Abstract
OBJECTIVE
The purpose of this article is to summarize advances in PET fluorescence resolution, agent design, and preclinical imaging that make a growing case for clinical PET fluorescence imaging.
CONCLUSION
Existing SPECT, PET, fluorescence, and MRI contrast imaging techniques are already deeply integrated into the management of cancer, from initial diagnosis to the observation and management of metastases. Combined positron-emitting fluorescent contrast agents can convey new or substantial benefits that improve on these proven clinical contrast agents.
Keywords: cancer, contrast medium, fluorescence, multimodality imaging, PET
We are limited in what we can do with our current imaging modalities [1, 2]. Fortunately, the limitations of our available imaging modalities are unique, allowing us to compensate by combining existing modalities into multimodality imaging probes. Not all multimodality combinations are useful. Instrumentation and chemical complications preclude the practicality of many combinations. However, certain imaging combinations stand out because they have highly synergistic properties: one such combination is the PET fluorescence imaging (PET/Fl) probe. Fluorescence contrast imaging nicely complements PET in terms of spatial resolution at the histologic and superficial levels [3]. Unlike PET probes, which rapidly decay, fluorescence probes are stable. PET, however, is superior to fluorescence imaging because of its usefulness in noninvasive quantitative resolution of structures through deep tissue. Both modalities can be used at nontoxic tracer quantities. These synergistic relationships are illustrated in Figure 1, which shows the complementary advantages of PET/Fl.
Fig. 1.
Compatibility between PET and fluorescence imaging in PET fluorescence images of 18F, cyanine 7–labeled macromolecule for sentinel node mapping. (Adapted with permission from [52]).
A, Noninvasive PET (red)/CT (blue) deep-tissue image in mouse shows injection site into foot-pad (i) and sentinel node (ii). PET is less invasive than fluorescence imaging, in which skin must be removed to clearly image sentinel nodes.
B, Cyanine-7 fluorescence image shows different mouse from that in A. Skin has been removed and submicron lymph track resolution (iii) is observable. This mouse has rare early lymph track bifurcation visualized with fluorescence imaging but not with PET. In this case, both popliteal and lumbar nodes are sentinel.
C and D, H and E (stained) (C) and fluorescence (D) histologic images show that sentinel lymph node in A does not homogeneously serve site of injection and that fluorescence can guide pathologist in generating section that likely serves injection site (iv). Without fluorescence guidance, pathologist may section area that does not serve injection site (v), resulting in false-negative diagnosis of micrometastatic disease. Both PET and fluorescence imaging can guide surgeon to node resection.
Improving Case for Imaging With PET Fluorescence Contrast Enhancement
PET/Fl combinations have the qualities of good clinical contrast agents, including lack of toxicity and the ability to image evidence of disease at high spatial and long temporal resolutions [1, 2]. Improvements in technology continue to push the boundaries of fluorescence imaging and PET. These advances strengthen the case for PET/Fl, which has the following advantages and limitations.
Spatial and Depth Resolution
In imaging, high spatial resolution is preferred, and progress at the in vitro, histologic, in vivo preclinical, and in vivo clinical levels contributes to the high resolutions of PET/Fl probes. Fluorescence probes are unparalleled in resolution at the in vitro level. Single-molecule fluorescence resolution can be achieved in live unfixed cells [4]. This is useful for probing the inner workings of free cancer cells but does not reliably translate into histologic imaging. In histologic analysis, fluorescence is easily resolved at the single-cell level, allowing imaging of advanced phenomena, such as intratumoral heterogeneity [5–7]. In vivo, single cells can be resolved with fluorescence [8], making imaging within an open surgical site [3, 9] and use with superficial cancers such as melanomas [10] practical. Unfortunately, in deep-tissue preclinical and clinical in vivo imaging, fluorescence imaging is less useful than ionizing and contrast-enhanced MRI because overlying tissues absorb and scatter exciting and emitted light, resulting in nonquantitative, distorted deep-tissue images [1, 11]. Fluorescent photon scattering and nonspecific absorption are especially pronounced through hair and bone. However, superficial fluorescence imaging is sufficient for qualitative preclinical analyses in mice and rats, in which investigators must use more expensive hairless mouse models to minimize these phenomena.
PET probes can be imaged with autoradiography at high spatial resolution in histologic samples [12], but the procedural requirements of these analyses in relation to fluorescence and diaminobenzidine-peroxidase histologic analyses prohibit routine clinical use, especially with short-lived isotopes. For in vivo analysis, PET is useful for visualizing superficial and deep-tissue cancers and metastases; however, the ability to image submicron structures, such as single cells, lymph vessels, and neuronal axons, has not been proven. In preclinical PET scanners, the choice and mean energy of an emitted positron (Table 1) can greatly affect the resolution of lesions [13]. Positron emitters with lesser average kinetic energies produce images of higher resolution than do emitters with greater positron energies [13, 14]. This difference in resolution is less noticeable with current clinical PET scanners. This may change with new instrumentation, such as small-area lutetium oxyorthosilicate arrays and positron-specific solid-state photo-multipliers that can rapidly alter the current resolution limits of PET [13].
TABLE 1.
Physical Properties of Suitable PET Isotopes for Incorporation Into PET Fluorescence Probes [80, 81]
| PET Isotope | Half-Life | β+ Purity of Decay (%) | Mean β+ Energy (keV) |
|---|---|---|---|
|
| |||
| 68Ga | 68 min | 89 | 783 |
| 18F | 108 min | 97 | 250 |
| 64Cu | 12.7 h | 18 | 278 |
| 89Zr | 78.4 h | 23 | 389 |
| 124I | 100.3 h | 23 | 840 |
Temporal Resolution
Fluorescence probes are superior to PET probes in terms of temporal stability. A fluorophore within a histologic sample can be indefinitely stable if properly frozen or fixed, allowing sample analysis and reanalysis at later dates. In microscopic histologic analyses in which fluorophores are subject to high-intensity illumination, fluorescence probes can be destroyed by overimaging (photobleaching), but this can be minimized (see Fluorophore Considerations section) [15]. Fluorophores are metabolized in vivo, as are other injectates. Fluorophores are sensitive to chemical degradation in local oxidative and acid-base environments. PET probes are less useful in histologic analysis. Although high-resolution autoradiography has been performed on histologic samples [12], positron emission autoradiography must be performed immediately because isotope decay continues to occur in frozen tissue, making reanalysis of a section less accurate or impossible when performed at a later date. For in vivo imaging, the half-life of an isotope limits the time over which a PET emitter can be imaged (Table 1). In preclinical studies, any PET emitter with a half-life longer than the event being imaged can be used for imaging. In clinical settings, to limit patient exposure to ionizing radiation, isotopes are generally chosen with half-lives that match the instance of maximal absolute signal-to-noise ratios at the site of interest [16].
