Abstract
As we experience the world, we must decide not only when and how to act based on input from the environment, but also when to avoid responding in situations where acting could lead to a detrimental outcome. The ability to regulate behavior in this way requires flexible cognitive control, as the same stimulus may call for a response in one context but not in another. In this sense, explicit non-responding can be characterized as an active, goal-directed cognitive process. Little is known about the mechanisms by which a currently active goal state modulates information processing to support the avoidance of undesired responding. In the present study, participants executed or withheld responses to a color target based whether its color matched that of a cue at the beginning of each trial. Behavioral and neural responses to task-irrelevant stimuli appearing as distractors were examined as a function of their relationship to the currently response-relevant color indicated by the cue. We observed a robust pattern in which stimuli possessing the currently response-irrelevant feature activate the default mode network, which was associated with a behavioral cost on trials in which this stimulus competed with a response-relevant target. Our findings reveal a role for the default mode network in goal-directed cognitive control, facilitating active disengagement based on contextually-specific task demands.
Keywords: cognitive control, inhibition, default mode network, fMRI
1. Introduction
The same stimulus may demand a response in one situation and the withholding of that response in another. For example, certain roadway intersections contain a stop sign that only applies to drivers intending to turn in a particular direction (e.g., stop except for right turn). In order to behave effectively, organisms must be able to flexibly modulate whether and how they respond to environmental input based on such contextually-specific demands. The coordination of information processing that supports goal-directed behavior in this way is broadly referred to as cognitive control.
Cognitive control is typically examined in the context of selecting and executing a response based on a set of currently relevant task rules (Badre, & D'Esposito, 2007; Koechlin, Basso, Pietrini, Panzer, & Grafman, 1999; Koechlin, Corrado, Pietrini, & Grafman, 2000). Research using this approach highlights the important role of the prefrontal cortex (PFC) in flexibly configuring information processing to support goal-directed behavior (Badre, & D'Esposito, 2007; Koechlin et al., 1999, 2000). The need to refrain from responding, however, may similarly require consideration of currently relevant task rules. Under such conditions, explicit non-responding can also be thought of as an active, goal-directed cognitive process.
The cancelation of a planned action has been investigated using the stop signal task, which implicates the inferior frontal and insular cortex and pre-supplementary motor area (pre-SMA) in response inhibition (e.g., Aron & Poldrack, 2006; Aron et al., 2003; Cai et al., 2014; Sharp et al., 2010). Similar findings have been observed using a simple go/no-go (GNG) task in which the no-go stimulus is consistent across trials (e.g., Liddle, Kiehl, & Smith, 2001; Watanabe et al., 2002). More flexible and cognitively demanding response inhibition has also been investigated using a variant of the sustained attention to response (SART) task in which immediately repeated stimuli require the withholding of a prepotent response, which implicates additional areas of prefrontal cortex reflecting greater demand for cognitive control (e.g., Garavan, Ross, Kaufman, & Stein, 2003; Garavan, Ross, & Stein, 1999; Hester et al., 2004; Kelly et al., 2004; Simmonds, Pekar, & Mostofsky, 2008).
In contrast to such overt response inhibition, less is known about the mechanisms by which cognitive control processes flexibly configure information processing to support the avoidance of responding in error. Contextually dependent response preparation and selection has been investigated using the AX variant of the continuous performance task (AX-CPT; e.g., Braver et al., 2001; Braver & Cohen, 2001; Paxton, Barch, Racine, & Braver, 2008). In the AX-CPT paradigm, participants respond to an ‘X’ probe differently based on whether it was immediately preceded by an ‘A’ cue or a different cue. However, as it has typically been used in the neuroimaging literature, the AX-CPT paradigm requires selecting between responses and not explicitly withholding from responding in certain contexts. In the present study, we focus on situations in which an individual can prepare in advance to refrain from responding to a particular stimulus based on contextual information, and how the activation of the corresponding goal state changes how this response-irrelevant stimulus is processed.
The process of deciding how to respond requires the ability to ignore irrelevant information and maintain focus on the stimuli and rules that dictate the correct course of action. A network of brain regions referred to as the default mode network (DMN), which includes the posterior cingulate cortex (PCC), medial PFC, and ventral precuneus, often appears to be suppressed during the performance of a variety of tasks involving cognitive control (Shulman et al., 1997). As activation within task-positive brain networks increases, activation within the DMN typically decreases, suggesting a competitive relationship between the processing of external stimulus information and the DMN (e.g., Greicius, Krasnow, Reiss, & Menon, 2003; Uddin, Kelly, Biswal, Castellanos, & Milham, 2009). Importantly, the DMN has been strongly linked to information processing in the absence of a task (e.g., Raichle et al., 2001; Shulman et al., 1997), the activation of which is often hypothesized to reflect internal thought (e.g., Sheline et al., 2009). More recent evidence suggests the activity within the DMN has consequences for goal-directed behavior. Greater activation within this network is associated with mind wandering (Weissman, Roberts, Visscher, & Woldorff, 2006), “zoning out,” and diminished performance during a demanding task (Esterman, Noonan, Rosenberg, & DeGutis, 2013).
