Abstract
Purpose
To identify the risk factors for progression to renal replacement therapy (RRT) and all-cause mortality in patients who underwent renal artery (RA) stent placement for atherosclerotic renal artery stenosis (RAS).
Materials and Methods
A retrospective study was performed from June 1996 to June 2009 that identified 1052 patients that underwent RA stent placement. The glomerular filtration rate at the time of RA stent placement was estimated from the serum creatinine level and divided into renal disease stages 1–5. Univariate and multivariable Cox proportional hazards models were used to determine which factors were associated with each endpoint.
Results
The times to progression to all-cause mortality and RRT were similar for chronic kidney (CKD) stages 1/2/3A and served as the reference group. In multivariable analysis, high-grade proteinuria (P<.001), higher CKD stage [stage 5 vs. 1-3A, (P<.001)], stage 4 vs. 1-3A, (P<.001), stage 3B vs. 1-3A, (P=.02)] remained independently associated with increased risk of progression to RRT. Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEi/ARB) use was associated with decreased risk of progression to RRT (P=0.03). Higher CKD stage [stage 5 vs. 1-3A, (P<.001), stage 4 vs. 1-3A (P=.004), carotid artery disease (P<.001), diabetes mellitus (P=.002), and high-grade proteinuria (P<.001) remained independently associated with all-cause mortality. Statin use was associated with decreased risk of all-cause mortality (P< 0.001).
Conclusion
Based on this analysis, patients with atherosclerotic RAS who undergo RA stenting who have high-grade proteinuria and CKD stage 3B, 4 or 5 have an increased risk of progression to RRT. Patients with high-grade proteinuria, CKD stage 3B, 4 or 5, carotid disease or diabetes have an increased risk for all-cause mortality after renal artery stenting. ACEi/ARB use in this patient population has a decreased risk of progression to RRT and patients on statins have a decreased risk of all-cause mortality.
Keywords: RAS, stents, hypertension, chronic kidney disease
Introduction
Atherosclerotic renal artery stenosis (RAS) is a recognized contributor to uncontrolled hypertension and/or worsening of renal function [1, 2]. Restoring blood flow with either surgical or endovascular revascularization can improve management of refractory renovascular hypertension and can sometimes salvage renal function [3–6]. However, identifying patients likely to benefit from endovascular intervention remains a major challenge. In recent years, prospective, randomized controlled trials have failed to demonstrate a major additional benefit for patients who have been treated with angioplasty and/or stent placement versus medical therapy for the treatment of RAS [7–11]. Several weaknesses have been identified with these trials including the small number of patients, cross over, selection bias, and potential of under powering of the trials [8, 12, 13]. Observational studies and results of registries suggest that technically successful revascularization confers a substantial survival benefit to some "high risk" patient subsets [5, 6, 14–20]. Because this population often includes older subjects with the presence of extensive comorbid cardiovascular disease, identifying predictors for all-cause mortality and/or progression to renal replacement therapy (RRT with hemodialysis, peritoneal dialysis, or kidney transplantation) is an important goal in planning therapy.
The purpose of the current study was to evaluate the factors that contribute to progression to RRT and all-cause mortality in patients with chronic kidney disease (CKD) with atherosclerotic renal artery stenosis who have undergone renal artery stent placement using the resources of the United States Renal Data System (USRDS) and the Social Security Death Index. It is hypothesized that patients with advanced chronic renal disease (CKD stage 3B or greater) would have worse outcomes as compared to stage 1, 2, and 3A. This information is important for interpreting completed and ongoing clinical trials, designing future clinical trials, and for clinical management of patients with comorbid risks to better determine which patients may benefit from renal artery stent placement.
Methods and Materials
Institutional review board approval was obtained prior to conducting this retrospective study. All patients undergoing stent placement for the treatment of atherosclerotic renal artery stenosis for either hypertension or worsening renal function from June 1996 to June 2009 were included in the data set. Demographic data, comorbidity data, pre-procedural and post-procedural laboratory data, and catheter-related angiographic data through October 2011 were obtained from medical records. Embolic protection devices were not used on any of the patients in this study. A total of 1222 unique patients were treated for renovascular disease. Patients were excluded if they had concomitant fibromuscular dysplasia (FMD) and/or were treated for non-atherosclerotic renal artery disease. The study group comprised of 1052 patients after applying the exclusion criteria. Of these patients, 526 were female (50%) with a total mean age of 72.4 ± 9.2 years (range: 42–93). The median follow-up period was 3.8 years (range: 0–10.79 years, mean = 2.2 ± 2.0). The patient demographics are displayed in Table 1.
Table 1.
