Abstract
Background
Individuals with previous nonmelanoma skin cancer (NMSC) are at increased risk for subsequent skin cancer, and should therefore limit UV exposure.
Objective
To determine whether individuals with previous NMSC engage in better sun protection than those with no skin cancer history.
Methods
We pooled self-reported data (2005 and 2010 National Health Interview Surveys) from US non-Hispanic white adults (758 with and 34,161 without previous NMSC). We calculated adjusted prevalence odds ratios (aPOR) and 95% confidence intervals (95% CI), taking into account the complex survey design.
Results
Individuals with previous NMSC versus no history of NMSC had higher rates of frequent use of shade (44.3% versus 27.0%; aPOR=1.41; 1.16–1.71), long sleeves (20.5% versus 7.7%; aPOR=1.55; 1.21–1.98), a wide-brimmed hat (26.1% versus 10.5%; aPOR=1.52; 1.24–1.87), and sunscreen (53.7% versus 33.1%; aPOR=2.11; 95% CI=1.73–2.59), but did not have significantly lower odds of recent sunburn (29.7% versus 40.7%; aPOR=0.95; 0.77–1.17). Among subjects with previous NMSC, recent sunburn was inversely associated with age, sun avoidance, and shade but not sunscreen.
Limitations
Self-reported cross-sectional data and unavailable information quantifying regular sun exposure.
Conclusion
Physicians should emphasize sunburn prevention when counseling patients with previous NMSC, especially younger adults, focusing on shade and sun avoidance over sunscreen.
Keywords: Nonmelanoma skin cancer, photo-protection, sunscreen, sunburn, UV exposure, multimodal sun protection, skin cancer prevention
INTRODUCTION
Nonmelanoma skin cancers (NMSC) are the most common of all human malignancies.1 Individuals with previous NMSC are at increased risk for subsequent NMSC2–5 and melanoma.6–12 UV radiation and sunburn are the main avoidable contributing factors to NMSC and melanoma.1,13–15 Thus, it is advisable for individuals with previous NMSC to engage in sun-protective practices to help avoid harmful sun exposure and potentially prevent subsequent skin cancers. Two previous US studies suggest that subjects report engaging in better sun-protective practices after NMSC treatment;16,17 however, evidence suggests that certain photo-protective practices may not necessarily translate into sunburn prevention.18
Our study uses nationally representative US data to examine (1) whether subjects with previous NMSC engage in better photo-protection than subjects without history of skin cancer and (2) correlates of recent sunburn among subjects reporting previous NMSC.
METHODOLOGY
Study population
We obtained data from the National Health Interview Survey (NHIS), a nationally representative cross-sectional survey of the US civilian non-institutionalized population that involves a complex survey design and population-based weights.19 As detailed in Supplemental Figure 1, we pooled self-reported data from 2005 (N=31,428) and 2010 (N=27,157), the most recent survey years with our questions of interest. Final adult response rates for the 2005 and 2010 NHIS were 69.0%20 and 60.8%,19 respectively. Analyses were restricted to non-Hispanic whites (N=35,648), as NMSC primarily affects this population.21 We excluded individuals reporting previous melanoma (N=323) regardless of history of NMSC, reporting skin cancer of unknown kind (N=352), or with missing responses to questions about previous cancer or NMSC (N=54). Our study population thus included 758 subjects reporting previous NMSC and 34,161 subjects without history of skin cancer. This study was exempt from Johns Hopkins University IRB review.
Definitions of covariates
Intuitive categories for age at interview were chosen with consideration for the low incidence of NMSC at younger ages. Survey responses for gender, region of the US, survey year, family history of skin cancer in any first-degree relative, highest level of education, and previous full-body skin examination were reported. Sun sensitivity was characterized as the effect of going out into the sun for an hour without photo-protection on the skin after several months of not being in the sun very much. Included responses were (a) “get a severe sunburn with blisters,” (b) “have a moderate sunburn with peeling,” (c) “burn mildly with some or no darkening/tanning,” (d) “turn darker without sunburn,” (e) “nothing would happen to my skin,” or (f) “do not go out in the sun.” Survey questions quantifying sun exposure were not available, thus we included surrogate variables (BMI and physical activity level). For physical activity level, we summed the reported minutes of mild-to-moderate and vigorous activity per week and created categories (0, <180, 180–360, 360+ minutes/week) using cut-points corresponding approximately to the 50th and 75th percentiles among subjects reporting physical activity.
Definitions of outcome variables
Survey respondents were asked four separate questions regarding how often they (1) stay in the shade, (2) wear a hat that shades their face, ears, and neck, such as a hat with a wide brim all around, (3) wear a long sleeved shirt, and (4) use sunscreen, when they go outside on a warm sunny day for more than one hour. For each of the four questions, we included responses of always, most of the time, sometimes, rarely, never, or “do not go out in the sun”. We defined sun avoidance as a response of “do not go out in the sun” to any of five questions regarding sunscreen, long sleeves, hat, shade, or sun sensitivity. Among the remaining sun-exposed individuals (did not avoid the sun), we examined responses for questions 1–4 categorized as frequent (always or most of the time), sometimes, or rare (rarely or never). We defined individuals with frequent multimodal sun protection as sun-exposed individuals who reported frequent use of at least two of the four examined sun-protective methods (questions 1–4). Recent sunburn was characterized as sunburn within the last 12 months.