Such prior demonstrations suggest that activation within the DMN is detrimental to the execution of cognitive control processes, interfering with the ability to carry out goal-directed behavior. Under certain conditions, however, it might be advantageous to disengage from task-related information processing, particularly when the desired outcome is to avoid responding. We hypothesized that when the task involves the need to refrain from responding contingent on a flexibly configured goal state, activation within the DMN might serve to support contextually-appropriate non-responding. Here, we examine the neural correlates of processing a response-irrelevant stimulus.
We recently developed a paradigm for investigating flexible, goal-contingent response inhibition (Anderson & Folk, 2014; see also Anderson & Folk, 2012a). Participants report the identity of a centrally-presented target letter only if its color matches that of a cue at the beginning of each trial. Immediately preceding the target, irrelevant flanker letters are presented. These flankers can be either compatible or incompatible with the target-associated response, and can be rendered in either the cued (response-relevant) or the uncued (response-irrelevant) color. Despite the fact that the flankers never require a response and are thus task-irrelevant, the processing of these flankers is strongly modulated by whether their color is response-relevant. When the flankers are rendered in the response-relevant color, they elicit a compatibility effect indicative of the activation of their associated response, whereas when they are rendered in the response-irrelevant color, they elicit a robust reverse compatibility effect indicative of the inhibition of their associated response.
In the present study, we investigated the brain systems underlying the processing of response-irrelevant compared to response-relevant stimuli. By including flanker-only trials during which the target was omitted, we isolated the modulatory impact of current goals on stimulus processing from overt action/inaction (see Serences et al., 2005, for a similar design concept in the context of contingent attentional capture). Our findings reveal a role for the DMN in goal-contingent response inhibition, thereby actively supporting human cognitive control.
2. Material and Methods
2.1. Participants
Eighteen neurologically healthy adult volunteers (18-22 years of age, mean = 19.9, 8 females) with normal or corrected-to-normal visual acuity and color vision were recruited from the Johns Hopkins University community to participate. Written informed consent was obtained for each participant. All procedures were approved by the Johns Hopkins Medicine Institutional Review Board.
2.2. Behavioral Task and Procedure
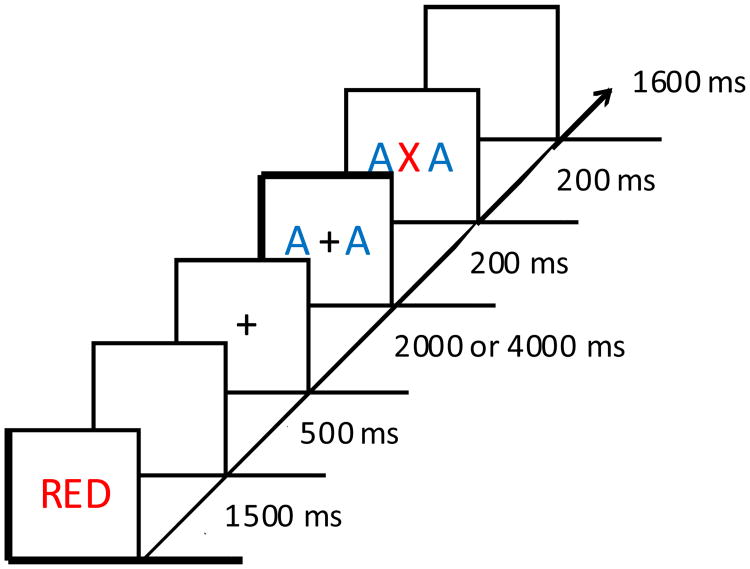
The experimental design was closely modeled after that of Anderson and Folk (2014). Each trial began with a color-word cue presented in the center of the screen for 1500 ms (Figure 1). Each letter was rendered in the color indicated by the word, which designated the color that would serve as the response-relevant color on that trial. Following a 500 ms blank screen, a white fixation cross (1.8° × 1.8° visual angle) was presented for either 2000 or 4000 ms (equally often, randomly determined on each trial). Next, two flanker letters (each 2.75° × 1.4°) were presented to the left and right of the fixation cross (each 2.6° center-to-center from fixation) for 200 ms. The flankers required no response, and were completely irrelevant to the task and unpredictive of the upcoming target color or identity.
Figure 1.
Sequence and timing of events for a trial. In this example, the target color matches the cued color, and so the participant would identify the target letter with a button press. Had the target been presented in blue, the participant would have withheld from responding. This is a trial on which the flankers were rendered in the uncued color.
On target present trials, a target letter (2.75° × 1.4°) replaced the fixation cross while the flankers remained onscreen for an additional 200 ms. On flanker only (target absent) trials, the flankers remained on screen for the same amount of time but the fixation cross remained in place of the target. This was followed by 1600 ms blank screen during which responses were recorded, and then by a variable inter-trial-interval of 2000 ms or 4000 ms (equally-often, randomly determined on each trial).
The target, flankers, and cue could be either red or blue. The letters ‘A’ and ‘X’ were used for the targets and flankers. The experiment consisted of 8 runs of 48 trials. Two-thirds of trials were target present trials, and the remaining third were flanker only (target absent) trials. On target present trials within each run, cue color, target color, target identity, flanker color, and flanker compatibility were fully crossed and counterbalanced. On flanker only trials, cue color, flanker color, and flanker identity were fully crossed and counterbalanced. Trials were presented in a random order.
