Abstract
T cells from HTLV-1-infected individuals have a decreased ability to proliferate after stimulation with recall antigens. This abnormality may be due to the production of regulatory cytokine or a dysfunctional antigen presentation. The aims of this study were to evaluate the antibody production and cytokine expression by lymphocytes before and after immunization with tetanus toxoid (TT) and to evaluate the immune response of monocytes after stimulation with TT and frequency of dendritic cells (DC) subsets. HTLV-1 carriers (HC) and uninfected controls with negative serology for TT were immunized with TT, and the antibody titers were determined by ELISA as well as the cell activation markers expression by monocytes. The frequencies of DC subsets were determined by flow cytometry. Following immunization, the IgG anti-TT titers and the frequency of CD4+ T cells expressing IFN-γ, TNF and IL-10 in response to TT were lower in the (HC) than in the controls. Additionally, monocytes from HC did not exhibit increased HLA-DR expression after stimulation with TT, and presented low numbers of DC subsets, therefore, it’s necessary to perform functional studies with antigen-presenting cells. Collectively, our finding suggests that HC present an impairment of the humoral and CD4+ T cell immune responses after vaccination.
Keywords: HTLV-1, antibody, cytokines, antigen-presenting cells, recall antigen
1. Introduction
The human T cell lymphotropic virus type 1 (HTLV-1) is a retrovirus that infects 10 to 20 million people worldwide [1]. Adult T-cell leukemia/lymphoma (ATLL) [2] and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [3] are the main diseases associated with this virus. However, a large percentage of infected individuals remain only HTLV-1 carriers (HC) [4]. HTLV-1 predominantly infects CD4+ T cells but also infects CD8+ T cells, dendritic cells, monocytes and B cells, leading to spontaneous lymphocyte proliferation and cytokine production in the absence of exogenous stimuli [5–9], such as interferon-gamma (IFN-γ), tumor necrosis factor (TNF) and interleukin-6 (IL-6) [10–12]. In vitro studies have shown that HC present a higher cytokine production compared with uninfected controls (UCs) [13].
HTLV-1 modifies the immune response to other infectious agents and increases susceptibility to other infectious diseases, such as strongyloidiasis [14], tuberculosis [15–17] and severe scabies [18]. The exacerbated production of Th1 cytokines may downregulate Th2 cell activation, and this imbalance may explain not only the increased susceptibility to Strongyloides stercoralis infection but also the increased frequency of recurrent and disseminated strongyloidiasis in HTLV-1-infected individuals [19, 20]. However, the increased susceptibility of HTLV-1-infected subjects to develop tuberculosis and fungal infections is intriguing, because the control of these infections is dependent on the activation of phagocytes mediated by IFN-γ [15, 21]. In Peru, HTLV-1-infected individuals have a twofold increased chance of acquiring tuberculosis [22], and in Salvador, Bahia, which is an endemic area for tuberculosis and HTLV-1, HTLV-1-infected subjects have a 2.6-fold greater risk of acquiring an infection with Mycobacterium tuberculosis [23]. It is known that HTLV-1-infected individuals present an impaired lymphoproliferative response to M. tuberculosis antigens, tetanus toxoid (TT), cytomegalovirus and Candida albicans antigens [24]. Possible factors that may contribute to this suppression include the decreased abilities of antigen-presenting cells (APC) to present antigen and/or an increasing in regulatory cytokines production. In patients with ATLL, a decreased expression of HLA-DR on dendritic cells has been documented [25, 26]. Additionally, it has been shown that IL-12 enhances lymphocyte proliferation and IFN-γ production in HTLV-1-infected subjects [27]. In addition, HC exhibit high IL-10 production [13]. Because a direct correlation between IFN-γ and IL-10 production is observed in HC, it is possible that this attempt to down-modulate the exaggerated immune response induced by the virus through the production of regulatory cytokines, may decrease the immune response to other antigens.
Although the T cell response has been widely studied in HTLV-1 infection, there are scattered studies regarding APCs in this viral infection. It is known that HTLV-1 can infect the myeloid cell lineage [7, 8, 28], and few studies have shown abnormalities in APCs that could lead to a decreased adaptative immune response to a biased antigen [28]. In this study, we hypothesized that HTLV-1-infected subjects have impairments in the humoral and cellular immune responses following vaccination with tetanus toxoid (TT), and this could be related to an increased production of regulatory cytokines or a decreased frequency or function of APCs. Thus, we evaluated the anti-TT antibody production and frequency of CD4+ and CD8+ T cells expressing cytokines (IFN-γ, TNF and IL-10) before and after immunization. Furthermore, we evaluated the frequency of plasmacytoid and myeloid dendritic cells and the ability of the monocytes to express costimulatory molecules (CD80 and CD86) and HLA-DR after stimulation with TT.
2. Materials and methods
2.1. Study design
This mixed-type study comprises a cohort study with the participation of HTLV-1 carriers (HC) and uninfected controls (UC) aimed to compare the immunological responses in these two groups after vaccination with tetanus toxoid (TT) and a cross-sectional study comparing the frequency of dendritic cells in HC (n = 20) and UC (n = 10).
2.2. Study population
For the cohort study, HC were selected from the HTLV-1 Clinic at the Hospital Universitário Professor Edgard Santos, Federal University of Bahia, Brazil. The diagnosis of HTLV-1 was performed by ELISA (Murex Biotech Limited, Dartford, UK) at the Blood Bank located in Salvador, Bahia, Brazil, and confirmed by western blot (HTLV blot 2.4, Genelabs, Singapore) in our laboratory. Since 2004, our HTLV-1 Clinic has conducted a cohort study, where the patients are followed by clinical and immunological evaluations (cytokines and proviral load determination). The uninfected controls were blood donors and the serum from these individuals were collected and stored at −20°C.
