Abstract
The cardiovascular safety of nicotine is an important question in the current debate on the benefits vs risks of electronic cigarettes and related public health policy. Nicotine exerts pharmacologic effects that could contribute to acute cardiovascular events and accelerated atherogenesis experienced by cigarette smokers. Studies of nicotine medications and smokeless tobacco indicate that the risks of nicotine without tobacco combustion products (cigarette smoke) are low compared to cigarette smoking, but are still of concern in people with cardiovascular disease. Electronic cigarettes deliver nicotine without combustion of tobacco and appear to pose low cardiovascular risk, at least with short term use, in healthy users.
Keywords: Cigarette smoking, electronic cigarettes, nicotine, cardiovascular disease
Introduction
Worldwide in the 21st century, tobacco smoke from cigarettes remains the single largest preventable cause of morbidity and premature death, including from cardiovascular disease (CVD), and is an urgent public health target (1, 2). As medical advances have shifted the global burden of disease from etiologies like infection and trauma to chronic disease, decreasing use of manufactured products that cause or exacerbate chronic disease is the most logical population intervention. Promoting the use of products that deliver nicotine but not combustion products has been advocated as one approach to promoting smoking cessation and reducing the harm from smoking. The objectives of this paper are to review data on the cardiovascular pharmacology and toxicology of nicotine and to assess the likelihood that products that deliver nicotine without combustion of organic materials, such as nicotine medications or electronic cigarettes, are likely to cause or aggravate cardiovascular disease.
Cigarette smoking and cardiovascular disease
To understand the potential adverse cardiovascular effects of nicotine, it is necessary to consider what we know about cigarette smoking and CVD. Cigarette smoking is one of the major causes of premature CVD around the world (1-3). Smoking markedly increases the risk of acute coronary and cerebrovascular events, including myocardial infarction, stroke and sudden death. Smoking accelerates atherogenesis producing premature atherosclerosis in epicardial coronary arteries, the aorta, carotid, and cerebral arteries, as well as peripheral circulation. Other cardiovascular effects of smoking include aggravation of stable angina pectoris, intermittent claudication, vasospastic angina, and restenosis after thrombolysis or angioplasty of coronary or peripheral arteries. Cigarette smoking also promotes progression/aggravation of heart failure, chronic kidney disease and cardiovascular morbidity and mortality in people with chronic kidney disease and increases the risk of developing atrial fibrillation.
Table 1 summarizes various adverse effects of cigarette smoking on cardiovascular health. Smokers experience acute myocardial infarction on average at a younger age than nonsmokers, and myocardial infarction is associated with more thrombus and less severe underlying atherosclerosis. Paradoxically, smokers who quit smoking after myocardial infarction have a much better prognosis than non-smokers because they have less severe underlying atherosclerosis and multiple reversible pathophysiological adverse effects caused by smoking.
Table 1.
Cardiovascular Disorders Causing by Cigarette Smoking
| Vascular Disease | Myocardial Disease |
|---|---|
| Accelerated atherosclerosis | Increases risk and aggravation of heart failure |
| Acute myocardial infarction | Hypertensive heart disease |
| Shorter exercise time to angina | |
| Coronary spasm | Inducing Cardiac Risk Factors |
| Stroke | Diabetes, type 2 |
| Aortic Aneurysm | Dyslipidemia |
| Peripheral obstructive arterial disease | Hypertension, including malignant hypertension |
| Stent thrombosis after PCI | Hypertensive renal disease |
| Graft occlusion after coronary bypass surgery | |
| Others | |
| Arrhythmias | Impaired wound healing |
| Sudden cardiac death | Erectile dysfunction |
| Atrial fibrillation | Reproductive disorders |
| Implantable debrillator shocks | Macular degeneration |
Mechanisms by which smoking causes cardiovascular disease
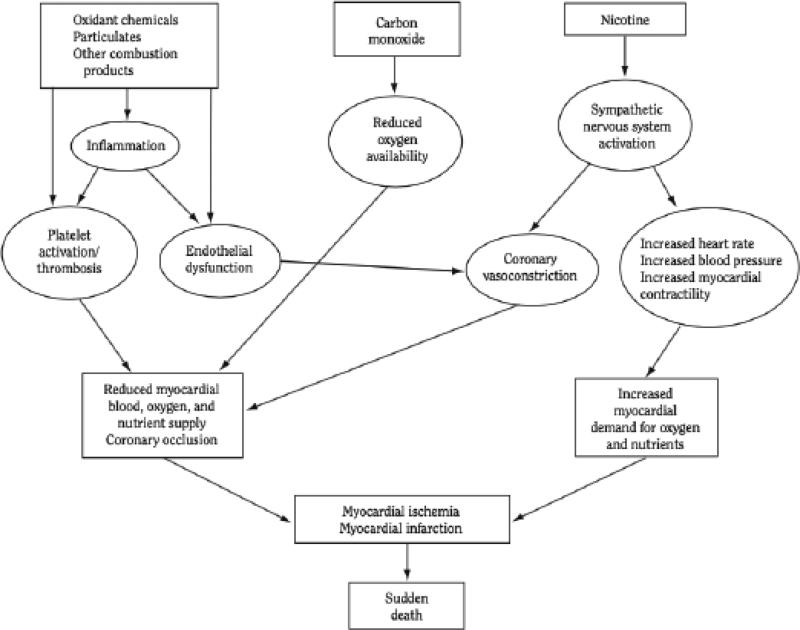
Several excellent recent reviews have examined the mechanisms by which smoking causes cardiovascular disease (3-8). In brief, the major mechanisms of smoking-induced CV disease (many of which are overlapping) are 1) oxidative injury, 2) endothelial damage and dysfunction, 3) enhanced thrombosis, 4) chronic inflammation, 5) hemodynamic stress, 6) adverse effects on blood lipids, 7) insulin resistance and diabetes, 8) reduced oxygen delivery by red blood cells, and 9) arrhythmogenesis. Enhanced angiogenesis has also been of concern, although its relevance to human cardiovascular disease has not yet been established.