Toxicity, Sensitivity, and Dose
The most attractive aspect of a small-molecule, PET/Fl combination is lack of expected chemical toxicity (assuming that the ligand being imaged is not deliberately toxic). One can image a PET/Fl agent at trace and nanomolar-to-subpicomolar quantities, concentrations that are unlikely to trigger patient biologic reactions [1, 17, 18]. At these concentrations, the modification of a fluorophore with a PET agent would conceivably transform a fluorophore into a PET drug, a class of agents with special U.S. Food and Drug Administration (FDA) status. The FDA recognizes PET drugs as useful in doses that are safe and effective. For this reason, these agents can be used without FDA investigational new drug application submission for basic science use in humans [19] (these studies are still subject to approval by a Radioactive Drug Research Committee). At higher concentrations, fluorophores are also nontoxic. Numerous FDA-approved fluorescence agents already exist, including indocyanine green, fluorescein, photofrin, and methylene blue [3, 20]. For this reason, fluorescence agents can be considered over contrast CT and contrast MRI multimodality agents, which, in rare instances, have been reported to cause neurotoxicity and nephrogenic systemic fibrosis [21, 22]. Immediate life-threatening contraindications do not exist in PET.
PET and nuclear medicine techniques do not cause great radiation exposure. Exposures are equal to or less than those of standard CT. PET emitters on fluorescence probes require less activity than does SPECT [23–25] for two reasons. First, PET isotopes have neutron-deficient nuclei and generally have approximately equal or shorter half-lives than their SPECT counterparts with current clinical precedent, for example: 111In (67.3 hours) and 201Tl (73.1 hours). Second, PET also entails a practical form of quantum computation, making PET potentially more sensitive than SPECT and reducing required radiation doses as much as an order of magnitude on some preclinical equipment. For example, Siemens Healthcare Inveon SPECT requires higher activity to compensate for photons lost in collimation, the process of interrogating each individual emitted photon for linear momentum. PET registers all detectable photons then determines vectoral information after the fact by pairing photons that have related (≈180°) linear momentums and shared temporal data (time of annihilation).
Current Preclinical Application
Contrast agents are necessary in medicine because they help differentiate physiologic processes from anatomic ones. They also allow us to noninvasively identify or exclude volumes that without contrast enhancement would be missed or incorrectly and invasively explored as incidentalomas. For this reason, there is a demand for higher-resolution, higher-sensitivity imaging probes. Although PET/F1 is in its infancy and no PET/F1 agent has been approved by the FDA, there are numerous preclinical applications of PET/F1 [26–28]. We discuss a sampling of these applications that entails use of a range of PET isotopes and fluorophores as representative targets in imaging.
Direct Tumor Imaging: PET Fluorescence Biomarker Imaging
PET fluorescence antibodies
A popular way to mark cancerous tissue is to target cancer cells directly, through the imaging of specific biomarkers expressed on the extra-cellular membrane of cancer cells. Fluorescence-labeled monoclonal antibodies (mAbs) are used regularly in histology, and systemic applications of both mAbs and peptides have been used in PET and SPECT in vivo.
Copper-64 is traditionally the most popular isotope for systemic mAb imaging. Representative mAb applications [26, 29–31] are shown in Table 2. Two applications, a trastuzumab and an EpCAM application [30, 31], are notable for imaging metastases. The targeting of CD133 expressing U251/glioblastomas by use of separately labeled PET (64Cu–triazacyclononanetriacetic acid [NOTA]) and fluorescent (Alexa Fluor 680, Molecular Probes) AC133 mAbs allows imaging of orthotopic tumors implanted in the brain. Instead of PET/FI mAb, Gaedicke et al. [32] used a mixture of individually labeled PET and fluorescence mAbs (Fig. 2), which give slightly different subcutaneous tumor uptakes. This result shows that PET/Fl mAb probes differ in distribution from PET mAb and fluorescence mAb probe mixtures.
TABLE 2.
Applications of Monoclonal Antibodies and Biologic Properties of PET Fluorescence Probes
| Antibody | Antigen | Cell Line, Cancer | PET Emitter | Chelator | Fluorophore | Time (h) | Percentage Injected Dose per Gram |
Model | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| Optimal (Tumor) | Blood | |||||||||
|
| ||||||||||
| NuB2 | CD20 | Raji, lymphoma | 64Cu | DOTA | Alexa Fluor 750 (Molecular Probes) | 48 | 16.3 ± 2.7 | 10 | Subcutaneous flank xenograft | [29] |
| Trastuzumab | ErbB-2a | 4T1, breast | 64Cu | DOTA | IRDye 800 (LI-COR) | Metastatic | [30] | |||
| Anti-EpCAM | EpCAM | PC3a, prostate | 64Cu | DOTA | IRDye 800CW (LI-COR) | Lymph node metastasis | [31] | |||
| AC133 | CD133 | U251, glioblastoma | 64Cu | NOTA | Alexa Fluor 680 (Molecular Probes) | 48 | 56 ± 16 | 8.16 ± 2.35 | Subcutaneous flank xenograft | [32] |
| TRC105 | CD105 | 4T1, breast | 89Zr | DFO | IRDye 800CW (LI-COR) | 24 | 10.9 ± 0.5 | Lung metastasis | [34] | |
| Trastuzumab | ErbB-2a | 18F | Bodipy (Molecular Probes) | [39] | ||||||
Note—DOTA = tetraazacyclododecanetetraacetic acid, NOTA = triazacyclononanetriacetic acid, DFO = deferoxamine.
Also known as HER2/neu.
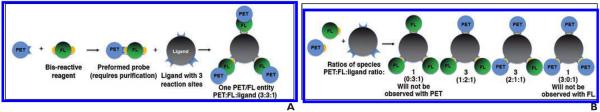
Fig. 2.
Schematic shows two options for generating PET fluorescence (FL) probes on ideal ligand with three possible reaction sites.
A, Preforming reactive PET/FI probe and then isolating it before reaction with trisubstitutable ligand gives single agent with maximum possible ratio of PET to FI to ligand (3:3:1).
