Abstract
Background
Accurate pathologic nodal staging mandates effective collaboration between surgeons and pathologists. The American College of Surgeons Oncology Group Z0030 trial (ACOSOG Z0030) tightly controlled surgical lymphadenectomy practice, but not pathology examination practice. We tested the survival impact of the thoroughness of pathologic examination (using the number of examined lymph nodes as a surrogate).
Methods
We re-analyzed the mediastinal lymph node dissection arm of ACOSOG Z0030, using logistic regression and Cox-proportional hazards models.
Results
Of 513 patients, 435 were pN0, 60 pN1, and 17 pN2. The mean number of mediastinal lymph nodes examined was 13.5, 13.1, and 17.1; station 10 lymph nodes were 2.4, 2.7, and 2.6; station 11–14 nodes were 4.6, 6.1, and 6.7; total lymph nodes were 19.7, 21.3, 25.4 respectively. The pN-category and histology were associated with increased number of examined intrapulmonary lymph nodes. Patients with pN1 had more non-hilar N1 nodes than patients with pN0, those with N2 had more N2 nodes examined than those with pN0 or pN1. Patients with pN0 had better survival with examination of more N1 nodes; those with pN1, had better survival with increased mediastinal nodal examination; the likelihood of discovering N2 disease was significantly associated with increased examination of mediastinal and non-hilar N1 lymph nodes.
Conclusions
Despite rigorously standardized surgical hilar/mediastinal lymphadenectomy, the number of lymph nodes examined was associated with the likelihood of detecting nodal metastasis, and survival. This may indicate an effect of incomplete pathologic examination.
Keywords: lung cancer, lymph nodes, metastasis, quality improvement, survival
Variation in the thoroughness and accuracy of pathologic nodal staging has been suggested as a cause of within-stage variation in survival of patients after curative-intent resection of non-small cell lung cancer [1,2,3]. Thorough pathologic nodal staging requires effective collaboration between the surgery and pathology teams. Failure of processes in one area may impair the ability to provide an accurate assessment of the pathologic nodal stage and post-operative prognosis. Retrieval of hilar and mediastinal lymph nodes (stations 2 – 10) entirely depends on the surgical team, whereas retrieval of intrapulmonary lymph nodes (stations 11–14) is usually performed during gross dissection of the lung resection specimen in the pathology laboratory. Irrespective of surgical practice, the thorough microscopic examination of surgically provided lymph node specimens entirely depends on the pathology team [4].
Efforts to improve the quality of pathologic staging of lung cancer mostly focus on improving the quality of the surgical lymph node harvest. American College of Surgeons Oncology Group (ACOSOG) Z0030 was a randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy in patients with clinical N0 or N1 (less than hilar), non-small cell lung cancer [5]. ACOSOG is now part of the Alliance for Clinical Trials in Oncology. In this trial, which revealed no difference in survival between the two arms, all patients underwent a thorough systematic sampling procedure, including surgical harvest of hilar (station 10) lymph nodes, and were only eligible for randomization after all nodes were found to be negative. Patients in arm 1 had no further lymph node dissection, while those in arm 2 went on to a standardized, carefully defined, and rigorously applied mediastinal nodal dissection to minimize variation in the quality of surgical nodal staging [5,6]. However, the study did not control for pathology practice.
Despite evidence that pathology practice contributes to variation in staging quality, there has been relatively little direct attention paid to the influence of pathology processes on patient survival [7,8]. Studies of discrepancies between surgeon and pathology claims of the thoroughness of nodal examination have usually adopted the pathology report as the best evidence of actual events [9]. Because surgical nodal retrieval processes were carefully controlled, but pathology processes (gross retrieval of intrapulmonary nodes and complete microscopic examination of directly provided lymph node specimens) were not controlled in this trial, ACOSOG Z0030 provides a natural experiment to test hypotheses on the potential impact of pathology processes on patient outcomes.
Our aims were to describe the distribution of N1 lymph node examination in the Z0030 cohort; identify factors associated with low and high N1 nodal count; examine the association between the number of lymph nodes examined and survival in patients with completely resected pN0 and pN1; and finally, to determine the association between the number of lymph nodes examined and discovery of unexpected N2 disease.
