Abstract
Background
Diabetes and obesity are associated with an increased risk of arrhythmia and sudden cardiac death. Abnormal lipid accumulation is observed in cardiomyocytes of obese and diabetic patients, which may contribute to arrhythmia, but the mechanisms are poorly understood. A transgenic mouse model of cardiac lipid overload, the PPARg cardiac overexpression mouse, has long QT and increased ventricular ectopy.
Objective
We evaluated the hypothesis that the increase in ventricular ectopy during cardiac lipid overload is caused by abnormalities in calcium handling due to increased mitochondrial oxidative stress.
Methods
Ventricular myocytes were isolated from adult mouse hearts to record sparks and calcium transients. Mice were implanted with heart rhythm monitors for in vivo recordings.
Results
PPARg cardiomyocytes have more frequent triggered activity and increased sparks compared to control. Sparks and triggered activity are reduced by mitotempo, a mitochondrial-targeted antioxidant. This is explained by a significant increase in oxidation of RyR2. Calcium transients are increased in amplitude and SR calcium stores are increased in PPARg cardiomyocytes. Computer modeling of the cardiac action potential demonstrates that long QT contributes to increased SR calcium. Mitotempo decreased ventricular ectopy in vivo.
Conclusions
During cardiac lipid overload, mitochondrial oxidative stress causes increased SR calcium leak by oxidizing RyR2 channels. This promotes ventricular ectopy, which is significantly reduced in vivo by a mitochondrial-targeted anti-oxidant. These results suggest a potential role for mitochondrial-targeted anti-oxidants to prevent arrhythmia and sudden cardiac death in obese and diabetic patients.
Keywords: arrhythmia, oxidative stress, calcium, lipid metabolism
Introduction
Obese and diabetic humans have increased rates of atrial fibrillation, ventricular ectopy, and long QT (LQT) 1–3. More importantly, diabetes and obesity are associated with an increased risk of sudden cardiac death, which is often caused by ventricular arrhythmias 4–6. The increased risk of sudden cardiac death is greater than the increased risk of myocardial infarction, suggesting that arrhythmic events are increased more than coronary events in obese and diabetic patients. For people with non-ischemic causes of sudden cardiac death, the cardiomyopathy of obesity is currently the most common autopsy finding 7.
Abnormal lipid accumulation is observed in the cardiomyocytes of obese and diabetic patients and is thought to contribute to an increased risk of arrhythmia. This non-ischemic cardiomyopathy has been termed lipotoxic cardiomyopathy 8, and is likely to become more prevalent as the demographics of Western societies shift to an older average age. However, the molecular mechanisms that cause arrhythmias during cardiac lipid overload have not been identified. Transgenic mouse models of cardiac lipid overload can provide mechanistic insight into the pathophysiology. Mice with cardiac-specific overexpression of peroxisome proliferator-activated receptor gamma (PPARg) accumulate lipids in their cardiomyocytes, without the systemic abnormalities of obesity or diabetes. PPARg is a transcription factor that regulates lipid metabolism. PPARg promotes lipid uptake by cells and is upregulated in the hearts of humans with metabolic syndrome 9. PPARg mice have increased cardiac lipid content and abnormal cardiac mitochondrial morphology 10. Specifically, PPARg mice have significantly increased cardiac triglycerides and free fatty acids 11. Our previous work characterized the abnormal electrophysiology of PPARg transgenic mice, demonstrating that they have LQT caused by a decrease of voltage-gated potassium channels, with increased action potential duration (APD), frequent premature ventricular complexes (PVCs), and sudden cardiac death from spontaneous ventricular tachycardia as young adults 12.
Since abnormalities of intracellular calcium homeostasis are a feature of several forms of arrhythmia, we hypothesized that the pathophysiology of ventricular ectopy in the lipotoxic heart would involve abnormalities in calcium handling. We used the PPARg mouse model to determine if abnormalities of intracellular calcium homeostasis play a causal role in ventricular ectopy in lipotoxic cardiomyopathy, and if abnormalities of calcium homeostasis are due to increased mitochondrial oxidative stress.
Methods
Mouse breeding and husbandry
Experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals. The Columbia University institutional animal care review board gave approval. PPARg mice on the C57/BL genetic background were bred and genotyped as previously described 10. Mice were euthanized by anesthesia overdose (inhaled isoflurane) followed by cervical dislocation.
Spark recording and analysis
Sparks were recorded with a Leica SP2 confocal microscope equipped with a 63x1.4 NA objective. Isolated cardiac myocytes experiments were performed at room temperature. Cardiomyocytes were loaded with fluo-4 (5 •M 10 min), in modified Tyrode’s solution containing 1 mM calcium. Line scan images were recorded with Leica TCS software and quantified with the Sparkmaster plugin of ImageJ.
In vivo heart rhythm telemetry
Mice were implanted with heart rhythm monitors as previously described (Data Sciences International, model EA-F20) 12. Isoflurane was used for general anesthesia. Mice recovered for one week after surgery before starting recordings. ECG recordings were made using Ponemah 3 software. A blinded observer tallied arrhythmia counts manually. Mitotempo was filter sterilized and given at 700 nanograms per gram of body weight as a daily intraperitoneal injection.