Patient characteristics
| CKD STAGE | 1/2/3a | 3b | 4 | 5 | Total |
|---|---|---|---|---|---|
| Age, mean (SD) | |||||
| Number | 445 | 339 | 220 | 48 | 1052 |
| Age | 70.2 (10.1) | 73.9 (7.8) | 74.4 (7.9) | 72.9 (10.3) | 72.4 (9.2) |
| Follow-up Time | |||||
| Number | 297 | 177 | 79 | 13 | 566 |
| Median (years) | 3.0 | 4.1 | 6.1 | 7.8 | 3.8 |
| Female | 231 (51.9%) | 176 (51.9%) | 100 (45.5 %) | 19 (39.6%) | 526 (50.0%) |
| Male | 214 (48.1%) | 163 (48.1%) | 120 (54.5 %) | 29 (60.4%) | 526 (50.0%) |
| GFR at Baseline | |||||
| Number | 445 | 339 | 220 | 48 | 1052 |
| GFR at baseline | 58.9 (12.6) | 37.2 (4.2) | 23.5 (4.2) | 12.0 (2.0) | 42.4 (17.8) |
| Laterality | |||||
| Missing | 67 (15.1%) | 28 (8.3%) | 2 (0.9%) | 3 (6.3%) | 100 (9.5%) |
| Unilateral | 248 (55.7%) | 184 (54.3%) | 103 (46.8 %) | 27 (56.3%) | 562 (53.4%) |
| Bilateral | 130 (29.2%) | 127 (37.5%) | 115 (52.3 %) | 18 (37.5%) | 390 (37.1%) |
| Peak RI (SD) | 0.7 (0.1) | 0.8 (0.7) | 0.8 (0.1) | 0.8 (0.1) | 0.8 (0.4) |
| Proteinuria | |||||
| Missing | 90 (20.2%) | 64 (18.9%) | 47 (21.4% ) | 9 (18.8%) | 210 (20.0%) |
| Low (AER<300) | 285 (64.0%) | 196 (57.8%) | 91 (41.4% ) | 3 (6.3%) | 575 (54.7%) |
| High (AER>300) | 70 (15.7%) | 79 (23.3%) | 82 (37.3% ) | 36 (75.0%) | 267 (25.4%) |
|
Baseline Blood Pressure Medicati ons |
|||||
| Number | 357 | 317 | 212 | 45 | 931 |
| Mean (SD) | 2.7 (1.4) | 2.8 (1.2) | 3.3 (1.2) | 2.9 (1.3) | 2.9 (1.3) |
| Baseline DBP | |||||
| Number | 290 | 231 | 168 | 38 | 727 |
| Mean (SD) | 78.3 (14.5) | 76.4 (15.2) | 74.4 (14.3 ) | 78.9 (14.4) | 76.8 (14.8) |
| Baseline SBP | |||||
| Number | 291 | 231 | 168 | 38 | 728 |
| Mean (SD) | 157.4 (28.4) | 156.6 (29.2) | 152.7 (27. 7) | 162.0 (26.8 ) | 156.3 (28.5) |
| Smoking Status | |||||
| Missing | 35 (7.9%) | 56 (16.5%) | 53 (24.1% ) | 14 (29.2%) | 158 (15.0%) |
| Never | 4 (0.9%) | 6 (1.8%) | 4 (1.8%) | 1 (2.1%) | 15 (1.4%) |
| Quit | 342 (76.9%) | 209 (61.7%) | 103 (46.8 %) | 19 (39.6%) | 673 (64.0%) |
| Current | 64 (14.4%) | 68 (20.1%) | 60 (27.3% ) | 14 (29.2%) | 206 (19.6%) |
| PAD | |||||
| Missing | 12 (2.7%) | 7 (2.1%) | 1 (0.5%) | 0 (0.0%) | 20 (1.9%) |
| No | 167 (37.5%) | 129 (38.1%) | 95 (43.2% ) | 25 (52.1%) | 416 (39.5%) |
| Yes | 266 (59.8%) | 203 (59.9%) | 124 (56.4 %) | 23 (47.9%) | 616 (58.6%) |
| Carotid Disease | |||||
| Missing | 9 (2.0%) | 2 (0.6%) | 0 (0.0%) | 0 (0.0%) | 11 (1.0%) |
| No | 278 (62.5%) | 178 (52.5%) | 127 (57.7 %) | 31 (64.6%) | 614 (58.4%) |
| Yes | 158 (35.5%) | 159 (46.9%) | 93 (42.3% ) | 17 (35.4%) | 427 (40.6%) |
| Diabetes | |||||
| Missing | 5 (1.1%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) | 6 (0.6%) |
| No | 322 (72.4%) | 228 (67.3%) | 136 (61.8%) | 37 (77.1%) | 723 (68.7%) |
| Yes | 118 (26.5%) | 110 (32.4%) | 84 (38.2% ) | 11 (22.9%) | 323 (30.7%) |
|
Coronary arterial disease |
|||||
| Missing | 16 (3.6%) | 3 (0.9%) | 0 (0.0%) | 0 (0.0%) | 19 (1.8%) |
| No | 177 (39.8%) | 123 (36.3%) | 75 (34.1% ) | 13 (27.1%) | 388 (36.9%) |
| Yes | 252 (56.6%) | 213 (62.8%) | 145 (65.9 %) | 35 (72.9%) | 645 (61.3%) |
| Stroke | |||||
| Missing | 9 (2.0%) | 2 (0.6%) | 0 (0.0%) | 0 (0.0%) | 11 (1.0%) |
| No | 353 (79.3%) | 266 (78.5%) | 163 (74.1 %) | 40 (83.3%) | 822 (78.1%) |
| Yes | 83 (18.7%) | 71 (20.9%) | 57 (25.9% ) | 8 (16.7%) | 219 (20.8%) |
| Hypertension | |||||
| Missing | 4 (0.9%) | 3 (0.9%) | 2 (0.9%) | 0 (0.0%) | 9 (0.9%) |
| No | 16 (3.6%) | 4 (1.2%) | 6 (2.7%) | 5 (10.4%) | 31 (2.9%) |
| Yes | 425 (95.5%) | 332 (97.9%) | 212 (96.4 %) | 43 (89.6%) | 1012 (96.2%) |
| Hyperlipidemia | |||||
| Missing | 8 (1.8%) | 2 (0.6%) | 1 (0.5%) | 0 (0.0%) | 11 (1.0%) |
| No | 102 (22.9%) | 57 (16.8%) | 46 (20.9% ) | 14 (29.2%) | 219 (20.8%) |
| Yes | 335 (75.3%) | 280 (82.6%) | 173 (78.6 %) | 34 (70.8%) | 822 (78.1%) |
| Primary endpoint | |||||
| Dialysis | 5 (1.1%) | 25 (7.4%) | 53 (24.1% ) | 22 (45.8%) | 105 (10.0%) |
| Transplant | 0 (0.0%) | 0 (0.0%) | 4 (1.8%) | 2 (4.2%) | 6 (0.6%) |