Patients and Methods
Study population
With a waiver of the informed consent requirement for this retrospective analysis of pre-existing, anonymized clinical trial data, we re-analyzed the mediastinal lymph node dissection (arm 2) cohort of ACOSOG Z0030. Details of the study population and the outcomes of the primary clinical trial have been published [5,6,10]. We limited analysis to arm 2 because of missing critical details in the systematic sampling arm of the trial. We also excluded 11 (2.1%) patients who had received an incomplete resection to avoid confounding the survival analysis.
Hypotheses
We hypothesized that despite thorough surgical hilar and mediastinal nodal retrieval, variation in the thoroughness of pathologic nodal examination (indicated by the number of lymph nodes examined) would have a survival impact. To test this central hypothesis, we made the following sub-hypotheses: the number of non-hilar N1 lymph nodes examined will cluster around the low end of the spectrum (proving that sub-optimal pathology practice was prevalent); because the number of N1 nodes examined will be directly proportional to the likelihood of correctly identifying patients with pN0 disease, there will be a sequential improvement in survival of patients with pN0 as more N1 lymph nodes are examined; pN0 patients with more non-hilar N1 (stations 11 – 14) lymph nodes examined will have a more favorable prognosis; pN1 patients will have more N1 lymph nodes examined than pN0 patients (supporting the notion that more thorough examination yields more discovery of nodal metastasis); survival will improve as the number of lymph nodes examined increases.
Statistical analysis plan
We summarized the distribution of lymph nodes examined from the mediastinum (stations 1–9), hilum (station 10), within the lung resection specimen (stations 11 – 14) as mean, median, and ranges of number of nodes. Treating the nodal count as a continuous variable, we used linear regression to examine factors associated with nodal count. We also dichotomized select nodal counts as “high” (≥median) versus “not high” (<median) for association analyses. We used Cox proportional hazards models to determine the association between the number of lymph nodes examined and survival of patients with pN0 and pN1 disease. Survival included overall survival (OS) and recurrence-free survival (RFS), and the analysis was performed without and with adjustment for age, sex, performance status, and T-category. Finally, we used logistic regression to examine the association between the number of lymph nodes examined and the discovery of unexpected N2 nodal metastasis. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. All analyses were based on the study database frozen on September 1, 2009.
Results
Patient population
Of the 513 eligible patients, the median follow-up in those not known to be deceased was 6.8 years. Forty-three percent had adenocarcinoma, and 27% had squamous histology. Ninety seven percent of patients had pT1 or T2 tumors, 85% had no nodal metastasis, 12% had pN1 and 3% had unexpected pN2 disease (Table 1).
Table 1.
Patient population (N = 513)*
| N | % | |
|---|---|---|
|
| ||
| Age in years, Mean (range) | 66.7 (9.1) 37–87 |
|
|
| ||
| Male | 267 | 52 |
|
| ||
| Performance status | ||
| 0 | 340 | 66 |
| 1 | 160 | 31 |
| 2 | 13 | 3 |
|
| ||
| Histology | ||
| Squamous | 139 | 27 |
| Adenocarcinoma | 219 | 43 |
| Large cell | 22 | 4 |
| Bronchoalveolar | 32 | 6 |
| Other | 98 | 19 |
|
| ||
| T-category | ||
| 1 | 238 | 47 |
| 2 | 254 | 50 |
| 3 | 9 | 2 |
| 4 | 10 | 2 |
|
| ||
| N-category | ||
| 0 | 435 | 85 |
| 1 | 60 | 12 |
| 2 | 17 | 3 |
|
| ||
| Extent of resection | ||
| R0 | 511 | 100 |
Not all numbers add to 513 due to missing data for certain characteristics.
Lymph node counts
In the whole cohort, the median number of lymph nodes examined from all stations was 19 (interquartile range [IQR] 13–24), including 12 (IQR 8–17) from mediastinal stations, 2 (IQR 1–3) from station 10, and 4 (IQR 2–7) non-hilar N1 nodes (Appendix figures 1a–d). Nodal distributions for patients with pN0, pN1, and pN2 were significantly different (Table 2).