Participants were instructed to report the target identity as quickly as possible while minimizing errors, pressing a button in the right hand for an ‘A’ and a button in the left hand for an ‘X’, but only when the target color matched the cue color. Participants were also informed that the flankers were irrelevant to the task and did not predict the upcoming target, and that they should focus exclusively on preparing for the upcoming target when the flankers appeared. False alarms (responses to uncued targets), misses (failing to respond to a cued target within the allotted time), and incorrect responses to cued targets were all considered errors. Participants were informed of their accuracy at the conclusion of each run. Halfway between the experimental session, participants were provided a brief rest period during which a high-resolution anatomical image of their brain was acquired.
Prior to scanning, participants completed an identical session of the experimental task in order to familiarize them with the stimuli and procedures. Only participants performing above 85% were allowed to proceed to the MRI session and were included in the study.
2.3. Stimulus Presentation and Response Recording
The stimuli were displayed using an Epson PowerLite 7600p projector with a custom zoom lens onto a screen mounted at the end of the magnet bore behind the participant's head. Participants viewed the screen using a mirror mounted to the head coil. Stimulus displays were generated using Matlab software with Psychophysics Toolbox extensions (Brainard, 1997), and responses were recorded using two custom-built, fiber-optic push button boxes.
2.4. MRI Data Acquisition
Images were acquired using a 3-Tesla Philips Gyroscan MRI scanner and a 32-channel transmit/receive sensitivity encoding (SENSE) head coil at the F. M. Kirby Research Center for Functional Brain Imaging located in the Kennedy Krieger Institute, Baltimore, MD. High-resolution whole-brain anatomical images were acquired using a T1-weighted magnetization-prepared rapid gradient echo pulse sequence [voxel size = 1 mm isotropic, repetition time (TR) = 8.1 ms, echo time (TE) = 3.7 ms, flip angle = 8°, acquisition matrix = 212 × 172, 150 axial slices, 0 mm gap, SENSE factor = 2]. Whole-brain functional images were acquired using a T2*-weighted echoplanar imaging (EPI) pulse sequence (voxel size = 2.5 mm isotropic, TR = 2000 ms, TE = 30 ms, flip angle = 70°, acquisition matrix = 76 × 76, 36 axial slices, 0.5 mm gap, SENSE factor = 2). Each EPI pulse sequence began with 4 dummy pulses that were not recorded in order allow magnetization to reach steady-state. Each run of the task lasted 8.2 min during which 242 volumes were acquired.
2.5. Data Analysis
2.5.1. Behavior
For the behavioral data, performance was combined from the two identical experimental sessions (one during and one prior to scanning). Only correct responses were included in the computation of mean response time (RT).
2.5.2. MRI
All preprocessing and analysis was conducted using the AFNI software package (Cox, 1996) except where otherwise noted. Each EPI run for each participant was slice-time corrected and then motion corrected using the last image prior to the anatomical scan as a reference. EPI images were then coregistered to the corresponding anatomical image for each participant. Using ANTs (Avants et al., 2011) nonlinear warping software, the images for each participant were warped to the Talairach brain (Talairach & Tournoux, 1988). Finally, the EPI images were converted to percent signal change normalized to the mean of each run, and then spatially smoothed using a 5 mm full-width half-maximum Gaussian kernel.
Data for one participant was not further analyzed due to severe motion during scanning (frequent movements exceeding the width of one voxel). Data for each of the remaining participants were subjected to a general linear model (GLM) that included six regressors of interest corresponding to the relationship between the flanker, target, and cue color: flanker in (1) cued and (2) uncued color with no target, flanker in (3) cued and (4) uncued color with target in cued color, and flanker in (5) cued and (6) uncued color with target in uncued color. These regressors were modeled using a canonical hemodynamic response function (HRF), each being represented by a simple HRF (without convolving with a particular duration) onset to the appearance of the flankers. Regressors of non-interest included the presentation of the cue (also modeled using a canonical HRF), six motion parameters, and drift in the scanner signal.
The resulting beta-weight estimates were analyzed using a three-way analysis of variance (ANOVA) with target condition (absent, cued color, uncued color) and flanker condition (cued vs uncued color) as fixed effects and subject as a random effect. The ANOVA was followed by a planned contrast examining the response to flankers in the cued vs uncued color specifically on target absent trials, which were included to isolate the response to the flankers (i.e., a biasing signal) in the absence of overt action/inaction. The results of the ANOVA and contrast were assessed for statistical significance (α = .05) using the AFNI program AlphaSim, which determines the probability of the observed cluster sizes occurring in synthetic data randomly generated to match the smoothness and spatial extent of the actual data (n iterations = 10,000 with voxelwise p = .005, clusters defined using nearest neighbor method, minimum cluster size at p < .05 = 54 voxels).
To better characterize the observed flanker-evoked activations, two follow-up analyses were performed. First, we repeated the planned contrast using independently identified DMN regions. Three spheres were created corresponding to the medial PFC, PCC, and ventral precuneus. The center of each sphere was taken directly from Table 1 of Laird et al. (2009), and the radius was determined such that the resulting volume most closely matched that reported by Laird et al. (2009). Second, we performed generalized psychophysiological interaction (PPI) analysis (McLaren et al., 2012) implemented in AFNI (http://afni.nimh.nih.gov/sscc/gangc/CD-CorrAna.html) using mean activity across the voxels within the three DMN areas observed in the planned contrast as the seed. A GLM was performed in which activity within this seed was included as a regressor, in addition to its interaction with each of the three trial types (cued target, uncued target, and target absent). A regressor for each of these three trial types as well as the cue presentation were modeled using a canonical HRF, and nuisance regressors for motion and scanner drift were included as in the main GLM. Follow-up contrasts compared the resulting beta weights for the uncued target and target absent interaction regressors against zero, as well as for cued target trials against these other two trial types.
