Abstract
Effective uptake of tumor cell-derived antigens by antigen-presenting cells is achieved pre-clinically by in situ labeling of tumor with α-gal glycolipids that bind the naturally occurring anti-Gal antibody. We evaluated toxicity and feasibility of intratumoral injections of α-gal glycolipids as an autologous tumor antigen-targeted immunotherapy in melanoma patients (pts). Pts with unresectable metastatic melanoma, at least one cutaneous, subcutaneous, or palpable lymph node metastasis, and serum anti-Gal titer ≥1:50 were eligible for two intratumoral α-gal glycolipid injections given 4 weeks apart (cohort I: 0.1 mg/injection; cohort II: 1.0 mg/injection; cohort III: 10 mg/injection). Monitoring included blood for clinical, autoimmune, and immunological analyses and core tumor biopsies. Treatment outcome was determined 8 weeks after the first α-gal glycolipid injection. Nine pts received two intratumoral injections of α-gal glycolipids (3 pts/cohort). Injection-site toxicity was mild, and no systemic toxicity or autoimmunity could be attributed to the therapy. Two pts had stable disease by RECIST lasting 8 and 7 months. Tumor nodule biopsies revealed minimal to no change in inflammatory infiltrate between pre- and post-treatment biopsies except for 1 pt (cohort III) with a post-treatment inflammatory infiltrate. Two and four weeks post-injection, treated nodules in 5 of 9 pts exhibited tumor cell necrosis without neutrophilic or lymphocytic inflammatory response. Non-treated tumor nodules in 2 of 4 evaluable pts also showed necrosis. Repeated intratumoral injections of α-gal glycolipids are well tolerated, and tumor necrosis was seen in some tumor nodule biopsies after tumor injection with α-gal glycolipids.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-016-1846-1) contains supplementary material, which is available to authorized users.
Keywords: Immunotherapy, α-Gal glycolipids, Cancer vaccines, Melanoma
Introduction
The American Cancer Society projects 76,380 new melanoma cases and 10,130 deaths due to melanoma in the USA in 2016 [1]. While melanoma is usually incurable once metastatic to distant sites, several laboratory insights about melanoma are changing clinical practice [2–5]. Germane to this study is the insight that melanomas express antigens that can be recognized by T cells, some of which are patient-specific mutant epitopes (i.e., neoantigens) [6]. This is clinically important as T cell-directed immunotherapies, such as treatment with immune checkpoint blockade, can mediate durable tumor regression in some melanoma patients [7–9]. However, durable antitumor responses are only realized in a minority of melanoma patients receiving these treatments. The genomic landscape in human melanoma from individual patients [10, 11], like that in other human tumors [12, 13], demonstrates multiple non-synonymous substitutions, deletions, and insertions in protein-coding sequences that alter amino acid sequences in tumor cells. Whether these mutations appear in driver or passenger genes, it is reasonable to assume that such de novo protein sequences may serve as target autologous tumor-associated antigens (TAA) for induction of protective immune responses. Treatments that stimulate an effective immune response against multiple autologous TAA could provide important clinical benefit for patients with advanced melanoma as well as patients with other types of cancers.
One of the main prerequisites for eliciting an effective antiautologous TAA immune response is the targeting of the TAA for effective uptake by APC. This was found to be feasible by intratumoral injection of α-gal glycolipids [14–16]. α-Gal glycolipids are a mixture of glycolipids carrying a carbohydrate antigen called the “α-gal epitope” with the structure Galα1-3Galβ1-4GlcNAc-R. The α-gal epitope is the ligand of the natural anti-Gal antibody which is the most abundant natural antibody in humans constituting ~1 % of serum immunoglobulins in healthy individuals as well as in cancer patients [17–20]. Whereas the anti-Gal antibody is naturally produced only in humans, apes, and Old World monkeys, the α-gal epitope is naturally synthesized and millions of epitopes per cell are expressed on most tissues in non-primate mammals, prosimians, and New World monkeys [17, 18, 21–23].
When injected into tumor lesions, α-gal glycolipids insert spontaneously by their “fatty acid tail” into tumor cell membranes and bind the natural anti-Gal antibody. This antigen/antibody interaction results in activation of complement and recruitment of cells able to induce antibody-dependent cell-mediated cytotoxicity (ADCC), as well as recruitment of APC such as dendritic cells (DCs) and macrophages, into treated lesion [14]. The interaction between the Fc portion of anti-Gal bound to α-gal glycolipids inserted into the tumor cell membranes and Fc receptors of the effector cells and APC results in ADCC as well as internalization into APC of tumor cells or tumor cell membranes coated with anti-Gal. The APC transport the TAA of the internalized tumor cells to draining lymph nodes (LNs) where they process and present immunogenic TAA peptides for the activation of tumor specific T cells. This treatment thus results in anti-Gal-mediated destruction of tumor cells in the injected tumor lesions and conversion of such lesions into an autologous cancer vaccine without having to characterize the various TAA in the treated patient [14, 16].
The primary objectives of this study were to (1) determine the toxicity of α-gal glycolipids injected intratumorally in advanced melanoma patients and (2) identify the maximum tolerated dose (MTD) of α-gal glycolipids in this patient population. The secondary objectives of this study included (1) assess response (both treated and untreated lesions) to intratumoral injection of α-gal glycolipids in advanced melanoma patients using RECIST and (2) evaluate, in HLA-A2+ treated patients, the development of an immune response to common TAAs.
