Abstract
Background
Previous work has shown that patients with conduct problems show impairments in reinforcement-based decision-making. However, studies with patients have not previously demonstrated any relationships between impairment in any of the neuro-computations underpinning reinforcement-based decision-making and specific symptom sets (e.g., level of conduct problems (CP) and/or callous-unemotional (CU) traits).
Methods
Seventy-two youth (20 female, mean age=13.81 [standard deviation= 2.14], mean IQ= 102.34 [standard deviation= 10.99]) from a residential treatment program and the community completed a passive avoidance task while undergoing functional MRI.
Results
Greater levels of CP were associated with poorer task performance. Reduced representation of expected values (EV) when making avoidance responses within bilateral anterior insula cortex/inferior frontal gyrus (AIC/iFG) and striatum was associated with greater levels of CP but not CU traits.
Conclusions
The current data indicate that difficulties in the use of value information to motivate decisions to avoid sub-optimal choices are associated with increased levels of CP (though not severity of CU traits). Moreover, they account for the behavioral deficits observed during reinforcement-based decision-making in youth with CP. In short, an individual’s relative failure to utilize value information within AIC/iFG to avoid bad choices is associated with elevated levels of CP.
Keywords: Conduct problems, decision-making, anterior insula, expected value, prediction error
Introduction
Reinforcement-based decision-making involves making responses to cues on the basis of expectations of reinforcement (reward or punishment). Youth with conduct problems (CP) show impairments in reinforcement-based decision-making (Blair, Colledge, & Mitchell, 2001; Budhani, Richell, & Blair, 2006; Schutter, van Bokhoven, Vanderschuren, Lochman, & Matthys, 2011). Recently, these impairments have been related to specific neural systems (in particular, ventromedial and dorsomedial frontal cortex, anterior insula cortex and striatum; Crowley et al., 2010; Finger et al., 2011; Finger et al., 2008; Rubia et al., 2009). Importantly, recent computational model-based fMRI research in healthy samples has allowed the identification of at least partially neurally distinct computational processes involved in reinforcement-based decision-making (Clithero & Rangel, 2014; O'Doherty, 2011; O'Doherty, Hampton, & Kim, 2007). This work allows the possibility of refining our understanding of reinforcement-based decision-making impairments in youth with CP, potentially relating specific symptom sets to impairment in specific neuro-computational processes. This, in turn, should aid assessment and the identification of treatment targets for future interventions.
Previous computational fMRI research has suggested that successful decision-making involves at least two critical computations: representation of expected value (EV) and prediction error (PE; Clithero & Rangel, 2014; O'Doherty, 2011; O'Doherty et al., 2007). EV is the subjective value associated with an action or stimulus. PE is the difference between the EV of the stimulus/response and the value of the feedback received. PEs signal that learning should occur leading to a revision of EV and thus more appropriate choice behavior in the future (Rescorla & Wagner, 1972). Regions particularly responsive to PE and EV include ventromedial frontal cortex (vmPFC), posterior cingulate cortex and striatum (Clithero & Rangel, 2014; O'Doherty, 2004; O'Doherty, 2011). Early work suggests that both the representation of PE and EV is compromised in youth with conduct problems (White et al., 2013). This impairment is thought to result in poorer reinforcement-based decision-making, increasing the probability of future frustration and potential consequent aggression (Blair, White, Meffert, & Hwang, 2014).
Decisions to not choose objects/actions are associated with activity in anterior insula cortex/inferior frontal gyrus (AIC/iFG), dorsal anterior cingulate cortex (dACC; Kuhnen & Knutson, 2005; Liu et al., 2007) and caudate (Casey et al., 2001). These regions have been implicated in the avoidance of sub-optimal choices (Budhani, Marsh, Pine, & Blair, 2007; Casey et al., 2001) and show increased activity as a function of the expected punishment or expected loss of reward associated with the choice. It has been hypothesized that dysfunction within these regions results in poorer avoidance of sub-optimal choices increasing the risk of choosing antisocial behavioral responses to environmental provocations (Blair et al., 2014). Previous work has also shown that youth with CP show impaired representation of EV information within AIC/iFG, dACC and caudate when making avoidance choices (White et al., 2014; White et al., 2013).
In short, youth with conduct problems show impairment in neuro-computations underpinning reinforcement-based decision-making (White et al., 2014; White et al., 2013). However, studies with patients have not previously demonstrated any relationships between impairment in any of the neuro-computations underpinning reinforcement-based decision-making and specific symptom sets. The goal of the current study is to determine whether level of impairment might be associated with level of severity of two specific symptom sets. The first of these is CP. Previous work has speculated that increased impairment in EV and PE representation will increase the risk of choosing antisocial behavioral options and consequently be associated with increased conduct problems (Blair et al., 2014). The second of these is callous-unemotional (CU) traits (reduced guilt and empathy). CU traits predict a unique developmental trajectory to CP (Frick & White, 2008) and are associated with greater levels of aggression, antisocial behavior and mental health problems (Frick, Ray, Thornton, & Kahn, 2014). Previous work has speculated that level of reinforcement-based decision-making impairments will be positively associated with CU traits (Blair, 2007). However, recent studies have reported no significant relationships between extent of impairment and level of CU traits (White et al., 2014; White et al., 2013). These studies, however, used an extreme groups approach resulting in relatively limited Ns (<20) and limited variability of CU traits (White et al., 2014; White et al., 2013), raising the possibility that these null findings are the result of type II error.