Statistical analysis
We performed multivariate logistic regression to estimate prevalence odds ratios (PORs) for the outcome variables using previous NMSC (compared to no history of skin cancer) as the main exposure variable. We selected covariates for adjustment a priori based on their known association with the primary outcomes and/or UV-exposure behaviors, including age, sex, region, family history of skin cancer, sun sensitivity, education, BMI, and physical activity.22–26 Refused, not ascertained, and unknown responses were coded collectively as missing for the above analysis. Frequencies of missing subjects were reported for descriptive (Supplemental Table 1) and sun-protective variables (Table 1). Additional logistic regression was performed for stratified analyses by descriptive variables and for subpopulation analyses restricted to subjects reporting previous NMSC with complete data (additionally adjusting for previous full-body skin examination). As a portion of survey respondents reported previous skin cancer of unknown type, we conducted sensitivity analyses looking at subjects reporting previous NMSC and skin cancer of unknown type together compared to subjects without history of skin cancer to examine whether point estimates for photo-protection would change if all skin cancers of unknown type had also been NMSC. We computed the Ptrend using the Wald statistic treating categorized exposure variables as continuous. Sample weights were adjusted by dividing each sample weight by 2 (the number of pooled years).19 All analyses were performed (SAS 9.4, Cary, NC) taking into account the adjusted sample weights, complex survey design, and differing 2005 and 2010 sample designs.19
Table 1.
Weighted percentages (Wt %) of various sun-protective practices and recent sunburn according to reported history of previous nonmelanoma skin cancer (NMSC) among non-Hispanic white subjects
| No history of skin cancer
|
Previous NMSC
|
|||
|---|---|---|---|---|
| N | Wt % (SE)a | N | Wt % (SE)a | |
| Among all subjects (N=34919) | ||||
| Recent sunburnsd | ||||
| 0 | 18868 | 52.6 (0.3) | 525 | 66.3 (2.0) |
| 1 | 6393 | 20.0 (0.3) | 96 | 14.0 (1.6) |
| 2+ | 6618 | 20.7 (0.3) | 103 | 15.7 (1.5) |
| Missing | 2282 | 6.7 (0.2) | 34 | 4.0 (0.7) |
| Sun avoidance | ||||
| No | 28603 | 85.2 (0.3) | 613 | 82.0 (1.7) |
| Yes | 2985 | 7.3 (0.2) | 100 | 12.8 (1.4) |
| Missing | 2573 | 7.5 (0.2) | 45 | 5.2 (0.9) |
| Among sun-exposed subjects (N=31834) | ||||
| Shadeb | ||||
| Rare | 8777 | 28.8 (0.3) | 116 | 18.0 (1.6) |
| Sometimes | 11374 | 36.9 (0.4) | 208 | 33.1 (2.1) |
| Frequent | 8732 | 27.0 (0.3) | 299 | 44.3 (2.2) |
| Missing | 2293 | 7.3 (0.2) | 35 | 4.6 (0.8) |
| Long sleevesb | ||||
| Rare | 21956 | 71.9 (0.3) | 359 | 56.1 (2.1) |
| Sometimes | 4359 | 13.4 (0.3) | 122 | 19.1 (1.7) |
| Frequent | 2625 | 7.7 (0.2) | 144 | 20.5 (1.7) |
| Missing | 2236 | 7.1 (0.2) | 33 | 4.3 (0.8) |
| Wide-brimmed hatb | ||||
| Rare | 21833 | 71.2 (0.3) | 340 | 52.0 (2.2) |
| Sometimes | 3603 | 11.3 (0.2) | 112 | 17.6 (1.8) |
| Frequent | 3516 | 10.5 (0.2) | 173 | 26.1 (1.9) |
| Missing | 2224 | 7.1 (0.2) | 33 | 4.3 (0.8) |
| Sunscreenb | ||||
| Rare | 12208 | 38.9 (0.4) | 171 | 25.3 (1.8) |
| Sometimes | 6401 | 21.0 (0.3) | 104 | 16.7 (1.7) |
| Frequent | 10361 | 33.1 (0.4) | 351 | 53.7 (2.2) |
| Missing | 2206 | 7.0 (0.2) | 32 | 4.3 (0.8) |
| Frequent protection practicesc | ||||
| 0 | 12519 | 41.2 (0.4) | 124 | 20.3 (1.8) |
| 1 | 10013 | 32.1 (0.3) | 200 | 30.7 (2.1) |
| 2 | 4367 | 13.5 (0.2) | 170 | 26.0 (2.0) |
| 3+ | 2010 | 5.9 (0.2) | 131 | 18.7 (1.6) |
| Missing | 2267 | 7.2 (0.2) | 33 | 4.3 (0.8) |
Pooled data from the 2005 and 2010 National Health Interview Surveys (NHIS)
Percentages are weighted according to US census data and reported with standard error (SE) measurements; weighted percentages (Wt %) may not add up to 100% due to rounding.
Self-reported frequency of the sun-protective practice on a warm sunny day.
Number of the four examined sun protection techniques that sun-exposed subjects reported frequently using on a warm sunny day.
Number of sunburns reported in the last year.
