Abstract
The Wnt signaling pathway is known as one of the important molecular cascades that regulate cell fate throughout lifespan. The Wnt signaling pathway is further separated into the canonical signaling pathway that depends on the function of β-catenin (Wnt/β-catenin pathway) and the noncanonical pathways that operate independently of β-catenin (planar cell polarity pathway and Wnt/Ca2+ pathway). The Wnt/β-catenin signaling pathway is complex and consists of numerous receptors, inhibitors, activators, modulators, phosphatases, kinases and other components. However, there is one central, critical molecule to this pathway, β-catenin. While there are at least 3 receptors, LRP 4, 5 and 6, and over twenty activators known as the wnts, and several inhibitors such as sclerostin, dickkopf and secreted frizzled-related protein, these all target β-catenin. These regulators/modulators function to target β-catenin either to the proteasome for degradation or to the nucleus to regulate gene expression. Therefore, the interaction of β-catenin with different factors and Wnt/β-catenin signaling pathway will be the subject of this review with a focus on how this pathway relates to and functions in the formation and maintenance of bone and teeth based on mainly basic and pre-clinical research. Also in this review, the role of this pathway in osteocytes, bone cells embedded in the mineralized matrix, is covered in depth. This pathway is not only important in mineralized tissue growth and development, but for modulation of the skeleton in response to loading and unloading and the viability and health of the adult and aging skeleton.
Keywords: β-catenin, wnts, bone, osteocytes, teeth, mechanosensation
Introduction
The protein β-catenin is the central target and an essential component of the Wnt/β-catenin signaling pathway. This pathway is involved in numerous aspects of growth and development in many organs and tissues, ranging from cell fate determination, polarity and differentiation to migration, proliferation, and function (Moon et al., 2002, Visweswaran et al., 2015). For example, during embryonic development, Wnt/β-catenin critically contributes to the establishment of the body axis and the orchestration of tissue and organ development. After development, Wnt/β-catenin has been shown to play an essential role in self-renewing tissues such as the hair follicle, the intestinal crypt, and the hematopoietic system. Under pathological conditions, mutations resulting in the activation of this pathway are commonly observed in cancers, such as colon cancer, hair follicle tumors, and leukemia (Clevers, 2006) (For review see Baron and Kneissel 2013). As several reviews have summarized and updated the progress of the Wnt signaling pathway in human skeletal disease (For review see Rudnicki and Williams 2015, Baron and Kneissel 2013), the focus in this review will be on the role of this pathway in mineralized tissue, both bone and tooth, development and function based on the recent discoveries using animal models and cell lines. This pathway not only plays a critical role in growth and development but in the maintenance of the mature skeleton and response to conditions of loading such as exercise and conditions of unloading such as space flight and patient immobilization.
2. Discovery and structure of β-catenin
Catenin, beta 1 (β-catenin; Ctnnb1) was first named in the late 1980s by Ozawa and colleagues along with α-catenin and γ-catenin as these proteins linked E-cadherin to cytoskeletal structures in Ca2+-dependent cell adhesion (Ozawa et al., 1989). It was reported as a component of a mammalian cell adhesion complex even though the protein can also be located in both the cytoplasm and nucleus (McCrea et al., 1991). The gene int 1 (integration 1) in mouse and the gene wg (wingless) in drosophila were reported, found to be homologues and later named as Wnt (Clevers, 2006). In the middle 1990s, several groups independently found that b-catenin in the nucleus triggered Wnt-mediated transcription via T-cell factor/Lymphoid enhancer-binding factor (TCF/LEF) transcription factors (Valenta et al., 2012). The studies eventually supported a dual function of β-catenin, one of crucial importance in the cadherin adhesion complex and a second playing a central role in the Wnt-signaling pathway.
The crystal structure of β-catenin was determined in 2008 (Xing et al., 2008). The structure includes the N-terminal region, the armadillo or Arm domain (total 13 amino acid repeat), the far C-terminal region adjacent to the Arm domain, the end of C-terminal region, Helix C, and unstructured sequences distal to Helix C, each with specific functions. The N-terminal region mediates the degradation of β-catenin, the inner surface of the Arm domain serves as a ligand binding site, the C-terminal segment acts as a strong transactivator by recruiting both effectors and inhibitors, and the Helix C is required for Wnt signaling by potentially recruiting various coactivators. The function of β-catenin closely relies on the molecular structure, though the binding domain or amino acids may vary in the different β-catenin signaling pathways. It is not clear how the unstructured sequences contribute to signaling, therefore studies using new techniques and approaches are still needed to explore the dynamic structure of β-catenin (Gottardi and Peifer, 2008).
3. Components of the canonical Wnt/β-catenin pathway
There are several pathways in which Wnt plays a role such as the planar cell polarity pathway, the Wnt/Ca+2 pathway and a Protein Kinase A pathway involving CREB, but we will be addressing the best studied pathways referred to as the canonical pathway or the Wnt/β-catenin signaling pathway (Bonewald and Johnson, 2008). Under normal homeostasis, phosphorylated β-catenin is part of a degradation complex consisting of adenomatous polyposis coli (APC), glycogen synthase kinase 3β (GSK3β), and axin where it becomes ubiquinated and targeted to the proteasome for degradation. However, with receptor activation (the low density lipoprotein receptor-related proteins, LRP, 4, 5 or 6), β-catenin is freed from its degradation complex in a monomeric form for translocation to the nucleus to initiate gene transcription. The secreted Wnts initiate receptor activation and signaling by binding to the transmembrane receptor Frizzled and LRP coreceptors 4, 5, or 6, inducing phosphorylation of the cytoplasmic tail of the LRPs which recruits axin from the degradation complex to bind to this phosphorylated site. With axin removed, the degradation complex is dissociated by cytoplasmic protein disheveled (DVL) to release β-catenin. Thus, β-catenin accumulates in the cytoplasm and ultimately translocates to the nucleus where it binds with TCF/LEF, leading to the activation of target genes. Antagonists of the Wnt receptors, including Sclerostin (SOST), Dickkopf (DKK) 1, 2, and 3, and secreted frizzled-related protein 1 (sFRP1) protein prevent β-catenin translocation by binding to Wnt proteins (as sFRP1) or by interfering with interactions between Wnt proteins and their receptors and coreceptors (as Sclerostin and Dkk-1)(Burgers and Williams, 2013, Canalis, 2013, Chen et al., 2007, Dallas et al., 2013). In the absence of wnts, the cytoplasmic levels of β-catenin remain low (Aberle et al., 1997).