The current study thus extends the previous literature by examining CP and CU continuously in a relatively large sample. On the basis of the previous theoretical work, we predicted that level of CP and/or CU traits would be inversely associated with i) EV representation within AIC/iFG, dACC and striatum during choice; and ii) PE representation within vmPFC and striatum.
Methods
Participants
Seventy-two subjects participated. Demographic information is presented in Table 1. Youths were drawn from a residential treatment program and the community. The youths recruited from the treatment program had been referred for behavioral and mental health problems. These youth were invited to participate based on the results of an intake assessment. Participants from the community were recruited through flyers. Parents completed a telephone screen to determine potential eligibility. Clinical assessment took place at the first visit for community youth. A statement of informed assent and consent was obtained from participating children and parents. Boys Town National Research Hospital Institutional Review Board approved this study.
Table 1.
Participant demographic information
| Total Participants | male | female | |
|---|---|---|---|
| N= 72 | N= 52 (72.22%) | N= 20 (27.78%) | |
| Mean (Std. Dev.) | Mean (Std. Dev.) | Mean (Std. Dev.) | |
| Age | 13.81 (2.19) | 13.73 (2.13) | 14.02 (2.34) |
| IQ | 102.78 (10.99) | 103.85 (10.32) | 100.00 (12.41) |
| CBCL CP Levels | 61.73 (11.40) | 61.27 (11.24) | 62.93 (12.05) |
| ICU scores | 29.18 (14.26) | 30.04 (14.37) | 26.95 (14.07) |
Std. Dev. = Standard Deviation; no significant differences were observed between males and females.; CBCL= Child Behavior Checklist; CP= Conduct Problems; ICU= Inventory of Callous-Unemotional Traits
Parents completed the Child Behavior Checklist (CBCL; Achenbach, 2009). The CBCL has been found to be reliable (Achenbach, 2009) and effective in both identifying clinical disorders and in quantifying the severity of psychopathology in children and adolescents (Achenbach, Dumenci, & Rescorla, 2003). IQ was assessed with the Wechsler Abbreviated Scale of Intelligence (WASI; two-subtest form; Wechsler, 1999). Exclusion criteria were pervasive developmental disorder, Tourette’s syndrome, lifetime history of psychosis, neurological disorder, history of head trauma, on-going, non-psychiatric medical illness requiring medication that may have psychotropic effects such as: beta blockers or steroids and IQ<80. In addition, parents completed the Inventory of Callous-Unemotional Traits (Frick, 2004). CP in the current study was operationalized as an average of t-scores from the Oppositional Defiant Disorder and Conduct Disorder subscales the CBCL. CP was not significantly related to age [r=.147, p=.218] or IQ [r=-.184, p=.122]. Levels of CP did not differ between males and females [F(1,70)=.301, p=.585] or between minority and non-minority participants [F(1,70)=.911, p=.440]. CU traits were not significantly related to IQ [r=−.118, p=.324], but were significantly associated with age [r=.257, p=.029]. Levels of CU traits did not differ between males and females [F(1,70)=.674, p=.414] or between minority and non-minority participants [F(1,70)=.853, p=.470]. CU traits and CP were highly correlated [r=.779, p<.001].
Passive avoidance task
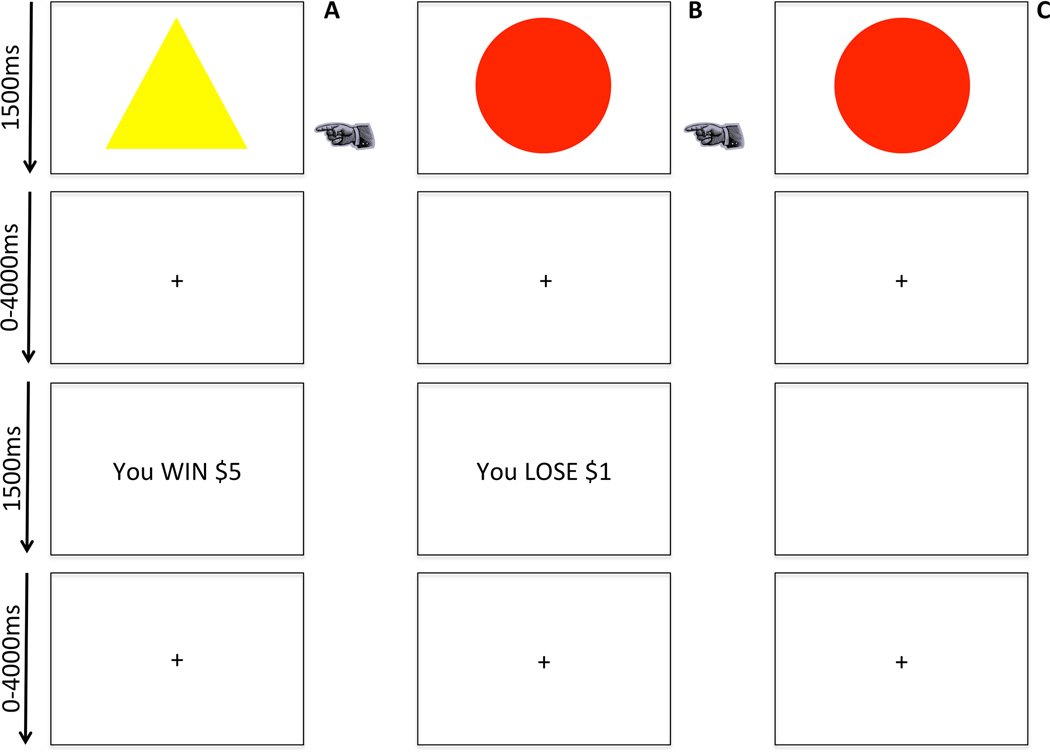
Participants completed a passive avoidance task where four images were presented (Figure 1). Trials began with a 1500ms image presentation, where participants either responded to the object or chose not to respond. The image presentation was followed by a randomly jittered fixation (0–4000ms) and by a 1500ms feedback presentation. Upon responding to an object, participants received one of four outcomes: win $5, win $1, lose $1 or lose $5. Each object could engender each of these outcomes. However, the feedback was probabilistic such that one object should result in a gain of $18.57 over 10 trials, one a gain of $7.14, one a loss of $18.57 and one a loss of $7.14. Feedback was followed by another randomly jittered fixation (0–4000ms). Participants completed 108 trials (36 trials/object) over 3 runs. Choosing not to respond resulted in no feedback.