| Death | 145 (32.6%) | 140 (41.3%) | 96 (43.6% ) | 16 (33.3%) | 397 (37.7%) |
| Censor | 295 (66.3%) | 174 (51.3%) | 67 (30.5%) | 8 (16.7%) | 544 (51.7%) |
|
Status at Last Follow-up |
|||||
| Alive | 297 (66.7%) | 177 (52.2%) | 79 (35.9% ) | 13 (27.1%) | 566 (53.8%) |
| Dead | 148 (33.3%) | 162 (47.8%) | 141 (64.1 %) | 35 (72.9%) | 486 (46.2%) |
The primary endpoint was the progression to RRT. The secondary endpoint was all-cause mortality. The information on the need for RRT was obtained by querying the United States Renal Data System (USRDS) in October 2011. All-cause mortality data were obtained in August 2011 by querying the death data present in the United States Social Security Death Index (SSDI) and the health system medical records. RRT was defined as needing hemodialysis, peritoneal dialysis, or renal transplantation at anytime after stent placement or undergoing renal transplantation.
Definitions
Glomerular filtration rate (GFR) was estimated using the serum creatinine level prior to the stent procedure using the MDRD (“modification of diet in renal disease”) formula: estimated GFR (eGFR)= 186 X (Serum creatinine)−1.54 X Age− 0.203 X 0.742 (Females) X 1.210 (African Americans) [21]. Baseline creatinine data were collected and analyzed based on the latest creatinine within 30 days before the procedure. Each patient’s CKD stage was based on his or her GFR at the time of the first RAS procedure. Patients were stratified into stages of renal disease based on the Kidney Dialysis Outcomes Quality Initiative (KDOQI) classification. Stage 1 was defined as a GFR greater 90 mL/min/m2; stage 2 was defined as GFR between 60–89 mL/min/m2; stage 3A was defined as GFR between 45–59 mL/min/m2; stage 3B was defined as GFR as 30–44 mL/min/m2; stage 4 was defined as GFR between 15–29 mL/min/m2; and stage 5 was defined as GFR less than 15 mL/min/m2. Prediction of the 24 hour urinary protein excretion rate was obtained from a randomly collected urine specimen prior to the renal artery intervention and made using the following formula: 24 hr predicted proteinuria (gm/L) per 1.73 m2 body surface area= (Urine [protein] X 0.088) ÷ Urine [creatinine] [22]. This was determined by the last available urine sample in the patient chart prior to intervention obtained within 30 days of the procedure date. High-grade proteinuria was defined as >300 mg/24 hour. Low-grade proteinuria was defined as ≤ 300 mg/24 hour.
Preliminary Kaplan-Meier plots of cumulative survival revealed that patients with stages 1, 2, and 3A had very similar risk of all-cause mortality and progression to RRT. For this reason, these stages were combined in the analyses. As this study was retrospective, all available data were used for each analysis. The sample size for each model was different for this reason and is included in the summary tables.
Refractory hypertension was defined as uncontrolled blood pressure despite the patient being on three anti-hypertensive medications including diuretic therapy, a systolic pressure greater than 140 mm Hg or diastolic pressure greater than 90 mm Hg [23]. Anti-anginal medications other than β-blockers were not defined as blood pressure medications for the purposes of this study. Patients without refractory hypertension were not excluded from this study as an indication for renal artery stent placement because in this population, renal artery stent placement was performed for preserving kidney function.
Medication usage at the time of stent placement was included in the analysis as well. Along with baseline hypertensive medications, usage of statins, angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARB), calcium channel blockers, beta blockers, aspirin, clopidogrel, warfarin, and insulin were recorded. Given similar mechanisms of ACEi and ARB, for the purpose of the analysis, their usage was combined. Specific medication data was only available for analysis in 951 of the 1052 patients.