3. Results
3.1. Behavior
Mean RT to correctly identified cued targets was subjected to a 2 × 2 ANOVA, with flanker color (cued vs uncued) and flanker compatibility (compatible vs incompatible) as within-subjects factors. This analysis revealed a significant main effect of flanker color, F(1,16) = 11.78, p = .003, ηp2 = .424, but no main effect of compatibility, F < 1. Importantly, there was a significant interaction between flanker color and flanker compatibility, F(1,16) = 34.65, p < .001, ηp2 = .684 (Figure 2). Planned comparisons indicated that uncued flankers produced a significant reverse-compatibility effect, t(16) = -3.79, p = .002, d = .92, whereas cued flankers produced a positive compatibility effect, t(16) = 4.01, p = .001, d = .97. This suggests that participants either activated or inhibited the flanker-evoked response based on the currently activated color-response rule, even though the flankers were known to be irrelevant to the task. The same ANOVA on accuracy revealed a main effect of color, F(1,16) = 7.32, p = .016, ηp2 = .314; the main effect of compatibility and the interaction were not significant, Fs < 1.32, ps > .26. The observed pattern in accuracy generally mirrored that in RT (flanker in cued color: congruent = 95.5%, incongruent = 94.3%; flanker in uncued color: congruent = 93.0%, incongruent = 93.4%). False alarms and misses occurred infrequently (< 5%).
Figure 2.
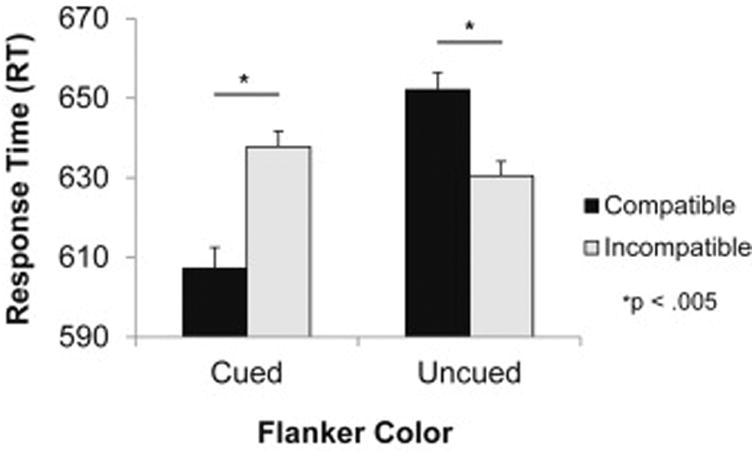
Response time in target identification as a function of flanker color on compatible and incompatible trials. *p < .005
3.2. Neuroimaging
A main effect of target condition was evident across bilateral inferior parietal cortex, insula, cingulate gyrus, thalamus, caudate, substantial nigra, and cerebellum (see Table 1), owing to differences in motor demands and the task-relevance of the central stimulus (absent, response-relevant, response-irrelevant). In all clusters exhibiting this main effect, cued targets were associated with significantly greater activation than uncued targets, each of which was associated with significantly greater activation than target-absent trials (ps < .05). The main effect of flanker color did not produce any significant regions of activation. However, it is possible that a difference in flanker processing based on color (cued vs uncued), reflecting the biasing signal related to the behavioral effect, was present but the associated activation was obscured by voluntary target-related processing. We anticipated this possibility, and therefore included target absent trials in the experimental design in order to better isolate potential biasing signals arising from the response-relevance of stimuli in the absence of voluntary selection and corresponding overt action/inaction. Limiting comparison to these trials, reliable differences emerged1. Specifically, flankers in the uncued color produced greater activation in the medial PFC, PCC, ventral precuneus, right middle frontal gyrus (MFG), and left superior temporal gyrus (STG) (Figure 3a and Table 2).
Table 1.
Brain areas in which a significant main effect of target condition was identified. Coordinates reflect the peak voxel for each region.
| region | Talairach coordinates (x, y, z) | volume (ml) |
|---|---|---|
| left insula | 40, 6, 13 | 39.88* |
| right insula | -37, 16, -2 | 7.48 |
| left thalamus | 16, 16, 7 | 39.88* |
| right thalamus | -13, 20, 10 | 39.88* |
| left caudate | 13, -8, -2 | 39.88* |
| right caudate | -16, -10, 3 | 39.88* |
| left substantia nigra | 11, 20, -11 | 39.88* |
| right substantia nigra | -8, 20, -8 | 39.88* |
| left inferior patietal lobule | 37, 28, 40 | 21.23 |
| right inferior parietal lobule | -47, 35, 37 | 9.68 |
| cingulate gyrus | 1, -8, 34 | 18.21 |
| culmen of cerebellum | 1, 54, -5 | 2.87 |
these brain regions all formed one contiguous cluster
Figure 3.