For the cross-sectional study, a total of twenty asymptomatic HTLV-1-infected individuals followed at the HTLV-1 Reference Development of Science Foundation (Salvador, Bahia, Brazil) were included in the study. Uninfected controls were blood donors.
The inclusion criteria were individuals of both genders, 18 to 65 years of age, HTLV-1 carriers, absence of HTLV-1-associated neurological manifestation, and immunized for TT for more than ten years. All of the subjects agreed to participate in the study and signed an informed consent form. The absence of neurological disease was determined by evaluation of motor dysfunction by Osame’s motor disability score (OMDS) [29], and neurological involvement was assessed by the expanded disability status scale (EDSS) [30]. Only subjects with OMDS = 0 and EDSS = 0 were included. The exclusion criteria were use of antiviral drugs or immunomodulators in the previous 90 days, helminthic infection, coinfection with HIV, HCV or HBV or immunization for TT for less than 10 years. Information of immunization with TT or documentation of immunization was based on a vaccination card. Informed consent was obtained from all of the enrolled patients, and the Institutional Research Board from the Federal University of Bahia approved this study (protocol number 154/2009).
2.3. Immunization protocol and serology to tetanus toxoid
Two doses of TT (40 I.U. of TT per dose, using thimerosal as a stabilizer and aluminum hydroxide as an adjuvant) were administered by intramuscular route, and the second dose was administered 30 days after the first dose. The heparinized peripheral blood and serum samples were obtained before and 60 days after immunization. Immunological evaluations were performed on day 0 (prior to TT vaccination) and on day 60.
The serology for TT was performed using an enzyme-linked immunosorbent assay (ELISA). Briefly, a 96-well plate was coated with TT (0.1 Lf/mL) in coating buffer (sodium carbonate and sodium bicarbonate, pH 9.6) overnight at 4°C. The sera from HCs and UCs were diluted (1:100) in PBS at pH 7.2 supplemented with 0.05% Tween 20 and incubated for 1 h at 37°C. Goat anti-human IgG alkaline phosphatase conjugate (Sigma Chemicals, St. Louis, MO, USA) was diluted (1:1,000) in PBS pH 7.2 supplemented with 0.05% Tween 20 and incubated for 1 h at 37°C, and 1 mg/mL p-nitrophenyl phosphate (pNPP, Sigma Chemicals, St. Louis, MO, USA) was used to reveal the reaction. The plate was read at 405 nm.
To evaluate the IgG isotypes, a 96-well plate was coated with TT (0.1 Lf/mL) in coating buffer overnight at 4°C. The plate was blocked with phosphate buffer saline plus 1% bovine albumin serum (PBS + 1% BSA) for 1 h at 37°C. The sera from HC and UC were added (1:100 dilution in PBS + 1% BSA) for 1 h at 37°C. The biotinylated antibodies (mouse anti-human IgG1, mouse anti-human IgG2, mouse anti-human IgG3 and mouse anti-human IgG4; Sigma Chemicals, St. Louis, MO, USA) were added for 1 h at 37°C. Horseradish-peroxidase streptavidin (Invitrogen, Carlsbad, CA, USA) was added for 30 min at room temperature (RT). The reactions were revealed by incubation with tetramethylbenzidine (TMB: Invitrogen, Carlsbad, CA, USA) for 20 min at room temperature. Finally, the reactions were stopped with 2 N H2SO4. The plate was read at 450 nm.
2.4. PBMC isolation and flow cytometry assays
PBMCs from HC and UC were obtained from heparinized venous blood samples by Ficoll-Hypaque density gradient centrifugation. Cultures of 4 x 105 PBMCs (30 μL/well) were prepared in RPMI 1640 plus 10% heat-inactivated fetal bovine serum (Sigma Chemical Co., St. Louis, MO, USA), antibiotics and glutamine. To evaluate the cytokine expression by lymphocytes before and after immunization, freshly preparated PBMCs were cultured for 20 h with TT (0.5 Lf/mL). Brefeldin A (1 μg/mL) was added for the last 4 h of the incubation. The cells were labeled with anti-CD4-FITC and fixed with formaldehyde. The cells were then permeabilized with 0.5% saponin solution in PBS and labeled with anti-IFN-γ-PE, anti-TNF-PE and anti-IL-10-PE for 30 min at room temperature. Cells not stimulated with TT were also labeled: frequency of CD4+ T cells expressing IFN-γ were 0.2 ± 0.1% (HC) and 0.2 ± 0.2% (UC). CD4+ T cells expressing TNF were 0.1 ± 0.08% (HC) and 0.2 ± 0.17% (UC). IL-10 expression by CD4+ T cells were 0.2 ± 0.2% (HC) and 0.27 ± 0.17% (UC). These background status had no statistical difference.
To evaluate the expression of costimulatory molecules and HLA-DR in monocytes from HC and UC before immunization with TT, PBMCs were cultured for 6 h with and without TT (0.5 Lf/mL). The cells were then incubated with FITC, PE or PE-Cy5-labeled monoclonal antibodies (Ig controls, CD14, HLA-DR, CD80 and CD86) for 20 min at 4°C. All of the above-mentioned reagents were from BD Pharmingen, (San Diego, CA, USA). After staining, the cells were washed with phosphate-buffer saline (PBS), fixed with 2% formaldehyde in PBS and maintained at 4°C until data acquisition. The values are expressed as mean of fluorescence intensity (MFI).
In all cases, 100,000 gated events were acquired with a FACScanto II cytometer, and the analysis was performed using the FlowJo software (version 7.6.1).
