Abstract
Objective
Recent studies suggesting clinical superiority of linezolid over vancomycin in the treatment of methicillin-resistant Staphylococcus aureus (MRSA) pneumonia led to a change in our institution’s clinical pathway/order form for hospital-acquired pneumonia, positioning linezolid as the preferred agent. Our objective was to assess the impact of this change within our institution.
Design
Retrospective electronic medical records review
Methods
The analysis for this observational study included eligible patients admitted to our medical center between May 1, 2011 and August 31, 2014 , with ICD-9 codes for MRSA and pneumonia. Included patients were at least 18 years of age and had vancomycin or linezolid initiated at least 2 days after admission and continued for at least 2 consecutive days. The primary endpoints were extent of antibiotic use before and after order form change and length of stay (LOS) and hospital charges in the two treatment groups. A secondary aim was to detect any gross discrepancies in patient outcomes such as treatment duration, mechanical ventilation duration, all-cause mortality rate, nephrotoxicity, and 30-day readmission between the two treatment groups.
Measurements and Main Results
Outcomes in 227 patients were assessed. Linezolid use increased 16.2% subsequent to the change in the order form. Although not statistically significant, the median hospital admission charge was $6,200 lower in patients treated with linezolid compared with those treated with vancomycin ($25,900 vs. $32,100). Hospital LOS was significantly associated with Charlson comorbidity index (P < 0.001), the type of treatment (p = 0.032), duration of treatment (p < 0.001), mechanical ventilation (p < 0.001), and ICU admission (p < 0.001). All-cause mortality favored linezolid treatment and these patients were more likely to be discharged (shorter LOS).
Conclusions
Although linezolid use increased markedly with this pathway/order form change, no negative institutional consequences or unfavorable patient outcomes were detected, justifying the change in policy from these perspectives.
Keywords: linezolid, methicillin-resistant staphylococcus aureus, pneumonia, vancomycin
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the most frequently identified pathogens associated with nosocomial pneumonia1, the second most prevalent nosocomial infection in the United States.2 The most recent Infectious Disease Society of America (IDSA) MRSA infection clinical practice guidelines recommend either intravenous vancomycin or oral/intravenous linezolid for the treatment of MRSA pneumonia.3 Vancomycin has been the standard of care for nosocomial MRSA pneumonia infections, but several recent studies have suggested that linezolid may be superior to vancomycin in treating these infections4–7, which could be due to suboptimal penetration of vancomycin into the lungs at therapeutic doses.8, 9
Based on these recent studies, our corresponding institutional clinician order forms were modified at our academic medical center in May 2013 to place linezolid physically above vancomycin as an antibiotic choice for the empiric treatment of adult hospital-acquired pneumonia, ventilator-associated pneumonia, and healthcare-associated pneumonia, hereafter collectively referred to as hospital-associated pneumonia (HAP), connoting preference. The selection of either agent on the order form allows 72 hours of empiric usage, beyond which a new order is required. Additional use criteria of either agent beyond 72 hours is restricted to patients with MRSA cultured from sputum, mini-bronchoalveolar lavage (mBAL), or BAL cultures, approval from a formal infectious diseases consultation, or approval from the Antimicrobial Stewardship Team.
The goal of this retrospective cohort research study was to evaluate the impact of utilizing linezolid as the preferred agent for treating MRSA pneumonia within our institution and to detect any gross signals of suboptimal patient outcomes due to a shift in antibiotic choice.
Methods
Study Design and Setting
This was a single-center, retrospective, cohort study conducted via electronic medical record system review of all patients with known or suspected MRSA pneumonia from May 1, 2011 to August 31, 2014. In May 2013, linezolid was moved above vancomycin on the HAP order form and a 72-hour stop time was added for both agents to encourage de-escalation of therapy in instances when patients presented with negative cultures. By collecting data from both before and after the modification, we were able to associate the utilization data with the change in order form. The study was reviewed and approved by the Institutional Review Board for Human Research at the Medical University of South Carolina (MUSC). MUSC is a tertiary and quaternary academic medical center and South Carolina’s only Level 1 trauma center, has over 700 licensed beds, an average daily census of 627, and over 36,000 annual inpatient admissions. The average adult inpatient length of stay (LOS), age, and case-mix index are 6.2 days, 52 years, and 1.9642, respectively. Per medical standards in our institution, the target serum trough vancomycin concentration for MRSA pneumonia is generally 15–20 μg/mL. Vancomycin dosing and concentrations are routinely monitored by clinical pharmacy staff and recommendations for dosing are routinely provided and implemented to achieve these target concentrations.
