Abstract
Context
Dexamethasone is often used to treat dyspnea in cancer patients but evidence is lacking.
Objectives
We determined the feasibility of conducting a randomized trial of dexamethasone in cancer patients, and estimated the efficacy of dexamethasone in the treatment of dyspnea.
Methods
In this double-blind, randomized, controlled trial, patients with dyspnea ≥4 were randomized to receive either dexamethasone 8 mg twice daily × four days then 4 mg twice daily × three days or placebo for seven days, followed by an open-label phase for seven days. We documented the changes in dyspnea (0-10 numeric rating scale [NRS]), spirometry measures, quality of life and toxicities.
Results
A total of 41 patients were randomized and 35 (85%) completed the blinded phase. Dexamethasone was associated with a significant reduction in dyspnea NRS of -1.9 (95% confidence interval [CI] -3.3 to -0.5, P=0.01) by day 4 and -1.8 (95% CI -3.2 to -0.3, P=0.02) by day 7. In contrast, placebo was associated with a reduction of -0.7 (95% CI -2.1 to 0.6, P=0.38) by day 4 and -1.3 (95% CI -2.4 to -0.2, P=0.03) by day 7. The between-arm difference was not statistically significant. Drowsiness improved with dexamethasone. Dexamethasone was well tolerated with no significant toxicities.
Conclusion
A double-blind, randomized, controlled trial of dexamethasone was feasible with a low attrition rate. Our preliminary data suggest that dexamethasone may be associated with rapid improvement in dyspnea and was well tolerated. Further studies are needed to confirm our findings.
Keywords: dexamethasone, dyspnea, neoplasms, pharmacologic therapy, pilot study, quality of life, randomized controlled trial
Introduction
Dyspnea is the “subjective awareness of difficulty breathing, which may be associated with the distressing sensation of suffocation” (1). It is one of the most common and devastating symptoms among cancer patients, is difficult to treat, and often worsens in the last months of life (2-4). In addition to treatment of underlying disease and reversible causes, the current management of dyspnea involves various pharmacologic and non-pharmacologic measures using an interdisciplinary approach (5-8).
Patients with advanced cancer often have an elevated inflammatory response, which could contribute to dyspnea both peripherally and centrally (9). Corticosteroids have potent anti-inflammatory activity, and thus may modulate the sensation of dyspnea. Although corticosteroids are often used for the palliation of dyspnea in cancer patients, only a few retrospective clinical studies have examined the efficacy of corticosteroids for dyspnea in the oncology setting (10, 11). Matso et al. surveyed 120 Japanese palliative care physicians about their use of steroids; 37% of physicians perceived the positive effect of steroids on dyspnea to take place within 24 hours, 38% within 1-2 days, and 24% within 3-7 days (12). The main perceived predictors of steroid efficacy were lymphangitic carcinomatosis, airway obstruction, and multiple lung metastases. A systematic review on dyspnea interventions found no randomized controlled trials on corticosteroids, and concluded that high quality studies are needed (13).
Dexamethasone is a synthetic, long-acting, potent corticosteroid with minimal mineralocorticoid activity that is commonly used in the oncology setting for management of fatigue, anorexia and nausea and vomiting (14, 15). An improved understanding of the efficacy of dexamethasone may allow us to better manage dyspnea in cancer patients and to enhance their function and quality of life. We determined the feasibility of conducting a double-blind, parallel randomized placebo-controlled trial of dexamethasone in cancer patients with dyspnea. We also examined the effects of dexamethasone and placebo on the intensity of dyspnea, physiologic parameters and adverse events.
Methods
Patients
Patients were eligible if they had a diagnosis of cancer with clinical or radiologic evidence of lung involvement (e.g., metastatic disease, lymphangitic carcinomatosis), age 18 or older, able to communicate in English, average dyspnea numeric rating scale intensity of ≥4/10 over the past week, Karnofsky performance status ≥40%, and seen at the Thoracic Medical Oncology or Supportive Care Clinics at M. D. Anderson Cancer Center. Patients with delirium, oxygen saturation <90% despite supplemental oxygen >6L/min, allergic reactions to dexamethasone, diagnosis of diabetes mellitus uncontrolled on oral hypoglycemic agents or insulin, severe anemia (hemoglobin <7g/L) not corrected prior to study enrollment, megestrol acetate use at the time of study enrollment, open wound that has not been healed, infection requiring antibiotics within the past two weeks, major surgery within the past two weeks, absolute neutrophil count <1000/mm3, chronic obstructive pulmonary disease (COPD) exacerbation, heart failure exacerbation and active or recent chronic systemic corticosteroid use (>14 days) were excluded. Patients who were receiving chemotherapy or expected to start within one week of study enrollment also were excluded because of the elevated risk of immunosuppression and the fact that they often use dexamethasone for nausea and vomiting. However, radiation therapy was permissible during the study. The Institutional Review Board at M. D. Anderson Cancer Center approved this study. All patients provided written informed consent.
