Abstract
Background
Higher Gleason grade is associated with prostate cancer mortality; however there is significant heterogeneity in this association. We evaluated whether vessel morphology, a biomarker of angiogenesis, aided in distinguishing mortality risks among men with high Gleason grading
Methods
We characterized vessel morphology (area and irregularity) among 511 patients diagnosed with prostate cancer during 1986 to 2000, re-reviewed Gleason grade, and followed men through 2012. Men were grouped according to integrated vessel lumen irregularity and vessel area across Gleason grade. The more angiogenic group was identified as those with more irregular vessel lumen and smaller vessel area. Crude rates (95% confidence intervals) and survival probability were estimated across Gleason grade and vessel morphology.
Results
During a median 14-year follow-up, 62 men developed bone metastases or died of prostate cancer. Lethality rates were uniformly low within Gleason grade categories 6 and 7 (3+4), regardless of vessel morphology. However, among men with Gleason grades of 7 (4+3) or 8–10, the more angiogenic group was associated with 4-fold higher risk of lethal outcomes compared to those with less angiogenic potential. Ten-year survival probability ranged from 95% to 74% according to the extent of vessel morphology (p<0.0001, log-rank test).
Conclusions
Vessel morphology may aid Gleason grading in predicting prostate cancer mortality risks among men diagnosed with high-grade Gleason cancers.
Keywords: vessel morphology, angiogenesis, Gleason grade, prostate cancer mortality
INTRODUCTION
Gleason grade is one of the strongest histopathologic predictors of prostate cancer mortality (1). However, there is significant heterogeneity in the biologic progression even among patients with cancers categorized as Gleason 8 or greater (1–3). Thus, high-grade cancer is an inadequate surrogate for metastatic potential (4). In particularly, among men with high-grade but apparently organ-confined cancer treated with curative intent, some are disease free for decades but some develop lethal prostate cancer within a few years after treatment. Thus, although the cancers appear histologically similar (high grade) and apparently localized, prognosis is highly variable and factors that influence this variable prognosis are poorly understood. Many prognostic factors are highly correlated with Gleason grade, and it has been challenging to identify those features that are independent of Gleason grade. In fact, most research has focused on prognostic factors measured within the neoplastic cell, and thus it is not surprising that such prognostic factors would be inherently correlated with Gleason grade.
Angiogenesis is a feature associated with the propensity of tumors to metastasize (5). The vessels are themselves not neoplastic and less correlated with Gleason grade, that is, it is not unusual to find high-grade prostate cancers with low angiogenic potential as well as low-grade prostate cancer with high angiogenic potential. We previously reported that morphologic measures of blood vessels in prostate cancer are strong predictors of lethal disease, with a 10-fold greater risk of developing lethal outcomes among tumors with greater vessel irregularity (a feature of high angiogenesis) compared to those with the most regularly shaped vessels (6). In this report, with extended follow-up, we sought to determine whether data on vascular morphology could distinguish mortality risks among men with lower-grade and higher-grade Gleason prostate cancer separately up to 26 years after diagnosis.
PATIENTS AND METHODS
Patients and Samples
The hypothesis was tested within that same dataset among patients diagnosed with clinically localized prostate cancer (T1/T2) from 1986 to 2000 and who had a radical prostatectomy in the Health Professionals Follow-up Study (HPFS) cohort with extended follow-up. The participants in HPFS, aged 40–75 years at enrollment in 1986, completed biennial postal questionnaires to collect lifestyle and medical information. Incident prostate cancer was initially identified through self-report and then confirmed by review of medical records, pathology reports and death certificate. Clinical course and information including disease diagnosis and deaths, pathologic stage, and prostate specific antigen at diagnosis were abstracted from medical records and death certificates. Study pathologists undertook a standardized histopathologic review including Gleason grading (1). We contacted hospital pathology departments to retrieve archival formalin-fixed paraffin-embedded prostatectomy specimens (6, 7). Of 1,593 men who underwent prostatectomy, 64% blocks were retrieved and the morphologic assessment of angiogenesis was completed among 572 samples. The clinical characteristics for these men were representative of the entire HPFS prostatectomy cohort (6, 8). The current analyses were based on 511 samples having complete re-reviewed Gleason grade and vessel morphology measures. We obtained written, informed consent from all participants. The research was approved by the institutional review boards of Harvard T.H. Chan School of Public Health and Partners Healthcare.
