Abstract
Dynamic-Contrast-Enhanced Magnetic Resonance Imaging (DCE-MRI) has been used widely for clinical applications. Pharmacokinetic modeling of DCE-MRI data that extracts quantitative contrast reagent/tissue-specific model parameters is the most investigated method. One of the primary challenges in pharmacokinetic analysis of DCE-MRI data is accurate and reliable measurement of the arterial input function (AIF), which is the driving force behind all pharmacokinetics. Because of effects such as inflow and partial volume averaging, AIF measured from individual arteries sometimes require amplitude scaling for better representation of the blood contrast reagent (CR) concentration time-courses. Empirical approaches like blinded AIF estimation or reference tissue AIF derivation can be useful and practical, especially when there is no clearly visible blood vessel within the imaging field-of-view (FOV). Similarly, these approaches generally also require magnitude scaling of the derived AIF time-courses. Since the AIF varies among individuals even with the same CR injection protocol and the perfect scaling factor for reconstructing the ground truth AIF often remains unknown, variations in estimated pharmacokinetic parameters due to varying AIF scaling factors are of special interest. In this work, using simulated and real prostate cancer DCE-MRI data, we examined parameter variations associated with AIF scaling. Our results show that, for both the fast-exchange-limit (FXL) Tofts model and the water exchange sensitized fast-exchange-regime (FXR) model, the commonly fitted CR transfer constant (Ktrans) and the extravascular, extracellular volume fraction (ve) scale nearly proportionally with the AIF, whereas the FXR-specific unidirectional cellular water efflux rate constant, kio, and the CR intravasation rate constant, kep, are both AIF scaling insensitive. This indicates that, for DCE-MRI of prostate cancer and possibly other cancers, kio and kep may be more suitable imaging biomarkers for cross-platform, multicenter applications. Data from our limited study cohort show that kio correlates with Gleason scores, suggesting that it may be a useful biomarker for prostate cancer disease progression monitoring.
Keywords: Dynamic-Contrast-Enhanced, water exchange, arterial input function, contrast reagent, AIF scaling, prostate cancer
Graphical abstract
INTRODUCTION
Dynamic-contrast-enhanced MRI [DCE-MRI] is widely used in the study of cancer and other pathologies. It provides high-resolution anatomical [lesion size, shape], microvascular [tumor blood volume, capillary permeability] [1–6] and, potentially metabolic [Na+,K+ ATPase activity] information [7,8]. The quantitative estimation of DCE-MRI pharmacokinetic parameters requires knowledge of the time-dependences of the plasma and interstitial compartmental contrast reagent (CR) concentrations, [CRp](t) and [CRo](t), respectively. The former is commonly referred to as the arterial input function [AIF]. In practice, direct determination of either concentration time-course is not straightforward. Classical methods using radiotracer-labeled CR require frequent blood sampling for [CRp](t) and sacrificial autoradiography for [CRo](t) determinations [9,10]. These make them cumbersome and impossible, respectively, for human studies. Furthermore, blood sampling is usually from a large vessel, not a capillary adjacent to the tissue of interest, and autoradiographic estimation of the tissue interstitial distribution volume has considerable systematic uncertainty.
Thus, the common practice is to estimate [CRp](t) and [CRo](t) noninvasively, from the DCE-MRI data themselves. However, the accuracy of AIF derived from DCE-MRI data can be influenced by several inherent experimental conditions, such as: 1) partial volume effect (signal from the image voxel, ideally containing only blood, is contaminated with signal from other non-blood tissues); 2) in-flow effect (signals generated by water molecules in the blood that were originally outside of the imaging volume flow in during signal acquisition and impact the final “blood” signal detected); 3) flow-profile dependency (when vessel cross-section contains more than one image voxel, measured voxel blood CR concentration is flow-profile dependent); and 4) no visible vessel in the imaging field-of-view (FOV). These factors can cause errors in the AIF amplitudes computed directly from blood vessel signals, and a scaling factor may be applied to reduce the errors [5,6]. Alternative methods mitigating the challenges of AIF quantification from blood signal have been proposed. Examples of these include reference tissue methods [11–13] and blinded AIF estimation [14–16]. Unfortunately, these approaches also require AIF amplitude scaling after derivation of the AIF time-course curves. The scaling factor (β) is often derived from literature reported tissue properties and generally does not account for individual variations. The AIF scaling uncertainties propagate to errors in the derived DCE-MRI pharmacokinetic modeling parameters. Thus, it is important to understand the effect of AIF scaling variations on modeled parameter values.
In this work, simulated DCE-MRI time-courses based on prostate data were subjected to repeated model fittings with the β of an AIF being the only variable. Repeated fittings of actual prostate region-of-interest (ROI) data were also performed to confirm the simulation findings. We used both the standard Tofts model and the Shutter-Speed model for data fitting. The former ignores intercompartmental water exchange (equivalent to assuming infinitely fast water exchange kinetics [the fast-exchange-limit (FXL)] pertains throughout the CR time-course), while the latter accounts for the effects of intercompartmental water exchange. The FXR (fast-exchange-regime) version of the Shutter-Speed Paradigm (SSP) was used in this study with the parameter kio (unidirectional equilibrium cellular water efflux rate constant) accounting for transcytolemmal water exchange. Both models return the Ktrans (rate constant for plasma/interstitium contrast agent transfer) and ve (extravascular, extracellular volume fraction) parameters. When comparing all results with the common practice (β=1.0; i.e., using the AIFs determined from reported methods as the reference), our results show that the commonly modeled Ktrans and ve values scale nearly proportionally with β, indicating that cautions should be used comparing Ktrans and ve values among different subjects or across different platforms. By contrast, the kep and kio (SSP specific) variations are much smaller in response to the same β variations. In addition, we show that kio values correlate with Gleason scores in our limited data set. This indicates that the AIF-scaling insensitive kio may offer a quantitative way for prostate cancer disease aggressiveness detection or monitoring.
MATERIAL and METHODS
Prostate DCE-MRI data were acquired from 13 subjects under a study protocol approved by the Portland VA Medical Center (PVAMC) and Oregon Health & Science University (OHSU) institutional review boards (IRBs). Each subject was referred for biopsy as standard care and volunteered for our DCE-MRI protocol before that procedure occurred. A prior biopsy was an exclusion criterion. All participants gave written informed consent. Additional details are reported in [5,6].
MRI
All prostate MRI data were acquired with a Siemens TIM Trio (3T) system. RF transmission utilized the instrument whole body coil, while reception employed a combination of flexible body and spine matrix coil arrays. Multi-slice T2-weighted images (Turbo Spin Echo, TR/TE/FA, 5000 ms/102 ms/90°) were acquired for anatomic contrast and ROI selection [a 320*180*16 matrix size and 360*360 mm2 transverse FOV resulted in nominal (1.1)2 mm2 in-plane resolution: slice thickness, 3 or 3.2 mm]. A near proton density (PD) image of the prostate gland was obtained using a 3D fast low-angle shot (FLASH) pulse sequence with a 256*144*16 matrix size and a 360*203 mm2 transverse FOV, resulting in a nominal in-plane resolution of (1.4)2 mm2. Other parameters were: slice thickness, 3 or 3.2 mm; TR/TE/FA, 200 ms/1.56 ms/8°. For DCE-MRI, the same FLASH sequence was run with the only differences being TR = 5.0 ms and a 15° flip angle, which yielded a 6.28 s imaging inter-sampling interval. The total DCE-MRI acquisition time was ten and a half minutes. A bolus of 0.1 mmol/kg Prohance (Bracco, Inc.) was administered in an antecubital vein ~ 38 s after commencing the DCE-MRI acquisition. It was delivered in 5 to 10 s, at 3.0 mL/s, followed by a 20 mL saline flush.