Study Design and Interventions
This was a double-blind, parallel, placebo-controlled, randomized trial for seven days with an open-label extension phase for another seven days. Randomization was performed using permuted blocks. Allocation was concealed by using a secured website that was only accessible to the study pharmacist after patient enrollment, who then assigned patients to the study intervention. Both the patient and research staff conducting the study assessments were blinded to the randomization sequence and the study intervention. Eligible patients were randomly assigned in a 1:1 ratio to receive either dexamethasone 8 mg (2 capsules of 4 mg) orally twice a day for four days, then 4 mg given orally twice a day for three days or identical-appearing placebo capsules (both prepared by Green Park Compounding Pharmacy, Houston, TX using United States Pharmacopeia grade materials). Patients were stratified according to FEV1/FVC ratio (<0.8 vs. ≥0.8). After one week, patients received dexamethasone 4 mg orally twice a day for seven days in an open-label fashion.
Study Outcomes and Endpoints
Our primary outcome was the proportion of patients who completed the blinded phase of this study. We assessed dyspnea using several questionnaires at baseline, day 4±2, day 7±2, and day 14±2. The Edmonton Symptom Assessment System (ESAS) is a validated symptom battery that assesses the average intensity of dyspnea and nine other symptoms over the past 24 hours (pain, fatigue, nausea, depression, anxiety, anorexia, drowsiness, well-being and sleep), each with a single 11-point numeric rating scale that ranges between 0 and 10, with 0 being no symptom at all and 10 being worst possible (16). The Minimal Clinically Important Difference (MCID) was ≥1 point for all symptoms (17). We also used an 11-point numeric rating scale similar to ESAS to assess the intensity of dyspnea “now” on a daily basis (18, 19).
As part of our exploratory analysis, we also assessed dyspnea “now” using the Modified Dyspnea Borg Scale, which ranges from 0 (no dyspnea) to 10 (worst dyspnea) (20, 21). In contrast to the numeric rating scale in ESAS, this is intended as a ratio scale, with four points being twice as severe as two points. The MCID for this validated scale is 1 point.(22)
The Cancer Dyspnea Scale is a validated 12-item questionnaire specifically designed to assess the quality of dyspnea in cancer patients during the past few days (23). Each item has a score between 1 and 5, with three subscores for sense of effort, anxiety and discomfort, and a total score. A higher score indicates a greater intensity of dyspnea.
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Core 30 (EORTC QLQ-C30), a well-validated quality of life questionnaire, consists of 30 items that encompasses three symptom scales (pain, fatigue, nausea/vomiting), six single-item symptom items, five functional scales (physical, cognitive, role, emotional, and social), and one scale assessing global health status/quality of life. Each scale comprises 2-5 items (24). All items have four response categories (1=not at all, 2=a little, 3=quite a bit, 4=very much), except for two items assessing overall health status/quality of life, which use a seven-point scale. This questionnaire includes one item to assess dyspnea during the past week (“Were you short of breath?). The four-point ordinal scale was transformed to 0-100 points using the formula ([raw score – 1]/3 ×100]), with higher scores indicating worse dyspnea (24).
The National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03 was used to assess adverse effects during the study.
The MicroLoop Spirometer (Micro Direct Inc., Lewiston, ME) was used at baseline to obtain forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), FEV1/FVC, peak inspiratory flow, and peak expiratory flow. Patients also were asked to use the portable Microlife PF 100 Peak Flow Meter (Microlife, Clearwater, FL) daily to measure peak flow and FEV1 while on study.
At the end of the first week, we asked patients about their change in dyspnea (better/same/worse) using the Global Symptom Evaluation (25, 26). We also assessed blinding by asking patients to guess their treatment assignment (“dexamethasone,” “placebo,” or “do not know”).
Statistical Analysis
This pilot study was based on a convenience sample of 20 subjects per arm. We considered a priori that the study was feasible if at least 50% of patients completed the study. This is based on historical data in which approximately 50% of patients did not complete their interventional clinical trial, and that cancer patients with dyspnea were more likely to drop out of study (27). For the secondary objective, 20 patients per arm provided 80% power to detect an effect size as small as 0.66 within arms with a two-tailed α of 0.05. This study was not powered for a direct comparison between dexamethasone and placebo.
We summarized the baseline demographics using descriptive statistics, including means, standard deviations (SDs), ranges, 95% confidence intervals (CIs) and frequencies. To estimate the effect size, we calculated the within-arm mean differences between baseline and day 4, 7 and 14 along with 95% CI for dyspnea and applied the Wilcoxon signed-rank test. We conducted similar testing for other secondary outcome variables. As an exploratory analysis, we also determined the mean differences with 95% CIs between study arms according to intention-to-treat analysis. A two-sided P-value of <0.05 was considered to be statistically significant.
