Abstract
Spectrofluorometric and emission peak titration and timed studies of OliGreen (OG) and PicoGreen (PG) were conducted in Tris EDTA (TE) buffer, pooled rat and fetal bovine serum with two different aptamers of 72 and 192 bases in length to determine if OG or PG were suitable for aptamer pharmacokinetic (PK) studies in sera. Results indicated that OG and PG detected the single-stranded (ss) and double- stranded (ds) stem-loop structures of the two aptamers quite well in TE with reliable standard curves having exponential character (or several linear detection regions) up to 1 μg/ml of aptamer DNA with detection limits of ~ 1 ng/ml. The intensity of OG and PG staining appeared to correlate with the number and percentage of ss and ds bases in each aptamer. OG and PG fluorescence in pooled rat serum or fetal bovine serum (FBS) did not titer as a function of DNA aptamer concentration from 1 μg/ml to 1 ng/ml. This lack of OG or PG aptamer assays in serum is contrary to most published reports of OG or PG assays for ss antisense oligonucleotides, ds PCR amplicons or other types of DNA in serum or plasma. Further studies suggested that the lack of OG and PG assay titration in serum might not be entirely due to aptamer degradation from nucleases in serum since the fluorescence signals in serum appeared relatively stable over time from 30 minutes to four hours. A hypothesis is presented which attributes the inability of OG or PG to assay aptamers in serum to a combination of high blue-green autofluorescence in serum with possible serum nuclease degradation of aptamers over time and the changing aptamer to serum protein ratio coupled to nonspecific binding of serum proteins to aptamers thereby possibly changing aptamer conformations as a function of aptamer concentration during titration experiments.
Keywords: aptamer, double-stranded, electrophoresis, OliGreen, PicoGreen, serum, single-stranded
Introduction
Tracking therapeutic nucleic acid concentrations in blood samples for pharmacokinetic (PK) studies has traditionally been approached by the use of radiolabeled DNA or RNA. Unfortunately, the use of radioisotopes presents safety and regulatory hurdles which make such methods very unattractive for many laboratories. Other methods including microplate assays to capture modified oligonucleotides from serum samples and quantify them by enzyme-linked colorimetric means have been effectively used for PK studies [1, 2], but such methods are relatively laborious.
Numerous investigators over the past two decades have turned to the use of OliGreen® (OG) for single-stranded (ss) DNA and PicoGreen® (PG) for double-stranded (ds) DNA detection and quantitation in buffer for quantitative polymerase chain reaction (QPCR) [3–6] and other buffer-based biosensor [7–11] and other assay applications [12–22]. OG and PG have been used to track and quantify ss and ds DNA in serum or plasma [15, 17–19] despite the high autofluorescence background of serum in the blue-green region of the spectrum [23] where these dyes emit, because these dyes can exhibit huge increases in fluorescence (e.g., a greater than 1,000-fold increase for PG [24]) when combined with DNA in solution. Investigators have reported ultrasensitive sub-ng/ml detection limits and linear detection of ss or ds DNA over several logs of concentration [15, 20, 22]. Some researchers have also reported the use of OG or PG to assess DNA aptamer levels in solution [8–11] regardless of the hybrid ss/ds nature of aptamer stem-loop structures. Hence, we set out to test two of our DNA aptamers (one short aptamer measuring 72 bases and one longer aptamer at 192 bases) to determine if OG and PG might be useful tracking and quantitative dyes for these aptamers in buffer and animal sera with low detection limits (low ng range) and a broad linear dynamic range from at least ng/ml to μg/ml aptamer levels.
Materials and Methods
Aptamers, Dyes and Other Materials
Sequences of the previously reported aptamers against an E. coli outer membrane protein (OMP; Eco 3R) [25] and a cancer biomarker protein (Extracellular Signal-Regulated Kinase; ERK2 or MAPK1) [26] are given below. These aptamers were synthesized and purified by desalting at Integrated DNA Technologies, Inc. (IDT, Coralville, IA) and their concentrations were reported by IDT. The authors validated the stock aptamer concentrations by absorbance readings of samples diluted in 10 mM Tris-HCl and 1 mM EDTA (TE) buffer at 260 nm in a quartz cuvette.