B, Simple and more common labeling strategy is to use commercial reagents. This scheme gives complicated mixture of products with lesser ratios of PET or FI to ligand. Approval for clinical application of complicated mixture of these products will be more challenging to gain.
A protocol exists for generating PET/Fl (89Zr-DFO/CW800) labeled antibodies for 89Zr imaging [33]. This isotope is popular in mAb imaging because of its pure decay and longer half-life [29]. In a study of PET/Fl TRC105 mAb, Hong et al. [34] used 89Zr gamma emission to quantify organ fluorescence within tissue imaged ex vivo. In this process, a quantitative calibration curve for future imaging with fluorescence alone is generated in ex vivo tissue.
Many PET/Fl mAb studies have shown observable intratumoral heterogeneity in ex vivo fluorescence imaging. A key experiment that, to our knowledge, has not been performed is a comparison of these data (the pharmacokinetic mAb delivery profile identifying antigen that can be accessed by a systematically delivered antibody) with fluorescence histologic findings obtained through ex vivo fluorescence antibody staining (which identifies total expressed receptors within a solid tumor or total antigen content). This analysis would identify the degree to which an mAb penetrates and delivers itself homogeneously to all expressed receptors within a solid tumor. This information would not necessarily be accurately obtained with antibody fragments or reengineered antibodies [35–38], which have different pharmacokinetic distributions.
One disadvantage in long-lived 64Cu and 89Zr mAb imaging is that one must wait for isotope decay and clearance before a different antibody can be imaged with PET. A fluorescence isotope allows imaging of multiple antibody probes containing different fluorescence values on multiple antibodies simultaneously through fluorescence endoscopy guided by PET or fluorescence histologic analysis of resected tissue. These procedures are arguably invasive. Alternatively, one could use a short-lived PET isotope that would rapidly decay, allowing prompt imaging of another antibody. Of note, a singular 18F-PET fluorescence application of 18F and boron-dipyrromethene (Bodipy, Molecular Probes) on trastuzumab [39] was not explored in a comprehensive biodistribution analysis of this mAb on a tumor model expressing ErbB-2, also known as HER2/neu. The half-life of 18F precludes generation of an optimal signal-to-noise ratio in a tumor for observation with PET. However, with a PET/Fl probe, a PET emitter may decay but would leave behind a stable fluorophore that could be used to verify a lesser-quality PET image and would be useful in fluorescence-guided surgery. Data collected with 18F-labeled mAbs could also be used in innovative methods being used by physicians who consider the time domain in image processing [40].
PET fluorescence peptides
Peptides have more rapid pharmacokinetic clearance than mAbs do. Peptides clear within 24 hours. This must be considered in image-guided surgical procedures. For this reason, 18F and 68Ga isotopes more appropriately match the targeting half-lives of peptides, as in targeting of the SSTR class of somatostatin receptors [2], and PET/Fl probes for BBR gastrin receptors [41]. A recent 64Cu GLP-1R peptide receptor application is of particular interest for its utility in imaging of 64Cu-Cy5–labeled exendin-4 peptide at receptors in 916–1 insulinomas in 2–4 hours. The pre-formulation of a tetraazacyclododecanetetraacetic acid (DOTA)–fluorescence probe is noted for its compatibility with mAb PET/Fl and would result in a homogeneously labeled PET/Fl agent (Fig. 2A). For this reason, this 64Cu chelator is superior to the applications shown in Table 2 for PET/Fl.
Indirect Tumor Imaging
Angiogenesis, enhanced permeation, and retention
One can use PET/Fl probes to image indirect anatomic changes in noncancerous tissue that are a result of a tumor or metastasis. PET/Fl strategies for doing this exist and are especially attractive if they highlight a tumor specifically and nonlinearly. Many such nontargeted agents include nanoparticles, which are untargeted and accumulate in a tumor owing to enhanced permeation and retention or target new or irregular vasculature with 18F, 64Cu, and 124I isotopes that are linked to protoporphyrins, cypates, or Bodipy fluorophores [28]. Cyclic Arg-Gly-Asp (cRGD) is a popular target that is PET/Fl labeled as 18F-rhodamine [42]. This agent incorporates multiple cRGD integrin-specific ligands with increased affinity for integrin receptors.
Mapping
PET/Fl agents can be used to predict routes that a delivered agent or cancer cells travel. This information can be important in cancer staging, which dictates the severity of a patient's prognosis, whether a cancer is palliative or curative in nature, and the toxicity of the drugs a patient will receive in the foreseeable future. The presence of micrometastases in sentinel nodes is important in staging [43]. Current sentinel node contrast agents based on stand-alone SPECT [44–48] and fluorescence imaging [49] lack tomographic potential, fluorescent resolution, or lymphatic tissue specificity. Without tomography, nodes are often missed. Without proper targeting or suborganelle resolution, contrast agents can often flow past sentinel nodes, requiring a surgeon to resect more nodes than necessary. The result is lymphedema and unnecessary, time-consuming work for pathologists.
Containing tilmanocept, a new clinical agent for mapping, two agents stand out for their ability to highlight PET/Fl in near-immediate clinical settings. Through the modification of an FDA-approved precursor [46, 50], 68Ga [51], and 18F [52] PET/Fl probes can yield more accurate identification and resection of sentinel lymph nodes. The PET component of the modified lymph node agent allows 3D tomography of lymph nodes while the fluorescence component provides lymph node and track connectivity data. A surgeon can thus differentiate a sentinel node from more distal nodes in preclinical models. Lymph vessels are submillimeter in width and are too small to be visualized with even preclinical PET. Additionally, fluorescence signal persists in histologic analysis, allowing one to determine the exact fraction of a lymph node that serves the site of injection in fluorescence histologic sample preparations (Fig. 1).
Rapid and accurate sentinel node visualization is necessary because sentinel nodes are generally acquired during primary tumor biopsy or resection in which a single surgical site is used to obtain primary tumor and sentinel node samples. Both modalities can be used to verify successful surgery. Sentinel nodes cannot be acquired after primary tumor resection because surgical intervention changes agent clearance at the tumor site.
New PET Fluorescence Probe Design
In designing a PET/Fl agent, radiochemical, synthetic chemical, and parallel screening considerations must be properly considered. The following is a guide that can help simplify the design of new PET/Fl probes.