New components of this pathway are constantly being discovered and therefore, understanding how this pathway operates is not completely known. This pathway has been expanding in number of Wnts, Wnt receptors, coreceptors, soluble inhibitors, modulators, and other molecules thereby increasing the complexity of this pathway. More than one pathway is believed to be activated by Wnt receptor activation. The β-catenin signaling pathway was found to cross talk with bone morphogenic protein 2 (BMP2) signaling to regulate osteoblastic anabolic function in bone (Zhang et al., 2013a). In addition, factor signaling pathways such as estrogen, prostaglandin and parathyroid hormone have been reported to crosstalk with Wnt signaling, which also increases the difficulty of studying and understanding this pathway (Logan and Nusse, 2004). However, one component, β-catenin, is not duplicated and remains a single constant target of this pathway.
4. Importance of the Wnt/β-catenin pathway in bone formation and maintenance
The Wnts are essential for skeletal formation and development and are involved in a variety of processes from limb patterning and formation to chondrogenesis, the differentiation, proliferation and synthesis of bone matrix by osteoblasts as well as the differentiation and function of osteoclasts during development (Bonewald and Johnson, 2008, Glass II and Karsenty, 2006). Genome wide association studies, GWAS, have shown that many members of this signaling pathway are associated with bone mineral density and susceptibility to fracture (Hsu and Kiel, 2012). In every study, LRP5 and SOST, the gene coding for sclerostin, an inhibitor of bone formation, are associated in with low bone mineral density, osteoporosis, and susceptibility to fracture. Also LRP4, DKK1, Wnt4, Wnt16 and ctnnb1 (the gene for β-catenin), are also highly associated with bone phenotype. During the early stages of fracture repair, β-catenin is required for pluripotent mesenchymal cells to differentiate into either osteoblasts or chondrocytes and in later stages of repair for preosteoblasts to differentiate into osteoblasts. Agents that are known to activate the β-catenin pathway are being used to accelerate bone healing along with other uses. Lithium, an approved pharmacologic agent, known to activate β-catenin, appears to enhance bone formation and may be used to improve fracture healing (Chen, Whetstone, 2007). Therapeutics such as the anti-sclerostin antibody are being developed to not only enhance bone formation but hopefully reverse the detrimental bone loss due to osteoporosis (Clarke, 2014, Desiderio et al., 2014, Iniguez-Ariza and Clarke, 2015, McClung and Grauer, 2014).
To determine if β-catenin is critical to osteoblast, osteoclast, or the function of both cells, targeted deletion has been performed in bone cells as the global deletion is lethal. In the postnatal and mature skeleton, the Wnt/β-catenin signaling pathway is essential for bone mass maintenance by regulating the activity of the bone-forming osteoblasts and indirectly, the bone-resorbing osteoclasts (Glass II and Karsenty, 2006). Several in vitro assays and in vivo mouse models have been used to determine the function of the β-catenin signaling pathway in these bone cell types. Mouse models have been generated using the global or targeted deletion of β-catenin or conversely global or targeted activation of β-catenin. By flanking the exon 2–6 with loxp sequences, β-catenin can be inactivated after crossing with Cre recombinase because these sequences encode the unique N-terminal domain (N) and armadillo 1–4. To overexpress active β-catenin, a similar method is used to flox exon 3. This exon encodes for the N-terminal and allows its phosphorylation by glycogen synthase kinase 3β and its subsequent degradation via ubiquitination. When this exon is deleted it creates a constitutively active molecule (Harada et al., 1999). Constitutively active β-catenin (ca-β-catenin) mouse models for deletion of both exons 3–6 and exons 3–5 have been made (Brault et al., 2001, Hu et al., 2005, Huelsken et al., 2000, Huelsken et al., 2001).
The benefits of the numerous tissue-specific mice models and in vitro cell lines have brought a better understanding of the function of β-catenin in bone metabolism. The macrophage-like cell line Raw264.7, Fzd8-deficient mice and mice with targeted deletion of β-catenin in osteoclasts were used to study the mechanism of action of β-catenin in osteoclasts. Increased bone resorption was observed suggesting that β-catenin negatively regulates osteoclasts independent of osteoblasts (Albers et al., 2013) To explore how β-catenin function in osteoclasts, studies focused on nuclear factor kappa B ligand (RANKL) have been performed. One study showed that β-catenin does not affect the receptor activator of RANKL induced osteoclastogenesis through the classic canonical wnt pathway as opposed to the non canonical β-catenin-independent pathway, since deletion of β-catenin in bone marrow macrophages did not affect their normal differentiation into osteoclasts in response to RANKL (Sapir-Koren and Livshits, 2014). However, another study found that β-catenin may upregulate RANKL through sclerostin (Wijenayaka et al., 2011). In summary, the mechanism of β-catenin function in osteoclasts is still unclear and further studies are still needed.