Figure 1 illustrates some of the mechanisms contributing to acute cardiovascular events. Each mechanism will be discussed in more detail in relation to the effects of nicotine later in this review.
Fig 1.
Overview of mechanisms by which cigarette smoking causes an acute cardiovascular event
Constituents of tobacco smoke that contribute to CVD
Cigarette smoke contains more than 9,000 chemicals, and greater than 69 known carcinogens, the vast majority of which are products of tobacco combustion (3, 6). Constituents of most concern with respect to cardiovascular disease are: 1) oxidizing chemicals, 2) carbon monoxide, 3) volatile organic compounds 4) particulates, 5) heavy metals and 6) nicotine.
Oxidizing chemicals such as free radicals (a puff of cigarette contains 1017), reactive oxygen species, and reactive nitrogen species are thought to be the main contributors to atherogenesis and thrombogenesis from cigarette smoke. Oxidative damage occurs by endothelial cell activation, dysfunction and damage (both reducing bioavailability of nitric oxide [NO] and depleting endogenous antioxidants), inflammation, platelet activation, and lipid abnormalities (3, 7).
Chronic exposure to carbon monoxide, which can produce a carboxyhemoglobin concentration as high as 10% in heavy smokers, produces a functional anemia as it binds more readily to hemoglobin than oxygen, outcompeting for and blocking oxygen-binding sites, and impairing release of the oxygen that does bind. Carbon monoxide- related hypoxemia not only worsens preexisting conditions (angina pectoris, CHF, intermittent claudication, or COPD), it contributes to smoking-related thrombogenesis via increased blood viscosity as the body compensates by increasing red blood cell mass.
Toxic organic chemicals in cigarette smoke include polycyclic hydrocarbons (PAHs) and reactive aldehydes such as acrolein, formaldehyde and acetaldehyde. PAHs accelerate atherosclerosis in some animal models. Aldehydes form reactive oxygen species with downstream effects described above (3, 6, 7). Additionally, acrolein co-localizes with intimal atherosclerotic macrophages, modifies apolipoprotien A-1, the major protein in HDL, contributes to endothelial damage, enhances thrombogenesis (by activating platelets and inhibiting antithrombin), and causes coronary vasospasm in animal studies.
Cigarette smoke consists of an aerosol of particulates carried in a gas phase. Smoke particulates are droplets containing solid carbonaceous materials, with characteristic diameter-dependent airway deposition patterns (9). In diameters <2.5 μm they are known as “fine” and “ultra-fine” (PM2.5 and PM0.1 respectively), deposit in alveoli, and can even pass into the pulmonary and systemic circulations. Inhalation of particulates results in oxidative injury, vascular inflammation, platelet activation, increased blood viscosity, and altered cardiac autonomic function. Chronic particulate inhalation, from cigarette smoke as well as from environmental pollution, results in non-linear dose-response increases in cardiovascular risk, and is considered a modifiable risk factor for cardiovascular morbidity and mortality.
Potentially cardiotoxic metals in tobacco smoke include lead, cadmium and arsenic. Metals can oxidize intracellular proteins their deposition in serum and aortic walls contributes to endothelial damage (3, 6).
Basic Pharmacology of Nicotine
Actions of Nicotinic Cholinergic Receptors (nAChRs)
Nicotine acts on pentameric nAChRs throughout the nervous system (brain, autonomic nervous system, and skeletal-muscle), as well as some non-neuronal sites (2, 3). The subunit composition of nAChRs varies by tissue and conveys different agonist binding and electrophysiologic characteristics. Nicotine binds to the outside of the channel and activates the release of various neurotransmitters, including catecholamines. The α4 β2 receptor is thought to mediate nicotine addiction. α3 β4 receptors, present in autonomic ganglia and the adrenal gland, mediate cardiovascular responses. α7 homomeric receptors, are present not only in the brain but also in non-neuronal tissue such as endothelial cells, airway epithelial cells, inflammatory cells (lymphocytes and macrophages) and keratinocytes.