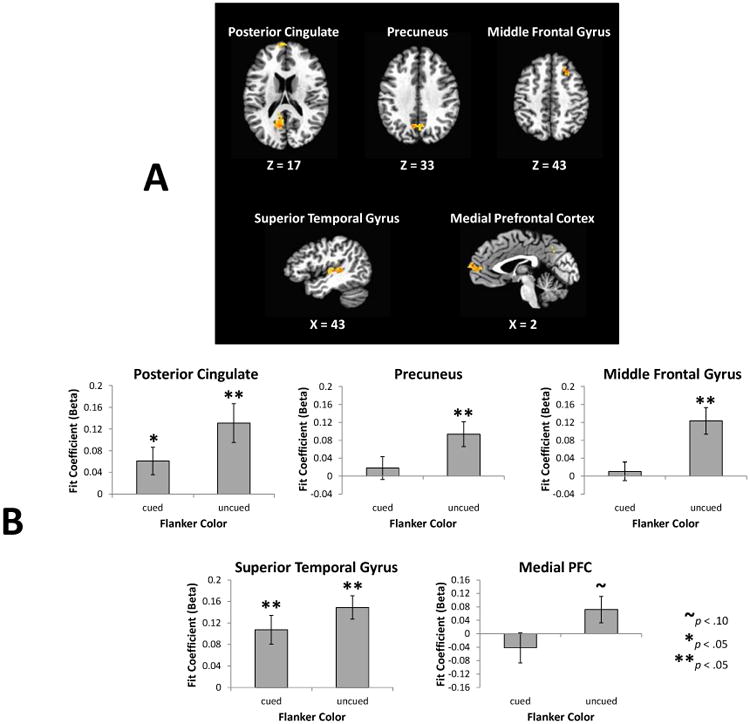
Imaging results from the planned contrast. (A) Brain regions in which the response to the irrelevant flankers differed as a function of their color (cued vs uncued) on target absent trials. Significantly greater activation in response to flankers in the uncued color was evident in the five areas shown. (B) Activations from panel A broken down by condition and compared to baseline (unmodeled variance). Areas were defined using a leave-one-subject-out procedure (Esterman et al., 2010) to preserve independence.
Table 2.
Brain areas in which the response to the irrelevant flankers differed as a function of their color (cued vs uncued) on target absent trials, being greater for uncued flankers. Coordinates reflect the peak voxel for each region.
| region | Talairach coordinates (x, y, z) | volume (ml) |
|---|---|---|
| PCC | 6, 61, 16 | 1.95 |
| medial PFC | 1, -61, 13 | 1.69 |
| left STG | 42, 23, 4 | 1.58 |
| precuneus | 8, 61, 31 | 1.20 |
| right MFG | -23, -23, 43 | 1.01 |
In order to better characterize these differences, we compared each flanker condition to baseline (Figure 3b), using a leave-one-subject-out procedure that preserves independence (Esterman, Tamber-Rosenau, Chiu, & Yantis, 2010). This analysis confirmed that the significant contrasts observed in Figure 3a reflected stronger activation (above-baseline) of these regions by response-irrelevant stimuli rather than reduced (below-baseline) suppression. To verify that the observed PCC, precuneus, and medial PFC activations are consistent with the DMN, we independently defined these regions using spheres, the center and spatial extent of which were based off of a meta-analysis of the DMN (Laird et al., 2009); using these regions, the difference between flankers in the cued and uncued color is significant, t(16) = 2.76, p = .014, d = .67.
To examine the relationship between such flanker-evoked activity and the inhibition evident in behavior, we tested for two potential correlations. Analyses focused on cued target trials, as these were the trials on which behavioral responses were made, the speed of which should be modulated by corresponding neural processing of the flankers. We compared the difference in cued vs uncued flanker-evoked activity in each of the five regions identified in Figure 3a to (1) the main behavioral effect of the flankers (RT on cued – uncued flanker trials) and (2) the interaction (compatibility effect on cued flanker trials – compatibility effect on uncued flanker trials). If flanker-evoked activity in any of these regions were related to the observed behavioral effects, relatively greater activity evoked by uncued vs cued flankers would be associated with a larger behavioral effect, resulting in a positive correlation. Such correlations with the main effect of the flankers were evident in the PCC, left STG, precuneus, and right MFG, rs > .493, ps < .022 (one-tailed) (see Figure 4b), and with the interaction in PCC and left STG, rs > .416, ps < .048 (one-tailed) (see Figure 4a).
Figure 4.
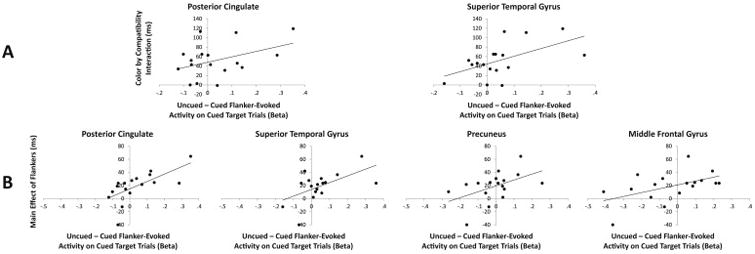
Scatterplots depicting the relationship between uncued minus cued flanker-evoked activity on cued target trials (x-axis) and the effects of the flankers on behavior for those trials (y-axis). Panel A shows the interaction between flanker color and flanker compatibility on the y-axis (compatibility effect on cued minus uncued flanker trials) and Panel B shows the main effect of the flankers on the y-axis (RT on uncued minus cued flanker trials).
