Abstract
An on-column approach for protein entrapment was developed to immobilize alpha1-acid glycoprotein (AGP) for drug-protein binding studies based on high-performance affinity chromatography. Soluble AGP was physically entrapped by using microcolumns that contained hydrazide-activated porous silica and by employing mildly oxidized glycogen as a capping agent. Three on-column entrapment methods were evaluated and compared to a previous slurry-based entrapment method. The final selected method was used to prepare 1.0 cm × 2.1 mm I.D. affinity microcolumns that contained up to 21 (± 4) μg AGP and that could be used over the course of more than 150 sample applications. Frontal analysis and zonal elution studies were performed on these affinity microcolumns to examine the binding of various drugs with the entrapped AGP. Site-selective competition studies were also conducted for these drugs. The results showed good agreement with previous observations for these drug-protein systems and with binding constants that have been reported in the literature. The entrapment method developed in this study should be useful for future work in the area of personalized medicine and in the high-throughput screening of drug interactions with AGP or other proteins.
Keywords: Alpha1-acid glycoprotein, High-performance affinity chromatography, Drug-protein binding, Entrapment, Immobilization method, Affinity microcolumn
Graphical abstract
INTRODUCTION
The pharmacokinetics (i.e., absorption, distribution, metabolism, and excretion) and pharmacodynamics (i.e., biochemical and physiological effects) of many drugs are influenced by interactions of these drugs with serum proteins [1–5]. One protein that is involved in such interactions is alpha1-acid glycoprotein (AGP) [2,6–8]. AGP, which has a normal concentration in serum of 0.5–1.0 g/L [6–8], is a major binding agent for many drugs in the blood stream [7,8]. For instance, AGP is the principal transport protein for numerous basic and neutral drugs [7,9–17]. Examples of these drugs are lidocaine [9,10], verapamil [11], imipramine [12], propranolol [11,13], and carbamazepine [16]. AGP also binds some acidic drugs (e.g., warfarin) [16,18].
The interactions of drugs with AGP have previously been studied with methods such as ultrafiltration [19], equilibrium dialysis [20–22], liquid chromatography (e.g., the Hummel-Dreyer method) [23], circular dichroism [24], surface plasmon resonance spectroscopy [25], and capillary electrophoresis [26]. High-performance affinity chromatography (HPAC) is another technique that has been used to study the binding of drugs with AGP [27–30]. This latter method uses columns that contain immobilized AGP to examine binding by this protein to applied drugs or target solutes [27–30]. Advantages noted for HPAC in this type of drug-protein binding study have included its speed, precision, ease of automation, ability to be combined with various detectors, need for only a relatively small amount of a drug or protein, and capability of using the same immobilized protein preparation for many samples or binding experiments [2,5].
Past work in HPAC has typically involved the use of covalent immobilization to place AGP and other proteins (e.g., human serum albumin, or HSA) in columns for use in drug binding studies and in applications such as chiral separations [2,5,25,29,31,32]. For example, HPAC columns have been made through the adsorption of oxidized AGP to diethylaminoethyl silica, followed by crosslinking of the oxidized AGP [32]. AGP has also been covalently immobilized through thiol groups to carboxymethyldextran supports [25]. For these particular supports, poor correlation has been noted when comparing the binding behavior of drugs with these AGP columns and the behavior seen for soluble AGP [25,31]. More recently, good correlation with the binding properties of soluble AGP have been obtained in HPAC by coupling mildly oxidized AGP to hydrazide-activated silica [29]. However, this approach does requires some initial modification of the carbohydrates residues on AGP (i.e., oxidation) before immobilization can occur [29].
Entrapment has been explored in some studies as an alternative to covalent coupling methods for proteins. For instance, hydrogels and sol-gel encapsulation have been used to immobilize large biomolecules like enzymes or antibodies for applications such as protein-based photonic devices, biosensors, and chromatographic columns [2,33–35]. In these reports, the biomolecule is physically entrapped during the formation of the support. Entrapment can result in a high activity for the immobilized biomolecule by avoiding problems such as improper orientation, steric hindrance, and multi-site attachment that can occur during covalent immobilization [36–38]. In addition, no modification of the protein is required before its immobilization. However, many of the materials that are currently used for entrapment are not suitable for use under the pressure or flow conditions that are often present in HPLC systems [36,38]. Also, these entrapment methods can lead to slow mass transfer as an applied analyte attempts to interact with a biomolecule that may be held deep within the support [39].
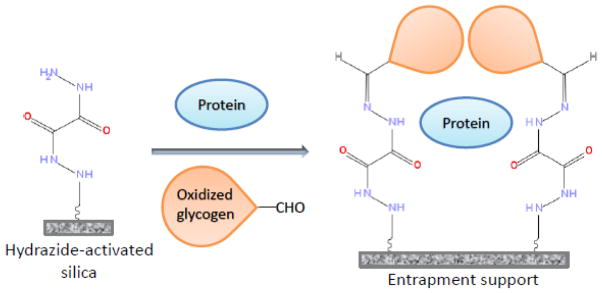
An alternative method has recently been reported that makes use of glycogen-capped and hydrazide-activated silica to entrap proteins (see Figure 1). This method has been utilized in a slurry-based format with HPLC-grade silica to entrap HSA and to create supports and columns that are suitable for use in HPAC [2,36,40]. It has also been demonstrated that this slurry-based method can be adapted for work with AGP [36,38]. This approach retains the ability of entrapment to provide an immobilized protein in a soluble and active form, while also overcoming the slow mass transfer effects and flow or pressure limitations that are often present when using hydrogels or sol-gel entrapment [2,36,38–40]. However, the extent of immobilization for proteins in this slurry-based method is limited by the overall solution volume, since only those proteins that are present within the pores or near the surface of the support will be entrapped during this process [38]. This feature also places some practical limits on this approach in terms of the total amount of protein that is required for entrapment.
Figure 1.
General scheme for protein entrapment based on the use of hydrazide-activated silica and oxidized glycogen as a capping agent.
In this study, an on-column method for entrapment will be explored as an alternative and more efficient means for preparing AGP microcolumns (i.e., columns with volumes in the mid-to-low microliter range) [41]. These AGP microcolumns will then be examined for use in HPAC and drug-binding studies. Various formats for on-column entrapment will be considered and compared with each other, as well as with the previous slurry-based method. The resulting AGP microcolumns will be evaluated in terms of their protein content and by using both frontal analysis and zonal competition experiments to examine the binding of these microcolumns with various drugs that are known to interact with AGP. These drugs will include propranolol (a basic drug; pKa, 9.6), carbamazepine (i.e., a “neutral” drug; pKa, 7) [3,17], and warfarin (an acidic drug; pKa, 5) [3]. The potential advantages and limitations of this entrapment approach will then be considered, as well as the possible extension of this method to other drug-protein systems.
MATERIALS AND METHODS
Reagents
The glycogen (from bovine liver, type IX; total glucose ≥ 85%; catalog no. G0885), AGP (from pooled human serum, lyophilized; ≥ 99% pure; catalog no. G9885), carbamazepine, R-propranolol, S-propranolol, and R-warfarin were purchased from Sigma-Aldrich (St. Louis, MO, USA). The Nucleosil Si-300 silica (particle size, 7 μm; pore size, 300 Å) was from Macherey-Nagel (Düren, Germany). All buffers and aqueous solutions were prepared using water from a Nanopure system (Barnstead, Dubuque, IA, USA) and were filtered using 0.20 μm nylon filters from Millipore (Billerica, MA, USA).