Computer Modeling of Action Potentials
A previously described model of the electrophysiology of the rat ventricular myocyte was adapted to study mouse electrophysiology, with updated sodium current model 13–15. PPARg overexpression genotype was simulated as a 30% reduction in Kv current density based on our prior publication. All results for action potentials represent steady state data for WT cardiomyocytes. In the model, PPARg cardiomyocytes showed oscillations of membrane potential and early afterdepolarizations, with no true steady state.
Statistical Analysis
Results are presented as mean +SEM. The unpaired t-test was used for comparisons of two means; a 2-tailed value of P < 0.05 was considered statistically significant. For groups of 2 or more ANOVA was used with post-hoc testing (Prism v5, GraphPad Software).
Additional details about the methods are available in the online supplement.
Results
PPARg cardiomyocytes have increased oxidative stress
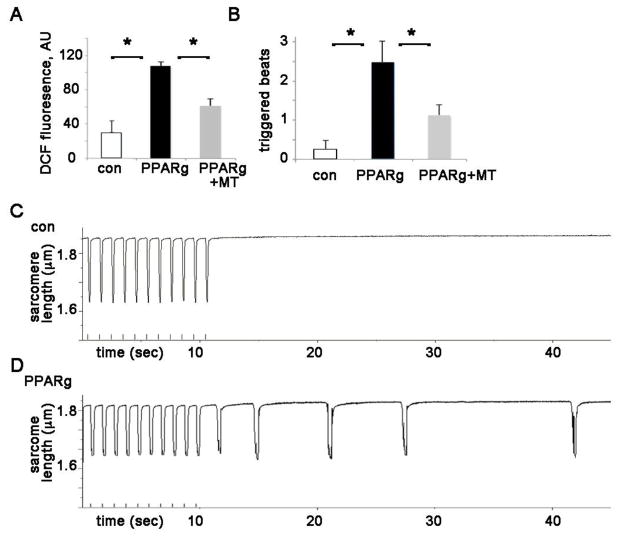
We predicted that cardiac lipid overload would cause increased oxidative stress in cardiomyocytes. To confirm increased cardiac reactive oxygen species (ROS) in our mouse model 11, we used the ROS indicator dihydrodichlorofluorescein diacetate (H2DCF-DA). DCF fluorescence is a widely used as a measure of general oxidative stress in live cells 16. We compared the ROS levels in live adult mouse ventricular myocytes from WT control and PPARg mice. Isolated cardiomyocytes from PPARg mice had significantly increased DCF signal compared to control (figure 1A), indicating that PPARg cardiomyocytes have significantly greater ROS. Mitochondria are a major source of ROS in cardiomyocytes, and we found that the mitochondrial targeted antioxidant mitotempo reduced mitochondrial superoxide in the PPARg cardiomyocytes (figure 1B).
Figure 1. PPARg cardiomyocytes have increased ROS and triggered activity.
A. ROS in isolated cardiomyocytes; height is DCF fluorescence minus background. ROS is reduced by mitotempo in PPARg cardiomyocytes. MT = mitotempo 20 μM. The means are significantly different by ANOVA, *= sig different from control by post-hoc test. Readings were performed in triplicate with 2000 cells/well from 3 animals of each type.
B. Graph of triggered activity from PPARg and control cardiomyocytes. The means are significantly different by ANOVA, *= sig different from control by post-hoc test, n=21–35 cells from 3 animals of each type.
C. Representative example of isolated control cardiomyocyte, paced without subsequent activity
D. Representative example of isolated PPARg cardiomyocyte with triggered activity after pacing.
PPARg cardiomyocytes have increased spontaneous contractions that are dependent on mitochondrial ROS
To determine if the increased ventricular ectopy that we previously reported in PPARg mice could be observed at the level of the individual cardiomyocyte, we evaluated the frequency of spontaneous contractions after a short period of pacing in isolated cardiomyocytes, as described previously 17. After a short period of pacing at 1 Hz, PPARg cardiomyocytes that were not spontaneously contracting at baseline have significantly more contractions (fig 1B,C,D). This pattern is consistent with a triggered activity mechanism of ventricular ectopy. Since we suspected that increased oxidative stress could contribute to the mechanism, we inhibited mitochondrial ROS with the mitochondrial targeted antioxidant mitotempo for one hour. This treatment significantly decreased spontaneous contractions in PPARg cardiomyocytes (figure 1B) demonstrating that mitochondrial ROS promotes spontaneous contractions. We performed additional experiments with WT cardiomyocytes and mitotempo, which show that mitotempo does not have an effect on spontaneous contractions in control cardiomyocytes (sup figure 1).