Eco 3R aptamer: 5′-ATCCGTCACACCTGCTCTGTCTGCGAGCGGGGCGCGGGCCCGGCGGGGGATGCGTGGTGTTGGCTCCCGTAT-3′
ERK aptamer: 5′-ATACGGGAGCCAACACCACCAAGGATGGACTAGTCCAACCTGATCTAATACCCCAAGCCAAACACCTAGTAACATT CAATGCATGCATACAATAGCTTACCCCCCAATAGGGAATAAATAAAAACAAAAGCATACACTCCCATATAAACCAC GATGCTCTCATTTAATTGCCCGAGAGCAGGTGTGACGGAT-3′
Quant-iT™ OG and PG kits were purchased from Molecular Probes, Inc. (Thermo-Fisher, Inc., Pittsburgh, PA). Normal rat serum and fetal bovine serum (FBS) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO) and aliquots were pooled to eliminate any possible effects of nonhomogeneous lots of sera from different animals on the fluorescence results. Recombinant human ERK2 protein was purchased from Novus Biologicals Inc. (Littleton, CO) and kept frozen at −20°C until thawed at room temperature (RT ~ 25°C) just prior to use in experiments.
Conduct of the Fluorescence Titration and Other Fluorescence Studies
Titration studies were essentially performed according to Molecular Probes, Inc.’s instructions. Briefly, two ml of TE buffer made in sterile nuclease-free deionized water (NFW) were added to clear polystyrene cuvettes (Thermo Fisher No. 14-955-129). Four μl of each aptamer stock at 1 mg/ml were added to two ml of TE buffer to yield a 2 μg/ml aptamer solution after mixing with a pipette tip. One ml of this solution was then transferred to 1 ml of TE buffer in second cuvettes and mixed to yield a 1 μg/ml aptamer solution and so on using a serial two-fold dilution (aptamer titration) scheme to achieve sub-ng/ml levels. Blanks consisting of 1 ml of TE buffer without aptamers were also prepared. A 1:200 dilution of stock frozen and thawed OG or PG was then made in TE and 1 ml of this diluted OG or PG solution was added to each cuvette (2 ml final volume in each cuvette) including the blanks. Cuvettes were capped and inverted several times followed by gentle mixing on an orbital shaker for 10 min at RT. For experiments involving rat serum or FBS, the sera were thawed and warmed to RT immediately before use and the same dilution and ten-fold or two-fold serial dilution (titration) schemes where used except that TE was replaced by pooled serum.
Fluorescence spectra and single point emission readings were acquired using a Cary-Varian Eclipse™ spectrofluorometer at the photomultiplier tube (PMT) voltages indicated in the text with 5 nm slits for excitation and emission apertures. For OG excitation was at 500 nm and emission spectra were scanned from 505 nm to 600 nm. For PG excitation was at 480 nm and emission spectra were scanned from 500 to 600 nm. Peak emissions were measured at 526 nm for OG and 530 nm for PG with 0.1 sec read times. Peak emissions were measured five times in succession and mean fluorescence plus and minus 2X standard deviations of the means are reported in some of the figures. In some cases, timed studies were conducted with fluorescence measurements taken at the indicated times after the 10 min mixing period. Limits of detection (LODs) were determined as the first concentration of aptamer which significantly differed from the blank (no aptamer) having no overlap between the means plus or minus 2X standard deviations. In one case, 500 ng/ml of recombinant human ERK2 protein was added in 100 μl of TE buffer to each of the ERK aptamer plus OG or PG samples to determine if changes to the fluorescence spectra could be detected as evidence of possible conformational changes associated with specific aptamer-protein binding.
Determination of Secondary Stem-Loop Structures of the Aptamers
Secondary stem-loop structures of the aptamers were determined using free internet UNAFold software (http://www.idtdna.com/Unafold/). Parameters for linear ss DNA using 0 mM Mg2+, 145 mM Na+, default 50% suboptimality and 25°C. Only the dominant folded (lowest free energy) stem-loop structures both each aptamer are reported herein.