Prostate Biopsy and Pathology
Subsequent to the DCE-MRI acquisition, each subject underwent a standard ten core “geographic” prostate biopsy procedure performed using (exclusively) ultrasound guidance; i.e., with no MRI input. Pathology examinations of biopsy core specimen fragments revealed Gleason scores ranging from 6 to 8 for five of the subjects. No malignancy was found for the other eight subjects.
Data Preparation
For DCE-MRI analysis, one ROI was selected for each of the 13 subjects [6] resulting in five malignant and eight benign ROI DCE-MRI time-courses. Since the precise biopsy track positions were not recorded, the spatial relationships between the DCE-MRI ROIs and the biopsy loci were restricted to only unilaterality. The experimental AIF was determined from voxels within a femoral artery visible in the FOV [6]. An individual AIF time-course was measureable for each subject except one, due to severe motion during the DCE acquisition in the latter case. A population-averaged AIF, based on the individual AIFs from six other subjects, was used for that individual.
Pharmacokinetic Analyses
In DCE-MRI, ΔR1t(t) describes the time-course of the ΔR1t [≡ R1t(t) - R1t(0)] value during the bolus CR passage; where R1t(t) is the measured tissue 1H2O longitudinal relaxation rate constant time-course and R1t(0) denotes the value prior to CR administration. Individual R1t(0) values were calculated numerically with the Gradient Recalled Echo (GRE) signal expression (Eq. 7) using the average of DCE baseline images and that of the near PD-weighted image. With R1t(0) determined, the R1t(t) time-course can be calculated from the DCE-MRI signal time-course using the signal mean from DCE baseline for PD computation. To enhance the kio detection capability, especially for the fast wash-out malignant lesion cases, only the first 70 time points (7.3 min total) were included in the model fitting for the prostate data. One critical step in quantitative DCE-MRI data analysis is to relate the measured ΔR1t(t) time-course to the time-course of the interstitial CR concentration time-course, [CRo](t).
The Shutter-Speed Paradigm
Because most water is inside cells but the CR visits at most the interstitial space, the kinetics of the equilibrium exchange of water molecules across the cell membranes must be taken into account. In this study we used the fast-exchange-regime (FXR)-allowed shutter-speed paradigm model that takes into account this exchange but assumes a single exponential recovery for the 1H2O signal [5,17,18]. This is expressed in
| (1) |
where R1i and R1o0 are, respectively, the longitudinal relaxation rate constants for the intra- and extracellular 1H2O signals (in the absence of exchange and CR); r1o is the interstitial CR relaxivity; kio is the unidirectional equilibrium cellular water efflux rate constant (reciprocal of the mean intracellular water lifetime, τi); and vi and ve are the respective intra- and extracellular volume fractions. The absolute difference in R1 between the extra- and intra-cellular space (|R1o–R1i|) is defined as the “shutter-speed”, τ1−1 [≡ κ1] [17].
Using the Kety-Schmitt pharmacokinetic rate law, the interstitial CR concentration time course, [CRo](t), is expressed by Equation (2), where Ktrans is the CR transfer rate constant
| (2) |
and [CRp](t), the blood plasma [CR] time-course, is the AIF. The MRI experimentally determined AIF can be expressed as in Equation (3):
| (3) |
where R1b(t) is the capillary blood 1H2O R1 time-course, R1b(0) is the pre-CR blood R1, r1p is the longitudinal relaxivity for CR in blood plasma and fixed at a literature value of 3.8 mM−1s−1 [19], and hv (fixed at 0.45) is the microvascular hematocrit.
To systematically account for AIF amplitude scaling, we rewrite Eq. (2) to include the time-independent AIF scaling factor, β, as Equation (4):
| (4) |
where β is a time-independent scaling factor that quantifies AIF amplitude variations.
The differential form of Eq. (4) is given in Equation (5).
| (5) |
It is important to point out that there is no “extra” β term in any pharmacokinetic model equations. Here, the β factor is added for the purpose of simulating AIF scaling uncertainties, with β = 1.0 representing the “ground truth” AIF. The use of β makes it easier to illustrate parameter changes associated with AIF scaling. That is “β·[CRp]” is the actual AIF used in each fitting. In Eq. (5), the Ktrans/ve term appearing in Eq. (4) is replaced by kep according to its definition.
Standard mathematical approach to solve an inhomogeneous first-order differential equation like Eq. (5) starts with solving its associated homogenous equation (i. e., Eq. (5) with the right-hand-side set to zero, or [CRp] =0). This immediately reveals that the rate constant (kep) is not impulse function ([CRp]) dependent. Specific to DCE-MRI, as long as the curve shape of [CRp](t) doesn’t change, then kep is independent of β. This observation is associated with the Kety-Schmitt pharmacokinetic rate law and thus applies to both the FXL and FXR models used in this study. In practice, since the AIFs are often determined numerically without an analytical expression, there might be small variations of kep following a β change.
The Tracer Paradigm
Very often in the literature the MRI CR is treated as a tracer (i.e., as if it’s directly detected) and, when the blood 1H2O contribution to the tissue signal is negligible, the tissue R1 change, ΔR1t(t), can be expressed using [CRo](t), as in Equation (6):
| (6) |
which is simpler than Eq. (1). However, Eq. (6) implies a linear [CRo]-dependence of ΔR1t, which is experimentally untrue [20,21]. It further implies that equilibrium transcytolemmal water exchange kinetics are effectively infinitely fast [kio → ∞] [21]. This is usually an incorrect over-simplification. The direct consequences of using tracer paradigm (TP) models are systematic errors in the estimated pharmacokinetic parameters such as Ktrans [example below] and ve [1–3,5–7]. Nevertheless, since tracer models find common use in the literature because of their relative simplicity, we also used one, the standard, fast-exchange-limit (FXL)-constrained Tofts tracer model [22], in addition to the FXR-SSP model in assessing the effects of AIF scaling uncertainty. The kio parameter is unique to the SSP models and cannot be accessed by any tracer paradigm model because such models effectively assume kio to approach infinity [kio → ∞]. Mathematically, Eq. (1) simplifies to the FXL Eq. (6) when κ1 → 0 [21]. Thus, the SSP-FXR automatically simplifies to the Tofts FXL model if κ1 (the shutter-speed) never rises sufficiently during a DCE time-course. However, this does not mean that kio → ∞, only that kio is indeterminate.
Simulations
The [CRo] expression in Eq. (4) was used by both the SSP and TP approaches. For a given Ktrans, ve, and kio (SSP only) parameter set, R1t(t) time courses are influenced by AIF scaling through the associated input function β·[CRp], where [CRp] (the reference or “true” AIF when β = 1.0) used for the simulation was based on our experimental measurements and further smoothed to mimic a well-determined AIF (see Figure 1A). Ktrans, ve, and kio values were selected based on those from the prostate data. Unless otherwise stated, we simulated R1t time-courses using the conventional β =1.0. After the R1t calculation, Equation (7) suitable for gradient-echo sequence was used to calculate the signal time-courses.