The Statistical Analysis System (SAS v. 9.2, SAS Institute, Inc., Cary, NC) was used for statistical analysis.
Results
Study Feasibility
Patients were recruited between January 22, 2013 and May 21, 2015. Among 77 patients eligible for this study, we enrolled 52 patients (68%). Eleven patients did not proceed to randomization because they became ineligible (n=5) or declined to continue (n=6). The remaining 41 patients were randomized to the dexamethasone arm or placebo arm, with 35 (85%) completing the blinded phase of this study (Fig. 1).
Fig. 1. CONSORT diagram.
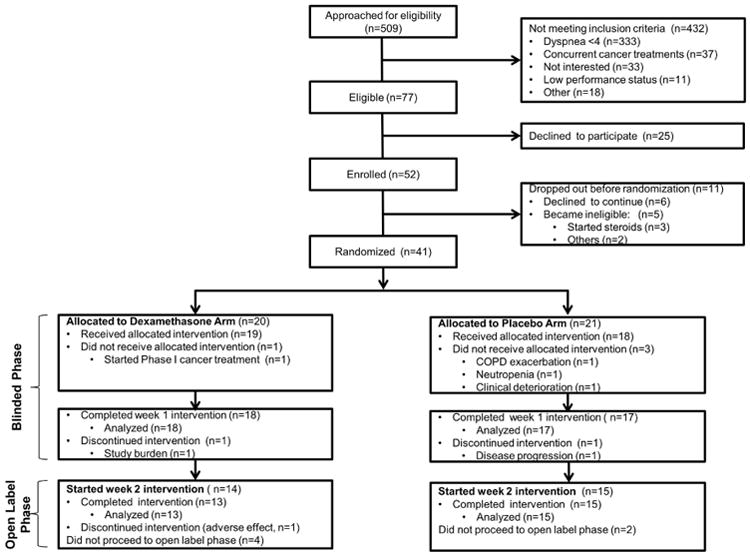
When asked to speculate which treatment they received after the first week, 7, 2 and 9 patients on the dexamethasone arm selected “dexamethasone,” “placebo,” and “do not know,” respectively, compared to 3, 7 and 7 patients on the placebo arm. No statistical significance was detected (P=0.12).
Patient Characteristics
As shown in Table 1, among those enrolled in the study, the average age was 63 years, 25 (61%) were female, and 27 (66%) were Caucasian. A majority had advanced cancer (n=36, 88%), with lung cancer (n=33, 81%) being the most common diagnosis. We found no significant differences in patient characteristics and co-interventions between the two groups at baseline.
Table 1. Baseline Patient Characteristics.
| Dexamethasone n=20 n (%)a |
Placebo n=21 n (%)a |
Total N=41 n (%)a |
|
|---|---|---|---|
| Average age (range) | 62 (49-71) | 64 (48-78) | 63 (48-78) |
| Female sex | 11 (55) | 14 (67) | 25 (61) |
| Race | |||
| Caucasian | 14 (70) | 13 (62) | 27 (66) |
| Black | 5 (25) | 6 (29) | 11 (27) |
| Hispanic | 1 (5) | 1 (5) | 2 (5) |
| Asian | 0 | 1 (5) | 1 (2) |
| Education | |||
| High school or less | 16 (80) | 18 (86) | 34 (83) |
| Some college | 1 (5) | 3 (14) | 4 (10) |
| Completed college | 3 (15) | 0 | 3 (7) |
| Cancer type | |||
| Mesothelioma | 1 (5) | 3 (14) | 4 (10) |
| Non-small cell lung cancer | 14 (70) | 17 (81) | 31 (76) |
| Small cell lung cancer | 2 (10) | 0 | 2 (5) |
| Other | 3 (15) | 1 (5) | 4 (10) |
| Cancer stage | |||
| Localized | 4 (20) | 1 (5) | 5 (12) |
| Locally advanced | 3 (15) | 4 (19) | 7 (17) |
| Metastatic/recurrent | 13 (65) | 16 (76) | 29 (71) |
| Comorbidities | |||
| COPD | 2 (10) | 7 (33) | 9 (22) |
| Asthma | 1 (5) | 2 (10) | 3 (7) |
| Concurrent therapies | |||
| Opioids, regular b | 6 (30) | 9 (43) | 15 (37) |
| Opioids, as needed | 9 (45) | 11 (52) | 20 (49) |
| Bronchodilators, regular | 1 (5) | 3 (14) | 4 (10) |
| Bronchodilators, as needed | 4 (20) | 4 (19) | 8 (20) |
| Supplemental oxygen, regular | 0 | 3 (14) | 3 (7) |
| Supplemental oxygen, as needed | 0 | 1 (5) | 1 (2) |
| Reasons for dyspnea c | |||
| Lung parenchymal lesions | 12 (60) | 11 (52) | 23 (56) |
| Post-radiation changes | 8 (40) | 6 (29) | 14 (34) |
| Pleural effusion | 4 (20) | 8 (38) | 12 (29) |
| Obstructive intrinsic lung disease | 5 (25) | 6 (29) | 11 (27) |
| Post-surgical changes | 4 (20) | 1 (5) | 5 (13) |
| Pleural lesions | 1 (5) | 5 (24) | 6 (15) |
| Other | 0 | 2 (10) | 2 (5) |
| Karnofsky Performance Status, mean (SD) | 74 (11) | 71 (11) | 72 (11) |
| Bedside spirometry measures | |||
| FEV1 | 1.7 (0.5) | 1.4 (0.6) | 1.6 (0.6) |
| FEV1 % predicted | 58.7 (17.1) | 55.1 (18.1) | 56.9 (17.5) |