RESULTS
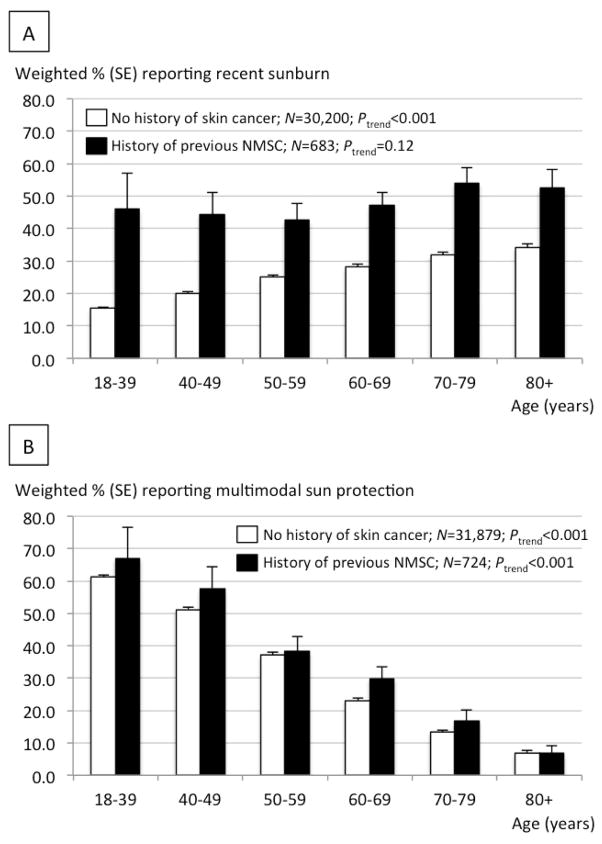
Descriptive characteristics among subjects with and without previous NMSC are reported in Supplemental Table 1. Table 1 shows the percentage of all subjects with versus without previous NMSC reporting recent sunburn (29.7% versus 40.7%) and sun avoidance (12.8% versus 7.3%). Among sun-exposed individuals, we report the percentage of subjects with versus without previous NMSC reporting frequent use of shade (44.3% versus 27.0%), long sleeves (20.5% versus 7.7%), a wide-brimmed hat (26.1% versus 10.5%), sunscreen (53.7% versus 33.1%), and multi-modal sun protection (44.7% versus 19.4%; Table 1). The crude percentage difference in sunburn between subjects with and without previous NMSC disappeared after stratification by age (Figure 1a). Approximately two-thirds (66.9%) of individuals aged 18–39 with previous NMSC reported recent sunburn (Figure 1a). The proportion of individuals reporting recent sunburn decreased with age among subjects with and without previous NMSC (Ptrend<0.001 for both groups; Figure 1a), while use of multimodal sun protection increased significantly with age among subjects reporting no history of skin cancer (Ptrend<0.001; Figure 1b). Among individuals employing multimodal sun protection, frequent sunscreen use decreased with age (Ptrend<0.001; Supplemental Table 2).
Figure 1.
A. Percentage of subjects reporting sunburn in the last year according to age. B. Percentage of subjects reporting frequent multimodal sun protection according to age. Data are pooled from the 2005 and 2010 NHIS. Percentages are weighted according to US census data and reported with standard error (SE) measurements. P-values for linear trend (Ptrend) were calculated using logistic regression treating age categories as a continuous variable. Point estimates are reported in Supplemental Table 3 (sunburn) and Supplemental Table 4 (frequent multimodal sun protection).
Among sun-exposed individuals, previous NMSC (compared to no history of skin cancer) was associated with higher odds of frequent use (versus rare or sometimes use) of shade (adjusted prevalence odds ratio (aPOR)=1.41; 95% CI=1.16–1.71), long sleeves (aPOR=1.55; 1.21–1.98), a wide-brimmed hat (aPOR=1.52; 1.24–1.87), and sunscreen (aPOR=2.11; 1.73–2.59) on a warm sunny day (Table 2). Previous NMSC was also associated with multimodal sun protection (aPOR=2.49; 1.93–3.22). Previous NMSC was not significantly associated with higher odds of sun avoidance (aPOR=1.30; 0.77–2.19) or lower odds of recent sunburn (aPOR=0.95; 0.77–1.17) among all subjects after multivariate adjustment (Table 2). The association between previous NMSC and sunburn remained non-significant even after stratification by age, sex, region, family history of skin cancer, sun sensitivity, education, BMI, physical activity, and survey year (results not shown). Sensitivity analysis looking at subjects reporting previous NMSC and skin cancer of unknown type together compared to subjects with no history of skin cancer did not substantially impact point estimates (results not shown).
Table 2.
Reported history of NMSCa as a predictor of recent sunburn and various sun-protective practices
| PORb | (95% CI) | |
|---|---|---|
| Among all subjects (N=34919) | ||
| Recent sunburnc | 0.95 | (0.77–1.17) |
| Sun avoidanced | 1.30 | (0.77–2.19) |
| Among sun-exposed subjects (N=31834) | ||
| Shadee | 1.41 | (1.16–1.71) |
| Long sleevese | 1.55 | (1.21–1.98) |
| Wide-brimmed hate | 1.52 | (1.24–1.87) |
| Sunscreene | 2.11 | (1.73–2.59) |
| Multimodal sun protectionf | 1.98 | (1.62–2.41) |
The analysis was conducted using pooled data from the 2005 and 2010 NHIS among non-Hispanic white subjects
Subjects who reported no history of skin cancer were used as the reference group
Prevalence odds ratio (POR) estimates were adjusted for age (18–39, 40–49, 50–59, 60–69, 70–79, 80+ years), sex, region (Northeast, Midwest, South, West), family history of skin cancer, sun sensitivity (severe sunburn, moderate sunburn, mild sunburn, no sunburn, no change), highest level of education (less than high school, high school diploma, some college, college degree or higher), BMI (<18.5, 18.5–<25.0, 25.0–<30.0, 30.0+ kg/m2), physical activity level (0, <180, 180–<360, 360+ min/wk), and survey year (2005, 2010).
Outcome variable: at least one sunburn in the last year versus none; this model included all study subjects and thus the covariate of sun sensitivity included the additional response of “do not go into sun.”
Outcome variable; model not adjusted for sun sensitivity because the covariate was used to create the outcome variable
Outcome variable: frequent use versus rare or sometimes use on a warm sunny day
Outcome variable: frequent use of at least two of the four sun protection methods examined among sun-exposed subjects versus fewer than two methods
Among all subjects with previous NMSC, recent sunburn was associated with younger age, residence outside of the South, previous full-body skin examination, and increasing sun sensitivity (Table 3). Recent sunburn was inversely associated with sun avoidance (aPOR=0.3; 0.1–0.9) among all subjects with previous NMSC (Table 4). Among sun-exposed individuals with previous NMSC, recent sunburn was associated with less frequent use of shade (Ptrend<0.001) and long sleeves (Ptrend=0.02), but not a wide-brimmed hat (Ptrend=0.06) or sunscreen (Ptrend=0.75; Table 4). Using more modes of frequent photo-protection was associated with decreasing odds of recent sunburn (Ptrend=0.001; Table 4).