2.5. Quantification of dendritic cell subsets
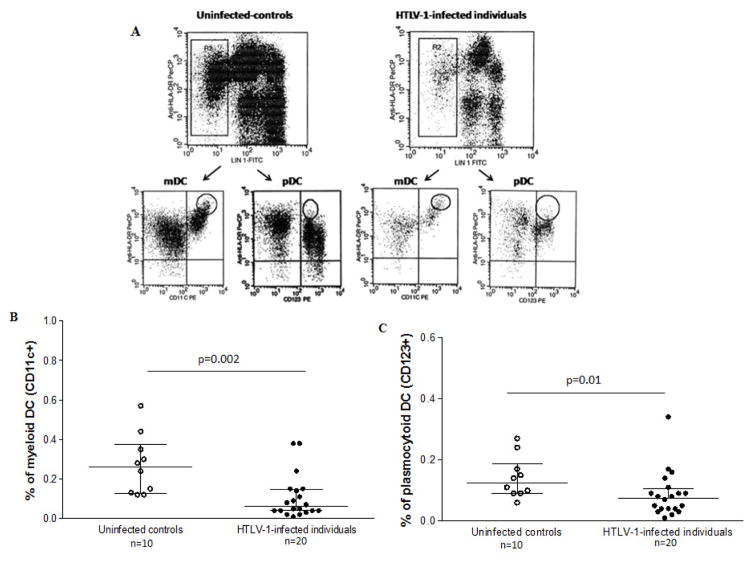
The peripheral blood dendritic cells were measured through a flow cytometry analysis of fresh whole blood within 12 h of sampling. Briefly, whole blood cells were incubated for 30 min in the presence of the following monoclonal antibodies (mAbs) at room temperature: cocktail lineage conjugated with fluorescein isothiocyanate (Lin 1-FITC) (Caltag, Burlingame, CA, USA), anti-HLA-DR conjugated with PE-Cy5 (Caltag, Burlingame, CA, USA), anti-CD11c+ conjugated with PE (Caltag, Burlingame, CA, USA) and anti-CD123 conjugated with PE (Caltag, Burlingame, CA, USA). The erythrocytes were lysed using FACS lysing solution (BD Biosciences, San Jose, CA, USA) at room temperature. The cells (50,000 events) were acquired by FACScan and analyzed using the Cell Quest software (Becton-Dickinson). The dendritic cells were identified as Lin− HLA-DR+ cells within a monocyte gate. The CD11c and CD123 expression was determined within Lin−HLA-DR+ cells to define the myeloid DC (CD11c+CD123−) and plasmacytoid DC (CD11c−CD123+) subsets.
2.6. Statistical analysis
The IgG anti-TT titers are presented as the median of optical density (O.D.) values. The flow cytometry data are presented as the means ± standard deviation.
To compare antibody production, frequency of cells expressing surface molecules, cytokines expression and HTLV-1 proviral load before and after immunization were performed using the Wilcoxon signed-rank test. The Mann-Whitney U test was used to compare the results between the patients and healthy subjects. The analyses were performed using GraphPad Prism version 5.0 (San Diego, CA, USA), and differences with p < 0.05 were considered significantly different.
3. Results
3.1. Study population and humoral immune response against TT (Total IgG and IgG isotypes)
The demographic characteristics of the participants and the proviral load as well as IFN-γ and TNF production in HTLV-1 infected subjects is shown on Table 1. The mean age of the HC was 47 ± 10.3 years old, and for the UC, 31 ± 8.8 years old (p = 0.002). The gender distribution among UC was similar to that observed in HC: 6 males and 6 females. Most of UC were non-white race. The TT vaccination period of the UC was similar to that of the HC. The median HTLV-1 proviral load before immunization with TT was 24,792 copies/106 cells (interquartile range: 4,639 – 129,914 copies/106 cells) and after immunization was 34,584 (IQR 5,394 – 159,175 copies/106 cells).
Table 1.
Demographic features from HTLV-1 carriers and uninfected controls. Proviral load, IFN-γ and TNF production from 14 HTLV-1 carriers enrolled in TT immunization protocol.
| HTLV-1 carriers (n = 14) | Uninfected controls (n = 12) | p value | |
|---|---|---|---|
| Age (mean ± S.D.) | 47 ± 10.3 | 31 ± 8.8 | 0.002* |
| Gender (M/F) | 6/8 | 6/6 | n.s.** |
| Human race: | n.s.** | ||
| White | 10% | 16% | |
| Non-white | 90% | 84% | |
| HTLV-1 Proviral load (copies/106 cells) (median [I.Q. range]) | 24,792 (4,639 – 129,914) | ---- | ---- |
| IFN-γ (pg/mL)a (median [I.Q. range]) | 1,540 (56 – 2,373) | ---- | ---- |
| TNF (pg/mL)a (median [I.Q. range]) | 597 (193 – 1,430) | ---- | ---- |
Concentration determined by ELISA using supernatant from culture of unstimulated peripheral blood mononuclear cells before immunization with TT.
Unpaired t test.
n.s. = not significant (Fisher’s exact test).
The background value in the assay to evaluate humoral immune response presented low optical density (O.D. = 0.051). Before immunization with TT, there was no difference in the IgG anti-TT titer between the HC (0.091) and UC (0.182) (p = 0.3). Following immunization, increased antibody production was observed in both HC (0.091 vs. 0.485, p = 0.001) and UC (0.182 vs. 0.804, p = 0.002). However, the antibody titer in the HC was lower than that found in the UC (p = 0.007) (Fig. 1).
Figure 1. Total IgG anti-TT production before and after immunization with TT.
Production of IgG anti-TT from HTLV-1 carriers and uninfected controls before and after immunization. The line represents the median.