Patient Population
Adult patients of at least 18 years of age admitted to MUSC between May 1, 2011 and August 31, 2014 were included in the study. Patients with MRSA pneumonia were identified as those with an International Classification of Diseases (ICD)-9 code for both MRSA (038.12, 041.12, 482.42, or V09.0) and pneumonia (482.40, 482.41, 482.42, 482.49, 482.89, 482.9, 484.8, 485, 486, 510.0, 510.9, 513.0, or 513.1), because ICD-9 does not include a code that specifically defines MRSA pneumonia. Eligible patients had vancomycin or linezolid initiated at least 2 days after admission and had received a minimum of 2 consecutive days of therapy. Exclusion criteria included mortality within 48 hours of either linezolid or vancomycin initiation, receipt of both linezolid and vancomycin during their admission (either concurrently or switched from one to the other). Additional exclusion criteria included or exposure to other antibiotic agents with anti-MRSA activity, including trimethoprim-sulfamethoxazole, clindamycin, minocycline, doxycycline, tetracycline, tigecycline, daptomycin, quinupristin/dalfopristin, ceftaroline, telavancin, rifampin, nitrofurantoin, during hospital admission.
Outcomes of Interest
The primary endpoints reflecting institutional consequences were the extent of overall antibiotic utilization (i.e., all linezolid and vancomycin use regardless of indication) before and after the order-form change, as well as median LOS and total hospital charges in patients who received either linezolid or vancomycin independent of temporal relationship with order form change. Linezolid and vancomycin use during the second quarter of 2013 was considered the pre-order form change baseline; usage in the second quarter of 2014 was considered the post-implementation quarterly amount. Secondary outcomes such as ICU LOS, treatment duration, mechanical ventilation duration, nephrotoxicity (defined as a 0.5-mg/mL increase or a 50% increase from baseline, serum creatinine measured before vancomycin treatment initiation), thrombocytopenia (defined as platelet count below 150,000 cells/mm3 or a 50% decrease from baseline platelet count [platelet count measured before linezolid treatment initiation]), all-cause mortality rate, 30-day readmission, and total hospital charges were evaluated between treatment groups, independently of temporal relationship to order form change, to identify if the order-form change had an impact on patient outcomes.
Data Collection
Antibiotic utilization data were obtained from the Department of Pharmacy Services’ drug use database, which reflects doses administered. Use was converted to census-normalized defined daily doses, which, for our purposes were 3 and 1.2 grams for vancomycin and linezolid, respectively. Total hospital charges were obtained through billing records from our electronic medical record system only, and do not represent the final reimbursement or negotiated charge. The following data were also captured from electronic medical records: gender, age, ethnicity, comorbidities, facility-administered medications (including all antibiotics administered during admission), hospital LOS, duration and dose of linezolid/vancomycin therapy, platelet count, serum creatinine, ICU LOS, days on mechanical ventilation, occurrence of readmission within 30 days, and duration from admission to death (if applicable). Charlson comorbidity index (CCI) was determined for each patient.