Renal artery stent placement
A description of the interventional procedure has been detailed elsewhere [24]. Three hundred and ninety patients (37.1%) had bilateral stents placed and 562 patients (53.4%) had unilateral stents placed.
Statistical Methods
SAS version 9.3 software (SAS Institute Inc., Cary, North Carolina, USA) was used for the statistical analyses. In data summaries, categorical variables were expressed as percentages and continuous variables were expressed as means ± standard deviation (SD) or medians (range) as appropriate. The primary endpoint and secondary endpoint were analyzed in two stages. First, univariate Cox-proportional hazards models were used to estimate the hazard ratios (HR) associated with pre-interventional clinical and angiographic variables [proteinuria, current smoking status, peripheral artery disease, carotid disease, diabetes, coronary artery disease (CAD), family history of CAD, stroke, cerebrovascular disease, hypertension, hyperlipidemia, medication usage, unilateral versus bilateral RAS treatment, and baseline CKD stage]. Next, variables with information present in at least 95% of patients, i.e. greater than or equal to 1000 patients and also the variable presence of high-grade proteinuria (>300 mg/24hour) were entered into a backward multivariable selection method (for each of the endpoints). Due to a lack of pattern with the missing data, the inclusion of variables with less than N=1000 observations led to lower sample sizes in the multivariable model building process. Proteinuria was included despite being missing in 20% of patients (N=842 out of 1052) because it was felt to be an important marker of renal disease. Given that only 951 patients had specific medication data, a separate multivariate analysis was performed only using these 951 patients. Beginning point for the analysis (time = 0) was the time of stent placement for each patient. A P-value < .05 was used to denote statistical significance. Kaplan-Meier plots versus CKD stage were used to supplement these analyses for each of the endpoints.
Results
Hypertension and hypertensive medications
The mean systolic blood pressure at baseline was 156 ± 29 mm Hg (range 90–225, median = 154) and the mean diastolic blood pressure was 76.8 ± 14.8 mm Hg (range 40–187, median = 77) while the mean number of blood pressure medications was 2.9 ± 1.3 (range 0–8, median =3, Table 1). The mean systolic blood pressure decreased by 17.6 mm Hg when compared from baseline to the last follow-up [95% CI (−20.9 to −14.2), P<.001] and the mean diastolic blood pressure decreased 7.9 mm Hg as well [95% CI (−9.8 to −6), P<.001]. The number of blood pressure medications decreased by .18 [95% CI (−.27 to −.09), P<.001].
Baseline renal disease characteristics
The distribution of baseline CKD Stages was: stages 1-3A (445 patients, 42.3%), stage 3B (339 patients, 32.3%), stage 4 (220 patients, 20.9%), and stage 5 (48 patients, 4.6%). Two hundred and sixty-seven patients had high-grade proteinuria (25.4%) and 575 had low-grade proteinuria (54.7%). During the follow-up period, 105 patients progressed to requiring hemodialysis while 6 patients underwent renal transplantation. When evaluating the cohort of patients that progressed to requiring hemodialysis based on baseline CKD stage, there were five patients within the CKD Stage 1-3A group, 25 patients in Stage 3B, 53 patients in Stage 4, and 22 in stage 5.
Predictors of progression to renal replacement therapy alone
Univariate and multivariable analyses were used to evaluate what risk factors may be associated with risk for progression to RRT. In the univariate analysis using CKD stage 1/2/3A as the reference group, CKD stage 3B, [HR=6.2, 95% CI (2.4–16.2), P<.001], CKD stage 4 [HR=25, 95% CI (10–62.4), P<.001], and CKD stage 5 [HR=84.3, 95% CI (32–222), P<.001] were associated with progression to RRT. Other factors included baseline high-grade proteinuria [HR=8.0, 95% CI (5–13), P<0.001]; current smoker [HR=2.8, 95% CI (1.8–4.4), P<0.001]; and bilateral renal artery stenosis [HR=1.5, 95% CI (1.0–2.1), P=.04]. Patients on calcium channel blockers had a significantly increased risk for progression to RRT [HR=1.99, 95% CI (1.03–3.84), P=.04].
