Abstract
Purpose
To evaluate the effect of chronic kidney disease (CKD) on all-cause mortality, major adverse limb event (MALE), MALE + POD (post-procedure death), and amputation after endovascular treatment of superficial femoral (SFA) or popliteal arterial atherosclerotic disease.
Materials and Methods
A retrospective review from January 2002 to October 2011 was performed of four hundred forty patients who underwent endovascular treatment of symptomatic SFA or popliteal atherosclerotic disease for claudication (N=251) or critical limb ischemia (CLI) (N=267). CKD stage was divided based on the Kidney Dialysis Outcomes Quality Initiative (KDOQI) classification. The outcomes and factors associated with amputation, MALE, and MALE + POD were determined.
Results
Patients with a diagnosis of diabetes [HR=2.2; 95% CI (1.3–3.6), P=0.002] and a run off score of 0 or 1 [HR=2.0; 95% CI (1.2–3.4), P=0.01] relative to run off score of 3 were at increased risk of amputation. A patient with a baseline GFR of less than 45 had a 17% increase in amputation for every 5 point decrease less than 45 [95% CI (1.09–1.26), P<0.001]. An increase of 10 years in age, [HR=1.9, 95% CI (1.5–2.3), P<0.001], a TASC class of C/D relative to A/B [HR=1.6, 95% CI (1.1–2.2), P=0.01], and CLI [HR=2.4, 95% CI (0.5–0.9), P<0.001] were associated with increased mortality. A female gender was associated with a decreased risk of mortality [HR=0.7, 95% CI (0.5–0.9), P=0.01)].
Conclusion
Worsening CKD is associated with higher amputation rates, all-cause mortality, and MALE + POD in patients undergoing endovascular treatment of SFA or popliteal disease.
Introduction
Peripheral arterial disease (PAD) is common, with disease prevalence ranging from 3–10% in the United States and 15–20% in those over 70 years old (1). Although noninvasive methods such as risk factor modification are initially recommended for patients with PAD, these patients are increasingly undergoing percutaneous angioplasty or stent implantation as a first-line invasive intervention. Percutaneous treatment has been established as a safe and effective alternative to surgical bypass while being more cost-effective and less invasive (2–5). Patients also report improved quality of life after endovascular treatment (6). Although infrainguinal angioplasty and stenting have been established as an appropriate treatment in many patients, some questions remain unanswered. Individualized approaches to patients should encompass anatomic considerations, the type of endovascular treatment and the effects of patient comorbidities on the potential success of the treatment.
Several studies have been conducted to investigate patient comorbidities and their relationship to patient outcomes. Bakken et al showed that diabetes mellitus is a significant risk factor of amputation in those patients undergoing superficial femoral artery angioplasty (7). An association between chronic kidney disease (CKD) and PAD has been established, however there are limited data available on the effect of CKD on outcomes of endovascular treatment of patients with SFA disease with respect to all-cause mortality, amputation, major adverse limb events (MALE), and MALE + POD (post-procedural death). In postmenopausal women, CKD has been shown to be an independent risk factor for future peripheral artery disease (8,9). Additional evidence supports that those patients on chronic hemodialysis have worse outcomes after primary angioplasty than those who do not receive hemodialysis (10–12). A recent study demonstrates increased rates of death and amputation in those patients with critical limb ischemia with severe (stage 4 or 5) CKD (13). The effect of moderate stages of CKD on patient outcomes after endovascular interventions is not well understood. The purpose of this study was to investigate the effect of different stages of CKD on amputation, all-cause mortality, MALE, MALE + POD, and restenosis in patients undergoing endovascular intervention for symptomatic femoral-popliteal atherosclerotic disease.
Materials and Methods
Prior to performing this retrospective study, institutional review board was obtained. This is a single-center study, which analyzed 440 patients (58% male, 72.3 ± 10.7 years old) in 518 femoral-popliteal atherosclerotic limbs treated with either percutaneous angioplasty or stent placement from 1/25/2002 to 10/10/2011 for claudication or critical limb ischemia. This group does not include patients who underwent concurrent procedures for inflow disease (e.g. iliac stenting), concurrent endarterectomy of the superficial femoral artery or popliteal arteries, or tibial artery treatment. The median duration of follow-up was 4.3 years (inter-quartile range from 1.4 to 4.8 years). Patient demographics and comorbidities of the 518 limbs in 440 patients are included in Table 1. For the purpose of this study, dissections that did not limit blood flow (N=36), some of which were treated with stents, were not considered to be complications.
Table 1.