| FVC | 2.4 (0.8) | 1.9 (0.7) | 2.2 (0.8) |
| FVC % predicted | 62.8 (15) | 55.4 (19) | 59.2 (17.2) |
| FEV1/FVC ratio (%) | 73.6 (13) | 75.6 (16.1) | 74.5 (14.4) |
COPD = chronic obstructive pulmonary disease; ECOG = Eastern Oncology Cooperative Group; NRS = numeric rating scale; SD = standard deviation
Unless otherwise specified
Only one patient in the placebo group had an increase in opioid during the first seven days of the study.
Some patients have more than one reason for dyspnea. If patients had a clinical diagnosis of radiation-induced fibrosis requiring systemic steroids, they were not eligible for this study.
However, some patients without this clinical diagnosis had variable degrees of fibrotic change in the lungs labeled as post-radiation changes, which may be contributing to dyspnea.
Changes in Dyspnea
We estimated the efficacy of dexamethasone and placebo within each study arm. Dexamethasone was associated with a significant reduction in ESAS dyspnea NRS of -1.9 (95% CI -3.3 to -0.5, P=0.01) by day 4 and -1.8 (95% CI -3.2 to -0.3, P=0.02) by day 7 (Table 2). In contrast, placebo was associated with a reduction of -0.7 (95% CI -2.1 to 0.6, P=0.38) by day 4 and -1.3 (95% CI -2.4 to -0.2, P=0.03) by day 7. We did not find a statistical significance between the two study arms at both time points, although this study was not powered for this comparison. After one week of open-label treatment, both arms experienced an improvement in dyspnea by day 14 (dexamethasone: mean -2.1 [95% CI -3.5 to -0.6], P=0.01; placebo: mean -1.7 [95% CI, 2.7 to -0.7], P=0.004).
Table 2. Change in Dyspnea in Dexamethasone and Placebo Arms a.
| Variable | Dexamethasone | Placebo | Mean Difference | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Change from Baseline (95% CI) | N | Mean (SD) | Change from Baseline (95% CI) | Between Arms (95% CI) | |
| ESAS Dyspnea (average 24 h) | |||||||
| Baseline | 19 | 5.0 (2.1) | - | 19 | 4.7 (1.5) | - | - |
| Day 4 | 15 | 3.3 (1.8) | -1.9 (-3.3, -0.5) | 15 | 3.7 (2) | -0.7 (-2.1, 0.6) | -1.2 (-3, 0.6) |
| Day 7 | 16 | 3.6 (2.6) | -1.8 (-3.2, -0.3) | 14 | 3.3 (2.1) | -1.3 (-2.4, -0.2) | -0.5 (-2.2, 1.2) |
| Day 14 | 13 | 3.2 (2.2) | -2.1 (-3.5, -0.6) | 15 | 2.9 (1.5) | -1.7 (-2.7, -0.7) | -0.4 (-2, 1.2) |
| Dyspnea numeric rating scale (now) | |||||||
| Baseline | 19 | 4.2 (1.7) | - | 19 | 4.6 (1.6) | - | - |
| Day 4 | 18 | 4.2 (1.9) | 0 (-1, 1) | 17 | 4.4 (2.1) | -0.1 (-1.1, 1) | 0.1 (-1.2, 1.4) |
| Day 7 | 18 | 3.1 (2.2) | -1.1 (-2.4, 0.2) | 17 | 4.2 (2.4) | -0.2 (-1.6, 1.2) | -0.9 (-2.7, 0.9) |
| Day 14 | 12 | 2.6 (1.5) | -1.6 (-3, -0.2) | 14 | 3.0 (1.8) | -1.5 (-2.5, -0.5) | -0.1 (-1.6, 1.4) |
| EORTC QLQ-C30 Dyspnea (past week) | |||||||
| Baseline | 19 | 57.9 (29.1) | - | 19 | 49.1 (20.4) | - | - |
| Day 4 | 15 | 46.7 (16.9) | -15.6 (-29.3, -1.8) | 15 | 46.7 (24.6) | 0 (-14, 14) | -15.6 (-33.5, 2.3) |
| Day 7 | 16 | 47.9 (17.1) | -10.4 (-21.1, 0.3) | 13 | 43.6 (16) | -5.1 (-19, 8.8) | -5.3 (-21.2, 10.6) |
| Day 14 | 13 | 46.1 (16.9) | -7.7 (-22.3, 6.9) | 15 | 40 (18.7) | -6.7 (-19.2, 5.8) | -1 (-18.4, 16.4) |
CI = confidence interval; EORTC QLQ-C30 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core-30; SD = standard deviation.