A crucial attribute of nicotinic receptors is desensitization, which results in the development of acute tolerance. In the presence of nicotine, nAChRs transition to an inactive state during which time they cannot be reactivated by nicotine. The effects of desensitization or tolerance are important in considering whether acute nicotine exposure studies, in-vitro or in animals, can be generalized to human exposure of nicotine over a prolonged period of time.
Pharmacokinetics of Nicotine
Nicotine in cigarette smoke is inhaled primarily in the particulate phase, followed by a rapid diffusion of nicotine into the vapor phase within the lungs. Nicotine from cigarette smoke is absorbed quickly and reaches the brain in 15-20 seconds after a puff, providing rapid feedback on desired psychological effects. Each puff of a cigarette delivers about 100-150ug of nicotine (but with considerable variability from person to person), and a cigarette normally delivers 1-2mg of nicotine to the systemic circulation. The result of taking a puff is a spike in arterial nicotine levels, and followed by a rapid fall off between cigarettes.
Nicotine has an average terminal half-life of two hours. With regular dosing blood nicotine levels rise over 6-8 hours, then plateau throughout the rest of the day during smoking. The pattern of human exposure is a combination of intermittent peaks and troughs throughout the day, with gradually declining nicotine exposure overnight. Typically average blood nicotine concentrations are 10-40ng/ml. Levels in the brain or heart are 2-3 fold higher.
Caveats in extrapolating nicotine basic science studies to human disease
Several caveats should be considered in extrapolating effects of nicotine in vitro and in animal studies to its effects on human disease. 1) Nicotine acts on receptors that are normally activated by endogenous acetylcholine. The cholinergic nervous system modulates major physiological functions in the context of multiple other modulating inputs and feedbacks. Showing that nicotine has an effect in an isolated test system in-vitro does not mean that such effect will occur in-vivo where normal homeostatic mechanisms are operative. 2) Many responses to nicotine exhibit complex dose- response relationships, such that lower doses activate and higher doses depress responses. Thus, doses of nicotine used in pre-clinical studies need to be considered carefully when extrapolating to effects in people. Optimally nicotine concentrations in blood or other fluids acting on receptor systems should be measured and shown to be relevant to exposures in humans]. 3) Acute desensitization or tolerance needs to be considered. An acute response in a model system may not be relevant to the effects of repeated nicotine exposure in people who regularly use nicotine products. 4) Animal vs human pharmacokinetic differences need to be considered. Nicotine from cigarettes is generally taken as intermittent doses which result in peaks in arterial blood and various body organs, followed by a decline, during which brain and tissue levels fall. Oral nicotine replacement products and smokeless tobacco result in more gradual and sustained rises in blood nicotine concentrations and result in less fluctuation between doses. The rate and pattern of nicotine metabolism varies markedly across species. Thus, the relevance of the route, rate and pattern of nicotine administration in animal studies needs to be considered in relating effects to those in humans.
Cardiovascular actions of Nicotine
In this section we consider evidence regarding actions of nicotine that could contribute to smoking-induced CVD. Some of these effects could influence acute ischemic events, such as shown in Fig 1. Other effects, such on lipids, insulin resistance and diabetes and hypertension, are more relevant to accelerated atherogenesis, but there is considerable overlap across mechanisms and type of CVD risk.
Systemic Hemodynamic Effects of Nicotine
The systemic hemodynamic effects of nicotine are mediated primarily by activation of the sympathetic nervous system. Nicotine releases norepinephrine from adrenergic neurons and increases adrenal release of epinephrine (3). Sympathetic stimulation is thought to be a result of activation of nAChRs in the peripheral nervous system, such as the carotid chemoreceptor, as well as by central nervous system nAChR activation.
Cigarette smoking causes a >150% increase in plasma epinephrine and acutely increases cardiac work by stimulating heart rate (as much as 10-15 bpm acutely and on average 7 bpm throughout the day), myocardial contractility, and blood pressure (acute increase 5-10 mm Hg) (3). Heart rate and blood pressure increase regardless of whether the route of administration is tobacco-smoke or nicotine (intravenous, intranasal, chewing gum or smokeless tobacco).
Cardiac output increases as a result of increased heart rate, enhanced cardiac contractility and enhanced cardiac filling, the latter due to systemic venoconstriction. Nicotine constricts blood vessels, including those in the skin and coronary blood vessels, but dilates blood vessels in skeletal muscle. Vasoconstriction of the skin results in reduced skin blood flow and reduced fingertip skin temperature. Actions of nicotine that reduce blood flow in microvascular beds may contribute to impaired wound healing, macular degeneration, progressive renal disease and placental dysfunction during pregnancy.
Nicotine and coronary blood flow
Nicotine can decrease coronary blood flow by acting on vascular smooth muscle α1-adrenergic receptors to constrict coronary arteries, but can also increase coronary blood flow by increasing cardiac output, causing subsequent flow-mediated dilation (FMD), and directly stimulating coronary artery β2-receptor for coronary vasodilation. Thus the net effect of an acute exposure on coronary blood flow is the balance of the two actions, typically a blunting of the expected FMD increase in coronary blood flow that would be expected from the nicotine-induced increase in cardiac output and increased myocardial oxygen demand.