Considerably more information is available on the role of β-catenin in the osteoblast lineage. β-catenin is necessary for differentiation of mesenchymal precursor cells into osteoblasts (Day et al., 2005) and osteoblast survival (Glass II and Karsenty, 2006). β-catenin deletion in the osteoblast lineage using alpha 1 type 1 collage-Cre (Col1a1-Cre) (Glass II et al., 2005) or in late osteoblasts using Osteocalcin-Cre (Holmen et al., 2005) resulted in mice will a low bone mass phenotype due to increased osteoclastic bone resorption due to decreased expression of the RANKL inhibitor osteoprotegerin (OPG). However, these mice died early due to an extremely fragile skeleton. Time and site specific expression of the constitutively active β-catenin in osteoblasts using TM-inducible Cre fusion protein showed that constitutively active β-catenin at different postnatal stages can increase vertebral bone volume by increasing bone formation and decreasing bone resorption (Jia et al., 2013). These mice were much smaller than controls due to the closure of the growth plate but were active and viable. When constitutively activated β-catenin was expressed in the osteoblasts using Col1a1 Cre × Ctnnb+/lox (exon 3) mice, a leukemia phenotype was observed (Kode et al., 2014). However, the leukemia phenotype was not observed in the DMP1-8kb-Cre × Ctnnb+/lox (exon 3) mice (Tu et al, 2015) but instead a bone anabolic response.
5. Role of β-catenin in osteocyte viability and function
Osteocytes are the most abundant bone cells and are located inside mature bone. They are originally derived from osteoprogenitors though osteoblast differentiation. Osteoblasts after producing osteoid that will mineralize to form bone have one of three fates, they can die due to senescence, degeneration/necrosis, apoptosis, and/or osteoclastic engulfment (Knothe Tate et al., 2004), they can become osteocytes or they can become lining cells (Manolagas, 2000). During their long lifespan, which varies from species to species, osteocytes are suggested to play a crucial biological and functional role in maintenance of the skeleton. Numerous studies has proposed that the osteocyte may function to 1) modify its extracellular microenvironment, 2) regulate bone remodeling by translating the mechanical stimuli into biochemical signals, 3) initiate and maintain bone mineralization, 4) regulate phosphate and calcium homeostasis and 5) also serve as a endocrine cell to target organs such as the kidney (Bonewald, 2005, Bonewald et al., 2013, Bonewald and Wacker, 2013, Xiong et al., 2014, Zhang et al., 2006) (Capulli et al., 2014, Sheng et al., 2013). Although the molecular mechanisms responsible for these osteocyte functions have not been completely elucidated, significant progress is being made to unravel the signaling pathways that play a role in osteocyte regulation of bone homeostasis. It also appears that osteocytes can play a role in disease. Understanding osteocyte function has led to new targets to treat bone diseases like osteoporosis, bone fracture healing, hypophosphatemic rickets, chronic kidney disease and others to maintain healthy bone it is necessary to maintain healthy osteocytes (Clarke, 2014, Dallas, Prideaux, 2013, Iniguez-Ariza and Clarke, 2015).
β-catenin is a central molecule that is necessary to maintain bone homeostasis and mechanotransduction through maintenance of osteocyte viability (Kramer et al., 2010). Dying osteocytes are necessary for bone repair such as occurs with microdamage (Kennedy et al., 2012). Bone that has no live osteocytes, called osteonecrotic or dead bone does not remodel suggesting that osteocytes are necessary for bone remodeling. Several factors have been identified that prevent osteocyte apoptosis such as bisphosphonates (Plotkin et al., 1999), prostaglandin (Kitase et al., 2010), muscle factors (Jahn et al., 2012) and estrogen (Tomkinson et al., 1998). However, one of the most potent inhibitors of osteocyte apoptosis is the application of fluid flow shear stress (Kitase, Barragan, 2010). Shear stress is thought to mimic the flow of the bone fluid through the osteocyte lacunacanalicular network in response to loading or exercise. It is thought to be necessary not only to maintain osteocyte viability (Davidson et al., 2012), but also osteocyte communication (Hu et al., 2015).
Fluid flow shear stress has also been shown to synergize with anti-apoptotic factors to protect osteocytes against cell death by a number of apoptotic agents such as dexamethasone. Fluid flow shear stress is a very potent inducer of prostaglandin E2 (PGE2) production by osteocytes (Kamel et al., 2010). In vitro experiments performed using the MLO-Y4 osteocyte like cell line, showed that the protective effects of shear stress induced PGE2 against dexamethasone was mediated through β-catenin (Kitase, Barragan, 2010). Muscle factors have also been shown to protect osteocytes against dexamethasone induced apoptosis (Jahn, Lara-Castillo, 2012). C2C12 differentiated myotubes secrete factors that induce the nuclear translocation of β-catenin to protect the cell against the apoptotic effect of glucocorticoid (Jahn, Lara-Castillo, 2012). This work shows that muscle tissue produces factors that maintain osteocyte viability and therefore skeletal health.
Bellido and colleagues showed that β-catenin is required for FOXO mediated transcription, and there is competition between FOXO and the β-catenin activated transcription factor T-cell factor (TCF) mediated transcription (Bellido, 2010). Using MLO-Y4 osteocyte like cell culture, they suggested that the bidirectional crosstalk between the caveolin-1/ERK and Wnt/β-catenin pathways was important for osteocyte survival in mechanotransduction (Gortazar et al., 2013). They also showed that shear stress induced PGE2 release in MLO-Y4 cells leads to the increase in nuclear accumulation of β-catenin through activation of both PI3K/Akt and cAMP-PKA signaling. β-catenin mediated the transduction of Caveolin-1-dependent vascular endothelial growth factor receptor 2 (VEGFR2), which is shown to promote the viability of osteocytic MLO-Y4 cells exposed to mechanical stimuli (de Castro et al., 2015). Jiang and colleagues showed that β-catenin binds to the connexin 43 (Cx43) promoter, stimulating Cx43 expression and functional gap junctions between osteocytes (Xia et al., 2010). These findings support the hypothesis that β-catenin is also involved in both cell viability and communication. In vitro studies have greatly aided investigations in understanding the function of β-catenin in osteocytes. However, in vitro studies cannot mimic the complexity of in vivo environment especially with regards to developing clinical diagnosis and treatment. In vivo, mechanical stimuli have been shown to protect osteocytes from undergoing apoptosis (Kalajzic et al., 2013). Experiments performed where animals were generated with the dentin matrix acidic phosphoprotein 1 (Dmp1) promoter driving the diphtheria toxin receptor also showed that viability of osteocytes is important for skeletal health and response to load. When diphtheria toxin was given to these mice killing all Dmp1 positive cells, early osteocytes, the animal experienced increased osteoclast activation, bone loss, and lost response to mechanical loading (Komori, 2014).