Materials and methods
Clinical protocol and patients
From September 2010 through August 2013, nine patients with advanced melanoma participated in this trial [University of Wisconsin (UW) Carbone Cancer Center Protocol CO08604]. The UW Human Subjects Committee and the FDA approved the study (IND 12946). All patients signed informed consent forms and registered with the Biostatistics Registration Desk prior to treatment. All patients had advanced unresectable histologically proven melanoma (recurrent stage III or stage IV) that was refractory to therapy or without known effective or curative therapy and with at least one readily resectable cutaneous, subcutaneous, or readily palpable lymph node metastasis. Measurable disease other than the lesion selected for serial biopsy was desirable but not required. Patients could have received any prior number of therapies. Patients needed to have an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–2. Patient needed to have adequate end organ function defined as the following: total bilirubin < 1.5 the upper limit of normal (ULN); AST and ALT < 2.5 × ULN; Creatinine < 1.5 × ULN; hemoglobin > 10.0, platelet count > 100,000, and WBC > 3000/mm3. Patients were excluded from this study if they had a history of autoimmune disease, required treatment with chemotherapy or steroids, or had significant intercurrent illnesses. Patients needed to have a serum anti-Gal titer above 1:50 as assayed by ELISA with synthetic α-gal epitopes linked to BSA as solid-phase antigen [24].
α-Gal glycolipid description, preparation, and delivery
α-Gal glycolipids were extracted at University of Massachusetts Medical School from rabbit red cell membranes as previously described [14]. Batches of 1 l of packed rabbit red cells (Pelfreez Biologicals, AK) were lysed by hypotonic shock and washed to remove hemoglobin. The red cell membranes were mixed by stirring in a solution of 600 ml chloroform and 1200 ml methanol for 20 h. After filtration for removal of particulate material, 400 ml of pyrogen-free, sterile, distilled water was gradually added to generate “Folch Partition” [25] that separates between ~600 ml lower organic phase (containing chloroform, methanol, phospholipids, cholesterol, and glycolipids with three carbohydrate chain lacking α-gal epitopes) and ~1500 ml upper aqueous phase. The aqueous phase contained most of the α-gal glycolipids with 5–40 carbohydrate units and having 1–8 carbohydrate branches (antennae) all capped with α-gal epitopes [14] and was dried in a rotary evaporator. The α-gal glycolipids were dissolved in sterile water as micelles and brought to a concentration of 10 mg/ml. The α-gal glycolipid micelles were sonicated to disperse large micelles and underwent final sterilization by filtration through a 0.2 μm Amicon filter. Between 300 and 400 mg of α-gal glycolipids could be prepared from each 1 l of packed rabbit red cells.
The planned injection volume was 1.0 ml for each of the three dose levels. The concentrations were 0.1, 1.0, and 10 mg/ml of α-gal glycolipids micelles. However, patients with a small tumor that could not be injected with 1 ml could receive the planned dose in an injection of 0.5 ml of 0.2, 2.0, or 20 mg/ml in each dose level. The dose (0.1, 1.0, or 10 mg) was injected throughout the target lesion in 3–10 aliquots.
Study design and biopsy schedule
Patients received a total of two injections of α-gal glycolipids administered 4 weeks apart (Supplementary Fig. S1). Blood samples for clinical, immunological, and autoimmune monitoring were obtained pre-treatment and 4 weeks after each treatment. In addition, clinical laboratories were obtained 24 h and 2 weeks after each treatment (Supplementary Fig. S1). Prior to the second treatment of α-gal glycolipids, patients received an intradermal injection in an extremity with 10 μg of α-gal glycolipids given as 0.1 ml from a concentration of 0.1 mg/ml and then observed for 1 h to test for allergic reaction to the treatment. A second α-gal glycolipids injection at the assigned dose level was then administered into the tumor lesion. The treated lesions were evaluated by physical examination prior to each treatment and 2 and 4 weeks following each injection. Core biopsies were obtained from the treated lesion prior to the first injection, 2 weeks following the first injection and 4 weeks following the second injection. The sizes of the treated lesions, as well as the sizes of selected untreated lesions, were monitored after the patient completed treatment in the study during regular clinic visits. Other lesions, in addition to the treated lesion, could be biopsied for evaluation of antitumor activity at sites distant from the treated lesion.
Toxicity grading and assessment
Both local and systemic toxicity to the α-gal glycolipids injections was assessed according to the NCI-CTC, version 3.0. A history and physical examination were obtained for each patient at baseline, then again on Days 1, 2, and 15 during each of the two 4-week treatment courses. Standard laboratory toxicities were assessed regularly throughout the study period. To assess local toxicity due to the therapy, injection sites were examined regularly while patients were on study and the sites were graded for “injection-site reactions.” Long-term follow-up and toxicity assessments were planned at 3, 6, and 12 months after completion of the study, after which patients were followed clinically for disease progression and survival.
Response criteria
The response of the tumor directly injected with the α-gal glycolipids to the treatment was evaluated 2, 4, and 8 weeks after injection, by directly measuring size of the injected tumor. Overall antitumor response (evaluating all sites of tumor per RECIST 1.0) included physical examination and CT scan measurements; criteria were applied to measurements obtained 8 weeks following initial injection with α-gal glycolipids [26]. Evaluation of antitumor responses included physical examination and CT scan measurements.
Clinical and laboratory immunological monitoring
Autoimmunity: Clinical evidence of an autoimmune response included rash, arthritis, arthralgia, serositis, vasculitis, and vitiligo. In addition, sera were obtained from patients to monitor for erythrocyte sedimentation rate (ESR) and antinuclear antibodies (ANA) pre-treatment, Day 15, Day 29, Day 43, Day 57, and at the 12 week, 24 week, and 1 year time points.
Processing and histological analyses of injection-site biopsies: Core biopsies were obtained from treated and untreated lesions (see biopsy schedule in Supplementary Fig. S1). Biopsies were bisected, half kept frozen, in OCT for further potential studies, and half fixed in 10 % buffered formalin and processed for H&E staining and immunohistochemistry studies. The expression of PD-L1 (mAb clone 5H1) [27] on the surface of tumor cells and on infiltrating macrophage/DC-like cells in tumor biopsies was assessed in targeted and non-targeted tumor biopsies pre-treatment as well as 2 weeks after the first injection and 4 weeks following the second injection.