Statistical Analysis
For the outcome of antibiotic utilization before and after the order form was changed, descriptive statistics (percent change) were used. Hospital charges associated with the anti-MRSA agent received were compared between patients receiving linezolid and vancomycin using the Wilcoxon rank sum test. Prior to examining the association between treatment options and different patient outcomes, the associations between the treatments and all potential predictor variables were evaluated using chi-square tests (categorical variables) and two sample t-tests or Wilcoxon rank sum test (continuous variables), as appropriate, to determine if the treatment groups were similar. All statistical assumptions were checked graphically and non-parametric tests were used when assumptions were not met. Hospital LOS was considered as time-to-event data and was analyzed using the competing risk regression approach. A competing risk regression approach was utilized for these outcomes, treating death as a competing risk because LOS could not be quantified in subjects who died. All predictors with a univariate p-value < 0.20 for the association with hospital LOS were considered in multivariate competing risk regression models. Step-wise selection was used to select the final multivariate models for each outcome, retaining all variables significant at p < 0.05 in the multivariate model. Univariate associations between mortality or 30-day readmission and all categorical variables were evaluated using chi-square tests and associations with continuous variables were evaluated using 2-sample t-tests or Wilcoxon rank sum tests as appropriate. For readmission within 30 days, patients that expired during their first hospital stay were excluded as they could not be readmitted to the hospital.
Univariate associations between time to death and all candidate predictor variables were evaluated using a series of Cox proportional hazards models to control for potential confounders. In these models, patients for whom time to death was not observed were treated as censored with time-to-event equivalent to their hospital LOS. All predictors with a univariate p-value < 0.20 were considered in the multivariate competing risk, logistic, or Cox regression model. Stepwise selection was used to select the final multivariate models for each outcome, retaining all variables significant at p < 0.05 in the multivariate model. The proportional hazards assumption was checked using the Grambsch-Therneau test and transformations were considered for variables for which the proportional hazards assumption was not met. All analyses were conducted using SAS 9.3 (Cary, NC).
Results
Patient Characteristics
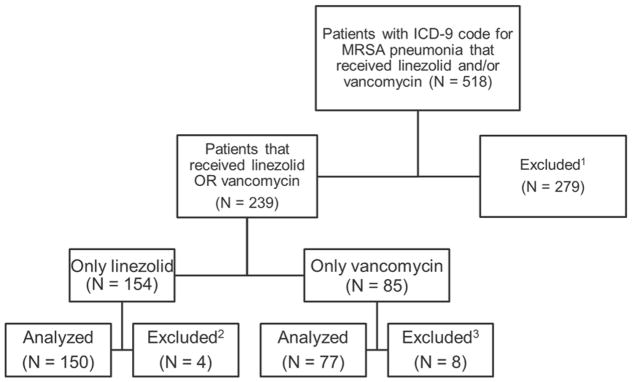
We analyzed data from 518 patients, of whom 227 met the inclusion criteria, with 150 (66%) patients receiving linezolid and 77 (34%) patients receiving vancomycin. Patients were excluded due to either concomitant or sequential treatment with other anti-MRSA agents or with concomitant or sequential linezolid and vancomycin therapy (n = 270) or due to failure to meet the minimum duration requirement of linezolid or vancomycin therapy (n = 12; Figure 1). Baseline characteristics of patients included in the study are presented in Table 1. The median age, gender, ethnicity, CCI, percent ICU admissions, and percent of patients on mechanical ventilation were similar in the two study groups. The most frequently identified comorbidities among patients included in this study were coronary artery disease, hypertension, diabetes, and asthma. Although CCI was identical in both groups, patients receiving linezolid had a narrower range of values compared with patients receiving vancomycin (0–9 vs. 0–13).
Figure 1.
Study Flow.
1 Excluded due to concurrent therapy of linezolid and vancomycin and/or exposure to other anti-MRSA antibiotics during hospital admission
2Excluded due to mortality within 48 hours of linezolid initiation and/or received less than 2 consecutive days of therapy
3Excluded due to mortality within 48 hours of vancomycin initiation and/or received less than 2 consecutive days of therapy
Table 1.