Apparatus
The chromatographic studies were carried out on a Jasco 2000 HPLC system (Easton, MD, USA) that contained a DG-2080–53 three solvent degasser, two PU-2080 isocratic pumps, an AS-2057 autosampler equipped with a 100 μL sample loop (operated in the partial loop injection mode), and a UV-2075 absorbance detector. The microcolumns were kept at 37°C by using a Jasco CO-2060 column oven. A Rheodyne Advantage PF six-port switching valve (Cotati, CA, USA) was used for alternating passage of the drug and buffer solutions through the microcolumns during the frontal analysis studies. The system components were controlled by a Jasco LC-Net II/ADC system and EZChrom Elite software v3.2.1 (Scientific Software, Pleasanton, CA, USA). The breakthrough times for the frontal analysis data and the central peak moments for the zonal elution data were determined by using PeakFit 4.12 (SeaSolve Software, San Jose, CA, USA). Linear and non-linear regression were conducted by using DataFit 8.1.69 (Oakdale Engineering, PA, USA).
The microcolumns were packed by using a Prep 24 pump from ChromTech (Apple Valley, MN, USA). The on-column immobilization method employed a Harvard Apparatus PHD ULTRA syringe pump (Holliston, MA, USA). Glycogen purification was accomplished by using a Vivaspin 6 ultrafiltration spin column (30 kDa molecular weight cutoff; Sartorious Stedim, Goettingen, Germany) and a 5702RH temperature-controlled centrifuge (Eppendorf, New York, NY, USA) that contained a fixed-angle centrifuge rotor from VWR (West Chester, PA, USA).
Microcolumn preparation and on-column entrapment
Diol-bonded silica was prepared from Nucleosil Si-300 and converted into a hydrazide-activated form according to a previously-established method [42]. The hydrazide-activated support was downward slurry packed at 4000 psi (28 MPa) into 1.0 cm × 2.1 mm I.D. stainless steel microcolumns using pH 7.4, 0.067 M potassium phosphate buffer as the packing solution. These microcolumns were stored in the same buffer at 4°C both prior to their use for immobilization and after the entrapment of AGP.
The initial conditions used for AGP entrapment were adapted from the slurry-based procedure that is described in Refs. [36,40] for AGP and HSA. Three approaches were developed and tested for the on-column entrapment of AGP; further details on each approach are provided in the Results and Discussion. In the final selected method for on-column entrapment, 400 μL of a 20 mg/mL AGP solution (prepared in pH 5.0, 0.10 M potassium phosphate) was first introduced into a microcolumn by a syringe pump at an infusion rate of 50 μL/min. The same pump was then used to draw this solution back through the microcolumn at 50 μL/min. This cycle of infusion/drawing of the AGP solution was repeated for a total of 4 h at room temperature. The AGP solution was then changed to a mixture of 1.76 mg/mL glycogen and 20 mg/mL AGP in pH 5.0, 0.10 M potassium phosphate. This mixture was circulated through the microcolumn by using the same cycle of infusion and solution drawing (i.e., 400 μL passed through the microcolumn at 50 μL/min) which was repeated over the course of 24 h at room temperature. A 400 μL portion of 0.3 mg/mL oxalic dihydrazide in pH 5.0, 0.10 M potassium phosphate was then applied for 1 h to the microcolumn in the same manner. Control microcolumns containing hydrazide-activated silica were prepared in the same manner by using only pH 5.0, 0.10 M potassium phosphate (i.e., in place of the AGP solution) or the same solution with oxidized glycogen present during microcolumn preparation.
Chromatographic studies
The chromatographic experiments were performed at 37°C using pH 7.4, 0.067 M potassium phosphate buffer as the mobile phase. All drug and solute solutions were prepared in this mobile phase and filtered through 0.20 μm nylon filters, followed by a 15 min degassing step, prior to use. The solutions and samples of carbamazepine and S-propranolol were used within two weeks of preparation, and the solutions of warfarin were used within one week [28,43]. When not in use, these solutions were stored in the dark at 4°C. The following detection wavelengths were employed in the chromatographic experiments: 280 nm, carbamazepine; 225 nm, S- or R-propranolol; 308 nm, R-warfarin; and 205 nm, sodium nitrate.
Frontal analysis was carried out at 0.10 mL/min by first equilibrating an AGP microcolumn with pH 7.4, 0.067 M phosphate buffer. The mobile phase was then switched to a solution containing a known concentration of carbamazepine in the same buffer. These solutions included up to eleven concentrations of carbamazepine that ranged from 0.1 to 25 μM. Each drug solution was continuously applied to the AGP microcolumn until a breakthrough curve with a level plateau was produced. The retained drug was then eluted and the microcolumn was regenerated by switching back to the pH 7.4, 0.067 M phosphate buffer. This process was repeated three times at each drug concentration. The central location of each breakthrough curve was determined by using a Savitzky-Golay first derivative algorithm for smoothing, followed by fitting the first derivative to an exponentially-modified Gaussian (EMG) curve. Identical studies were performed on a control microcolumn to correct for any non-specific binding of carbamazepine to the support, in a manner similar to that described for carbamazepine and other drugs on columns containing covalently immobilized AGP [27–30].
Zonal elution studies were also performed on the AGP microcolumns. These experiments were carried out using flow rates of 0.50 mL/min or less and 5 μL injections of samples containing 5 μM for each drug or solute. The measured retention factors showed no significant variations as the conditions were altered over the given range of flow rates or as the sample concentration was decreased, as has been noted in prior work with other HPAC columns containing AGP or HSA [29,40]. The central moment of each peak obtained from the zonal elution studies was determined by using a fit to an EMG curve. The retention factor (k) of an injected solute in the zonal elution experiments was calculated by using the relationship k = (tR – tM)/tM, where tR was the retention time (i.e., the central moment) for the solute and tM was the column void time [2,5].
In the zonal elution-based competition studies, the mobile phase was pH 7.4, 0.067 M phosphate buffer that contained various concentrations of a competing agent. These studies involved 5 μL injections of 5 μM carbamazepine in the presence of mobile phases containing 0–20 μM S-propranolol. Zonal elution was also used to examine the overall binding of several drugs with AGP. In these experiments, the mobile phase was pH 7.4, 0.067 M phosphate buffer into which 5 μL injections were made of samples containing 5 μM carbamazepine, R-propranolol, S-propranolol, or R-warfarin. The void time of each microcolumn was determined by making 5 μL injections of 20 μM sodium nitrate (i.e., a solute which has been shown to be an effective marker for the non-retained volume on comparable AGP columns) [38]. The extra-column void time was determined in a similar manner by making injections of sodium nitrate when a zero-volume union was used in place of the microcolumn.
RESULTS AND DISCUSSION
Strategies Considered for On-Column Entrapment
Three strategies were initially developed and tested for use in the on-column entrapment of AGP. Each of these methods employed the same three basic steps. First, a pH 5.0 solution containing AGP was passed through a microcolumn containing hydrazide-activated silica to allow some AGP to enter the pores of the support. This step was followed by application of a pH 5.0 solution containing a mixture of oxidized glycogen and AGP to the microcolumn to entrap the AGP in the support through the coupling of the oxidized glycogen with the support’s hydrazide groups. A pH 5.0 solution of oxalic dihydrazide was then passed through the microcolumn to react with any aldehyde groups on the support that remained from the synthesis of the hydrazide-activated silica or that were still present on the bound and oxidized glycogen.