Demographics by patient
| Age, mean (SD) | 72.3 (10.7) |
|
| |
| Gender | |
| Male | 299 (58) |
| Female | 219 (42) |
|
| |
| Smoking Status, n (%) | |
| Never | 154 (30) |
| Former | 254 (49.0) |
| Current | 110 (21) |
|
| |
| Chronic kidney disease, n (%) | |
| Yes | 145 (28) |
| No | 368 (72) |
|
| |
| Diabetes, n (%) | |
| Yes | 283 (55) |
| No | 234 (45) |
|
| |
| Coronary arterial disease, n (%) | |
| Yes | 325 (63) |
| No | 193 (3) |
|
| |
| Hypertension, n (%) | |
| Yes | 456 (88) |
| No | 62 (12) |
|
| |
| Hyperlipidemia, n (%) | |
| Yes | 405 (78) |
| No | 112 (22) |
|
| |
| KDOQI CKD Stage, n (%) | |
| 1 | 61 (12) |
| 2 | 172 (33) |
| 3A | 141 (27) |
| 3B | 80 (16) |
| 4 | 20 (4) |
| 5 | 43 (8) |
| Claudication | CLI | P-value | |
|---|---|---|---|
|
| |||
| CKD Stage, n (%) | <0.001 | ||
| 1 | 25 (10%) | 36 (14%) | |
| 2 | 110 (44%) | 62 (23%) | |
| 3A | 76 (30%) | 65 (24%) | |
| 3B | 32 (13%) | 48(18%) | |
| 4 | 7 (3%) | 13 (5%) | |
| 5 | 0 (0%) | 43 (16%) | |
| missing | 1 | 0 | |
Endovascular treatment
Endovascular treatment of the superficial femoral and popliteal arteries was performed as described briefly. Usually, the contralateral common femoral artery was punctured and a 6–8 F vascular sheath (Terumo, Somerset, NJ) was placed. After performing a diagnostic pelvic angiogram, the contralateral femoral artery was selected and a lower extremity diagnostic angiogram was obtained. The patient was systemically heparinized using a bolus of 5000 units of unfractionated heparin (Baxter Healthcare, Deerfield, IL). The diseased femoral artery was then crossed using a 4-F glide head catheter (Terumo) and a 0.035” soft angled glide wire (Terumo). The diseased femoral artery was treated with either plain old balloon angioplasty (POBA) or typically a self-expanding stent chosen at the discretion of the treating physician. The length and diameter of the balloon or stent used were based on the length of the stenosis and the diameter of the non-diseased artery distal to the stenosis. Balloon expandable stents were used in a minority of the cases. The following stents were used: Herculink and Absolute stent (Abbott Vascular, Redwood City, CA), Conformex and Luminex stent (Bard Peripheral Vascular Inc., Tempe, AZ), Zilver stent (Cook Medical Inc., Bloomington, IN), S.M.A.R.T. and Precise stent (Cordis Corporation, Bridgewater, NJ), Protégé stent (ev3 Endovascular Inc., Plymouth, MN), and Racer stent (Medtronic Inc., Minneapolis, MN). Pressure gradients were obtained only if there was a question of lesion severity. A gradient was considered hemodynamically significant when it was greater than 10% of the peak systolic pressure when measured proximal and distal to the stenosis. A completion angiogram was performed to determine for the presence of a dissection, rupture, or embolus (14,15). Four limbs lacked available images to properly designate Trans-Atlantic Inter-Society Consensus (TASC) II severity. Tibial run-off vessels were described as patent if they provided in-line flow to the foot or occluded if they contained proximal or mid-level occlusions with or without distal refilling. Again, patients included in this cohort did not have any tibial vessels treated. After the procedure, patients were started on anti-platelet therapy using either 81-mg daily oral dose of aspirin or 300-mg initial oral dose of clopidogrel (Sanofi-Aventis, Bridgewater, NJ), followed by 75 mg daily oral dose for a minimum of three months followed by an 81-mg daily oral dose of aspirin for life.
The images were reviewed for TASC II classification and runoff arteries to the foot. Runoff scores of 0, 1, 2, and 3 were then designated based on the total number of arteries that were patent to the foot. Table 2 provides the number of patients in each category (POBA or stent). The patients were categorized into those who presented with claudication (N=251) and those who presented with critical limb ischemia (CLI) (N=267). The primary endpoints of the study were ipsilateral amputation, all-cause mortality, major adverse limb event (MALE), and major adverse limb event + post-procedure death (MALE + POD).
Table 2.
Procedural details by limb
| Treatment, n (%) | |
| Plain old balloon angioplasty | 268 (52) |
| Stent | 250 (48) |
|
| |
| Runoff to the foot, n (%) | |
| 0 | 7 (1) |
| 1 | 133 (26) |
| 2 | 212 (41) |
| 3 | 163 (32) |
|
| |
| TASC II, n (%) | |
| A | 119 (23) |
| B | 245 (48) |
| C | 140 (27) |
| D | 10 (2) |
Post-procedural follow-up was dictated by the providing physician, but generally included a routine acquisition of ankle-brachial indices, transcutaneous oxygen pressure measurement (TcPO2) measurements, and Doppler ultrasound (US), with cross-sectional imaging [computed tomographic angiography (CTA) and magnetic resonance angiography (MRA)] and conventional angiography being less common. Follow-up was advised at one month and then at three, six, and twelve months, and then yearly after the procedure. At each time point, clinical evaluation was performed. If indicated, additional follow-up was obtained including non-invasive vascular studies such as ankle-brachial index, Duplex ultrasound, computed tomography angiography, or conventional angiography, as needed to determine the patency of the treated arteries.
Ankle-brachial index (ABI)
Standard ABI technique was performed according to the guidelines from the Inter-Society Consensus for the Management of Peripheral Arterial Disease document (16).
Duplex ultrasound (US)
Duplex ultrasound was used for follow-up of the femoro-popliteal stenoses after treatment. Baseline and follow-up duplex ultrasound studies were performed using a 9.0 L MHz phased-array transducer (GE Logiq E9, GE Healthcare, Wauwatosa, WI). Peak systolic velocity ratio of greater than 2.5 fold was considered a significant restenosis in the treated vessel.