Isotope Choice
Traditionally, a PET isotope is chosen with a half-life that matches the process being imaged [53, 54], with the expectation of generating a high signal-to-background ratio at the site or event of interest. For example, systemic injection of an intact antibody is traditionally imaged with 64Cu or 89Zr to observe maximum tumor-to-background ratios at a tumor site. In preclinical small animal scanners, an isotope with a large positron mean energy can produce artifacts that can occlude signal from nearby tumors. For this reason, animals with flank tumors are preferred over orthotopic models because they can be xenografted in locations that are removed from the organs through which tracers accumulate in a disease-nonspecific manner.
Fluorine-18 is preferred because of current clinical use of 18F-FDG. FDG is the most used PET agent and comprises more than 95% of current clinical PET studies. However, 18F radiochemistry can be difficult to manipulate at the radiochemical level, and many forgo 18F for simpler chelation strategies, such as 64Cu or 68Ga capture [55–58].
The choice of PET isotope may be based on the versatility of a chelator. For example, DOTA can be interchangeably substituted with 64Cu and 68Ga for PET, 111In for SPECT, and 90Y for radiotherapy. This is ideal if one wants to synthesize a single precursor for both imaging and therapy.
Fluorophore Considerations
Fluorophores can generate infinitely more signal than a PET emitter, especially when fluorescence agents are imaged at longer time points. PET emitters indirectly emit two photons from an unstable nucleus before it is transformed into another element that cannot emit photons of the same quality. A fluorophore, on the other hand, draws its energy from an external energy source, and as long as there is an infinite supply of exciting light, a single fluorophore can fluoresce over and over again, generating infinite signal. In reality, fluorophores have limited photo stabilities and decompose after just a few excitations and emissions. This is likely to change because many researchers are intensely interested in more photostable fluorophores, and new modifications that stabilize a fluorophore to photo bleaching have been reported [59]. There are numerous fluorophores to choose from, and they are generally inexpensive to synthesize or can be purchased [15] (Table 3).
TABLE 3.
Common Commercially Available Fluorophores and Their Properties
| Fluorophore | Maximum Excitation (nm) | Maximum Emission (nm) | Extinction Coefficient (cm−1M−1) | Quantum Yield | Approximate Costper Milligram ($)a |
|---|---|---|---|---|---|
|
| |||||
| Pacific blue | 416 | 451 | 46,000 | 0.75 | 50 |
| Alexa Fluor 488b | 494 | 517 | 73,000 | 0.92 | 250 |
| FITC | 492 | 518 | 75,000 | 0.92 | 0.5 |
| Alexa Fluor 532b | 530 | 555 | 81,000 | 0.61 | 250 |
| Cy3 | 555 | 570 | 150,000 | 0.31 | 20–100 |
| Cy3.5 | 591 | 604 | 116,000 | 0.35 | 20–100 |
| Cy5 | 646 | 662 | 250,000 | 0.2 | 20–100 |
| Cy5.5 | 673 | 707 | 209,000 | 0.2 | 20–100 |
| Cy7 | 750 | 773 | 199,000 | 0.3 | 20–100 |
| Cy7.5 | 788 | 808 | 223,000 | 20–100 | |
Note—FITC = fluorescein isothiocyanate, Cy = cyanine.
Common suppliers include Thermo Fisher, Sigma-Aldrich, Lumiprobe, Click Chemistry Tools, Cyandye, and BioActs.
Molecular Probes.
Of commercial fluorophores, rhodamines, fluorescein, boron-dipyrromethene, and cyanine fluorophores are common and interchangeably used (Table 2). Radiation can bleach fluorophores, but this can be prevented through the removal of oxygen and radical species during high-activity radiosynthesis. The use of nitrogen atmospheres or radical scavengers, such as hydroquinone or phenylenediamine [15], can be added to increase fluorophore stability. Fluorophores with quantum yields that are close to unity and have large extinction coefficients are better fluorophores. However, these two quantities must often be juxtaposed on the choice of wavelength: long-wavelength fluorescent signal is less affected by scattering and non-useful absorption through tissue, allowing deeper imaging through tissue [1, 11]. Depending on the application, long-wavelength fluorescence may not be an issue in a PET/F1 probe, especially when PET compensates for lack of depth penetration in fluorescence. Not all agents are candidates for PET/F1. The modification of a small molecule with a PET trap and a fluorophore results in added molecular weight, charge, and phobicity characteristics of the PET/F1 probe on a small molecule. This may result in an agent with vastly different pharmacokinetic distribution than the intended product. This is a more minor issue when considering higher-molecular-weight agents such as mAbs and peptides.
Synthetic Chemical Considerations
Because of the nature of radioisotope handling, the short half-lives of PET tracers, and the stability of fluorophores, the ideal PET/F1 probe would incorporate a radiolabel in a final radiochemical step. Strategies that require incorporation of a PET isotope followed by multiple synthetic manipulations are possible but less amenable to PET/F1 probe generation. This means that PET/F1 chemistry is most suited to last-step chelation techniques suited for 64Cu [2, 60, 61], 68Ga [55–58, 62, 63], 89Zr [64] trapping, and advanced water-insensitive isotope trapping and exchange strategies in 18F capture [65–77].
Having decided on a PET emitter and fluorophore, one must consider two possible ways to synthesize PET/F1 probes (Fig. 2). In the first, one would first conjugate a PET trap with a fluorophore before the resulting complex reacted with a ligand. This approach requires a bis-functionalized PET trap or a bis-functionalized fluorophore and is preferred on ligands with limited conjugation sites. This strategy gives exact, quantifiable ratios of fluorophore to PET emitter to ligand. It also results in the most efficient use of conjugatable sites, the greatest loading of fluorophore-PET emitter per ligand, and a single chemical entity (assuming filling of all conjugatable sites). Unfortunately, these prereacted fluorophore PET synthons (Fig. 2A) are not yet commercially available and require synthetic chemistry expertise to make [41, 52].
An alternative way of generating PET/F1 probes on ligands with multiple conjugation sites is to react a ligand with an activated mixture of PET trap and fluorophore (Fig. 2B). This approach is much simpler than the first method described and is preferred in mAb applications (Table 2) because PET/F1 probes can be performed rapidly with commercially available products (Tables 3 and 4) for quick proof-of-principal experiments. Unfortunately, this approach results in complicated mixtures of chemical entities and a lower utility of fluorophore and PET emitter per ligand. These mixtures can prove problematic for immediate clinical translation.
TABLE 4.