Immunological monitoring: PBMC (HLA-A2+ patients) were incubated for 10 min at 25 °C with 1 μg each of the following HLA-A*0201 unlabeled TAA pentamers: gp100209–217(210M), gp100154–162, MelanA/MART-126–35(27L), NY-ESO-1157–165, and Tyrosinase369–377(371D) (ProImmune). Cells stained with CMV pp65495–504 or HTLV-1 Tax11–19 served as positive or negative controls, respectively. Cells were washed and counterstained with the viability dye ViViD (Invitrogen), Alexa 700-conjugated anti-CD3 (UCHT1), FITC-conjugated anti-CD8 (LT8, ProImmune), APC-Cy7-conjugated anti-CD4 (RPA-T4, BD), Pacific Blue-conjugated anti-CD14 (M5E2), eFluor450-conjuagted anti-CD19 (HIB19), plus 2.5 tests of APC-labeled Fluorotag (ProImmune) for 20 min on ice. Flurotag is an APC tagged protein that binds specifically to unlabeled pentamers and is used as a secondary reagent. Reagents were from eBioscience, unless otherwise indicated. Data were acquired on a LSR II cytometer and analyzed with FlowJo 9.6.4. Following exclusion of doublets, CD4+, CD14+, CD19+, dye aggregates and dead cells, CD3+ CD8+ pentamer+ cells were examined.
Statistical methods
The study design was for escalating doses of α-gal glycolipids to be administered intratumorally in cohorts of three patients. If no dose-limiting toxicity (DLT) was observed in the first three patients by Day 57, the next dose level was to be accrued. If one DLT occurred, three additional patients needed to be accrued at the same dose level. If no additional DLTs were observed, the next dose level was accrued. However, if two or more DLTs were observed in a given dose level, the MTD was defined as the next lowest dose level.
Regarding the immunological monitoring, logistic regression analysis was used to analyze the rates of TAA pentamer+ T cells after gates were constructed from background measurements. Factors in the logistic regression analysis included patient identifier (eight patients), cell condition (background/TAA+) and tissue sampling time (pre-/post-), and the logistic regression was fitted to data using glm in R [28]. Regarding the tumor necrosis data, McNemar’s test was used to assess effects of treatment. The analyses of PD-L1 expression were exploratory.
Results
Patient characteristics
Nine patients were entered into this study between September 2010 and May 2013; their pre-treatment characteristics are given in Table 1. The median patient age at the time of enrollment was 63 years (range 42–77 years). Four of the nine patients were women. All nine patients had an ECOG Performance Status of 0 or 1. All nine patients had been treated surgically before entering the study, two had received prior treatment with a BRAF inhibitor and MEK inhibitor, and one had received prior ipilimumab. The most common sites of metastatic disease were the lungs (n = 7) and distant LNs, skin, or subcutaneous tissue (n = 6 with at least one of the above). One patient had recurrent stage III disease at the time of study entry.
Table 1.
Patient demographics
| No. of patients | |
|---|---|
| Total no. of patients | 9 |
| Sex | |
| Male | 5 |
| Female | 4 |
| Primary site | |
| Cutaneous | 7 |
| Acral | 1 |
| Ocular | 1 |
| Prior therapy | |
| Surgery | 9 |
| Isolated limb perfusion | 1 |
| Radiation | 4 |
| Adjuvant interferon | 4 |
| Temozolomide | 3 |
| BRAF inhibitor | 2 |
| BRAF inhibitor + MEK inhibitor | 2 |
| Ipilimumab | 1 |
| hu14.18-IL2 | 1 |
| Sites of metastatic disease | |
| Regional metastases alone | 1 |
| Distant node, skin, subcutaneous | 6 |
| Lung | 7 |
| Other visceral metastasesa | 3 |
| Disease status | |
| III, recurrent | 1 |
| M1ab | 1 |
| M1bc | 4 |
| M1 cd | 3 |
| Performance Statuse | |
| 0 | 6 |
| 1 | 3 |
Median age, 63 years; range 42–77 years
aAdrenal, bone, liver, pleura
bMetastatic disease to distant nodes, skin, or subcutaneous lesions
cMetastatic disease to lung
dMetastatic disease to other visceral sites or M1a, M1b disease with elevated lactate dehydrogenase
eEastern Cooperative Oncology Group Performance Status
Treatment summary
The pre-treatment anti-Gal titers, α-gal dose levels, and administered treatment volumes of α-gal glycolipids are given in Supplementary Table S1. All doses were administered according to the pre-determined schedule. None of the patients experienced allergic reaction to the α-gal glycolipids injection, DLT, or Grade 3 or higher treatment-related toxicities. Grade 1 and 2 toxicities were not prospectively collected. The local toxicities at the sites of α-gal glycolipid injections were mild and included erythema, swelling, pain, and tenderness (Table 2). No clinical autoimmune toxicity was detected, based on the absence of clinical rash, arthritis, arthralgia, serositis, vasculitis or vitiligo, and no significant increase in laboratory evidence of ESR or ANA (data not shown).
Table 2.
Treatment summary
| Patient ID | Best overall responseb | Duration of best overall response (months) | Target lesion measurement (mm)a | Site reactions | |||
|---|---|---|---|---|---|---|---|
| Baseline | 4 wk. post-first injection | 4 wk. post-second injection | First injection | Second injection | |||
| UW001 | SD | 8.2c | 33 | 31 | 31 | S | E, S |
| UW002 | PD | 23 | 31 | 44 | E, S | E, T | |
| UW003 | PD | 20 | 19 | 24 | |||
| UW004 | PD | 20 | 22 | 34 | |||
| UW005 | PD | 22 | 22 | 32 | P | S | |
| UW006 | SD | 7.5c | 9 | 9 | 10 | E | |
| UW007 | PD | 9 | 14 | 22 | E, S, P | E, S, T | |
| UW008 | PD | 12 | 10 | 10 | E, S | ||
| UW009 | PD | 10 | 15 | 15 | S, T | S, T | |
There were no Grade 3 or higher treatment-related toxicities. Grade 1 and 2 toxicities were not prospectively collected
There was no clinical autoimmunity and no induction of pathological autoantibodies
PD progressive disease (per RECIST 1.0), SD stable disease (per RECIST 1.0), S swelling, E erythema, T tenderness, P pain
aThe treated tumor lesions were the target lesions
bPatients had a physical examination including evaluation of treated tumor lesions and repeat radiological imaging on Day 57 (±3 business days)
cFrom Day 57 scan
Antitumor effects
The best clinical response and the duration of that response are given in Table 2. All nine patients had clinically evaluable disease at the time of study enrollment; two patients maintained stable disease for 8.2 months (UW001) and 7.5 months (UW006) after receiving two injections of α-gal glycolipids administered 4 weeks apart; the remaining seven patients had disease progression. The injected lesions (target lesions) were individually measured at baseline as well as 4 weeks after each injection of α-gal glycolipids. Three of the injected lesions were stable in size 4 weeks after the second of α-gal glycolipids injection, and the remaining six lesions had at least a 20 % increase in measurement of the longest diameter 4 weeks after the second α-gal glycolipids injection (Table 2).