Definitions
Glomerular filtration rate (GFR) was calculated using the serum creatinine levels obtained prior to the procedure (available in all but one patient) and patient demographic information using the modification of diet in renal disease (MDRD) equation: estimated GFR (eGFR)= 186 × (Serum creatinine)−1.54 × Age−0.203 × 0.742 (Females) × 1.210 (African Americans) (17,18). Patients were stratified into stages of renal disease based on the Kidney Dialysis Outcomes Quality Initiative (KDOQI) classification. Stage 1 was defined as a GFR greater 90 mL/min/1.73m2; stage 2 was defined as GFR between 60–89 mL/min/1.73m2; stage 3A was defined as GFR between 45–59 mL/min/1.73m2; stage 3B was defined as GFR between 30–44 mL/min/1.73m2; stage 4 was defined as GFR between 15–29 mL/min/1.73m2; and stage 5 was defined as GFR less than 15 mL/min/1.73m2.
Femoro-popliteal stenoses and occlusions were defined according to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) document (16). Patients were divided based on treatment approach into groups receiving angioplasty or stent and into a claudication or CLI groups. Technical success of the procedure was defined by less than 30% residual stenosis by visual estimate of the completion angiogram. Clinical success of the procedure was defined as freedom from amputation, an absence of restenosis, or revascularization of the target artery after index procedure. In-stent restenosis was defined as more than 50% blockage by visual estimate on follow-up conventional angiography or CT, or 2.5 times the increment in the translesional peak systolic velocities obtained with at a Doppler angle of < 600 based on US. All complications were recorded according to the guidelines published by the Society of Interventional Radiology (19). MALE was defined as above ankle amputation, new bypass graft, jump/interposition graft revision, thrombectomy, or thrombolysis. MALE-POD was defined as MALE plus post-procedural death within 30 days of index procedure.
Statistical methods
Descriptive statistics are reported as number (percent) and as mean (SD) or median (range) when appropriate. Kaplan-Meier survival was used to estimate amputation, MALE-POD, and all-cause mortality. The association of GFR measurement with the risk of each outcome was visualized using a smoothing spline to display the risk. Cox proportional hazard regression was used to assess patient and disease characteristics for association with each of the outcomes of amputation, all-cause mortality, and restenosis. A multiple variable Cox model was assessed using the backward selection method to identify the final models. The alpha level was set at 0.05 for statistical significance.
Results
Endovascular treatment
Limbs were treated for claudication (n=251, 48.5%) and CLI (n=267, 51.5%). There were 119 (23.2%) SFA/popliteal arteries treated for TASC A, 245 (47.7%) for TASC B, 140 (27.2%) for TASC C, and 10 (2.0%) for TASC D (Table 2). The runoff score of 0 was seen in 7 (1.4%), 1 in 133 (25.8%), 2 in 212 (41.2%) and 3 in 163 (31.6%). Stent placement of the SFA occurred in 250 limbs (48.3%) while POBA in 268 (51.7%). Patients with CLI had worse baseline CKD stage (3B/4/5) (P<0.001) when compared to those with claudication.
Factors associated with risk of amputation
A total of 100 limbs required an amputation during the follow-up period, 92 in limbs with CLI and 8 with claudication. A total of 59 limbs required a major amputation during the follow-up period, 54 in limbs with CLI and 5 presenting as claudication. The 5-year freedom from any amputation was 54%, [95% CI (45%–64%)]; in the claudication group it was 89% [95% CI (75%–99%)] and in the CLI limbs it was 31% [95% CI (21%–43%)]. A limb with CLI relative to claudication was at a 12-fold increased risk of amputation [95% CI (5.8–24.7), P<0.0001].
The majority of the amputations were in patients with CLI and therefore the assessment of clinical factors for association with amputation risk was limited to these patients with 267 limbs presenting with CLI. There was a linear association for GFR and amputation for a patient with a baseline GFR less than 45 [HR=1.22 for each 5 points less than 45, 95% CI (1.04–1.3), P<0.001, Table 3]. Factors univariately associated with an increased risk of either a major or minor amputation included a CKD stage of 5 relative to 1/2/3A [HR=4.1, 95% CI (2.5–6.8), P<0.001], diagnosis of diabetes [HR=3.2; 95% CI (2.0–5.2), P<0.001], a run off score of 0 or 1 [HR=2.4; 95% CI (1.5–4.0), P<0.001], and run off score of 2 [HR=1.3; 95% CI (0.8–2.1), P<0.001] relative to run off score of 3.
Table 3.
Cox Proportional Hazards for outcome of Amputation
| Univariate Model | Multiple Variable Model | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| CKD Stage | ** | |||
| 5 | 4.1 (2.5–6.8) | <0.001 | ||
| 4/3b | 1.6 (0.95–2.6) | 0.08 | ||
| 1/2/3a | 1.0 (reference) | |||
|
| ||||
| Gender | ** | |||
| Female | 1.3 (0.9–2.0) | 0.14 | ||
| Male | 1.0 (reference) | |||
|
| ||||
| Smoking Status | ** | |||
| Current | 0.4 (0.2–0.8) | 0.004 | ||
| Former | 0.5 (0.3–0.8) | 0.005 | ||
| Never | 1.0 (reference) | |||
|
| ||||
| Diabetes | ||||
| Yes | 3.2 (2.0–5.2) | <0.001 | 2.2 (1.3–3.6) | |
| No | 1.0 (reference) | 1.0 (reference) | 0.002 | |
|
| ||||
| Coronary arterial disease | ** | |||
| Yes | 1.5 (0.99–2.4) | 0.06 | ||
| No | 1.0 (reference) | |||
|
| ||||
| TASC Class | ** | |||
| C or D | 1.0 (0.6–1.6) | 0.98 | ||
| A or B | 1.0 (reference) | |||
|
| ||||
| Treatment | ** | |||
| Plain old balloon angioplasty | 1.4 (0.9–2.1) | 0.09 | ||
| Stent | 1.0 (reference) | |||
|
| ||||
| Run-off Score | ||||
| 0 or 1 | 2.4 (1.5–4.0) | <0.001 | 2.0 (1.2–3.4) | 0.01 |
| 2 | 1.3 (0.8–2.1) | <0.001 | 1.2 (0.7–2.1) | 0.43 |
| 3 | 1.0 (reference) | 1.0 (reference) | ||
|
| ||||
| GFR, per 5 units <45 | 1.22 (1.14–1.30) | <0.001 | 1.17 (1.09–1.26) | <0.001 |
Variable not retained using backward selection method, alpha-level = 0.05 for retention.
