclusions
In the past three decades, extensive research has led to the neurohormonal model of heart failure and four classes of medications, beta-blockers3,4, angiotensin receptor blockers35, ACE inhibitors5,6, and aldosterone antagonists7, that have been the core of guideline-directed medical therapy for heart failure with reduced ejection fraction and have dramatically lowered the morbidity and mortality associated with this disease. This has led to a decrease in hospitalizations and improvement in survival after diagnosis despite a relatively constant incidence of heart failure with reduce ejection fraction1. Ivabradine and sacubitril/valsartan represent the first new-in-class medications in the efforts to further improve the outcomes for patients with heart failure with reduced ejection fraction. Sacubitril/valsartan showed a clear benefit over the current standard of care of an ACE inhibitor and thus may replace the ACE inhibitor or angiotensin receptor blockers in eligible patients with heart failure with reduced ejection fraction. In select patients, ivabradine can further decrease hospitalizations for heart failure in patients in sinus rhythm with a resting heart rate greater than 70bpm on maximally tolerated beta-blockers. These new drugs for heart failure with reduced ejection fraction represent important therapeutic advances. Nevertheless, additional therapies to further improve outcomes in heart failure with reduced ejection fraction are needed. In addition, there is a critical need to identify medical therapies that can improve outcomes in patients with heart failure with preserved ejection fraction.
Table 1.
Prospective Randomized Clinical Trials of New Heart Failure Medications
| Generic Name | Sacubitril/Valsartan | Ivabradine |
|---|---|---|
| Brand Name (US) | Entresto | Corlanor |
| FDA-approved Indications | To reduce the risk of cardiovascular death or heart failure hospitalization. | To reduce the risk of hospitalization for worsening heart failure in patients with LVEF ≤ 35% and HR ≥ 70bpm (sinus) on maximally tolerated dose of beta-blockers |
| Dosing |
Starting: 49/51mg BID or 24/26mg BID if any of the following: moderate hepatic impairment (Child-Pugh B), severe renal impairment (eGFR < 30), or currently on low-dose or no ACE inhibitor or ARB Goal: Double the dose every 2–4 weeks to target dose of 97/103mg BID |
Starting: 5mg BID or 2.5mg BID if either: conduction defects or concerns of potential hemodynamic compromise from bradycardia Goal: After 2 weeks, if HR > 60bpm, increase by 2.5mg BID to a maximum of 7.5mg BID, if HR 50–60bpm, maintain current dose, and if HR < 50bpm, reduce by 2.5mg BID |
| Contraindications | Pregnancy, history of angioedema with ACE/ARB, concomitant use of ACE (must stop 36 hours before sacubitril/valsartan start), concomitant use of aliskiren in patients with diabetes | Decompensated heart failure, severe hepatic impairment, resting HR < 60bpm, pacemaker dependent (i.e. near 100% pacing burden), sick sinus syndrome, SA block, or 3rd degree AV block without a pacemaker, BP < 90/50 mmHg, pregnancy |
| Adverse Effects | Hypotension, hyperkalemia, renal impairment, cough, | Bradycardia, hypertension, atrial fibrillation, and luminous phenomena (phosphenes) |
Table 2.
FDA-approved Doses and Indications for New Heart Failure Medications. Adapted from the FDA-approved label information.