Figure 1. The passive avoidance task.
Participants chose to respond or not respond to four objects. Reinforcement was probabilistic. Over the course of the task, the selection of two objects would result in profit, and selection of the other two objects would result in loss. In column A, a participant chooses to respond and receives rewarding feedback. In column B, a participant chooses to respond and receives punishing feedback. In column C, a participant refuses to respond and receives no feedback.
 = participant chooses object
= participant chooses object
A learning curve, based on participants’ behavioral data, was modeled establishing EVs and PEs for each object. EV for the first trial of each object was 0. PE was then calculated based on the feedback (F), which was coded 5 ($5 win), 1 ($1 win), −1 ($1 loss) or −5 ($5 loss) with the formula:
where the PE for the current trial(t) equaled the feedback value for the current trial minus the EV for the current trial. EV was updated via the following formula:
where the EV of the current trial (t) equals the EV of the previous trial (t−1) plus the PE of the previous trial multiplied by the learning rate (α). The learning rate of α=0.228 was established by taking the average value of the learning rate for each participant as established via a model-fitting procedure (see supplementary Appendix S1).
MRI parameters
Participants were scanned using a 1.5-Tesla Toshiba Vantage Titan scanner. A total of 93 functional images per run were taken with a gradient echo planar imaging (EPI) sequence (repetition time=3000 milliseconds; echo time=45 milliseconds; 64×64 matrix; 83° flip angle; 25cm field of view). Whole-brain coverage was obtained with 32 axial slices (thickness, 3mm; 1mm spacing; in-plane resolution, 3.91×3.91mm). A high-resolution anatomical scan (3-dimensional spoiled gradient recalled acquisition in a steady state, repetition time=12 milliseconds, echo time= 5 milliseconds, 256mm field of view, 20° flip angle, 78 axial slices, thickness, 2 mm, 256×256 matrix) in register with the EPI data set was obtained covering the whole brain.
Imaging data preprocessing
Imaging data were preprocessed and analyzed in Analysis of Functional Neuroimages (AFNI; Cox, 1996). At the individual level, functional images from the first 5 repetitions, collected prior to equilibrium magnetization, were discarded. Functional images from the 3 time series were motion corrected and spatially smoothed with a 6-mm full-width half-maximum Gaussian filter. All time series were normalized by dividing the signal intensity of a voxel at each point by the mean signal intensity of that voxel for each run and multiplying the result by 100. Resultant regression coefficients therefore represented a percentage of signal change from the mean.
Following this, four regressors were generated: objects chosen, objects refused, reward received, punishment received. Furthermore, the EV values during the choice-phase and PE values during the feedback-phase were used to modulate the percent signal change at each voxel and time point. All regressors were created by convolving the train of stimulus events with a gamma variate hemodynamic response function to account for the slow hemodynamic response. The participants’ anatomical scans were then individually registered to the Talairach and Tournoux atlas (Talairach & Tournoux, 1988). The individuals’ functional EPI data were then registered to their Talairach anatomical scan. Linear regression modeling was performed using the eight regressors described above plus regressors to model a first-order baseline drift function. This produced a modulated and unmodulated β coefficient and associated t statistic for each voxel and regressor.
fMRI data analysis
The modulated coefficients from the individual subject analyses were entered into a series of one-way Analyses of Covariance (ANCOVA). In the choice-phase, CP was entered as a covariate in a one-way (choice: approach, refuse) ANCOVA conducted on blood-oxygen-level-dependent (BOLD) data from the choice-phase modulated by EV. In the feedback-phase, CP was entered as a covariate in a one-way (feedback: rewarding, punishing) ANCOVA conducted on BOLD data from the feedback-phase modulated by PE. These analyses were repeated using CU traits as the covariate in place of CP levels. In any contrast where significant main effects or interactions involving CP or CU traits were observed, the ANCOVA was re-run using both CP and CU traits as covariates, in order to test whether the observed relationship was specific to the original covariate or was better accounted by the second covariate. The results of analyses using the un-modulated coefficients can be found in the online Appendix S1.