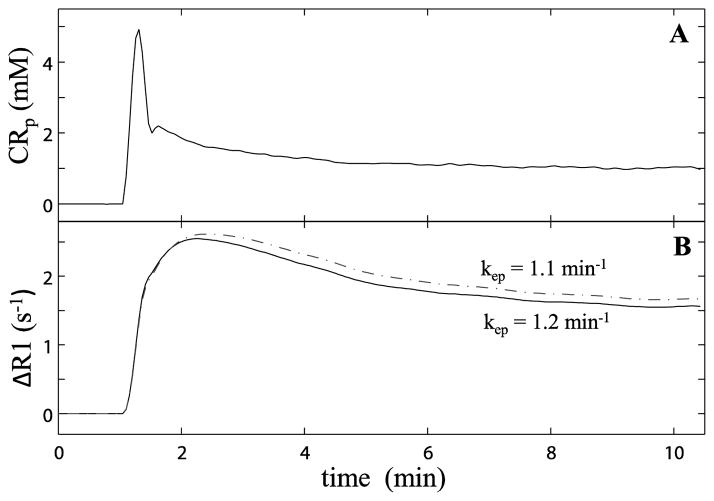
Figure 1.
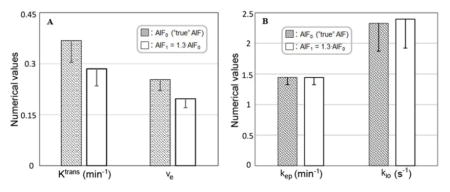
Figure 1A plots the averaged blood plasma concentration ([CRp]) time-course derived from five individually determined arterial input functions [AIFs]. Under the condition of β =1.3 (i.e., 130% the 1A AIF amplitude), the 1B solid curve plots the ΔR1(t) time-course calculated with Ktrans = 0.36 min−1, ve = 0.3 (kep =1.2 min−1), τi = 0.6. The dashed curve traces ΔR1(t) with a different parameter set: Ktrans = 0.35 min−1, ve = 0.32 (kep =1.1 min−1), τi = 0.6 s.
| [7] |
Here, S0 is proportional to the equilibrium magnetization, TR is the pulse sequence repetition time, α the read pulse flip angle. Noiseless signal time-courses as well as those with added Gaussian white noise were both used to investigate parameter variations under different β values.
Simulated time-courses were fitted with different AIFs defined as β·[CRp]. Eq. (4) suggests that β interacts inversely with Ktrans and proportionately with ve in data fitting. To investigate parameter variations caused by different AIF scaling, simulations were carried out by fitting pharmacokinetic parameters with β fixed at 13 different values from 0.8 to 2.0 with a step-size of 0.1. A larger than 100% β variation was included for model fittings so that parameter trends in response to AIF scaling can be better observed.
Fitting the simulated time-courses yielded Ktrans, ve, and τi (≡ 1/kio, SSP only) values. In addition, kep [≡ Ktrans/ve] [22], and the intracellular volume fraction vi [≡ 1 – ve] values were determined. While ve defines the CR accessible volume fraction, the vi parameter equals the cell density [ρ] and mean individual cell volume (<V>) product [ρ •<V>], within the ROI or voxel [7].
To avoid local minima in each optimization procedure, 20 different initial guess parameter value sets were used for each β step and each model. Parameters from the best fitting of the 20 trials for each combination were then selected as the fitted results. In addition, two-dimensional grid searches [3,23] were also performed to investigate the Ktrans-ve parameter space to gain insights into the fitting approaches and to provide assurance in avoiding potential local minima during fitting.
RESULTS
Figure 1A plots the averaged blood plasma concentration, [CRp], time-course derived from five individual AIFs [6]. Under the condition of β =1.3 (i.e., 130% the AIF amplitude as show in 1A), the solid curve in 1B plots the ΔR1(t) time-course predicted by the shutter-speed approach for kep = 1.2 min−1, vi = 0.70, and kio = 1.7 s−1 [Ktrans = 0.36 min−1, ve = 0.30, τi = 0.60s]. The 1B dashed curve shows the prediction for kep = 1.1 min−1, vi = 0.68, and kio = 1.7 s−1 [Ktrans = 0.35 min−1, ve = 0.32, τi = 0.60 s]. As expected, the ΔR1(t) wash-out portion is rather sensitive to an only 8% kep reduction [there is also a 3% vi reduction]. Also as expected, the simulated ΔR1(t) time-courses with either parameter set are lower than the 1B curves when β=1.0 (using the AIF in 1A; not shown).
To investigate Ktrans and ve (and thus kep and vi) parameter sensitivity to AIF scaling, a grid search [23] rather than a non-linear data fitting was selected as the first step of data analysis. Although the former can be tedious and slow, a large searching range can often provide the global solution and avoid local minima that could trap a non-linear optimization.
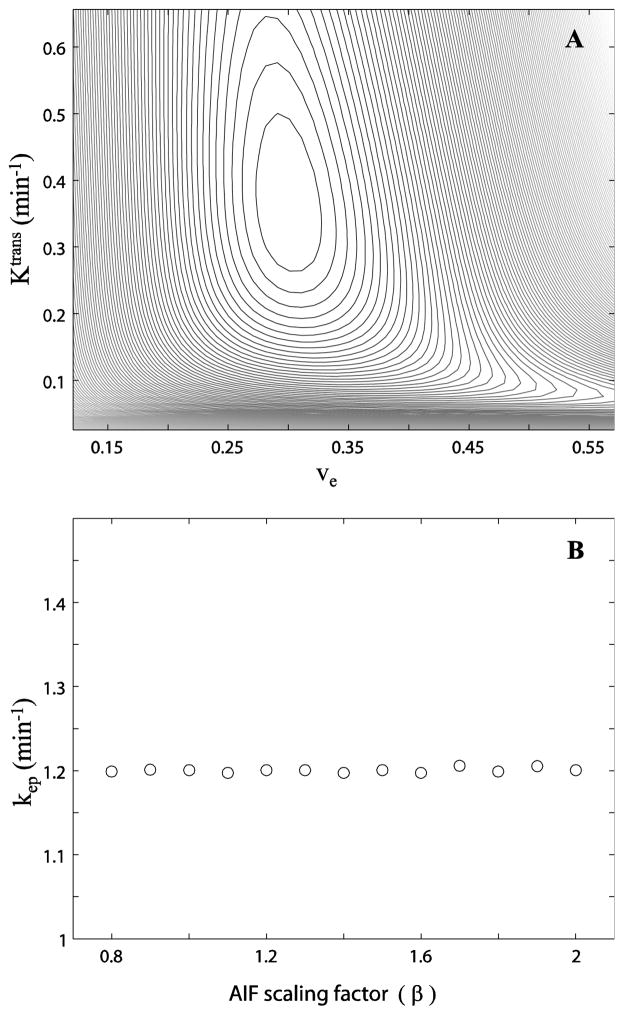
For demonstration, Figure 2A shows a search of the Ktrans, ve error surface. Using the Fig. 1A AIF, and the parameter set of Ktrans = 0.36 min−1, ve = 0.30, and kio = 1.7 s−1, the reference R1t and its related reference signal time-course, Sref(t), were generated. Then, the simulated R1(t) for each grid point (Ktrans,ve coordinate pair; kio = 1.7 s−1) and Sgrid(t) time-course was calculated and this was compared with Sref(t). The constant difference square sum {χ2 = Σt[Sref(t) – Sgrid(t)]2 [3]} value contours decrease to a global minimum at the coordinates Ktrans = 0.36 min−1 and ve =0.30 (kep = 1.2 min−1), matching the inputs used in generating the reference curve as expected. The contour lines measure the χ2 value (“goodness”) of comparisons with the reference curve of different model curves calculated for the parameter values given by the coordinates [3]. The χ2 value decreases as one moves on the surface towards the closed contours. Figure 2B shows the best fitted kep values determined at different β. A near constant kep [1.2 min−1] is observed over the range of β tested. [Slight kep deviations are most likely due to the finite numbers of Ktrans and ve steps (500–600) used in the grid search.]