Table 3.
Weighted percentage and odds of recent sunburna according to descriptive characteristics among non-Hispanic white subjects reporting a history of previous NMSC
| Recent sunburna
|
PORc (95% CI) | P-value | ||
|---|---|---|---|---|
| No, N=492
|
Yes, N=188
|
|||
| Wt % (SE)b | Wt % (SE)b | |||
| Age (years) | ||||
| 18–39 | 2.0 (0.7) | 8.1 (2.3) | 3.8 (1.3–10.8) | |
| 40–49 | 8.4 (1.7) | 25.7 (4.0) | 2.3 (1.2–4.6) | |
| 50–59 | 18.7 (2.1) | 25.4 (3.7) | 1.0 (Ref) | |
| 60–69 | 28.2 (2.3) | 27.4 (3.7) | 0.7 (0.4–1.3) | |
| 70–79 | 23.5 (2.1) | 10.1 (2.4) | 0.3 (0.2–0.6) | |
| 80+ | 19.2 (2.0) | 3.2 (1.2) | 0.2 (0.1–0.4) | <0.001e |
| Gender | ||||
| Male | 50.4 (2.7) | 55.9 (4.1) | 1.4 (0.9–2.2) | |
| Female | 49.6 (2.7) | 44.1 (4.1) | 1.0 (Ref) | 0.11 |
| Region | ||||
| South | 47.3 (2.6) | 28.1 (3.7) | 1.0 (Ref) | |
| Northeast | 11.3 (1.8) | 17.9 (3.5) | 3.0 (1.5–5.9) | 0.002 |
| Midwest | 21.9 (2.0) | 29.4 (4.1) | 2.3 (1.3–4.1) | 0.004 |
| West | 19.5 (2.0) | 24.6 (3.3) | 2.2 (1.2–3.8) | 0.007 |
| Family history of skin cancer | ||||
| No | 73.0 (2.4) | 66.3 (4.0) | 1.0 (Ref) | |
| Yes | 27.0 (2.4) | 33.7 (4.0) | 1.4 (0.9–2.4) | 0.17 |
| Sun sensitivity | ||||
| Severe sunburn | 13.7 (1.8) | 18.5 (3.3) | 5.6 (2.6–12.4) | <0.001 |
| Moderate sunburn | 29.3 (2.4) | 36.9 (4.0) | 3.7 (1.8–7.5) | <0.001 |
| Mild sunburn | 22.1 (2.0) | 35.8 (4.0) | 4.9 (2.3–10.4) | <0.001 |
| No sunburnd | 22.2 (2.1) | 6.5 (1.7) | 1.0 (Ref) | |
| Do not go into the sun | 12.7 (1.8) | 2.3 (1.1) | 1.3 (0.3–4.9) | 0.75 |
| Highest level of education | ||||
| Less than high school | 10.0 (1.5) | 5.5 (1.9) | 1.0 (0.4–4.9) | |
| High school diploma | 25.3 (2.2) | 20.2 (3.2) | 0.8 (0.5–1.5) | |
| Some college | 26.3 (2.3) | 23.3 (3.2) | 0.9 (0.5–1.6) | |
| College degree or higher | 38.4 (2.7) | 51.0 (4.3) | 1.0 (Ref) | 0.62e |
| Body mass index (kg/m2) | ||||
| <25.0d | 37.3 (2.4) | 36.0 (3.9) | 1.0 (Ref) | |
| 25–<30 | 38.3 (2.4) | 41.6 (4.1) | 1.4 (0.8–2.3) | |
| 30+ | 24.4 (2.3) | 22.4 (3.5) | 1.0 (0.6–1.7) | 0.85e |
| Physical activity level (min/wk) | ||||
| None | 32.2 (2.3) | 16.4 (3.0) | 1.0 (Ref) | |
| <180 | 28.5 (2.3) | 31.9 (4.0) | 1.6 (0.9–2.8) | |
| 180–<360 | 19.4 (2.0) | 24.4 (3.5) | 1.5 (0.8–2.3) | |
| 360+ | 20.0 (1.9) | 27.3 (3.5) | 2.2 (1.2–4.1) | 0.03e |
| Previous full-body skin check | ||||
| No | 25.7 (2.3) | 35.5 (4.2) | ||
| Yes | 74.3 (2.3) | 64.5 (4.2) | 0.4 (0.3–0.7) | <0.001 |
| Survey year | ||||
| 2005 | 39.3 (2.4) | 40.2 (3.9) | 1.0 (Ref) | |
| 2010 | 60.7 (2.4) | 59.8 (3.9) | 1.1 (0.8–1.7) | 0.53 |
Analyses were conducted on a subset of non-Hispanic white subjects reporting a history of previous NMSC with complete data, using pooled data from the 2005 and 2010 National Health Interview Survey (NHIS).
At least one sunburn reported in the last year
Percentages are weighted according to U.S. census data and reported with standard error (SE) measurements; weighted percentages (Wt %) may not add up to 100% due to rounding
The model used to generate the prevalence odds ratio (POR) estimates included all listed covariates
Certain response categories were collapsed (sun sensitivity of “no sunburn” and “no change”; BMI of <18.5 and 18.5–<25.0) in this analyses for stable estimates
P-value for linear trend was computed using the Wald statistic, treating the categorized exposure variable as a continuous variable.
Table 4.