Table 2.
Distribution of number of lymph nodes examined in patients with pN0, pN1, and pN2 non-small cell lung cancer.*
| Stations | Mean | Median | IQR | Range | P |
|---|---|---|---|---|---|
|
| |||||
| Mediastinal (2–9)† | |||||
| All patients, n=513 | 13.6 | 12 | 8–17 | 1–64 | 0.19 |
| pN0, n=435 | 13.5 | 11 | 8–17 | 1–64 | Ref |
| pN1, n=60 | 13.1 | 12 | 7–15 | 2–42 | 0.68 |
| pN2, n=17 | 17.1 | 17 | 11–23 | 6–30 | 0.079 |
|
| |||||
| Hilar (10)† | |||||
| All patients, n=489 | 2.4 | 2 | 1–3 | 1–14 | 0.6 |
| pN0, n=415 | 2.4 | 2 | 1–3 | 1–14 | Ref |
| pN1, n=57 | 2.7 | 2 | 1–3 | 1–14 | 0.33 |
| pN2, n=16 | 2.6 | 1 | 1–3 | 1–9 | 0.75 |
|
| |||||
| Hilar/mediastinal (2–10)† | |||||
| All patients, n=513 | 15.9 | 14 | 10–20 | 1–71 | 0.24 |
| pN0, n=435 | 15.8 | 14 | 10–20 | 1–71 | Ref |
| pN1, n=60 | 15.6 | 14 | 9.5–19 | 2–44 | 0.88 |
| pN2, n=17 | 19.5 | 18 | 12–25 | 7–39 | 0.094 |
|
| |||||
| Non-hilar N1 (11–14)‡ | |||||
| All, n=445 | 4.8 | 4 | 2–7 | 1–30 | 0.007 |
| pN0, n=373 | 4.6 | 4 | 2–6 | 1–28 | Ref |
| pN1, n=56 | 6.1 | 4 | 3–7 | 1–30 | 0.01 |
| pN2, n=15 | 6.7 | 5 | 3–9 | 1–19 | 0.046 |
|
| |||||
| N1 (10–14) | |||||
| All, n=507 | 6.6 | 5 | 3–9 | 1–37 | 0.002 |
| pN0, n=430 | 6.3 | 5 | 3–8 | 1–37 | Ref |
| pN1, n=59 | 8.3 | 7 | 4–11 | 1–34 | 0.001 |
| pN2, n=17 | 8.3 | 8 | 4–10 | 1–25 | 0.078 |
|
| |||||
| All stations (2–14) | |||||
| All, n=513 | 20.1 | 19 | 13–24 | 1–83 | 0.066 |
| pN0, n=435 | 19.7 | 18 | 13–24 | 1–83 | Ref |
| pN1, n=60 | 21.3 | 19 | 14–25 | 2–64 | 0.3 |
| pN2, n=17 | 25.4 | 23 | 17–28 | 10–53 | 0.031 |
Not all numbers add to 513 due to missing data for certain characteristics;
dependent on surgical collection;
dependent on pathology processes.
Factors associated with differences in the number of lymph nodes examined
Of all clinical and demographic factors, histology and pathologic nodal stage were the only factors associated with the number of intrapulmonary lymph nodes examined (Appendix Table 1). Patients with squamous cell lung cancer had significantly more non-hilar N1, and total N1 lymph nodes examined, but not more hilar or mediastinal lymph nodes, than those with other types of non-small-cell lung cancer. The number of lymph nodes examined was associated with the pathologic nodal stage. Patients with pN0 had significantly fewer non-hilar N1 lymph nodes examined than those with pN1 or pN2 (Table 2). Patients with pN2 had a strong trend toward having more mediastinal lymph nodes examined. Ultimately, patients with pN1 had on average 1.5 more lymph nodes examined, and those with pN2 had 5.7 more lymph nodes than those with pN0. There was no significant difference in the hilar lymph node count between stage groups.