Figure 2.
Figure 2A shows a Ktrans vs. ve error surface. The contour plot shows the chi square statistic, χ2 = Σ[Sref(t) – Sgrid(t)]2, for simulated DCE-MRI time-courses from parameters equal to the Ktrans,ve coordinates. The χ2 value decreases as the contour lines move towards the center of the closed loops. B shows the best kep (≡Ktrans/ve) values determined at different β values. A near constant kep is observed within the entire β range tested.
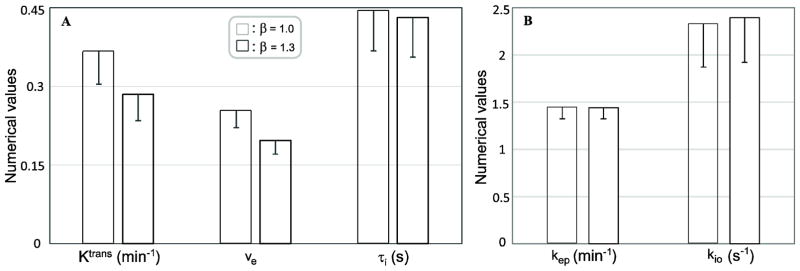
Using shutter-speed simulated data and analyses, Figure 3A shows Ktrans, ve, and τi changes associated with a 30% AIF scaling change. The reference parameter values (cross-hatched bars) are: Ktrans = 0.36 min−1, ve = 0.25, and τi = 0.45 s. Monte Carlo simulations (using Fig. 1A AIF as the β =1.0 reference) were used to estimate Ktrans, ve, and τi accuracy (mean) and precision (SD). 400 fittings were performed with random Gaussian noise (SNR = 10 at baseline, slightly better than the median baseline voxel SNR of 5.3 estimated from the prostate ROI slices of all subjects) added to the simulated time-course each time, and each fitting started with a different initial guess parameter value set. Mean and SD values of kep and kio derived from the 3A fittings are plotted in Figure 3B. Fig. 3A clearly shows that Ktrans and ve decrease proportionally (~ 30%) with the 30% β increase, while the τi change is negligibly small (< 3%), well below the standard deviation (~10%) associated with the data SNR. Defined as Ktrans/ve, kep is insensitive to β variation (Fig. 3B) as expected. Being the τi reciprocal, the rate constant kio has a similar relative measurement error as τi. Except for having different numerical means, vi and ve have the same SD values because vi ≡1-ve. That is, error propagation for both ve and vi is exactly the same. Results of kio (or τi) and kep being less sensitive to AIF scaling variations indicate that the values of these parameters are likely to be more consistent despite difficulties in accurate AIF determination in many experimental conditions. We use results from real prostate ROI data in the following figures to illustrate additional aspects also seen with simulations. This provides some validations of the simulation results and adds insights on actual data processing when AIF scaling uncertainty plays a factor in pharmacokinetic model parameters.
Figure 3.
Figure 3A depicts the Ktrans, ve, and τi changes associated with a 30% change in β. Monte Carlo simulations were used to estimate Ktrans, ve, and τi accuracy (mean) and precision (SD) changes resulting from a 30% β increase. It is seen that Ktrans and ve change proportionately (~ 30%) with β, while the τi change is negligibly small (< 3%), well below the standard deviation (>10%) associated with the data SNR. 3B shows the similar plots for kep and kio. Defined as Ktrans/ve, kep is insensitive to β variation as discussed in the text. Being the τi reciprocal, the rate constant kio has the same relative error as that of τi.
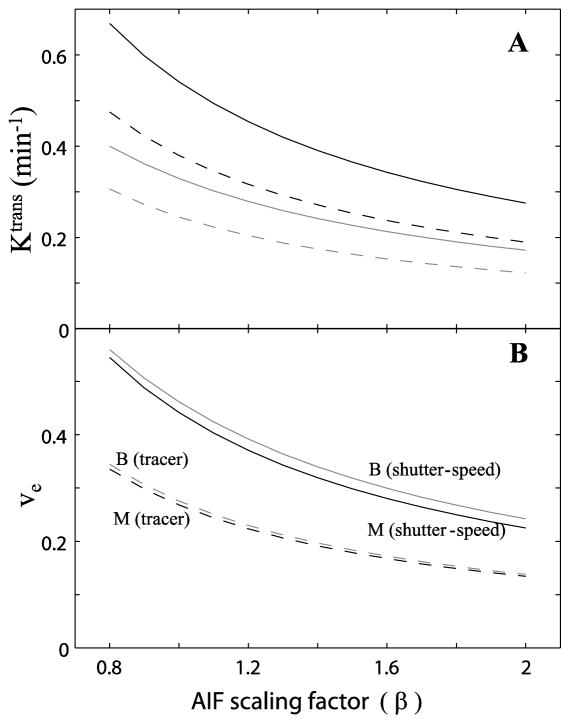
Figure 4A shows representative ROI Ktrans variations associated with AIF scaling uncertainty, for malignant (black curves) and benign (gray curves) tissues. The individual AIF (= β·[CRp]) was used for fitting at each β value, mimicking AIF scaling uncertainty seen in experimental AIF derivation. Each ROI time-course was fitted with shutter-speed (solid curves) and tracer (dashed curves) analyses. The best fitted values returned from the 20 different initial parameter guess sets are plotted. The Ktrans values are rather AIF scaling dependent, decreasing with increasing β. Similar findings are observed for the ve parameter (Figure 4B). Both Ktrans and ve are the most commonly reported parameters in pharmacokinetic model fitting.
Figure 4.
Figure 4A shows representative Ktrans changes for malignant (black curves) and benign (gray curves) tissues with increasing β. Each ROI data set was fitted with models from the shutter-speed (solid curves) and tracer (dashed curves) paradigms. The best fitted values from 20 different initial guesses are plotted. The Ktrans values decrease with β increase. 4B shows the same trends for ve.
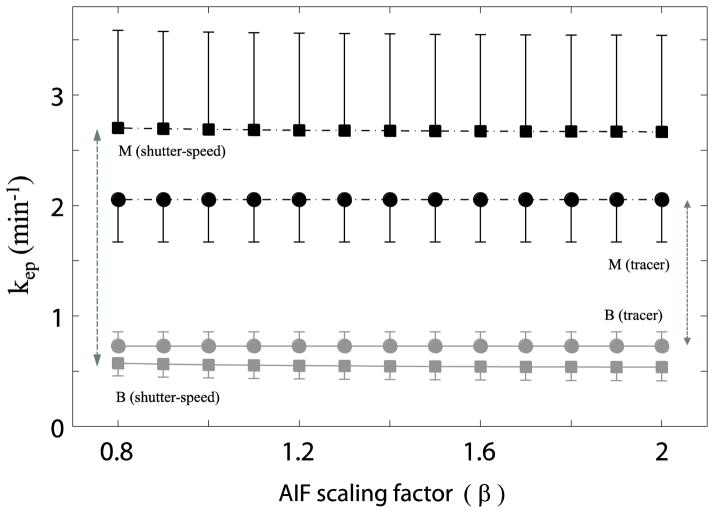
The simulated results in Fig. 2B indicate that kep appears to be independent of β scaling. This is confirmed by results from real prostate data (Figure 5). Averaged kep values of eight benign and five malignant ROIs (with standard errors), respectively, are plotted versus β values used in this study. For each signal-averaged ROI at each β step, 20 fittings with different initial guess each time were performed and the one with the smallest fitting error was then selected for that ROI. The results from shutter-speed analyses are plotted as square points and those from tracer analyses as circular points. The malignant ROIs are represented by black color vs. gray color for the benign group. Regardless of the analysis paradigm and tissue type, the kep within each analysis remains nearly constant at different β values, indicating kep is basically unaffected by AIF scaling uncertainty. The apparent malignant/benign discrimination [kep(M) – kep(B)] is increased with the shutter-speed paradigm (17,18) [double-headed vertical arrows in Fig. 5]. This trend is shown because when compared to their FXL counterparts, kep values determined by the FXR often increase for malignant cases and decrease for benign cases. Although this systematic behavior may not be covered by generic statistical tools, we report the Hedges’ g effect size for malignant/benign separation by kep to provide a primitive numerical comparison. The computed Hedges’ g values for kep mean differences are 1.15 (95% CI: 0.24, 2.07) and 0.82 (95% CI: −0.06, 1.70) for the FXL and FXR approaches, respectively. Future investigation with larger subject cohort is needed to address this issue more thoroughly.