Weighted percentage (Wt %) and odds of recent sunburna according to sun-protective behaviors among non-Hispanic white subjects with a reported history of previous NMSC
| Recent sunburna
|
PORc (95% CI) | P-value | ||||
|---|---|---|---|---|---|---|
| No | Wt % (SE)b | Yes | Wt % (SE)b | |||
| Sun avoidanced | ||||||
| No | 407 | 82.4 (2.2) | 180 | 96.1 (1.6) | 1.0 (Ref) | |
| Yes | 85 | 17.6 (2.2) | 8 | 4.0 (1.6) | 0.3 (0.1–0.9) | 0.03 |
| Shadee | ||||||
| Rare | 68 | 16.4 (2.1) | 44 | 23.0 (3.4) | 1.0 (Ref) | |
| Sometimes | 129 | 32.5 (2.7) | 73 | 40.4 (3.9) | 0.6 (0.3–1.1) | |
| Frequent | 215 | 51.1 (2.9) | 69 | 36.6 (3.8) | 0.3 (0.2–0.6) | <0.001g |
| Long sleevese | ||||||
| Rare | 224 | 55.8 (2.8) | 119 | 66.4 (3.8) | 1.0 (Ref) | |
| Sometimes | 77 | 19.8 (2.3) | 38 | 21.2 (3.4) | 0.8 (0.4–1.4) | |
| Frequent | 106 | 24.4 (2.3) | 23 | 12.4 (2.7) | 0.5 (0.2–0.9) | 0.02g |
| Wide-brimmed hate | ||||||
| Rare | 218 | 52.2 (2.7) | 106 | 60.6 (4.2) | 1.0 (Ref) | |
| Sometimes | 69 | 18.2 (2.5) | 41 | 21.1 (3.3) | 1.0 (0.6–1.7) | |
| Frequent | 120 | 29.7 (2.4) | 33 | 18.3 (3.3) | 0.6 (0.3–1.01) | 0.06g |
| Sunscreene | ||||||
| Rare | 121 | 29.6 (2.5) | 38 | 20.9 (3.4) | 1.0 (Ref) | |
| Sometimes | 63 | 16.0 (2.1) | 38 | 21.4 (3.7) | 1.3 (0.7–2.5) | |
| Frequent | 223 | 54.4 (2.8) | 104 | 57.7 (4.3) | 1.0 (0.5–1.8) | 0.75g |
| Frequent protectionf | ||||||
| 0 methods | 74 | 19.2 (2.2) | 45 | 26.3 (3.9) | 1.0 (Ref) | |
| 1 method | 126 | 30.7 (2.7) | 66 | 36.8 (4.2) | 0.8 (0.4–1.4) | |
| 2 methods | 110 | 28.4 (2.7) | 51 | 25.8 (3.5) | 0.4 (0.2–0.9) | |
| 3+ methods | 97 | 12.7 (2.2) | 18 | 11.1 (2.8) | 0.3 (0.1–0.7) | 0.001g |
Analyses were conducted on a subset of non-Hispanic white subjects reporting a history of previous NMSC for which there was a complete set of data, using pooled data from the 2005 and 2010 National Health Interview Survey (NHIS).
At least one sunburn reported in the last year
Percentages are weighted according to U.S. census data and reported with standard error (SE) measurements; weighted percentages (Wt %) may not add up to 100% as variables with missing responses are not reported.
Prevalence odds ratio (POR) estimates were adjusted for age (18–39, 40–49, 50–59, 60–69, 70–79, 80+ years), sex, region (Northeast, Midwest, South, West), family history of skin cancer, sun sensitivity (severe sunburn, moderate sunburn, mild sunburn, no sunburn), highest level of education (less than high school, high school diploma, some college, college degree or higher), BMI (<25.0, 25.0–<30.0, 30.0+ kg/m2), physical activity level 0, <180, 180–<360, 360+ min/wk), previous full-body skin check, and survey year (2005, 2010).
Analysis among all subjects reporting previous NMSC with complete data; model not adjusted for sun sensitivity because this covariate was used to create the variable of sun avoidance.
Self-reported frequency of sun-protective practice on a warm sunny day; analysis among sun-exposed individuals reporting previous NMSC with complete data
Subjects who frequently used at least two of the four examined sun protection practices on a warm sunny day; analysis among sun-exposed individuals reporting previous NMSC with complete data
P-value for linear trend was computed using the Wald statistic, treating the categorized exposure variable as a continuous variable.
DISCUSSION
Individuals reporting previous NMSC reported similar relative odds of recent sunburn compared to individuals without history of skin cancer, despite having better sun-protective practices. Our findings are supported by a recent study showing no significant difference in reported rates of sunburn between subjects with and without any previous skin cancer.27 Smaller studies looking at patients before and four months after NMSC treatment have similarly shown significant improvements in the use of shade,16 protective clothing,16 hats,16,17 and sunscreen.17
Approximately 30% of non-Hispanic whites reporting previous NMSC identified having recently sunburned. In recent literature, similar rates of sunburn were found in a Canadian study post-melanoma,28 while higher rates were reported among northern Australian men post-NMSC.29 We observed even higher rates of sunburn in the younger population. Previous studies have observed associations of previous NMSC with subsequent skin cancer,2–12 cumulative sun exposure with risk of NMSC,30–34 and sunburn at any age with melanoma.13–15 Thus, these high rates of sunburn are concerning in the young post-NMSC population, as younger subjects have more years to accumulate harmful sun exposure. We generally observed increasing use of multimodal photo-protection with age, despite the oldest populations possibly experiencing less relative benefit with regard to increased longevity. Our findings suggest that the observed decrease in sunburn with age may result from increased photo-protection in addition to less engagement in activities with sun exposure.35 Among users of multimodal photo-protection, frequent sunscreen use decreased with age, which may reflect that sunscreen application is often a more active decision in the older population than dressing in protective clothing.