Common Commercially Available Activated Chelators
| Chelator | PET Isotopes |
|---|---|
|
| |
| DOTA | 64Cu, 68Ga |
| NOTA | 64Cu, 68Ga, 18F |
| DFO-SCN | 89Zr |
| EDTA | 89Zr, 68Ga |
| DTPA | 89Zr, 64Cu, 68Ga |
| TETA | 64Cu |
| NETA | 64Cu |
Note—Common suppliers include Sigma-Aldrich and Macrocyclics. DOTA = tetraazacyclododecanetetraacetic acid, NOTA = triazacyclononanetriacetic acid, DFO-SCN = deferoxamine-thiocyanate, EDTA = ethylenediaminetetraacetic acid, DTPA = diethylenetriaminepentaacetic acid, TETA = triethylenetetramine, NETA = tetraethylenetetramine.
Parallel Screening
Fluorophores have ex vivo utility that PET does not, such as compatibility in 96-well microarray scanners, florescence activity cell counters, and sorters. Advanced fluorescence techniques such as Förster resonance energy transfer (FRET) can yield information about ligand-target binding, and pH-and oxidation state–sensitive fluorophores can yield subcellular information that more clearly defines the state of a cancer [15]. In vitro and in vivo, multiple fluorophores can be used and imaged simultaneously. Multiple PET agents cannot be imaged simultaneously. One must wait for an isotope to decay before a second image can be acquired. This can prove problematic for repeat scanning with long-lived isotopes: one would ideally use an isotope before, during, and after intervention to most accurately gauge the effectiveness of an intervention.
Background Uptake, Isotope Dissociation
In imaging, probe instability due to isotope dissociation, transchelation [78], or background uptake by nondiseased tissue is of debatable concern. Ideally, one would want a ligand for PET/F1 with which these non–disease-specific signals are zero. These highly specific radiotracers require mandatory hybrid imaging because highly specific PET data are difficult to interpret without anatomic landmarking. On the other hand, 18F-FDG scans display discernable quantities of background uptake by nondiseased tissue, allowing anatomic landmarking in PET. Because of this background uptake, the presentation of an FDG PET scan is often simplified by displaying it without a CT or other form of anatomic image. Regardless of data presentation, the acquisition and reconstruction of PET images are performed simultaneously with CT or MRI for CT- or MRI-based attenuation correction during image reconstruction, which greatly improves the resolution of a PET image.
Summary
This article describes the current state of PET/F1 multimodality probe development in oncology. We highlight the current limits of PET/F1 with the understanding that these limits will quickly be outdated by new advances realized though the multidisciplinary collaboration between the chemical, physical, engineering, radiologic, pathologic, oncologic, and surgical fields that are necessary in PET/F1 probe development. Current preclinical PET/F1 agents are also briefly discussed. Because the existing PET/F1 agents are too numerous for adequate discussion, we focus on two extremes: direct cancer imaging at the molecular level through direct tumor-antigen and tumor-biomarker targeting and non–cancer-specific agents that improve patient prognoses through enhanced permeation and retention or tissue mapping. Having established that there are no set rules in PET/F1 cancer imaging, we finally describe starting points and highlight considerations that should be addressed in novel PET/F1 agent design.
Future of PET Fluorescence Imaging
The current extent of oncologic PET/F1 applications has been to readapt known SPECT agents, antibodies, peptides, and mapping tools into higher-resolution PET/F1 equivalents. Although these applications would translate into more accurate medical diagnoses, the full potential of combined PET and fluorescence imaging is yet to be realized.
One example requires one to consider that a PET/F1 multimodality imaging probe misleadingly implies the use of only two imaging modalities. In reality, PET/F1 requires the use of additional, anatomic modes of imaging to accurately interpret PET/F1 data (CT, transmission, and MRI for PET or light-imaging strategies for fluorescence imaging). Light detection and ranging (LIDAR) is an intensely watched light-imaging strategy noted for its utility in near-instantaneous digitalization and mapping. Like fluorescence, LIDAR cannot pass opaque tissue. One solution to this problem is a PET/F1 probe, which forces coregistration of PET and fluorescence signals through molecular association, leading one to immediately contemplate advanced applications such as bright-field–fluorescence–PET documentation of surgical procedures that are guided by artificial intelligence or automated in sealed environments. The next generation of PET/F1 probes will include agents that can be controlled to a certain degree. This means optogenetic-specific PET and photodynamic PET/F1 probes. Other topics in future PET/F1 development include further minimization of the molecular footprint of a PET/F1 probe so that theragnostic pharmaceutical drugs can be designed with more favorable absorption, distribution, metabolism, excretion, and toxicity properties. This would give rise to pharmaceutical design that is aided or accomplished through the use of image guidance, could reduce attrition rates, and would more quickly help identify contraindications in drug development. Finally, molecular PET/F1 probes designed for use in oncology will not be limited to oncology and will have broad-reaching, immediate application to other fields, such as neurology [79] and cardiology [67].
Acknowledgments
R. Ting, F. F. An, and H. Kommidi are funded by NIH K99/R00 grant (4R00EB013904-03) from the National Institute of Biomedical Imaging and Bioengineering.