Histology of tumor biopsies
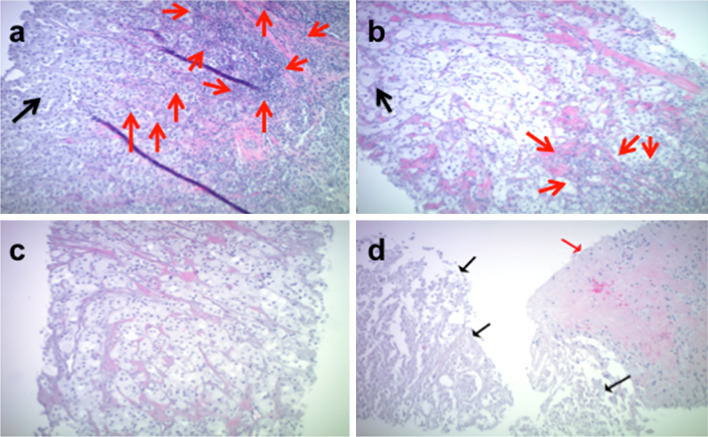
Tumor nodules that were targeted for α-gal glycolipids injection, and where available, tumor nodules not targeted for injection, were needle core biopsied pre-treatment and evaluated on H&E stained sections. Targeted and non-targeted nodules were re-biopsied 2 weeks after the first injection and at 8 weeks (4 weeks following the second injection). Representative histological analyses from a single patient (UW002) are shown in Fig. 1, and the tumor biopsy summary from all patients is given in Table 3. Minimal to no changes in inflammatory infiltrate were noted between biopsies. Tumor cell necrosis, however, appeared to increase following therapy (Table 3). In some cases, this appeared as small foci of tumor necrosis and apoptosis amidst otherwise viable melanoma cells, while in others large diffuse areas of tumor necrosis were noted, which did not appear to be associated with either neutrophilic or lymphocytic inflammatory responses. Overall, an increased tumor necrosis was observed in the injected lesion in five of nine patients 2 weeks after the first injection. The degree of necrosis was decreased, maintained, or increased, in two, two, or one, respectively, of these five patients 4 weeks after the second injection. The remaining four patients did not exhibit necrosis in the injected lesion. However, one of these four patients had an intense inflammatory infiltrate that obscured tumor cell evaluation at both post-treatment time points. Biopsies of non-targeted lesions were collected 4 weeks post-second injection and two of four patients had evidence of necrosis. One patient had focal necrosis in a non-targeted lesion at pre-treatment; this lesion was not evaluable at later time points. Interestingly, the melanoma cells of the patient (UW002) who did not show frank necrosis showed a peculiar ballooning type of degeneration with a frothy, ground-glass-appearing cytoplasm at both biopsy time points in the target lesion, as well as a diffuse necrosis of all tumor cells in the non-target lesion 4 weeks after the second injection (Fig. 1).
Fig. 1.
Tumor biopsy histology. Representative histological analyses (H&E) from UW002 (×10 magnification). Target lesion, panels (a–c): a pre-treatment; nests of tumor cells with foamy, gray cytoplasm (black arrow). Lymphocytic inflammation is primarily at the edge of the tumor, but also infiltrates within the tumor cells (red arrows). b 2 weeks post-first injection; melanoma cells exhibit ballooning of their cytoplasm which now appears “foamy” (black arrow). A small patch of lymphoid cells is present in the tumor (red arrows). c 4 weeks post-second injection; Tumor cells with atypical ballooned, foamy appearance in the absence of inflammation or necrosis. d Non-target lesion, 4 weeks post-second injection; the tumor cells are necrotic (black arrows) with an area of collagenous fibrous tissue infiltrated by lymphocytes (red arrow)
Table 3.
Tumor biopsy necrosis
| Patient ID | Pre-treatment | 2 wk. post-first injection | 4 wk. post-second injection | |||
|---|---|---|---|---|---|---|
| Target | Non-target | Target | Non-target | Target | Non-target | |
| UW001a,b | F | ND | D | ND | F | ND |
| UW002a,c | Neg | N/A | B | ND | B | D |
| UW003a,d | Neg | Neg | F | ND | D | Neg |
| UW004a,c | Neg | Neg | F | ND | F | F |
| UW005a,b | Neg | N/A | F | ND | Neg | Neg |
| UW006 | Neg | Neg | Neg | ND | Neg | ND |
| UW007 | Neg | F | Neg | ND | Neg | ND |
| UW008 | Neg | ND | HI | ND | HI | ND |
| UW009 | Neg | ND | Neg | ND | Neg | ND |
Tumor biopsies were obtained to assess effects of treatment in both injected “target” and non-injected “non-target” lesions. No significant differences were found using necrosis as an indicator of treatment-associated effect in target lesions pre-treatment vs. 2 wk. post-first injection or vs. 4 wk. post-second injection (McNemar’s exact binomial test)
F small areas of focal necrosis, ND biopsy not done, D larger areas of diffuse necrosis, Neg no necrosis, N/A inadequate tissue for staining, B ballooning degeneration of tumor cells with necrosis, HI heavy inflammatory infiltrate, difficult to assess the presence of tumor cells
aIncreased necrosis 2 wk. post-first injection vs. pre-treatment
bDecreased necrosis at 4 wk. post-second injection vs. 2 wk. post-first injection
cNo change in necrosis at 4 wk. post-second injection vs. 2 wk. post-first injection
dIncreased necrosis at 4 wk. post-second injection vs. 2 wk. post-first injection
Exploratory analyses of the expression of PD-L1 on the surface of tumor cells and on infiltrating macrophage/DC-like cells in tumor biopsies demonstrated that three of eight evaluable patients in this study had increased PD-L1 expression on infiltrating macrophage/DC-like cells following treatment with α-gal glycolipids (Supplementary Table S2a). Two of eight evaluable biopsies contained PD-L1+ tumor cells at pre-treatment, whereas six of eight were negative. Of these six negative biopsies, one patient (UW003) had some PD-L1+ tumor cells following treatment with α-gal glycolipids (both time points) (Supplementary Table S2b). This same patient showed strong up-regulation of PD-L1 expression on tumor cells in a distant, non-injected tumor nodule following treatment (Supplementary Fig. S2).