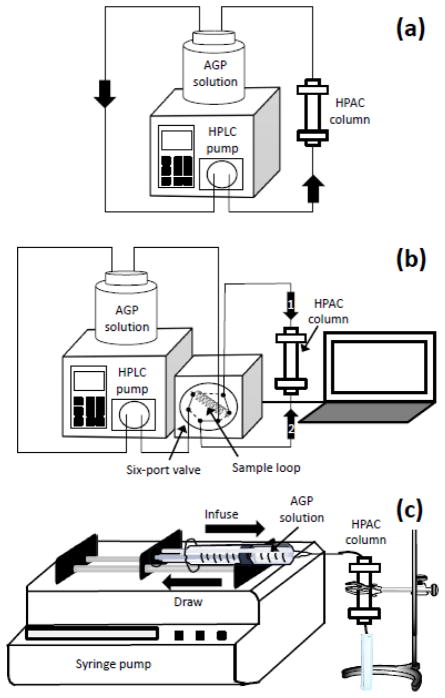
In the first on-column entrapment method (i.e., Method 1), 5 mL of a pH 5.0 solution containing 4.35 mg/mL AGP was applied to the microcolumn through circulation of the AGP solution through the microcolumn in a single direction for 4 h and at 0.1 mL/min by using an HPLC pump. This approach is illustrated in Figure 2(a). A 5 mL portion of a pH 5.0 solution containing 1.76 mg/mL glycogen and 4.35 mg/mL AGP was then passed for 24 h through the microcolumn at 0.1 mL/min, followed by application of 10 mL of a pH 5.0 solution containing 0.3 mg/mL oxalic dihydrazide for 1 h at 0.1 mL/min.
Figure 2.
On-column entrapment of AGP by using (a) Method 1, (b) Method 2, or (c) Method 3. Further details on each method can be found in the text.
The next approach that was considered (i.e., Method 2) utilized an automated switching valve and a sample loop to reduce the solution volumes that were needed for entrapment. This method is illustrated in Figure 2(b). In this procedure, 1 mL of a pH 5.0 solution containing 12 mg/mL AGP was introduced into the microcolumn by using a sample loop that was connected to an electronically-controlled six-port valve. This valve was used to change the direction of the solution going through the microcolumn, which was altered every 5–10 s for 4 h. Next, 1 mL of a pH 5.0 solution containing 1.76 mg/mL glycogen and 12 mg/mL AGP was introduced and circulated through the microcolumn in the same fashion for 24 h. This was followed by the application of 1 mL of a pH 5.0 solution containing of 0.3 mg/mL oxalic dihydrazide, which was carried out in the same manner for 1 h.
The third on-column entrapment method (i.e., Method 3) used a syringe pump to deliver the reagent solutions. This method, which is shown in Figure 2(c), made it possible to further reduce the solution volumes that were needed for entrapment. The syringe pump was used with a programmable sequence that allowed the repeated infusion and drawing of a small volume of solution through the microcolumn at a set flow rate. In this case, 400 μL of a pH 5.0 solution containing 20 mg/mL AGP was first introduced into the microcolumn at 50 μL/min. The same pump was then used to draw this solution back through the microcolumn at the same flow rate. This process of infusion/drawing of the solution was repeated over the course of 4 h. The solution that was being passed through the microcolumn was then changed to a pH 5.0 mixture of 1.76 mg/mL glycogen and 20 mg/mL AGP, which was circulated through the microcolumn using the same cycle of infusion/drawing over 24 h. A 400 μL portion of a pH 5.0 solution containing 0.3 mg/mL oxalic dihydrazide was then passed through the microcolumn in the same manner for 1 h.
Comparison of On-column Entrapment Methods
Each of the on-column entrapment methods for AGP was evaluated and compared with the other techniques, as well as with the slurry-based entrapment method [38]. The results are summarized in Table 1. The relative amount of AGP that was entrapped in each type of microcolumn was estimated and compared by measuring the retention factors for S-propranolol, a drug that is known to bind with AGP with an affinity in the range of 0.63–4.2 × 106 M−1 at pH 7.4 and 37ºC [11,13,29]. The retention factors for S-propranolol on control microcolumns that did not contain AGP were also determined to correct for non-specific binding of this drug to the support.
Table 1.
Comparison of AGP supports and microcolumns prepared by several entrapment methods
| Entrapment methoda | ||||
|---|---|---|---|---|
| On-column method 1 | On-column method 2 | On-column method 3 | Slurry-based method [38] | |
| Concentration of AGP used for entrapment (mg/mL) | 4.35 | 12.0 | 20.0 | 10.0 |
| Amount of AGP used for entrapment (mg) | 21.8 | 12.0 | 8.0 | 10.0 |
| Volume of AGP solution used for entrapment (mL) | 5.0 | 1.0 | 0.4 | 1.0 |
| Amount of silica used for entrapment (mg) | 15.6 | 15.6 | 15.6 | 90 |
| Effective reaction volumeb | 12.5 μL | 12.5 μL | 12.5 μL | 2.5 mL |
| Specific retention factor of S-propranololc | 9.9 (± 0.8) | 7.9 (± 0.2) | 33 (± 1) | 3.2 (± 0.4)d |
| Estimated amount of active AGP (× 10−10 mol)c,e | 1.6 (± 0.3) | 1.2 (± 0.2) | 5.2 (± 1.0) | 0.5 (± 0.1) |
All of these supports were prepared using HPLC-grade silica with a particle size of 7 μm and a pore size of 300 Å. The size of each final microcolumn was 1.0 cm × 2.1 mm i.d.
The column void volume was used as the reaction volume for Methods 1–3, because the reaction took place within the column. The reaction volume shown for the slurry-based method was the total volume of the silica slurry, AGP solution, and oxidized glycogen solution.
The values shown in parentheses represent a range of ± 1 S.D, as based on triplicate measurements made at pH 7.4 and 37°C and a flow rate of 0.10 mL/min for Methods 1–3 or at 0.50 mL/min for the slurry-based method. A correction for the non-specific binding by S-propranolol to the support was made by using retention data collected from a control column; this correction was 62% of the total retention seen on the entrapped AGP columns prepared using Methods 1 and 2, and 26% on the column made using Method 3.
This is the result that was obtained when using a 5:1 ratio of oxalic dihydrazide versus aldehyde groups during the synthesis of the hydrazide-activated silica, which were the same conditions used in this current study.
The value given for Method 3 is the moles of active AGP that were measured for Method 3 by using frontal analysis and carbamazepine as an applied solute, as described in the main body of the text. The values for the other methods are calculated based on the moles of active AGP in Method 3 and the ratio of the specific retention factor for each other method versus Method 3. Frontal analysis based on S-propranolol for the column made by Method 3 gave an mL value of 3.0 (± 0.7) × 10−10 mol. Use of this second mL value would lower the estimated amount of active AGP in each of the columns by 42%, but the results would follow the same relative order for their AGP content as shown above.
The difference in the total retention measured for S-propranolol on an AGP microcolumn and the retention observed on the corresponding control microcolumn gave a specific retention factor (k) that was directly proportional to the amount of active protein in the AGP microcolumn. This is illustrated by the general expression in Eq. (1) [41].
| (1) |
In this equation, Ka represents the association equilibrium constant for an injected drug with an immobilized binding agent such as AGP, mL is the total moles of binding sites for the drug on the immobilized agent, and VM is the void column volume. Eq. (1) describes a situation in which there is only one type of interaction site for the drug with the immobilized protein or binding agent. In the case where multi-site binding is present, Eq. (2) gives a similar relationship that relates k to mL and the apparent association equilibrium constant (or global affinity constant, nKa’) for the interaction between the drug and immobilized binding agent [41].
| (2) |
In Eq. (2), the terms Ka1 through Kan are the association equilibrium constants for the drug or injected target at each of its individual binding sites in the column, while n1 through nn represent the relative number of each site per binding agent or protein.