Measures of Vessel Morphology
We characterized prostate cancer morphology as previously described (6). A study pathologist identified all prostatectomy blocks that contained cancer and assessed between one and nine blocks with cancer per case. Semi-automated image analysis was accomplished using Image ProPlus 4.5 software (Media Cybernetics, Silver Spring, MD) under the pathologist’s supervision. A Spot RT Slider Camera (Diagnostic Instruments, Sterling Heights, MI) was used to capture slide images under high-powered fields (x200), which automated and evaluated image processing for the quantification of microvessel size and architecture. Two measures were used in the current analysis: vessel area (μm2) and irregularity of the vessel lumen (calculated as perimeter2/4π ×area). For vessel irregularity, a value of 1.0 indicates a perfect circle and values greater than 1.0 indicate increasing irregularity. Smaller and less regularly shaped vessels are associated with a poor prognosis in prostate cancer (6, 9). Microvessel density, which did not appear to be an independent predictor of lethality (6), was not included in the current analysis. During morphologic evaluation the laboratory remained blinded to lethal outcome status.
Statistical Analysis
We classified men into four Gleason grade categories: 6, 7(3+4), 7(4+3), and 8–10 (1). We also classified men into two groups according to the median values of vessel irregularity (more irregular vs. less irregular) and vessel area (smaller vs. larger), respectively. Within each category of Gleason grade, we then generated a combined morphology score based on the two groups of vessel irregularity and vessel area: the more angiogenic group including men with both more irregular vessel lumen and smaller vessel area, the less angiogenic group including men with less irregular vessel lumen and larger vessel area, and the moderately angiogenic group including the rest of the patients. To examine individual vessel morphology measure, we grouped men according to tertiles of irregularity of vessel lumen and vessel area, separately. Lethal prostate cancer is our primary outcome, defined as development of distant metastases or death due to prostate cancer during up to 26 years of follow-up. Person-time was calculated from date of diagnosis to development of metastases, cancer death, or censored at time of death from other causes or end of follow-up (January 1, 2012). Assignment of cause of death was based on medical records, and all available information. Crude rates, 95% confidence interval (CI), as well as 10-year and 20-year survivals using Kaplan-Meier methods were calculated within each group. We also tested the associations between vessel morphology and lethal prostate cancer among men with high Gleason grade (7(4+3) and greater). Cox proportional hazard regression model was used to estimate the hazard ratio (HR) and 95%CI of prostate cancer mortality by different groups of combined morphology score. The multivariable model included age at diagnosis (years, continuous), body mass index at diagnosis (kg/m2, continuous), smoking status at diagnosis (never, past, current), and vigorous exercise at diagnosis (hr/week, continuous). All the statistical analyses were carried out using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
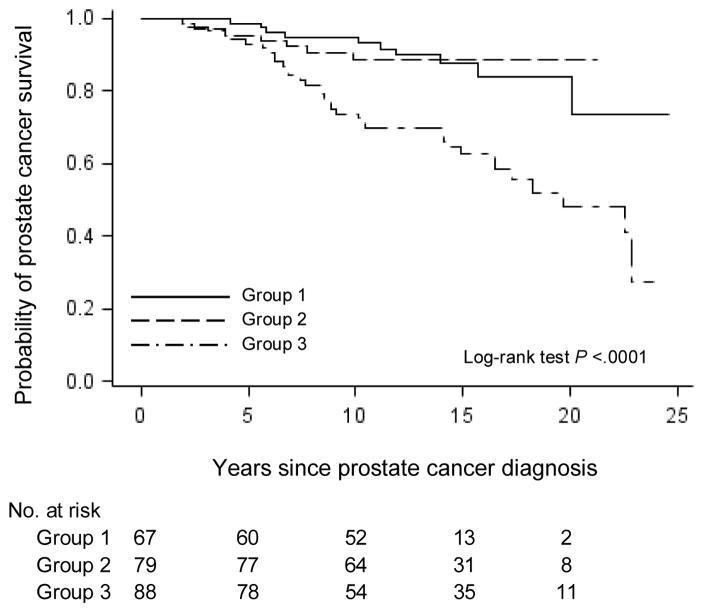