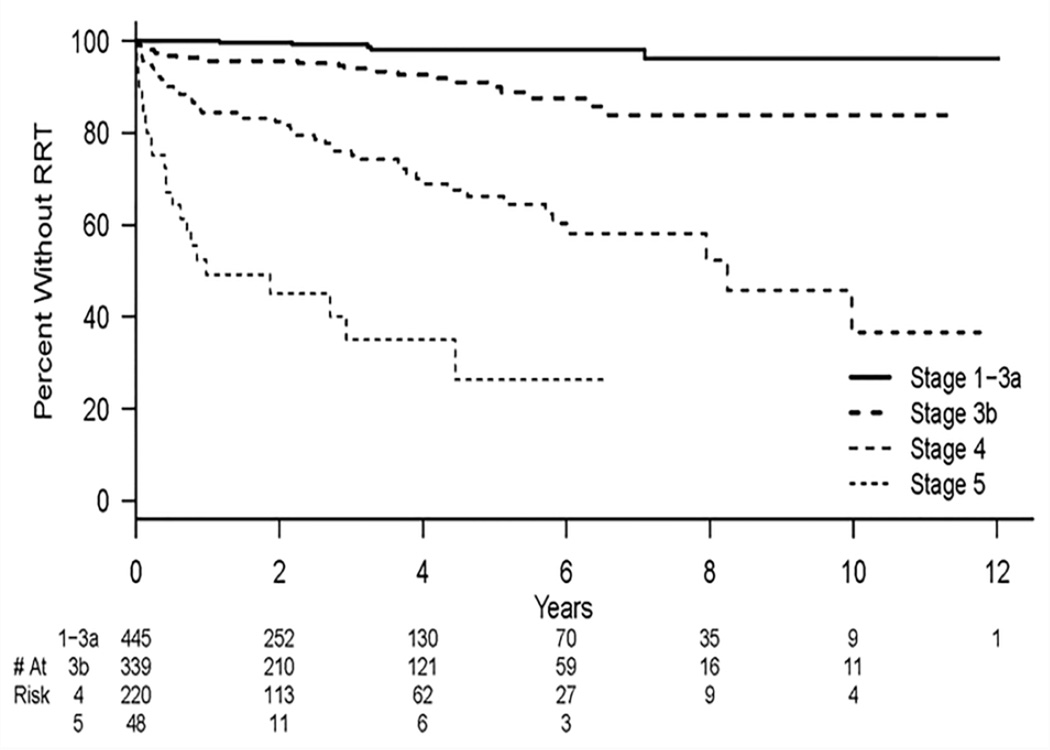
In multivariable analysis, CKD stages 1/2/3A as the reference group, CKD stage 3B [HR=3.4, 95% CI (1.3–9.4), P<.02], CKD stage 4 [HR=14.4, 95% CI (5.6–36.7), P<.001], and CKD stage 5 [HR 33.9, 95% CI (12.3–93.7), P<.001] remained independently associated with progression to RRT. Figure 1 shows a Kaplan-Meier plot of this endpoint. In addition, baseline high-grade proteinuria [HR=3.8, 95% CI (2.3–6.3), P<.001] and calcium channel blocker usage [HR=2.2, 95% CI (1.1– 4.1), P= 0.03] remained independently associated with RRT. Patients who used ACEi/ARB were less likely to progress to RRT [HR=0.57, 95% CI (0.34–0.95), P= 0.03]. All univariate and multivariable results are listed in Table 2.
Figure 1.
Kaplan-Meier estimates for time to renal replacement therapy (RRT) for different baseline CKD stages. There is a significant difference between each of the CKD stages (P<0.001).
Table 2.
Cox Proportional Hazards Models [Progression to renal replacement therapy, (RRT)]
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Risk factors (variables in each factor being compared (detailed counts in Table 1) |
Number of patients with data |
HR (95% CI) |
p-value | HR (95% CI) |
p-value |
| Stage 5 vs. 1/2/3A 4 vs. 1/2/3A 3b vs. 1/2/3A |
1052 |
84.3 (32.0–222.0) 25.0 (10.0–62.4) 6.2 (2.4–16.2) |
<.001 <.001 <.001 |
33.9 (12.3–93.7) 14.4 (5.6–36.7) 3.4 (1.3–9.4) |
<.001 <.001 .02 |
| Diabetes Mellitus (Y vs. N) |
1046 | 1.2 (0.8–1.8) | .38 | ||
| Hypertension (Y vs. N) |
1043 | 0.6 (0.2–1.7) | .37 | ||
| Hyperlipidemia (Y vs. N) |
1041 | 0.9 (0.6–1.4) | .63 | ||
| Carotid Artery Disease (Y vs. N) |
1041 | 1.1 (0.7–1.6) | .73 | ||
| Stroke (Y vs. N) |
1041 | 1.2 (0.8–1.8) | .43 | ||
| Coronary Arterial Disease [CAD, (Y vs. N)] |
1033 | 1.4 (0.9–2.0) | .13 | ||
| Peripheral Arterial Disease (Y vs. N) |
1032 | 1.0 (0.7–1.4) | .85 | ||
| Proteinuria (Y vs. N) |
842 | 8.0 (5.0–13.0) | <.001 | 3.8 (2.3–6.3) | <.001 |
| Family History of CAD (Y vs. N) |
966 | 0.8 (0.6–1.2) | .35 | ||
| Bilateral RAS (vs Unilateral) |
952 | 1.5 (1.0–2.1) | .04 | ||
| Current Smoker (Y vs. N) |
894 | 2.8 (1.8–4.4) | <.001 | ||
| Statin (Y vs. N) |
951 | 1.14 (0.69–1.89) | 0.62 | ||
| ACEi/ARB (Y vs. N) |
951 | 0.67 (0.40–1.11) | 0.12 | 0.57 (0.34–0.95) | 0.03 |
| Calcium Channel Blocker (Y vs. N) |
951 | 1.99 (1.03–3.83) | 0.04 | 2.1 (1.09–4.1) | 0.03 |
| Beta Blocker (Y vs. N) |
951 | 1.0 (0.58 – 1.74) | 1.0 | ||
| Aspirin (Y vs. N) |
951 | 1.29 (0.67–2.49) | 0.45 | ||
| Plavix (Y vs. N) |
951 | 1.0 (0.65–1.54) | 0.99 | ||
| Warfarin (Y vs. N) |
951 | 1.10 (0.72–1.68) | 0.65 | ||
| Insulin (Y vs. N) |
951 | 1.51 (0.98–2.3) | 0.06 | ||
Definitions: HR- hazard ratio, CI- confidence interval
Predictors of all-cause mortality
There were far more patient deaths (397, 37.7%) than patients requiring RRT (111, 10.6%). In addition, there were an additional 89 deaths that occurred after the patients had been started on dialysis or transplanted, thus the all-cause mortality analysis included 486 deaths.