Results
Behavioral results
A 3 (run: 1–3) by 2 (error type: commission errors, omission errors) repeated-measures ANCOVA using CP as a covariate was conducted on the error data to test for the influence of CP on task performance over the three runs of the task. A main effect of CP was the only significant effect observed [F(1,70)=4.378, p=.040]. Greater CP was negatively associated with task performance [r=−.244, p=.039]. No significant main effects of error type [F(1,70)=.306, p=.582] or run [F(1,70)=1.113, p=.332] were observed. No significant effects of either the CP-by-error type, CP-by-run, error type-by-run or CP-by-error type-by-run interactions were observed [F(2,69)<1.000, p>.371]. When using CU traits as a covariate, a main effect of error type was the only significant effect observed [F(1,70)=6.337, p=.014]; there were significantly more commission than omission errors. There was no significant modulation of performance by CU traits either as a main effect [F(1,70)=1.302, p=.258] or in interaction with run or error type [F(1,70)<.794, p>.376].
To test the validity of the learning model, we examined the extent to which EV predicted whether a participant would respond to an object. Consistent with the model, there was a significant relationship between EV and choosing to respond [average correlation: r= .37, one-sample t-test (null: r=0), t=11.15, p<.01]. Furthermore, the degree to which EV predicted choosing to respond was negatively associated with CP [r= −.249, p=.035], but was unrelated to CU traits [r= −.179, p=.133].
fMRI results
The goal of the current study was to examine the extent to which either CP or CU symptom levels modulated the BOLD response associated with EV and/or PE signaling. Two one-way (Choice: approach, refuse) ANCOVAs were conducted on the EV modulated BOLD data from the choice-phase (one with CP and one with CU level as the covariate). Two one-way (Feedback: reward, punishment) ANCOVAs were conducted on the PE modulated BOLD data from the feedback-phase (one with CP and one with CU level as the covariate). Regions showing a main effect of choice and feedback are described in online Appendix S1.
Choice-phase data
ANCOVA on choice-phase data modulated by EV using CP levels as a covariate
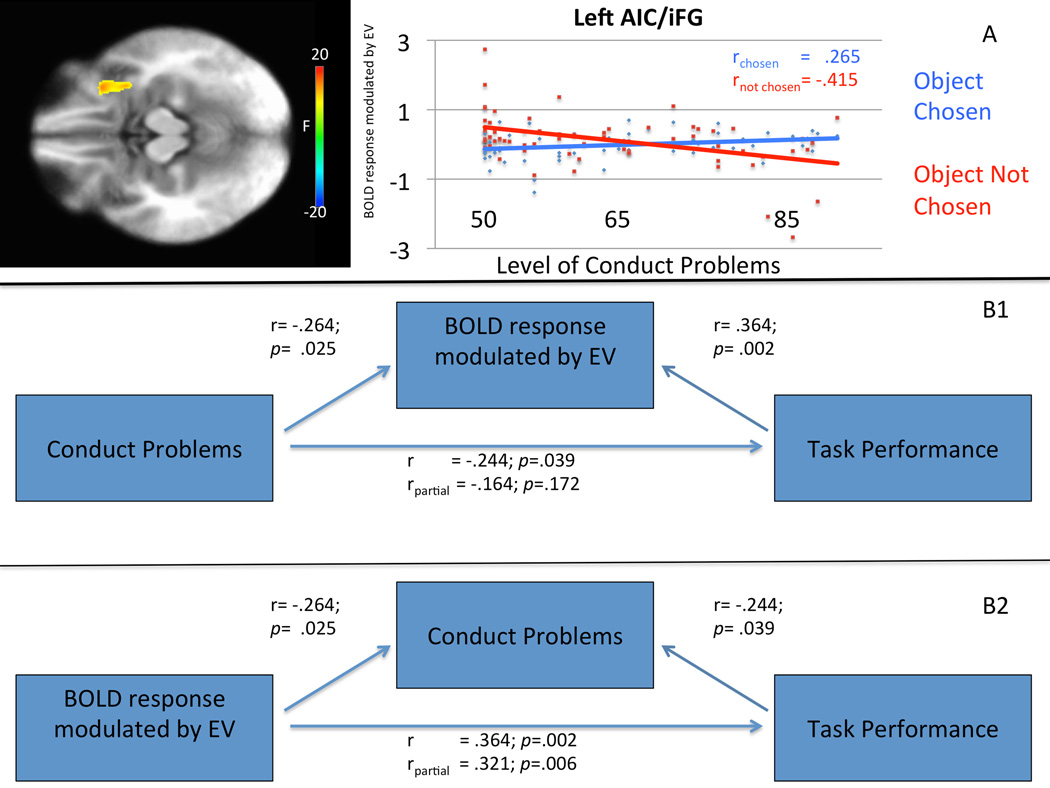
Significant choice-by-CP level interactions were observed within regions including bilateral AIC/iFG, striatum and right middle temporal/occipital gyrus (see Figure 2A/Table 2). In all regions, greater CP levels were associated with reduced modulation of activity by EV when refusing an object relative to choosing an object. In other words, individuals with higher CP symptom levels were poorer at using EV information to guide their avoidance behavior. A planned follow-up analysis was conducted including both CU traits and CP level as covariates simultaneously to test whether the observed relationship between CP levels and AIC/iFG activation remained significant when including CU traits in the model. Activation within bilateral AIC/iFG remained significant when CU traits were added as a covariate to the model. No regions showed a significant main effect of the CP level covariate.
Figure 2. Choice-by-conduct problem level interaction co-varying conduct problem levels in left anterior insula cortex/inferior frontal gyrus in 72 adolescents.
A. Greater conduct problem levels were associated with reduced modulation of activity by expected value when refusing an object relative to choosing an object in left anterior insula cortex/inferior frontal gyrus.