References
- 1.Tsien RY. Imagining imaging's future. Nat Rev Mol Cell Biol. 2003 Sep;(suppl):SS:16–SS21. [PubMed] [Google Scholar]
- 2.Edwards WB, Xu B, Akers W, et al. Agonist-antagonist dilemma in molecular imaging: evaluation of a monomolecular multimodal imaging agent for the somatostatin receptor. Bioconjug Chem. 2008;19:192–200. doi: 10.1021/bc700291m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen QT, Tsien RY. Fluorescence-guided surgery with live molecular navigation: a new cutting edge. Nat Rev Cancer. 2013;13:653–662. doi: 10.1038/nrc3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernández-Suárez M, Ting AY. Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol. 2008;9:929–943. doi: 10.1038/nrm2531. [DOI] [PubMed] [Google Scholar]
- 5.Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–364. doi: 10.1038/nature12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Starczynski J, Atkey N, Connelly Y, et al. HER2 gene amplification in breast cancer: a rogues'. gallery of challenging diagnostic cases—UKNEQAS interpretation guidelines and research recommendations. Am J Clin Pathol. 2012;137:595–605. doi: 10.1309/AJCPATBZ2JFN1QQC. [DOI] [PubMed] [Google Scholar]
- 7.Perez K, Walsh R, Brilliant K, et al. Heterogeneity of colorectal cancer (CRC) in reference to KRAS proto-oncogene utilizing WAVE technology. Exp Mol Pathol. 2013;95:74–82. doi: 10.1016/j.yexmp.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu AP, Whitney MA, Crisp JL, Friedman B, Tsien RY, Nguyen QT. Improved facial nerve identification with novel fluorescently labeled probe. Laryngoscope. 2011;121:805–810. doi: 10.1002/lary.21411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellebust A, Richards-Kortum R. Advances in molecular imaging: targeted optical contrast agents for cancer diagnostics. Nanomedicine (Lond) 2012;7:429–445. doi: 10.2217/nnm.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu SP, Lee I, Ghoroghchian PP, et al. Near-infrared optical imaging of B16 melanoma cells via low-density lipoprotein–mediated uptake and delivery of high emission dipole strength tris[(porphinato)zinc(II)] fluorophores. Bioconjug Chem. 2005;16:542–550. doi: 10.1021/bc0497416. [DOI] [PubMed] [Google Scholar]
- 11.Weissleder R, Ntziachristos V. Shedding light onto live molecular targets. Nat Med. 2003;9:123–128. doi: 10.1038/nm0103-123. [DOI] [PubMed] [Google Scholar]
- 12.Dence CS, Ponde DE, Welch MJ, Lewis JS. Auto-radiographic and small-animal PET comparisons between (18)F-FMISO, (18)F-FDG, (18)F-FLT and the hypoxic selective (64)Cu-ATSM in a rodent model of cancer. Nucl Med Biol. 2008;35:713–720. doi: 10.1016/j.nucmedbio.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Crespo A. Comparison of Gallium-68 and Fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62. doi: 10.1016/j.apradiso.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Disselhorst JA, Brom M, Laverman P, et al. Image-quality assessment for several positron emitters using the NEMA NU 4-2008 standards in the Siemens Inveon small-animal PET scanner. J Nucl Med. 2010;51:610–617. doi: 10.2967/jnumed.109.068858. [DOI] [PubMed] [Google Scholar]
- 15.Tsien RY, Waggoner A. Fluorophores for confocal microscopy: photophysics and photochemistry. In: Pawley JB, editor. Handbook of biological confocal microscopy. Springer Science+Buisness Media; New York, NY: 2006. pp. 267–278. [Google Scholar]
- 16.van de Watering FC, Rijpkema M, Perk L, Brink-mann U, Oyen WJ, Boerman OC. Zirconium-89 labeled antibodies: a new tool for molecular imaging in cancer patients. Biomed Res Int. 2014;2014:203601. doi: 10.1155/2014/203601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rischpler C, Park MJ, Fung GS, Javadi M, Tsui BM, Higuchi T. Advances in PET myocardial per-fusion imaging: F-18 labeled tracers. Ann Nucl Med. 2012;26:1–6. doi: 10.1007/s12149-011-0552-5. [DOI] [PubMed] [Google Scholar]
- 18.Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507–518. doi: 10.1038/nrclinonc.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research website [Accessed March 29, 2016];Guidance: investigational new drug applications for positron emission tomography (PET) drugs. 2012 Dec; www.fda.gov/ownloads/drugs/guidancecomplianceregulatory-information/guidances/ucm291573.pdf.
- 20.Gibbs SL. Near infrared fluorescence for image-guided surgery. Quant Imaging Med Surg. 2012;2:177–187. doi: 10.3978/j.issn.2223-4292.2012.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR. 1994;15:1401–1407. discussion, 1408–1411. [PMC free article] [PubMed] [Google Scholar]
- 22.Zou Z, Zhang HL, Roditi GH, Leiner T, Kucharczyk W, Prince MR. Nephrogenic systemic fibrosis: review of 370 biopsy-confirmed cases. JACC Cardiovasc Imaging. 2011;4:1206–1216. doi: 10.1016/j.jcmg.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Hendee WR, O'Connor MK. Radiation risks of medical imaging: separating fact from fantasy. Radiology. 2012;264:312–321. doi: 10.1148/radiol.12112678. [DOI] [PubMed] [Google Scholar]
- 24.Amis ES, Jr, Butler PF, Applegate KE, et al. American College of Radiology white paper on radiation dose in medicine. J Am Coll Radiol. 2007;4:272–284. doi: 10.1016/j.jacr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Mettler FA, Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263. doi: 10.1148/radiol.2481071451. [DOI] [PubMed] [Google Scholar]
- 26.Azhdarinia A, Ghosh P, Ghosh S, Wilganowski N, Sevick-Muraca EM. Dual-labeling strategies for nuclear and fluorescence molecular imaging: a review and analysis. Mol Imaging Biol. 2012;14:261–276. doi: 10.1007/s11307-011-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lütje S, Rijpkema M, Helfrich W, Oyen WJ, Boerman OC. Targeted radionuclide and fluorescence dual-modality imaging of cancer: preclinical advances and clinical translation. Mol Imaging Biol. 2014;16:747–755. doi: 10.1007/s11307-014-0747-y. [DOI] [PubMed] [Google Scholar]