Overall, subjects reporting previous NMSC more frequently engage in photo-protective practices reducing chronic UV exposure, which may help reduce the risk of subsequent NMSC.30–34 However, their sun-protective practices may still not be consistent enough to prevent acute episodes of UV exposure leading to sunburns, which are associated with risk of melanoma and BCC.13–15 Physicians should thus continue to emphasize the importance of sunburn prevention, especially among younger individuals with previous NMSC. It is important to consider that individuals with previous NMSC likely had higher UV exposure compared to the general population before NMSC diagnosis, and thus improved sun-protective practices post-NMSC may have contributed to returning their rates of sunburn back to rates comparable to the general population.
Among individuals reporting previous NMSC, risk factors associated with recent sunburn included younger age, residence outside of the South, increasing sun sensitivity, and lack of previous full-body skin examination. This association with age is consistent with previous studies.29,25 The observed association with previous full-body skin examination may be due in part to counseling from health care providers about photo-protection, or conversely if subjects who engage in photo-protection are more proactive about skin cancer screening. The association between region and recent sunburn may be explained in part by better photo-protection counseling at lower latitudes in the U.S.36
Sun avoidance and frequent use of shade were associated with the largest reduction in odds of recent sunburn among subjects with previous NMSC; however, we observed the smallest magnitudes of association between previous NMSC and these behaviors. Interestingly, while frequent use of sunscreen was the most commonly employed sun-protective method, it was not associated with lower odds of recent sunburn among subjects with previous NMSC, suggesting that individuals may not necessarily be effectively applying sunscreen. Other studies similarly suggest that sunscreen alone may not provide adequate protection from harmful sun exposure,18,37,38 as sunscreen use correlates with more overall sun exposure,39,40 sunscreen application may be too thin,39,41 areas of skin may be left unprotected after application.40,42 Additionally, intermittent sunscreen use may result in skin that is not as hardened from chronic UV exposure and more susceptible to acute episodes of sun exposure resulting in sunburn. As the most effective methods of photo-protection may not be the most common methods used among subjects with previous NMSC, this discrepancy may help explain the similar relative odds of recent sunburn in subjects with versus without previous NMSC. Thus, use of effective protective methods such as sun avoidance and shade should be advised. Additionally, we believe that emphasizing patient counseling about proper sunscreen application could increase effectiveness of sunscreen application in preventing sunburn. As sunscreen is the most common form of photo-protection employed, improving the effectiveness of sunscreen could have a large impact on decreasing unnecessary sunburn. Multimodality of sun protection was associated with lower odds of sunburn and thus may offer a highly beneficial approach to prevent sunburns in individuals with previous NMSC.
Strengths of this study include the large sample size using a nationally representative sample. Pooling multiple years of data allowed for increased precision of estimates, especially among smaller subpopulations.
Limitations include the cross-sectional study design with reliance on current sun-protective practices and lack of data quantifying sun exposure (e.g., at midday) in the survey questionnaire, although we attempted to address residual confounding by examining physical activity level and BMI. Additionally, pooling multiple years of data may make findings less generalizable to a specific year, and as much of the data is old (up to 10 years), the accuracy of the data representing the current US population may be limited. The data is self-reported and recall bias is possible, as those with previous NMSC may better remember sunburn or photo-protection if their physician advised being mindful of photo-protection; however, our estimates are consistent and agree with smaller studies with short-term follow-up.16,17 An additional limitation is the use of non-confirmed NMSC cases, as the NHIS is thought to underreport cases.43,44 While melanoma is generally recognized as serious, certain individuals may perceive their NMSC diagnosis as less important and may therefore be less likely to report NMSC. This would further underscore the need to better emphasize sun-protection in this at-risk population. Survey information on subtype of NMSC and sunburn severity was not available. It is possible that despite similar odds of recent sunburn in subjects with and without previous NMSC, sunburn among subjects with previous NMSC was less severe. Prospective studies are still needed to examine relative photo-protective behaviors and risk of sunburn post-NMSC in the context of both prior photo-protective behaviors and current UV exposures and to examine whether proper photo-protection and sunburn prevention will help reduce the risk of subsequent skin cancers in the population with previous NMSC.
CONCLUSIONS
Among non-Hispanic whites, we observe that individuals with previous NMSC engage in better sun-protective practices but not sunburn prevention compared to subjects with no history of skin cancer, suggesting that photo-protection may still not be consistent enough to prevent acute sun exposure leading to sunburn. Physicians should thus continue to emphasize the importance of sunburn prevention, especially among younger individuals with previous NMSC. Sun avoidance (including shade) may be a more effective means of photo-protection than use of sunscreen in subjects with previous NMSC, and should thus be emphasized when counseling patients. However, due to the commonness of sunscreen use, counseling efforts on ways to improve sunscreen effectiveness could also have a large public health impact in preventing sunburn.
Supplementary Material
Acknowledgments
FUNDING:
This publication was made possible in part by the Dr. Thomas Provost Young Investigator Fund of the Johns Hopkins Department of Dermatology; the Dean’s Summer Research Fund of the Johns Hopkins University School of Medicine; and the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by Grant Number TL1 TR001078 from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Funding for the statistical analysis was supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health through Grant Number 1UL1TR001079.
The authors would like to thank Rosa Crum, MD, MHS for helpful discussions.
ABBREVIATIONS
- NMSC
non-melanoma skin cancer
- UV
ultraviolet
- POR
prevalence odds ratio
- aPOR
adjusted prevalence odds ratio
- 95% CI
95% confidence interval
- US
United States
- NHIS
National Health Interview Survey
- IRB
Institutional Review Board
Footnotes
IRB: The study was exempt from Johns Hopkins University IRB review.
Conflicts of interest: The authors have no known conflicts of interest to declare.
Statement on prior presentation: A modified version of these findings were presented at the 2014 Society for Investigative Dermatology Annual Meeting.