The multiple variable model analysis demonstrated that a history of diabetes, runoff score, and baseline GFR was associated with an increased risk of either a major or minor amputation. Patients with a diagnosis of diabetes [HR=2.2; 95% CI (1.3–3.6), P=0.002] and a run off score of 0 or 1 [HR=2.0; 95% CI (1.2–3.4), P=0.01] relative to run off score of 3 were at increased risk of amputation. There was a linear association between GFR and amputation. A patient with a baseline GFR of less than 45 had a 17% increase in amputation for every 5 point decrease less than 45 [95% CI (1.09–1.26), P<0.001, Table 3].
Factors associated with major adverse limb events (MALE) and MALE + POD (post-procedure death)
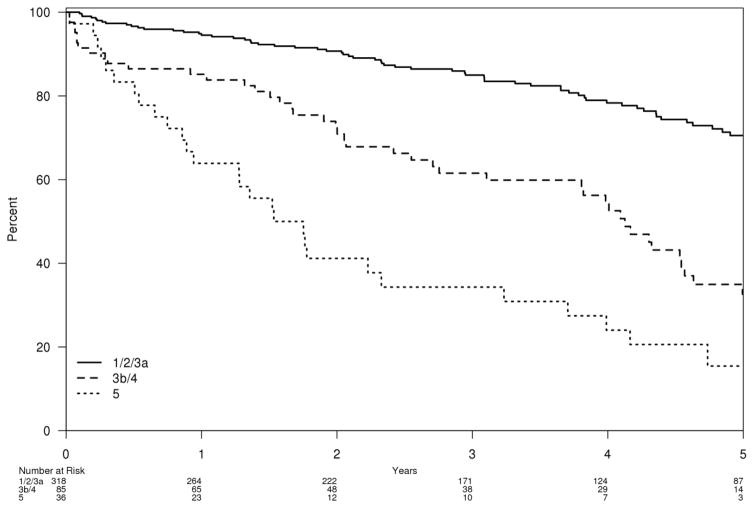
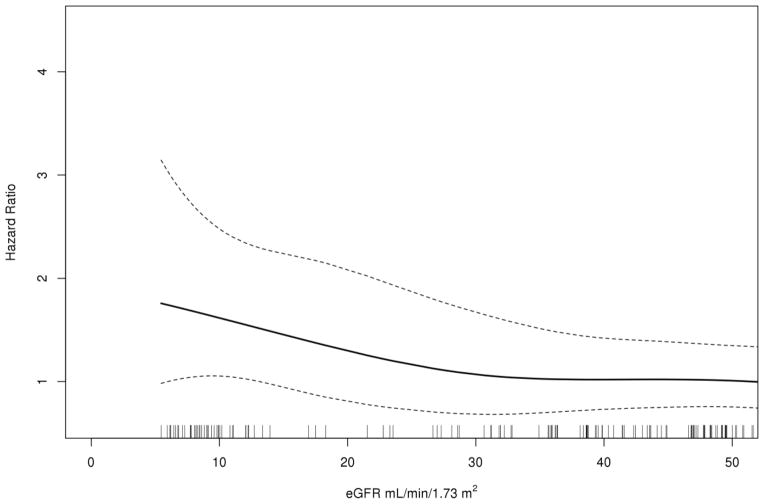
The 30-day MALE for CLI patients was 12% and for patients with claudication, it was 1.5%. Factors univariately associated with an increased risk of MALE included a history of coronary arterial disease [HR=1.8, 95% CI (1.01–3.3), P=0.048], Table 4]. The 30-day MALE + POD for patients with CLI was 17% and for claudication 1.5%. Factors univariately associated with an increased risk of MALE + POD included a history of coronary arterial disease [HR=1.8, 95% CI (1.03–3.1), P=0.04]. When compared to CKD stage 1/2/3A, patients with stage 5 CKD disease had two fold increase in risk of MALE + POD [95% CI (1.1–3.6), P=0.02, (Table 5 and Fig. 1)]. There was a linear association between GFR and MALE + POD in patients with CLI. A patient with a baseline GFR of less than 45 had a 6% increase in MALE + POD for every 5 point decrease less than 45 [95% CI (1.00–1.12), P=0.035, Fig. 2]. In patients with claudication, there was a significant linear association for GFR and MALE + POD with approximately a 20% increase for each average baseline GFR of 5 points or less than 45 [95% CI (1.05–1.37), P=0.035].
Table 4.