Comparative analysis of node number and survival
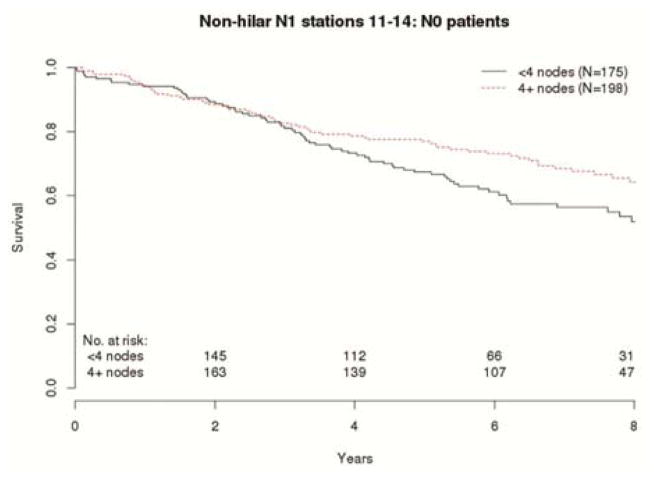
OS and RFS of patients within the same pN-category was consistently associated with the number of lymph nodes examined (Table 3). The risk of death in patients with pN0 decreased with increasing number of non-hilar N1 lymph nodes examined (hazard ratio [HR] 0.96; 95% confidence interval [CI] 0.91 – 1.01; p=0.098]. This relationship is best illustrated in the OS comparison of pN0 patients with non-hilar lymph node counts above and below the median (Fig 1). With adjustment for age, sex, performance status and T-category, the HR was 0.95; CI 0.9 – 1.01; p=0.092. There was no such trend with the number of N2 lymph nodes examined in patients with pN0 (Table 3).
Table 3.
Stage-stratified association between the number of lymph nodes examined and survival.
| OS (Adjusted) | RFS (Adjusted) | |||
|---|---|---|---|---|
|
| ||||
| HR [95% CI] | P | HR [95% CI] | ||
|
| ||||
| pN0, n=435; 162 deaths, 188 recurrences | ||||
|
| ||||
| Stations | ||||
| Mediastinal, 2 – 9 | 1.01 [0.99 – 1.03] | 0.5 | 1 [0.98 – 1.02] | 0.95 |
| 1 [0.99 – 1.03] | 0.57 | 1 [0.98 – 1.02] | 0.99 | |
| Hilar, 10 | 1.03 [0.96 – 1.11] | 0.45 | 1.04 [0.97 – 1.11] | 0.28 |
| 1.07 [0.99 – 1.15] | 0.095 | 1.06 [0.99 – 1.14] | 0.11 | |
| Non-hilar N1, 11 – 14 | 0.96 [0.91–1.01] | 0.098 | 0.97 [0.92 – 1.01] | 0.17 |
| 0.95 [0.90 – 1.01] | 0.092 | 0.97 [0.92 – 1.01] | 0.15 | |
| Non-hilar N1, ≥4 nodes* | 0.71 [0.51–1.0] | 0.053 | 0.8 [0.58–1.09] | 0.15 |
| 0.68 [0.48–0.96] | 0.029 | 0.78 [0.57–1.07] | 0.13 | |
| N1, 10 – 14 | 0.97 [0.93 – 1.01] | 0.099 | 0.98 [0.94 – 1.01] | 0.22 |
| 0.97 [0.94 – 1.02] | 0.022 | 0.98 [0.95 – 1.02] | 0.36 | |
| All, 2 – 14 | 0.99 [0.98 – 1.01] | 0.83 | 0.99 [0.98 – 1.01] | 0.61 |
| 1 [0.98 – 1.02] | 0.89 | 1 [0.98 – 1.01] | 0.66 | |
|
| ||||
| pN1, n= 60;39 deaths, 45 recurrences | ||||
|
| ||||
| Stations | ||||
| Mediastinal, 2 – 9 | 0.95 [0.90 – 0.99] | 0.035 | 0.94 [0.90 – 0.99] | 0.015 |
| 0.95 [0.9 – 0.99] | 0.044 | 0.94 [0.90 – 0.99] | 0.015 | |
| Hilar, 10 | 0.98 [0.87 – 1.11] | 0.77 | 0.94 [0.82 – 1.07] | 0.34 |
| 0.98 [0.85 – 1.13] | 0.76 | 0.94 [0.81 – 1.09] | 0.39 | |
| Non-hilar N1, 11 – 14 | 0.98 [0.93 – 1.04] | 0.5 | 0.97 [0.91 – 1.03] | 0.33 |
| 0.99 [0.93 – 1.05] | 0.63 | 0.96 [0.9 – 1.03] | 0.28 | |
| N1, 10 – 14 | 0.97 [0.92 – 1.03] | 0.33 | 0.95 [0.89 – 1.01] | 0.13 |
| 0.98 [0.92 – 1.04] | 0.43 | 0.94 [0.88 – 1.01] | 0.1 | |
| All, 2 –14 | 0.97 [0.94 – 1.0] | 0.055 | 0.96 [0.93 – 0.99] | 0.015 |
| 0.97 [0.94 – 1.0] | 0.074 | 0.95 [0.92 – 0.99] | 0.012 | |
Primary analysis for number of lymph nodes is unadjusted; secondary analysis adjusts for age, sex, performance status and T-stage.