Statistically significant values based on 95% confidence intervals are bolded.
The dyspnea numeric rating scale examining dyspnea “now” showed similar trends favoring dexamethasone, although it only reached statistical significance on day 14 (Table 2). EORTC dyspnea also showed significant improvements in dyspnea in the dexamethasone arm by day 4 (mean -15.6, 95% CI -29.3 to -1.8, P=0.04) (Table 2). No statistically significant differences were found in other exploratory outcomes such as the Modified Dyspnea Borg scale and Cancer Dyspnea Scale (Supplementary Table, available at jpsmjournal.com).
Changes in Non-Dyspnea Outcomes
ESAS drowsiness improved in the dexamethasone arm by day 4 (mean change -2.2 vs. 0.7, P=0.03) and day 7 (mean change -1.8 vs. 1.1, P=0.01) but not day 14 (mean change -1.2 vs. 0.2, P=0.51); however, the baseline level of drowsiness was higher in the dexamethasone arm (3.7 vs. 1.8, P=0.03). Otherwise, we did not detect any significant differences in other ESAS symptoms and EORTC QLQ-C30 domains (Table 3).
Table 3. Change in Selected Edmonton Symptom Assessment Scale Symptoms in Dexamethasone and Placebo Arms.
| Variable | Dexamethasone | Placebo | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Change from Baseline (SD) | N | Mean (SD) | Change from Baseline (SD) | ||
| ESAS Fatigue | |||||||
| Baseline | 19 | 5.1 (2.3) | - | 19 | 3.4 (2.8) | - | - |
| Day 4 | 15 | 4.7 (2.4) | -0.7 (3.1) | 15 | 3.3 (2.1) | 0.4 (3.4) | 0.3 |
| Day 7 | 16 | 4.3 (2.2) | -1.2 (2.3) | 14 | 3.4 (2.2) | 0.5 (3.3) | 0.08 |
| Day 14 | 13 | 4.6 (1.7) | -0.8 (1.9) | 15 | 3.3 (1.9) | 0.5 (2.8) | 0.18 |
| ESAS Drowsiness | |||||||
| Baseline | 19 | 3.7 (2.7) | - | 19 | 1.8 (2.2) | - | - |
| Day 4 | 15 | 2.1 (2.2) | -2.2 (2.9) | 15 | 2.3 (2.6) | 0.7 (2.9) | 0.03 |
| Day 7 | 16 | 2.3 (2.3) | -1.8 (3.1) | 14 | 2.4 (1.9) | 1.1 (2.1) | 0.01 |
| ESAS Appetite | |||||||
| Baseline | 19 | 3.1 (2.6) | - | 19 | 3.3 (2.8) | - | - |
| Day 4 | 15 | 2.9 (3.6) | -0.4 (4.4) | 15 | 2.2 (2.2) | -1.1 (3.2) | 0.87 |
| Day 7 | 16 | 3.1 (3.3) | -0.2 (4.5) | 14 | 1.8 (1.9) | -1.4 (2.8) | 0.58 |
| Day 14 | 13 | 1.9 (2.2) | -1.5 (3.5) | 15 | 2.1 (2.7) | -0.9 (3.7) | 0.53 |
CI = confidence interval; ESAS = Edmonton Symptom Assessment System; SD =standard deviation.
Adverse Effects
Dexamethasone at the doses given in the study was well tolerated, with no documented grade 3 toxicities (Table 4). By the end of the first week, the dexamethasone group had 11 possible/probable grade 1-2 events and no grade 3 event, compared to the placebo group with 16 possible/probable grade 1-2 events and one grade 3 event.