TAA pentamer+ T cells detected pre- and post-treatment with α-gal glycolipids
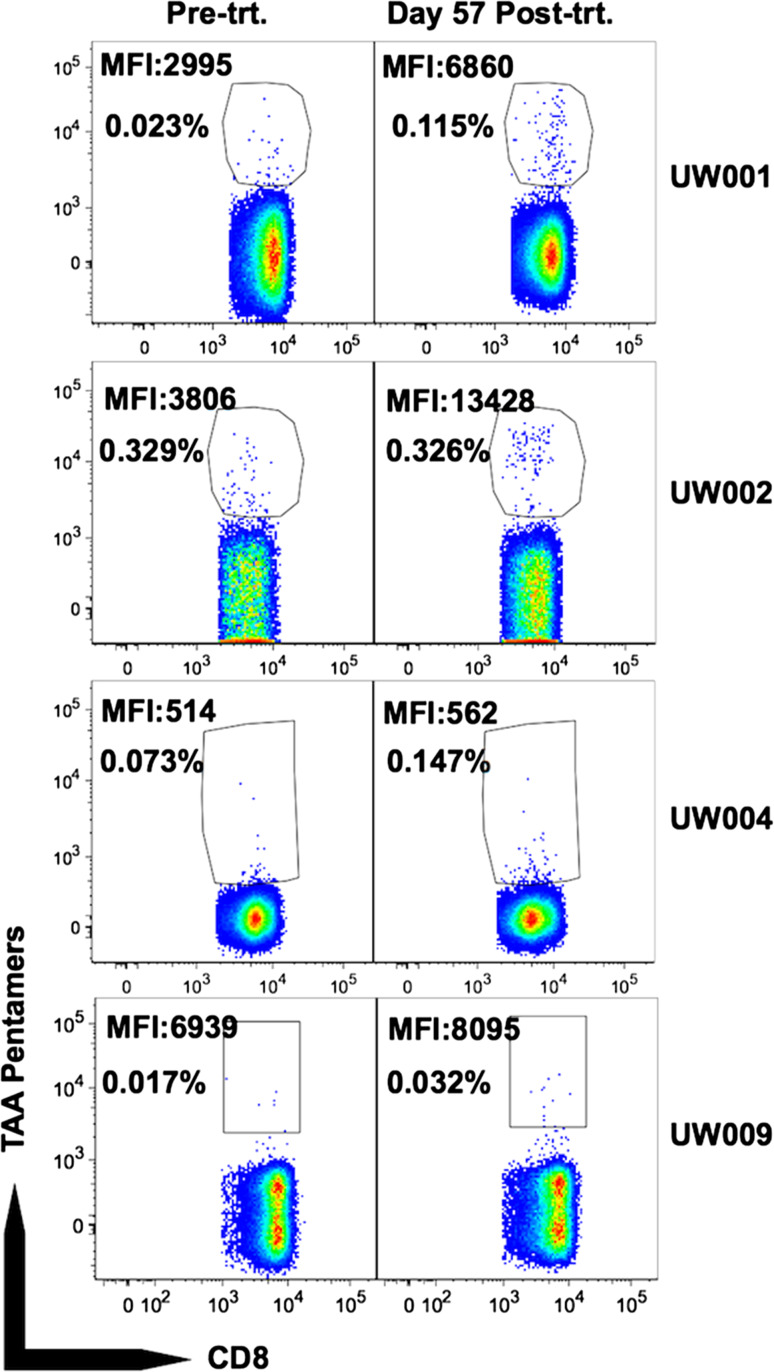
Pre-treatment and Day 57 post-treatment PBMC from HLA-A2+ patients (eight of nine treated patients are HLA-A2+; UW008 is HLA-A2−) were incubated with pooled TAA pentamers. An HTLV-1 pentamer was used as a negative control to establish the pentamer+ gate. TAA pentamer+ cells were observed in all patients, both pre- and post-treatment, and their frequency was above that following staining with the negative control HTLV-1 reagent. An approximate two- to fivefold increase in the frequency of TAA pentamer+ cells post- versus pre-treatment was observed for three of eight patients (UW001, UW004, and UW009) (Fig. 2; Table 4). In the remainder of patients, the frequency of TAA pentamer+ cells post-treatment was not substantially boosted (UW002, UW003 and UW007), or was decreased (UW005 and UW006), as compared to pre-treatment (Fig. 2; Table 4). The median fluorescence intensity (MFI) of the TAA pentamer+ T cells was markedly higher post-treatment as compared to pre-treatment for both UW001 and UW002, and slightly less so for UW009 (Fig. 2; Table 4). Logistic regression analysis (methods) indicated a significantly higher rate of TAA pentamer+ T cells among samples stained with TAA pentamers (p < 0.0001), as one would expect; it also indicated that post-treatment samples had slightly higher rates of TAA+ cells, though the observed increase was not statistically significant (p = 0.26).
Fig. 2.