- 28.Seibold U, Wangler B, Schirrmacher R, Wangler C. Bimodal imaging probes for combined PET and OI: recent developments and future directions for hybrid agent development. Biomed Res Int. 2014;2014:153741. doi: 10.1155/2014/153741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paudyal P, Paudyal B, Iida Y, et al. Dual functional molecular imaging probe targeting CD20 with PET and optical imaging. Oncol Rep. 2009;22:115–119. doi: 10.3892/or_00000413. [DOI] [PubMed] [Google Scholar]
- 30.Sampath L, Kwon S, Hall MA, Price RE, Sevick-Muraca EM. Detection of cancer metastases with a dual-labeled near-infrared/positron emission tomography imaging agent. Transl Oncol. 2010;3:307–317. doi: 10.1593/tlo.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall MA, Kwon S, Robinson H, et al. Imaging prostate cancer lymph node metastases with a multimodality contrast agent. Prostate. 2012;72:129–146. doi: 10.1002/pros.21413. [DOI] [PubMed] [Google Scholar]
- 32.Gaedicke S, Braun F, Prasad S, et al. Noninvasive positron emission tomography and fluorescence imaging of CD133+ tumor stem cells. Proc Natl Acad Sci USA. 2014;111:E692–E701. doi: 10.1073/pnas.1314189111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen R, Vugts DJ, Stigter-van Walsum M, Visser GW, van Dongen GA. Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single- or dual-mode fluorescence and PET imaging. Nat Protoc. 2013;8:1010–1018. doi: 10.1038/nprot.2013.054. [DOI] [PubMed] [Google Scholar]
- 34.Hong H, Zhang Y, Severin GW, et al. Multimodality imaging of breast cancer experimental lung metastasis with bioluminescence and a monoclonal antibody dual-labeled with 89Zr and IRDye 800CW. Mol Pharm. 2012;9:2339–2349. doi: 10.1021/mp300277f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg PK, Garg S, Zalutsky MR. Fluorine-18 labeling of monoclonal antibodies and fragments with preservation of immunoreactivity. Bioconjug Chem. 1991;2:44–49. doi: 10.1021/bc00007a008. [DOI] [PubMed] [Google Scholar]
- 36.Cai W, Olafsen T, Zhang X, et al. PET imaging of colorectal cancer in xenograft-bearing mice by use of an 18F-labeled T84.66 anti-carcinoembryonic antigen diabody. J Nucl Med. 2007;48:304–310. [PubMed] [Google Scholar]
- 37.Otsuka FL, Welch MJ, Kilbourn MR, Dence CS, Dilley WG, Wells SA., Jr Antibody fragments labeled with fluorine-18 and gallium-68: in vivo comparison with indium-111 and iodine-125-labeled fragments. Int J Rad Appl Instrum B. 1991;18:813–816. doi: 10.1016/0883-2897(91)90023-e. [DOI] [PubMed] [Google Scholar]
- 38.Knowles SM, Wu AM. Advances in immuno-positron emission tomography: antibodies for molecular imaging in oncology. J Clin Oncol. 2012;30:3884–3892. doi: 10.1200/JCO.2012.42.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendricks JA, Keliher EJ, Wan D, Hilderbrand SA, Weissleder R, Mazitschek R. Synthesis of [18F]BODIPY: bifunctional reporter for hybrid optical/positron emission tomography imaging. Angew Chem Int Ed Engl. 2012;51:4603–4606. doi: 10.1002/anie.201107957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schillaci O. Use of dual-point fluorodeoxyglucose imaging to enhance sensitivity and specificity. Semin Nucl Med. 2012;42:267–280. doi: 10.1053/j.semnuclmed.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Paulus A, Desai P, Carney B, et al. Development of a clickable bimodal fluorescent/PET probe for in vivo imaging. EJNMMI Res. 2015;5:120. doi: 10.1186/s13550-015-0120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, Radtke MA, Wong MQ, Lin KS, Yapp DT, Perrin DM. Dual mode fluorescent F-18-PET tracers: efficient modular synthesis of rhodamine-[cRGD](2)-[F-18]-organotrifluoroborate, rapid, and high yielding one-step F-18-labeling at high specific activity, and correlated in vivo PET imaging and ex vivo fluorescence. Bioconjug Chem. 2014;25:1951–1962. doi: 10.1021/bc5003357. [DOI] [PubMed] [Google Scholar]
- 43.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. Springer; New York. NY: 2002. [Google Scholar]
- 44.Mariani G, Gipponi M, Moresco L, et al. Radioguided sentinel lymph node biopsy in malignant cutaneous melanoma. J Nucl Med. 2002;43:811–827. [PubMed] [Google Scholar]
- 45.Mariani G, Moresco L, Viale G, et al. Radioguided sentinel lymph node biopsy in breast cancer surgery. J Nucl Med. 2001;42:1198–1215. [PubMed] [Google Scholar]
- 46.Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: Tc-99m-DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–959. [PubMed] [Google Scholar]
- 47.Vera DR, Wallace AM, Hoh CK. [Tc-99m] MAG(3)-mannosyl-dextran: a receptor-binding radiopharmaceutical for sentinel node detection. Nucl Med Biol. 2001;28:493–498. doi: 10.1016/s0969-8051(01)00218-9. [DOI] [PubMed] [Google Scholar]
- 48.Schöder H, Glass EC, Pecking AP, et al. Molecular targeting of the lymphovascular system for imaging and therapy. Cancer Metastasis Rev. 2006;25:185–201. doi: 10.1007/s10555-006-8498-0. [DOI] [PubMed] [Google Scholar]
- 49.Hirsch JI, Tisnado J, Cho SR, Beachley MC. Use of isosulfan blue for identification of lymphatic vessels: experimental and clinical evaluation. AJR. 1982;139:1061–1064. doi: 10.2214/ajr.139.6.1061. [DOI] [PubMed] [Google Scholar]
- 50.Vera DR, Wisner ER, Stadalnik RC. Sentinel node imaging via a nonparticulate receptor-binding radiotracer. J Nucl Med. 1997;38:530–535. [PubMed] [Google Scholar]
- 51.An C, Shi Y, Li P, et al. Molecular dialogs between the ischemic brain and the peripheral immune system: dualistic roles in injury and repair. Prog Neurobiol. 2014;115:6–24. doi: 10.1016/j.pneurobio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ting R, Aguilera TA, Crisp JL, et al. Fast 18F labeling of a near-infrared fluorophore enables positron emission tomography and optical imaging of sentinel lymph nodes. Bioconjug Chem. 2010;21:1811–1819. doi: 10.1021/bc1001328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kenanova V, Wu AM. Tailoring antibodies for radionuclide delivery. Expert Opin Drug Deliv. 2006;3:53–70. doi: 10.1517/17425247.3.1.53. [DOI] [PubMed] [Google Scholar]
- 54.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 55.Decristoforo C, Pickett RD, Verbruggen A. Feasibility and availability of (6)(8)Ga-labelled peptides. Eur J Nucl Med Mol Imaging. 2012;39(suppl 1):S31–S40. doi: 10.1007/s00259-011-1988-5. [DOI] [PubMed] [Google Scholar]