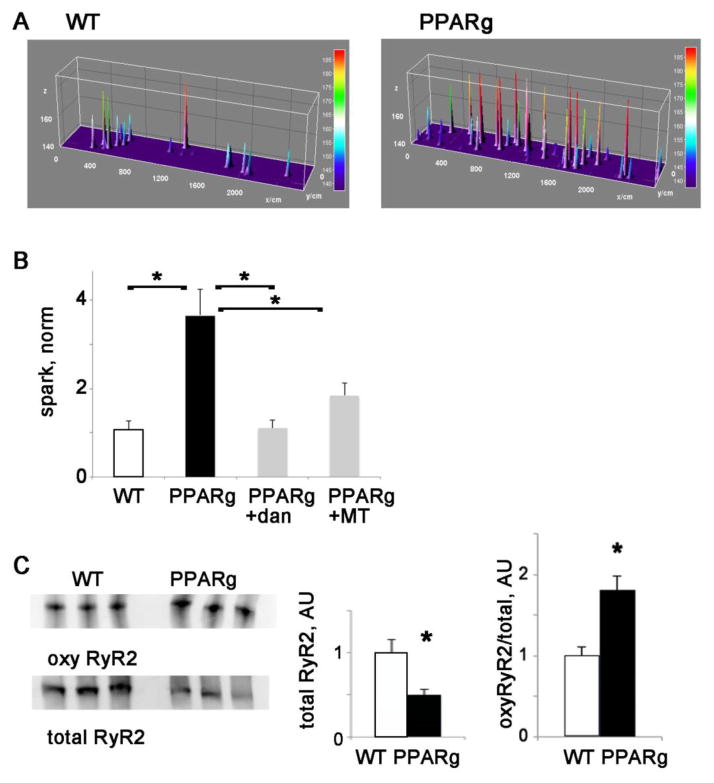
PPARg cardiomyocytes have increased spark frequency and oxidation of RyR2
To directly evaluate SR calcium release in the PPARg heart, we measured spark frequency in isolated PPARg and WT control cardiomyocytes 18, 19. PPARg cardiomyocytes had significantly more sparks than those from control animals (figure 2). This is significantly decreased by dantrolene, an inhibitor of RyR channels. To evaluate the role of mitochondrial ROS, we treated PPARg cardiomyocytes with the antioxidant mitotempo, which decreased sparks significantly in PPARg cardiomyocytes (figure 2B).
Figure 2. PPARg cardiomyocytes have increase calcium sparks.
A. Representative examples of sparks raw data from control and PPARg cardiomyocytes
B. Graph of sparks in WT control and PPARg cardiomyocytes, normalized to control. Dantrolene and mitotempo reduce spark frequency. n=11–20 cells each from 3 animals of each type, the means are significantly different by ANOVA, *= sig different by post-hoc test, dan=dantrolene 1 μM, MT = mitotempo 20 μM
C. Western blots of oxy-RyR2 and total RyR2, and graphs of mean total RYR2 and oxyRyR2 normalized to total RyR2. *= p<0.05
The increase in sparks indicates that PPARg cardiomyocytes have increased spontaneous calcium release. Increased SR calcium leak can be caused by increased phosphorylation or oxidation of ryanodine receptor RyR channels 20–22. Ventricular lysates were used to evaluate RyR2 oxidation status. We found a decrease in total RyR2 protein levels, but a significant increase in oxidation of RyR2 (figure 1E). Phosphorylation of RyR2 was not increased (sup figure 2).
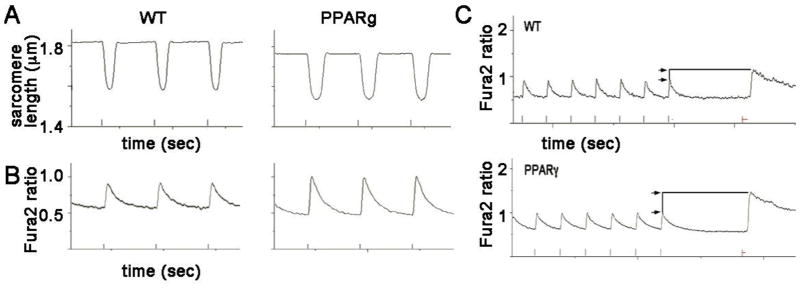
PPARg cardiomyocytes have increased calcium transient amplitude and increased SR calcium
To evaluate contractility and calcium homeostasis in the lipotoxic cardiomyocytes, we measured sarcomere length and calcium transients using isolated cardiomyocytes, paced by field stimulation. Sarcomere length measurements show that relaxation is significantly prolonged in PPARg cardiomyocytes, as measured by the time constant tau of the return-to-baseline curve (figure 3 and table 1). This is consistent with the prolonged action potential duration that we have previously reported in PPARg cardiomyocytes using patch clamp methods 12. Contractility, measured by fractional shortening, is similar in both groups, as is contraction velocity. Overall, isolated cardiomyocytes from young adult PPARg mice have properties consistent with normal systolic function and delayed relaxation.
Figure 3. Abnormal relaxation and calcium transients in PPARg cardiomyocytes.