Tumor antigen-specific T cells pre- and post-treatment. Pre-treatment (Pre-trt.) and Day 57 post-treatment (Post-trt.) PBMC from indicated patients were stained and gated as described in “Materials and methods” section. Values in plots are MFI of pentamer+ T cells, and % pentamer+ of CD8+ T cells after background subtraction
Table 4.
Pentamer data
| Patient ID | Pre-treatment | 4 wk. post-second injection | ||
|---|---|---|---|---|
| % Pentamer+a,b | MFI pentamer+c | % Pentamer+a,b | MFI pentamer+c | |
| UW001 | 0.023 | 2995 | 0.115 | 6860 |
| UW002 | 0.329 | 3806 | 0.326 | 13,428 |
| UW003 | 0.007 | 2460 | 0.011 | 3078 |
| UW004 | 0.073 | 514 | 0.147 | 562 |
| UW005 | 0.173 | 538 | 0.07 | 521 |
| UW006 | 0.112 | 758 | 0.06 | 585 |
| UW007 | 0.104 | 5792 | 0.095 | 5972 |
| UW008d | ND | ND | ND | ND |
| UW009 | 0.017 | 6939 | 0.032 | 8095 |
MFI median fluorescence intensity, ND not done
a% TAA pentamer+ minus % negative control HTLV-1 Tax11–19 pentamer+
bFrequency of TAA pentamer+ T cells was significantly higher than that of control pentamer+ T cells (p < 0.0001, logistic regression analysis). Frequency of TAA pentamer+ T cells was slightly higher post-treatment versus pre-treatment, but this difference was not statistically significant (p = 0.26, logistic regression analysis)
cMFI of TAA pentamer+
dUW008 is HLA-A2−; therefore, pentamer assay is not done
Discussion
This study is the first report of the clinical safety and biologic activity of repeated intratumoral injections with α-gal glycolipids as means for converting tumor lesions into autologous vaccines. A recent study demonstrated clinical safety of a single intratumoral injection with α-gal glycolipids [29]. However, it was essential to determine if repeated injections of α-gal glycolipids were safe and to biopsy injected tumor nodules before and after treatment as it was anticipated that induction of inflammation in melanoma lesions, recruitment of APC to the treated lesions, and effective transport of TAA to draining LNs by the APC would require multiple intratumoral injections with α-gal glycolipids. We now report that two intratumoral injections of α-gal glycolipids, separated in time by 4 weeks and at a dose up to 10 mg/lesion, are well tolerated. No dose-limiting toxicity was seen in any patient. Toxicity to this immunotherapy was limited to local injection-site reactions. The MTD of α-gal glycolipids for intratumoral injections was not determined, as no Grade 3 or higher treatment-associated toxicity was seen at the doses evaluated in this study. We also detected tumor necrosis in the majority of melanoma tumor nodules after intratumoral injection with α-gal glycolipids. However, we did not observe dramatic changes in cell infiltrates between pre- and post-treatment biopsies in this study. Patients who received chemotherapy, immunotherapy, or other investigational therapy within the preceding 4 weeks were excluded from this study, so concomitant treatments did not mediate changes in cell infiltrates in these patients. It could be that by the time the biopsies were performed (2 weeks following the first injection and 4 weeks following the second injection), most of the recruited APC migrated with the internalized tumor cell membranes to draining LNs.
We identified no clinical toxicities to suggest treatment-associated autoimmune toxicity. We followed serial ANA levels as a relatively non-specific marker of autoimmunity, and we did not see any evidence of induction of pathologic autoantibodies in this study population. One patient (UW001) had a diagnosis of paraneoplastic necrotizing myopathy after presenting with worsening fatigue, muscle weakness, and whole-body arthralgias at the time of significant melanoma progression approximately 8 months after intratumoral injection with α-gal glycolipids. The necrotizing myopathy diagnosis was considered unlikely to be related to the patient’s prior treatment with α-gal glycolipids due to (1) delayed timing related to prior protocol therapy; (2) lack of an inflammatory cell infiltrate in the muscle biopsy; and (3) lack of detectable autoantibodies to striated muscle as assayed by ELISA with striated muscle tissue homogenate as solid-phase antigen [29].
The immunological monitoring performed in this study suggested a modest melanoma-specific cellular immune response. In a few patients, we observed, albeit low, increases in the proportion of T cells binding TAA pentamers, or activated to produce IFN-γ (data not shown). Importantly, however, it should be noted that these assays were done without prior in vitro stimulation to increase the precursor frequency of responding cells, but rather from freshly thawed PBMC. While we hypothesize that α-gal glycolipids injection may potentiate responses to patient-specific neoantigens, we needed to use known immunodominant A2-restricted peptides and pentamers for our studies due to the lack of autologous tumor for the functional studies [30, 31]. Thus, the finding of modest melanoma-specific cellular immune responses in our study is not entirely surprising.
Since the natural anti-Gal antibody is produced throughout life as the most abundant antibody, harnessing it for targeting vaccinating antigens that express α-gal epitopes to APC has been proposed as an effective tool in cancer immunotherapy [16, 32]. Immunization of anti-Gal producing mice with HIV [33] and influenza virus [34] vaccines engineered to have α-gal epitopes resulted in 30–100-fold increases in immunogenicity compared to vaccines without α-gal epitopes. Accordingly, the efficacy of anti-Gal in eliciting protective antimelanoma immune response was demonstrated in anti-Gal producing mice (i.e., α1,3 galactosyltransferase knockout mice) immunized with B16 melanoma cells engineered to express α-gal epitopes [35]. This immunotherapy was further validated in the mouse melanoma model with a melanoma cell line transfected with retrovirus vector containing this gene [36] and with melanoma cell line expressing the α-gal epitope and producing human MUC1 [37].
In an attempt to induce anti-Gal-mediated targeting of autologous TAA to APC, clinical trials were performed in patients with pancreatic and hepatocellular carcinomas by in vitro enzymatic synthesis of α-gal epitopes on tumor cell membranes [38, 39]. Anti-Gal further targeted these tumor cell membranes for in vitro uptake by autologous DCs that were subsequently injected, together with the tumor cell membranes, as an autologous tumor vaccine into the treated cancer patients.