6. Role of β-catenin in osteocyte mechanotransduction
Wolff’s Law is well known in the bone biology field and states that bone has the capacity to adapt to loading or unloading by increasing or decreasing bone mass. However, it is not clear what is responsible for bone’s capacity to sense mechanical loading or lack of mechanical stimuli and then how the mechanical stimuli is translated into biochemical activity. Evidence has accumulated in the last two decades that osteocytes, the most abundant bone cell (95% of the cellular component in mature adult bone) is the main mechanosensory cell that responds to mechanical loading or lack of mechanical stimuli (Bonewald, 2011, Dallas, Prideaux, 2013, Kalajzic, Matthews, 2013). Their stellate shaped cell bodies, dendritic processes and/or primary cilia are thought to contribute to the mechanosensory function. Gap junctions allow intracellular communication between osteocytes within the lacunocanalicular network and with cells such as osteoblasts, lining cells and osteoclasts on the bone surface. The dendritic processes of these cells can extend into the bone marrow space and the vascular space potentially mediating communication with non-bone cells (Bonewald and Johnson, 2008, Dallas, Prideaux, 2013, Kalajzic, Matthews, 2013, Malone et al., 2007, Verbruggen et al., 2014). The Wnt/β-catenin pathway has been identified using in vitro and in vivo approaches to be an important pathway in osteocytes to sense and transduce the signals of mechanical stimuli to bone cells.
Cell culture experiments using osteocyte-like cell lines and primary osteocytes have provided insight showing that β-catenin is one of the most important molecules in the osteocyte response to mechanotransduction. In vitro when exposed to fluid flow shear stress, osteocyte-like MLO-Y4 cells showed obvious nuclear translocation of β-catenin at very low levels of shear stress to do not elicit responses in other cell types such as osteoblasts (Kamel, Picconi, 2010). With the development of transgenic models and new techniques, Wnt/β-catenin signaling has been identified as a normal physiological response to load and activation of the Wnt/β-catenin pathway enhances the sensitivity of osteoblasts/osteocytes to mechanical loading (Robinson et al., 2006). One of the most useful models developed to study the role of β-catenin in osteocytes has been the 10kb Dmp1-Cre promoter crossed with floxed β-catenin (Kramer, Halleux, 2010). Dentin-matrix protein, Dmp1, is expressed as the bone cell is starting to embed or become surrounded by mineral and therefore a good marker for the early osteocyte was utilized to delete β-catenin in osteocytes. Mice lacking both alleles of β-catenin were normal when born but developed progressive loss of bone density and an increase of osteoclast activity throughout the skeleton (Kramer, Halleux, 2010). This was in contrast to deletion of β-catenin using either the Col1a1-Cre (Glass II, Bialek, 2005) or the osteocalcin-Cre (Holmen, Zylstra, 2005). Those mice showed a perinatal defect and died around a month of age whereas the Dmp1-Cre knock-outs were viable for a longer period of time, 3–4 months. Their skeleton displayed a ‘moth eaten’ appearance with holes bored through the cortical bone by osteoclasts. The heterozygotes lacking one allele of β-catenin appeared normal at 2 months of age with only a small reduction in trabecular bone.
The effect of loss-(exon 2–6) or gain-of-function (exon 3) of β-catenin in osteocytes have been shown to decrease or increase bone mass, supporting the hypothesis that β-catenin in osteocytes is essential for normal bone mass. The decrease in activity of β-catenin results in less expression of OPG and low bone mass due to increased osteoclast activity (Glass II, Bialek, 2005, Holmen, Zylstra, 2005). Bellido and colleagues recently used the Dmp1-8kb-Cre crossed with the gain-of-function Ctnnb+/lox (exon 3) mice (Tu et al., 2015). Similar to the activation of β-catenin in osteoblasts, these mice exhibit an increased bone mineral density and decreased bone resorption. Furthermore, the Notch signaling was activated and interacted with activation of β-catenin in osteocytes. However, they were much smaller than controls due to early closure of the growth plate but viable for longer than 12 months of age. In stark contrast, activation of β-catenin in osteoblasts using the osteocalcin-Cre results in anemia, expansion of myeloid cells and death at 6 weeks of age (Kode, Manavalan, 2014) which was not observed with deletion in osteocytes. This shows that osteocytes most likely regulate bone growth-probably through regulation of the osteoblast.
Not only have studies been used to show that β-catenin is necessary for normal osteocyte function postnatally but also to demonstrate the role of β-catenin in the response of the skeleton to load and unloading. Using the 10kb Dmp1-Cre promoter, when both alleles of β-catenin are conditionally deleted the result is very fragile bone, whereas when one allele of β-catenin is deleted the skeleton is relatively normal. However, when 5 month old male mice were subjected to anabolic loading, no response was observed in the Dmp1-Cre β-catenin heterozygote mice suggesting that β-catenin is essential for osteocyte response to loading (Javaheri et al., 2014). These findings suggest that one allele of β-catenin is necessary for normal bone, and both alleles are necessary for the bone anabolic response. Recently we have shown that β-catenin haplo insufficient male mice do not lose bone in response to hindlimb unloading, whereas female mice show a greater response to unloading compared to controls (abstract and paper submitted). The male mice lost bone with tail attachment and no suspension suggesting stress had a greater effect on the transgenic animals than their controls. Additional bone was not lost with unloading. The data suggest a protective effect of β-catenin against the effects of stress in males and partial protection against unloading in females.