Table 4. Adverse Events.
| Attribution | Dexamethasone a | Placebo b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days 1-7 | Days 8+ | Days 1-7 | Days 8+ | |||||||||
|
| ||||||||||||
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| Possible | 2 | 2 | 0 | 1 | 0 | 0 | 7 | 3 | 1 | 2 | 3 | 2 |
| Probable | 3 | 4 | 0 | 3 | 0 | 0 | 3 | 3 | 0 | 4 | 2 | 0 |
| Unlikely | 2 | 2 | 0 | 1 | 0 | 0 | 8 | 0 | 0 | 2 | 1 | 0 |
| Unrelated | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||||||||
| Total | 7 | 9 | 0 | 5 | 0 | 0 | 19 | 6 | 1 | 8 | 6 | 2 |
The possible/probable grade 1 and 2 events in the dexamethasone arm were as follows: anxiety/irritability (n=2), diarrhea (n=1), dyspepsia (n=1), halitosis (n=1) edema (n=3), hiccups (n=1), hot flashes (n=1), hyperglycemia (n=1), hyperhidrosis (n=1), insomnia (n=2) and myalgia (n=1).
The possible/probable grade 1 and 2 events in the placebo arm included blurred vision (n=2), dyspepsia (n=2), edema (n=1), fatigue (n=3), flashing (n=1), mouth sores (n=1), taste changes (n=1), hyperglycemia (n=1), fever (n=1), insomnia (n=4), myalgia (n=1), nausea/vomiting (n=4), pain (n=2), rash (n=1) and sore throat (n=1). The grade 3 events in the placebo arm were gastric hemorrhage/ulcer (n=2) and insomnia (n=1).
Discussion
We successfully completed a double-blind, randomized, controlled trial of dexamethasone versus placebo in cancer patients. Our preliminary data support that dexamethasone was well tolerated and may be associated with a rapid improvement in dyspnea. Larger studies are needed to confirm our findings.
One of the major challenges in conducting studies in dyspnea is related to dropout because cancer patients with dyspnea often have a poor prognosis and are in acute distress. In a study combining data from multiple randomized controlled trials in supportive cancer care, we previously reported that higher baseline dyspnea intensity was associated with higher attrition rates (27). In this pilot study, we tried to minimize dropout through several mechanisms, including careful design of eligibility criteria, enrollment from thoracic oncology clinics, minimizing study duration, and daily phone calls to provide careful monitoring. We achieved a high completion rate of 85% by week 1 (blinded phase) and 71% by week 2 (open label phase), suggesting that this study design was feasible.
In advanced cancer, the host inflammatory response is often upregulated and produces cytokines with both systemic and peripheral effects (9). Similar to COPD, cancer is characterized by a significant inflammatory component, including airway wall infiltration of macrophages and T lymphocytes, increased lung tumor necrosis factor-alpha (TNFα) and interleukin (IL)-8, increased lung IL-6 during exacerbations, elevated serum IL-6, TNFα, C-reactive protein and fibrinogen, and increased peripheral neutrophil activation (28). Corticosteroids have been shown to improve dyspnea in obstructive lung diseases by modulating the inflammatory response (29, 30). More recently, a randomized controlled trial on an IL-8 monoclonal antibody also found significant improvement in the sensation of dyspnea (31). Given that inflammation is a significant mediator of dyspnea in cancer patients, we hypothesize that corticosteroids could have a therapeutic benefit in the oncology setting.
Our study population is representative of patients with dyspnea in the oncology setting. They had low percent predicted FEV1 and FVC, and relative normal FEV1/FVC ratio suggesting restrictive lung disease pattern rather than obstructive disease. Indeed, many pathophysiologic changes such as parenchymal lung lesions, radiation-induced lung changes, and pleural effusion contribute to a restrictive pattern. Of note, a small proportion of patients with specific etiologies for dyspnea such as radiation-induced fibrosis, large pleural effusions, and malignant airway obstruction were specifically excluded from this study because they had definitive interventions to reverse the changes. Thus, our study focused on a large majority of cancer patients who had chronic dyspnea and no easily reversible diagnoses.
Consistent with our hypothesis, we found that ESAS dyspnea improved by 1.9 points after only four days of dexamethasone during the blinded phase of this study. Because the minimal clinically important difference is one point (17), this magnitude of change may be clinically significant. Other dyspnea measures such as dyspnea numeric rating scale “now” and EORTC dyspnea showed similar trends, albeit non-statistically significant. When interpreting the findings, it is important to note that the sample size was small. In post-hoc sample size determination, we calculated that 33 patients are required per arm to detect a within-arm difference of 1/10 point with 80% power and alpha of 0.05, assuming a standard deviation of 2. Thus, the lack of statistical significance does not necessarily rule out an efficacy. The other exploratory dyspnea measures such as the Cancer Dyspnea Scale generally showed some improvement with dexamethasone, although within-arm and between-arm differences mostly did not reach statistical significance. The small sample size likely contributes to “false negative”. Furthermore, the responsiveness of these scales needs to be further examined.
To our knowledge, this is the first randomized controlled trial to specifically examine the effect of corticosteroids for dyspnea in cancer patients. Our findings are in line with other case series. Elsayem et al. reported significant improvement in dyspnea after administration of high dose steroids among patients with upper airway obstruction (10). In a prospective series involving 13 patients, Hardy et al. found that five advanced cancer patients experienced some improvement with dexamethasone, six had no change and two did worse (32).