Univariate and multivariable analyses were used to predict factors associated with all-cause mortality. In the univariate analysis using CKD stage 1/2/3A as the reference group, CKD stage 3B [HR=1.4, 95% CI (1.1–1.7), P=.007], CKD stage 4 [HR=1.9, 95% CI (1.5–2.4), P<.001], and CKD stage 5 [HR=3.8, 95% CI (2.6–5.5), P<.001] remained associated with increased risk of all-cause mortality. Other factors included baseline high grade proteinuria [HR=2.2, 95% CI (1.8–2.7), P<.001]; current smoker [HR=1.3, 95% CI (1.1–1.6), P=.02]; history of peripheral arterial disease [HR=1.5, 95% CI (1.2–1.8), P<.001]; history of diabetes mellitus [HR=1.3, 95% CI (1.1–1.6), P=.006]; history of carotid artery disease [HR=1.3, 95% CI (1.1–1.6), P=.001]; history of coronary arterial disease [HR=1.4, 95% CI (1.2–1.7), P<.001]; and treatment of bilateral renal artery stenosis [HR=1.3, 95% CI (1.0–1.5), P=.02] (Table 3). Patients who were using statins at the time of renal artery stent placement [HR=0.71, 95% CI (0.57–0.87), P=0.001]; ACEi/ARB [HR=0.75, 95% CI (0.59–0.96), P=0.02]. or calcium channel blockers [HR=0.77, 95% CI (0.61–0.98), P=.03] remained associated with a decreased risk of all-cause mortality. However, patients using insulin had associated increased risk of all-cause mortality [HR=1.3, 95% CI (1.06–1.61), P= 0.01].
Table 3.
Cox Proportional Hazards Models (All-cause mortality)
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Risk factors (variables in each factor being compared (detailed counts in Table 1)) |
Number of patients with data |
HR (95% CI) |
p-value | HR (95% CI) |
p-value |
| Stage 5 vs. 1/2/3A 4 vs. 1/2/3A 3b vs. 1/2/3A |
1052 |
3.8 (2.6–5.5) 1.9 (1.5–2.4) 1.4 (1.1–1.7) |
<.001 <.001 .007 |
3.0 (1.9–4.7) 1.5 (1.1–2.0) 1.1 (0.8–1.4) |
<.001 .004 .63 |
| Diabetes Mellitus (Y vs. N) |
1046 | 1.3 (1.1–1.6) | .006 | 1.4 (1.1–1.7) | .002 |
| Hypertension (Y vs. N) |
1043 | 0.9 (0.5–1.5) | .58 | ||
| Hyperlipidemia (Y vs. N) |
1041 | 0.9 (0.7–1.1) | .38 | ||
| Carotid Artery Disease (Y vs. N) |
1041 | 1.3 (1.1–1.6) | .001 | 1.5 (1.2–1.8) | <.001 |
| Stroke (Y vs. N) |
1041 | 1.2 (1.0–1.5) | .08 | ||
| Coronary Arterial Disease [CAD (Y vs. N)] |
1033 | 1.4 (1.2–1.7) | <.001 | ||
| Peripheral Arterial Disease (Y vs. N) |
1032 | 1.5 (1.2–1.8) | <.001 | ||
| Proteinuria (Y vs. N) |
842 | 2.2 (1.8–2.7) | <.001 | 1.8 (1.4–2.2) | <.001 |
| Family History of CAD (Y vs. N) |
966 | 1.1 (0.9–1.3) | .46 | ||
| Bilateral RAS (vs. Unilateral) |
952 | 1.3 (1.0–1.5) | .02 | ||
| Current Smoker (Y vs. N) |
894 | 1.3 (1.1–1.6) | .02 | ||
| Statin (Y vs. N) |
951 | 0.7 (0.57–0.87) | <.001 | 0.55 (0.42–0.70) | <0.001 |
| ACEi/ARB (Y vs. N) |
951 | 0.75 (0.59–0.96) | 0.02 | 0. 82 (0.63–1.06) | 0.12 |
| Calcium Channel Blocker (Y vs. N) |
951 | 0.77 (0.61–0.98) | 0.03 | 0.88 (0.68–1.13) | 0.31 |
| Beta Blocker (Y vs. N) |
951 | 1.0 (0.78–1.29) | 0.98 | ||
| Aspirin (Y vs. N) |
951 | 0.87 (0.67–1.12) | 0.28 | ||
| Plavix (Y vs. N) |
951 | 0.97 (0.82–1.22) | 0.97 | ||
| Warfarin (Y vs. N) |