Cox Proportional Hazards for outcome of major adverse limb events
| Univariate Model | Multiple Variable Model | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| CKD Stage | ** | |||
| 5 | 1.8 (0.96–3.2) | 0.07 | ||
| 4/3b | 0.7 (0.4–1.4) | 0.29 | ||
| 1/2/3a | 1.0 (reference) | |||
|
| ||||
| Gender | ** | |||
| Female | 0.8 (0.5–1.2) | 0.28 | ||
| Male | 1.0 (reference) | |||
|
| ||||
| Smoking Status | ** | |||
| Current | 1.1 (0.5–2.5) | 0.79 | ||
| Former | 1.2 (0.8–2.3) | 0.28 | ||
| Never | 1.0 (reference) | |||
|
| ||||
| Diabetes | ** | |||
| Yes | 1.7 (0.9–3.1) | 0.10 | ||
| No | 1.0 (reference) | |||
|
| ||||
| Coronary arterial disease | ** | |||
| Yes | 1.8 (1.01–3.3) | 0.048 | ||
| No | 1.0 (reference) | |||
|
| ||||
| TASC Class | ** | |||
| C or D | 1.3 (0.8–2.2) | 0.37 | ||
| A or B | 1.0 (reference) | |||
|
| ||||
| Treatment | ** | |||
| Plain old balloon angioplasty | 0.9 (0.6–1.5) | 0.81 | ||
| Stent | 1.0 (reference) | |||
|
| ||||
| Run-off Score | ** | |||
| 0 or 1 | 1.4 (0.7–2.9) | 0.35 | ||
| 2 | 1.1 (0.5–2.2) | 0.87 | ||
| 3 | 1.0 (reference) | |||
|
| ||||
| GFR, per 5 units <45 | 1.04 (0.98–1.10) | 0.21 | ** | |
Variable not retained using backward selection method, alpha-level = 0.05 for retention.
Table 5.
Cox Proportional Hazards for outcome of MALE + POD (post-procedure death)
| Univariate Model | Multiple Variable Model | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| CKD Stage | ** | |||
| 5 | 2.0 (1.1–3.6) | 0.02 | ||
| 4/3b | 1.05 (0.6–1.9) | 0.87 | ||
| 1/2/3a | 1.0 (reference) | |||
|
| ||||
| Gender | ** | |||
| Female | 0.8 (0.5–1.2) | 0.31 | ||
| Male | 1.0 (reference) | |||
|
| ||||
| Smoking Status | ** | |||
| Current | 1.0 (0.5–2.2) | 0.92 | ||
| Former | 1.2 (0.8–2.1) | 0.36 | ||
| Never | 1.0 (reference) | |||
|
| ||||
| Diabetes | ** | |||
| Yes | 1.4 (0.8–2.4) | 0.22 | ||
| No | 1.0 (reference) | |||
|
| ||||
| Coronary arterial disease | ** | |||
| Yes | 1.8 (1.03–3.1) | 0.04 | ||
| No | 1.0 (reference) | |||
|
| ||||
| TASC Class | ** | |||
| C or D | 1.3 (0.8–2.1) | 0.30 | ||
| A or B | 1.0 (reference) | |||
|
| ||||
| Treatment | ** | |||
| Plain old balloon angioplasty | 1.0 (0.7–1.7) | 0.82 | ||
| Stent | 1.0 (reference) | |||
|
| ||||
| Run-off Score | ** | |||
| 0 or 1 | 1.6 (0.8–3.3) | 0.19 | ||
| 2 | 1.4 (0.6–2.6) | 0.50 | ||
| 3 | 1.0 (reference) | |||
|
| ||||
| GFR, per 5 units <45 | 1.06 (1.00–1.12) | 0.04 | 1.06 (1.00–1.12) | 0.04 |
Variable not retained using backward selection method, alpha-level = 0.05 for retention.
Figure 1.
Survival free estimate of all-cause mortality by CKD stage 1/2/3A, 3B/4, or 5
Figure 2.
In CLI patients, smoothing spline of GFR for the outcome of MALE + POD
Factors associated with all-cause mortality
The 30-day mortality for CLI patients was 3.0% and for patients with claudication, it was 0%. A total of 156 of the 440 patients died over the follow-up period. The overall 5-year survival subsequent to the initial procedure was 57% [95% CI=51%–63%]. There was a significant linear association for GFR and all-cause mortality, with an increased risk of approximately 20% for each average baseline GFR of 5 points or less than 45 [95% CI (1.14–1.24), P<0.001, Table 6]. Factors univariately associated with an increased risk of all-cause mortality included increasing age [HR=1.8 per 10 years, 95% CI (1.5–2.2), P<0.001], a TASC class of C/D relative to A/B [HR=1.4; 95% CI (1.02–2.0), P=0.04], a diagnosis of diabetes [HR=1.7, 95% CI (1.2–2.3), P=0.002], and a diagnosis of coronary arterial disease [HR=1.6, 95% CI (1.1–2.3), P=0.01]. When compared to a patient with CKD stage 1/2/3A, a patient with stage 3B/4 was at a significantly increased risk [HR=2.8, 95% CI (2.0–4.1), P<0.001] as was a patient with stage 5 [HR=5.7; 95% CI (3.7–8.9), P<0.001]. Relative to a run-off score of 3, a runoff score of 0/1 was at a significantly increased risk [HR=2.1, 95% CI (1.3–3.3), P=0.001) as was a runoff score of 2 [HR=2.1; 95% CI (1.4–3.2), P<0.001].
Table 6.