The analysis of the IgG isotypes revealed high IgG1 production following immunization in both groups: 0.025 vs. 1.0 for HC (p = 0.0001) and 0.028 vs. 2.744 for UC (p = 0.0005). After immunization, the IgG1 anti-TT production in HC was lower than that observed in the UC group (p = 0.0006) (Fig. 2A). The IgG2 anti-TT production increased after immunization in the HC (0.195 vs. 0.351, p = 0.0009) and UCs (0.162 vs. 0.591, p = 0.002) (Fig. 2B). The same pattern was found for IgG3 anti-TT production: 0.027 vs. 0.049 in HCs (p = 0.009); 0.019 vs. 0.053 in UCs (p = 0.04) (Fig. 2C). IgG4 anti-TT production was higher after immunization in both groups: 0.053 vs. 0.267 in HC (p = 0.004); 0.048 vs. 0.206 in UCs (p = 0.0005) (Fig. 2D).
Figure 2. IgG anti-TT isotype production before and after immunization with TT.
Production of IgG1 (A), IgG2 (B), IgG3 (C) and IgG4 (D) anti-TT from HTLV-1 carriers and uninfected controls before and after immunization. The open and closed circles represent the results before immunization, and the open and closed triangles represent the results after immunization.
3.2. IFN-γ, TNF and IL-10 expression by lymphocytes
The frequency of CD4+ T cells expressing IFN-γ remained the same before and after immunization (0.2 ± 0.1%, p = 0.8) in the HC. There was no significant increase in the expression of this cytokine by CD4+ T cells from the UC after immunization (0.4 ± 0.3% to 1.0 ± 2.0%, p = 0.8). After immunization, the frequency of CD4+ T cell expressing this cytokine was lower in the HC compared with the UC (p = 0.01) (Fig. 3B).
Figure 3. Cytokine expression by lymphocytes stimulated with TT before and after immunization.
(A) Representative plots of the gating strategy used to define the lymphocyte population, the CD4+ T cells expressing cytokines obtained through the analysis of mononuclear cells from one HTLV-1 carrier. (B) IFN-γ, (C) TNF-α and (D) IL-10 expression by TT-stimulated CD4+ from HTLV-1 carriers and uninfected controls before and after immunization. The white bars represent the lymphocytes stimulated with TT before immunization, and the crosshatched bars represent the lymphocytes stimulated with TT after immunization.
The analysis of TNF revealed that the percentage of CD4+ T cells expressing TNF from the HC was similar before and after immunization (0.3 ± 0.2% vs. 0.3 ± 0.1%, p = 0.7). Alternatively, in the UC, there was an increase in the percentage of cells expressing TNF from 0.3 ± 0.1% to 0.8 ± 0.9% (p = 0.02). The comparison of the frequency of cells expressing TNF revealed that the HC had lower frequency than the UC (p = 0.02) (Fig. 3C).
The IL-10 expression by CD4+ T cells from the HC did not increase after immunization (0.3 ± 0.3% vs. 0.2 ± 0.1%, p = 1). The analysis of the UC revealed a slight increase in IL-10 expression by CD4+ T cells after immunization, but this difference was not significant (0.3 ± 0.1% vs. 1.3 ± 2.9%, p = 0.08). The frequency of these cells was lower in HCs compared with UC (p = 0.01) (Fig. 3D).
Regarding the expression of these cytokines in CD8+ T cells, there was no statistical difference when compared cells stimulates with TT before and after immunization, both in HC and in UC (data not shown).
3.3. HLA-DR, CD80 and CD86 expression by monocytes
The HLA-DR expression by CD14+ monocytes from HC did not increase after stimulation with TT (781 ± 873 vs. 793 ± 761, p = 0.3). However, the monocytes from the UC exhibited increased HLA-DR expression after stimulation with TT from 681 ± 615 to 898 ± 1,055 (p = 0.005) (Fig. 4B). After stimulation, the monocytes from the HC did not exhibit increased CD80 expression (1,167 ± 1,410 vs. 1,579 ± 2,272, p = 0.07). The same result was observed with the monocytes from the UC (936 ± 1,318 vs. 1,176 ± 1,890, p = 0.2) (Fig. 4C). The analysis of CD86 expression showed that the monocytes from the HC did not increase after stimulation with TT (3,628 ± 4,447 vs. 3,394 ± 4,529, p = 0.07). The same finding was obtained for the monocytes from the UC (2,686 ± 3,240 vs. 2,647 ± 3,169, p = 0.5) (Fig. 4D).
Figure 4. Surface cell molecule expression by monocytes with or without stimulation.
(A) Representative plots of the gating strategy used to set the monocyte population and CD14+ cells. Representative histograms of one HTLV-1-infected individual and one uninfected control show HLA-DR, CD80 and CD86 in unstimulated cells (gray line) and cells stimulated with tetanus toxoid (black line). (B) HLA-DR, (C) CD80 and (D) CD86 expression by CD14+ monocytes from HTLV-1 carriers and uninfected controls with or without stimulation with TT. The white bars represent the non-stimulated monocytes, and the black bars represent the monocytes stimulated with TT. MFI = mean of fluorescence intensity.
3.4. Quantification of DC subsets
The median frequencies of both myeloid (mDC) and plasmacytoid (pDC) circulating DCs in the HC (0.06%, range: 0.01–0.4, and 0.08%, range: 0.01–0.3, respectively) were significantly lower than those found in the UC (0.3%, range: 0.1–0.6, and 0.1%, range: 0.06–0.3, respectively) (p = 0.002 and p = 0.01 for mDC and pDC, respectively) (Fig. 5B and C).
Figure 5. Frequency of dendritic cell subsets from HTLV-1 carriers (n = 20) and uninfected controls (n = 10).
(A) The DCs were identified within gate R2 as lineage fluorescein isothiocyanate (FITC) cocktail (anti-CD3, CD14, CD16, CD19, CD20, and CD56)-negative and peridinin chlorophyll (PerCP) HLA-DR-positive. Detection of HLA-DR+CD11c+ myeloid DCs (mDCs) and HLA-DR+CD123+ plasmacytoid DCs (pDCs), respectively. (B) Percentages of (mDCs) and (C) pDCs from HTLV-1 carriers and uninfected controls. The data represent the medians and ranges. The differences between HTLV-1-infected patients and controls were determined by the Mann–Whitney U test.