Electrophoresis, Staining and Gel Documentation
Thirty μl rat serum samples with 1 μg/ml of each aptamer in OG or PG were taken from cuvettes following the timed studies and run with 6 μl of 6X loading buffer in a 12% polyacrylamide SDS/HEPES mini Pierce Precise™ gel (Thermo Fisher) at 125V for 1 h in ice cold SDS/HEPES running buffer. The unstained polyacrylamide gel was removed from its cassette and digitally photographed on a UV transilluminator using a UVP, LLC (Upland, CA), DigiDoc-It® system. The polyacrylamide gel was then placed in 100 ml deionized water and stained with 5 μl of 1% w/v ethidium bromide (EtBr) for 10 min followed by decanting of the EtBr solution and a 10 min rinse in deionized water. The gel was than photographed again on the UV transilluminator using an orange emission filter. A 1% agarose gel in cold Tris Acetate EDTA (TAE) buffer was also run with 5 μl (5 μg) of the stock 1 mg/ml Eco 3R and ERK aptamers with 5 μl of 6X loading buffer at 100V for 1 h, stained with EtBr and photographed as before. Both gels were run with one lane containing 7 μl of Bio-Rad 50–2,000 base pair (bp) Amplisize® DNA ladder standards.
Results and Discussion
Figure 1 reveals the secondary stem-loop structures of the two DNA aptamers used in these studies. Not only do the aptamers differ in length (72 vs. 192 bases), but they differ in the number and percentages of bases in ss and ds regions, as noted in Figure 1. Although the difference in percentages of ss and ds bases is relatively small (i.e., 69.8% – 61.1% = 8.7% for bases in ss regions and 38.9% – 30.2% = 8.7% for bases in ds regions), the absolute number differences are large (134 – 44 = 90 ss bases and 58 – 28 = 30 ds bases). These differences in the numbers of ss and ds bases allowed comparison of the effects of ss and ds regions on detection by OG and PG.
Fig. 1.
Secondary stem-loop structures of the Eco 3R and ERK aptamers. Structures were determined by free energy minimization using web-based UNAFold software. The dominant lowest free energy structures are depicted using 145 mM sodium ion, 0 mM magnesium ion, 50% suboptimality, 25°C and linear DNA parameters. The numbers and percentages of nucleotide bases involved in ss and ds regions for each aptamer are also shown.
Figure 2 illustrates that when OG and PG interact with each aptamer at the same w/v concentration (not the same molarity) and the same PMT settings (700V), brighter fluorescence results from the OG interaction with both aptamers than with the PG interaction (i.e., compare panel 2A with 2C and 2B and 2D). It is quite clear from Figure 2 that each aptamer was more detectable with OG than with PG in TE buffer when assays were conducted according to Molecular Probes’ instructions. This may be due to the fact that both aptamers consist more of ss regions (61.1% to 69.8%) which will bind OG (Figures 2A and 2B) vs. ds regions which interact with PG (Figs. 2C and 2D). If one wishes to compare the fluorescence intensities of the shorter 72 base Eco 3R (Figure 2A) aptamer with that of the longer 192 base ERK aptamer (Figure 2A) it appears that at 1,000 ng/ml or 1 μg/ml the longer ERK aptamer with 90 more ss bases is off scale and brighter than the shorter Eco 3R aptamer with OG, but the other aptamer concentrations gave comparable fluorescence with OG regardless of aptamer type. In terms of molarity, the heavier ERK aptamer was less concentrated than the lighter Eco 3R aptamer and yet gave brighter fluorescence with OG at the 1,000 ng/ml level. Both aptamers gave comparable fluorescence intensity at 1,000 ng/ml with PG (Figures 2C and 2D). However, PG fluorescence was noticeably reduced for ERK vs. Eco 3R aptamers at or below the 500 ng/ml level (Figures 2C vs. 2D), despite the ERK aptamer having 30 more bases in ds regions. The suppressed ERK aptamer fluorescence vs. Eco 3R aptamer could be due to the lower molarity of the heavier ERK aptamer in this comparison.
Fig. 2.
Comparison of emission spectra for the Eco 3R and ERK aptamers in TE buffer with OG and PG as a function of DNA aptamer concentration in ng/ml. Excitations were as described in the text and all emission spectra were scanned from 500 or 505 nm to 600 nm with a 700V PMT setting to enable intensity comparisons.