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS, or NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Karagas M, Weinstock M, Nelson H. Keratinocyte carcinomas (basal and squamous cell carcinomas of the skin) In: Schottenfeld D, Fraumeni JJ, editors. Cancer Epidemiology and Prevention. 3. New York: Oxford University Press; 2006. pp. 1230–1250. [Google Scholar]
- 2.Flohil SC, Koljenović S, De Haas ERM, Overbeek LIH, De Vries E, Nijsten T. Cumulative risks and rates of subsequent basal cell carcinomas in the Netherlands. Br J Dermatol. 2011;165(4):874–881. doi: 10.1111/j.1365-2133.2011.10488.x. [DOI] [PubMed] [Google Scholar]
- 3.Marcil I, Stern RS. Risk of developing a subsequent nonmelanoma skin cancer in patients with a history of nonmelanoma skin cancer: a critical review of the literature and meta-analysis. Arch Dermatol. 2000;136(12):1524–1530. doi: 10.1001/archderm.136.12.1524. [DOI] [PubMed] [Google Scholar]
- 4.Revenga F, Paricio JF, Vazquez MM, Del Villar V. Risk of subsequent non-melanoma skin cancer in a cohort of patients with primary basal cell carcinoma. J Eur Acad Dermatol Venereol. 2004;18(4):514–515. doi: 10.1111/j.1468-3083.2004.00956.x. [DOI] [PubMed] [Google Scholar]
- 5.Wehner MR, Linos E, Parvataneni R, Stuart SE, Boscardin WJ, Chren M-M. Timing of subsequent new tumors in patients who present with basal cell carcinoma or cutaneous squamous cell carcinoma. JAMA Dermatology. 2015;151(4):382–388. doi: 10.1001/jamadermatol.2014.3307. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Ruczinski I, Jorgensen TJ, Yenokyan G, Yao Y, Alani R, Liegeois NJ, Hoffman SC, Hoffman-Bolton J, Stricklant PT, Helzlsouer KJ, Alberg AJ. Nonmelanoma skin cancer and risk for subsequent malignancy. J Natl Cancer Inst. 2008;100(17):1215–1222. doi: 10.1093/jnci/djn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flohil SC, Van Der Leest RJT, Arends LR, De Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: A systematic review and meta-analysis. Eur J Cancer. 2013;49(10):2365–2375. doi: 10.1016/j.ejca.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Ong ELH, Goldacre R, Hoang U, Sinclair R, Goldacre M. Subsequent primary malignancies in patients with nonmelanoma skin cancer in england: A national record-linkage study. Cancer Epidemiol Biomarkers Prev. 2014;23(3):490–498. doi: 10.1158/1055-9965.EPI-13-0902. [DOI] [PubMed] [Google Scholar]
- 9.Rees JR, Zens MS, Gui J, Celaya MO, Riddle BL, Karagas MR. Non melanoma skin cancer and subsequent cancer risk. PLoS One. 2014;9(6):e99674. doi: 10.1371/journal.pone.0099674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robsahm TE, Karagas MR, Rees JR, Syse A. New malignancies after squamous cell carcinoma and melanomas: a population-based study from Norway. BMC Cancer. 2014;14(1):210. doi: 10.1186/1471-2407-14-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song F, Qureshi AA, Giovannucci EL, Giovannucci EL, Fuchs CS, Chen WY, Stampfer MJ, Han J. Risk of a second primary cancer after non-melanoma skin cancer in white men and women: a prospective cohort study. PLoS Med. 2013;10(4):e1001433. doi: 10.1371/journal.pmed.1001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheless L, Black J, Alberg AJ. Nonmelanoma skin cancer and the risk of second primary cancers: a systematic review. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1686–1695. doi: 10.1158/1055-9965.EPI-10-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(1–3):8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 14.Dennis LK, Vanbeek MJ, Beane Freeman LE, Smith BJ, Dawson DV, Coughlin JA. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis Ann Epidemiol. 2008;18(8):614–627. doi: 10.1016/j.annepidem.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veierod MB, Weiderpass E, Thorn M, Hansson J, Lund E, Armstrong B, Adami HO. A Prospective Study of Pigmentation, Sun Exposure, and Risk of Cutaneous Malignant Melanoma in Women. JNCI J Natl Cancer Inst. 2003;95(20):1530–1538. doi: 10.1093/jnci/djg075. [DOI] [PubMed] [Google Scholar]
- 16.Rhee JS, Davis-Malesevich M, Logan BR, Neuburg M, Burzynski M, Nattinger AB. Behavior modification and risk perception in patients with nonmelanoma skin cancer. WMJ. 2008;107(2):62–68. [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee JS, Matthews BA, Neuburg M, Smith TL, Burzynski M, Nattinger AB. Quality of life and sun-protective behavior in patients with skin cancer. Arch Otolaryngol Head Neck Surg. 2004;130(2):141–146. doi: 10.1001/archotol.130.2.141. [DOI] [PubMed] [Google Scholar]
- 18.Linos E, Keiser E, Fu T, Colditz G, Chen S, Tang JY. Hat, shade, long sleeves, or sunscreen? Rethinking US sun protection messages based on their relative effectiveness. Cancer Causes Control. 2011;22(7):1067–1071. doi: 10.1007/s10552-011-9780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics. Data File Documentation, National Health Interview Survey, 2010 (machine Readable Data File and Documentation) National Center for Health Statistics, Centers for Disease Control and Prevention; Hyattsville, Maryland: 2011. [Google Scholar]
- 20.National Center for Health Statistics. Data File Documentation, National Health Interview Survey, 2005 (machine Readable Data File and Documentation) National Center for Health Statistics, Centers for Disease Control and Prevention; Hyattsville, Maryland: 2006. [Google Scholar]