4. Discussion
It is well known that HTLV-1 may impair the immune response to other pathogens and increases the susceptibility to other infectious diseases [15, 18, 31–33]. However, the mechanisms involved in this immunosuppression are not completely understood. In this study, we showed that the antibody production and the frequency of cells expressing cytokines after immunization with TT were lower in HTLV-1-infected individuals than in the uninfected controls. These data indicate that both the antibody production and cell-mediated immunity in HTLV-1-infected subjects are impaired in response to a biased antigen. Moreover, we showed that monocytes from HTLV-1-infected individuals exhibit a lower HLA-DR expression after TT stimulation and there was a lower frequency of the DC subsets in these individuals than the healthy subjects. These findings may indicate a possible involvement of APCs in abnormal immune response to TT in HTLV-1-infected subjects.
This study extends previous observations regarding the immune response after immunization with TT in HTLV-1-infected subjects because, in addition to antibody production, we evaluated the T cells and the APC response. In a previous study evaluating the anti-TT antibody production in HTLV-1-infected subjects, it was found that the HC produced a high amount of neutralizing anti-TT antibody [34]. In this study, we showed that the level of antibody production (total anti-TT IgG) in HTLV-1 carriers was lower than that observed in UC after immunization with TT. In this study, the TT immunization schedule, the evaluation time of the immune response after immunization and the determination of IgG production were different from those presented by Biasutti et al. [34]. However, although contradictory, we are comfortable with our results because the evaluation of IgG1 production revealed a similar pattern as that found for the total anti-TT IgG production. Another important issue is the age difference between the groups. The mean of age in UC was significantly lower than that of HTLV-1 carriers. It is well known that the humoral immune response gradually decreases with age, especially in individuals older than 60 years old [35, 36]. However, in biological terms we believe that the difference in age was not a strong factor that influenced directly on the results from this study, since the antibody levels were similar in the 2 groups before vaccination and both groups increased antibody production after TT vaccination. Additionally, it has been showed that after booster immunization with this recall antigen the anti-TT production in elderly was similar to that found among young people [37].
A decrease in IgG production after vaccination with TT has also been found in HIV-infected individuals compared with healthy subjects [38]. The present study provides the first observation showing that HTLV-1-infected individuals present impaired antibody production after vaccination, which is a relevant finding for public health strategies.
Regarding the influence of the viral load in the immune response, it was documented that immunization for influenza virus induced an increased HIV-1 proviral load [39, 40], in our study, the HTLV-1 proviral load did not change after immunization with TT.
Despite we found that the frequency of CD4 T cells expressing IFN-γ upon stimulation with TT was lower than in uninfected control it is known that IFN-γ produced by CD4+ and CD8+ T cells are higher in HTLV-1 infected individuals than in uninfected controls [41]. As IFN-γ induces IgG production [42] and down-modulates the IgG1 response we evaluated the IgG isotypes before and after immunization with TT. The IgG2, IgG3 and IgG4 titers were similar in both HCs and UCs before and after immunization. However, the IgG1 production presented the same pattern as that observed for total IgG anti-TT. After immunization, the HCs presented lower levels of IgG1 anti-TT compared with the UC. Because IgG1 is the predominant IgG subclass anti-TT antibody [43], this result confirms the finding from the analysis of the total IgG data, which revealed that HTLV-1-infected individuals present an impairment to produce anti-TT antibody after immunization.
During HTLV-1 infection, immunological abnormalities have been documented in both APC and T cells [41, 44], which could impair the immunological response to other antigens. The increased susceptibility to S. stercoralis in HTLV-1-infected individuals has been associated with a decrease in the Th2 immune response against parasite antigens [32] and with an increase in regulatory T cells [45]. Additionally, it has been shown that patients with HTLV-1 and strongyloidiasis present a negative correlation between the IFN-γ and IL-5 levels and between the IFN-γ levels and the total parasite-specific IgE [45, 46]. We showed that the frequency of CD4+ T cells expressing IFN-γ and IL-10 and the frequency of CD4+ T cells expressing TNF were lower in cultures of cells from HC than in UC after stimulation with TT. This finding indicates that the production of both pro-inflammatory and regulatory cytokines is decreased after immunization with TT in HTLV-1 infection. The impairment of T cells to produce cytokines and stimulate antibody production after immunization with the TT antigen may be due to a defect in APCs. Because HTLV-1 predominantly infects T cells, very little attention has been given to the role of monocytes/macrophages and DCs in this viral infection. However, HTLV-1 may infect monocytes/macrophages and DCs [7, 8]. Moreover, it has been shown that DCs from HTLV-1-infected subjects express less HLA-DR [25] and that they present an impairment in the differentiation of monocytes to dendritic cells [26]. In contrast, it has been reported that the Tax protein is effective for the differentiation and activation of monocyte-derived dendritic cells from ATLL patients by the induction of efficient antigen presentation and T cell stimulation [47, 48]. It is relevant to note that these previous studies included patients with HAM/TSP and/or ATLL, who are known to have an array of immunological abnormalities.[41, 49]. In the present study, all of the participants were HTLV-1 carriers, and we showed that these individuals exhibit impaired expression of HLA-DR. It is possible that a decrease in the intensity of HLA-DR expression may impair antigen presentation to T cells, contributing to the decreased antibody levels and attenuated cell-mediated immunity observed in HTLV-1-infected subjects after immunization.