Figure 5.
Figure 5 plots the β-dependence of the mean kep values for the five malignant (black) and eight benign (gray) cases. The shutter-speed kep values have square symbols while the tracer values have circle symbols. Regardless of the pharmacokinetic paradigm and tissue characteristics, the kep value for each model remains constant, indicating kep is insensitive to AIF scaling.
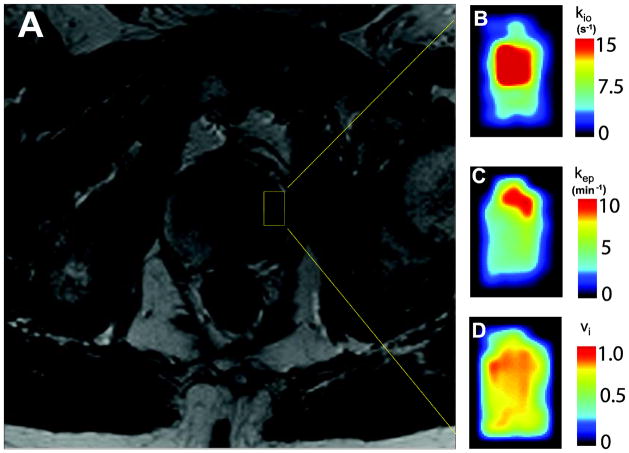
Figure 6A shows a portion of an axial T2-weighted image of a 66 year old subject with prostate malignancy. Figures 6B–6D show zoomed pharmacokinetic parametric maps (kio, kep, and vi, respectively) of the lesion area within the prostate, which is generally indicated by the yellow rectangle in Fig. 6a. The black borders in Figs. 6B–6D provide some contrast to the generally cold color of normal-appearing glandular tissue within the rectangular ROI encompassing the lesion. The parametric maps were obtained as the best results from 30 fittings with different initial guess sets. Elevated kio values are seen in part of the lesion, possibly indicating increased cellular metabolic activity [7] associated with malignancy. Noticeably higher kep values reflecting increased vascular leakage are seen only in the anterior portion within the lesion ROI. Besides being on the “warm” portion of the color map (often brighter contrast), vi is directly proportional to cell density.
Figure 6.
Figure 6A shows a portion of an axial pelvic T2-weighted image of a 66 year old subject with left prostate malignancy (anterior up). Figures 6B–6D show zoomed parametric maps of a tumor-encompassing ROI in the left prostate indicated by the 6A yellow rectangle. Figure 6B is the kio map, which displays metabolic activity. Significantly elevated kio is clearly visible in the anterior half of the ROI. Figure 6C shows increased kep in only the most anterior portion of the ROI. The kep parameter measures capillary CR permeability. Figure 6D shows vi slightly elevated in the anterior half of the ROI. This most likely represents increased cell density. Black borders are added in 6B–6D to provide contrast with the elevated parametric color maps.
In Figure 7, lesion ROI-averaged in vivo SSP-only kio values are plotted against the independently-obtained ex vivo Gleason scores for the five malignant cases. Each voxel within each ROI was fitted 30 times with different initial guess values using both the shutter-speed paradigm and tracer paradigm. The best fitted results from each paradigm were recorded. Then, the commonly used model selection approach, the Akaike information criterion (AIC), was used to determine which model better described the specific voxel time-course. In Figure 7, only those voxels favored by AIC for the shutter-speed paradigm were included in the averaging calculation [the tracer paradigm leaves kio indeterminate]. Using the lesion ROI (indicated by the yellow rectangle) from Figure 6 as an example, kio was averaged for 36 out of 38 voxels. The numbers of AIC-favored over the total ROI voxels for the remaining four malignant cases are: 23/56, 13/16, 8/11, and 20/28. A clear trend of increasing kio with Gleason score (Spearman’s correlation coefficient, r = 0.91; p = 0.03) is observed (the empirical blue dashed curve is intended only to guide the eye). The kio error bars show standard errors for the analysis of each small lesion. The vertical error bars also reflect our unknown registration of MRI ROI and biopsy locus. That is, without clear knowledge of the latter, voxels from benign tissues can be accidentally included in the lesion ROIs. As CR extravasation is generally lower in non-cancerous tissue, kio uncertainty increases because of decreased shutter speed [17], which results in larger error bar length compared to an ROI containing more malignant tissue. The horizontal error bars show calculated differences between clinical Gleason scores reported in another study, and the weighted mean Gleason scores given by an expert reviewer [24]. These bars partially illustrate the uncertainties in Gleason pattern reporting (e.g., if no reported Gleason scores differ by two categorical points or larger, a 0.1 bar length then represents 10% of the cases having different Gleason scores). Other fitted parameter values from the same patient group were reported previously [6]. None of the fitted Ktrans and ve values from either paradigm showed a clear correlation with Gleason scores.
Figure 7.
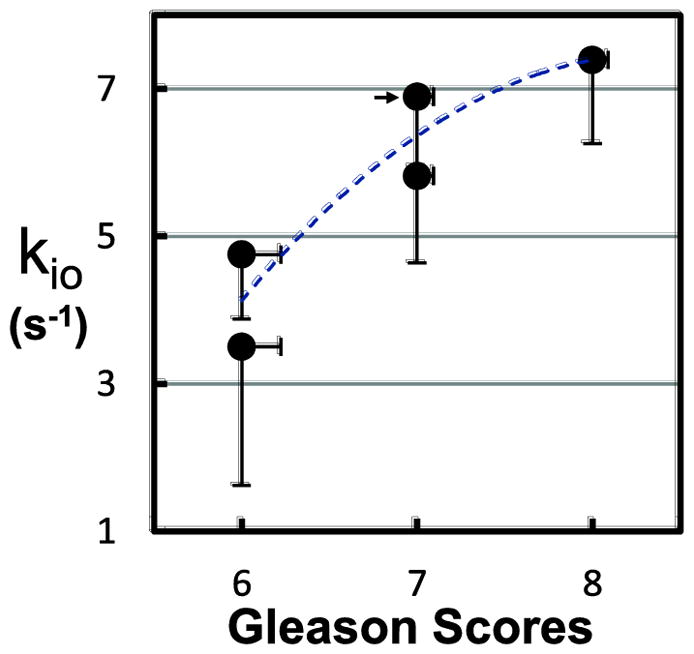
Figure 7 plots lesion ROI-averaged in vivo kio values against independently obtained ex vivo Gleason scores for the five malignant cases. A clear trend of increasing kio with Gleason score (Spearman’s correlation coefficient, r = 0.91; p = 0.03) is observed (the empirical blue dashed curve is intended only to guide the eye). The error bars show kio standard errors and estimated Gleason score errors (see text).