B1. The relationship between greater levels of conduct problems and poorer task performance was mediated by BOLD response modulated by expected value in left anterior insula cortex/inferior frontal gyrus.
B2. The relationship between BOLD response modulated by expected value in left anterior insula cortex/inferior frontal gyrus and poorer task performance was not mediated by level of conduct problems.
Table 2.
Brain regions demonstrating differential BOLD response modulated by expected value/prediction error co-varying conduct problems level during a passive avoidance task in 72 youth
| Coordinates of Peak Activation b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Regiona | Left/Right | BA | x | y | z | F | p | Voxels |
| Choice-Phase | ||||||||
| Conduct Problem Level-by-Choice Interaction | ||||||||
| AIC/iFG | Left | 47 | −31.5 | 16.5 | −15.5 | 17.09 | <.0001 | 46 |
| AIC/iFG | Right | 47 | 46.5 | 22.5 | −0.5 | 16.85 | .0001 | 22 |
| striatum | Right | 4.5 | 1.5 | 11.5 | 16.25 | .0001 | 75 | |
| middle temporal/occipital gyrus | Right | 19 | 37.5 | −79.5 | 20.5 | 13.47 | .0005 | 24 |
| Feedback-Phase | ||||||||
| Conduct Problem Level-by-Feedback Type Interaction | ||||||||
| cuneus | Left | 30 | −13.5 | −67.5 | 8.5 | 19.40 | <.0001 | 132 |
| Main Effect of Feedback Type | ||||||||
| medial frontal gyrus | Right | 6 | 22.5 | 4.5 | 50.5 | 13.57 | .0005 | 20 |
| superior frontal gyrus | Right | 6 | 13.5 | 22.5 | 50.5 | 17.23 | <.0001 | 19 |
| Main Effect of CU Traits | ||||||||
| superior temporal gyrus | Right | 22 | 58.5 | −40.5 | 17.5 | 17.10 | <.0001 | 18 |
According to the Talairach Daemon Atlas (http://www.nitrc.org/projects/tal-daemon/).
Based on the Tournoux & Talairach standard brain template, BA= Brodmann’s Area
AIC/iFG= anterior insula cortex/inferior frontal gyrus
ANCOVA on choice-phase data modulated by EV using CU traits as a covariate
No regions were identified showing either a choice-by-CU traits interaction or a main effect of the CU traits covariate.
Feedback-phase data
ANCOVA on feedback-phase data modulated by PE using CP levels as a covariate
A significant CP level-by-feedback type interaction was observed in left cuneus (Table 2). The association between levels of CP and modulated activation to rewarding feedback was larger and more positive than the relationship between CP levels and punishing feedback. A significant main effect of CP level was observed in right superior and medial frontal gyri. In both regions, greater levels of CP were associated with reduced modulated activation.
ANCOVA on feedback-phase data modulated by PE using CU traits as a covariate
No significant CU traits-by-feedback type interaction was observed; however, a significant main effect of CU traits was observed in right superior temporal gyrus, where greater levels of CU traits were associated with increased modulated activation.
Relationship of behavioral performance to modulated BOLD response
To test the extent to which atypical behavioral performance on the task might relate to atypical modulation of activity within bilateral AIC/iFG and striatum, we conducted three correlational analyses. Activation in neither striatum nor right AIC/iFG was associated with task performance [r=.121 & .181, p>.129]. However, a significant association between behavioral performance and modulated activation in left AIC/iFG [r=.364, p=.002] was observed. Given the significant correlations between task performance, BOLD response modulated by EV and CP, mediation analyses were conducted (Baron & Kenny, 1986). BOLD response modulated by EV in left AIC/iFG significantly mediated the relationship between CP and behavioral performance (see Figure 2B).
Discussion
The current study sought to determine the extent to which levels of CP and CU were associated with aberrant modulation of BOLD signal by EV and/or PE. There were four main findings. First, greater levels of CP, but not CU traits, were associated with poorer task performance. Second, greater levels of CP were associated with reduced representation of EV when not choosing relative to choosing an object in striatum and bilateral AIC/iFG. Third, greater representation of EV in left AIC/iFG was associated with improved task performance. Fourth, the representation of EV in left AIC/iFG mediated the relationship between CP and task performance.
Consistent with predictions, increasing levels of CP were associated with poorer behavioral performance. Similar to previous findings, greater CP was associated with both higher error rates (Blair et al., 2001; Budhani et al., 2006; Crowley et al., 2010; Finger et al., 2011; Finger et al., 2008; Rubia et al., 2009) and with a reduced relationship between EV and choosing an object (White et al., 2014; White et al., 2013). Also consistent with predictions, increasing levels of CP were associated with reduced representation of EV in bilateral AIC/iFG and striatum. It should be noted that the predicted inverse association between CP and representation of EV in dACC was only weakly observed at a less stringent threshold (p=.02; k=9). AIC/iFG, dACC and striatum have been implicated in the avoidance of sub-optimal choices (Budhani et al., 2007; Casey et al., 2001) as a function of EV (Kuhnen & Knutson, 2005; Liu et al., 2007). Notably, greater representation of EV in left AIC/iFG was associated with better task performance and this association mediated the relationship between CP and task performance. In our previous work, we have observed that patients with CP (diagnoses of ODD or CD) show significant impairment in the representation of EV within AIC/iFG when making avoidance responses. The current paper extends these findings by showing that the extent of this impairment relates to the severity of CP. Not all patients with ODD/CD show impairment in the representation of EV within AIC/iFG. However, for those that do, the extent of this impairment is associated with an exacerbation of the CP symptom set.