vs. non-hilar N1 with <4 nodes (below median).
Figure 1.
Overall survival of pathologic node-negative patients with above or below median non-hilar lymph node counts.
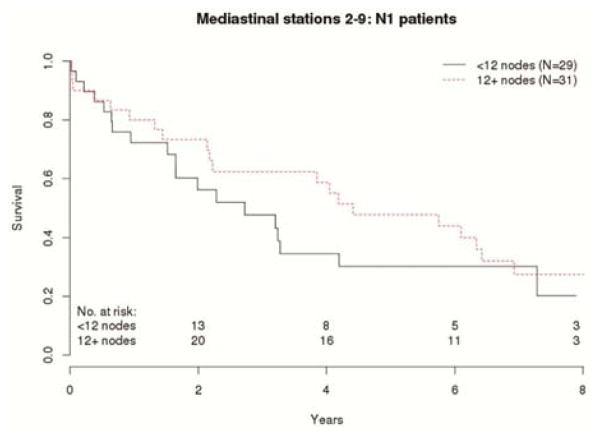
In the 60 patients with pN1 disease, there was a strong trend associating the number of mediastinal and total number of lymph nodes examined with OS. Patients with pN1 who had more mediastinal lymph nodes examined had a lower risk of death than those with fewer mediastinal lymph nodes (HR 0.95, CI 0.90–0.99, p=0.035 unadjusted; HR 0.95, CI 0.90–0.99, p= 0.044 adjusted). The risk of death or recurrence (RFS) was even more strongly associated with the number of mediastinal lymph nodes examined (HR 0.94, CI 0.90–0.99, p= 0.015 unadjusted; HR 0.94, CI 0.90–0.99, p= 0.015 adjusted). This survival difference is clearly illustrated when patients with mediastinal lymph node counts above or below the median are compared (Figure 2). The total number of examined lymph nodes was also associated with survival (Table 3).
Figure 2.
Overall survival of pN1 patients with above or below median mediastinal lymph node counts.
Lymph node number and discovery of unexpected N2 disease
The discovery of N2 disease was associated with the number of mediastinal lymph nodes examined (odds ratio [OR] 1.04, CI 0.99 – 1.09, p= 0.079 (Table 4). There was a trend toward association between the number of non-hilar N1 nodes examined and discovery of N2 lymph nodes (OR 1.09, CI 0.99 – 1.19, p=0.077. Ultimately, there was a significant association between the total lymph node count and discovery of N2 disease (OR 1.04, CI 1.0 – 1.07, p= 0.041).
Table 4.