| Trial Name | Year | Therapies | Study Population | Study Size | Follow-Up | Outcomes | Reference |
|---|---|---|---|---|---|---|---|
| PARADIGM-HF | 2014 | LCZ696 200mg BID v. enalapril 10 mg BID | EF ≤ 40%, NYHA II–IV, recent hospitalization or elevated BNP or NT-proBNP, with no drop out during run-in period | 8,442 | 27 months (median) | HR 0.80 (0.73–0.87) for cardiac death or heart failure hospitalization [similar in individual components], HR 0.84 (0.76–0.93) for all cause mortality | #8 |
| BEAUTIFUL | 2008 | Ivabradine 7.5mg BID v. placebo BID | Age > 55, known coronary disease, EF ≤ 40%, sinus rhythm, HR > 60bpm | 10,917 | 19 months (median) | HR 1.0 (0.91 – 1.1) for cardiac death, heart failure or myocardial infarction (MI) admission [similar in individual components], HR 0.78 (0.62–0.97) for admission for MI or unstable angina admission if baseline HR> 70bpm | #22 |
| SHIFT | 2010 | Ivabradine 7.5mg BID v. placebo BID | Sinus rhythm, HR > 70bpm, EF≤ 35%, hospitalized for heart failure in last year | 6,558 | 22.9 months (median) | HR 0.89 (0.82–0.96) for all cause hospital admission, HR 0.74 (0.58–0.94) for heart failure death, HR 0.90 (0.80–1.02) for all cause mortality | #9 |
| OVERTURE | 2002 | Omapatrilat 40mg daily v. enalapril 10 mg BID | NYHA II–IV or EF ≤ 30%, hospitalized for heart failure in last year | 5,770 | 14.5 months (mean) | HR 0.94 (0.86–1.03) for all cause mortality or heart failure hospitalization requiring IV therapy, HR 0.89 (0.82 to 0.98) for any heart failure admission | #29 |
Acknowledgments
Funding: J. Gordin is supported by the NIH Cardiovascular Scientist Training Program (T32 HL007895).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. AHA statistical update. Heart disease and stroke statistics—2013 update. A report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, et al. AHA policy statement. Forecasting the future of cardiovascular disease in the United States. A policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in-Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–7. [PubMed] [Google Scholar]
- 4.Packer M, Fowler MB, Roecker EB, Coats AJS, Katus HA, Krum H, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survical (COPERNICUS) study. Circulation. 2002;106:2194–9. doi: 10.1161/01.cir.0000035653.72855.bf. [DOI] [PubMed] [Google Scholar]
- 5.The Consensus Trial Study Group. Effects of Enalapril on Mortality in Severe Congestive Heart Failure. N Engl J Med. 1987;316:1429–35. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 6.The SOLVD Investigators. Effect of Enalapril on Survival in Patients with Reduced Left Ventricular Ejection Fractions and Congestive Heart Failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effects of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 8.Swedberg K, Komajda M, Bohm M, Borer JS, Ford I, Dubost-Brama A, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–85. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 9.McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-Neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 10.Ma TKW, Kam KKH, Yan BP, Lam Y. Renin-angiotensin-aldosterone system blockade for cardiovascular disease: current status. Br J Pharmacol. 2010;160:1273–92. doi: 10.1111/j.1476-5381.2010.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lymperopoulous A, Rengo G, Koch WJ. Adrenergic nervous sytem in heart failure: pathophysiology and therapy. Circ Res. 2013;113:739–753. doi: 10.1161/CIRCRESAHA.113.300308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med. 1991;325:303–10. doi: 10.1056/NEJM199108013250502. [DOI] [PubMed] [Google Scholar]
- 13.Banka G, Heidenreich PA, Fonarow GC. Incremental cost-effectiveness of guideline-directed medical therapies for heart failure. J Am Coll Cardiol. 2013;61:1440–6. doi: 10.1016/j.jacc.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Ho JF, Larson MG, Ghorbani A, Cheng S, Coglianese EE, Vasan RS, et al. Long-term cardiovascular risks associated with an elevated heart rate: the Framingham heart study. J Am Heart Assoc. 2014;3:e000668. doi: 10.1161/JAHA.113.000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fragasso G, De Cobelli F, Spoladore R, Esposito, Salerno A, Calori G, et al. Resting cardiac energy metabolism is inversely associated with heart rate in healthy young men. Am Heart J. 2011;162:136–41. doi: 10.1016/j.ahj.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Fiuzat M, Wojdyla D, PIna I, Adams K, Whellan D, O’Connor CM. Heart rate or beta-blocker dose? Association with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACITON trial. J Am Coll Cardiol HF. 2015 doi: 10.1016/j.jchf.2015.09.002. epub. [DOI] [PubMed] [Google Scholar]