7. Role of β-catenin in tooth formation
The morphogenesis of teeth is very complicated and must occur with precision for formation of normal dentition. Several distinct, different signaling pathways such as BMPs (Vainio et al., 1993), Sonic hedgehog (Shh) (Khan et al., 2007) and fibroblast growth factors (FGFs) (Bei, 2009) are involved in the epithelial-mesenchymal interactions during tooth initiation tooth specific cell proliferation, migration and differentiation. Different mice models have been used to study them (Munne et al., 2009, Murashima-Suginami et al., 2008). Among these pathways, Wnt/β-catenin signaling is dynamically active and involved in various stages of tooth morphogenesis (Liu and Millar, 2010). Stabilized epithelial β-catenin in the dental initiation promotes continuous ectopic dental development in embryos. Conversely, deletion of epithelial β-catenin inhibits or arrests tooth development at the early bud stage (Liu et al., 2008). From the bud stage to the cap stage, gain-of-function of β-catenin in the mutant embryos has a positive effect on the formation of enamel knots (Chen et al., 2009), whereas lost-of-function of mesenchymal β-catenin is negative for the development of enamel knots (Jarvinen et al., 2006). Inactivation of β-catenin in developing odontoblasts caused a deficiency in the molar roots and aberrantly thin incisors (Kim et al., 2013, Zhang et al., 2013b). Overall, β-catenin is essential for tooth development.
As both teeth and bone are mineralized tissue, it is not surprising that similar or related phenotypes would be present with deletion or activation of β-catenin in mice (Chen, Lan, 2009, Clevers, 2006). With the Col1a1-Cre, activation of β-catenin led to increased ossification and decreased osteoclast number in the long bone and vertebrae (Glass II, Bialek, 2005). With regards to the teeth in these mice, tooth eruption was delayed and aberrant maxillofacial formation was observed (Glass II, Bialek, 2005, Kim et al., 2012). The phenotype in long bone, vertebrae and teeth may be due to the same cellular and molecular mechanisms, as the number and activity of osteoclasts are decreased in β-catenin knock-outs due to the reduced RANKL production (Li et al., 2000, Lu et al., 2009). Similar results were observed using the 8kb Dmp1 Cre; β-catenin activation mouse model (Tu, Delgado-Calle, 2015). Recently, it has been shown that constitutively active β-catenin in the dental mesenchyme leads to excessive cementum formation. Activation of Wnt/β-catenin signaling may stimulate cementoblast differentiation and cementum formation (Kim et al., 2011). Gain- or loss-of-function of β-catenin through different Cre models cause a hyper- or hypo- genesis in both teeth and bone (Holmen, Zylstra, 2005, Liu, Chu, 2008). These findings support the concept that there are similar effects of β-catenin on both teeth and bone during postnatal life due to the embryonic homology of odontoblasts, cementoblasts, ameloblasts, and osteoblasts.
8. Role of β-catenin signaling in the periodontal ligament (PDL) and dental mechanotransduction
During postnatal life, the occlusal force produced by daily chewing maintains maxillofacial bone and protects it against bone resorption. The bone turnover rate in alveolar bone is considerably higher than that in long bones, 30–35 % vs. 2–5% per year (Clarke, 2008, Huja et al., 2006). Also during orthodontic treatment, mechanical stimuli initiate both bone formation and resorption around the tooth root. It is not clear if β-catenin is responsible for bone maintenance in response to loading, plays a role in mechanotransduction in alveolar bone around teeth, or plays a role in the functions of the PDL. Supporting evidence shows β-catenin expression in the PDL in mice (Lim et al., 2014, Premaraj et al., 2011) in addition to being present in osteocytes and cementocytes. A recent study using PDL stem cells showed that β-catenin could regulate osteogenic differentiation in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway, which is similar to the regulation by β-catenin of bone homeostasis (Liu et al., 2011). With the osteocalcin-Cre; Wntless fl/fl mice (Wntless, a conserved membrane protein gene dedicated to the secretion of Wnt proteins), a pathological widening of the periodontal ligament space is observed with this elimination of Wntless in teeth (Lim, Liu, 2014). An in vivo study using human PDL cells showed responses to the activation of β-catenin via LiCl, leading to intensive β-catenin nuclear translocation (Premaraj, Souza, 2011). With loading on the human PDL cell, the activation of β-catenin was increased and inhibition of FAK suppressed β-catenin, suggesting that β-catenin mediated mechanotransduction in PDL (Premaraj et al., 2013). It would be of interest to perform in vivo experiments on transgenic mice models with targeted deletion or constitutive activation of β-catenin in specific dental tissues to further investigate the function of β-catenin in tooth movement. Unlike the appendicular skeleton, less is known about β-catenin function in craniofacial growth, development and function. With the tools and recent advances in the bone field, the role of β-catenin in oral and craniofacial bone and teeth will be available for investigation.
9. Conclusion and perspectives
Clearly, the wnt/β-catenin signaling pathway is critical for normal bone and tooth formation and development. But its effects are not limited to growth and development, as β-catenin also clearly plays a role in mechanosensation and transduction. In the osteocyte, this pathway is essential for viability, protection against apoptotic factors, for communication, for normal bone formation, in addition to being essential for sensing mechanical loading.
There are still many questions to be answered. It appears that osteocytes can orchestrate the activity of both osteoblasts and osteoclasts to initiate new bone turnover balance with physiological loading. However, how do osteocytes sense the mechanical signal and translate the mechanical signals into chemical signaling? Which structures are critical for signal transduction, the osteocyte cell body, their dendritic processes, pericellular matrix or a combination? Are other signaling pathways involved in the signal transduction? Also, as osteocytes are endocrine cells, what are the effects of loading on this function of osteocytes?