The major clinical and lifestyle characteristics among 511 patients by combined morphology score are in Supplementary Table 1. Among 511 patients, Gleason score 6, 7(3+4), 7 (4+3) and 8–10 accounted for 16%, 38%, 27% and 19%, respectively (Table 1). Little correlation between vessel morphology and lethal prostate cancer was observed among men with Gleason grade 6 cancers, with one lethal event during follow-up in 1,264 person years. Nine lethal events occurred during 2,753 person-years of follow-up in men with Gleason 7 (3+4) cancers and vessel morphology did not provide any further discrimination, though our sample size was limited (Table 1). However, among men with higher Gleason grade cancer (7(4+3) and higher), the crude rates per 1,000 person-years for lethal prostate cancer increased considerably as the angiogenic potential of vessel morphology increased - with a 4-fold gradient in risk of lethality comparing the more with the less angiogenic groups (Table 1). Ten-year survivals were 95% for the cancers with both more irregular vessel lumen and smaller vessel area compared to 74% for the group with less angiogenic potential (p<.0001, log-rank test) (Figure 1). At 20 years, the proportions free of lethal prostate cancer ranged from 84% to 48% according to the extent of vessel morphology. In the basic Cox model, patients with more angiogenic potential had a 3-fold higher risk of dying from prostate cancer than those with less angiogenic cancer (HR=3.42, 95%CI=1.51, 7.76) (Table 2). Further adjustment for potential confounders did not considerably change the association. We also observed statistically significantly elevated prostate cancer mortality for more irregular vessel lumen and smaller vessel area, separately (Supplementary tables and figures). The proportions of men free of lethal prostate cancer at 10 years were 75% for more irregular vessel lumen (p=0.002, log rank test), and 78% for smaller vessel area (p=0.002, log rank test), in comparison with 92% for less irregular vessel lumen and 95% for larger vessel area.
Table 1.
Crude rates of lethal prostate cancer by Gleason grade and combined morphology score among 511 men diagnosed with prostate cancer in Health Professionals Follow-up Studya
| Gleason Grade | Combined morphology score | N | Deaths/Person-years | Crude Rate (95% CI) per 1,000 person-years | 10-year Survival | 20-year Survival |
|---|---|---|---|---|---|---|
| 6 | Group 1: Less angiogenic | 29 | 0/394 | 0 | 100% | 100% |
| Group 2: Moderately angiogenic | 28 | 1/417 | 2.40 (−2.30, 7.11) | 98% | 95% | |
| Group 3: More angiogenic | 26 | 0/453 | 0 | 100% | 100% | |
| 7(3+4) | Group 1: Less angiogenic | 77 | 1/1042 | 0.96 (−0.92, 2.84) | 99% | 98% |
| Group 2: Moderately angiogenic | 58 | 4/842 | 4.75 (0.10, 9.41) | 95% | 90% | |
| Group 3: More angiogenic | 59 | 4/869 | 4.60 (0.09, 9.12) | 95% | 91% | |
| 7(4+3) | Group 1: Less angiogenic | 43 | 1/572 | 1.75 (−1.68, 5.18) | 98% | 97% |
| Group 2: Moderately angiogenic | 51 | 3/681 | 4.41 (−0.58, 9.40) | 96% | 91% | |
| Group 3: More angiogenic | 43 | 16/566 | 28.27 (14.42, 42.12) | 72% | 43% | |
| 8–10 | Group 1: Less angiogenic | 24 | 6/255 | 23.51 (4.70, 42.33) | 76% | 53% |
| Group 2: Moderately angiogenic | 28 | 7/402 | 17.4 (4.51, 30.29) | 83% | 65% | |
| Group 3: More angiogenic | 45 | 19/541 | 35.11 (19.32, 50.90) | 65% | 30% |
We categorized vessel irregularity and vessel area into two groups according to the median values. Men were categorized into the more angiogenic group if they had both more irregular vessel lumen and smaller vessel area; men were categorized into the less angiogenic group if they had both less irregular vessel lumen and larger vessel area. The rest of men were grouped into the moderately angiogenic group.