A. Representative sarcomere length recordings from isolated myocytes from PPARg and WT littermates. Note the slower return to diastolic baseline in the PPARg cell. Marks at bottom show pacing (1 Hz) for time scale.
B. Representative calcium imaging from isolated myocytes from PPARg and WT littermates loaded with fura-2. The tracings show increased amplitude and slower return to baseline in PPARg cardiomyocytes, indicated increased calcium SR release and decreased SERCA2 function.
C. Representative caffeine-evoked transients from WT and PPARg cardiomyocytes. Cells were paced to achieve steady-state calcium levels, and then caffeine (20 mM) was rapidly perfused. The height of the caffeine-induced calcium signal, relative to the paced transient, is greater in the PPARg cell, indicating greater SR calcium content.
Table 1. Contractility and calcium transient data.
For cell shortening, n= 38–40 cells, from 3–4 animals each group. For calcium transients, n= 40–70 cells from 3–4 animals each group.
| mean + SEM | con | PPARg | |
|---|---|---|---|
| Cell Shortening | |||
| diastolic SL | 1.82 + 0.01 | 1.80 + 0.01 | |
| % FS | 11.67 + 0.58 | 12.53 + 0.46 | |
| tau | 0.075 + 0.005 | 0.085+ 0.006 | |
| Ca2+ transient | |||
| diastolic signal | 0.60 + 0.01 | 0.55 + 0.02 | |
| peak height, %baseline | 57.7 + 3.0 | 100.9 + 4.0* | |
| tau (sec) | 0.21 + 0.01 | 0.25 + 0.01* | |
sig different from control by t-test
Calcium transients were visualized in PPARg cardiomyocytes using fura-2. PPARg calcium transients are significantly increased in amplitude (figure 3B and table 1). This is consistent with increased SR calcium content. The application of caffeine, to release sarcoplasmic reticulum stores, shows a greater amount of calcium release from PPARg cells after controlling for baseline transient amplitude (figure 3C). The mean peak caffeine-evoked height normalized to paced transient height is 1.47 AU for WT, 2.12 for PPARg, (p=0.02 by t-test). This indicates a significant increase in sarcoplasmic reticulum (SR) calcium stores in PPARg cardiomyocytes.
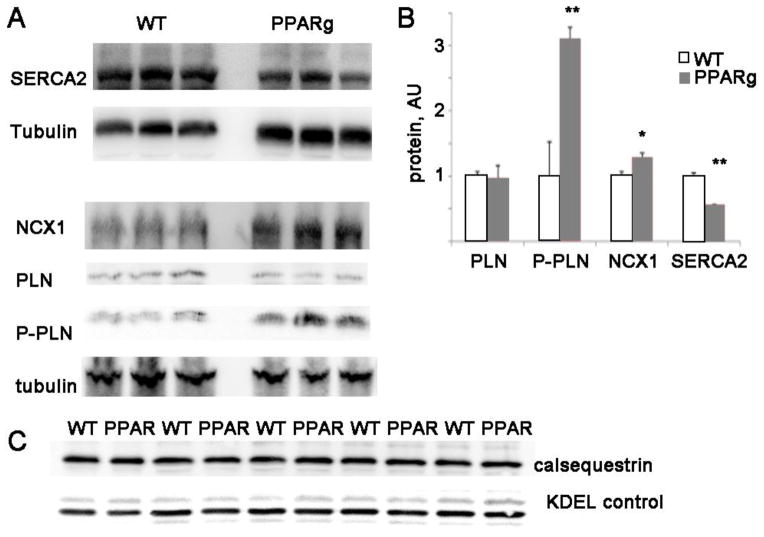
PPARg hearts have abnormal levels of calcium handling proteins
Although an increase in L-type current could cause increased SR calcium loading, our prior work has shown that PPARg cardiomyocytes have normal L-type current densities and current-voltage relationships 12. We have also shown that PPARg cardiomyocytes do not have increased late sodium current 12. To better understand the molecular mechanisms of the observed abnormalities in calcium homeostasis, we evaluated the levels of cardiac proteins that are critical for calcium handling. Since SERCA2 pumps calcium back into the sarcoplasmic reticulum 23, an increase in SERCA could cause an increase in SR calcium load. However, SERCA2 protein is significantly reduced in the PPARg ventricles (figure 4). Phospholamban, which inhibits SERCA2 activity, has similar protein levels to control but phosphorylation was increased which would tend to increase SERCA2 activity, compensating for the decrease in SERCA protein level.
Figure 4. PPARg mice have abnormal levels of calcium handling proteins.
A. Representative immunoblots of SERCA2 NCX1, and PLN proteins
B. Graph of protein levels normalized to control levels after adjusting for tubulin loading, AU= arbitrary units. n=4 animals of each type, t-test: *= p<0.05, **= p<0.01
C. Membrane preparation western blot from PPARg and WT littermate hearts show equivalent amounts of calsequestrin protein. KDEL is a loading control from the same membrane.