Also, better tools are needed to study osteocytes in bone and teeth such as cementocytes-all thought to be mechanosensory cells. More osteocyte-specific Cre models are needed for future studies. Technology has allowed dramatic advances to take place in understanding the role of β-catenin in osteocyte function. It will be important to use new technologies such as the CRISPR/CAS gene editing approach to study osteocytes and the role of β-catenin in their function.
Figure 1.
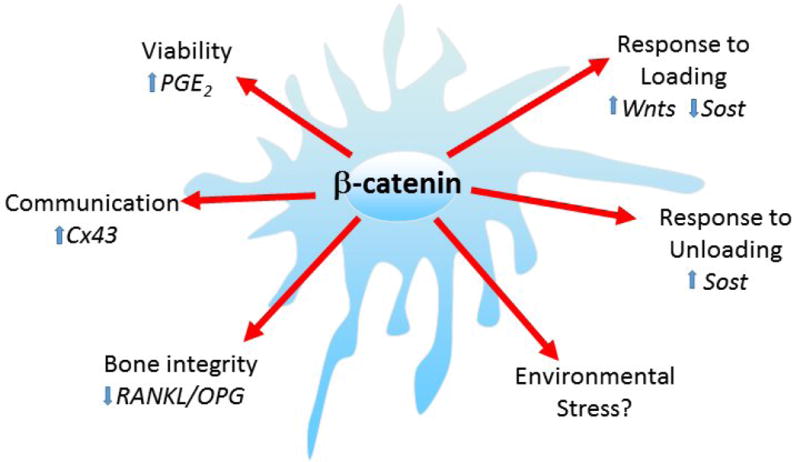
In the osteocyte, β-catenin is essential for 1). Maintenance of osteocyte viability and protection from apoptotic factors such as glucocorticoids through PGE2 (Kitase et al, 2010); 2). Communication between osteocytes potentially through the regulation of Connexin 43 (Xia et al, 2009); 3). Bone integrity as loss of β-catenin in osteocytes leads to elevated bone resorption through the increase of RankL/OPG ratios (Kramer et al, 2010); 4). Response to anabolic loading through the upregulation of Wnts (Javaheri et al, 2014); 5). Response to unloading leads to an increase in inhibitors of the wnt/β -catenin pathway such as sclerostin (Robling et al, 2008) and 6). Gender effects are observed in response to unloading and potentially environmental stress (Maurel and Duan et al; 2016). The target gene involved in sensing environment stress is still unclear. It will be important to determine if β-catenin also plays a role in these functions in other cells embedded in a mineralized matrix such as cementocytes.
Acknowledgments
This work was funded by NIH NIA grants PO1AG039355.
Abbreviations
- APC
adenomatous polyposis coli
- BMP
bone morphogenic protein
- Col1a1
alpha 1 type 1 collage
- Cx43
connexin 43
- DKK
Dickkopf
- Dmp1
Dentin matrix acidic phosphoprotein 1
- DVL
disheveled
- FGFs
fibroblast growth factors
- GSK3β
glycogen synthase kinase 3β
- int 1
integration 1
- LRP
low density lipoprotein receptor-related protein
- OPG
osteoprotegerin
- PCP
planar cell polarity
- PDL
periodontal ligament
- PGE2
prostaglandin E2
- RANKL
receptor activator of nuclear factor kappa B ligand
- sFRP1
secreted frizzled-related protein 1
- Shh
Sonic hedgehog
- SOST
Sclerostin
- TCF/LEF
T-cell factor/Lymphoid enhancer-binding factor
- VEGFR2
vascular endothelial growth factor receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers J, Keller J, Baranowsky A, Beil FT, Catala-Lehnen P, Schulze J, Amling M, Schinke T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J Cell Biol. 2013;200:537–549. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bei M. Molecular genetics of ameloblast cell lineage. J Exp Zool B Mol Dev Evol. 2009;312b:437–444. doi: 10.1002/jez.b.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T. Antagonistic interplay between mechanical forces and glucocorticoids in bone: a tale of kinases. J Cell Biochem. 2010;111:1–6. doi: 10.1002/jcb.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF. Summary–Osteocytes and mechanotransduction. J Musculoskelet Neuronal Interact. 2005;5:333–334. [PubMed] [Google Scholar]
- Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF, Kneissel M, Johnson M. Preface: the osteocyte. Bone. 2013;54:181. doi: 10.1016/j.bone.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Bonewald LF, Wacker MJ. FGF23 production by osteocytes. Pediatr Nephrol. 2013;28:563–568. doi: 10.1007/s00467-012-2309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Burgers TA, Williams BO. Regulation of Wnt/beta-catenin signaling within and from osteocytes. Bone. 2013;54:244–249. doi: 10.1016/j.bone.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canalis E. Wnt signalling in osteoporosis: mechanisms and novel therapeutic approaches. Nat Rev Endocrinol. 2013;9:575–583. doi: 10.1038/nrendo.2013.154. [DOI] [PubMed] [Google Scholar]
- Capulli M, Paone R, Rucci N. Osteoblast and osteocyte: games without frontiers. Arch Biochem Biophys. 2014;561:3–12. doi: 10.1016/j.abb.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol. 2009;334:174–185. doi: 10.1016/j.ydbio.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke BL. Anti-sclerostin antibodies: utility in treatment of osteoporosis. Maturitas. 2014;78:199–204. doi: 10.1016/j.maturitas.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Dallas SL, Prideaux M, Bonewald LF. The osteocyte: an endocrine cell … And more. Endocr Rev. 2013;34:658–690. doi: 10.1210/er.2012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EH, Reformat DD, Allori A, Canizares O, Janelle Wagner I, Saadeh PB, Warren SM. Flow perfusion maintains ex vivo bone viability: a novel model for bone biology research. J Tissue Eng Regen Med. 2012;6:769–776. doi: 10.1002/term.478. [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739–750. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- de Castro LF, Maycas M, Bravo B, Esbrit P, Gortazar A. VEGF Receptor 2 (VEGFR2) Activation Is Essential for Osteocyte Survival Induced by Mechanotransduction. J Cell Physiol. 2015;230:278–285. doi: 10.1002/jcp.24734. [DOI] [PubMed] [Google Scholar]
- Desiderio V, Tirino V, Papaccio G, Paino F. Bone defects: Molecular and cellular therapeutic targets. Int J Biochem Cell Biol. 2014;51:75–78. doi: 10.1016/j.biocel.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Glass DA, II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, et al. Canonical Wnt Signaling in Differentiated Osteoblasts Controls Osteoclast Differentiation. Dev Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Glass DA, II, Karsenty G. Molecular bases of the regulation of bone remodeling by the canonical Wnt signaling pathway. Curr Top Dev Biol. 2006;73:43–84. doi: 10.1016/S0070-2153(05)73002-7. [DOI] [PubMed] [Google Scholar]