As is shown in Table 1, all of the on-column entrapment methods gave higher specific retention factors for S-propranolol than the slurry-based entrapment method [38]. This increase in retention ranged from 2.5-fold to over 10-fold and depended on both the type of method that was used for on-column entrapment and the concentration/solution volume of AGP that was used for this process. For example, Method 2 used the same volume of the AGP solution as the slurry-based method and only a slightly (1.2-fold) higher concentration and mass of AGP; however, Method 2 resulted in a support that had a 2.5-fold higher specific retention for S-propranolol. It is believed that this improvement was due to an increase in the ratio of the support’s pore volume versus the total reaction volume for the on-column method. This increase would have resulted in the pore volume of the support (or the stagnant mobile phase volume in the column) making up a larger portion of the total reaction volume, leading to more effective utilization of the soluble AGP for entrapment.
Even larger improvements in the retention for S-propranolol were seen for the other on-column entrapment methods. When compared to the slurry-based method, Method 1 used a 56% lower concentration of AGP and a 5-fold higher AGP solution volume, or an overall 2.18-fold higher mass of AGP; however, Method 3 also produced about a 3-fold increase in the specific retention for S-propranolol. The best overall result was obtained in Method 3, which used a 2-fold higher concentration of AGP and a 60% lower AGP solution volume (or a 20% lower mass of AGP) than the slurry-based method, while also giving an increase of 10.3-fold in the specific retention for S-propranolol. In each of the on-column methods, the observed increase in specific retention was a reflection of the increase in the amount of active AGP that was present in the microcolumns, as is indicated by Eqs. (1–2). In this particular case, the amount of entrapped AGP was estimated to be in the range of 1.2–5.2 × 10−10 mol for Methods 1–3 when using 1.0 cm × 2.1 mm i.d. microcolumns.
Table 1 also compares some other features of these entrapment methods. For instance, the most efficient use of the soluble AGP occurred in Method 3, which utilized the smallest amount of AGP for entrapment but resulted in the largest amount of entrapped AGP. This method also required the smallest volume of the AGP solution for use in entrapment. Method 2 gave the second highest level of immobilization efficiency, followed by Method 1 and the slurry-based method (Note: the column prepared by Method 1 had slightly more entrapped AGP and gave greater drug retention than that obtained by Method 2, which was probably due to the much greater amount of AGP that was used for entrapment in Method 1 versus Method 2). The differences in the solution volumes that were needed were a result of the way in which the reagent solutions were applied to the microcolumns. The use of an HPLC pump to apply and cycle these solutions through the microcolumn required the largest solution volume for the entrapment process, while the use of a slurry, switching valve or syringe pump needed much smaller solution volumes. Method 3 was chosen for use in all further studies in this report due to the relatively high retention and protein content it produced, as well as the fairly small volume of the AGP and reagent solutions that it needed for entrapment. Microcolumns that were prepared by this approach could be made with a variation of 15% or less in the column-to-column protein content [44]. This method also resulted in good stability for the entrapped AGP and reproducible retention factors, with a variation of less than < 3% being noted for S-propranolol over the course of more than 150 sample applications and approximately 6 months of operation.
Evaluation of AGP Microcolumns by Frontal Analysis
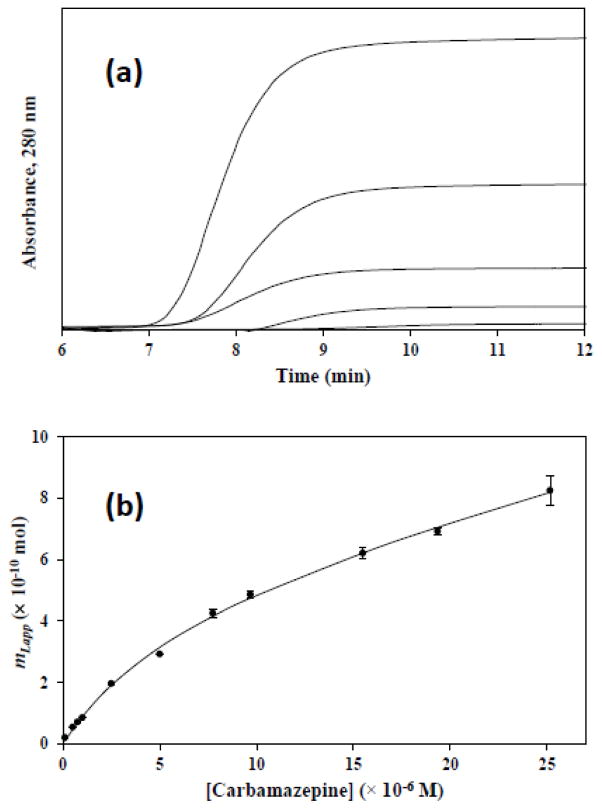
Microcolumns that were prepared by Method 3 were examined in more detail by frontal analysis to evaluate their binding to carbamazepine, another model drug with known binding to AGP [30]. Figure 3(a) provides some typical breakthrough curves that were generated for this drug at 0.10 mL/min. Breakthrough times were obtained within 8–11 min under these conditions, and stable plateaus were produced within 9–12 min at these particular drug concentrations. At 0.50 mL/min, equivalent curves could be generated with plateaus that appeared within 1.8–2.4 min.
Figure 3.
Frontal analysis results obtained for carbamazepine at 0.1 mL/min on a 1 cm × 2.1 mm I.D. microcolumn containing entrapped AGP. The plots in (a) show typical breakthrough curves that were obtained for solutions of 0.1, 0.5, 1.0, 2.5 or 5.0 μM carbamazepine (bottom-to-top). The graph in (b) shows the fit of the frontal analysis data for carbamazepine to a two-site binding model, as obtained by using Eq. (4) and using for one of the two sites the association equilibrium constant that had been measured for carbamazepine on a control microcolumn. Each point in (b) represents the average of triplicate runs; the error bars represent a range of ± 1 SD, with a typical relative precision in the range of ± 0.3−6.6%. The correlation coefficient for the fit in (b) was 0.999 (n = 11).
The moles of the applied drug (mLapp) that were required to reach the mean position for each breakthrough curve at a given concentration of the drug, [D], were used to generate binding isotherms for carbamazepine on the microcolumns. One way the data for carbamazepine were analyzed was by preparing a double-reciprocal plot of 1/mLapp versus 1/[D], as is illustrated in Figure 4. A linear response is expected in this type of plot for a system in which the drug binds reversibly to the column at only one type of site, as is demonstrated in Eq. (3).