Furthermore, in this study, we demonstrated that the HTLV-1 carriers showed lower levels of pDCs and mDCs compared with the uninfected controls. This result is similar to previous studies, which found lower levels of these DCs in ATL patients [50]. The decreasing of DC may impair immune response and consequently the antigen presentation. For instance, it has been demonstrated that the absence of pDCs in PBMC stimulated with influenza virus decreased the anti-influenza IgG production [51].
The cytokines produced by APCs, such as IL-12 and IL-10, play a pivotal role in the development of an immune response. We have previously shown that HTLV-1 carriers produce more IL-10, which is important to down-modulate the immunological response in humans, compared with uninfected controls [13]. In addition, it has been shown that the IL-12 levels are reduced in the supernatants of cultures of cells from HTLV-1-infected subjects with tuberculosis stimulated with PPD [52]. Furthermore, the exogenous addition of IL-12 to lymphocyte cultures of HTLV-1-infected subjects enhances lymphocyte proliferation in response to PPD [27]. However, our data do not show impairment in IL-12 nor a higher expression of IL-10 by monocytes (data not shown). Therefore, we cannot rule out a role for regulatory cytokines in the impairment of the immune response in HTLV-1-infected subjects because our functional studies were performed only in monocytes. Nonetheless, we showed that decreases in the frequency of DCs and in the expression of HLA-DR occurred. These findings may play an important role in the decreased immune response to a biased antigen and may explain the increasing susceptibility of HTLV-1-infected subjects to develop other infectious diseases.
In conclusion, HTLV-1-infected individuals have a decreased humoral and CD4+ T cell immune responses against TT after immunization. These abnormalities may be partly explained by the failure to increase HLA-DR expression on monocytes and the low frequency of DC subsets observed in these individuals. However, it’s necessary studies addressing the functional ability of DC to present antigen to T cells need to be performed.
Acknowledgments
We are grateful to Cristiano Franco for his secretarial assistance.
We are grateful to Lucia Passos and Gloria Orge for helping with the selection of the patients.
We are grateful to Cristina Toledo-Cornell for providing language help.
This work was supported by the CNPq (Brazilian Council for Scientific and Technological Development) and the NIH Training Grant R01 AI079238A (EMC).
AS, SS, LPC and EMC are funded by the CNPq.
Footnotes
Authors’ contribution
AS, SS, LPC, MFRG and EMC participated equally in the study design.
AS and EMC drafted the manuscript.
AS and SS performed PBMC isolation.
AS and LPC performed flow cytometry.
MFRG performed flow cytometry for dendritic cells.
All authors read and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edlich RF, Arnette JA, Williams FM. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I) J Emerg Med. 2000;18:109. doi: 10.1016/s0736-4679(99)00173-0. [DOI] [PubMed] [Google Scholar]
- 2.Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980;77:7415. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 4.Hollsberg P, Hafler DA. Seminars in medicine of the Beth Israel Hospital, Boston. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson S, Gupta A, Mattson D, Mingioli E, McFarlin DE. Immunological studies in tropical spastic paraparesis. Ann Neurol. 1990;27:149. doi: 10.1002/ana.410270209. [DOI] [PubMed] [Google Scholar]
- 6.Kramer A, Jacobson S, Reuben JF, Murphy EL, Wiktor SZ, Cranston B, et al. Spontaneous lymphocyte proliferation in symptom-free HTLV-I positive Jamaicans. Lancet. 1989;2:923. doi: 10.1016/s0140-6736(89)91591-2. [DOI] [PubMed] [Google Scholar]
- 7.de Revel T, Mabondzo A, Gras G, Delord B, Roques P, Boussin F, et al. In vitro infection of human macrophages with human T-cell leukemia virus type 1. Blood. 1993;81:1598. [PubMed] [Google Scholar]
- 8.Knight SC, Macatonia SE, Cruickshank K, Rudge P, Patterson S. Dendritic cells in HIV-1 and HTLV-1 infection. Adv Exp Med Biol. 1993;329:545. doi: 10.1007/978-1-4615-2930-9_91. [DOI] [PubMed] [Google Scholar]
- 9.Yamano Y, Cohen CJ, Takenouchi N, Yao K, Tomaru U, Li HC, et al. Increased expression of human T lymphocyte virus type I (HTLV-I) Tax11-19 peptide-human histocompatibility leukocyte antigen A*201 complexes on CD4+ CD25+ T Cells detected by peptide-specific, major histocompatibility complex-restricted antibodies in patients with HTLV-I-associated neurologic disease. J Exp Med. 2004;199:1367. doi: 10.1084/jem.20032042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kubota R, Kawanishi T, Matsubara H, Manns A, Jacobson S. Demonstration of human T lymphotropic virus type I (HTLV-I) tax-specific CD8+ lymphocytes directly in peripheral blood of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by intracellular cytokine detection. J Immunol. 1998;161:482. [PubMed] [Google Scholar]
- 11.Nishimoto N, Yoshizaki K, Eiraku N, Machigashira K, Tagoh H, Ogata A, et al. Elevated levels of interleukin-6 in serum and cerebrospinal fluid of HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neurol Sci. 1990;97:183. doi: 10.1016/0022-510x(90)90217-b. [DOI] [PubMed] [Google Scholar]