Molecular Probes cites a potential detection limit of 100 pg/ml for its 18 base linear DNA oligonucleotide standard with the OG kit. However, using the 72 base Eco3R aptamer, an LOD of only 1 ng/ml was obtained with OG in TE buffer as shown in Figure 3. In addition, the titration curve from 1 ng/ml to zero was clearly not entirely linear, but exponential (at least from 0 to 125 ng/ml as in Figures 3D and 4D), in nature unlike the linear fluorescence data that Molecular Probes reports in its OG kit instructions for its 18mer from 0 to 1 μg/ml. Assessment of the OG plus Eco 3R titration emission spectra (Figures 3A and 3B) was aided by single peak fluorescence reads at 526 nm in Figures 3C and 3D to reveal an LOD of 1 ng/ml of Eco 3R, but in each case the readings had to be segregated into linear high (≥ 125 ng/ml) and parabolic low (≤ 125 ng/ml) range Eco 3R concentrations in TE to reasonably plot all of the data. PMT settings (voltages) were adjusted to 900V to achieve the greatest spread between spectra for LOD determination in the low concentration range (Figure 3B).
Fig. 3.
Analysis of the Eco 3R aptamer as a function of aptamer concentration in TE buffer with OG by spectrofluorometry with emission scanning from 505 to 600 nm and plotting of mean emission peak heights of five readings taken at 526 nm ± 2X standard deviation error bars to determine the LOD.
Fig. 4.
Analysis of the Eco 3R aptamer as a function of aptamer concentration in TE buffer with PG by spectrofluorometry with emission scanning from 500 to 600 nm and plotting of mean emission peak heights of five readings taken at 530 nm ± 2X standard deviation error bars to determine the LOD.
Data in Figure 4 for Eco 3R interacting with PG were very similar to those observed in Figure 3. Again, the LOD for Eco 3R was 1 ng/ml and a more parabolic titration curve was noted for the low concentration (≤ 125 ng/ml) range. Again, the PMT voltage was adjusted to 1,000V to achieve the greatest spread between spectra in the low range (Figure 4B) for LOD determination.
Figure 5 illustrates a similar fluorescence behavior for the ERK aptamer with OG and PG (Figures 5A and 5C) in that the aptamer gave brighter fluorescence upon interacting with OG than with PG, perhaps due to the greater ss nature of the ERK aptamer. When a 500 ng/ml (~ 11.5 nM) final concentration of the cognate recombinant human ERK2 protein (~ 43.5 kDa) in 100 μl of TE was added to 500 ng/ml of the ~ 59 kDa ERK2 aptamer (~ 8 nM concentration; close to a 1:1 aptamer to protein ligand ratio) and mixed for 15 min, a small increase in fluorescence was seen in Figure 5D (denoted by the asterisk) versus the same spectrum in Figure 5C without the added ERK2 protein. This observation was of interest because a decrease in fluorescence was expected due to dilution as the cuvette volume increased from 2.0 to 2.1 ml. Instead, the fluorescence appeared to increase (arrow and asterisk in Figure 5D versus arrow in Figure 5C). Unfortunately, this increased fluorescence effect was not reproducible in subsequent recombinant ERK2 protein addition experiments (data not shown). However, this result is reported because it may represent a transient or short-lived interaction of the aptamer with its cognate protein target which has proven somewhat labile in other experiments conducted by the authors (data not shown).
Fig. 5.
Spectrofluorometric comparison of the ERK aptamer titration in TE buffer with OG and PG without (panels A and C) and with 500 ng/ml of recombinant human ERK2 protein (panels B and D) added in 100 μl of TE buffer to each cuvette following the readings taken in panels A and C and mixing for 15 min at RT. The increase in fluorescence indicated by the asterisk was not reproducible in subsequent experiments (data not shown), but is reported because it may have been transient. All emission scans were from 500 or 505 nm to 600 nm.
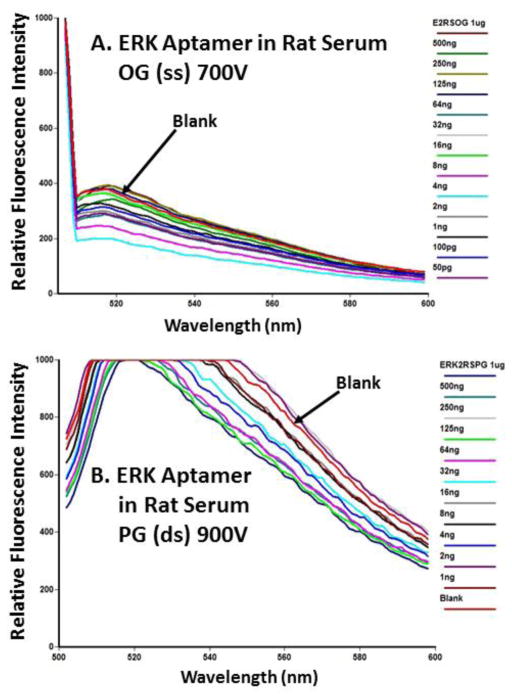
Figures 6 and 7 show that neither aptamer titered well in normal rat serum with OG or PG. In these figures, there is a general decrease in fluorescence from 1 μg/ml (1,000 ng/ml) to low ng or pg levels of each aptamer, but the curves either overlap very closely or the blank actually exhibits stronger fluorescence than serum samples containing added aptamers. We observed this lack of fluorescence titration behavior in several other experiments with rat serum or FBS (data not shown), thus indicating that neither OG or PG would be of value for tracking and quantifying aptamers in animal sera for PK studies.