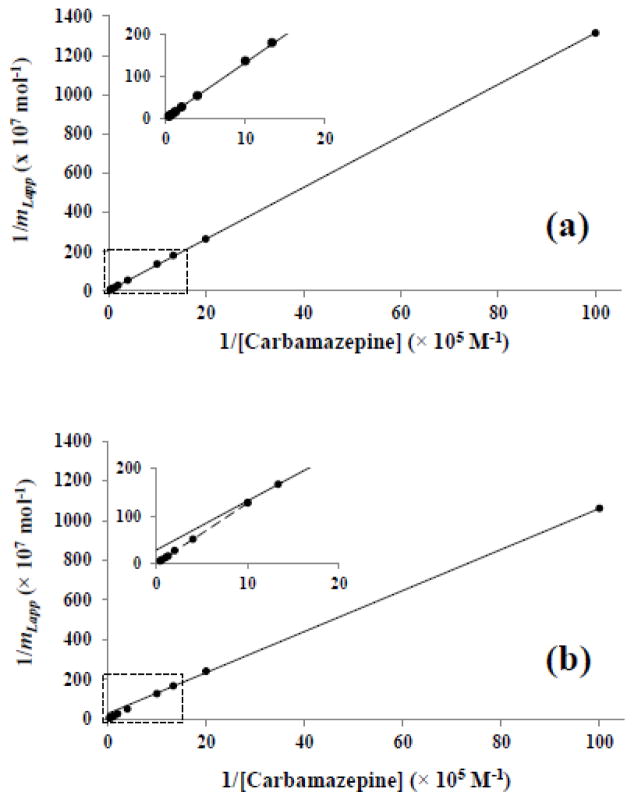
Figure 4.
Double-reciprocal plots obtained for data obtained at 0.10 mL/min for (a) carbamazepine on a 1 cm × 2.1 mm I.D. control microcolumn or (b) a 1 cm × 2.1 mm I.D. microcolumn containing entrapped AGP column. The inset shows an expanded view of the response that was obtained at low values of 1/[Carbamazepine] versus the best-fit line that was noted at higher values for this same parameter. The correlation coefficients for these fits were (a) 0.9999 (n = 11) and (b) 0.9994 (n = 10) for all of the data points or 0.9999 (n = 4) for the four data points at the highest values of 1/[Carbamazepine].
| (3) |
The slope and intercept of this line can then be used to obtain both the association equilibrium constant (Ka) and moles of binding sites (mL) for this interaction [5,45].
Figure 4(a) shows a double-reciprocal plot that was prepared according to Eq. (3) for carbamazepine on the control microcolumn. This plot gave linear behavior with a correlation coefficient of 0.999. Such behavior indicated that carbamazepine had some non-specific interactions with the support, which could be described by a single-site binding model. This binding was found to have a Ka value of 9.8 (± 5.1) × 102 M−1 and a value for mL of 8.0 (± 4.0) × 10−7 mol. When a similar double-reciprocal plot was prepared for carbamazepine on the AGP microcolumn, deviations from linearity were seen at high drug concentrations, or low values of 1/[D], as is shown in Figure 4(b). This behavior suggested that carbamazepine had at least two types of binding sites on the AGP microcolumn (e.g., one involving non-specific binding with the support, as noted on the control microcolumn, and the other involving interactions with AGP) [30,46]. The same type of behavior has been seen in previous frontal analysis studies that have been conducted with carbamazepine on columns containing covalently immobilized AGP and a similar support material [30].
A two-site model and non-linear regression were next employed to examine the binding of carbamazepine to the AGP microcolumn [30]. This was accomplished by fitting the data to Eq. (4) [30,46].
| (4) |
In this expression, the terms Ka1 and mL1 are the association equilibrium constant and moles of the first type of binding site, while Ka2 and mL2 are the corresponding terms for the second type of binding site [46]. Figure 3(b) shows the resulting plot of mLapp versus [D] for carbamazepine on the AGP microcolumn and the fit of these results to Eq. (4). This fit was obtained by using the previously-measured association equilibrium constant for carbamazepine on the control microcolumn as Ka2, and with the values mL2, Ka1 and mL1 being determined from the best-fit line in Figure 3(b). The resulting plot had a correlation coefficient of 0.999 (n = 11) and gave a random distribution of the residuals for the individual data points.
The value of Ka1 that was determined from Figure 3(b) for the binding of carbamazepine with the entrapped AGP was 1.6 (± 0.4) × 105 M−1 at pH 7.4 and 37º C. This result was statistically equivalent, at the 95% confidence level, to an association equilibrium constant of 1.0 (± 0.1) × 105 M−1 for carbamazepine hat has been reported at the same pH and temperature for a highly active form of covalently immobilized AGP [30]. The estimated value for Ka1 was also within the general range of 104–105 M−1 that has been observed in several prior binding studies that have used carbamazepine and soluble AGP [16,30,47].
The amount of active and entrapped AGP that was present in the microcolumn was also determined from Eq. (4) and the fit of this equation to Figure 3(b). This fit gave a value for mL1 of 5.2 (± 1.0) × 10−10 mol, which represented the moles of AGP that could bind to carbamazepine in the microcolumn. This amount corresponded to 21 (± 4) μg of AGP, as calculated by using an average molar mass of 41 kDa for this protein [48].
Zonal Elution Studies Examining the Overall Binding of Drugs with AGP
The next group of studies investigated the use of an entrapped AGP microcolumn to screen the overall binding of several drugs to AGP and to estimate the binding constants for these compounds. This work was carried out by using R-propranolol, S-propranolol and R-warfarin as model drugs that are known to bind to AGP [11,13]. Results were obtained for each drug in a relatively short time and using only retention factor measurements. For instance, R-warfarin, a compound with an approximate association equilibrium constant of 2.1 (± 0.3) × 106 M−1 for AGP at 37ºC and pH 7.0 [23], eluted within 1.8–2.0 min from the AGP microcolumn at 0.5 mL/min and within 7–8 min at 0.1 mL/min. A similar range of retention times was seen for the other drugs that are listed in Table 2. As a result, even triplicate experiments over a wide range of flow rates could be carried out in a short amount of time with this approach.
Table 2.
Binding constants measured for various drugs by zonal elution on columns containing entrapped AGP
| Drug | Specific retention factora | Estimated Ka or nKa’ (M−1) | Literature values (M−1)b |
|---|---|---|---|
|
| |||
| S-Propranolol | 38.9 (± 5.5) | 1.6 (± 0.5) × 106 | 1.4 (± 0.7) × 106 [27] |
| 4.2 (± 0.3) × 106 [29] | |||
| 1.04 (± 0.09) × 106 [49] | |||
|
| |||
| R-Propranolol | 30.0 (± 4.0) | 1.2 (± 0.4) × 106 | 1.1 (± 0.6) × 106 [27] |
| 2.7 (± 0.2) × 106 [29] | |||
| 0.8 (± 0.2) × 106 [49] | |||
|
| |||
| R-Warfarin | 25.6 (± 3.5) | 1.0 (± 0.3) × 106 | 2.1 (± 0.3) × 106 [23] |
Each value in parentheses represents a range of ± 1 S.D., as based on triplicate measurements (n = 3). The specific retention factor was obtained by taking the difference between the overall retention factor on an entrapped protein column and the retention factor due to non-specific interactions for the same solute on the control column, with the latter values accounting for 6.5–7.4% of the total retention for warfarin and 65–71% for propranolol.
The specific retention factors that were determined for each of the model drugs on the AGP microcolumn are listed in Table 2. The overall retention factors that were measured on the AGP microcolumn and control microcolumn gave relative variations of only ± 2–7% over flow rates ranging from 0.05 to 0.5 mL/min. The resulting specific retention factors that were calculated from this data also had good precisions, with typical variations of ± 13–14%. Based on these specific retention factors, each of the injected drugs could be easily ranked in terms of their overall binding strength to AGP. For instance, S-propranolol had the highest specific retention factor and strongest binding to AGP, followed by R-propranolol and R-warfarin. The order of elution for the propranolol enantiomers agreed with what has been noted under the same mobile phase conditions when using columns that contained highly active and covalently immobilized AGP [27,29].