The sodium-calcium exchanger NCX1 removes calcium from the cytosol by extruding calcium from the cell. NCX1 protein is increased significantly in the PPARg heart, approximately 30% more than WT littermates. An increase in NCX1 may partially compensate for the increase in calcium entry from the prolonged APD. Increased NCX1 has been described in other forms of cardiomyopathy and may have a role in arrhythmia by depolarizing cardiomyocytes and initiating delayed afterdepolarizations 24, 25. To evaluate another possible cause of SR calcium overload, we isolated SR membranes from mouse hearts and quantified calsequestrin protein, which is the major SR calcium binding protein in cardiomyocytes. Calsequestrin levels are similar in PPARg hearts and those from WT littermates, so this cannot explain the difference in SR calcium content.
Computer modeling supports that LQT can cause SR calcium overload
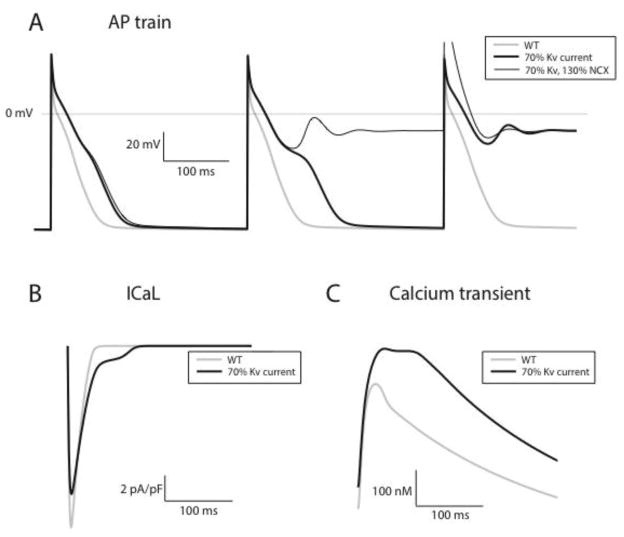
We suspected that the LQT phenotype of the PPARg model could be responsible for the increased intracellular calcium. Computer modeling was performed to investigate the relationship between long QT and SR calcium load. Our model shows that a minimal model of PPARg overexpression, simulated by a 30% reduction of WT Kv current alone, produces early afterdepolarizations during pacing at 200 BPM (Figure 5, panel A). We also explored the impact of concomitant NCX upregulation, by increasing NCX density by 30% in the PPARg model. With this enhanced model, abnormalities in repolarization occur earlier in the drive train, suggesting that NCX serves a modulatory role in producing early afterdepolarizations (panel A, thin black trace).
Figure 5. Computational modeling of rodent myocyte electrophysiology predicts abnormal repolarization and calcium handling in the PPARg mouse.
Panel A shows action potentials generated at 200 BPM for three models: wild type (gray trace), minimal model of PPARg overexpression incorporating only Kv current downregulation (thick black trace), second model incorporating both Kv current downregulation and NCX upregulation (thin black trace). For pacing to steady state, the WT model does not produce EADs. In contrast, for both PPAR gamma models, early afterdepolarizations are observed, with failure of the action potential to repolarize. NCX upregulation modulates the generation of EADs, as failure to repolarize occurs earlier in the pacing drive train. Panels B and C show the L type current and the calcium transient for the beat immediately preceding EAD formation, for the PPAR gamma minimal model (thick black trace) and corresponding WT action potential (gray trace). Substantially higher plateau inward current is noted (panel B), with larger calcium transients (panel C).
Studying the beat immediately preceding afterdepolarization formation reveals that in PPARg cells, a significant increase in the plateau influx of calcium through L-type channels occurs (figure 5, panel B). This in turn causes an increase in cytoplasmic calcium (panel C) and ultimately early afterdepolarizations. This supports the hypothesis that a decrease in Kv current on the order of what we have observed experimentally can substantially prolong APD, as well as produce changes in calcium cycling and repolarization that are potentially pro-arrhythmic.
Mitochondrial anti-oxidant prevents ventricular ectopy in vivo
We hypothesized that the SR calcium overload contributed to arrhythmia in vivo. To test this, we implanted PPARg mice with heart rhythm telemetry monitors, allowing long-term heart rhythm recordings in unrestrained, non-sedated animals. We treated the mice with mitotempo. Mice were treated for 2 days during telemetry monitoring. Mitotempo resulted in a decrease in PVC frequency (figure 6). Mitotempo treatment did not change the QT (sup table 1). This in vivo telemetry experiment indicates that decreasing mitochondrial superoxide rapidly resets calcium handling to a less arrhythmogenic state.
Figure 6. Mitotempo improves heart rhythm in PPARg mice.