- 12.Osame M. Pathological mechanisms of human T-cell lymphotropic virus type I-associated myelopathy (HAM/TSP) J Neurovirol. 2002;8:359. doi: 10.1080/13550280260422668. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho EM, Bacellar O, Porto AF, Braga S, Galvao-Castro B, Neva F. Cytokine profile and immunomodulation in asymptomatic human T-lymphotropic virus type 1-infected blood donors. J Acquir Immune Defic Syndr. 2001;27:1. doi: 10.1097/00126334-200105010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Neva FA, Murphy EL, Gam A, Hanchard B, Figueroa JP, Blattner WA. Antibodies to Strongyloides stercoralis in healthy Jamaican carriers of HTLV-1. N Engl J Med. 1989;320:252. doi: 10.1056/NEJM198901263200416. [DOI] [PubMed] [Google Scholar]
- 15.de Lourdes Bastos M, Osterbauer B, Mesquita DL, Carrera CA, Albuquerque MJ, Silva L, et al. Prevalence of human T-cell lymphotropic virus type 1 infection in hospitalized patients with tuberculosis. Int J Tuberc Lung Dis. 2009;13:1519. [PMC free article] [PubMed] [Google Scholar]
- 16.Pedral-Sampaio DB, Martins Netto E, Pedrosa C, Brites C, Duarte M, Harrington W., Jr Co-Infection of Tuberculosis and HIV/HTLV Retroviruses: Frequency and Prognosis Among Patients Admitted in a Brazilian Hospital. Braz J Infect Dis. 1997;1:31. [PubMed] [Google Scholar]
- 17.Verdonck K, Gonzalez E, Henostroza G, Nabeta P, Llanos F, Cornejo H, et al. HTLV-1 infection is frequent among out-patients with pulmonary tuberculosis in northern Lima, Peru. Int J Tuberc Lung Dis. 2007;11:1066. [PubMed] [Google Scholar]
- 18.Brites C, Weyll M, Pedroso C, Badaro R. Severe and Norwegian scabies are strongly associated with retroviral (HIV-1/HTLV-1) infection in Bahia, Brazil. Aids. 2002;16:1292. doi: 10.1097/00002030-200206140-00015. [DOI] [PubMed] [Google Scholar]
- 19.Porto MA, Alcantara LM, Leal M, Castro N, Carvalho EM. Atypical clinical presentation of strongyloidiasis in a patient co-infected with human T cell lymphotrophic virus type I. Am J Trop Med Hyg. 2005;72:124. [PubMed] [Google Scholar]
- 20.Porto AF, Santos SB, Muniz AL, Basilio V, Rodrigues W, Jr, Neva FA, et al. Helminthic infection down-regulates type 1 immune responses in human T cell lymphotropic virus type 1 (HTLV-1) carriers and is more prevalent in HTLV-1 carriers than in patients with HTLV-1-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2005;191:612. doi: 10.1086/427560. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves DU, Guedes AC, Proietti AB, Martins ML, Proietti FA, Lambertucci JR. Dermatologic lesions in asymptomatic blood donors seropositive for human T cell lymphotropic virus type-1. Am J Trop Med Hyg. 2003;68:562. doi: 10.4269/ajtmh.2003.68.562. [DOI] [PubMed] [Google Scholar]
- 22.Verdonck K, Gonzalez E, Schrooten W, Vanham G, Gotuzzo E. HTLV-1 infection is associated with a history of active tuberculosis among family members of HTLV-1-infected patients in Peru. Epidemiol Infect. 2008;136:1076. doi: 10.1017/S0950268807009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lourdes Bastos M, Osterbauer O, Mesquita DL, Carrera CA, Albuquerque MJ, Silva L, et al. Prevalence of human T-cell lymphotropic virus type 1 infection in hospitalized patients with tuberculosis. Int J Tuberc Lung Dis. 2009;13(12):1519. [PMC free article] [PubMed] [Google Scholar]
- 24.Mascarenhas RE, Brodskyn C, Barbosa G, Clarencio J, Andrade-Filho AS, Figueiroa F, et al. Peripheral blood mononuclear cells from individuals infected with human T-cell lymphotropic virus type 1 have a reduced capacity to respond to recall antigens. Clin Vaccine Immunol. 2006;13:547. doi: 10.1128/CVI.13.5.547-552.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makino M, Wakamatsu S, Shimokubo S, Arima N, Baba M. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implications for the dendritic cell defect in adult T cell leukemia. Virology. 2000;274:140. doi: 10.1006/viro.2000.0445. [DOI] [PubMed] [Google Scholar]
- 26.Nascimento CR, Lima MA, de Andrada Serpa MJ, Espindola O, Leite AC, Echevarria-Lima J. Monocytes from HTLV-1-infected patients are unable to fully mature into dendritic cells. Blood. 2011;117:489. doi: 10.1182/blood-2010-03-272690. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki M, Dezzutti CS, Okayama A, Tachibana N, Tsubouchi H, Mueller N, et al. Modulation of T-cell responses to a recall antigen in human T-cell leukemia virus type 1-infected individuals. Clin Diagn Lab Immunol. 1999;6:713. doi: 10.1128/cdli.6.5.713-717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macatonia SE, Cruickshank JK, Rudge P, Knight SC. Dendritic cells from patients with tropical spastic paraparesis are infected with HTLV-1 and stimulate autologous lymphocyte proliferation. AIDS Res Hum Retroviruses. 1992;8:1699. doi: 10.1089/aid.1992.8.1699. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa M, Nakahara K, Maruyama Y, Kawabata M, Higuchi I, Kubota H, et al. Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neurovirol. 1996;2:345. doi: 10.3109/13550289609146899. [DOI] [PubMed] [Google Scholar]
- 30.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 31.Carvalho EM, Da Fonseca Porto A. Epidemiological and clinical interaction between HTLV-1 and Strongyloides stercoralis. Parasite Immunol. 2004;26:487. doi: 10.1111/j.0141-9838.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 32.Porto AF, Santos SB, Alcantara L, Guerreiro JB, Passos J, Gonzalez T, et al. HTLV-1 modifies the clinical and immunological response to schistosomiasis. Clin Exp Immunol. 2004;137:424. doi: 10.1111/j.1365-2249.2004.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdonck K, Gonzalez E, Schrooten W, Vanham G, Gotuzzo E. HTLV-1 infection is associated with a history of active tuberculosis among family members of HTLV-1-infected patients in Peru. Epidemiol Infect. 2008;136:1076. doi: 10.1017/S0950268807009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biasutti C, Moraes-Pinto MI, Segurado AC. Antibody response after vaccination with tetanus and diphtheria toxoids in human T-cell lymphotropic virus type 1 asymptomatic carriers. Vaccine. 2008;26:2808. doi: 10.1016/j.vaccine.2008.03.068. [DOI] [PubMed] [Google Scholar]