In healthy people smoking a cigarette increases coronary blood flow by up to 40%, but smokers with CVD show decreases in cardiac reserve and increased coronary vascular resistance proportional to the severity of their CAD (3). In patients with stable angina cigarette smoking causes silent impairment of coronary blood supply, mimicking exercise-induced ischemia. Cigarette smoking can cause coronary spasm and is a risk factor for vasospastic angina. It is unclear to what extent these adverse effects of smoking on coronary blood flow are due to nicotine vs adverse effects of smoke constituents on endothelial function.
Nicotine and Myocardial Remodeling
Cigarette smoking and smokeless tobacco use have been associated with an increased risk of heart failure. Heart failure occurs in the context of tissue remodeling. Beta adrenergic stimulation enhances tissue remodeling, including hypertrophy and fibrosis (10). Nicotine acting via persistent sympathetic activation could contribute to myocardial remodeling. Nicotine has been shown in some animals to promote remodeling and fibrosis (11).
Nicotine and arrhythmogenesis
The risk of sudden cardiac death in smokers compared to non-smokers is disproportionately increased compare to risks of myocardial infarction. This might result from the combined effects of ischemia and the arrhythmogenic effects of nicotine. Catecholamine release from nicotine could contribute to fatal ventricular tachycardia and fibrillation. Animal studies find that nicotine decreases the ventricular fibrillation threshold and promotes the development of ventricular fibrillation after experimental myocardial infarction. Smoking is also associated with an increased risk of atrial fibrillation. This is likely due to a combination of atrial fibrosis and remodeling and systemic catecholamine release, both of which could be contributed to by actions of nicotine. Smokers with implanted cardiac defibrillators have a higher risk of inappropriate shocks compared to non-smokers, and this could also be contributed to by nicotine mediated catecholamine release.
Nicotine and thrombogenesis
Nicotine, presumably by releasing epinephrine, activates platelets acutely in some animal studies, but in long term studies in rodents reduced platelet activation is observed. However, recent in-vitro and in-vivo studies comparing cigarettes with normal and low nicotine content suggest that nicotine may decrease platelet aggregability in people (12). Most studies of nicotine medication and smokeless tobacco users do not indicate that nicotine activates platelets based on urinary TxA2 metabolite excretion. (3, 8)
Nicotine and endothelial dysfunction
Endothelial cells synthesize and release NO, which dilates blood vessels, inhibits inflammation and prevents platelet activation. The vasodilatory response to increase local blood flow, FMD, is dependent on NO release. Endothelial dysfunction (impaired FMD) is consistently seen in both active cigarette smokers and passive smokers (3, 5). The main mediators of endothelial dysfunction appear to be oxidative stress and chronic inflammation. The mechanisms include inactivation and reduced bioavailability of nitric oxide. Nicotine infused locally and the use of nicotine nasal spray in people can impair endothelial function, but it is unclear how important nicotine is compared to the powerful effects of oxidants and pro-inflammatory agents.
Nicotine and Inflammation
Exposure to tobacco smoke induces a chronic systemic inflammatory response via multiple, interrelated pathways (3, 5, 6). Inflammation plays a key role in atherogenesis as well as in acute ischemic events. An atherosclerotic plaque forms when activated monocytes adhere at the site of damaged endothelium, migrate into the sub-endothelium, differentiate into macrophages and eventually foam cells. A chronic inflammatory state ensues with macrophages secreting inflammatory mediators which promote spread, destabilization, and ultimately rupture of the plaque resulting in local vasoconstriction and thrombosis. Markers of inflammation, including elevated leukocyte counts, C-reactive protein (CRP), and fibrinogen, all of which are seen in smokers, are powerful predictors of future CV events.
Nicotine may act on the immune system directly, acting on nAChRs that modulate immune function, or indirectly, by activating the sympathetic nervous system. There is a cholinergic immune system, mediated by non-neuronal nAChRs, primarily homomeric alpha 7 type. In general, stimulation of this system has an anti-inflammatory effect.(13) In animal models of systemic inflammatory disease, such as sepsis, acute lung injury and viral myocarditis systemic administration of nicotine suppresses inflammation and has a beneficial effect on mortality.
However nicotine might also have pro-inflammatory effects. Nicotine has been reported to be a chemotactic agent for the attraction and extravasation of neutrophils into the subendothelium. In mouse models of atherosclerosis, acute myocardial infarction activates the sympathetic nervous system, which acts primarily via beta 3 receptors to induce an inflammatory state, liberating hematopoietic stem and progenitor cells from bone marrow, boosting monocyte production and accelerating atherosclerosis (14). Thus, the sympathetic activating effects of nicotine are of concern with respect to chronic inflammation as a contributor to ischemic vascular disease. Smoking cessation studies using nicotine medications have shown significant improvement in inflammatory markers over time. It appears on balance that nicotine is not a major contributor to the chronic inflammatory state caused by smoking.
Nicotine and angiogenesis
Nicotine acting on α7 nAChRs acutely enhances angiogenesis, promoting endothelial cell migration, proliferation and survival, and enhances the angiogenic response to inflammation, ischemia, and atherosclerosis.(15) On the other hand chronic nicotine treatment in rodents found impairment rather than enhanced angiogenesis mediated by downregulation of vascular nAChRs.(16). Thus the clinical importance of nicotine-mediated angiogenesis is questionable.