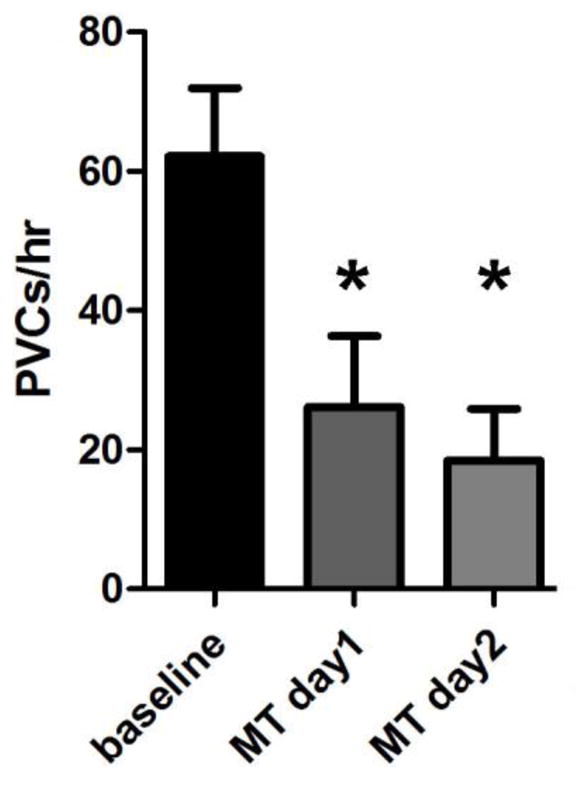
Mean PVC count from PPARg mice, at baseline and during mitotempo treatment. N = 4 animals. The means are significantly different by ANOVA, *= sig different by post-hoc test.
Discussion
Since abnormalities of intracellular calcium homeostasis are a common feature of many forms of cardiomyopathy and arrhythmia, we hypothesized that the pathophysiology of ventricular ectopy in lipotoxic hearts would be caused by perturbations of calcium handling. We demonstrate that lipotoxic cardiomyocytes have increased ROS and abnormal calcium homeostasis, manifest by increased spark frequency and SR calcium overload. We also show that cardiac lipotoxicity causes increased spontaneous contractions, which are improved by inhibition of mitochondrial ROS. These cellular abnormalities promote ventricular ectopy, which is improved in vivo by treatment with the mitochondrial anti-oxidant mitotempo.
Calcium handling and ROS
We show that sparks and spontaneous contractions are improved with inhibition of mitochondrial ROS, identifying mitochondrial ROS is a mechanistic link between lipotoxicity and abnormal calcium homeostasis. Prior work had proposed that ROS increases SR calcium leak in isolated cardiomyocytes, using cells from aged animals (which have increased ROS) or using pharmacologic manipulations to increase ROS 26, 27. Our work shows that this mechanism is important for cardiac lipid overload. Furthermore, we show that the mechanism of increased RyR2 calcium leak is oxidation of the RyR2 channel rather than increased phosphorylation.
SR calcium and LQT
The abnormalities identified in this study are in contrast to those using systolic heart failure models, which typically have decreased SR calcium content and lower amplitude calcium transients 28. Paradoxically, despite the increase in SR calcium leak, we show a significant increase in SR calcium in the transgenic model. Young adult PPARg mice represent an early form of cardiomyopathy, with compensated systolic function. We had previously shown that PPARg cardiomyocytes have prolonged APD due to decreased voltage-gated potassium channel currents, with normal L-type current densities and current-voltage relationships 12. It is likely that the LQT phenotype contributes to increased SR calcium. Our minimal computational model demonstrates that when the reduction in potassium current density is simulated in isolation, the consequent APD prolongation and corresponding increase in calcium influx during the action potential can increase SR load and calcium transient amplitudes, providing an explanation for pathologic calcium overload. There is precedent for this model. Prior work by others has shown than prolongation of the APD can cause increase SR calcium load 29. LQT may promote ventricular tachycardia by increasing calcium entry during the prolonged repolarization phase; prolongation of the action potential duration increases calcium entry into the cardiomyocyte cytosol 30. Prolonged QT is an independent risk factor for cardiovascular mortality in heart failure patients 31 and also in diabetics without heart failure 32. Obese humans have prolonged QT compared to normal-weight controls, highlighting the potential clinical relevance of our findings 1.
The increase in NCX1 may contribute to ventricular ectopy. Although SERCA2 function is the dominant mechanism for calcium extrusion in healthy mouse myocytes, during calcium overload, an increase in NCX1 could be protective by extruding excess calcium. However, during spontaneous calcium release events, a larger NCX current can theoretically promote arrhythmias by producing the depolarizing transient inward current that is postulated to be the ionic basis of delayed afterdepolarizations 24, 33, 34. Our findings of altered calcium cycling with PPARg overexpression are broadly consistent with prior experiments using Kv current blockade in a canine preparation 35.