Number of lymph nodes examined and discovery of unexpected N2 disease.
| Discovery of N2 disease
|
|||
|---|---|---|---|
| OR | 95% CI | P | |
| Mediastinal stations (2–9) | 1.04 | 0.99–1.09 | 0.079 |
| Hilar (10) | 1.03 | 0.84–1.27 | 0.79 |
| Non-hilar N1 (11–14) | 1.09 | 0.99–1.19 | 0.077 |
| N1 (10–14) | 1.06 | 0.98–1.15 | 0.13 |
| All stations (2–14) | 1.04 | 1.0–1.07 | 0.041 |
17 N2 subjects
Comment
ACOSOG Z0030 revealed similar survival in clinical T1 or T2, N0 (or non-hilar N1) non-small-cell lung cancer patients irrespective of the extent of lymphadenectomy beyond a standardized systematic sampling procedure [6]. Patients were randomized after frozen-section documentation of the absence of hilar and mediastinal nodal metastasis on systematic sampling; the surgical staging procedures were carefully defined and rigorously implemented. Consequently, the mediastinal lymph node yield was superb [10]. Because the trial did not implement a similarly rigorous pathology examination process, it provided an opportunity to test hypotheses about pathology processes.
Studies in community practices have demonstrated a problem in the thoroughness of examination of lung resection specimens, especially with regard to gross retrieval of intrapulmonary lymph nodes [8,11]. The generalizability of these findings is questionable. In the community-based Memphis Metropolitan Area Quality of Surgical Resection cohort, a median of 4 N1 lymph nodes were retrieved from routine pathology examination; redissection of discarded lung resection specimens yielded a median of 6 additional lymph nodes, providing a median of 11 N1 lymph nodes in total [8]. In the Z0030 mediastinal lymph node dissection arm, a median of 5 N1 lymph nodes were examined [10], including those directly retrieved by surgeons during the standardized lymphadenectomy [5,6,10]. The similarity between the community-based cohort and the Z0030 population suggests a similar rate of inadvertent loss of intrapulmonary lymph nodes in both cohorts, raising the possibility that sub-optimal pathology practice of nodal retrieval and examination was prevalent in Z0030, involving many of the best institutions in North America. By comparison, only a median of 5 mediastinal lymph nodes were retrieved in the community cohort [8], compared to 12 in the ACOSOG Z0030 cohort [10].
Patients with pN0 had fewer non-hilar lymph nodes examined than those with pN1 (median 4 [interquartile range 2–6] v 4 [3–7]), whereas there was no difference in the (surgically collected) hilar lymph node count (2 [1–3] v 2 [1–3]) (Table 2). Even more striking was the difference in the mediastinal lymph node counts. Patients with pN0, pN1, and pN2 disease had a median of 11, 12, and 17 mediastinal lymph nodes, respectively. Overall, patients with pN0, pN1 and pN2 had a median of 18, 19, and 23 total lymph nodes examined, respectively. This pattern is remarkably similar to previous reports in a community-based series, in which the number of lymph nodes examined was strongly associated with the final pathologic stage [12].
Even with careful surgical nodal harvest, there was an association between the number of lymph nodes examined and survival. For patients with pN0, there was a consistent trend toward a survival advantage from examination of more intrapulmonary lymph nodes, but not from examination of hilar or mediastinal nodes. This association was stronger with adjustment for T-category, suggesting a link with the innate risk of nodal metastasis, such risk being higher in more advanced tumors [13,14]. In patients with pN1, the association between mediastinal nodal count and prognosis was also linked with T-category. It is well established that patients with pN1 disease have a higher risk for N2 disease [15]. It seems plausible that the more thoroughly mediastinal lymph nodes are examined, the lower the risk of missing N2 disease. This is supported by the data, revealing an association between increased examination of lymph nodes and discovery of unexpected N2 disease (Table 4).
Our findings are consistent with previous reports suggesting that the number of lymph nodes examined (or with metastasis) has independent prognostic value, which might augment the current TNM staging system [16–19]. However, others have disputed this, suggesting that the number of lymph nodes present in a lung resection specimen is bell-shaped in well-staged populations [20]. It is possible that the survival association of nodal counts is an epiphenomenon. For example, it is possible that a more vigorous immune response to cancer causes lymph nodes to be more prominent and therefore easier to identify for examination. It is impossible to answer such a question from retrospective analyses.