Baseline Demographics and Clinical Characteristics by Study Group.
| Characteristics | Linezolid (n = 150) | Vancomycin (n = 77) | P value |
|---|---|---|---|
| Age, mean (median, range) | 56.5 (61, 18–92) | 56.6 (60, 19–93) | 0.982 |
| Gender (% Male) | 73 (48.6%) | 37 (48.1%) | 0.930 |
| Ethnicity (% Caucasian) | 76 (50.7%) | 41 (53.3%) | 0.713 |
| Charlson Comorbidity Index, median (standard deviation, range) | 2 (2.1, 0–9) | 2 (2.8, 0–13) | 0.160 |
| ICU admission, n (%) | 73 (48.6%) | 37 (48.1%) | 0.930 |
| Mechanical ventilation, n (%) | 57 (38.0%) | 28 (36.4%) | 0.809 |
Institutional Consequences
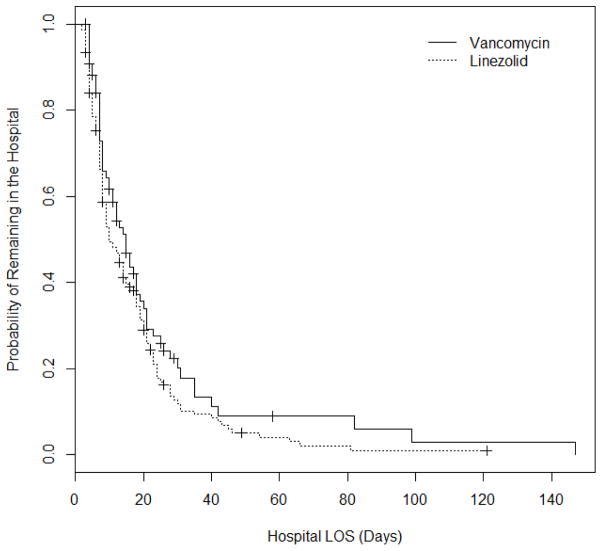
The change in antibiotic usage is presented in Table 2. Assessment of antibiotic usage between pre-implementation and the end of study period revealed that linezolid use increased 16.2%, while vancomycin use (all indications) decreased 2.5%. As for total hospital charges, linezolid-treated patients accrued a median charge of $25,900 (range: $4,240-$495,400) while vancomycin-treated patients accrued a median charge of $32,100 (range: $5,790–$437,700) during admission, although the difference was not statistically significant (p = 0.311). The difference in hospital LOS was not statistically significant (p = 0.318). However, when treated as time-to-event data in the initial statistical analysis, likelihood of discharge (time to discharge) was significantly associated with linezolid treatment (p = 0.032).
Table 2.
Pre-implementation and post-implementation utilization of linezolid and vancomycin.
| Time Period | Linezolid (DDD/1000PD) | Vancomycin (DDD/1000PD) |
|---|---|---|
| 2013 (2nd Quarter) | 50.3 | 160.7 |
| 2014 (2nd Quarter) | 58.5 | 156.7 |
| Change (%) | 16.2% | −2.5% |
DDD/1000PD: defined daily doses per 1000 patient days
Patient Outcomes
Results of the initial patient outcomes analysis are presented in Table 3. No significant difference was observed across the two treatment groups in any of the outcomes of interest except for all-cause mortality, occurring in 10% of linezolid- and 19.5% of vancomycin-treated patients (p = 0.046) at the end of hospitalization. Table 4 presents the subdistribution hazard ratios (SHR) for probability of remaining in the hospital estimated from univariate and multivariate competing risk regression models of hospital LOS. When hospital LOS was evaluated as a time-to-event outcome, patients that were treated with linezolid had a significantly higher likelihood of being discharged, inferring a shorter hospital stay. We did not find a significant univariate or multivariate association between hospital LOS and age, gender, ethnicity, or the occurrence of thrombocytopenia or nephrotoxicity. However, both models showed that hospital LOS was significantly associated with CCI, the type of treatment, duration of treatment, mechanical ventilation, and ICU admission, meaning that generally sicker patients stayed in the hospital longer.
Table 3.