Cox Proportional Hazards for outcome of All-Cause Mortality
| Univariate Model | Multiple Variable Model | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| CKD Stage | ||||
| 5 | 5.7 (3.7–8.9) | <0.001 | 6.2 (3.9–9.7) | <0.001 |
| 4/3b | 2.8 (2.0–4.1) | <0.001 | 2.1(1.4–3.1) | <0.001 |
| 1/2/3a | 1.0 (reference) | 1.0 (reference) | ||
|
| ||||
| Age, per 10 years | 1.8 (1.5–2.2) | <0.001 | 1.9 (1.5–2.3) | <0.001 |
|
| ||||
| Gender | ||||
| Female | 0.9 (0.6–1.2) | 0.40 | 0.7 (0.5–0.9) | |
| Male | 1.0 (reference) | 1.0 (reference) | 0.02 | |
|
| ||||
| Smoking Status | ** | |||
| Current | 0.4 (0.2–0.6) | <0.001 | ||
| Former | 0.8 (0.6–1.1) | 0.18 | ||
| Never | 1.0 (reference) | |||
|
| ||||
| Diabetes | ** | |||
| Yes | 1.7 (1.2–2.3) | 0.002 | ||
| No | 1.0 (reference) | |||
|
| ||||
| Coronary arterial disease | ** | |||
| Yes | 1.6 (1.1–2.3) | 0.01 | ||
| No | 1.0 (reference) | |||
|
| ||||
| TASC Class | ||||
| C or D | 1.4 (1.02–2.0) | 0.04 | 1.6 (1.1–2.2) | 0.01 |
| A or B | 1.0 (reference) | 1.0 (reference) | ||
|
| ||||
| Treatment | ** | |||
| Plain old balloon angioplasty | 1.1 (0.8–1.5) | 0.09 | ||
| Stent | 1.0 (reference) | |||
|
| ||||
| Run-off Score | ** | |||
| 0 or 1 | 2.1 (1.3–3.3) | 0.001 | ||
| 2 | 2.1 (1.4–3.2) | <0.001 | ||
| 3 | 1.0 (reference) | |||
|
| ||||
| GFR, per 5 units <45 | 1.19 (1.14–1.24) | <0.001 | ** | |
Variable not retained using backward selection method, alpha-level = 0.05 for retention.
The multiple variable model for all-cause mortality included age, gender, CKD stage, TASC class, and CLI/claudication status. An increase of 10 years in age, [HR=1.9, 95% CI (1.5–2.3), P<0.001], a TASC class of C/D relative to A/B [HR=1.6, 95% CI (1.1–2.2), P=0.01], and CLI [HR=2.4, 95% CI (0.5–0.9), P<0.001] were associated with increased mortality. A female gender was associated with a decreased risk of mortality [HR=0.7, 95% CI (0.5–0.9), P=0.01)]. When compared to CKD stage 1/2/3A, stage 3B/4 disease had a 2.1 fold increased risk of all-cause mortality [95% CI (1.4–3.1), P<0.001] and stage 5 disease had a 6.2 fold increased risk [95% CI (3.9–9.7), P<0.001].
Factors associated with Restenosis
Restenosis was reported in a total of 199 limbs over the follow-up period. The overall 5-year freedom from restenosis was 28% [95% CI (21%–36%), Table 7]. Two factors were univariately associated with an increased risk of restenosis, female gender [HR=1.5, 95% CI (1.1–1.9), P=0.007] and a TASC class of C/D relative to A/B [HR=1.4; 95% CI (1.03–1.9), P=0.03]. The multiple variable model assessed factors associated with restenosis and identified the same variables: female gender [HR=1.5, 95% CI (1.2–2.0), P=0.003) and a TASC class of C/D relative to A/B [HR=1.4, 95% CI (1.02–1.9), P=0.04].
Table 7.
Cox Proportional Hazards for outcome of restenosis
| Univariate Model | Multiple Variable Model | |||
|---|---|---|---|---|
|
| ||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||
| CKD Stage | ** | |||
| 5 | 1.4 (0.8–2.3) | 0.26 | ||
| 4/3b | 0.9 (0.6–1.3) | 0.61 | ||
| 1/2/3a | 1.0 (reference) | |||
|
| ||||
| Age, per 10 years | 1.0 (0.9–1.2) | 0.61 | ** | |
|
| ||||
| Gender | ||||
| Female | 1.5 (1.1–1.9) | 0.007 | 1.5 (1.2–2.0) | |
| Male | 1.0 (reference) | 1.0 (reference) | 0.003 | |
|
| ||||
| Smoking Status | ** | |||
| Current | 0.9 (0.6–1.3) | 0.52 | ||
| Former | 0.7 (0.5–0.99) | 0.047 | ||
| Never | 1.0 (reference) | |||
|
| ||||
| Diabetes | ** | |||
| Yes | 1.2 (0.9–1.6) | 0.15 | ||
| No | 1.0 (reference) | |||
|
| ||||
| Coronary arterial disease | ** | |||
| Yes | 0.9 (0.7–1.2) | 0.57 | ||
| No | 1.0 (reference) | |||
|
| ||||
| TASC Class | ||||
| C or D | 1.4 (1.03–1.9) | 0.03 | 1.4 (1.02–1.9) | 0.04 |
| A or B | 1.0 (reference) | 1.0 (reference) | ||
|
| ||||
| Treatment | ** | |||
| Plain old balloon angioplasty | 1.0 (0.8–1.4) | 0.82 | ||
| Stent | 1.0 (reference) | |||
|
| ||||
| Run-off Score | ** | |||
| 0 or 1 | 1.1 (0.8–1.6) | 0.45 | ||
| 2 | 0.9 (0.7–1.3) | 0.67 | ||
| 3 | 1.0 (reference) | |||
Variable not retained using backward selection method, alpha-level = 0.05 for retention.