- Gortazar AR, Martin-Millan M, Bravo B, Plotkin LI, Bellido T. Crosstalk between caveolin-1/extracellular signal-regulated kinase (ERK) and beta-catenin survival pathways in osteocyte mechanotransduction. J Biol Chem. 2013;288:8168–8175. doi: 10.1074/jbc.M112.437921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Peifer M. Terminal regions of beta-catenin come into view. Structure. 2008;16:336–338. doi: 10.1016/j.str.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO. Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem. 2005;280:21162–21168. doi: 10.1074/jbc.M501900200. [DOI] [PubMed] [Google Scholar]
- Hsu YH, Kiel DP. Clinical review: Genome-wide association studies of skeletal phenotypes: what we have learned and where we are headed. J Clin Endocrinol Metab. 2012;97:E1958–1977. doi: 10.1210/jc.2012-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- Hu M, Tian GW, Gibbons DE, Jiao J, Qin YX. Dynamic fluid flow induced mechanobiological modulation of in situ osteocyte calcium oscillations. Arch Biochem Biophys. 2015;579:55–61. doi: 10.1016/j.abb.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-Catenin Controls Hair Follicle Morphogenesis and Stem Cell Differentiation in the Skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1243–1249. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez-Ariza NM, Clarke BL. Bone biology, signaling pathways, and therapeutic targets for osteoporosis. Maturitas. 2015;82:245–255. doi: 10.1016/j.maturitas.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Jahn K, Lara-Castillo N, Brotto L, Mo CL, Johnson ML, Brotto M, Bonewald LF. Skeletal muscle secreted factors prevent glucocorticoid-induced osteocyte apoptosis through activation of beta-catenin. Eur Cell Mater. 2012;24:197–209. doi: 10.22203/ecm.v024a14. discussion 209–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvinen E, Salazar-Ciudad I, Birchmeier W, Taketo MM, Jernvall J, Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2006;103:18627–18632. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri B, Stern AR, Lara N, Dallas M, Zhao H, Liu Y, Bonewald LF, Johnson ML. Deletion of a Single beta-Catenin Allele in Osteocytes Abolishes the Bone Anabolic Response to Loading. J Bone Miner Res. 2014;29:705–715. doi: 10.1002/jbmr.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, Chen S, Zhang B, Liang H, Feng J, Zong Z. Effects of constitutive beta-catenin activation on vertebral bone growth and remodeling at different postnatal stages in mice. PLoS One. 2013;8:e74093. doi: 10.1371/journal.pone.0074093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalajzic I, Matthews BG, Torreggiani E, Harris MA, Divieti Pajevic P, Harris SE. In vitro and in vivo approaches to study osteocyte biology. Bone. 2013;54:296–306. doi: 10.1016/j.bone.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel MA, Picconi JL, Lara-Castillo N, Johnson ML. Activation of beta-catenin signaling in MLO-Y4 osteocytic cells versus 2T3 osteoblastic cells by fluid flow shear stress and PGE2: Implications for the study of mechanosensation in bone. Bone. 2010;47:872–881. doi: 10.1016/j.bone.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy OD, Herman BC, Laudier DM, Majeska RJ, Sun HB, Schaffler MB. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 2012;50:1115–1122. doi: 10.1016/j.bone.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Seppala M, Zoupa M, Cobourne MT. Hedgehog pathway gene expression during early development of the molar tooth root in the mouse. Gene Expr Patterns. 2007;7:239–243. doi: 10.1016/j.modgep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kim TH, Bae CH, Jang EH, Yoon CY, Bae Y, Ko SO, Taketo MM, Cho ES. Col1a1-cre mediated activation of beta-catenin leads to aberrant dento-alveolar complex formation. Anat Cell Biol. 2012;45:193–202. doi: 10.5115/acb.2012.45.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Bae CH, Lee JC, Ko SO, Yang X, Jiang R, Cho ES. beta-catenin is required in odontoblasts for tooth root formation. J Dent Res. 2013;92:215–221. doi: 10.1177/0022034512470137. [DOI] [PubMed] [Google Scholar]
- Kim TH, Lee JY, Baek JA, Lee JC, Yang X, Taketo MM, Jiang R, Cho ES. Constitutive stabilization of ss-catenin in the dental mesenchyme leads to excessive dentin and cementum formation. Biochem Biophys Res Commun. 2011;412:549–555. doi: 10.1016/j.bbrc.2011.07.116. [DOI] [PubMed] [Google Scholar]
- Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe Tate ML, Adamson JR, Tami AE, Bauer TW. The osteocyte. Int J Biochem Cell Biol. 2004;36:1–8. doi: 10.1016/s1357-2725(03)00241-3. [DOI] [PubMed] [Google Scholar]
- Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T. Mouse models for the evaluation of osteocyte functions. J Bone Metab. 2014;21:55–60. doi: 10.11005/jbm.2014.21.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol Cell Biol. 2010;30:3071–3085. doi: 10.1128/MCB.01428-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WH, Liu B, Cheng D, Williams BO, Mah SJ, Helms JA. Wnt signaling regulates homeostasis of the periodontal ligament. J Periodontal Res. 2014 doi: 10.1111/jre.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, et al. Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Millar SE. Wnt/beta-catenin signaling in oral tissue development and disease. J Dent Res. 2010;89:318–330. doi: 10.1177/0022034510363373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Shi S, Deng M, Tang L, Zhang G, Liu N, Ding B, Liu W, Liu Y, Shi H, et al. High levels of beta-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J Bone Miner Res. 2011;26:2082–2095. doi: 10.1002/jbmr.440. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu X, Rios HF, Jiang B, Xing L, Kadlcek R, Greenfield EM, Luo G, Feng JQ. A new osteopetrosis mutant mouse strain (ntl) with odontoma-like proliferations and lack of tooth roots. Eur J Oral Sci. 2009;117:625–635. doi: 10.1111/j.1600-0722.2009.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Maurel D, Duan P, Farr J, Cheng A, Johnson M, Bonewald L. Beta-catenin haplo insufficient male mice do not lose bone in response to hindlimb unloading (resubmitted) doi: 10.1371/journal.pone.0158381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung MR, Grauer A. Romosozumab in postmenopausal women with osteopenia. N Engl J Med. 2014;370:1664–1665. doi: 10.1056/NEJMc1402396. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- Munne PM, Tummers M, Jarvinen E, Thesleff I, Jernvall J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development. 2009;136:393–402. doi: 10.1242/dev.025064. [DOI] [PubMed] [Google Scholar]
- Murashima-Suginami A, Takahashi K, Sakata T, Tsukamoto H, Sugai M, Yanagita M, Shimizu A, Sakurai T, Slavkin HC, Bessho K. Enhanced BMP signaling results in supernumerary tooth formation in USAG-1 deficient mouse. Biochem Biophys Res Commun. 2008;369:1012–1016. doi: 10.1016/j.bbrc.2008.02.135. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest. 1999;104:1363–1374. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaraj S, Souza I, Premaraj T. Mechanical loading activates beta-catenin signaling in periodontal ligament cells. Angle Orthod. 2011;81:592–599. doi: 10.2319/090310-519.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaraj S, Souza I, Premaraj T. Focal adhesion kinase mediates beta-catenin signaling in periodontal ligament cells. Biochem Biophys Res Commun. 2013;439:487–492. doi: 10.1016/j.bbrc.2013.08.097. [DOI] [PubMed] [Google Scholar]
- Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, et al. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- Sapir-Koren R, Livshits G. Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption-formation cycles? Osteoporos Int. 2014;25:2685–2700. doi: 10.1007/s00198-014-2808-0. [DOI] [PubMed] [Google Scholar]
- Sheng MH, Zhou XD, Bonewald LF, Baylink DJ, Lau KH. Disruption of the insulin-like growth factor-1 gene in osteocytes impairs developmental bone growth in mice. Bone. 2013;52:133–144. doi: 10.1016/j.bone.2012.09.027. [DOI] [PubMed] [Google Scholar]
- Tomkinson A, Gevers EF, Wit JM, Reeve J, Noble BS. The role of estrogen in the control of rat osteocyte apoptosis. J Bone Miner Res. 1998;13:1243–1250. doi: 10.1359/jbmr.1998.13.8.1243. [DOI] [PubMed] [Google Scholar]
- Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, Taketo MM, Burr DB, Plotkin LI, Bellido T. Osteocytes mediate the anabolic actions of canonical Wnt/beta-catenin signaling in bone. Proc Natl Acad Sci U S A. 2015;112:E478–486. doi: 10.1073/pnas.1409857112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen SW, Vaughan TJ, McNamara LM. Fluid flow in the osteocyte mechanical environment: a fluid-structure interaction approach. Biomech Model Mechanobiol. 2014;13:85–97. doi: 10.1007/s10237-013-0487-y. [DOI] [PubMed] [Google Scholar]
- Visweswaran M, Pohl S, Arfuso F, Newsholme P, Dilley R, Pervaiz S, Dharmarajan A. Multi-lineage differentiation of mesenchymal stem cells – To Wnt, or not Wnt. Int J Biochem Cell Biol. 2015;68:139–147. doi: 10.1016/j.biocel.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One. 2011;6(10):e25900. doi: 10.1371/journal.pone.0025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX. Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol Cell Biol. 2010;30:206–219. doi: 10.1128/MCB.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Takemaru K, Liu J, Berndt JD, Zheng JJ, Moon RT, Xu W. Crystal structure of a full-length beta-catenin. Structure. 2008;16:478–487. doi: 10.1016/j.str.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Piemontese M, Thostenson JD, Weinstein RS, Manolagas SC, O’Brien CA. Osteocyte-derived RANKL is a critical mediator of the increased bone resorption caused by dietary calcium deficiency. Bone. 2014;66:146–154. doi: 10.1016/j.bone.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Barragan-Adjemian C, Ye L, Kotha S, Dallas M, Lu Y, Zhao S, Harris M, Harris SE, Feng JQ, et al. E11/gp38 selective expression in osteocytes: regulation by mechanical strain and role in dendrite elongation. Mol Cell Biol. 2006;26:4539–4552. doi: 10.1128/MCB.02120-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Oyajobi BO, Harris SE, Chen D, Tsao C, Deng HW, Zhao M. Wnt/beta-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone. 2013a;52:145–156. doi: 10.1016/j.bone.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Yang G, Wu X, Xie J, Yang X, Li T. Disruption of Wnt/beta-catenin signaling in odontoblasts and cementoblasts arrests tooth root development in postnatal mouse teeth. Int J Biol Sci. 2013b;9:228–236. doi: 10.7150/ijbs.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]