- 35.Grubeck-Loebenstein B, Wick G. The aging of the immune system. Adv Immunol. 2002;80:243. doi: 10.1016/s0065-2776(02)80017-7. [DOI] [PubMed] [Google Scholar]
- 36.Hainz U, Jenewein B, Asch E, Pfeiffer KP, Berger P, Grubeck-Loebenstein B. Insufficient protection for healthy elderly adults by tetanus and TBE vaccines. Vaccine. 2005;23:3232. doi: 10.1016/j.vaccine.2005.01.085. [DOI] [PubMed] [Google Scholar]
- 37.Kaml M, Weiskirchner I, Keller M, Luft T, Hoster E, Hasford J, et al. Booster vaccination in the elderly: their success depends on the vaccine type applied earlier in life as well as on pre-vaccination antibody titers. Vaccine. 2006;24:6808. doi: 10.1016/j.vaccine.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 38.Kroon FP, van Tol MJ, Jol-van der Zijde CM, van Furth R, van Dissel JT. Immunoglobulin G (IgG) subclass distribution and IgG1 avidity of antibodies in human immunodeficiency virus-infected individuals after revaccination with tetanus toxoid. Clin Diagn Lab Immunol. 1999;6:352. doi: 10.1128/cdli.6.3.352-355.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien WA, Grovit-Ferbas K, Namazi A, Ovcak-Derzic S, Wang HJ, Park J, et al. Human immunodeficiency virus-type 1 replication can be increased in peripheral blood of seropositive patients after influenza vaccination. Blood. 1995;86:1082. [PubMed] [Google Scholar]
- 40.Rosok B, Voltersvik P, Bjerknes R, Axelsson M, Haaheim LR, Asjo B. Dynamics of HIV-1 replication following influenza vaccination of HIV+ individuals. Clin Exp Immunol. 1996;104:203. doi: 10.1046/j.1365-2249.1996.25732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos SB, Porto AF, Muniz AL, de Jesus AR, Magalhaes E, Melo A, et al. Exacerbated inflammatory cellular immune response characteristics of HAM/TSP is observed in a large proportion of HTLV-I asymptomatic carriers. BMC Infect Dis. 2004;4:7. doi: 10.1186/1471-2334-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawano Y, Noma T, Yata J. Regulation of human IgG subclass production by cytokines. IFN-gamma and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J Immunol. 1994;153:4948. [PubMed] [Google Scholar]
- 43.Rubin RL, Tang FL, Lucas AH, Spiegelberg HL, Tan EM. IgG subclasses of anti-tetanus toxoid antibodies in adult and newborn normal subjects and in patients with systemic lupus erythematosus, Sjogren’s syndrome, and drug-induced autoimmunity. J Immunol. 1986;137:2522. [PubMed] [Google Scholar]
- 44.Manuel SL, Sehgal M, Khan ZK, Goedert JJ, Betts MR, Jain P. An altered maturation and adhesion phenotype of dendritic cells in diseased individuals compared to asymptomatic carriers of human T cell leukemia virus type 1. AIDS Res Hum Retroviruses. 2013;29:1273. doi: 10.1089/aid.2013.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montes M, Sanchez C, Verdonck K, Lake JE, Gonzalez E, Lopez G, et al. Regulatory T cell expansion in HTLV-1 and strongyloidiasis co-infection is associated with reduced IL-5 responses to Strongyloides stercoralis antigen. PLoS Negl Trop Dis. 2009;3:e456. doi: 10.1371/journal.pntd.0000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neva FA, Filho JO, Gam AA, Thompson R, Freitas V, Melo A, et al. Interferon-gamma and interleukin-4 responses in relation to serum IgE levels in persons infected with human T lymphotropic virus type I and Strongyloides stercoralis. J Infect Dis. 1998;178:1856. doi: 10.1086/314507. [DOI] [PubMed] [Google Scholar]
- 47.Jain P, Ahuja J, Khan ZK, Shimizu S, Meucci O, Jennings SR, et al. Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type 1. J Leukoc Biol. 2007;82:44. doi: 10.1189/jlb.1006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manuel SL, Schell TD, Acheampong E, Rahman S, Khan ZK, Jain P. Presentation of human T cell leukemia virus type 1 (HTLV-1) Tax protein by dendritic cells: the underlying mechanism of HTLV-1-associated neuroinflammatory disease. J Leukoc Biol. 2009;86:1205. doi: 10.1189/jlb.0309172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santos SB, Oliveira P, Luna T, Souza A, Nascimento M, Siqueira I, et al. Immunological and viral features in patients with overactive bladder associated with human T-cell lymphotropic virus type 1 infection. J Med Virol. 2012;84:1809. doi: 10.1002/jmv.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hishizawa M, Imada K, Kitawaki T, Ueda M, Kadowaki N, Uchiyama T. Depletion and impaired interferon-alpha-producing capacity of blood plasmacytoid dendritic cells in human T-cell leukaemia virus type I-infected individuals. Br J Haematol. 2004;125:568. doi: 10.1111/j.1365-2141.2004.04956.x. [DOI] [PubMed] [Google Scholar]
- 51.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 52.Bastos MD, Santos S, Souza A, Finkmoore B, Bispo O, Barreto T, et al. Influence of HTLV-1 on the clinical, microbiologic and immunologic presentation of tuberculosis. BMC Infect Dis. 2012;12:199. doi: 10.1186/1471-2334-12-199. [DOI] [PMC free article] [PubMed] [Google Scholar]