Discussion
In 2005–2010, 13.1% of United States population had some degree of CKD based on one time estimated GFR less than 60 ml/min/1.73 m2 or a urine albumin/creatinine ratio of 30 mg/g or higher, (20). CKD is associated with hypertension, dyslipidemia, hyperphosphatemia, hyperhomocysteinemia, and chronic inflammation (21). These risk factors have been hypothesized to lead to increased cardiovascular mortality in these patients (22). Peripheral arterial disease is more prevalent in patients with CKD (20). Nonetheless, there are limited data on the effects of underlying renal disease on the outcomes of endovascular interventions in this population.
Lacroix et al have shown that CKD is an independent predictor of 1-year mortality in patients hospitalized for PAD, however in their study, CKD was not an independent predictor of amputation (23). In patients with diabetes, CKD is associated with lower survival after amputation (24). Also, patients with diabetes have a strong association between stage of CKD and foot ulcers or lower-extremity amputation (25).
The OLIVE registry was a prospective multicenter study that revealed hemodialysis is associated with a poor prognosis for MALE in patients with CLI who underwent endovascular treatment. However, the effects of moderate CKD not requiring hemodialysis were not determined. Also, patients with claudication undergoing treatment were not included (26). Willenberg et al prospectively followed 208 patients who underwent endovascular treatment and revealed that renal insufficiency was an independent predictor of mortality in CLI patients undergoing endovascular treatment (27). Again, patients with claudication were not included in this study. Recently Patel et al evaluated the effect of severe (class 4 and 5) CKD on outcomes after infrainguinal endovascular interventions in 879 patients of which only 14% had severe CKD. This study revealed that severe CKD increased the risk of late mortality, or amputation (13). They were unable to establish an association between moderate CKD and adverse outcomes. In their cohort, there was a nonuniform distribution of disease severity with the 71.8% of patients with severe kidney disease presenting with CLI. In addition, although they mentioned that patients with severe CKD (stage 4 and 5) had an increased frequency of tibial disease and multilevel interventions, the number of distal runoff vessels and its effect on adverse outcomes is unclear.
The present study showed a linear association between baseline GFR of less than 45 ml/min/1.73m2 with a 17% increase in amputation or 6% increase in MALE + POD in CLI patients. The risk increases with a decrease in baseline GFR of 5 ml/min/1.73m2. With respect to the patients’ baseline CKD stage, patients with CKD stage 5 are at a 4.1 fold increase in risk of amputation after femoral-popliteal intervention when compared to stages 1/2/3A CKD when adjusted for treatment method (POBA or stent placement) or TASC classification. History of diabetes and poor tibial artery runoff were also identified as predictors of amputation. This study also demonstrates an association of increased risk of all-cause mortality in patients with GFR less than 45 ml/min/1.73m2 (stage 3B, 4 and 5).
Despite the significant association between CKD with the risk of amputation, there was no association noted between the stage of CKD and the risk of restenosis. There are multiple possible explanations for this finding. First, the increased risk of amputation without evidence of increased restenosis in patients with higher stage of CKD may be related to the presence of microvascular disease, which is a common finding in patients with CKD. Another explanation could be immune dysfunction associated with CKD, which leads to increased risk of infection and possibly delayed and incomplete healing (28).
Recently, Conte et al and the Society of Vascular Surgery have recommended additional performance measures including MALE and MALE + POD outcomes for patients undergoing lower extremity treatment (29). However, these performance measures do not have different outcomes based on baseline kidney function. In the present study, for an outcome of MALE + POD, there was a linear association of a decrease in baseline GFR < 45 which was statistically significant. There was trend towards worse outcomes for MALE in patients with baseline stage CKD stage 5 versus CKD stages1/2/3A. In addition, for outcomes of both MALE and MALE + POD, patients with history of coronary arterial disease had worse outcomes.
The present study has several limitations. These include being a retrospective study and a lack of standardized follow-up in which early asymptomatic restenosis may have been missed. The evaluation of restenosis on imaging modality varied (CTA, MRA, US, ABI) and each has different sensitivities and specificities for restenosis. The current study did not test all of the endpoints that may be considered a clinical success, which is different for patients with CLI or claudication.
In summary, the present study supports the notion that increasing severity of chronic kidney disease at the time of intervention worsens the prospect of amputation-free survival, MALE + POD, and overall survival of patients receiving endovascular treatment of symptomatic femoral-popliteal atherosclerotic disease. Future studies investigating the outcomes of patients with advanced CKD is needed to confirm these results.
Acknowledgments
This work was presented at Society of Interventional Radiology Annual Meeting in 2014. The work was funded by a NIH grant HL098967 (SM) from the National Heart, Lung, And Blood Institute.