Fig. 6.
Failure of Eco 3R aptamer titration using OG (panel A) or PG (panel B) in pooled normal rat serum. No clear titration pattern emerged and the fluorescence of some samples registered below that of the blank spectrum. All emission scans ranged from 500 or 505 nm to 600 nm.
Fig. 7.
Failure of ERK aptamer titration using OG (panel A) or PG (panel B) in pooled normal rat serum. No clear titration pattern emerged and the fluorescence of some samples registered below that of the blank spectrum. All emission scans ranged from 500 or 505 nm to 600 nm.
Figure 8 is similar to Figures 6 and 7 in that it plots aptamer OG or PG fluorescence as a function of aptamer concentration for both aptamers from other rat serum-based experiments, but peak height is plotted for simplicity. Figure 8 illustrates how wildly the peak fluorescence can fluctuate as a function of aptamer concentration in pooled rat serum, thereby making OG or PG unacceptable as fluorescent dyes to quantify or track aptamers in serum.
Fig. 8.
Peak height plots of Eco 3R and ERK aptamers in pooled normal rat serum with OG (panel A) and PG (panel B) showing unpredictable fluctuations in fluorescence as a function of aptamer concentration.
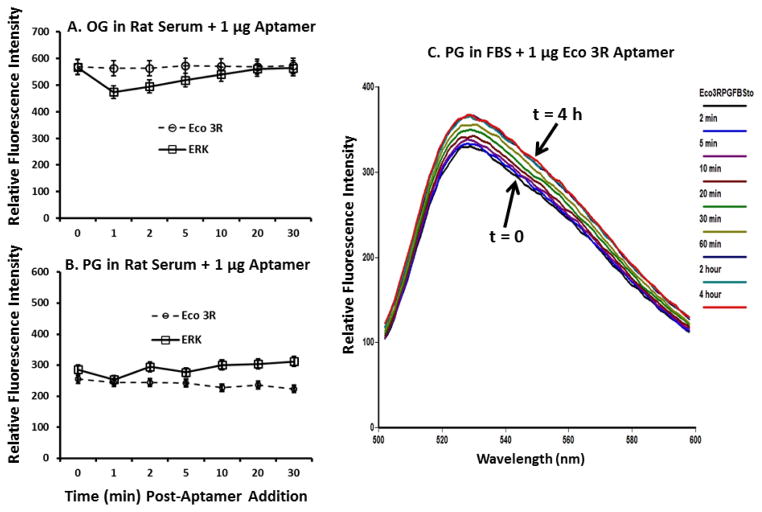
Figure 9 shows that the lack of aptamer titration in rat serum might not be due to aptamer breakdown into smaller fragments due to serum nucleases. This is because the OG and PG fluorescence signals were fairly stable in rat serum for 30 min (Figures 9A and 9B) and in FBS for as long as 4 h (Figure 9C).
Fig. 9.
Results of timed aptamer stability studies in pooled normal rat serum over 30 min using 1 μg/ml of each aptamer in OG (panel A) and PG (panel B) and Eco 3R in FBS with PG over 4 h (panel C).
Several attempts were made to identify intact 72 and 192 base aptamers in rat serum by electrophoresis at the end of these timed experiments as Bruno has successfully done previously for Alexa Fluor 647-labeled aptamers in serum [23]. However, no evidence of the ~ 30 ng of OG- or PG-stained aptamer bands in polyacrylamide gels with or without ethidium bromide (EtBr) staining was ever noted as typified in Figures 10A and 10B. Figures 10A and 10B also illustrate the intense blue-green background generated by proteins in normal rat serum making aptamer detection at 526 to 530 nm quite difficult. Detection in the so called “red window” of low serum fluorescence (~600–700 nm) is preferable and has worked in the past for Bruno when tracking aptamers in serum [23]. Figure 10C is a useful positive control showing 5 μg of each aptamer from the stock 1 mg/ml solutions after staining with EtBr staining in a 1% agarose gel. Aptamer stock concentrations were verified by IDT and by absorbance readings at 260 nm in the author’s laboratory prior to serial dilutions. Interestingly, the aptamers in Figure 10C also appear to form higher molecular weight concatamers or partial hybrids with themselves which may effect OG or PG fluorescence intensities.