Finally, in order to gain further insight into the nature of the observed DMN-related inhibition, we examined where in the brain task-evoked stimulus processing was modulated by activity within the DMN. If DMN-related activation served to facilitate disengagement from task-related information processing, then a contextually-dependent correlation between the DMN and areas involved in representing task-related information should be evident specifically on cued target trials (the trials on which a target-identification response was made that would be subject to inhibition). In this respect, the flankers serve to create variance in the DMN, and we sought to determine whether such variance was related to modulations in other regions. To this end, the three DMN areas activated by flankers in the response-irrelevant color (PCC, medial PFC, and precuneus in Figure 3a) served as the seed for psychophysiological interaction (PPI) analysis. On target absent and uncued target trials, in which participants were not required to make a target-identification response, no significant clusters exhibiting a contextually-dependent correlation between task-evoked stimulus processing and activity within the DMN were observed. Next, we contrasted these two trial types with the contextually-dependent correlations observed on cued target trials. Significantly greater contextual dependencies on cued target trials were observed in the left dorsolateral prefrontal cortex (DLPFC), right occipital cortex (cuneus), and right STG, in addition to left motor cortex (see Figure 5).
Figure 5.

Regions in which the contextually-dependent functional correlation between task-evoked stimulus processing and activity within the default mode network was greater on cued target trials than on uncued target and target absent trials, as revealed by psychophysiological interaction analysis.
4. Discussion
In the present study, we examined the neural mechanisms by which task-irrelevant stimuli are processed as a function of their relationship to the currently active response rule. Replicating previous behavioral findings (Anderson & Folk, 2014), task-irrelevant flankers had a strong influence on responses to a subsequent target that was facilitory when they matched the currently response-relevant color and inhibitory when they matched the currently response-irrelevant color. Examination of the neural correlates of this automatic but goal-contingent processing revealed the activation of the DMN by response-irrelevant stimuli.
4.1. Cognitive Control and Withholding Responses
The need to respond to a stimulus in one context but not in another places unique demands on information processing. The stimulus must be selected and evaluated in light of the current goal state, but once it is selected its associated response must be subsequently ignored in the event that it is determined to be response-irrelevant. In this case, refraining from responding cannot reflect passive ignoring but instead requires active monitoring of the context, the updating of corresponding goals, and the relating of incoming stimulus information to these goals.
Our findings suggest that such active non-responding involves goal-contingent disengagement from task-related information processing. The organism must engage in goal-directed processing in order to decide how a stimulus should influence behavior. In the event that the stimulus indicates the need to refrain from responding, such inaction is facilitated by disengaging attention from the currently activated task rules. To at least some degree, this disengagement is specific to the response rule activated by the stimulus: Behaviorally, such disengagement makes it more difficult to select the corresponding response in the event that a subsequent target calls for that response. Neuroanatomically, this goal-contingent disengagement was related to increased activation within the DMN.
Behaviorally relevant sensory events can elicit the reorienting of attention away from the current focus, a process mediated by the ventral attention network (Corbetta, Patel, & Shulman, 2008). Activation of the ventral attention network signals the dorsal attention network to shift attention away from the current focus and to the eliciting stimulus. However, it is at times advantageous to shift attention away from the current focus without necessarily selecting a different stimulus, particularly when the situation explicitly calls for inaction. Our findings suggest a corresponding role for the DMN in disengaging attention from task-related processing. This relationship was borne out in our task via a psychophysiological interaction analysis, which showed a contextually-dependent correlation between activity within the DMN and task-evoked stimulus processing within the DLPFC and occipital cortex specifically on trials in which a target identification response was required (cued target trials). These results are consistent with DMN-mediated selective disengagement from goal-contingent information processing, a form of non-motoric (cognitive) response inhibition.
4.2. The Role of the DMN in Cognitive Control
Differential neural processing of the task-irrelevant flankers based on cue color in the present study came exclusively in the form of increased activation on uncued vs cued flanker trials. This suggests that participants entered into a state of preparedness to respond in anticipation of the target, and inhibited or canceled this preparedness when a stimulus in the response-irrelevant color was detected. Such goal-contingent inhibition was associated with increased activation in the medial PFC, PCC, and ventral precuneus.
The medial PFC, PCC, and ventral precuneus comprise prominent areas of the DMN (e.g., Laird et al., 2009; Raichle et al., 2001). The DMN has traditionally been linked to internal thought, being more active in the absence of task-related processing (e.g., Raichle et al., 2001; Shulman et al., 1997). Activity in this network is also anti-correlated with activity in networks associated with the performance of a cognitive task (e.g., Greicius, et al., 2003; Uddin, et al., 2009). More recently, activation of the DMN has been linked to fluctuations in task performance related to mind wandering and “zoning out” (Esterman et al., 2013; Weissman et al., 2006). The findings of the present study suggest that the DMN plays an active, functional role in goal-directed cognition. When presented with a context in which responding would be inappropriate, activity within the DMN increases, which is associated with a behavioral signature of response inhibition. In this sense, when disengagement from the task is expected to facilitate appropriate behavior, the activation of the DMN fits the profile of an act of cognitive control.