- 21.Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, Roberts WE, Draelos ZD, Bhushan R, Taylor SC, Lim HW. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748–762. doi: 10.1016/j.jaad.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 22.Holman DM, Berkowitz Z, Guy GP, Hartman AM, Perna FM. The association between demographic and behavioral characteristics and sunburn among U.S. adults - National Health Interview Survey, 2010. Prev Med. 2014;63:6–12. doi: 10.1016/j.ypmed.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson JK, Rademaker AW, Ph D, Sylvester JA, Cook B. Summer Sun Exposure: Knowledge, Attitudes, and Behaviors of Midwest Adolescents. Prev Med. 1997;26(3):364–372. doi: 10.1006/pmed.1997.0156. [DOI] [PubMed] [Google Scholar]
- 24.Coups EJ, Manne SL, Heckman CJ. Multiple Skin Cancer Risk Behaviors in the U.S. Population. Am J Prev Med. 2008;34(2):87–93. doi: 10.1016/j.amepre.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 25.Brown TT, Quain RD, Troxel AB, Gelfand JM. The epidemiology of sunburn in the US population in 2003. J Am Acad Dermatol. 2006;55(4):577–583. doi: 10.1016/j.jaad.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 26.Hall HI, May DS, Lew RA, Koh HK, Nadel M. Sun protection behaviors of the U.S. white population. Prev Med. 1997;26(4):401–407. doi: 10.1006/pmed.1997.0168. [DOI] [PubMed] [Google Scholar]
- 27.Mallett KA, Ackerman S, Turrisi R, Robinson JK. Rates of sunburn among dermatology patients. JAMA Dermatology. 2015;151(2):231–232. doi: 10.1001/jamadermatol.2014.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee TK, Brazier AS, Shoveller Ja, Gallagher RP. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17(1):51–55. doi: 10.1097/CMR.0b013e3280112b98. [DOI] [PubMed] [Google Scholar]
- 29.Woolley T, Buettner PG, Lowe JB. Sunburn in Australian men with a history of non-melanoma skin cancer. Am J Health Behav. 2003;27(3):195–207. doi: 10.5993/ajhb.27.3.1. [DOI] [PubMed] [Google Scholar]
- 30.Gallagher RP, Hill GB, Bajdik CD, Coldman AJ, Fincham S, McLean DI, Threlfall WJ. Sunlight exposure, pigmentation factors, and risk of nonmelanocytic skin cancer. II. Squamous cell carcinoma. Arch Dermatol. 1995;131(2):164–169. [PubMed] [Google Scholar]
- 31.Gallagher RP, Hill GB, Bajdik CD, Fincham S, Coldman AJ, McLean DI, Threlfall WJ. Sunlight exposure, pigmentary factors, and risk of nonmelanocytic skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995;131(2):157–163. [PubMed] [Google Scholar]
- 32.Kricker A, Armstrong BK, English DR. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control. 1994;5(4):367–392. doi: 10.1007/BF01804988. [DOI] [PubMed] [Google Scholar]
- 33.Rosso S, Zanetti R, Martinez C, Tormo MJ, Schraub S, Sancho-Garnier H, Franceschi S, Gafa L, Perea E, Navarro C, Laurent R, Schrameck C, Talamini R, Tumino R, Wechsler J. The multicentre south European study “Helios” II: Different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73(11):1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Dam RM, Huang Z, Rimm EB, Weinstock MA, Spiegelman D, Colditz GA, Willett WC, Giovannucci E. Risk factors for basal cell carcinoma of the skin in men: results from the health professionals follow-up study. Am J Epidemiol. 1999;150(5):459–468. doi: 10.1093/oxfordjournals.aje.a010034. [DOI] [PubMed] [Google Scholar]
- 35.Sjogren K, Stjernberg L. A gender perspective on factors that influence outdoor recreational physical activity among the elderly. BMC Geriatr. 2010;10:34. doi: 10.1186/1471-2318-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winkelmann RR, Rigel DS. Assessing frequency and quality of US dermatologist sunscreen recommendations to their patients. J Am Acad Dermatol. 2015;72(3):557–558. doi: 10.1016/j.jaad.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Dobbinson S, Borland R. Reaction to the 1997/98 SunSmart Campaign: Results from a Representative Household Survey of Victorians. 2000 [Google Scholar]
- 38.Huncharek M, Kupelnick B. Use of topical sunscreens and the risk of malignant melanoma: A meta-analysis of 9067 patients from 11 case-control studies. Am J Public Health. 2002;92(7):1173–1177. doi: 10.2105/ajph.92.7.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thieden E, Philipsen Pa, Sandby-Møller J, Wulf HC. Sunscreen use related to UV exposure, age, sex, and occupation based on personal dosimeter readings and sun-exposure behavior diaries. Arch Dermatol. 2005;141(8):967–973. doi: 10.1001/archderm.141.8.967. [DOI] [PubMed] [Google Scholar]
- 40.Autier P, Boniol M, Doré JF. Sunscreen use and increased duration of intentional sun exposure: Still a burning issue. Int J Cancer. 2007;121(1):1–5. doi: 10.1002/ijc.22745. [DOI] [PubMed] [Google Scholar]
- 41.De Villa D, Da Silva Nagatomi AR, Paese K, Guterres S, Cestari TF. Reapplication improves the amount of sunscreen, not its regularity, under real life conditions. Photochem Photobiol. 2011;87(2):457–460. doi: 10.1111/j.1751-1097.2010.00856.x. [DOI] [PubMed] [Google Scholar]
- 42.Lademann J, Schanzer S, Richter H, Pelchrzim RV, Zastrow L, Golz K, Sterry W. Sunscreen application at the beach. J Cosmet Dermatol. 2004;3(2):62–68. doi: 10.1111/j.1473-2130.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 43.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279–282. doi: 10.1001/archdermatol.2010.4. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, Han J, Li WQ, Li T, Qureshi AA. Basal-cell carcinoma incidence and associated risk factors in U.S. women and men. Am J Epidemiol. 2013;178(6):890–897. doi: 10.1093/aje/kwt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.