Comparison of Patient Outcomes
| Characteristics | Linezolid (N = 150) | Vancomycin (N = 77) | P value |
|---|---|---|---|
| Hospital LOS, median days (range) | 10 (2–121) | 12 (3–147) | 0.318 |
| ICU LOS in all patients, median days (range) | 0 (0–28) | 0 (0–26) | 0.584 |
| ICU LOS in patients admitted to ICU, median days (range) | 4 (1–28) | 4 (1–26) | 0.199 |
| Mechanical ventilation days in all patients, median (range) | 0 (0–100) | 0 (0–73) | 0.627 |
| Mechanical ventilation days in mechanical ventilated patients, median (range) | 10 (2–100) | 7 (1–73) | 0.335 |
| Thrombocytopenia, n (%) | 8 (5.3%) | 3 (3.9%) | 0.754 |
| Nephrotoxicity, n (%) | 5 (3.3%) | 6 (8.9%) | 0.098 |
| 30-day readmission, n (%) | 31 (20.7%) | 14 (18.2%) | 0.657 |
| Time to readmission if readmitted, median (range) | 44.5 (2–1337) | 38 (2–933) | 0.464 |
| Mortality, n (%) | 15 (10.0%) | 15 (19.5%) | 0.046 |
| Time to death (if occurred), median days (range) | 14 (3–121) | 12 (3–58) | 0.789 |
| Duration of treatment, median days (range) | 4 (2–60) | 4 (2–38) | 0.894 |
| Hospital charge, median $ (range) | 25,900 (4,240–495,400) | 32,100 (5,790–437,700) | 0.311 |
Table 4.
Subdistribution Hazard Ratios (SHR) for Probability of Discharging from the Hospital Estimated from Univariate and Multivariate Competing Risk Regression Models of Hospital LOS.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Predictor | SHR (95% CI) | P value | SHR (95% CI) | P value |
| Treatment (linezolid vs. vancomycin) | 1.38 (1.03, 1.84) | 0.032 | 1.59 (1.11, 2.30) | 0.012 |
| CCI (1 point increase) | 0.78 (0.71, 0.86) | <0.001 | 0.82 (0.74, 0.91) | <0.001 |
| Admission to the ICU (Yes vs. No) | 0.31 (0.22, 0.43) | <0.001 | 0.52 (0.33, 0.81) | 0.004 |
| Use of a ventilator (Yes vs. No) | 0.34 (0.25, 0.46) | <0.001 | 0.55 (0.34, 0.88) | 0.013 |
| Duration of MRSA therapy (1 day increase) | 0.96 (0.93, 0.98) | <0.001 | N/A | |
Approximately 13% (n = 30/227) of study patients died prior to being discharged from the hospital. Regardless of treatment type, mortality was not associated with patient age, gender, ethnicity, occurrence of thrombocytopenia or nephrotoxicity, duration of therapy, or CCI score. Regardless of treatment type, both the univariate and multivariate regression models indicated that the odds ratio for mortality was positively associated with the use of vancomycin and admission to the ICU (Table 5). The final multivariate model included only treatment type and ICU admission, due to the small number of deaths observed. The results showed that patients who received vancomycin had a 52% increase in the odds of dying relative to patients on linezolid, controlling for ICU admission (p = 0.040, 95% CI, 1.02–2.28), and patients admitted to the ICU had 2.3 times the odds of dying relative to patients not admitted to the ICU, controlling for therapy type (p < 0.001, 95% CI 1.44–3.71). None of the patient covariates in the data were significantly associated with 30-day readmission in the univariate model.
Table 5.
Odds ratios (OR) for probability of dying estimated from univariate and multivariate logistic regression models of mortality.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Predictor | OR (95% CI) | P value | OR (95% CI) | P value |
| Treatment (vancomycin vs. linezolid) | 2.18 (1.00, 4.73) | 0.046 | 1.52 (1.02, 2.28) | 0.040 |
| Admission to the ICU (Yes vs. No) | 2.27 (1.42, 3.63) | <0.001 | 2.31 (1.44, 3.71) | <0.001 |
| Use of a ventilator (Yes vs. No) | 2.20 (1.45, 3.34) | <0.001 | N/A | N/A |
Discussion
This retrospective analysis assessed the extent of antibiotic utilization following the change of preferential antibiotic agent, as well as hospital charges and length of stay associated with treatment type for known or suspected MRSA pneumonia at our institution. In addition, although the intent of this analysis was not to perform a robust comparative efficacy analysis of linezolid and vancomycin but knowing that the change would increase the number of patients treated with linezolid, we were interested if treatment type was associated with any obvious, negative impact on patient outcomes. Although the change resulted in markedly increased linezolid usage, total hospital charges were not significantly different. These observations, along with an apparent increased risk of mortality with vancomycin treatment and shorter length of stay for linezolid-treated patients, suggest that aside from likely increases in the pharmacy budget, the change did not result in immediate negative consequences to the institution or patients.