Figure 1.
Kaplan-Meier curves show the probability of prostate cancer survival after diagnosis according to integrated vessel morphology measure among 234 patients with Gleason grade 7(4+3) and 8–10 in the Health Professionals Follow-up Study. Integrated morphology measure was combined irregularity of vessel lumen and vessel area. Group 1 is less angiogenic with more regular vessel lumen and larger vessel area; group 2 is moderately angiogenic; group 3 is more angiogenic with more irregular vessel lumen and smaller vessel area.
Table 2.
Relative risk of prostate cancer mortality by combined vessel morphology score among 234 patients with Gleason score 7 (4+3) and 8+
| Group 1: less angiogenic | Combined morphology scorea Group 2: moderately angiogenic | Group 3: more angiogenic | |
|---|---|---|---|
| Events/n | 7/67 | 10/79 | 35/88 |
| Follow-up time, py | 9,921 | 12,995 | 13,286 |
| Model 1 HR (95% CI) | 1.00 | 1.03 (0.39, 2.70) | 3.42 (1.51, 7.76) |
| Model 2 HR (95% CI)b | 1.00 | 1.04 (0.39, 2.76) | 3.32 (1.45, 7.60) |
Abbreviation: py, person years; HR, hazard ratio; CI, confidence interval.
We categorized vessel irregularity and vessel area into two groups according to the median values. Men were categorized into the more angiogenic group if they had both more irregular vessel lumen and smaller vessel area; men were categorized into the less angiogenic group if they had both less irregular vessel lumen and larger vessel area. The rest of men were grouped into the moderately angiogenic group.
Cox proportional hazards regression model adjusted for age at diagnosis (years, continuous), body mass index at diagnosis (kg/m2, continuous), smoking status at diagnosis (never, past, current), and vigorous exercise at diagnosis (hr/week, continuous).
DISCUSSION
Our results suggest that parameters of vascular morphology - the biomarker of angiogenesis - are important modifiers of risk conferred by grade, especially among high-grade cancer. Among men with low-grade prostate cancer at prostatectomy, the risk of progression to lethal disease is low. Although many of these tumors had highly angiogenic features, very few of these lesions appeared to have potential to progress. In contrast, although high-grade Gleason tumors are generally believed to have a high propensity to progress, it appeared that angiogenesis was an important limiting factor for progression. We found that only 7 cancers of Gleason grade 7 (4+3) or 8–10 progressed to lethality in 827 person-years in tumors with low angiogenic potential. This rate of progression is remarkably low, even up to 20 years after diagnosis, considering that these poorly differentiated lesions otherwise generally have the hallmarks of aggressive malignancy, including elevated growth signals, resistance to apoptosis and unlimited replicative potential (5). Our results imply that the progression of high-grade prostate lesions requires angiogenesis (10, 11), which is regulated by pro-angiogenic molecules such as vascular endothelial growth factor, fibroblast growth factor 2, TGFβ, and cyclooxygenase 2 (11). Low angiogenic potential may be an important factor in limiting progression of high grade prostate cancer.