Nicotine and dyslipidemia
On average, cigarette smokers have lower levels of HDL cholesterol (10-15% decreased overall) and higher levels of LDL cholesterol than nonsmokers (3). Nicotine induces lipolysis via catecholamine action at β-adrenoreceptors, increasing plasma free fatty acid concentrations which could result in enhanced synthesis of LDL and lowering of HDL (3, 17). HDL increases within 2 weeks of cessation of smoking, suggesting a short term pharmacologic effect, such as could be mediated by nicotine. Studies of nicotine gum use reported no effect on serum lipids, although a cross-sectional study of smokeless tobacco users found a higher prevalence of hypercholesterolemia. While it is reasonable to conclude that there is a link between nicotine and a more atherogenic lipid profile, multiple cessation studies using nicotine medications (NRT and nicotine nasal spray) report reduced dyslipidemia with significant improvement in HDL/LDL ratios (3, 18)
Nicotine and hypertension
Smoking a cigarette acutely increases blood pressure, an effect mediated by nicotine. However in regular smokers, smoking is not associated with higher blood pressure in most epidemiological studies. The measurement of blood pressure is usually made after short term abstinence from smoking when blood pressure effects have disappeared. Cigarette smoking transiently increases blood pressure many times throughout the day, and when blood pressure is assessed by ambulatory blood pressure monitoring, average blood pressures are higher when a person is smoking. Supporting a persistent effect on blood pressure is the observation that muscle sympathetic activation is chronically increased in smokers, presumably an effect of nicotine (19). A recent analysis of large cohorts of persons 55 years of age and older reported significant associations between smoking and hypertensive heart disease and hypertensive renal disease (20).
Progression of chronic hypertension to accelerated or malignant hypertension is more common in smokers. Nicotine could contribute to this progression by aggravating vasoconstriction, although other tobacco smoke toxin are also likely to be contributing to progressive vascular injury. The majority of smokeless tobacco studies do not support an increase in the incidence or prevalence of hypertension in users exposed to nicotine via this route (21).
Nicotine and insulin resistance
Cigarette smoking is an important risk factor for developing type 2 diabetes. Smokers have increased insulin resistance relative to non-smokers, although there is no evidence of an effect on insulin secretion (3). Nicotine could enhance insulin resistance via increased levels of insulin-antagonistic hormones (catecholamines, cortisol, and growth hormone). Nicotine also directly activates AMP-activated protein kinase via a7 nAChR effects in adipose to produce insulin resistance (22). Studies examining long term use of nicotine gum have noted a dose-response association between hyperinsulinemia and insulin resistance, suggesting nicotine is the primary constituent in tobacco smoke responsible for this finding in people (3).
Human Experimental and Epidemiologic studies Relating Nicotine to Cardiovascular Toxicity
It is difficult to distinguish the independent roles of nicotine vs tobacco combustion products in cigarette smokers because all smokers are exposed to both. We can however examine circumstances in which individuals are exposed to nicotine without combustion products – namely with the use of nicotine medications or smokeless tobacco.
Nicotine Medications and Cardiovascular Disease
Randomized clinical trials (RCT's) testing nicotine medication in cessation trials for CVD patients, case control studies, long term longitudinal studies, and meta-analysis have found no increased risk of serious CV events in patients given nicotine medication, even in the context of acute and chronic CVD. (2, 18, 23, 24) A RCT in 1996 randomized 584 smokers with high-risk pre-existing CVD to transdermal nicotine vs placebo and found no significant increase in CV events. (24) Another study treated 36 smokers awaiting elective coronary bypass surgery with nicotine patches (initially 14 then 21mg) and urged them to stop smoking (25). Participants underwent exercise nuclear perfusion studies and measurement of exhaled carbon monoxide, and plasma nicotine and cotinine levels at baseline and after patch use. By self-report most continued to smoke, but did reduce tobacco consumption while on the patch, which was confirmed by lowered carbon monoxide levels but higher serum nicotine and cotinine levels. Despite higher nicotine levels, tobacco reduction markedly improved myocardial perfusion, suggesting that combustion products rather than nicotine were the primary contributors to smoking-induced cardiac ischemia. A five year longitudinal study of 3,094 COPD users of nicotine gum in 1996 showed no association between rates of hospitalization for CVD conditions or deaths from CVD with use of nicotine gum, dosage of nicotine in gum, or dual use of nicotine with cigarettes (18). A network meta-analysis performed in 2013 analyzed 63 RCT of nicotine medications including 21 NRT studies, 28 bupropion studies and 18 varenicline studies and found no significant increase in major adverse CV events (death, myocardial infarction, or stroke) with any of the three therapies, but a slight increase in less serious CV events (e.g. tachycardia and arrhythmia) with NRT (23)
Smokeless Tobacco and Cardiovascular Disease