The change in our protocol was based mainly on a study which compared the safety and efficacy of linezolid and putatively dose-optimized vancomycin for the treatment of nosocomial MRSA pneumonia.4 The study assessed clinical efficacy following end of treatment and at end of study. Linezolid-treated patients had a higher rate of microbiologic success (81.9% vs 60.6%) and clinical cure (57.6% vs 46.6%), as well as a lower incidence of nephrotoxicity (8.4% v. 18.2%) in comparison with those treated with vancomycin. Since the initiation of our analysis, additional studies comparing linezolid and vancomycin have appeared in the literature using different methods of comparison, some with conflicting results. Another study compared the clinical success of linezolid versus vancomycin for the treatment of ventilator-associated pneumonia (VAP) due to MRSA.5 This was a retrospective observational study that included patients with culture-confirmed MRSA, and clinical success was defined as the improvement or resolution of signs and symptoms of pneumonia by day 14 of diagnosis. VAP patients with MRSA were more likely to experience clinical success (85% vs 69%) when treated with linezolid versus vancomycin, although differences between the two treatment groups were not statistically significant with respect to secondary outcomes including mortality, days on mechanical ventilation, ICU LOS, hospital LOS, and safety profile (e.g., nephrotoxicity, thrombocytopenia, and anemia). A meta-analysis included nine randomized trials and more than 4000 patients; the authors found no statistical difference between linezolid and vancomycin on mortality or clinical response.10 Another meta-analysis included nine randomized trials involving nearly 3000 patients found linezolid to be comparable with vancomycin for clinical cure in treating nosocomial pneumonia, including those cases caused by MRSA.11 Using a national health system database, a study compared the effectiveness of linezolid with that of vancomycin in more than 5,000 adult patients.6 The investigators observed similar individual clinical outcomes among MRSA pneumonia patients treated with linezolid compared with those treated with vancomycin, but found linezolid-treated patients to have a higher composite outcome of clinical success. Thus, the question of comparative efficacy of vancomycin vs linezolid for this indication may not yet be settled. Our results therefore provide additional perspective to other healthcare systems considering preferred treatment.
In our analysis, we observed a change in antibiotic usage between pre-order form change implementation and the end of observation period, in which linezolid use increased approximately 16% while vancomycin use decreased 2.5%. During this period, no other educational or procedural efforts related to linezolid/vancomycin use took place, no significant shortage occurred, and the types of infection reflected the historic infection mix at our institution. Hence, although these results are not indication specific, it is reasonable to believe that this change in linezolid utilization was associated with the revised MRSA pneumonia pathway/order form. The increased usage suggests a high level of influence of the revised order form in antibiotic selection. As vancomycin is generally used for most other serious MRSA-associated infections in our institution, it is not surprising that its use was not affected in a dramatic way.
As it was not our purpose to compare the efficacy of the two antibiotics in MRSA pneumonia, our methods are quite different from the large-scaled comparative trials mentioned above. For example, we excluded patients that received other anti-MRSA antibiotics during hospitalization, thus avoiding this potential cofounding variable. In addition, the baseline characteristics of our study population in the two groups were not statistically significantly different. Instead of the 30-day or 60-day mortality often seen in literature, we evaluated all-cause mortality at end-of-hospitalization as deaths occurred post-discharge were not entered into our electronic medical record system. In our analysis, we observed a significant difference in mortality rates between the two treatment groups (10% vs. 19.5%; P = 0.046). This result is encouraging in the sense that it suggests that the change in our institutional protocol did not lead to harmful outcomes for our patients, at least in regard to this outcome. At the same time, it must be emphasized that the number of patients expiring during treatment was small and this difference could be due to a number of factors beyond antibiotic use.