Ventricular Ectopy in Lipotoxic Cardiomyopathy
There is prior evidence that ventricular tachycardia in non-ischemic cardiomyopathy is caused by triggered activity rather than reentry, which is the major mechanism in scar-related ventricular tachycardia (such as after myocardial infarction) 36. The increase in spontaneous contractions in isolated cardiomyocytes from PPARg mice observed in this study is consistent with triggered activity. The increase in ventricular ectopy correlates with an increase in single-cell spontaneous contractions and increased spark frequency. In combination with increased spark frequency, the increase in calcium transient amplitude may contribute to ventricular tachycardia in the PPARg mice. The improvement in heart rhythm during short-term treatment with mitotempo supports the idea that mitochondrial ROS promotes ventricular ectopy during lipotoxicity. Identifying the molecular mechanisms causing electrophysiologic abnormalities and arrhythmias in lipotoxic cardiomyopathy may allow for targeted therapy to prevent arrhythmia and sudden cardiac death in obese and diabetic patients.
The emerging connections between cardiac metabolism, circadian rhythm, and heart rhythm are intriguing 37, 38. While this is beyond the scope of the present manuscript, the PPARg mouse model could be a useful tool for future investigation in this area.
Conclusions
During cardiac lipid overload, mitochondrial oxidative stress causes increased SR calcium leak by oxidizing RyR2 channels. This promotes ventricular ectopy, and heart rhythm is improved in vivo by a mitochondrial-targeted anti-oxidant. These results suggest a potential role for mitochondrial-targeted anti-oxidants to prevent arrhythmia and sudden cardiac death in obese and diabetic patients.
Supplementary Material
Acknowledgments
Sources of Funding: This work was supported by K08 HL105801 (JPM), K08 HL116790 (VI), and 1R01HL121253 (HMC).
Abbreviations
- APD
action potential duration
- DCF
dichlorofluorescein
- NCX
sodium calcium exchanger
- PPARg
peroxisome proliferator-activated receptor gamma
- PVC
premature ventricular complex
- ROS
reactive oxygen species
- RyR
ryanodine receptor channels
- SR
sarcoplasmic reticulum
- WT
wild-type
Footnotes
COI: none
Disclosures: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramirez AH, Schildcrout JS, Blakemore DL, Masys DR, Pulley JM, Basford MA, Roden DM, Denny JC. Modulators of normal electrocardiographic intervals identified in a large electronic medical record. Heart Rhythm. 2011 Feb;8:271–277. doi: 10.1016/j.hrthm.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messerli FH, Nunez BD, Ventura HO, Snyder DW. Overweight and sudden death. Increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med. 1987 Oct;147:1725–1728. doi: 10.1001/archinte.147.10.1725. [DOI] [PubMed] [Google Scholar]
- 3.Zemva A, Zemva Z. Ventricular ectopic activity, left ventricular mass, hyperinsulinemia, and intracellular magnesium in normotensive patients with obesity. Angiology. 2000 Feb;51:101–106. doi: 10.1177/000331970005100202. [DOI] [PubMed] [Google Scholar]
- 4.Filippi A, Sessa E, Jr, Mazzaglia G, Pecchioli S, Jr, Capocchi R, Jr, Caprari F, Scivales A, Cricelli C. Out of hospital sudden cardiac death in Italy: a population-based case-control study. J Cardiovasc Med (Hagerstown) 2008 Jun;9:595–600. doi: 10.2459/JCM.0b013e3282f2c9d0. [DOI] [PubMed] [Google Scholar]
- 5.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003 Apr 29;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 6.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999 Apr 20;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 7.Hookana E, Junttila MJ, Puurunen VP, Tikkanen JT, Kaikkonen KS, Kortelainen ML, Myerburg RJ, Huikuri HV. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011 Oct;8:1570–1575. doi: 10.1016/j.hrthm.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 8.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007 Oct 12;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 9.Marfella R, Di Filippo C, Portoghese M, Barbieri M, Ferraraccio F, Siniscalchi M, Cacciapuoti F, Rossi F, D’Amico M, Paolisso G. Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res. 2009 Nov;50:2314–2323. doi: 10.1194/jlr.P900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007 Oct;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Son NH, Yu S, Tuinei J, Arai K, Hamai H, Homma S, Shulman GI, Abel ED, Goldberg IJ. PPARgamma-induced cardiolipotoxicity in mice is ameliorated by PPARalpha deficiency despite increases in fatty acid oxidation. J Clin Invest. 2010 Oct;120:3443–3454. doi: 10.1172/JCI40905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrow JP, Katchman A, Son NH, et al. Mice With Cardiac Overexpression of Peroxisome Proliferator-Activated Receptor gamma Have Impaired Repolarization and Spontaneous Fatal Ventricular Arrhythmias. Circulation. 2011 Dec 20;124:2812–2821. doi: 10.1161/CIRCULATIONAHA.111.056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandit SV, Clark RB, Giles WR, Demir SS. A mathematical model of action potential heterogeneity in adult rat left ventricular myocytes. Biophysical journal. 2001 Dec;81:3029–3051. doi: 10.1016/S0006-3495(01)75943-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clancy CE, Rudy Y. Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999 Aug 5;400:566–569. doi: 10.1038/23034. [DOI] [PubMed] [Google Scholar]