Smokeless tobacco (ST) delivers as much nicotine to the systemic circulation as does cigarette smoking, albeit with slower absorption (21). Worldwide, ST exists in many forms, but the best epidemiologic studies of smokeless tobacco and CV health have been conducted in Sweden, where 25% of men use a form of ST called snus. Non-invasive studies of the extent of atherosclerosis, using carotid intimal wall thickness, found the expected increase among smokers, but no difference between snus users and non-smokers. Case control studies in Sweden have shown no increased risk of myocardial infarction or stroke, but a small and statistically significant increased case fatality rate for both among snus users compared to non-tobacco using controls. In contrast the a large cohort study in the United States and the InterHeart study of smokeless tobacco users in many countries around the world did find an association between ST and myocardial infarction (26, 27). The explanation for discrepant findings may be differences in ST products or other CV risk factors in various regions of the world. A recent Swedish study raises concern about nicotine safety in people with CVD. Among survivors of acute myocardial infarction who were snus users at the time of the event, those who continued to use snus after the event had a significantly higher mortality compared to those who quit.(28) This study suggests that nicotine may be hazardous in patient with CAD. Smokeless tobacco has also been reported to increase in the risk of heart failure, but unlike cigarette smoking does not increase the risk of atrial fibrillation. (29, 30) The American Heart Association reviewed the cardiovascular risk of ST and concluded that while ST most likely conveys less cardiovascular risk than smoking, it still poses some CV risk and recommended against it use in patients with cardiovascular disease. (21)
Cardiovascular Toxicity of Electronic Cigarettes
Electronic cigarettes (e-cigarettes) are battery-powered devices that heat a liquid composed of propylene glycol (PG) and/or vegetable glycerin (VG), nicotine and flavoring, to form an aerosol that is inhaled like a cigarette (31). Introduced to the US in 2007, and without effective regulation for manufacture or sale, devices have undergone rapid innovation and differentiation (>460 brands and >7,000 flavors in 2014). Public adoption of e-cigarettes has been rapid, and use is occurring both as an alternative to combustible cigarettes and for recreational use.
Nicotine delivery and pharmacokinetics from e-cigarettes
Important considerations in interpreting studies of the CV effects of e-cigarettes are the dose of nicotine delivered and the resultant nicotine blood levels. Using standardized puffing procedures simulating cigarette smoking, early cigarette-like devices (“cig-alikes”) deliver much less nicotine, but newer devices with high voltage batteries deliver higher doses of nicotine, in some cases similar to cigarettes; and the rate of nicotine absorption is rapid but somewhat slower than from cigarette smoke (31, 32). The fact that early devices delivered low levels of nicotine is important to consider in interpreting cardiovascular responses to such devices. It is also important to recognize that cigarette smokers typically take 8-10 puffs over 5-8 minutes resulting in an arterial spike of nicotine levels, whereas most e-cigarette use is intermittent throughout the day, resulting in lower and more stable nicotine levels and no arterial spikes. Since the rate of rise and peak levels of nicotine influence the intensity of pharmacologic effects, nicotine from e-cigarettes could have less cardiovascular impact than nicotine from tobacco cigarettes.
Cardiovascular effect of e-cigarettes
A few studies have examined the CV effects of e-cigarettes, as reviewed in recent publications. (33, 34) Studies of more advanced devices with effective nicotine delivery find that e-cigarette use produces the expected heart rate acceleration similar to that seen with cigarette smoking. Blood pressure effects are variable. Longitudinal studies report no significant changes in heart rate or blood pressure. An echocardiographic study of 40 subjects after vaping a second generation e-cigarette for 7 minutes found no significant changes, whereas cigarette smoking resulted in impaired myocardial relaxation (35). Overall, the acute cardiovascular effects of e-cigarettes reported to date are consistent with the expected effects of nicotine. No data are available on long term cardiovascular effects or effects in people with CVD.
Cardiovascular concerns from e-cigarette aerosol constituents other than nicotine
Low levels of PAHs, volatile organic compounds, phenolic compounds, acrolein, acetaldehyde, and formaldehyde have been found in some aerosols (36, 37). While levels are generally much lower than those generated by cigarettes, at high battery voltage and power, aldehyde generation can be as high as that from cigarettes. Particulates are generated by e-cigarettes and are present in concentration similar to those of conventional cigarettes. However, the particulates are primarily liquid, dissipate quickly, and are of uncertain relevance with respect to human disease. This is in contrast to particles from combustion that contain solid matter and are persistent in the environment. Heavy metals (cadmium, lead, nickel, silver) and silicates may be present in trace amounts due to device heating element rather than liquid. Novel compounds in e-cigarette vapor with unknown cardiovascular effects, include a wide variety of flavorings and fragrances (largely untested in inhaled products). Propylene glycol can be a pulmonary irritant, although chronic exposure studies in rodents report few significant pulmonary effects. If e-cigarettes do produce pulmonary injury and chronic inflammation, this could increase cardiovascular risk.