951 | 0.97 (0.80–1.18) | 0.78 | ||
| Insulin (Y vs. N) |
951 | 1.3 (1.06 – 1.61) | 0.01 | ||
Definitions: HR-hazard ratio, CI- confidence interval
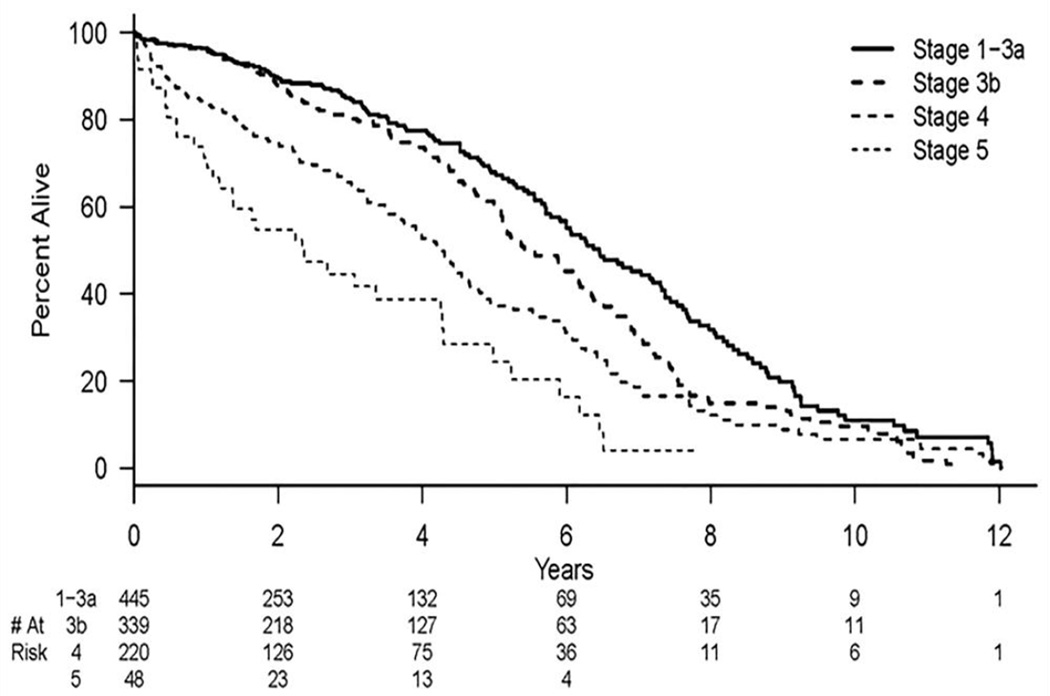
In the multivariable analysis, diabetes mellitus [HR=1.4, 95% CI (1.1–1.7), P=.002]; high-grade proteinuria [HR=1.8, 95% CI (1.4–2.2), P=.001]; history of carotid artery disease [HR=1.5, 95% CI (1.2–1.8), P<.001]; and higher CKD stage [stage 5 vs. 1-3A (HR 3.0, 95% CI (1.9–4.7), P<.001, stage 4 vs. 1-3A, HR 1.5, 95% CI (1.1–2.0), P=.004] remained independently associated with all-cause mortality. Statin use remained independently associated with all-cause mortality [HR=0.53, 95% CI (0.42–0.77), P< 0.001]. All univariate and multivariable results are listed in Table 3. Figure 2 is a Kaplan-Meier plot of all-cause mortality versus CKD stage.
Figure 2.
Kaplan-Meier estimates for freedom from death for different baseline CKD stages. There is a significant difference between CKD Stage 4 vs. CKD Stage 1/2/3A (P<.001) and CKD Stage 5 vs. CKD Stage 1/2/3A (P=0.004).
All-cause mortality or RRT
Table 1 displays a summary of how many patients progressed to RRT or died based on CKD stage. As can be seen, in low stage disease, patients are much more likely to die before they require RRT (145 deaths versus 5 requiring dialysis for the stage 1-3A group). The opposite was true in stage 5 patients where 24 required RRT compared to 16 deaths.
Complications
The 30-day all-cause mortality was 1.2% (13 deaths out of 1052 patients). The 30-day progression to RRT was 2.6% (27 patients with RRT out of 1052). Fifteen patients required hemodialysis within the first 30-days with three patients in CKD stage 3B, seven in CKD stage 4, and five in CKD stage 5. No patients underwent renal transplantation within the 30-days of the renal artery stent procedure.
Discussion
These data represent the largest cohort of patients with atherosclerotic renal artery stenosis who have undergone renal artery stenting followed longitudinally for outcomes of all-cause mortality and progressing to RRT with the resources of the Social Security Death index and USRDS, respectively. The data provide the pre-procedural relative risk for several important predictors of progression to RRT and all-cause mortality in patients with RAS treated with stent placement and CKD defined as eGFR < 45mL/min/1.73m2. This includes baseline CKD stage, diabetes, high baseline proteinuria, and carotid artery disease. There are different rates of progression to RRT and all-cause mortality in patients with different baseline CKD stage. In addition, certain medication usage can have a positive or negative impact on patients RAS and their progression to RRT and all-cause mortality. Statins decrease all-cause mortality in patients with stent placement for RAS and ACEi/ARB decrease progression to RRT. Quantitative estimates of post-procedural outcomes and which is likely to come first allow informed decisions regarding patient selection for revascularization and trial design.