- 17.Poole-Wilson PA, Swedberg K, Cleland JGF, Di Lenarda A, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 18.McAlister FA, Wiebe N, Ezekowtiz JA, Leung AA, et al. Meta-analysis: β-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med. 2009;150:784–94. doi: 10.7326/0003-4819-150-11-200906020-00006. [DOI] [PubMed] [Google Scholar]
- 19.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure: physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 20.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–46. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 21.DiFrancesco D. Cardiac pacemaker I(f) current and its inhibition by heart rate-reducing agents. Curr Med Res Opin. 2005;21:1115–22. doi: 10.1185/030079905x50543. [DOI] [PubMed] [Google Scholar]
- 22.Fox K, Ford I, Steg PG, Tendera M, Ferrari R, et al. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:807–16. doi: 10.1016/S0140-6736(08)61170-8. [DOI] [PubMed] [Google Scholar]
- 23.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of hear failure: A report of the American College of Cardiology Foundation/American Heart Association tast force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 24.Swedberg K, Komajda M, Bohm M, Borer J, Robertson M, Tavazzi L, et al. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose? J Am Coll Cardiol. 2012;59:1938–45. doi: 10.1016/j.jacc.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, et al. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376:886–94. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 26.Tardif J, O’Meara E, Komajda M, Bohm M, Borer JS, Ford I, et al. Effects of selective heart rate reduction with ivabradine on left ventricular remodeling and function: results from the SHIFT echocardiography substudy. Eur Heart J. 2011;32:2507–15. doi: 10.1093/eurheartj/ehr311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekman I, Chassany O, Komajda M, Bohm M, Borer JS, Ford I, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32:2395–2404. doi: 10.1093/eurheartj/ehr343. [DOI] [PubMed] [Google Scholar]
- 28.Rademaker MT, Charles CJ, Espiner EA, Nicholls G, Richards AM. Long-term adrenomedullin administration in experimental heart failure. Hypertension. 2002;40:667–72. doi: 10.1161/01.hyp.0000037132.90640.26. [DOI] [PubMed] [Google Scholar]
- 29.Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther. 2004;102:223–41. doi: 10.1016/j.pharmthera.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Cheng C, Onishi K, Ohte N, Suzuki M, Little W. Function effects of endogenous bradykinin in congestive heart failure. J Am Coll Cardiol. 31:1679–86. [PubMed] [Google Scholar]
- 31.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Roleau J, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the ompapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE) Circulation. 2002;106:920–6. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 32.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the omapatrilat cardiovascular treatment vs. enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–11. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Noe A, Chandra P, Al-Fayoumi S, Lingueros-Saylan M, Sarangapani R, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 34.Heran BS, Musini VM, Bassett K, Taylor RS, Weight JM. Angiotensin receptor blockers for heart failure (review) Cochrane Database of Systematic Reviews. 2012;(4):Art No.: CD003040. doi: 10.1002/14651858.CD003040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohn JN, Tognoni G for the Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 36.Pablos-Mendez A, Barr G, Shea A. Run-in periods in randomized trials: implications for the application of results in clinical practice. JAMA. 1998;279:222–5. doi: 10.1001/jama.279.3.222. [DOI] [PubMed] [Google Scholar]
- 37.Packer M, McMurray JJ, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2005;131:54–61. doi: 10.1161/CIRCULATIONAHA.114.013748. [DOI] [PubMed] [Google Scholar]
- 38.Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, et al. Predischarge b-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43:635–41. doi: 10.1016/j.jacc.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 39.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomized controlled trial. Lancet. 2012;380:1387–95. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 40.Fox K, Ford I, Steg PG, Tardif J, et al. Ivabradine in stable coronary artery disease without clinical heart failure. N Engl J Med. 2014;371:1091–9. doi: 10.1056/NEJMoa1406430. [DOI] [PubMed] [Google Scholar]