In contrast to the choice-phase data, predictions with respect to PE were not supported. CP levels were not associated with reduced representation of PE in vmPFC or striatum. However, it should be noted that no regions showed a significant main effect of feedback type. This is in contrast to previous work using a nearly identical paradigm (White et al., 2013). As such, while the current data suggest that individual differences in representation of PE in vmPFC/ striatum are not associated with severity of CP, it is possible that the failure to detect a relationship was associated with stochastic variables associated with this particular task variant or MRI scanner (e.g., a 1.5T rather than the 3T machines used in our previous work).
A significant feedback type-by-CP interaction was observed in visual cortex (cuneus/lingual gyrus) and a main effect of CP was observed in pre-motor cortex (medial and superior frontal cortex). A larger, more positive association between CP and PE was observed to rewarding feedback relative to punishing feedback in visual cortex, while increased CP was associated with generally reduced modulation of BOLD responses by PE within pre-motor cortex. Previous work has shown that EV/PE signaling is propagated to a number of brain regions, including visual and motor areas; however, it is argued that this signaling is part of generating appropriate behavioral choices, as opposed to the generation of EV/PE representations (see; O'Doherty, 2011). Given the absence of regions showing a significant effect of feedback-type and a lack of a priori hypotheses concerning pre-motor and visual cortices, these findings may represent type I error and should be interpreted with caution.
In contrast to level of CP, CU traits were not related either to behavioral performance or the representation of EV. This is consistent with two previous studies (White et al., 2014; White et al., 2013) and indicates that the previous absence of a significant relationship in this previous work did not reflect a type II error related to relatively limited Ns (<20) and/or limited variability of CU traits. While dysfunction in the amygdala’s response to distress cues, particularly fearful expressions, may be associated with increasing levels of the CU symptom set (e.g. Viding et al., 2012; White et al., 2012), dysfunction in the representation of EV does not appear to be.
Greater representation of PE was associated with increased levels of CU traits in superior temporal cortex. Temporal cortex has been implicated in the representation of PE (Spoormaker et al., 2011). However, this has generally been in conjunction with PE representation in vmPFC and striatum (O'Doherty, Buchanan, Seymour, & Dolan, 2006), which was not observed in the current study. Theoretical positions on psychopathy, of which CU traits are a facet (Frick & White, 2008), have suggested superior temporal cortex dysfunction (Anderson & Kiehl, 2012; Blair, 2008). Moreover, recent structural imaging work has identified a relationship between CU traits and reduced cortical thickness within a region of right superior temporal cortex (e.g. Wallace et al., 2014). However, a precise view regarding computations reliant on superior temporal cortex and how their dysfunction might lead to CU traits has not been provided. The current data reinforce the suggestion that the role of superior temporal cortex in CU traits needs greater attention.
One caveat should be considered with respect to the current results. As is often seen in the literature (c.f. Frick & Dickens, 2006), level of CU traits and conduct problems were very highly correlated in the current sample (r=0.78). This might suggest that either they are both consequent on the same underlying mechanisms or that it is necessary to proceed cautiously when distinguishing the dysfunctional processes underlying each. In some cases, this can be done by testing for a suppressor effect (e.g. Lozier, Cardinale, VanMeter, & Marsh, 2014). A suppressor effect exists where two positively correlated predictor variables have opposing correlations with the criterion variable (e.g. P1 positively correlates with P2, P1 positively correlates with C, P2 negatively correlates with C; Ludlow & Klein, 2014). In the current study, however, as both predictor variables (CP & CU) were expected to positively correlate with the neural criterion variable, testing for a suppressor effect would not be appropriate. The concern in the current study was that CU traits and CP would be two symptoms sets stemming from the same underlying pathophysiology. For this reason, we adopted an approach of conducting two ANCOVAs, one examining the relationship between level of CU traits and the other the level of CP and BOLD responses during reinforcement-based decision-making. If the same (or very similar) underlying dysfunction gave rise to both CU traits and CP, then the results of both ANCOVAs should have been very similar. Instead, greater dysfunction in EV representation in AIC/iFG is associated with greater CP and no relationship was found between atypical BOLD responses and level of CU traits. Moreover, the observed relationship between greater dysfunction in EV representation in AIC/iFG and greater CP remained significant even when conducting an ANCOVA using both level of CP and level of CU traits as covariates, suggesting that CU traits do not mediate the relationship between EV representation in AIC/iFG and CP.
These findings have clinical implications. They reinforce arguments to conceptualize psychiatric disorders dimensionally with reference to underlying neurobiological mechanisms rather than as categorical syndromes of co-occurring behaviors (Cuthbert & Insel, 2013; Insel & Cuthbert, 2015). The current study has identified a mechanism sensitive to EV information and involved in avoidance responses that, when dysfunctional, is associated with the CP symptom set. As such this mechanism can be considered a treatment target. We predict that if this treatment target is successfully addressed, there will be a corresponding reduction in CP (though not CU) symptoms. Moreover, while patients with ODD or CD may show impairment in the representation of EV within AIC/iFG, not all show the same level of impairment. Similarly, not all patients with ODD or CD show impairment in the amygdala’s response to distress cues, though severity of impairment is associated with increasing levels of CU traits and an increased risk for instrumental aggression (Lozier et al., 2014; Viding et al., 2012; White et al., 2012). In short, these data reinforce calls to develop individualized treatment approaches for psychiatric symptoms sets based on an individual’s specific neurocognitive impairments as opposed to treating groups of patients based on diagnostic labels (Cuthbert & Insel, 2013; Insel & Cuthbert, 2015).