It was possible with this same data to estimate the association equilibrium constants or global affinities for the interactions of these drugs with AGP. This was accomplished by using Eqs. (1–2) along with the specific retention factors that were measured for the drugs, the void volume of the microcolumn, and the amount of AGP that was present (e.g., 5.2 (± 1.0) × 10−10 mol, as estimated from the previous frontal analysis studies with carbamazepine and by assuming the same amount of AGP was accessible to each drug) [29]. The results that were obtained by this approach are summarized in Table 2. The binding constants of 1.6 (± 0.5) × 106 M−1 and 1.2 (± 0.4) × 106 M−1 that were determined for S-propranolol and R-propranolol, respectively, gave good agreement with values of 1.04–4.2 × 106 M−1 and 0.8–2.7 × 106 M−1 that have been reported for the same drugs with AGP at pH 7.4 and 37°C [23,27,29,49]. The value of 1.0 (± 0.3) × 106 M−1 that was obtained here for R-warfarin in pH 7.4, 0.067 M potassium phosphate buffer at 37°C was also consistent with a binding constant of 2.1 (± 0.3) × 106 that has been reported for the same system in pH 7.0, 0.010 M citrate phosphate buffer at 37°C [23].
Zonal Elution Competition Studies using AGP Microcolumns
Another application that was examined for the AGP microcolumns was their use in competition studies. Such studies were carried out by using carbamazepine as a competing agent while a small amount of S-propranolol was injected onto an AGP microcolumn. Such experiments can be used to determine if the injected probe compound (e.g., S-propranolol) shares a common binding site with the competing agent that is in the mobile phase (e.g., carbamazepine) [2,30].
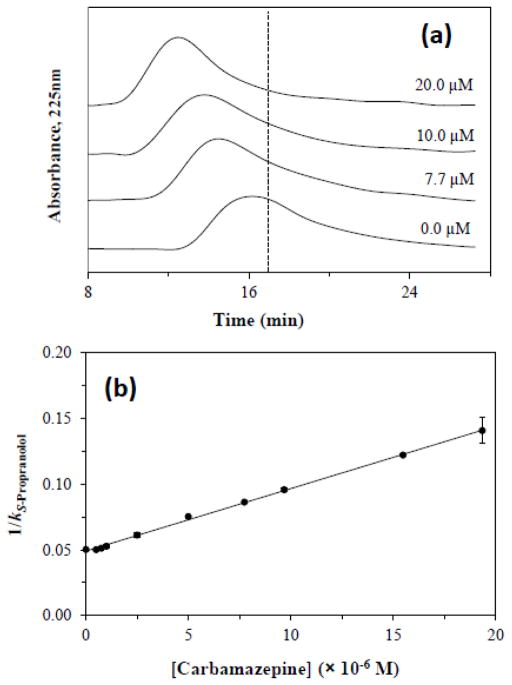
It was found in these studies that an increase in the concentration of carbamazepine in the mobile phase produced a decrease in the retention of S-propranolol, as is demonstrated in Figure 5(a). The observed change in the retention factor (k) for S-propranolol as the concentration of carbamazepine was varied was fit to Eq. (5) [2].
Figure 5.
Competition studies based on zonal elution on a 1 cm × 2.1 mm I.D. microcolumn containing entrapped AGP for the competition of 5 μL injected samples of S-propranolol in the presence of mobile phases containing a known concentration of carbamazepine. The plots in (a) show some typical chromatograms that were obtained in these experiments for mobile phases that contained 0.0, 7.7, 10.0 or 20.0 μM carbamazepine. The plot in (b) is the linear fit that was obtained when such data were analyzed according to Eq. (5). The dashed line in (a) is the approximate location of the central moment for the peak of S-propranolol in the absence of any carbamazepine in the mobile phase. Each point in (b) represents the average of triplicate measurements. The error bars in (b) represent a range of ± 1 S.D. and had relative precisions that ranged from ± 0.2%−7.1%. The correlation coefficient in (b) was 0.999 (n = 10).
| (5) |
In this equation, KaA and KaI represent the association equilibrium constants for the injected probe (A) and competing agent (I). The term [I] is the molar concentration of the competing agent in the mobile phase, and mL is the moles of common binding sites for the probe and competing agent in the column. Eq. (5) predicts that a plot of 1/k versus [I] should give a linear relationship with a positive slope for a system in which the injected probe has only one type of binding site in the column and there is direct competition of the probe with the competing agent at this site. In this situation, it is possible to use the slope and intercept of the best-fit line to obtain the association equilibrium constant for the competing agent, KaI, at the site of competition between A and I [2].
Figure 5(b) shows the results that were acquired when a plot of 1/kS-Propranolol versus [Carbamazepine] was fit to Eq. (5). Linear behavior with a positive slope was observed in this graph, with a correlation coefficient of 0.999 (n = 10) and only random variations being seen in the residuals for the individual data points about the best-fit response. This result confirmed the presence of direct competition between carbamazepine and S-propranolol on the entrapped AGP microcolumn, in agreement with previous observations that have been made on columns containing active and covalently immobilized AGP [30].
The best-fit of Eq. (5) to Figure 5(b) was used to determine the association equilibrium constant for carbamazepine at its site of competition with S-propranolol. This gave a KaI value of 1.01 (± 0.02) × 105 M−1 for carbamazepine, which was equivalent (at the 95% confidence level) with an association equilibrium constant of 1.0 (± 0.1) × 105 M−1 that has been measured for carbamazepine with AGP at the same pH and temperature in a previous study [30]. This result was also comparable, and consistent at the 98% confidence level, with the binding constant of 1.6 (± 0.4) × 105 M−1 that was measured earlier in this current study when using frontal analysis to examine the binding of carbamazepine with a microcolumn containing entrapped AGP.
CONCLUSIONS
This work examined various approaches for the on-column entrapment of AGP for use in affinity microcolumns and drug-protein binding studies. The conditions used in the final selected method required only 400 μL of an AGP solution for entrapment and gave over a 10-fold improvement in final protein content when compared to a previous slurry-based entrapment technique [36,38]. The on-column entrapment method was used to prepare 1.0 cm × 2.1 mm I.D. affinity microcolumns that contained up to 21 (± 4) μg AGP and that could be used over the course of more than 150 sample applications.
Frontal analysis and zonal elution studies were performed on these affinity microcolumns to examine their use in such applications as binding studies and the high-throughput screening of drug-protein interactions. These results were typically obtained in a matter of minutes and, in the case of the zonal elution experiments, could be conducted by using only simple retention factor measurements. In addition, the binding constants that were obtained with the AGP microcolumns for drugs such as carbamazepine, R- or S-propranolol and R-warfarin showed good agreement with previous observations for these drug-protein systems and with binding constants that have been reported in the literature [16,23,27,29,30,47,49]. The AGP microcolumns were also used in zonal elution-based competition studies between carbamazepine and S-propranolol, again giving good agreement with prior behavior noted in the literature [30].