One finding of our study requires comment. The median duration of therapy was 4 days and only about half of the study population received antimicrobial therapy for 5 days or more. The most likely explanation is that some patients may have been discharged and finished their course of therapy on an outpatient basis (we only quantified days of inpatient treatment). A switch from intravenous to oral linezolid may explain the greater likelihood for discharge in that treatment group and shorter LOS. Alternatively, although our patients had a DRG designation of MRSA and pneumonia, they may have ultimately been culture negative, resulting in discontinuation of their empirically initiated MRSA-directed therapy.
In an age of charge-conscious healthcare choices, it is common to compare acquisition charges of alternative antibiotic charges and clearly linezolid is costlier than vancomycin. The Average Wholesale Price (AWP) of a dose of linezolid is approximately 6 to 27 times that of vancomycin, based on the dosage form and assuming doses of 600 mg and 1000 mg, respectively.12, 13 Total hospital charges assessed in the present study, while not a pure surrogate for attributable charges of MRSA pneumonia treatment, suggest no cost advantage for vancomycin at the institutional level. This is not necessarily a unique finding. In a recently published health economics study, researchers evaluated healthcare resource use, cost of care, and cost-effectiveness associated with nosocomial MRSA-pneumonia patients treated with linezolid or vancomycin. 14 The results suggested that linezolid-treated patients experienced a lower incidence of renal failure and hence, had lower hospital costs. However, due to the higher medication cost associated with linezolid, the overall healthcare resource use and total cost were similar in linezolid- and vancomycin-treated patients. Another study compared the charge-effectiveness of linezolid and vancomycin in nosocomial pneumonia patients by retrospectively reviewing insurance claims data from a large health plan.15 The results demonstrated that due to the improved survival related to linezolid use, the higher cost of linezolid was offset by the reduction in healthcare costs. Consequently, linezolid and vancomycin appeared to be equally cost-effective in the treatment of nosocomial MRSA pneumonia. In our study, hospital charge information, which included all raw charges associated with inpatient services, medications, and laboratory tests, was collected for each patient. We did not find any significant difference between the charges incurred with linezolid- versus vancomycin-treated patients. However, the results might be considered economically important, as the median hospital admission charge was $6,200 lower in patients treated with linezolid compared with those treated with vancomycin ($25,900 vs. $32,100). At the same time, one must consider hospital charges versus implications for the pharmacy budget, which, as in our case, are different.
Several limitations exist in our study. This was a single-center, retrospective study, and thus the establishment of a causal relationship between treatments and outcomes could not be proven and our results may not apply to other institutions. The study included all patients with ICD-9 codes for both MRSA and pneumonia. Hence, it is theoretically possible that a patient diagnosed with both pneumonia (due to some organism other than MRSA) and a separate (not pneumonia) MRSA infection could be included in the studied population. This could have led to a situation whereby not every patient in the study actually had MRSA pneumonia. In addition, the true charge for either therapy may not be reflected due to the exclusion of patients who received both linezolid and vancomycin during hospitalization. Additionally, the study period was from May 1, 2011 to August 31, 2014, with the change in clinician order form in May, 2013 when linezolid became the preferred agent. The unequal time periods are a potential source of bias. Finally, the number of linezolid-treated patients was almost twice the number of vancomycin-treated patients, which could potentially impact the statistical analysis of this study. At the same time, this unequal distribution may be testimony to the effectiveness of the change in the order form.
Conclusion
In this retrospective study comparing institutional consequences and a variety of broad patient outcomes of preferred linezolid- vs vancomycin- treated MRSA pneumonia, statistically significant differences in mortality and length of hospital stay favoring linezolid were noted. Safety profiles of both agents appeared to be similar. The overall hospital charges were lower in linezolid- compared with vancomycin-treated patients, despite the higher acquisition charge of linezolid. Taken together, these results help justify our change in policy.