DISCUSSION
Accurate AIF quantification has been one of the primary challenges in quantitative pharmacokinetic analysis of DCE-MRI data. Many AIF derivation methods [5,6,11–16] require an AIF amplitude scaling in the process. Since the ground truth of the scaling factor is often unknown (for example, using muscle as a reference tissue for AIF scaling runs into the problem of varying pharmacokinetic properties of the muscle itself under different physiological conditions), this makes investigation of AIF scaling effects important. By explicitly including an AIF scaling factor (β) into the pharmacokinetic modeling, this work investigated the pharmacokinetic model parameter variations associated with AIF scaling uncertainty. Our results show that the kep and the SSP-specific kio parameters are insensitive to AIF scaling. In a theoretical scenario where kep is truly time and [CR] independent, its insensitivity to [CRp] scaling can be directly predicted from Eq. (5). That is, kep insensitivity to AIF scaling is a direct result of the Kety-Schmitt pharmacokinetic rate law. This is the reason that insensitivity of kep to AIF scaling is observed for both the TP and the SSP approaches [Fig. 5]. On the other hand, the relative insensitivity of the SSP-specific kio to AIF scaling is the result of multiple factors. First, kio quantifies water exchange, which is a separate process independent of CR pharmacokinetics; second, the interstitial CR concentration, [CRo], generally experiences a more slow-varying temporal feature and reaches its maximum at a much later time when compared with [CRp] (i.e., [CRo] is less sensitive to [CRp] first pass); third, kio does not appear with [CRp] in the same term in a pharmacokinetic equation or within a [CRp] expression. One the other hand, a larger [CRp] helps reduce kio uncertainty in CR-extravasating tissues by generating a larger [CRo] and thus a larger shutter speed [17].
Traditionally, the most common parameters extracted from quantitative DCE-MRI are Ktrans and ve. We found these parameters to be quite sensitive to the AIF scaling factor β over a range from 0.8 to 2.0 for both simulated (Fig. 3) and real prostate ROI data (Fig. 4). This may partially explain the no statistical difference findings [25,26] reported in literature when comparing pharmacokinetic parameters (Ktrans and ve) derived from population averaged AIFs vs. individually measured AIFs -- it is possible that the errors (specially AIF scaling uncertainty) in individually measured AIFs propagate to the fitted Ktrans and ve parameters and thus negatively impact their precisions as quantitative imaging biomarkers. Thus, when AIF amplitude uncertainty is high, the kio and kep parameters are more robust DCE-MRI biomarkers for their relative AIF-scaling insensitivity. As long as the AIF time-course curve shape can be well captured, kio and kep should be the more reproducible parameters than Ktrans and ve. With the recent discovery that kio is a measure of on-going Na+,K+-ATPase turnover [7], the capability to quantify kio using the SSP model potentially ushers DCE-MRI into the emerging field of high-resolution metabolic imaging [8], in addition to its well-known function of quantifying microvascular properties. The parameter kep could be a better measure of capillary CR permeation than Ktrans. The parameter Ktrans usually well-approximates the permeability coefficient capillary surface area product, PCR†•S [8,27]. Thus, it can have a significant contribution from the voxel blood volume surface area. In addition, Ktrans magnitude closely tracks the initial slope of the tissue enhancement time-course, a feature that describes similar characteristics of vb•[CRp] (vb is the blood volume). This is one reason that Ktrans accuracy depends heavily on AIF. On the other hand, kep quantification depends more on the washout period of the tissue enhancement time-course. As shown in Figure 1B, a 10% kep change causes noticeable difference in only the washout phase. In this case, the Ktrans difference is relatively small (~ 3 %) as the rising phase of the curves are nearly indistinguishable.
Figure 7 demonstrates the tumor grading potential of the new kio biomarker for prostate cancer. Despite the kio and Gleason score uncertainties shown by the error bars, the data show a strong correlation: kio increases “hyperbolically” with Gleason scores above 5, the traditional malignancy threshold. The smooth curve drawn through the points serves only to guide the eye. Therefore, the kio parameter may be a useful noninvasive imaging biomarker that can be potentially used to reduce the number of biopsies performed in current clinical practice.
It is worth noting that even though accurate AIF quantification remains challenging, especially during the CR first pass, an AIF scaling error of less than 30% can be managed from the washout phase, taking into account the total blood volume and CR elimination rate. It is important to point out that the precision in kio quantification is better when CR extravasation is extensive (large Ktrans) [7]. To insure kio accuracy, we used 30 initial guess sets for each voxel during fitting, and limited our kio report to voxels with AIC statistics favoring the shutter-speed paradigm.
The uncertainty in [CRo](t) quantification (because of AIF uncertainty) affects kio and vi accuracy, and the maximum [CRo] magnitude affects kio precision [6,7]. It has recently been suggested it might be possible to determine kio and vi without the use of CR [28]. Though this is not yet established, it would preclude parametric errors due to AIF uncertainty. However, the Ktrans and kep parameters are not accessible without the use of CR.
With faster imaging speed as a result of ever improving MRI scanner hardware and software, DCE-MRI data acquisition can achieve improved AIF temporal resolution and better tissue curve-shape quantifications. Still, intrinsic challenges, like the in-flow effect, associated with AIF quantification remain. These mostly affect the AIF magnitude. This study only examines the effects of this primary AIF scaling error. Implications of other AIF uncertainties on pharmacokinetic parameters will require separate investigation.
Using a mouse model, a recent study [29] utilizing X-ray fluorescence microscopy showed that gadodiamide (Omniscan) enters normal murine prostatic epithelia and lumens. Although it remains unclear whether CR also enters some tumor cells, this new finding may require to be accounted for with an additional compartment in prostate DCE-MRI pharmacokinetic modeling. It is unclear at this point whether the CR concentration differences between the prostatic epithelia, the lumens, and the EES are large enough to be separately resolved. Still, the new finding may partially explain the large ve values reported in prostate DCE-MRI literature.
It is interesting to point out that for extracellular Gadolinium based CRs with appreciable extravasation, CR molecules will present in blood plasma and extravascular extracellular space (EES) after a bolus IV injection. The associated compartment-specific relaxivities for the EES and blood plasma are r1o and r1p, respectively. In most DCE-MRI studies it is generally assumed that r1o and r1p are numerically equal. However, there are some indirect evidences suggesting potential differences [30,31] between the two. Under the condition that r1o and r1p are both time-and [CR]-independent, the effect of unequal r1o and r1p on pharmacokinetic model parameter estimations is very similar to that of DCE data modeled under different AIF scaling factors, as was undertaken in this study. The similarity is shown in the APPENDIX.
The data studied here have also been used in two earlier reports [6, 5]. The former showed the shutter-speed Ktrans and kep as well as the ΔKtrans [≡ Ktrans(FXR) - Ktrans(FXL)] imaging biomarkers exhibit improved malignant/benign tissue discrimination [6]. The latter paper showed the CA extravasation magnitude effect on commonly modeled DCE parameter (Ktrans, ve, vb τi, τb) precisions. Specifically, τi (1/kio) uncertainty and the feasibility of τi (1/kio) mapping of the entire normal prostate were demonstrated [5]. In this paper, after identifying kep and kio as the AIF-scaling-insensitive DCE parameters, the potential for kio (with its newly discovered metabolic activity nature) as a disease aggressiveness biomarker was shown: a kio Gleason score correlation.