The on-column entrapment method that was developed and tested in this report is a general one that could be extended to other soluble proteins [36,40]. Columns that are prepared by this approach should be useful in studies based on frontal analysis, zonal elution, or related methods to examine the interactions of other drugs or solutes with entrapped proteins [2]. Possible future applications for this method include the preparation of affinity microcolumns that contain specific preparations of modified proteins or with proteins that have been isolated from individual biological samples [36,40,50,51]. This type of tool should be valuable in fields such as personalized medicine and in detailed studies of how the modification of a protein can alter its ability to bind to drugs or other solutes.
Acknowledgments
This work was supported by the National Institutes of Health under grants R01 GM044931 and R01 DK069629. The authors also thank J. Vargas-Badilla for the information on the column-to-column reproducibility.
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare no conflict of interest in the work that is presented in this study. The general concept of the entrapment approach that was used in this work is described in U.S. Patent 8,268,570.
References
- 1.Cohen LH, Nicoll-Griffith DA. Plasma protein binding methods in drug discovery and development: bioanalysis. In: Lyubimov AV, editor. Encyclopedia of drug metabolism and interactions. Hoboken: Wiley; 2012. pp. 657–74. [Google Scholar]
- 2.Hage DS, Anguizola J, Barnaby O, Jackson A, Yoo MJ, Papastavros E, Pfaunmiller E, Sobansky M, Tong Z. Characterization of drug interactions with serum proteins by using high-performance affinity chromatography. Curr Drug Metab. 2011;12:313–28. doi: 10.2174/138920011795202938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kratochwil NA, Huber W, Muller F, Kansy M, Gerber PR. Predicting plasma protein binding of drugs: a new approach. Biochem Pharmacol. 2002;64:1355–74. doi: 10.1016/s0006-2952(02)01074-2. [DOI] [PubMed] [Google Scholar]
- 4.Vuignier K, Guillarme D, Veuthey JL, Carrupt PA, Schappler J. High performance affinity chromatography (HPAC) as a high-throughput screening tool in drug discovery to study drug-plasma protein interactions. J Pharm Biomed Anal. 2013;74:205–12. doi: 10.1016/j.jpba.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Hage DS. High-performance affinity chromatography: a powerful tool for studying serum protein binding. J Chromatogr B. 2002;768:3–30. doi: 10.1016/s0378-4347(01)00482-0. [DOI] [PubMed] [Google Scholar]
- 6.Ceciliani F, Pocacqua V. The acute phase protein α1-acid glycoprotein: a model for altered glycosylation during diseases. Curr Protein Pept Sci. 2007;8:91–108. doi: 10.2174/138920307779941497. [DOI] [PubMed] [Google Scholar]
- 7.Israili ZH, Dayton PG. Human alpha-1-glycoprotein and its interactions with drugs. Drug Metab Rev. 2001;33:161–235. doi: 10.1081/dmr-100104402. [DOI] [PubMed] [Google Scholar]
- 8.Tesseromatis C, Alevizou A, Tigka E, Kotsiou A. Acute-phase proteins: alpha-1-acid glycoprotein. In: Veas F, editor. Acute phase proteins - regulation and functions of acute phase proteins. Rijeka, Croatia: InTech; 2011. pp. 247–60. [Google Scholar]
- 9.Goolkasian DL, Slaughter RL, Edwards DJ, Lalka D. Displacement of lidocaine from serum α1-acid glycoprotein binding sites by basic drugs. Eur J Clin Pharmacol. 1983;25:413–7. doi: 10.1007/BF01037957. [DOI] [PubMed] [Google Scholar]
- 10.Routledge PA, Barchowsky A, Bjornsson TD, Kitchell BB, Shand DG. Lidocaine plasma protein binding. Clin Pharmacol Ther. 1980;27:347–51. doi: 10.1038/clpt.1980.46. [DOI] [PubMed] [Google Scholar]
- 11.Hanada K, Ohta T, Hirai M, Arai M, Ogata H. Enantioselective binding of propranolol, disopyramide, and verapamil to human α1-acid glycoprotein. J Pharm Sci. 2000;89:751–7. doi: 10.1002/(SICI)1520-6017(200006)89:6<751::AID-JPS6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 12.Freilich DI, Giardina EG. Imipramine binding to alpha-1-acid glycoprotein in normal subjects and cardiac patients. Clin Pharmacol Ther. 1984;35:670–4. doi: 10.1038/clpt.1984.93. [DOI] [PubMed] [Google Scholar]
- 13.Albani F, Riva R, Contin M, Baruzzi A. Stereoselective binding of propranolol enantiomers to human α1-acid glycoprotein and human plasma. Br J Clin Pharmacol. 1984;18:244–6. doi: 10.1111/j.1365-2125.1984.tb02462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaliszan R, Nasal A, Turowski M. Binding site for basic drugs on α1-acid glycoprotein as revealed by chemometric analysis of biochromatographic data. Biomed Chromatogr. 1995;9:211–5. doi: 10.1002/bmc.1130090504. [DOI] [PubMed] [Google Scholar]
- 15.Piafsky KM, Borga O. Plasma protein binding of basic drugs. II. Importance of alpha1-acid glycoprotein for interindividual variation. Clin Pharmacol Ther. 1977;22:545–9. doi: 10.1002/cpt1977225part1545. [DOI] [PubMed] [Google Scholar]
- 16.Lai CM, Moore P, Quon CY. Binding of fosphenytoin, phosphate ester pro drug of phenytoin, to human serum proteins and competitive binding with carbamazepine, diazepam, phenobarbital, phenylbutazone, phenytoin, valproic acid or warfarin. Res Commun Mol Pathol Pharmacol. 1995;88:51–62. [PubMed] [Google Scholar]
- 17.Kim Y, Choi K, Jung J, Park S, Kim PG, Park J. Aquatic toxicity of acetaminophen, carbamazepine, cimetidine, diltiazem and six major sulfonamides, and their potential ecological risks in Korea. Environ Int. 2007;33:370–5. doi: 10.1016/j.envint.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 18.Herve F, Gomas E, Duche JC, Tillement JP. Evidence for differences in the binding of drugs to the two main genetic variants of human α1-acid glycoprotein. Br J Clin Pharmacol. 1993;36:241–9. doi: 10.1111/j.1365-2125.1993.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leow KP, Wright AW, Cramond T, Smith MT. Determination of the serum protein binding of oxycodone and morphine using ultrafiltration. Ther Drug Monit. 1993;15:440–7. doi: 10.1097/00007691-199310000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Urien S, Albengres E, Pinquier JL, Tillement JP. Role of alpha-1 acid glycoprotein, albumin, and nonesterified fatty acids in serum binding of apazone and warfarin. Clin Pharmacol Ther. 1986;39:683–9. doi: 10.1038/clpt.1986.119. [DOI] [PubMed] [Google Scholar]
- 21.Urien S, Albengres E, Zini R, Tillement JP. Evidence for binding of certain acidic drugs to α1-acid glycoprotein. Biochem Pharmacol. 1982;31:3687–9. doi: 10.1016/0006-2952(82)90597-4. [DOI] [PubMed] [Google Scholar]
- 22.Urien S, Bree F, Testa B, Tillement JP. pH-dependence of warfarin binding to α1-acid glycoprotein (orosomucoid) Biochem J. 1993;289:767–70. doi: 10.1042/bj2890767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakagawa T, Kishino S, Itoh S, Sugawara M, Miyazaki K. Differential binding of disopyramide and warfarin enantiomers to human α1-acid glycoprotein variants. Br J Clin Pharmacol. 2003;56:664–9. doi: 10.1046/j.1365-2125.2003.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama T, Furuie MA, Hibino S, Otagiri M. Comparative study of interaction mode of diazepines with human serum albumin and α1-acid glycoprotein. J Pharm Sci. 1992;81:16–20. doi: 10.1002/jps.2600810104. [DOI] [PubMed] [Google Scholar]