The immunotherapy with α-gal glycolipids was developed in order to enable the in situ expression of α-gal epitopes on tumor cells, thereby converting them into autologous tumor vaccines targeted by anti-Gal to APC without the need of any in vitro manipulations. Immunotherapy with intratumoral injection of α-gal glycolipids can be investigated in many clinical situations, including neoadjuvant therapy in which the injected tumor serves as a temporary autologous vaccine before definitive resection of surgically resectable lesion. In addition, the simplicity of this therapy allows for intratumoral injection of α-gal glycolipids to be administered in most clinic settings without the need of specialized facilities for in vitro processing.
It is possible that α-gal glycolipids may synergize with other immunological treatments in combined immunotherapy to mediate antitumor activity in patients with advanced disease. For example, intratumoral injection of α-gal glycolipids may activate T cell clones specific to neoantigens on tumor cells that could be subsequently expanded following administration of immune checkpoint inhibitors. Recent evidence suggests that analysis of PD-L1 expression may identify melanoma patients most likely to benefit from immune checkpoint blockade [5, 7, 40]. While not an endpoint of this study, exploratory histological analyses were performed to evaluate whether the α-gal glycolipids injections impacted tumor cells and potential immune effector cells by up-regulating PD-L1 expression. Indeed, a proportion of patients showed up-regulation of PD-L1 on infiltrating macrophages in tumor nodules, and two patients showed up-regulation of PD-L1 expression on tumor cells. In addition to its presence on tumor cells, PD-L1 can be expressed on myeloid-derived DCs, monocytes, and MDSCs in humans [41–43]. Additional biomarker analysis of α-gal glycolipids treatment is needed.
In conclusion, repeated intratumoral injections with α-gal glycolipids injections are safe and well tolerated and do not elicit local or systemic allergic reactions following two injections given 4 weeks apart at a doses up to 10 mg/injection. The reason why some patients do not react to the therapy remains unknown and further investigations should address this critical issue. Because of the ubiquitous production of the natural anti-Gal antibody in humans and in view of the immunotherapeutic efficacy of α-gal glycolipids in melanoma bearing anti-Gal producing mice, we suggest future optimization of the treatment dose, alone and in combination with immune checkpoint blockade, as a strategy to enhance treatment efficacy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Michael D. Macklin for technical assistance and Laddie Johnson for assistance with manuscript preparation. The authors thank the UW Translational Research Initiatives in Pathology laboratory, in part supported by the UW Department of Pathology and Laboratory Medicine and the UW Carbone Cancer Center, for use of its facilities and services. We also thank the nurses on the UW Clinical Research Unit for outstanding nursing care and for clinical trial support.
Financial support
Support was provided by NIH Grants CA130295, P30 CA014520 from the National Cancer Institute, by resources at the William S. Middleton Memorial Veterans Hospital, Madison, WI, and by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), Grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the views of the Department of Veterans Affairs or the US Government. Additional support was provided by Ann’s Hope Foundation, the Tim Eagle Memorial, the Jay Van Sloan Memorial from the Steve Leuthold Family, the Gretchen and Andrew Dawes Melanoma Research Fund, and Agalimmune.
Abbreviations
- ADCC
Antibody-dependent cell-mediated cytotoxicity
- ANA
Antinuclear antibodies
- DLT
Dose-limiting toxicity
- ECOG
Eastern Cooperative Oncology Group
- ESR
Erythrocyte sedimentation rate
- MFI
Median fluorescence intensity
- MTD
Maximum tolerated dose
- PD
Progressive disease
- pt
Patient
- SD
Stable disease
- ULN
Upper limit of normal
- UW
University of Wisconsin
Compliance with ethical standards
Conflict of interest
The authors have the following financial or other conflicts of interests to disclose related to this publication: Uri Galili is the inventor of this immunotherapy and is a consultant to Agalimmune Inc. which further develops cancer immunotherapy with α-gal glycolipids. All other authors declare no financial or other conflicts of interests related to this publication.
Statement of human rights
The UW Human Subjects Committee and the FDA approved this study (IND 12946). Informed consent was obtained from all individual participants included in the study and all individual participants registered with the Biostatistics Registration Desk prior to treatment. The clinical trial registration number for this study is: NCT00668512. All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Callahan MK, Wolchok JD. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McArthur GA, Ribas A. Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol. 2013;31:499–506. doi: 10.1200/JCO.2012.45.5568. [DOI] [PubMed] [Google Scholar]
- 4.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 5.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rooij N, van Buuren MM, Philips D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 10.Berger MF, Hodis E, Heffernan TP, et al. Melanoma genome sequencing reveals frequent PREX2 mutations. Nature. 2012;485:502–506. doi: 10.1038/nature11071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu C, Zhang J, Nagahawatte P, et al. The genomic landscape of childhood and adolescent melanoma. J Invest Dermatol. 2015;135:816–823. doi: 10.1038/jid.2014.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kan Z, Jaiswal BS, Stinson J, et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature. 2010;466:869–873. doi: 10.1038/nature09208. [DOI] [PubMed] [Google Scholar]