Fig. 10.
(A) Unstained 12% polyacrylamide SDS gel showing lane 1; unstained Bio-Rad Amplisize® DNA ladder, lane 2; 30 ng of Eco 3R aptamer in rat serum with OG, lane 3; 30 ng of ERK aptamer in rat serum with OG, lane 4; 30 ng of Eco 3R aptamer in rat serum with PG, lane 5; 30 ng of ERK aptamer in rat serum with PG during UV illumination. (B) the same gel as in panel A following EtBr staining. (C) Lane 1; Bio-Rad Amplisize® DNA ladder, lane 2; 5 μg of stock Eco 3R aptamer in TE buffer and lane 3; 5 μg of stock ERK aptamer in TE buffer electrophoresed in a 1% agarose EtBr stained gel. DNA ladder standard lengths are shown in base pairs (bp).
In deference to other authors who have reported linear titration curves for various linear ss or ds DNA in sera with OG and/or PG [4, 13, 15, 17], we did not observe valid aptamer titrations in rat or fetal bovine serum using OG or PG despite multiple attempts. Only Park et al. [16] reported that QPCR was preferable to the use of PG for quantifying cell-free DNA in in plasma, thereby somewhat supporting our observations. It is true that the oligonucleotides studied with OG and PG in sera by other authors were generally linear and not convoluted with ss and ds stem-loop regions like aptamers. The more linear oligonucleotides could possibly be more highly decorated with OG or PG and therefore more detectable with these dyes against the intense blue-green autofluorescence background of sera. However, in our hands, OG and PG were useless as quantifying dyes in serum, despite showing excellent titration curves in TE buffer. These observations have led to a hypothesis that the failure of aptamers to be quantified in serum could be due to a variety of factors including: 1) high autofluorescence background in the blue-green region of the spectrum, 2) some nuclease activity resulting in aptamer breakdown over time, and 3) binding of the aptamers to serum proteins whether specific or not to change aptamer conformations [27]. In addition, nonspecific binding of the aptamers to serum proteins may change fluorescence according to the changing ratios of aptamers to proteins as a function of aptamer levels during the titration for each different type of aptamer (Figures 6–8). Sera represent complex milieus of blue-green fluorescing proteins, vitamins, and other solutes and may impact the 3-dimensional shape of aptamers differently when aptamers are more or less abundant in sera. Thus, changing aptamer to protein ratios might be expected to change the interactions with OG and PG as the percentage of ss and ds regions change and lead to seemingly random fluctuations in fluorescence (e.g., Figures 6–8).
Conclusions
OG and PG work well for detection of aptamers in TE buffer with OG giving stronger fluorescence than PG in the case of the two aptamers studied here. The stronger OG fluorescence may be related to the greater number and percentage of ss bases or regions in the two aptamers examined. Therefore, OG and PG appear quite good and appropriate for fluorescence-based quantitation in buffer applications such as QPCR [3, 5, 6] or some aptamer biosensor applications [8–11], although we failed to detect reliable aptamer binding of the ERK aptamer to its cognate ERK2 protein target which it is known to bind with a measured Kd of ~ 60 nM [26].
The failure of both aptamers to titer in pooled rat or fetal bovine serum may not appear to be related to aptamer stability in serum containing nucleases because the OG and PG signals were relatively stable over much greater time periods (Figure 9) than those used to conduct and measure the titrations. However, unlike past studies using red Alexa Fluor 647 fluorophore-labeled aptamers [23], intact full-length aptamers were not detected in aptamer-spiked rat serum by electrophoresis in this study. The lack of detection of aptamers by electrophoresis may be due to several factors including degradation of the aptamers, aptamer binding to various serum proteins, or stronger affinity of the aptamers for OG and PG which have reported pg detection limits versus EtBr with a low ng detection limit when bound to ds DNA [5]. The authors also hypothesize that the changing ratios of aptamers to serum proteins in such titration experiments may change the conformations of aptamers [27] and lead to unpredictable fluctuations in the OG or PG fluorescence output during such titrations. Hence, OG and PG cannot be recommended for aptamer quantitation in serum.