Conclusion
In summary, the current data add to the evidence indicating that CP are associated with problems in representing EV during decision-making. Specifically, reduced modulation of AIC/iFG when making avoidance responses is associated with greater levels of CP and mediated the relationship between level of CP and poorer task performance. Furthermore, atypical avoidance responses appear to be un-related to levels of CU traits. Thus, EV signaling when making avoidance decisions represents a treatment target for youth with CP.
Supplementary Material
Key points.
Youth with conduct problems (CP) exhibit poor decision-making skills and difficulty representing expected value (EV).
This study found that greater levels of CP were associated with poorer task performance and reduced representation of EV when not choosing relative to choosing an object in striatum and bilateral anterior insula cortex/inferior frontal gyrus (AIC/iFG).
Additionally, the representation of EV in left AIC/iFG mediated the relationship between CP and task performance.
EV signaling when making avoidance decisions represents a treatment target common to youth with CP.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (1-ZIA-MH002860), J.R.B principle investigator. This study was conducted under protocol number 05-M-0105, with ClinicalTrials.gov Identifier NCT00104039. S.W. and J.R.B. had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflicts of interest statement: No conflicts declared.
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Model-fitting.
Table S1. Brain regions demonstrating differential un-modulated BOLD response co-varying conduct problems level during the choice-phase of a passive avoidance task in 72 youth.
Table S2. Brain regions demonstrating differential un-modulated BOLD response co-varying callous-unemotional traits during the choice-phase of a passive avoidance task in 72 youth.
Table S3. Brain regions demonstrating differential un-modulated BOLD response co-varying conduct problem levels during the feedback-phase of a passive avoidance task in 72 youth.
Table S4. Brain regions demonstrating differential un-modulated BOLD response co-varying callous-unemotional traits during the feedback-phase of a passive avoidance task in 72 youth.
References
- Achenbach TM. The Achenbach System of Empirically Based Assessment (ASEBA): Development, Findings, Theory and Applications. Burlington, VT: University of Vermont Research Center for Children, Youth and Families; 2009. [Google Scholar]
- Achenbach TM, Dumenci L, Rescorla LA. DSM-oriented and empirically based approaches to constructing scales from the same item pools. J Clin Child Adolesc Psychol. 2003;32(3):328–340. doi: 10.1207/S15374424JCCP3203_02. [DOI] [PubMed] [Google Scholar]
- Anderson NE, Kiehl KA. The psychopath magnetized: insights from brain imaging. Trends Cogn Sci. 2012;16(1):52–60. doi: 10.1016/j.tics.2011.11.008. doi: S1364-6613(11)00241-5 [pii] 10.1016/j.tics.2011.11.008 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Fine cuts of empathy and the amygdala: dissociable deficits in psychopathy and autism. Q J Exp Psychol (Hove) 2008;61(1):157–170. doi: 10.1080/17470210701508855. [DOI] [PubMed] [Google Scholar]
- Blair RJ, White SF, Meffert H, Hwang S. Disruptive behavior disorders: taking an RDoC(ish) approach. Curr Top Behav Neurosci. 2014;16:319–336. doi: 10.1007/7854_2013_247. [DOI] [PubMed] [Google Scholar]
- Blair R, J R. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences. 2007;11(9):387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Blair R, J R, Colledge E, Mitchell D, G V. Somatic markers and response reversal: Is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? Journal of Abnormal Child Psychology: An official publication of the International Society for Research in Child and Adolescent Psychopathology. 2001;29(6):499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34(4):1754–1765. doi: 10.1016/j.neuroimage.2006.08.060. doi: S1053-8119(06)00919-0 [pii] 10.1016/j.neuroimage.2006.08.060 [doi] [DOI] [PubMed] [Google Scholar]
- Budhani S, Richell RA, Blair RJ. Impaired reversal but intact acquisition: probabilistic response reversal deficits in adult individuals with psychopathy. J Abnorm Psychol. 2006;115(3):552–558. doi: 10.1037/0021-843X.115.3.552. doi:2006-09167-016 [pii] 10.1037/0021-843X.115.3.552 [doi] [DOI] [PubMed] [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, Noll DC. Sensitivity of prefrontal cortex to changes in target probability: A functional MRI study. Human brain mapping. 2001;13(1):26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clithero JA, Rangel A. Informatic parcellation of the network involved in the computation of subjective value. Soc Cogn Affect Neurosci. 2014;9(9):1289–1302. doi: 10.1093/scan/nst106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. doi: S0010480996900142 [pii] [DOI] [PubMed] [Google Scholar]