Conclusions
Studies of the pharmacology and toxicology of nicotine in animals and some epidemiologic studies in people support the biological plausibility that nicotine contributes to acute cardiovascular events in smokers with underlying CVD, and exerts pharmacologic effects that could contribute to accelerated atherogenesis. Short-term nicotine use, such as nicotine medication to aid smoking cessation, appears to pose little cardiovascular risk, even to patients with known CVD. Longer term nicotine use, such as in ST users, appears not accelerate atherogenesis, but may contribute to acute cardiovascular events in the presence of CVD.
The cardiovascular safety of nicotine is an important element in the debate on the benefits vs risk of e-cigarette use and related public policy. Based on current knowledge, we believe that the cardiovascular risks of nicotine from e-cigarette use in people without CVD are quite low. We have concerns that nicotine from e-cigarettes could pose some risk for users with CVD. Other constituents of e-cigarette aerosol could theoretically pose some cardiovascular risk, but experimental evidence of such risk is lacking. While people with established CVD might incur some increased risk from e-cigarette use, the risk is certainly much less than that of smoking. If e-cigarettes can be substituted completely for conventional cigarettes, the harms from smoking would be substantially reduced and there would likely be a substantial net benefit for cardiovascular health.
While the role of e-cigarettes for smoking cessation is not established, it is clear that some smokers do quit successfully by using e-cigarettes (38). For smokers who present to physicians expressing interest in using e-cigarettes to quit smoking, we endorse the statement of the American Heart Association: “If a patient has failed initial treatment, has been intolerant to or refuses to use conventional smoking cessation medications, and wishes to use e-cigarettes to aid quitting, it a reasonable to supp0ort the attempt” (39). For patients with cardiovascular disease in particular, we recommend that when they are confident that they no longer need to use e-cigarettes to keep from smoking, that they discontinue e-cigarette use.
Acknowledgements
The preparation of this paper was supported by US Public Health Service grants P50 CA180890 (NLB) from the National Cancer Institute and Food and Drug Administration Center for Tobacco Products and the training grant R25 CA113710 (ADB). The authors thank Tyson Douglass for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict(s) of interest:
Dr. Benowitz is a consultant to several pharmaceutical companies that market medications to aid smoking cessation and has served as a paid expert witness in litigation against tobacco companies. The other authors have no conflicts to disclose.
References
- 1.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, et al. 21st-century hazards of smoking and benefits of cessation in the United States. New England Journal of Medicine. 2013;368(4):341–50. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services . A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. The Health Consequences of Smoking: 50 Years of Progress. [Google Scholar]
- 3.U.S. Department of Health and Human Services . How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2010. [Google Scholar]
- 4.Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, et al. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes: clinical perspectives from the prevention of cardiovascular disease section leadership council and early career councils of the american college of cardiology. Journal of the American College of Cardiology. 2015;66(12):1378–91. doi: 10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 5.Messner B, Bernhard D. Smoking and cardiovascular disease mechanisms of endothelial dysfunction and early atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2014;34(3):509–15. doi: 10.1161/ATVBAHA.113.300156. [DOI] [PubMed] [Google Scholar]
- 6.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nature Reviews Cardiology. 2013;10(4):219–30. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 7.Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(7):1460–7. doi: 10.1161/ATVBAHA.112.300154. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Progress in cardiovascular diseases. 2003;46(1):91–111. doi: 10.1016/s0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 10.van Berlo JH, Maillet M, Molkentin JD. Signaling effectors underlying pathologic growth and remodeling of the heart. The Journal of clinical investigation. 2013;123(1):37–45. doi: 10.1172/JCI62839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen K, Nizamutdinov D, Guerrier M, Afroze S, Dostal D, Glaser S. General mechanisms of nicotine-induced fibrogenesis. FASEB journal. 2012;26(12):4778–87. doi: 10.1096/fj.12-206458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girdhar G, Xu S, Bluestein D, Jesty J. Reduced-nicotine cigarettes increase platelet activation in smokers in vivo: a dilemma in harm reduction. Nicotine & tobacco research. 2008;10(12):1737–44. doi: 10.1080/14622200802443528. [DOI] [PubMed] [Google Scholar]
- 13.Filippini P, Cesario A, Fini M, Locatelli F, Rutella S. The Yin and Yang of non-neuronal α7-nicotinic receptors in inflammation and autoimmunity. Current drug targets. 2012;13(5):644–55. doi: 10.2174/138945012800399008. [DOI] [PubMed] [Google Scholar]