Increased activation was also observed in right MFG and left STG. In studies of involuntary attentional orienting, the right MFG is more active when an irrelevant distractor matches the stimulus properties used to define targets (e.g., Corbetta & Shulman, 2002; Serences et al., 2005), facilitating the involuntary selection of that stimulus (contingent attentional capture, see Folk, Remington, & Johnston, 1992). In the present study, we observed a similar profile underlying the involuntary inhibition of a response rule based on the mismatch between the distractor color and the cued color. Thus, in the present study, participants may have approached the task with a preparedness to respond to the target that was then canceled in the event that a response-irrelevant stimulus was detected. The left STG activation, corresponding to Wernicke's area, suggests enhanced processing of the meaning of the flankers when presented in the uncued color (Just, Carpenter, Keller, Eddy, & Thulborn, 1996). The magnitude of this STG activation was predictive of the behavioral interaction, which implies representation of the specific flanker letter identify. This and the fact that the activation was greater to uncued flankers suggest the active selection of these stimuli, mirroring the interpretation of the MFG activation. Broadly, such active selection is necessary for the system to know when to disengage from further processing.
4.3. Controlled and Automatic Processing Revisited
The differential processing of the flankers as a function of cue color cannot itself be explained as a voluntary act of cognitive control and instead reflects a biasing signal. All flankers were irrelevant to the task, and participants should have been equally motivated to ignore the flankers regardless of their color. Intentionally inhibiting or activating the flanker-associated response rule based on their color would not confer any performance benefit, as the flankers were unpredictive of the color and identity of targets in all cases. Similar logic is frequently used when examining the influence of task goals on involuntary attentional orienting (e.g., Anderson & Folk, 2012b; Folk et al., 1992; Serences et al., 2005). The findings thus have important implications for how we characterize the mechanisms by which goal-directed cognitive control is realized.
Cognitive control has traditionally been studied in situations where contextually-specific responses are intentionally executed under demanding conditions (Badre, & D'Esposito, 2007; Koechlin et al., 1999, 2000). For example, participants may be asked to classify a target stimulus based on a set of rules that jointly determine categorization (e.g., Badre, & D'Esposito, 2007) or to respond differently to an ambiguous stimulus based on the context in which the stimulus is experienced (e.g., Braver et al., 2001; Braver & Cohen, 2001; Paxton et al., 2008). This mode of responding can be contrasted with a more automatic, stimulus-driven mode in which responses are triggered by a cue (e.g., Berridge, 2012; Berridge & Robinson, 1998; Hikosaka, Yamamoto, Yasuda, & Kim, 2013; Shiffrin & Schneider, 1977).
A widely held assumption in the field of cognitive neuroscience is that cognitive control and automatic responding reflect distinct and opposing mental processes. This assumption is built into many of the paradigms frequently used to investigate the nature of cognitive control. Under these conditions, cognitive control processes are specifically recruited in the service of overcoming an automatic or otherwise prepotent response tendency (e.g., Miller & Cohen, 2001; Pardo, Pardo, Janer, & Raichle, 1990), or to respond to an otherwise ambiguous stimulus (e.g., Braver et al., 2001; Braver & Cohen, 2001; Paxton et al., 2008). Such a dichotomization echoes the seminal work by Shiffrin and Schneider (Schneider & Shiffrin, 1977; Shiffrin & Schneider, 1977) that outlined the conditions under which information processing is controlled and automatic. Automatic processing is held to be the product of consistent mapping in which a stimulus is responded to in the same manner over repeated trials. With sufficient experience, the stimulus will eventually come to trigger its associated response automatically even when responding in this way runs counter to current goals (Shiffrin & Schneider, 1977). The same is true of response inhibition when a stimulus is consistently mapped onto the need to withhold a response (Chiu, Aron, & Verbruggen, 2012; Lenartowicz, Verbruggen, Logan & Poldrack, 2011; Verbruggen & Logan, 2008). Inconsistent mapping, in contrast, demands controlled and effortful information processing (Schneider & Shiffrin, 1977; Shiffrin & Schneider, 1977), as experiential history will not favor any one particular response and the corresponding response rule must be actively represented.
The present study highlights the neural underpinnings of an act of cognitive control that can be automatically cue-triggered yet conditionalized on flexibly updated task goals, an example of conditional automaticity (Bargh, 1989). Such information processing stands in contrast to that which occurs under consistent mapping conditions through associative learning (Chiu et al., 2012; Lenartowicz et al., 2011; Schneider & Shiffrin, 1977; Shiffrin & Schneider, 1977; Verbruggen & Logan, 2008). Our findings suggest that cognitive control may not necessarily be as voluntary and effortful as previously thought. At the level of a single trial, the establishment of task-related priorities can flexibly preconfigure information processing such that a particular stimulus will automatically trigger a particular set of cognitive operations that bias decision-making.
4.4. Conclusions
Our findings provide evidence linking the DMN to human cognitive control processes. When a response-irrelevant stimulus is encountered, it biases the disengagement from task-related information processing, which is reflected in the activation of the DMN. Such disengagement serves in the interest of preventing responses when the response would be contextually inappropriate, resulting in behavioral inhibition of the undesired response. In this way, the DMN actively supports goal-directed behavior.
Acknowledgments
We thank Michelle DiBartolo for assistance with data collection.
Funding: This research was supported by R01-DA013165 to S.M.C. The funder played no role in the reported study beyond financial support.