- 13.McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Sci. 2016;351:1463–1469. doi: 10.1126/science.aaf1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galili U, Wigglesworth K, Abdel-Motal UM. Intratumoral injection of alpha-gal glycolipids induces xenograft-like destruction and conversion of lesions into endogenous vaccines. J Immunol. 2007;178:4676–4687. doi: 10.4049/jimmunol.178.7.4676. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Motal UM, Wigglesworth K, Galili U. Intratumoral injection of alpha-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity. Cancer Immunol Immunother. 2009;58:1545–1556. doi: 10.1007/s00262-009-0662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galili U. In situ conversion of tumors into autologous tumor-associated antigen vaccines by intratumoral injection of alpha-gal glycolipids. Oncoimmunology. 2013;2:e22449. doi: 10.4161/onci.22449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galili U, Rachmilewitz EA, Peleg A, Flechner I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med. 1984;160:1519–1531. doi: 10.1084/jem.160.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galili U, Macher BA, Buehler J, Shohet SB. Human natural anti-alpha-galactosyl IgG. II. The specific recognition of alpha(1–3)-linked galactose residues. J Exp Med. 1985;162:573–582. doi: 10.1084/jem.162.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buonomano R, Tinguely C, Rieben R, Mohacsi PJ, Nydegger UE. Quantitation and characterization of anti-Galalpha1-3Gal antibodies in sera of 200 healthy persons. Xenotransplantation. 1999;6:173–180. doi: 10.1034/j.1399-3089.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 20.Hamanova M, Zdrazilova Dubska L, Valik D, Lokaj J. Natural antibodies against alpha(1,3) galactosyl epitope in the serum of cancer patients. Epidemiol Mikrobiol Imunol. 2014;63:130–133. [PubMed] [Google Scholar]
- 21.Galili U, Clark MR, Shohet SB, Buehler J, Macher BA. Evolutionary relationship between the natural anti-Gal antibody and the Gal alpha 1–3Gal epitope in primates. Proc Natl Acad Sci USA. 1987;84:1369–1373. doi: 10.1073/pnas.84.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galili U, Shohet SB, Kobrin E, Stults CL, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem. 1988;263:17755–17762. [PubMed] [Google Scholar]
- 23.Teranishi K, Manez R, Awwad M, Cooper DK. Anti-Gal alpha 1–3Gal IgM and IgG antibody levels in sera of humans and old world non-human primates. Xenotransplantation. 2002;9:148–154. doi: 10.1034/j.1399-3089.2002.1o058.x. [DOI] [PubMed] [Google Scholar]
- 24.Stone KR, Abdel-Motal UM, Walgenbach AW, Turek TJ, Galili U. Replacement of human anterior cruciate ligaments with pig ligaments: a model for anti-non-gal antibody response in long-term xenotransplantation. Transplantation. 2007;83:211–219. doi: 10.1097/01.tp.0000250598.29377.13. [DOI] [PubMed] [Google Scholar]
- 25.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm0902-1039c. [DOI] [PubMed] [Google Scholar]
- 28.R Development Core Team (2015) R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org/. Accessed 15 March 2016
- 29.Whalen GF, Sullivan M, Piperdi B, Wasseff W, Galili U. Cancer immunotherapy by intratumoral injection of alpha-gal glycolipids. Anticancer Res. 2012;32:3861–3868. [PubMed] [Google Scholar]
- 30.Schaefer C, Butterfield LH, Lee S, Kim GG, Visus C, Albers A, Kirkwood JM, Whiteside TL. Function but not phenotype of melanoma peptide-specific CD8(+) T cells correlate with survival in a multiepitope peptide vaccine trial (ECOG 1696) Int J Cancer. 2012;131:874–884. doi: 10.1002/ijc.26481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appay V, Jandus C, Voelter V, et al. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177:1670–1678. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 32.Galili U, LaTemple DC. Natural anti-Gal antibody as a universal augmenter of autologous tumor vaccine immunogenicity. Immunol Today. 1997;18:281–285. doi: 10.1016/S0167-5699(97)80024-2. [DOI] [PubMed] [Google Scholar]
- 33.Abdel-Motal U, Wang S, Lu S, Wigglesworth K, Galili U. Increased immunogenicity of human immunodeficiency virus gp120 engineered to express Galalpha1-3Galbeta1-4GlcNAc-R epitopes. J Virol. 2006;80:6943–6951. doi: 10.1128/JVI.00310-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdel-Motal UM, Guay HM, Wigglesworth K, Welsh RM, Galili U. Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells. J Virol. 2007;81:9131–9141. doi: 10.1128/JVI.00647-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaTemple DC, Abrams JT, Zhang SY, Galili U. Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for alpha1,3galactosyltransferase. Cancer Res. 1999;59:3417–3423. [PubMed] [Google Scholar]
- 36.Rossi GR, Mautino MR, Unfer RC, Seregina TM, Vahanian N, Link CJ. Effective treatment of preexisting melanoma with whole cell vaccines expressing alpha(1,3)-galactosyl epitopes. Cancer Res. 2005;65:10555–10561. doi: 10.1158/0008-5472.CAN-05-0627. [DOI] [PubMed] [Google Scholar]
- 37.Deguchi T, Tanemura M, Miyoshi E, et al. Increased immunogenicity of tumor-associated antigen, mucin 1, engineered to express alpha-gal epitopes: a novel approach to immunotherapy in pancreatic cancer. Cancer Res. 2010;70:5259–5269. doi: 10.1158/0008-5472.CAN-09-4313. [DOI] [PubMed] [Google Scholar]
- 38.Qiu Y, Yun MM, Xu MB, Wang YZ, Yun S. Pancreatic carcinoma-specific immunotherapy using synthesised alpha-galactosyl epitope-activated immune responders: findings from a pilot study. Int J Clin Oncol. 2013;18:657–665. doi: 10.1007/s10147-012-0434-4. [DOI] [PubMed] [Google Scholar]
- 39.Qiu Y, Xu MB, Yun MM, Wang YZ, Zhang RM, Meng XK, Ou-Yang XH, Yun S. Hepatocellular carcinoma-specific immunotherapy with synthesized alpha1,3-galactosyl epitope-pulsed dendritic cells and cytokine-induced killer cells. World J Gastroenterol. 2011;17:5260–5266. doi: 10.3748/wjg.v17.i48.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24:207–212. doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan KR, Amaria RN, Ramirez O, Callihan EB, Gao D, Borakove M, Manthey E, Borges VF, McCarter MD. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol Immunother. 2013;62:1711–1722. doi: 10.1007/s00262-013-1475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan KR, Borges E, McCarter MD. Immunosuppressive myeloid-derived suppressor cells expressing PDL1 are increased in human melanoma tumor tissue. Cancer Res. 2014;74(19 Suppl):1671. doi: 10.1158/1538-7445.AM2014-1671. [DOI] [Google Scholar]
- 43.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.