- 14.Dutta P, Courties G, Wei Y, Leuschner F, Gorbatov R, Robbins CS, et al. Myocardial infarction accelerates atherosclerosis. Nature. 2012;487(7407):325–9. doi: 10.1038/nature11260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Cooke JP. Nicotine and pathological angiogenesis. Life sciences. 2012;91(21):1058–64. doi: 10.1016/j.lfs.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konishi H, Wu J, Cooke JP. Chronic exposure to nicotine impairs cholinergic angiogenesis. Vascular Medicine. 2010;15(1):47–54. doi: 10.1177/1358863X09106326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson K, Arner P. Systemic nicotine stimulates human adipose tissue lipolysis through local cholinergic and catecholaminergic receptors. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2001;25(8):1225–32. doi: 10.1038/sj.ijo.0801654. [DOI] [PubMed] [Google Scholar]
- 18.Murray RP, Bailey WC, Daniels K, Bjornson WM, Kurnow K, Connett JE, et al. Safety of nicotine polacrilex gum used by 3,094 participants in the Lung Health Study. CHEST Journal. 1996;109(2):438–45. doi: 10.1378/chest.109.2.438. [DOI] [PubMed] [Google Scholar]
- 19.Hering D, Kucharska W, Kara T, Somers VK, Narkiewicz K. Smoking is associated with chronic sympathetic activation in hypertension. Blood pressure. 2010;19(3):152–5. doi: 10.3109/08037051.2010.484150. [DOI] [PubMed] [Google Scholar]
- 20.Carter BD, Freedman ND, Jacobs EJ. Smoking and mortality--beyond established causes. The New England journal of medicine. 2015;372(22):2170. doi: 10.1056/NEJMc1503675. [DOI] [PubMed] [Google Scholar]
- 21.Piano MR, Benowitz NL, FitzGerald GA, Corbridge S, Heath J, Hahn E, et al. Impact of smokeless tobacco products on cardiovascular disease: implications for policy, prevention, and treatment a policy statement from the American Heart Association. Circulation. 2010;122(15):1520–44. doi: 10.1161/CIR.0b013e3181f432c3. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Song P, Zhang W, Liu J, Dai X, Liu Z, et al. Activation of AMPKalpha2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nature medicine. 2015;21(4):373–82. doi: 10.1038/nm.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.113.003961. CIRCULATIONAHA. 113.003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, et al. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. New England Journal of Medicine. 1996;335(24):1792–8. doi: 10.1056/NEJM199612123352402. [DOI] [PubMed] [Google Scholar]
- 25.Mahmarian JJ, Moyé LA, Nasser GA, Nagueh SF, Bloom MF, Benowitz NL, et al. Nicotine patch therapy in smoking cessation reduces the extent of exercise-induced myocardial ischemia. Journal of the American College of Cardiology. 1997;30(1):125–30. doi: 10.1016/s0735-1097(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364(9438):937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 27.Yatsuya H, Folsom AR. Risk of incident cardiovascular disease among users of smokeless tobacco in the Atherosclerosis Risk in Communities (ARIC) study. American journal of epidemiology. 2010;172(5):600–5. doi: 10.1093/aje/kwq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arefalk G, Hambraeus K, Lind L, Michaëlsson K, Lindahl B, Sundström J. Discontinuation of smokeless tobacco and mortality risk after myocardial infarction. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.007252. CIRCULATIONAHA. 113.007252. [DOI] [PubMed] [Google Scholar]
- 29.Arefalk G, Hergens M-P, Ingelsson E, Ärnlöv J, Michaëlsson K, Lind L, et al. Smokeless tobacco (snus) and risk of heart failure: results from two Swedish cohorts. European journal of preventive cardiology. 2012;19(5):1120–7. doi: 10.1177/1741826711420003. [DOI] [PubMed] [Google Scholar]
- 30.Hergens M- P, Galanti R, Hansson J, Fredlund P, Ahlbom A, Alfredsson L, et al. Use of Scandinavian moist smokeless tobacco (snus) and the risk of atrial fibrillation. Epidemiology. 2014;25(6):872–6. doi: 10.1097/EDE.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 31.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation. 2014;129(19):1972–86. doi: 10.1161/CIRCULATIONAHA.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St Helen G, Havel C, Dempsey D, Jacob P, 3rd, Benowitz NL. Nicotine delivery, retention, and pharmacokinetics from various electronic cigarettes. Addiction (Abingdon, England) 2015 doi: 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riley HE, Berry-Bibee E, England LJ, Jamieson DJ, Marchbanks PA, Curtis KM. Hormonal contraception among electronic cigarette users and cardiovascular risk: a systematic review. Contraception. 2015 doi: 10.1016/j.contraception.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Favaloro EJ, Meschi T, Mattiuzzi C, Borghi L, Cervellin G. E-cigarettes and cardiovascular risk: beyond science and mysticism. Seminars in thrombosis and hemostasis. 2014;40(1):60–5. doi: 10.1055/s-0033-1363468. [DOI] [PubMed] [Google Scholar]
- 35.Farsalinos KE, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: comparison with the effects of regular cigarettes. BMC cardiovascular disorders. 2014;14(1):78. doi: 10.1186/1471-2261-14-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng T. Chemical evaluation of electronic cigarettes. Tobacco control. 2014;23(suppl 2):ii11–ii7. doi: 10.1136/tobaccocontrol-2013-051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tayyarah R, Long GA. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regulatory Toxicology and Pharmacology. 2014;70(3):704–10. doi: 10.1016/j.yrtph.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Brown J, Beard E, Kotz D, Michie S, West R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction (Abingdon, England) 2014;109(9):1531–40. doi: 10.1111/add.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhatnagar A, Whitsel LP, Ribisl KM, Bullen C, Chaloupka F, Piano MR, et al. Electronic Cigarettes A Policy Statement From the American Heart Association. Circulation. 2014;130(16):1418–36. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]