Footnotes
The same comparison for the other two target conditions revealed no significant difference between cued and uncued flankers when presented with a cued target, and significantly greater activation for cued vs uncued flankers when presented with an uncued target in left parietal cortex only (x = 28, y = 61, z = 34).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian A. Anderson, Johns Hopkins University
Charles L. Folk, Villanova University
Susan M. Courtney, Johns Hopkins University
References
- Anderson BA, Folk CL. Contingent involuntary motoric inhibition: The involuntary inhibition of a motor response contingent on top-down goals. Journal of Experimental Psychology: Human Perception and Performance. 2012a;38:1348–1352. doi: 10.1037/a0030514. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Folk CL. Dissociating location-specific inhibition and attention shifts: Evidence against the disengagement account of contingent capture. Attention, perception, and Psychophysics. 2012b;74:1183–1198. doi: 10.3758/s13414-012-0325-9. [DOI] [PubMed] [Google Scholar]
- Anderson BA, Folk CL. Conditional automaticity in response selection: Contingent involuntary response inhibition with varied stimulus-response mapping. Psychological Science. 2014;25:547–554. doi: 10.1177/0956797613511086. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. Cortical and subcortical contributions to stop signal response inhibition: Role of the subthalamic nucleus. Journal of Neuroscience. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54:2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19:2082–2099. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Bargh JA. Conditional automaticity: Varieties of automatic influence on social perception and cognition. In: Uleman J, Bargh J, editors. Unintended Thought. New York: Guilford; 1989. pp. 3–51. [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. European Journal of Neuroscience. 2012;35:1124–1143. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:443–446. [PubMed] [Google Scholar]
- Braver TS, Barch DM, Keys BA, Carter CS, Cohen JD, Kaye JA, Janowksy JS, Taylor SF, Yesavage JA, Mumenthaler MS, et al. Context processing in older adults: Evidence for a theory relating cognitive control to neurobiology in healthy aging. Journal of Experimental Psychology: General. 130:746–763. [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Working memory, cognitive control, and the prefrontal cortex: Computational and empirical studies. Cognitive Processing. 2001;2:25–55. [Google Scholar]
- Cai W, Ryali S, Chen T, Li CSR, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: Evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analysis across multiple datasets. Journal of Neuroscience. 2014;34:14652–14667. doi: 10.1523/JNEUROSCI.3048-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Aron AR, Verbruggen F. Response suppression by automatic retrieval of stimulus-stop association: Evidence from transcranial magnetic stimulation. Journal of Cognitive Neuroscience. 2012;24:1908–1918. doi: 10.1162/jocn_a_00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for the analysis and visualization of function magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, DeGutis J. In the zone or zoning out?Tracking behavioral and neural fluctuations during sustained attention. Cerebral cortex. 2013;3:2712–2723. doi: 10.1093/cercor/bhs261. [DOI] [PubMed] [Google Scholar]
- Esterman M, Tamber-Rosenau BJ, Chiu YC, Yantis S. Avoiding non-independence in fMRI data analysis: Leave one subject out. NeuroImage. 2010;50:572–576. doi: 10.1016/j.neuroimage.2009.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Kaufman J, Stein EA. A midline dissociation between error-processing and response-conflict monitoring. Neuroimage. 2003;20:1132–1139. doi: 10.1016/S1053-8119(03)00334-3. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences, USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester RL, Murphy K, Foxe JJ, Foxe DM, Javitt DC, Garavan H. Predicting success: Patterns of cortical activation and deactivation prior to response inhibition. Journal of Cognitive Neuroscience. 2004;16:776–785. doi: 10.1162/089892904970726. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Yamamoto S, Yasuda M, Kim HF. Why skill matters. Trends in Cognitive Sciences. 2013;17:434–441. doi: 10.1016/j.tics.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. European Journal of Neuroscience. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J. Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proceedings of the National Academy of Sciences, USA. 2000;97:7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. Journal of Neuroscience. 2009;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenartowicz A, Verbruggen F, Logan GD, Poldrack RA. Inhibition-related activation in the right inferior frontal gyrus in the absence of inhibitory cues. Journal of Cognitive Neuroscience. 2011;23:3388–3399. doi: 10.1162/jocn_a_00031. [DOI] [PubMed] [Google Scholar]
- Liddle PF, Kiehl KA, Smith AM. Event-related fMRI study of responrse inhibition. Human Brain Mapping. 2001;12:100–109. doi: 10.1002/1097-0193(200102)12:2<100::AID-HBM1007>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage. 2012;61:1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proceedings of the National Academy of Sciences, USA. 1990;87:256–259. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cerebral Cortex. 2008;18:1010–1028. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences, USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W, Shiffrin RM. Controlled and automatic human information processing I: Detection, search, and attention. Psychological Review. 1977;84:1–66. [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychological Science. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, Mehta MA. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences, USA. 2010;107:6106–6111. doi: 10.1073/pnas.1000175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processing in depression. Proceedings of the National Academy of Sciences, USA. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Controlled and automatic human information processing II: Perceptual learning, automatic attending, and general theory. Psychological Review. 1977;84:127–190. [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Uddin LQ, Kelly AMC, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the default mode network components: Correlation, anticorrelation, and causality. Human Brain Mapping. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD. Automatic and controlled response inhibition: Associative learning in the go/no-go and stop signal paradigms. Journal of Experimental Psychology: General. 2008;137:649–672. doi: 10.1037/a0013170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]