One limitation of this study is that we investigated the effect of only AIF scaling uncertainty on estimations of pharmacokinetic parameters. Other confounding factors, like variable T2* effect during CR passage and in-flow effect, etc., can result in AIF curve shape variations, which will cause different variations in the derived kep values. Future studies will be needed to address these issues. It is encouraging that a recent multi-center prostate DCE-MRI data analysis study showed that the variations of the kep parameter were substantially smaller than those of the Ktrans parameter when they were derived from AIFs quantified with different methods, leading to both AIF curve-shape and amplitude differences [32].
CONCLUSIONS
AIF quantification remains a major challenge for DCE-MRI pharmacokinetics. Several AIF derivation approaches require AIF magnitude scaling, which generally suffers from the lack of ground truth knowledge. This simulation work demonstrates that the kep and the Shutter-Speed Paradigm specific kio (1/τi) parameters are nearly insensitive to AIF scaling. This supports the notion that both the kep and kio parameters are more robust DCE-MRI biomarkers.
Highlights.
In DCE-MRI pharmacokinetic modeling, arterial input function (AIF) scaling is often needed for AIF derivation.
Contrast reagent transfer constant (Ktrans) and extravascular, extracellular volume fraction (ve) model parameters are affected by AIF-scaling.
The contrast reagent intravasation rate constant (kep) is insensitive to AIF-scaling error.
The unidirectional rate constant for equilibrium cellular water efflux (kio) is insensitive to AIF-scaling error.
Acknowledgments
This work was supported in part by the Medical Research Foundation of Oregon and National Institute of Health grants: RO1-EB00422, U01-CA154602, R44 CA180425.
Abbreviations
- AIF
arterial input function
- AIF scaling factor
β
- CR
contrast reagent
- [CRp]
contrast reagent concentration in blood plasma
- [CRo]
contrast reagent concentration in the extravascular, extracellular space
- [CRt]
contrast reagent concentration in tissue
- DCE-MRI
dynamic contrast-enhanced magnetic resonance imaging
- FOV
field of view
- FXL
fast-exchange-limit
- FXR
fast-exchange-regime
- hv
microvascular hematocrit
- Ktrans
rate constant for plasma/interstitium contrast agent transfer
- kep
= Ktrans/ve, rate constant for contrast reagent intravasation
- kio
= 1/τi, unidirectional equilibrium cellular water efflux rate constant
- PD
proton density
- r1
longitudinal contrast reagent relaxivity
- r1o
longitudinal relaxivity for CR in the extravascular, extracellular space
- r1p
longitudinal relaxivity for CR in the blood plasma space
- R1b
= 1/T1b, blood longitudinal relaxation rate constant for blood 1H2O
- R1t
= 1/T1t, tissue longitudinal relaxation rate constant for tissue 1H2O
- ROI
region of interest
- ρ
cell number density [number of cells/unit volume]
- SD
standard deviation
- SNR
signal-to-noise ratio
- SSP
Shutter-Speed Paradigm
- SXR
slow-exchange-regime
- τi
mean intracellular water lifetime
- T1−1
= (|R1o –R1i|), the NMR R1“shutter-speed”, [≡ κ1]
- TP
Tracer Paradigm (Tofts model)
- ve
extravascular, extracellular volume fraction; the EES
- vi
intracellular volume fraction (= 1- ve, when ignoring blood volume fraction)
APPENDIX
The relaxivity of a contrast reagent (CR) is defined as the slope of the R1 and [CR] equation as expressed in Eq. (1a),
| (1a) |
where r1 is the relaxivity in unit of (mM−1s−1).
The r1 values are specific to the compartments occupied by CR molecules. In DCE-MRI, low molecular weight Gadolinium based CR can present in blood plasma and the extravascular, extracellular space (EES).
Eq. (3) can be used to convert measured blood R1 time-course to that of blood plasma CR concentration time-course, [CRp].
For the FXR and the FXL models, the relationships of tissue R1 and the EES CR concentration, [CRo], is given by Eq. (1) and Eq. (5), respectively.
Because r1 always appears in the form of r1•[CR] product in a relaxivity expression, the r1o•[CRo] term which quantifies EES R1 change can be expanded to:
| (2a) |
where r1o is the longitudinal CR relaxivity for the EES, and r1p is the longitudinal CR relaxivity for blood plasma. All other parameters are defined in MATERIALS and METHODS section.
It is common practice in DCE-MRI pharmacokinetic modeling to assume that r1o = r1p. However, there is some evidence indicating unequal r1o and r1p values [30,31].
When both r1o and r1p are [CR]- and time-independent, the r1o/r1p ratio can be moved to either side of Eq. (2a). That is, r1o/r1p ≠ 1.0 has a similar effect as that of employing an AIF scaling factor (β) in pharmacokinetic modeling. Here, the AIF is assumed to be well-characterized and the r1o/r1p ratio depends on the compartments the CR molecules occupy. When the r1o/r1p ratio is unknown or there is strong evidence indicating that r1o/r1p ≠ 1.0, then results from this work show that kep can be the parameter preferred over Ktrans for characterizing microvasculature. This is because of its insensitivity to AIF scaling uncertainties.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li X, Huang W, Morris EA, Tudorica LA, Seshan VE, Rooney WD, Tagge I, Wang Y, Xu J, Springer CS., Jr Dynamic NMR effects in breast cancer dynamic-contrast-enhanced MRI. Proc Natl Acad Sci U S A. 2008;105:17937–17942. doi: 10.1073/pnas.0804224105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang W, Li X, Morris EA, Tudorica LA, Seshan VE, Rooney WD, Tagge I, Wang Y, Xu J, Springer CS. The magnetic resonance shutter speed discriminates vascular properties of malignant and benign breast tumors in vivo. Proc Natl Acad Sci U S A. 2008;105:17943–17948. doi: 10.1073/pnas.0711226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X, Rooney WD, Varallyay CG, Gahramanov S, Muldoon LL, Goodman JA, Tagge IJ, Selzer AH, Pike MM, Neuwelt EA, Springer CS., Jr Dynamic-contrast-enhanced-MRI with extravasating contrast reagent: rat cerebral glioma blood volume determination. J Magn Reson. 2010;206:190–199. doi: 10.1016/j.jmr.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang W, Tudorica LA, Li X, Thakur SB, Chen Y, Morris EA, Tagge IJ, Korenblit ME, Rooney WD, Koutcher JA, Springer CS., Jr Discrimination of benign and malignant breast lesions by using shutter-speed dynamic contrast-enhanced MR imaging. Radiology. 2011;261:394–403. doi: 10.1148/radiol.11102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Priest RA, Woodward WJ, Siddiqui F, Beer TM, Garzotto MG, Rooney WD, Springer CS., Jr Cell membrane water exchange effects in prostate DCE-MRI. J Magn Reson. 2012;218:77–85. doi: 10.1016/j.jmr.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Priest RA, Woodward WJ, Tagge IJ, Siddiqui F, Huang W, Rooney WD, Beer TM, Garzotto MG, Springer CS., Jr Feasibility of shutter-speed DCE-MRI for improved prostate cancer detection. Magn Reson Med. 2013;69:171–178. doi: 10.1002/mrm.24211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Springer CS, Jr, Li X, Tudorica LA, Oh KY, Roy N, Chui SY, Naik AM, Holtorf ML, Afzal A, Rooney WD, Huang W. Intratumor mapping of intracellular water lifetime: metabolic images of breast cancer? NMR Biomed. 2014;27:760–773. doi: 10.1002/nbm.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rooney WD, Li X, Sammi MK, Bourdette DN, Neuwelt EA, Springer CS., Jr Mapping human brain capillary water lifetime: high-resolution metabolic neuroimaging. NMR Biomed. 2015;28:607–623. doi: 10.1002/nbm.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preston E, Foster DO. Diffusion into rat brain of contrast and shift reagents for magnetic resonance imaging and spectroscopy. NMR Biomed. 1993;6:339–344. doi: 10.1002/nbm.1940060510. [DOI] [PubMed] [Google Scholar]