- 25.Frostell-Karlsson A, Remaeus A, Roos H, Andersson K, Borg P, Hamalainen M, Karlsson R. Biosensor analysis of the interaction between immobilized human serum albumin and drug compounds for prediction of human serum albumin binding levels. J Med Chem. 2000;43:1986–92. doi: 10.1021/jm991174y. [DOI] [PubMed] [Google Scholar]
- 26.Amini A, Westerlund D. Evaluation of association constants between drug enantiomers and human α1-acid glycoprotein by applying a partial-filling technique in affinity capillary electrophoresis. Anal Chem. 1998;70:1425–30. doi: 10.1021/ac970766q. [DOI] [PubMed] [Google Scholar]
- 27.Mallik R, Xuan H, Guiochon G, Hage DS. Immobilization of α1-acid glycoprotein for chromatographic studies of drug-protein binding II. correction for errors in association constant measurements. Anal Biochem. 2008;376:154–6. doi: 10.1016/j.ab.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallik R, Xuan H, Hage DS. Development of an affinity silica monolith containing α1-acid glycoprotein for chiral separations. J Chromatogr A. 2007;1149:294–304. doi: 10.1016/j.chroma.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xuan H, Hage DS. Immobilization of α1-acid glycoprotein for chromatographic studies of drug-protein binding. Anal Biochem. 2005;346:300–10. doi: 10.1016/j.ab.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 30.Xuan H, Joseph KS, Wa C, Hage DS. Biointeraction analysis of carbamazepine binding to α1-acid glycoprotein by high-performance affinity chromatography. J Sep Sci. 2010;33:2294–301. doi: 10.1002/jssc.201000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SS, Mehta J. Measurement of drug-protein binding by immobilized human serum albumin-HPLC and comparison with ultrafiltration. J Chromatogr B. 2006;834:108–16. doi: 10.1016/j.jchromb.2006.02.053. [DOI] [PubMed] [Google Scholar]
- 32.Hermanson GT, Mallia AK, Smith PK. Immobilized affinity ligand techniques. San Diego: Academic Press; 1992. [Google Scholar]
- 33.Besanger TR, Brennan JD. Entrapment of membrane proteins in sol-gel derived silica. J Sol-Gel Sci Techn. 2006;40:209–25. [Google Scholar]
- 34.Keeling-Tucker T, Brennan JD. Fluorescent probes as reporters on the local structure and dynamics in sol-gel-derived nanocomposite materials. Chem Mater. 2001;13:3331–50. [Google Scholar]
- 35.Vera-Avila LE, Garcia-Salgado E, Garcia de Llasera MP, Pena-Alvarez A. Binding characteristics of bovine serum albumin encapsulated in sol-gel glasses: an alternative for protein interaction studies. Anal Biochem. 2008;373:272–80. doi: 10.1016/j.ab.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Jackson AJ, Xuan H, Hage DS. Entrapment of proteins in glycogen-capped and hydrazide-activated supports. Anal Biochem. 2010;404:106–8. doi: 10.1016/j.ab.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HS, Hage DS. Immobilization methods for affinity chromatography. In: Hage DS, editor. Handbook of affinity chromatography. 2. Boca Raton: CRC Press; 2006. pp. 35–78. [Google Scholar]
- 38.Bi C, Jackson A, Vargas-Badilla J, Li R, Rada G, Anguizola J, Pfaunmiller E, Hage DS. Entrapment of alpha1-acid glycoprotein in high-performance affinity columns for drug- protein binding studies. J Chromatogr B. doi: 10.1016/j.jchromb.2015.11.021. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monton MRN, Forsberg EM, Brennan JD. Tailoring sol-gel-derived silica materials for optical biosensing. Chem Mater. 2012;24:796–811. [Google Scholar]
- 40.Jackson AJ, Anguizola J, Pfaunmiller E, Hage DS. Use of entrapment and high-performance affinity chromatography to compare the binding of drugs and site-specific probes with normal and glycated human serum albumin. Anal Bioanal Chem. 2013;405:5833–41. doi: 10.1007/s00216-013-6981-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng X, Li Z, Beeram S, Podariu M, Matsuda R, Pfaunmiller E, White CJ, II, Carter N, Hage DS. Analysis of biomolecular interactions using affinity microcolumns: a review. J Chromatogr B. 2014;968:49–63. doi: 10.1016/j.jchromb.2014.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruhn PF, Garver S, Hage DS. Development of dihydrazide-activated silica supports for high-performance affinity chromatography. J Chromatogr A. 1994;669:9–19. doi: 10.1016/0021-9673(94)80332-3. [DOI] [PubMed] [Google Scholar]
- 43.Yoo MJ, Hage DS. Evaluation of silica monoliths in affinity microcolumns for high-throughput analysis of drug-protein interactions. J Sep Sci. 2009;32:2776–85. doi: 10.1002/jssc.200900346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vargas-Badilla J. PhD Dissertation. University of Nebraska-LIncoln; Lincoln, NE: 2015. Applications of high performance affinity chromatography with high capacity stationary phases made by entrapment. [Google Scholar]
- 45.Sobansky MR, Hage DS. Identification and analysis of stereoselective drug interactions with low-density lipoprotein by high-performance affinity chromatography. Anal Bioanal Chem. 2012;403:563–71. doi: 10.1007/s00216-012-5816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tweed SA, Loun B, Hage DS. Effects of ligand heterogeneity in the characterization of affinity columns by frontal analysis. Anal Chem. 1997;69:4790–8. doi: 10.1021/ac970565m. [DOI] [PubMed] [Google Scholar]
- 47.Bi C, Zheng X, Hage DS. Analysis of free drug fractions in serum by ultrafast affinity extraction and two-dimensional affinity chromatography using α1-acid glycoprotein microcolumns. J Chromatogr A. 2016;1432:49–57. doi: 10.1016/j.chroma.2015.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zsila F, Iwao Y. The drug binding site of human α1-acid glycoprotein: insight from induced circular dichroism and electronic absorption spectra. Biochim Biophys Acta. 2007;1770:797–809. doi: 10.1016/j.bbagen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Xuan H, Hage DS. Evaluation of a hydrazide-linked α1-acid glycoprotein chiral stationary phase: separation of R- and S-propranolol. J Sep Sci. 2006;29:1412–22. doi: 10.1002/jssc.200600051. [DOI] [PubMed] [Google Scholar]
- 50.Herve F, Duche JC, d'Athis P, Marche C, Barre J, Tillement JP. Binding of disopyramide, methadone, dipyridamole, chlorpromazine, lignocaine and progesterone to the two main genetic variants of human α1-acid glycoprotein: evidence for drug-binding differences between the variants and for the presence of two separate drug-binding sites on α1-acid glycoprotein. Pharmacogenetics. 1996;6:403–15. doi: 10.1097/00008571-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Herve F, Caron G, Duche JC, Gaillard P, Rahman NA, Tsantili-Kakoulidou A, Carrupt PA, d'Athis P, Tillement JP, Testa B. Ligand specificity of the genetic variants of human α1-acid glycoprotein: generation of a three-dimensional quantitative structure-activity relationship model for drug binding to the A variant. Mol Pharmacol. 1998;54:129–38. doi: 10.1124/mol.54.1.129. [DOI] [PubMed] [Google Scholar]