Acknowledgments
Financial support for this project was provided by NIH (NIGMS) SBIR Grant No. 2R44GM101712-02 and NCI SBIR Contract No. HHSN261201300075C.
References
- 1.Healy JM, Lewis SD, Kurz M, Boomer RM, Thompson KM, Wilson C, McCauley TG. Pharmacokinetics and biodistribution of novel aptamer compositions. Pharm Res. 2004;21:2234–2246. doi: 10.1007/s11095-004-7676-4. [DOI] [PubMed] [Google Scholar]
- 2.Yu RZ, Baker B, Chappell A, Geary RS, Cheung E, Levin AA. Development of an ultrasensitive noncompetitive hybridization-ligation enzyme-linked immunosorbent assay for the determination of phosphorothioate oligodeoxynucleotide in plasma. Anal Biochem. 2002;304:19–25. doi: 10.1006/abio.2002.5576. [DOI] [PubMed] [Google Scholar]
- 3.Chiminqgi M, Moutereau S, Pernet P, Conti M, Barbu V, Lemant J, Sacko M, Vaubourdolle M, Loric S. Specific real-time PCR vs. fluorescent dyes for serum free DNA quantification. Clin Chem Lab Med. 2007;45(8):993–995. doi: 10.1515/CCLM.2007.191. [DOI] [PubMed] [Google Scholar]
- 4.Szpechcinski A, Struniawska R, Zaleska J, Chabowski M, Orlowski T, Roszkowski K, Chorostowska-Wynimko J. Evaluation of fluorescence-based methods for total vs. amplifiable DNA quantification in plasma of lung cancer patients. J Physiol Pharmacol. 2008;59(Suppl 6):675–681. [PubMed] [Google Scholar]
- 5.Ahn SJ, Costa J, Emanuel JR. PicoGreen quantitation of DNA: effective evaluation of samples pre- or post-PCR. Nucl Acids Res. 1996;24(13):2623–2625. doi: 10.1093/nar/24.13.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhinn H, Scherman D, Escriou V. One-step quantification of single-stranded DNA in the presence of RNA using OliGreen in a real-time polymerase chain reaction thermocycler. Anal Biochem. 2008;372(1):116–118. doi: 10.1016/j.ab.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Gui H, Jin Q, Zhang Y, Wang X, Yang Y, Shao C, Cheng C, Wei F, Yang Y, Yang M, Song H. Development of an aptamer/fluorescence dye PicoGreen-based method for detection of fumonisin B1. Sheng Wu Gong Cheng Xue Bao. 2015;31(9):1393–1400. [PubMed] [Google Scholar]
- 8.Lv Z, Chen A, Liu J, Guan Z, Zhou Y, Xu S, Yang S, Li C. A simple and sensitive approach for ochratoxin A detection using a label-free fluorescent aptasensor. PLoS One. 2014;9(1):e85968. doi: 10.1371/journal.pone.0085968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lv Z, Liu J, Zhou Y, Guan Z, Yang S, Li C, Chen A. Highly sensitive fluorescent detection of small molecules, ions, and proteins using a universal label-free aptasensor. Chem Commun (Camb) 2013;49(48):5465–5467. doi: 10.1039/c3cc42801j. [DOI] [PubMed] [Google Scholar]
- 10.Oh BN, Lee S, Park HY, Baeg JO, Yoon MY, Kim J. Sensitive fluorescence assay of anthrax protective antigen with two new DNA aptamers and their binding properties. Analyst. 2011;136(16):3384–3388. doi: 10.1039/c0an00978d. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Yang C, Zhou X, Qin J. Label-free aptamer-based sensors for L-argininamide by using nucleic acid minor groove binding dyes. Chem Commun. 2011;47:3192–3194. doi: 10.1039/c0cc04844e. [DOI] [PubMed] [Google Scholar]
- 12.Blotta I, Prestinaci F, Mirante S, Cantafora A. Quantitative assay of total dsDNA with PicoGreen reagent and real-time fluorescent detection. Ann Ist Super Sanita. 2005;41(1):119–123. [PubMed] [Google Scholar]
- 13.Chen JA, Meister S, Urbonaviciute V, Rödel F, Wilhelm S, Kalden JR, Manger K, Voll RE. Sensitive detection of plasma/serum DNA in patients with systemic lupus erythematosus. Autoimmunity. 2007;40(4):307–310. doi: 10.1080/08916930701356317. [DOI] [PubMed] [Google Scholar]