The placebo arm also was associated with a significant within-arm reduction in dyspnea intensity, albeit with a relative delay (i.e. day 7) and a lower magnitude (i.e. 1.3 points) compared to the dexamethasone arm. This effect may be related to a combination of placebo effect, co-interventions, and obsequiousness bias. A study with a larger sample size may allow us to further examine the effects of co-interventions on dyspnea. This highlights the importance of including a placebo control arm in our pilot study to properly estimate the effect size (33). The between-arm difference was -1.2 by day 4 and -0.5 by day 7. The larger observed difference by day 4 may be related to the higher doses of dexamethasone administered in the first four days.
We found that dexamethasone of up to 16 mg/day was well tolerated among cancer patients enrolled in this study, with no grade 3 toxicities observed and generally fewer side effects than in the placebo arm. Future studies may examine the use of dexamethasone at higher doses and/or for longer durations.
Although corticosteroids have been found to reduce fatigue and improve appetite in our randomized trials (14, 15), these benefits were not observed in our study. The discrepancy may be partly explained by differences in patient population, medication doses, sample size and study settings. Our eligibility criteria did not specifically include nor exclude patients with moderate to high levels of fatigue and/or anorexia. Interestingly, ESAS drowsiness improved with dexamethasone without significant insomnia documented. Improved dyspnea may allow patients to be more active and less drowsy. Further research is needed to confirm this secondary finding.
Limitations
This study has several limitations. First, study participants were all recruited from a single tertiary care cancer center, which may limit its generalizability. Second, a majority of patients had lung malignancies in this study because of the higher prevalence of dyspnea in this population. Further studies are needed to examine the efficacy of corticosteroids in patients with other types of advanced cancer. Third, the study sample size was relatively small, and including many exploratory outcomes. Thus, our findings can only be considered preliminary. Fourth, we did not assess inflammatory markers which may be potentially predictive. Finally, because we did not power the study specifically for between-arm comparisons, no definitive conclusion can be drawn regarding the efficacy of dexamethasone compared to placebo.
Conclusion
Currently, there are limited options for palliation of dyspnea in cancer patients, and include systemic opioids, bronchodilators for patients with airflow obstruction, supplemental low and high flow oxygen for patients with hypoxemia, and non-invasive ventilation for patients with hypercapnia (6, 7, 34-36). The current study provides data to support that a double-blind, randomized, controlled trial of dexamethasone was feasible with a low attrition rate. Our preliminary data suggest that dexamethasone was associated with rapid improvement in dyspnea and was well tolerated. Specifically, it justifies larger randomized controlled trials utilizing high doses of dexamethasone and longer duration, which may result in a greater impact on dyspnea. Future studies with larger sample size also would allow stratification of putative predictors of response to corticosteroids, which could potentially facilitate a more personalized approach to the management of dyspnea.
Acknowledgments
This research is supported by an Institutional Research Grant from M. D. Anderson Cancer Center (Dr. Hui). Dr. Hui is supported in part by an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE) and a National Institutes of Health grant (R21CA186000-01A1). Dr. Bruera is supported in part by National Institutes of Health grants R01NR010162-01A1, R01CA122292-01, and R01CA124481-01. The funding sources were not involved in the conduct of the study or development of the submission.