The consistency of these relationships in the Z0030 cohort with extremely tightly-controlled surgical processes, but less well-controlled pathology processes, raises legitimate questions about the contribution of pathology processes to the lymph node number-outcome relationship. Despite all its well-recognized limitations, especially fragmentation of lymph nodes during surgical delivery, the number of lymph nodes examined does reflect the thoroughness of staging [16–19,21]. In this analysis, that would mean the thoroughness of pathologic examination of lymph nodes in resection specimens. The station of N1 nodal metastasis is independently prognostic. Patients with stations 13 and 14 involvement have a worse prognosis than those with pN0, but better prognosis than patients with stations 11 and 12 metastasis, whose prognosis is in turn better than patients with station 10 involvement [22,23]. Because the protocol mandated intraoperative dissection of station 10, it seems plausible that variation in the thoroughness of pathology practice mostly impacted discovery of station 11–14 nodal metastasis.
This unplanned retrospective analysis is hypothesis-generating, we acknowledge several other weaknesses. We limited analysis to the mediastinal lymph node dissection arm of Z0030 because we lacked critical details on lymph node examination in the systematic sampling arm. This had the practical effect of reducing the statistical power of our analyses, and probably prevented several trends from reaching statistical significance. The dataset did not provide information on the number of lymph nodes with metastasis. Therefore, we were unable to test whether patients with more N1 lymph node involvement (or a higher positive N1 node ratio) had worse survival than those with fewer N1 lymph nodes with metastasis (or a lower positive N1 node ratio). We were also unable to test the association between the number of positive N1 nodes (or higher positive N1 nodal ratio) and the likelihood of unexpected pN2 disease.
Our assumption that the number of lymph nodes examined is a surrogate for the thoroughness of pathologic nodal staging may be incorrect. Inter-individual variation in nodal counts exists. Besides, we have no direct evidence of variation in the gross specimen dissection, pathologic nodal extraction and examination processes in this dataset. However, comparison to the community-based Memphis Metropolitan Area Quality of Surgical Resection cohort, in which the evidence of missed nodal metastasis and its impact on the accuracy of pathologic nodal staging is clearly established, suggests the need to further explore this hypothesis in rigorous prospective studies.
Standard recommendations advocate submission of ‘every lymph node for pathology examination’ [4]. Gross dissection manuals recommend searching for lymph nodes in the peri-hilar soft tissue and lung parenchyma immediately surrounding the airways [24, 25]. A precise, step-wise, anatomically sound novel dissection method improves the intrapulmonary lymph node yield [26]. Such novel dissection methods need rigorous prospective evaluation. Studies of the survival impact of pathologic nodal staging should standardize pathology examination practices, in addition to surgical practice.
Supplementary Material
Acknowledgments
This work was supported by NCI U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology) and R01CA172253 (Osarogiagbon).
Footnotes
Set MMC box: The Appendices can be viewed in the online version of this article [INSERT article doi] on http://www.annalsthoracicsurgery.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Detterbeck F, Puchalski J, Rubinowitz A, Cheng D. Classification of the thoroughness of mediastinal staging of lung cancer. CHEST. 2010;137:436–42. doi: 10.1378/chest.09-1378. [DOI] [PubMed] [Google Scholar]
- 2.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the Surveillance, Epidemiology, and End Results database. J Thorac Oncol. 2012;7:1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 3.Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg. 2014;97:385–93. doi: 10.1016/j.athoracsur.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ADASP recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Modern Pathology. 2001;14:629–32. doi: 10.1038/modpathol.3880362. [DOI] [PubMed] [Google Scholar]
- 5.Allen MS, Darling GE, Pechet TTV, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg. 2006;81:1013–20. doi: 10.1016/j.athoracsur.2005.06.066. [DOI] [PubMed] [Google Scholar]