- 14.Gealy R, Wright-Bourque JL, Kraynak AR, McKelvey TW, Barnum JE, Storer RD. Validation of a high-throughput in vitro alkaline elution/rat hepatocyte assay for DNA damage. Mutat Res. 2007;629(1):49–63. doi: 10.1016/j.mrgentox.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Gray GD, Wickstrom E. Rapid measurement of modified oligonucleotide levels in plasma samples with a fluorophore specific for single-stranded DNA. Antisense Nucleic Acid Drug Dev. 1997;7(3):133–140. doi: 10.1089/oli.1.1997.7.133. [DOI] [PubMed] [Google Scholar]
- 16.Park JL, Kim HJ, Choi BY, Lee HC, Jang HR, Song KS, Noh SM, Kim SY, Han DS, Kim YS. Quantitative analysis of cell-free DNA in the plasma of gastric cancer patients. Oncol Lett. 2012;3(4):921–926. doi: 10.3892/ol.2012.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramachandran K, Speer CG, Fiddy S, Reis IM, Singal R. Free circulating DNA as a biomarker of prostate cancer: comparison of quantitation methods. Anticancer Res. 2013;33(10):4521–4529. [PubMed] [Google Scholar]
- 18.Reyderman L, Stavchansky S. Determination of single-stranded oligodeoxynucleotides by capillary gel electrophoresis with laser induced fluorescence and on column derivatization. J Chromatogr A. 1996;755(2):271–280. doi: 10.1016/s0021-9673(96)00605-x. [DOI] [PubMed] [Google Scholar]
- 19.Reyderman L, Stavchansky S. Quantitative determination of short single-stranded oligonucleotides from blood plasma using capillary electrophoresis with laser-induced fluorescence. Anal Chem. 1997;69(16):3218–3222. doi: 10.1021/ac970280+. [DOI] [PubMed] [Google Scholar]
- 20.Uddin MN, Do DP, Pai SB, Gayakwad S, Oettinger CW, D’Souza MJ. A methodology for quantitation and characterization of oligonucleotides in albumin microspheres. Analyst. 2009;134(7):1483–1489. doi: 10.1039/b823554f. [DOI] [PubMed] [Google Scholar]
- 21.Zhang SB, Yang S, Vidyasagar S, Zhang M, Casey-Sawicki K, Liu C, Yin L, Zhang L, Cao Y, Tian Y, Swarts S, Fenton BM, Keng P, Zhang L, Okunieff P. PicoGreen assay of circular DNA for radiation biodosimetry. Rad Res. 2015;183:188–195. doi: 10.1667/RR13556.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Analyt Biochem. 1997;249:228–238. doi: 10.1006/abio.1997.2177. [DOI] [PubMed] [Google Scholar]
- 23.Bruno JG. Strategies for maximizing fluorescence detection sensitivity in the red region of urine and serum. J Bionanosci. 2014;8:122–126. [Google Scholar]
- 24.Dragan AI, Casas-Finet JR, Bishop ES, Strouse RJ, Schenerman MA, Geddes CD. Characterization of PicoGreen interaction with dsDNA and the origin of its fluorescence enhancement upon binding. Biophys J. 2010;99(9):3010–3019. doi: 10.1016/j.bpj.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruno JG, Carrillo MP, Phillips T, Andrews CJ. A novel screening method for competitive FRET-aptamers applied to E. coli assay development. J Fluoresc. 2010;20(6):1211–1223. doi: 10.1007/s10895-010-0670-9. [DOI] [PubMed] [Google Scholar]
- 26.Bruno JG. Predicting the uncertain future of aptamer-based diagnostics and therapeutics. Molecules. 20:6866–6887. doi: 10.3390/molecules20046866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bruno JG, Carrillo MP, Phillips T, Edge A. Serum inverts and improves the fluorescence response of an aptamer beacon to various vitamin D analytes. Luminescence: Journal of Biological and Chemical Luminescence. 2012;27:51–58. doi: 10.1002/bio.1324. [DOI] [PubMed] [Google Scholar]