Footnotes
Clinicaltrials.gov registration: NCT01670097
Disclosures: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21:95–102. doi: 10.1016/s0885-3924(00)00258-x. [DOI] [PubMed] [Google Scholar]
- 3.Tishelman C, Petersson LM, Degner LF, Sprangers MA. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25:5381–5389. doi: 10.1200/JCO.2006.08.7874. [DOI] [PubMed] [Google Scholar]
- 4.Hui D, Dos Santos R, Chisholm G, Bruera E. Symptom expression in the last 7 days of life among cancer patients admitted to acute palliative care units. J Pain Symptom Manage. 2015;50:488–494. doi: 10.1016/j.jpainsymman.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Aharon I, Gafter-Gvili A, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review and meta-analysis. Acta Oncol. 2012;51:996–1008. doi: 10.3109/0284186X.2012.709638. [DOI] [PubMed] [Google Scholar]
- 6.Cranston JM, Crockett A, Currow D. Oxygen therapy for dyspnoea in adults. Cochrane Database Syst Rev. 2008;3:CD004769. doi: 10.1002/14651858.CD004769.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Higginson IJ, Bausewein C, Reilly CC, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Resp Med. 2014;2:979–987. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang XS, Shi Q, Williams LA, et al. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain Behav Immun. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsayem A, Bruera E. High-dose corticosteroids for the management of dyspnea in patients with tumor obstruction of the upper airway. Support Care Cancer. 2007;15:1437–1439. doi: 10.1007/s00520-007-0305-0. [DOI] [PubMed] [Google Scholar]
- 11.Lin RJ, Adelman RD, Mehta SS. Dyspnea in palliative care: expanding the role of corticosteroids. J Palliat Med. 2012;15:834–837. doi: 10.1089/jpm.2011.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuo N, Morita T, Iwase S. Efficacy and undesirable effects of corticosteroid therapy experienced by palliative care specialists in Japan: a nationwide survey. J Palliat Med. 2011;14:840–845. doi: 10.1089/jpm.2011.0002. [DOI] [PubMed] [Google Scholar]
- 13.Viola R, Kiteley C, Lloyd NS, et al. The management of dyspnea in cancer patients: a systematic review. Support Care Cancer. 2008;16:329–337. doi: 10.1007/s00520-007-0389-6. [DOI] [PubMed] [Google Scholar]
- 14.Yennurajalingam S, Frisbee-Hume S, Palmer JL, et al. Reduction of cancer-related fatigue with dexamethasone: a double-blind, randomized, placebo-controlled trial in patients with advanced cancer. J Clin Oncol. 2013;31:3076–3082. doi: 10.1200/JCO.2012.44.4661. [DOI] [PubMed] [Google Scholar]
- 15.Paulsen O, Klepstad P, Rosland JH, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial. J Clin Oncol. 2014;32:3221–3228. doi: 10.1200/JCO.2013.54.3926. [DOI] [PubMed] [Google Scholar]
- 16.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 17.Hui D, Shamieh O, Paiva C, et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: a prospective study. Cancer. 2015;121:3027–3035. doi: 10.1002/cncr.29437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hui D, Morgado M, Vidal M, et al. Dyspnea in hospitalized advanced cancer patients: subjective and physiologic correlates. J Palliat Med. 2013;16:274–280. doi: 10.1089/jpm.2012.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bausewein C, Farquhar M, Booth S, Gysels M, Higginson IJ. Measurement of breathlessness in advanced disease: a systematic review. Respir Med. 2007;101:399–410. doi: 10.1016/j.rmed.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Powers J, Bennett SJ. Measurement of dyspnea in patients treated with mechanical ventilation. Am J Crit Care. 1999;8:254–261. [PubMed] [Google Scholar]
- 21.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21:177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]
- 22.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82:800–805. doi: 10.1054/bjoc.1999.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 25.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol. 1996;49:1215–1219. doi: 10.1016/s0895-4356(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hui D, Glitza I, Chisholm G, Yennu S, Bruera E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2013;119:1098–1105. doi: 10.1002/cncr.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falk JA, Minai OA, Mosenifar Z. Inhaled and systemic corticosteroids in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:506–512. doi: 10.1513/pats.200707-096ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lapperre TS, Snoeck-Stroband JB, Gosman MM, et al. Effect of fluticasone with and without salmeterol on pulmonary outcomes in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2009;151:517–527. doi: 10.7326/0003-4819-151-8-200910200-00004. [DOI] [PubMed] [Google Scholar]
- 30.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361:449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 31.Mahler DA, Huang S, Tabrizi M, Bell GM. Efficacy and safety of a monoclonal antibody recognizing interleukin-8 in COPD: a pilot study. Chest. 2004;126:926–934. doi: 10.1378/chest.126.3.926. [DOI] [PubMed] [Google Scholar]
- 32.Hardy JR, Rees E, Ling J, et al. A prospective survey of the use of dexamethasone on a palliative care unit. Palliat Med. 2001;15:3–8. doi: 10.1191/026921601673324846. [DOI] [PubMed] [Google Scholar]
- 33.Hui D, Bruera E. The essential role of feasibility studies in supportive care: reply to Sanz Rubiales and del Valle. J Pain Symptom Manage. 2014;48:e5–6. doi: 10.1016/j.jpainsymman.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Aharon I, Gafter-Gvili A, Paul M, Leibovici L, Stemmer SM. Interventions for alleviating cancer-related dyspnea: a systematic review. J Clin Oncol. 2008;26:2396–2404. doi: 10.1200/JCO.2007.15.5796. [DOI] [PubMed] [Google Scholar]
- 35.Hui D, Morgado M, Chisholm G, et al. High-flow oxygen and bilevel positive airway pressure for persistent dyspnea in patients with advanced cancer: a phase II randomized trial. J Pain Symptom Manage. 2013;46:463–473. doi: 10.1016/j.jpainsymman.2012.10.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hui D, Xu A, Frisbee-Hume S, et al. Effects of prophylactic subcutaneous fentanyl on exercise-induced breakthrough dyspnea in cancer patients: a preliminary double-blind, randomized, controlled trial. J Pain Symptom Manage. 2014;47:209–217. doi: 10.1016/j.jpainsymman.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]