- Crowley TJ, Dalwani MS, Mikulich-Gilbertson SK, Du YP, Lejuez CW, Raymond KM, Banich MT. Risky decisions and their consequences: Neural processing by boys with antisocial substance disorder. PLoS ONE. 2010;5(9) doi: 10.1371/journal.pone.0012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, Blair RJ. Disrupted reinforcement signaling in the orbitofrontal cortex and caudate in youths with conduct disorder or oppositional defiant disorder and a high level of psychopathic traits. Am J Psychiatry. 2011;168(2):152–162. doi: 10.1176/appi.ajp.2010.10010129. doi: appi.ajp.2010.10010129 [pii] 10.1176/appi.ajp.2010.10010129 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger EC, Marsh AA, Mitchell DG, Reid ME, Sims C, Budhani S, Blair JR. Abnormal ventromedial prefrontal cortex function in children with psychopathic traits during reversal learning. Arch Gen Psychiatry. 2008;65(5):586–594. doi: 10.1001/archpsyc.65.5.586. doi:65/5/586 [pii] 10.1001/archpsyc.65.5.586 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick . The Inventory of Callous-Unemotional Traits. New Orleans: Department of Psychology. University of New Orleans; 2004. Unpublished Ratings Scale. [Google Scholar]
- Frick, Dickens Current perspectives on conduct disorder. Curr Psychiatry Rep. 2006;8(1):59–72. doi: 10.1007/s11920-006-0082-3. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Ray JV, Thornton LC, Kahn RE. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological bulletin. 2014;140(1):1–57. doi: 10.1037/a0033076. [DOI] [PubMed] [Google Scholar]
- Frick PJ, White SF. Research review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry. 2008;49(4):359–375. doi: 10.1111/j.1469-7610.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN. Medicine. Brain disorders? Precisely. Science. 2015;348(6234):499–500. doi: 10.1126/science.aab2358. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. doi: S0896-6273(05)00657-4 [pii] 10.1016/j.neuron.2005.08.008 [doi] [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE. Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. J Neurosci. 2007;27(17):4587–4597. doi: 10.1523/JNEUROSCI.5227-06.2007. doi:27/17/4587 [pii] 10.1523/JNEUROSCI.5227-06.2007 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71(6):627–636. doi: 10.1001/jamapsychiatry.2013.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow L, Klein K. Suppressor variables: The difference between 'is' versus 'acting as'. Journal of Statistics Education. 2014;22(2):1–19. [Google Scholar]
- O'Doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Curr Opin Neurobiol. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. doi: S0959-4388(04)00168-0 [pii] 10.1016/j.conb.2004.10.016 [doi] [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann N Y Acad Sci. 2011;1239:118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Buchanan TW, Seymour B, Dolan RJ. Predictive neural coding of reward preference involves dissociable responses in human ventral midbrain and ventral striatum. Neuron. 2006;49(1):157–166. doi: 10.1016/j.neuron.2005.11.014. doi: S0896-6273(05)00960-8 [pii] 10.1016/j.neuron.2005.11.014 [doi] [DOI] [PubMed] [Google Scholar]
- O'Doherty JP, Hampton A, Kim H. Model-based fMRI and its application to reward learning and decision making. In: Balleine BW, Doya K, O'Doherty J, Sakagami M, editors. Reward and decision making in corticobasal ganglia networks. Malden: Blackwell Publishing; 2007. pp. 35–53. [Google Scholar]
- Rescorla, Wagner . A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black A, Prokasy W, editors. Classical Conditioning II: Current Research and Theory. II. New York: Appleton Crofts; 1972. pp. 64–99. [Google Scholar]
- Rubia K, Smith AB, Halari R, Matsukura F, Mohammad M, Taylor E, Brammer MJ. Disorder-specific dissociation of orbitofrontal dysfunction in boys with pure conduct disorder during reward and ventrolateral prefrontal dysfunction in boys with pure ADHD during sustained attention. The American Journal of Psychiatry. 2009;166(1):83–94. doi: 10.1176/appi.ajp.2008.08020212. [DOI] [PubMed] [Google Scholar]
- Schutter DJ, van Bokhoven I, Vanderschuren LJ, Lochman JE, Matthys W. Risky decision making in substance dependent adolescents with a disruptive behavior disorder. Journal of abnormal child psychology. 2011;39(3):333–339. doi: 10.1007/s10802-010-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, Andrade KC, Schroter MS, Sturm A, Goya-Maldonado R, Samann PG, Czisch M. The neural correlates of negative prediction error signaling in human fear conditioning. Neuroimage. 2011;54(3):2250–2256. doi: 10.1016/j.neuroimage.2010.09.042. doi: S1053-8119(10)01223-1 [pii] 10.1016/j.neuroimage.2010.09.042 [doi] [DOI] [PubMed] [Google Scholar]
- Talairach, Tournoux . Co-planar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, McCrory EJ. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am J Psychiatry. 2012;169(10):1109–1116. doi: 10.1176/appi.ajp.2012.12020191. [DOI] [PubMed] [Google Scholar]
- Wallace GL, White SF, Robustelli B, Sinclair S, Hwang S, Martin A, Blair RJ. Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(4):456–465. e451. doi: 10.1016/j.jaac.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- White SF, Fowler KA, Sinclair S, Schechter JC, Majestic CM, Pine DS, Blair RJ. Disrupted expected value signaling in youth with disruptive behavior disorders to environmental reinforcers. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(5):579–588. e579. doi: 10.1016/j.jaac.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio C, Pope K, Blair RJ. Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to nonemotional features. Am J Psychiatry. 2012;169(7):750–758. doi: 10.1176/appi.ajp.2012.11081270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SF, Pope K, Sinclair S, Fowler KA, Brislin SJ, Williams WC, Blair RJ. Disrupted expected value and prediction error signaling in youths with disruptive behavior disorders during a passive avoidance task. Am J Psychiatry. 2013;170(3):315–323. doi: 10.1176/appi.ajp.2012.12060840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.