Figure 2.
Kaplan-Meier Curves for Hospital LOS by Therapy Type
Acknowledgments
Financial support: Funded in part by the South Carolina Clinical & Translational Research Institute (SCTR) at the Medical University of South Carolina which is funded by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant (UL1TR001450).
Footnotes
Presented, in part at the IDWeek Annual Meeting, San Diego, CA, October 7–11, 2015
References
- 1.Kollef M, Shorr A, Tabak Y, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:854–62. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 2.Emori TG, Gaynes RP. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin Microbiol Rev. 1993;6:428–42. doi: 10.1128/cmr.6.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Bayer A, Cosgrove S, et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:1–38. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 4.Wunderink R, Niederman M, Kollef M, et al. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A randomized, controlled study. Clin Infect Dis. 2012;54:621–9. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 5.Peyrani P, Wiemken T, Kelley R, et al. Higher clinical success in patients with ventilator-associated pneumonia due to methicillin-resistant Staphylococcus aureus treated with linezolid compared with vancomycin: Results from the IMPACT-HAP study. Crit Care. 2014;18:R118. doi: 10.1186/cc13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caffrey A, Morrill H, Puzniak L, et al. Comparative effectiveness of linezolid and vancomycin among a national Veterans Affairs cohort with methicillin-resistant Staphylococcus aureus pneumonia. Pharmacotherapy. 2014;34:473–80. doi: 10.1002/phar.1390. [DOI] [PubMed] [Google Scholar]
- 7.Holmes N, Tong S, Davis J, van Hal S. Treatment of methicillin-resistant Staphylococcus aureus: Vancomycin and beyond. Semin Respir Crit Care Med. 2015;36:17–30. doi: 10.1055/s-0034-1397040. [DOI] [PubMed] [Google Scholar]
- 8.Chastre J, Blasi F, Masterton R, Rello J, Torres A, Welte T. European perspective and update on the management of nosocomial pneumonia due to methicillin-resistant Staphylococcus aureus after more than 10 years of experience with linezolid. Clin Microbiol Infect. 2014;20(Suppl 4):19–36. doi: 10.1111/1469-0691.12450. [DOI] [PubMed] [Google Scholar]
- 9.Welte T, Pletz M. Antimicrobial treatment of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) pneumonia: Current and future options. Int J Antimicrob Agents. 2010;36:391–400. doi: 10.1016/j.ijantimicag.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Kalil A, Klompas M, Haynatzki G, Rupp M. Treatment of hospital-acquired pneumonia with linezolid or vancomycin: A systematic review and meta-analysis. BMJ Open. 2013:3e003912. doi: 10.1136/bmjopen-2013-003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zou Y, Xie J, et al. Linezolid versus vancomycin for the treatment of suspected methicillin-resistant Staphylococcus aureus nosocomial pneumonia: A systematic review employing meta-analysis. Eur J Clin Pharmacol. 2015;71:107–15. doi: 10.1007/s00228-014-1775-x. [DOI] [PubMed] [Google Scholar]
- 12.Linezolid. Lexicomp. Wolters Kluwer Health, Inc; Hudson, OH: [Accessed May 24, 2015]. Lexi-Drugs. Available at: http://online.lexi.com. [Google Scholar]
- 13.Vancomycin. Lexicomp. Wolters Kluwer Health, Inc; Hudson, OH: [Accessed May 24, 2015]. Lexi-Drugs. Available at: http://online.lexi.com. [Google Scholar]
- 14.Niederman M, Chastre J, Solem C, et al. Health economic evaluation of patients treated for nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus: Secondary analysis of a multicenter randomized clinical trial of vancomycin and linezolid. Clin Ther. 2014;36:1233–43. doi: 10.1016/j.clinthera.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Mullins C, Kuznik A, Shaya F, et al. Charge-effectiveness analysis of linezolid compared with vancomycin for the treatment of nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus. Clin Ther. 2006;28:1184–98. doi: 10.1016/j.clinthera.2006.08.016. [DOI] [PubMed] [Google Scholar]