Footnotes
Conflict of Interest Disclosure Statement form: The authors have none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 3.Haider SN, Kavanagh EG, Forlee M, et al. Two-year outcome with preferential use of infrainguinal angioplasty for critical ischemia. J Vasc Surg. 2006;43:504–512. doi: 10.1016/j.jvs.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 4.Lam RC, Shah S, Faries PL, McKinsey JF, Kent KC, Morrissey NJ. Incidence and clinical significance of distal embolization during percutaneous interventions involving the superficial femoral artery. J Vasc Surg. 2007;46:1155–1159. doi: 10.1016/j.jvs.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 5.Morgan JH, 3rd, Wall CE, Jr, Christie DB, Harvey RL, Solis MM. The results of superficial femoral, popliteal, and tibial artery stenting for peripheral vascular occlusive disease. Am Surg. 2005;71:905–909. discussion 909–910. [PubMed] [Google Scholar]
- 6.Sabeti S, Czerwenka-Wenkstetten A, Dick P, et al. Quality of life after balloon angioplasty versus stent implantation in the superficial femoral artery: findings from a randomized controlled trial. J Endovasc Ther. 2007;14:431–437. doi: 10.1177/152660280701400401. [DOI] [PubMed] [Google Scholar]
- 7.Bakken AM, Palchik E, Hart JP, Rhodes JM, Saad WE, Davies MG. Impact of diabetes mellitus on outcomes of superficial femoral artery endoluminal interventions. J Vasc Surg. 2007;46:946–958. doi: 10.1016/j.jvs.2007.06.047. discussion 958. [DOI] [PubMed] [Google Scholar]
- 8.O’Hare AM, Vittinghoff E, Hsia J, Shlipak MG. Renal insufficiency and the risk of lower extremity peripheral arterial disease: results from the Heart and Estrogen/Progestin Replacement Study (HERS) J Am Soc Nephrol. 2004;15:1046–1051. doi: 10.1097/01.asn.0000119574.27772.fd. [DOI] [PubMed] [Google Scholar]
- 9.O’Hare A, Johansen K. Lower-extremity peripheral arterial disease among patients with end-stage renal disease. J Am Soc Nephrol. 2001;12:2838–2847. doi: 10.1681/ASN.V12122838. [DOI] [PubMed] [Google Scholar]
- 10.Graziani L, Silvestro A, Bertone V, et al. Percutaneous transluminal angioplasty is feasible and effective in patients on chronic dialysis with severe peripheral artery disease. Nephrol Dial Transplant. 2007;22:1144–1149. doi: 10.1093/ndt/gfl764. [DOI] [PubMed] [Google Scholar]
- 11.Kumada Y, Aoyama T, Ishii H, et al. Long-term outcome of percutaneous transluminal angioplasty in chronic haemodialysis patients with peripheral arterial disease. Nephrol Dial Transplant. 2008;23:3996–4001. doi: 10.1093/ndt/gfn378. [DOI] [PubMed] [Google Scholar]
- 12.Smolock CJ, El-Sayed HF, Davies MG. Outcomes of femoropopliteal interventions for critical ischemia in the hemodialysis-dependent patient. Ann Vasc Surg. 2015;29:237–243. doi: 10.1016/j.avsg.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Patel VI, Mukhopadhyay S, Guest JM, et al. Impact of severe chronic kidney disease on outcomes of infrainguinal peripheral arterial intervention. J Vasc Surg. 2014;59:368–375. doi: 10.1016/j.jvs.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Misselt AJ, Zielinski MD, Medina OI, et al. Clinical outcomes after endovascular treatment of superficial femoral disease in patients with disabling claudication and critical limb ischemia: midterm analysis. Angiology. 2012;63:259–265. doi: 10.1177/0003319711414866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendes BC, Oderich GS, Fleming MD, et al. Clinical significance of embolic events in patients undergoing endovascular femoropopliteal interventions with or without embolic protection devices. J Vasc Surg. 2014;59:359–367. e1. doi: 10.1016/j.jvs.2013.07.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Journal of vascular surgery. 2007;45:S5–S67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Klahr S. The modification of diet in renal disease study. N Engl J Med. 1989;320:864–866. doi: 10.1056/NEJM198903303201310. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi S, Kamiyama T, Tomaru U, et al. Frequency and pattern of expression of the stem cell marker CD133 have strong prognostic effect on the surgical outcome of colorectal cancer patients. Oncol Rep. 2010;24:1201–1212. doi: 10.3892/or_00000973. [DOI] [PubMed] [Google Scholar]
- 20.Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63:A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Locatelli F, Bommer J, London GM, et al. Cardiovascular disease determinants in chronic renal failure: clinical approach and treatment. Nephrol Dial Transplant. 2001;16:459–468. doi: 10.1093/ndt/16.3.459. [DOI] [PubMed] [Google Scholar]
- 22.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 23.Lacroix P, Aboyans V, Desormais I, et al. Chronic kidney disease and the short-term risk of mortality and amputation in patients hospitalized for peripheral artery disease. J Vasc Surg. 2013;58:966–971. doi: 10.1016/j.jvs.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Lavery LA, Hunt NA, Ndip A, Lavery DC, Van Houtum W, Boulton AJ. Impact of chronic kidney disease on survival after amputation in individuals with diabetes. Diabetes Care. 2010;33:2365–2369. doi: 10.2337/dc10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care. 2008;31:1331–1336. doi: 10.2337/dc07-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iida O, Nakamura M, Yamauchi Y, et al. Endovascular treatment for infrainguinal vessels in patients with critical limb ischemia: OLIVE registry, a prospective, multicenter study in Japan with 12-month follow-up. Circ Cardiovasc Interv. 2013;6:68–76. doi: 10.1161/CIRCINTERVENTIONS.112.975318. [DOI] [PubMed] [Google Scholar]
- 27.Willenberg T, Baumann F, Eisenberger U, Baumgartner I, Do DD, Diehm N. Impact of renal insufficiency on clinical outcomes in patients with critical limb ischemia undergoing endovascular revascularization. J Vasc Surg. 2011;53:1589–1597. doi: 10.1016/j.jvs.2011.01.062. [DOI] [PubMed] [Google Scholar]
- 28.Vaziri ND, Pahl MV, Crum A, Norris K. Effect of uremia on structure and function of immune system. J Ren Nutr. 2012;22:149–56. doi: 10.1053/j.jrn.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conte MS, Geraghty PJ, Bradbury AW, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vasc Surg. 2009;50:1462–73. e1–3. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]