- 6.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 trial. J Thorac Cardiovasc Surg. 2011;141:662–70. doi: 10.1016/j.jtcvs.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gephardt GN, Baker PB. Lung carcinoma surgical pathology report adequacy. Arch Pathol Lab Med. 1996;120:922–7. [PubMed] [Google Scholar]
- 8.Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol. 2012;30:2823–8. doi: 10.1200/JCO.2011.39.2589. [DOI] [PubMed] [Google Scholar]
- 9.Osarogiagbon RU, Allen JW, Farooq A, Wu JT. Objective review of mediastinal lymph node examination in a lung cancer resection cohort. J Thorac Oncol. 2012;7:390–6. doi: 10.1097/JTO.0b013e31823e5e2d. [DOI] [PubMed] [Google Scholar]
- 10.Darling GE, Allen MS, Decker PA, et al. Number of lymph nodes harvested from a mediastinal lymphadenectomy. CHEST. 2011;139:1124–9. doi: 10.1378/chest.10-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osarogiagbon RU, Ramirez RA, Wang CG, et al. Size and histologic characteristics of lymph node material retrieved from tissue discarded after routine pathologic examination of lung cancer resection specimens. Ann Diagn Pathol. 2014;18:136–9. doi: 10.1016/j.anndiagpath.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osarogiagbon RU, Allen JW, Farooq A, Berry A, O’Brien T. Pathologic lymph node staging practice and stage-predicted survival after resection of lung cancer. Ann Thorac Surg. 2011;91:1486–93. doi: 10.1016/j.athoracsur.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Chen K, Yang F, Jiang G, Li J, Wang J. Development and validation of a clinical prediction model for N2 lymph node metastasis in non-small cell lung cancer. Ann Thorac Surg. 2013;96:1761–8. doi: 10.1016/j.athoracsur.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 14.Farjah F, Lou F, Sima C, Rusch VW, Rizk NP. A prediction model for pathologic N2 disease in lung cancer patients with a negative mediastinum by positron emission tomography. J Thorac Oncol. 2013;8:1170–80. doi: 10.1097/JTO.0b013e3182992421. [DOI] [PubMed] [Google Scholar]
- 15.Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85:211–5. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Wei S, Asamura H, Kawachi R, Sakurai H, Watanabe S. Which is the better prognostic factor for resected non-small cell lung cancer: the number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol. 2011;6:310–8. doi: 10.1097/JTO.0b013e3181ff9b45. [DOI] [PubMed] [Google Scholar]
- 17.Fukui T, Mori S, Yokoi K, Mitsudomi T. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1:120–5. [PubMed] [Google Scholar]
- 18.Ludwig MS, Goodman M, Miller DL, Johnstone PAS. Postoperative survival and the number of lymph nodes sample during resection of non-negative non-small cell lung cancer. CHEST. 2005;128:1545–50. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 19.Ou SI, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol. 2008;3:880–6. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 20.Riquet M, Legras A, Mordant P, et al. Number of mediastinal lymph nodes in non-small cell lung cancer: a Gaussian curve, not a prognostic factor. Ann Thorac Surg. 2014;98:224–31. doi: 10.1016/j.athoracsur.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Lin CJ, Hsu W, Huang B, Huang M, Wang L. Long-term results of pathological stage I non-small cell lung cancer: validation of using the number of totally removed lymph nodes as a staging control. Eur J Cardiothorac Surg. 2003;24:994–1001. doi: 10.1016/s1010-7940(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 22.Maeshima AM, Tsuta K, Asamura H, Tsuda H. Prognostic implication of metastasis limited to segmental (level 13) and/or subsegmental (level 14) lymph ndoes in patients with surgically resected nonsmall cell lung carcinoma and pathologic N1 lymph node status. Cancer. 2012;118:4512–8. doi: 10.1002/cncr.27424. [DOI] [PubMed] [Google Scholar]
- 23.Rena O, Boldorini R, Papalia E, et al. Metastasis to subsegmental and segmental lymph nodes in patients resected for non-small cell lung cancer: prognostic impact. Ann Thorac Surg. 2014;97:987–92. doi: 10.1016/j.athoracsur.2013.11.051. [DOI] [PubMed] [Google Scholar]
- 24.Lester SC. Manual of Surgical Pathology. 2. Elsevier Churchill; Livingstone: 2006. pp. 497–516. [Google Scholar]
- 25.Westra WH, Hruban RH, Phelps TH, et al. Surgical Pathology Dissection. 2. Springer; 2002. [Google Scholar]
- 26.Osarogiagbon RU, Eke R, Sareen S, et al. The impact of a novel gross dissection protocol on intrapulmonary lymph node retrieval from lung cancer resection specimens. Ann Diagn Pathol. 2014;18:220–6. doi: 10.1016/j.anndiagpath.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.