Our results may provide insights into the progression of high-grade prostate cancer. Although much research on tumor progression is focused on the cancer cells (e.g. molecular signatures, mRNA expression), determinants of angiogenesis are not clear. It is possible that extra-tumoral signals from the microenvironment, such as surrounding inflammation, might influence angiogenesis. We previously showed that frequent consumption of tomato products and greater estimated lycopene intake was associated with decreased angiogenesis markers and lethal prostate cancer, but not with the overall prevalence of high-grade prostate cancer, suggesting that some factors influence the propensity of high-grade lesions to progress but not their overall prevalence (12). Future approaches for prevention may be targeted on inhibiting angiogenesis and inhibiting progression of high-grade cancers.
This study has limitations. Our study only included patients treated with prostatectomy; we do not know the impact of angiogenesis among patients choosing other forms of treatments. In addition, this cohort includes mainly white health professionals in the United States. Our sample size was small, with only 52 lethal cases in men with high-grade prostate cancer. Nonetheless, the results were highly statistically significant and robust across integrated or individual measures of angiogenesis.
In conclusion, we report that the angiogenesis makers may help distinguish prostate cancer mortality risks among men diagnosed with high-grade Gleason cancer. Future studies should further investigate this finding, which, if confirmed could have useful clinical applications both for prognostication and potentially identifying targets for treatment (13–15).
Supplementary Material
Supplementary Figure 1. Kaplan-Meier curves show the probability of prostate cancer survival after diagnosis according to tumor vessel area among 511 patients with Gleason grade 7(4+3) and 8–10 in the Health Professionals Follow-up Study. Vessel area was categorized as tertiles (Tertile 1 = smaller vessel area, Tertile 2 = intermediate vessel area, Tertile 3 = larger vessel area). Smaller area is more angiogenic.
Supplementary Figure 2. Kaplan-Meier curves show the probability of prostate cancer survival after diagnosis according to tumor vessel lumen irregularity among 511 patients with Gleason grade 7(4+3) and 8–10 in the Health Professionals Follow-up Study. Vessel lumen irregularity was categorized as tertiles (Tertile 1 = more regular vessel lumen, Tertile 2 = intermediately regular vessel lumen, Tertile 3 = less regular vessel lumen). More irregular shape is more angiogenic.
Supplementary Table 1. Characteristics of 511 men diagnosed with prostate cancer in the Health Professional Follow-up (HPFS) Study, by combined morphology score
Supplementary Table 2. Crude rates of lethal prostate cancer by Gleason grade and vessel area among 511 men diagnosed with prostate cancer in Health Professionals Follow-up Studya
Supplementary Table 3. Relative risk of prostate cancer mortality by vessel area among 234 patients with Gleason score 7 (4+3) and 8+
Supplementary Table 4. Crude rates of lethal prostate cancer by Gleason grade and irregularity of vessel lumen among 511 men diagnosed with prostate cancer in Health Professionals Follow-up Studya
Supplementary Table 5. Relative risk of prostate cancer mortality by vessel irregularity among 234 patients with Gleason score 7 (4+3) and 8+
Acknowledgments
This study was supported by funding from the National Institutes of Health (grant No. PO1 CA055075, CA141298, and CA13389, UM1 CA167552), the US Army Prostate Cancer Research Program Idea Development Award PC060389, the DF/HCC Prostate SPORE Career Development Award NIH/NCI P50 CA90381 and the Ohio State University NIH P30 CA016058. JRR and LAM are Prostate Cancer Foundation Young Investigators. The funding bodies had no influence in the design or conduct of the study, analysis and interpretation of the data, or preparation of the article. We would like to thank the participants and staff of the Health Professionals Follow-up Study for their valuable contributions. We would also like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459–3464. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20–69: an autopsy study of 249 cases. In vivo. 1994;8:439–443. [PubMed] [Google Scholar]