- 15.Iyer V, Hajjar RJ, Armoundas AA. Mechanisms of abnormal calcium homeostasis in mutations responsible for catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2007 Feb 2;100:e22–31. doi: 10.1161/01.RES.0000258468.31815.42. [DOI] [PubMed] [Google Scholar]
- 16.Eruslanov E, Kusmartsev S. Identification of ROS using oxidized DCFDA and flow-cytometry. Methods Mol Biol. 2010;594:57–72. doi: 10.1007/978-1-60761-411-1_4. [DOI] [PubMed] [Google Scholar]
- 17.Respress JL, van Oort RJ, Li N, et al. Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res. 2012 May 25;110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Denegri M, Ruan Y, Avelino-Cruz JE, Perissi A, Negri S, Napolitano C, Coetzee WA, Boyden PA, Priori SG. Short communication: flecainide exerts an antiarrhythmic effect in a mouse model of catecholaminergic polymorphic ventricular tachycardia by increasing the threshold for triggered activity. Circ Res. 2011 Jul 22;109:291–295. doi: 10.1161/CIRCRESAHA.111.247338. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez-Velasco M, Rueda A, Rizzi N, Benitah JP, Colombi B, Napolitano C, Priori SG, Richard S, Gomez AM. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2009 Jan 30;104:201–209. 212. doi: 10.1161/CIRCRESAHA.108.177493. following 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011 Aug 3;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terentyev D, Gyorke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Gyorke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008 Dec 5;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, Wilson LD, Cardounel AJ, Laurita KR, Carnes CA, Billman GE, Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009 Dec 1;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kho C, Lee A, Hajjar RJ. Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy. Nat Rev Cardiol. 2012 Dec;9:717–733. doi: 10.1038/nrcardio.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012 May 1;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reppel M, Fleischmann BK, Reuter H, Pillekamp F, Schunkert H, Hescheler J. Regulation of Na+/Ca2+ exchange current in the normal and failing heart. Ann N Y Acad Sci. 2007 Mar;1099:361–372. doi: 10.1196/annals.1387.065. [DOI] [PubMed] [Google Scholar]
- 26.Yan Y, Liu J, Wei C, Li K, Xie W, Wang Y, Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc Res. 2008 Jan 15;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 27.Cooper LL, Li W, Lu Y, Centracchio J, Terentyeva R, Koren G, Terentyev D. Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. J Physiol. 2013 Dec 1;591:5895–5911. doi: 10.1113/jphysiol.2013.260521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo M, Anderson ME. Mechanisms of altered Ca(2)(+) handling in heart failure. Circ Res. 2013 Aug 30;113:690–708. doi: 10.1161/CIRCRESAHA.113.301651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sah R, Ramirez RJ, Kaprielian R, Backx PH. Alterations in action potential profile enhance excitation-contraction coupling in rat cardiac myocytes. J Physiol. 2001 May 15;533:201–214. doi: 10.1111/j.1469-7793.2001.0201b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sah R, Oudit GY, Nguyen TT, Lim HW, Wickenden AD, Wilson GJ, Molkentin JD, Backx PH. Inhibition of calcineurin and sarcolemmal Ca2+ influx protects cardiac morphology and ventricular function in K(v)4. 2N transgenic mice. Circulation. 2002 Apr 16;105:1850–1856. doi: 10.1161/01.cir.0000014211.47830.4d. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe E, Arakawa T, Uchiyama T, Tong M, Yasui K, Takeuchi H, Terasawa T, Kodama I, Hishida H. Prognostic significance of circadian variability of RR and QT intervals and QT dynamicity in patients with chronic heart failure. Heart Rhythm. 2007 Aug;4:999–1005. doi: 10.1016/j.hrthm.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Okin PM, Devereux RB, Lee ET, Galloway JM, Howard BV. Electrocardiographic repolarization complexity and abnormality predict all-cause and cardiovascular mortality in diabetes: the strong heart study. Diabetes. 2004 Feb;53:434–440. doi: 10.2337/diabetes.53.2.434. [DOI] [PubMed] [Google Scholar]
- 33.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001 Jun 8;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 34.Verkerk AO, Veldkamp MW, Baartscheer A, Schumacher CA, Klopping C, van Ginneken AC, Ravesloot JH. Ionic mechanism of delayed afterdepolarizations in ventricular cells isolated from human end-stage failing hearts. Circulation. 2001 Nov 27;104:2728–2733. doi: 10.1161/hc4701.099577. [DOI] [PubMed] [Google Scholar]
- 35.Schotten U, de Haan S, Verheule S, Harks EG, Frechen D, Bodewig E, Greiser M, Ram R, Maessen J, Kelm M, Allessie M, Van Wagoner DR. Blockade of atrial-specific K+-currents increases atrial but not ventricular contractility by enhancing reverse mode Na+/Ca2+-exchange. Cardiovasc Res. 2007 Jan 1;73:37–47. doi: 10.1016/j.cardiores.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 36.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995 Aug 15;92:1034–1048. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 37.Jeyaraj D, Haldar SM, Wan X, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012 Mar 1;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckle T, Hartmann K, Bonney S, et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012 May;18:774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.