- 56.Prata MI. Gallium-68: a new trend in PET radio-pharmacy. Curr Radiopharm. 2012;5:142–149. doi: 10.2174/1874471011205020142. [DOI] [PubMed] [Google Scholar]
- 57.Roesch F. Maturation of a key resource: the germanium-68/gallium-68 generator—development and new insights. Curr Radiopharm. 2012;5:202–211. doi: 10.2174/1874471011205030202. [DOI] [PubMed] [Google Scholar]
- 58.Lang L, Li W, Guo N, et al. Comparison study of [F-18]FAI-NOTA-PRGD2, [F-18]FPPRGD2, and [Ga-68]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjug Chem. 2011;22:2415–2422. doi: 10.1021/bc200197h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng Q, Juette MF, Jockusch S, et al. Ultra-stable organic fluorophores for single-molecule research. Chem Soc Rev. 2014;43:1044–1056. doi: 10.1039/c3cs60237k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu AM, Yazaki PJ, Tsai SW, et al. High-resolution microPET imaging of carcino-embryonic antigen-positive xenografts by using a copper-64-labeled engineered antibody fragment. Proc Natl Acad Sci USA. 2000;97:8495–8500. doi: 10.1073/pnas.150228297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pascu SI, Waghorn PA, Conry TD, et al. Designing Zn(II) and Cu(II) derivatives as probes for in vitro fluorescence imaging. Dalton Trans. 2007;43:4988–4997. doi: 10.1039/b705227h. [DOI] [PubMed] [Google Scholar]
- 62.Dimitrakopoulou-Strauss A, Hohenberger P, Haberkorn U, Macke HR, Eisenhut M, Strauss LG. 68Ga-labeled bombesin studies in patients with gastrointestinal stromal tumors: comparison with 18F-FDG. J Nucl Med. 2007;48:1245–1250. doi: 10.2967/jnumed.106.038091. [DOI] [PubMed] [Google Scholar]
- 63.Meyer GJ, Macke H, Schuhmacher J, Knapp WH, Hofmann M. 68Ga-labelled DOTA-derivatised peptide ligands. Eur J Nucl Med Mol Imaging. 2004;31:1097–1104. doi: 10.1007/s00259-004-1486-0. [DOI] [PubMed] [Google Scholar]
- 64.Deri MA, Zeglis BM, Francesconi LC, Lewis JS. PET Imaging with (89)Zr: from radiochemistry to the clinic. Nucl Med Biol. 2013;40:3–14. doi: 10.1016/j.nucmedbio.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, Guo J, Tang S, Lang L, Chen X, Perrin DM. One-step and one-pot-two-step radiosynthesis of cyclo-RGD-(18)F-aryltrifluoroborate conjugates for functional imaging. Am J Nucl Med Mol Imaging. 2013;3:44–56. [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Z, Li Y, Lozada J, et al. Kit-like 18F-labeling of RGD-19F-arytrifluroborate in high yield and at extraordinarily high specific activity with preliminary in vivo tumor imaging. Nucl Med Biol. 2013;40:841–849. doi: 10.1016/j.nucmedbio.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Liu S, Li D, Shan H, Gabbai FP, Li Z, Conti PS. Evaluation of (1)(8)F-labeled BODIPY dye as potential PET agents for myocardial perfusion imaging. Nucl Med Biol. 2014;41:120–126. doi: 10.1016/j.nucmedbio.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Burke BP, Clemente GS, Archibald SJ. Boron-(18) F containing positron emission tomography radio-tracers: advances and opportunities. Contrast Media Mol Imaging. 2015;10:96–110. doi: 10.1002/cmmi.1615. [DOI] [PubMed] [Google Scholar]
- 69.Liu Z, Pourghiasian M, Benard F, Pan JH, Lin KS, Perrin DM. Preclinical evaluation of a high-affinity F-18-trifluoroborate octreotate derivative for somatostatin receptor imaging. J Nucl Med. 2014;55:1499–1505. doi: 10.2967/jnumed.114.137836. [DOI] [PubMed] [Google Scholar]
- 70.Lindner S, Michler C, Leidner S, et al. Synthesis and in vitro and in vivo evaluation of SiFA-tagged bombesin and RGD peptides as tumor imaging probes for positron emission tomography. Bioconjug Chem. 2014;25:738–749. doi: 10.1021/bc400588e. [DOI] [PubMed] [Google Scholar]
- 71.Zhu J, Chin J, Wangler C, Wangler B, Lennox RB, Schirrmacher R. Rapid F-labeling and loading of PEGylated gold nanoparticles for in vivo applications. Bioconjug Chem. 2014;25:1143–1150. doi: 10.1021/bc5001593. [DOI] [PubMed] [Google Scholar]
- 72.Olasz EB, Lang L, Seidel J, Green MV, Eckelman WC, Katz SI. Fluorine-18 labeled mouse bone marrow-derived dendritic cells can be detected in vivo by high resolution projection imaging. J Immunol Methods. 2002;260:137–148. doi: 10.1016/s0022-1759(01)00528-2. [DOI] [PubMed] [Google Scholar]
- 73.Kuhnast B, de Bruin A, Hinnen F, Tavitian B, Dolle F. Design and synthesis of a new [F-18]fluoropyridine-based haloacetamide reagent for the labeling of oligonucleotides: 2-bromo-N-[3-(2-[F-18]fluoropyridin-3-yloxy)propyl]acetamide. Bioconjug Chem. 2004;15:617–627. doi: 10.1021/bc049979u. [DOI] [PubMed] [Google Scholar]
- 74.Ross TL, Ermert J, Hocke C, Coenen HH. Nucleophilic F-18-fluorination of heteroaromatic iodonium salts with no-carrier-added [F-18]fluoride. J Am Chem Soc. 2007;129:8018–8025. doi: 10.1021/ja066850h. [DOI] [PubMed] [Google Scholar]
- 75.Flavell RR, Kothari P, Bar-Dagan M, et al. Site-specific F-18-labeling of the protein hormone leptin using a general two-step ligation procedure. J Am Chem Soc. 2008;130:9106–9112. doi: 10.1021/ja801666z. [DOI] [PubMed] [Google Scholar]
- 76.Glaser M, Arstad E. “Click labeling” with 2-[F-18] fluoroethylazide for positron emission tomography. Bioconjug Chem. 2007;18:989–993. doi: 10.1021/bc060301j. [DOI] [PubMed] [Google Scholar]
- 77.Ducongé F, Pons T, Pestourie C, et al. Fluorine-18-labeled phospholipid quantum dot micelles for in vivo multimodal imaging from whole body to cellular scales. Bioconjug Chem. 2008;19:1921–1926. doi: 10.1021/bc800179j. [DOI] [PubMed] [Google Scholar]
- 78.Boswell CA, Sun XK, Niu WJ, et al. Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. J Med Chem. 2004;47:1465–1474. doi: 10.1021/jm030383m. [DOI] [PubMed] [Google Scholar]
- 79.Shimojo M, Higuchi M, Suhara T, Sahara N. Imaging multimodalities for dissecting Alzheimer's disease: advanced technologies of positron emission tomography and fluorescence imaging. Front Neurosci. 2015;9:482. doi: 10.3389/fnins.2015.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jadvar H, Parker JA. Clinical PET and PET/CT. Springer; London, UK: 2005. [Google Scholar]
- 81.Cherry SR, Sorenson JA, Phelps ME. Physics in nuclear medicine. 4th ed Elsevier/Saunders; Philadelphia, PA: 2012. [Google Scholar]