- 3.Andren O, Fall K, Franzen L, Andersson SO, Johansson JE, Rubin MA. How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol. 2006;175:1337–1340. doi: 10.1016/S0022-5347(05)00734-2. [DOI] [PubMed] [Google Scholar]
- 4.Giovannucci E. Commentary: Serum lycopene and prostate cancer progression: a reconsideration of findings from the prostate cancer prevention trial. Cancer Cause Control. 2011;22:1055–9. doi: 10.1007/s10552-011-9776-x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed HU, Arya M, Freeman A, Emberton M. Do low-grade and low-volume prostate cancers bear the hallmarks of malignancy? Lancet Oncol. 2012;13:e509–517. doi: 10.1016/S1470-2045(12)70388-1. [DOI] [PubMed] [Google Scholar]
- 6.Mucci LA, Powolny A, Giovannucci E, et al. Prospective study of prostate tumor angiogenesis and cancer-specific mortality in the health professionals follow-up study. J Clin Oncol. 2009;27:5627–5633. doi: 10.1200/JCO.2008.20.8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pettersson A, Graff RE, Bauer SR, et al. The TMPRSS2:ERG rearrangement, ERG expression, and prostate cancer outcomes: a cohort study and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21:1497–1509. doi: 10.1158/1055-9965.EPI-12-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graff RE, Pettersson A, Lis RT, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103:851–860. doi: 10.3945/ajcn.115.118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West AF, O’Donnell M, Charlton RG, Neal DE, Leung HY. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer. 2001;85:576–583. doi: 10.1054/bjoc.2001.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 11.Russo G, Mischi M, Scheepens W, De la Rosette JJ, Wijkstra H. Angiogenesis in prostate cancer: onset, progression and imaging. BJU Int. 2012;110:E794–808. doi: 10.1111/j.1464-410X.2012.11444.x. [DOI] [PubMed] [Google Scholar]
- 12.Zu K, Mucci L, Rosner BA, et al. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;106:djt430. doi: 10.1093/jnci/djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Cozzi PJ. Angiogenesis as a strategic target for prostate cancer therapy. Med Res Rev. 2010;30:23–66. doi: 10.1002/med.20161. [DOI] [PubMed] [Google Scholar]
- 14.Alhusban A, Al-Azayzih A, Goc A, Gao F, Fagan SC, Somanath PR. Clinically Relevant Doses of Candesartan Inhibit Growth of Prostate Tumor Xenografts In Vivo through Modulation of Tumor Angiogenesis. J Pharmacol Exp Ther. 2014;350:635–645. doi: 10.1124/jpet.114.216382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin CI, Merley A, Sciuto TE, et al. TM4SF1: a new vascular therapeutic target in cancer. Angiogenesis. 2014;17:897–907. doi: 10.1007/s10456-014-9437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Kaplan-Meier curves show the probability of prostate cancer survival after diagnosis according to tumor vessel area among 511 patients with Gleason grade 7(4+3) and 8–10 in the Health Professionals Follow-up Study. Vessel area was categorized as tertiles (Tertile 1 = smaller vessel area, Tertile 2 = intermediate vessel area, Tertile 3 = larger vessel area). Smaller area is more angiogenic.
Supplementary Figure 2. Kaplan-Meier curves show the probability of prostate cancer survival after diagnosis according to tumor vessel lumen irregularity among 511 patients with Gleason grade 7(4+3) and 8–10 in the Health Professionals Follow-up Study. Vessel lumen irregularity was categorized as tertiles (Tertile 1 = more regular vessel lumen, Tertile 2 = intermediately regular vessel lumen, Tertile 3 = less regular vessel lumen). More irregular shape is more angiogenic.
Supplementary Table 1. Characteristics of 511 men diagnosed with prostate cancer in the Health Professional Follow-up (HPFS) Study, by combined morphology score
Supplementary Table 2. Crude rates of lethal prostate cancer by Gleason grade and vessel area among 511 men diagnosed with prostate cancer in Health Professionals Follow-up Studya
Supplementary Table 3. Relative risk of prostate cancer mortality by vessel area among 234 patients with Gleason score 7 (4+3) and 8+
Supplementary Table 4. Crude rates of lethal prostate cancer by Gleason grade and irregularity of vessel lumen among 511 men diagnosed with prostate cancer in Health Professionals Follow-up Studya
Supplementary Table 5. Relative risk of prostate cancer mortality by vessel irregularity among 234 patients with Gleason score 7 (4+3) and 8+