- 10.Nagaraja TN, Ewing JR, Karki K, Jacobs PE, Divine GW, Fenstermacher JD, Patlak CS, Knight RA. MRI and quantitative autoradiographic studies following bolus injections of unlabeled and (14)C-labeled gadolinium-diethylenetriaminepentaacetic acid in a rat model of stroke yield similar distribution volumes and blood-to-brain influx rate constants. NMR Biomed. 2011;24:547–558. doi: 10.1002/nbm.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovar DA, Lewis M, Karczmar GS. A new method for imaging perfusion and contrast extraction fraction: input functions derived from reference tissues. J Magn Reson Imaging. 1998;8:1126–1134. doi: 10.1002/jmri.1880080519. [DOI] [PubMed] [Google Scholar]
- 12.Yang C, Karczmar GS, Medved M, Stadler WM. Estimating the arterial input function using two reference tissues in dynamic contrast-enhanced MRI studies: fundamental concepts and simulations. Magn Reson Med. 2004;52:1110–1117. doi: 10.1002/mrm.20243. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Karczmar GS, Medved M, Stadler WM. Multiple reference tissue method for contrast agent arterial input function estimation. Magn Reson Med. 2007;58:1266–1275. doi: 10.1002/mrm.21311. [DOI] [PubMed] [Google Scholar]
- 14.Schabel MC, Fluckiger JU, DiBella EV. A model-constrained Monte Carlo method for blind arterial input function estimation in dynamic contrast-enhanced MRI: I. Simulations. Phys Med Biol. 2010;55:4783–4806. doi: 10.1088/0031-9155/55/16/011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fluckiger JU, Schabel MC, DiBella EV. Toward local arterial input functions in dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2010;32:924–934. doi: 10.1002/jmri.22339. [DOI] [PubMed] [Google Scholar]
- 16.Kratochvila J, Jirik R, Bartos M, Standara M, Starcuk Z, Jr, Taxt T. Distributed capillary adiabatic tissue homogeneity model in parametric multi-channel blind AIF estimation using DCE-MRI. Magn Reson Med. 2016;75:1355–1365. doi: 10.1002/mrm.25619. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Rooney WD, Springer CS., Jr A unified magnetic resonance imaging pharmacokinetic theory: intravascular and extracellular contrast reagents. Magn Reson Med. 2005;54:1351–1359. doi: 10.1002/mrm.20684. [Erratum: Magn. Reson. Med. 55 (2006) 1217.] [DOI] [PubMed] [Google Scholar]
- 18.Yankeelov TE, Rooney WD, Li X, Springer CS., Jr Variation of the relaxographic “shutter-speed” for transcytolemmal water exchange affects the CR bolus-tracking curve shape. Magn Reson Med. 2003;50:1151–1169. doi: 10.1002/mrm.10624. [DOI] [PubMed] [Google Scholar]
- 19.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 20.Landis CS, Li X, Telang FW, Molina PE, Palyka I, Vetek G, Springer CS., Jr Equilibrium transcytolemmal water-exchange kinetics in skeletal muscle in vivo. Magn Reson Med. 1999;42:467–478. doi: 10.1002/(sici)1522-2594(199909)42:3<467::aid-mrm9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Wilson GJ, Woods M, Springer CS, Jr, Bastawrous S, Bhargava P, Maki JH. Human whole-blood (1)H2O longitudinal relaxation with normal and high-relaxivity contrast reagents: influence of trans-cell-membrane water exchange. Magn Reson Med. 2014;72:1746–1754. doi: 10.1002/mrm.25064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 23.Bevington PR. Data Reduction and Error Analysis for the Physical Sciences. McGraw-Hill; New York: 1969. [Google Scholar]
- 24.Goodman M, Ward KC, Osunkoya AO, Datta MW, Luthringer D, Young AN, Marks K, Cohen V, Kennedy JC, Haber MJ, Amin MB. Frequency and determinants of disagreement and error in gleason scores: a population-based study of prostate cancer. Prostate. 2012;72:1389–1398. doi: 10.1002/pros.22484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng R, Chang SD, Jones EC, Goldenberg SL, Kozlowski P. Comparison between population average and experimentally measured arterial input function in predicting biopsy results in prostate cancer. Acad Radiol. 2010;17:520–525. doi: 10.1016/j.acra.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukla-Dave A, Lee N, Stambuk H, Wang Y, Huang W, Thaler HT, Patel SG, Shah JP, Koutcher JA. Average arterial input function for quantitative dynamic contrast enhanced magnetic resonance imaging of neck nodal metastases. BMC Med Phys. 2009;9:4. doi: 10.1186/1756-6649-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Springer CS, Jr, Jerosch-Herold M. First-pass dynamic contrast-enhanced MRI with extravasating contrast reagent: evidence for human myocardial capillary recruitment in adenosine-induced hyperemia. NMR Biomed. 2009;22:148–157. doi: 10.1002/nbm.1293. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton JI, Deshmane A, Hougen S, Griswold M, Seiberlich N. Magnetic Resonance Fingerprinting with Chemical Exchange (MRF-X) for Quantification of Subvoxel T1, T2, Volume Fraction, and Exchange Rate. Proc Intl Soc Mag Reson Med. 2015;23:329. [Google Scholar]
- 29.Mustafi D, Gleber SC, Ward J, Dougherty U, Zamora M, Markiewicz E, Binder DC, Antic T, Vogt S, Karczmar GS, Oto A. IV Administered Gadodiamide Enters the Lumen of the Prostatic Glands: X-Ray Fluorescence Microscopy Examination of a Mouse Model. AJR Am J Roentgenol. 2015;205:W313–9. doi: 10.2214/AJR.14.14055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanisz GJ, Henkelman RM. Gd-DTPA relaxivity depends on macromolecular content. Magn Reson Med. 2000;44:665–667. doi: 10.1002/1522-2594(200011)44:5<665::aid-mrm1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Spiller M, Caravan P. Evidence for weak protein binding of commercial extracellular gadolinium contrast agents. Magn Reson Med. 2010;63:609–616. doi: 10.1002/mrm.22214. [DOI] [PubMed] [Google Scholar]
- 32.Huang W, Chen Y, Fedorov A, Li X, Jajamovich GH, Malyarenko DI, Aryal MP, LaViolette PS, Oborski MJ, O’Sullivan F, Abramson RG, Jafari-Khouzani K, Afzal A, Tudorica A, Moloney B, Gupta SN, Besa C, Kalpathy-Cramer J, Mountz JM, Laymon CM, Muzi M, Kinahan PE, Schmainda K, Cao Y, Chenevert TL, Taouli B, Yankeelov TE, Fennessy F, Li X. The Impact of Arterial Input Function Determination Variations on Prostate Dynamic Contrast-Enhanced Magnetic Resonance Imaging Pharmacokinetic Modeling: A Multicenter Data Analysis Challenge. Tomography. 2016;2:56–66. doi: 10.18383/j.tom.2015.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]