Prospective, randomized controlled studies comparing renal artery intervention employing either angioplasty or stent placement have shown little benefit for renal artery stent placement when compared to medical therapy including ASTRAL (Angioplasty and Stenting for Renal Artery Lesions), STAR (Stent Placement in Patients with Atherosclerotic Renal artery stenosis), CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions), and others [7, 8, 11, 25, 26]. Several limitations of these studies have been noted including possible under powering of the trial, selection bias, and the fact that a significant number of patients assigned to stent group did not undergo stent placement [13]. Importantly, widely differing mortality rates and subsequent outcomes suggest substantial selection differences for these trials as compared to the patients undergoing clinical management at other centers. Patient results from the present study differ from these randomized trials with regard to baseline characteristics and in outcomes for progression to RRT and all-cause mortality. ASTRAL was a renal preservation trial and its cumulative risk for patients progressing to RRT was 39 patients in 335 who were stented (11.6%) compared to 10.6% in this study. In CORAL, the progression to RRT was 3.5%. The all-cause mortality rate was reported at 30.7% in ASTRAL, 13.7% in CORAL, and 24% in STAR. Taken together, patients from the present study had greater mortality risk than any of the previous RCTs, emphasizing widely varying mortality rates among series of patients being treated with atherosclerotic renal vascular disease. Patient demographics from this cohort indicate more severe baseline renal dysfunction than patients enrolled in the prospective randomized trials noted above.
Previous studies with atherosclerotic renovascular disease have reported 7-year all-cause mortality rates in patients with RAS ranging from 63 to 73% [27, 28]. Patients with a baseline serum creatinine of >2.5 mg/dL have increased all-cause mortality [29, 30]. Recent studies have demonstrated that patients with CKD with high levels of urinary protein excretion rates have increased all-cause mortality when compared to those with low levels of proteinuria [31–33]. Small studies also suggest that proteinuria in patients with RAS is associated with a worse outcome after RA stent placement [34–37]. This was confirmed in a twenty patient study that showed the presence of increased proteinuria was associated with worse outcomes in patients with concomitant RAS [38]. Evidence also indicates that patients with baseline proteinuria less than 1 gm/day do better than those with larger quantities of proteinuria [34]. A separate study report showed that recovery of sufficient GFR to discontinue dialysis could occur in patients on hemodialysis treated for RAS with a stent with baseline proteinuria of <300 mg/24hour when compared >300 mg/24hour [3]. In the present study, we extend these observations to patients with advanced baseline CKD stage in particular CKD stage 3B, 4, and 5 undergoing renal artery stent placement when compared to CKD stage 1/2/3A. Baseline high-grade proteinuria was independently associated with all-cause mortality and progression to RRT when analyzed using univariate and multivariable analyses. As these data only examine patients who underwent renal artery stent placement, a definitive conclusion cannot be drawn as to whether patients with RAS, a higher CKD stage, and proteinuria may not benefit from stent placement. Further studies are needed to determine if all-cause mortality or progression to RRT is reduced with stent placement in patients with higher stage CKD and proteinuria with a treatable lesion as compared to patients without treatable lesions.
Recent CORAL trial results were presented which showed that patients with renal artery stenosis with a baseline urine albumin to creatinine ratio (UACR) < 22.5 mg/g with renal artery stenting were associated with significantly better event-free survival from the primary composite endpoint (73% vs. 59% at 5 years, P=.02), cardiovascular or renal death (93% vs. 85%, P<.01), progressive renal insufficiency (91% vs. 77%, P=.03), and overall survival (89% vs. 76%, P<.01). The authors concluded that “these findings suggest that low urine albumin: creatinine ratios may indicate a potential large subgroup of those with renal artery stenosis that experience improved event-free and overall-survival after renal artery stent placement plus optimal medical therapy compared with optimal medical therapy alone." [39].
Diabetes mellitus influenced the outcome of interventions in RAS in all CKD stages and was an independent risk factor for all-cause mortality, but not for RRT. The current literature contains conflicting reports on the role and efficacy of stent placement for the therapy of RAS in-patient with diabetes mellitus. [38, 40, 41]. The findings in this study are consistent with those that were observed in ASTRAL and CORAL. Finally, Perkovi et al also showed that stent placement was associated with a worse outcome for death in patients with diabetes and RAS [38].
Importantly, this study identifies an effect of certain medications after renal revascularization. Statins decreased the risk of all-cause mortality and ACEi/ARB decreased the risk of progression to RRT. The use of dihydropyridine calcium channel blocking drugs was associated with higher risk of progression to RRT. These results emphasize that whenever possible, patients should be treated with ACE/ARB therapy and statins which is consistent with experimental data showing the effect of ARB being protective in animal models with renal artery stenosis [7, 8, 11, 25, 26].
There are several limitations of the study. This was a retrospective study and was not performed prospectively with defined follow-up. There was no control group identified for comparison. In addition, there was no specific standardization of the procedures and the stent placement of RAS was performed at the discretion of the performing interventionist. A small percentage of the patients in the present study did not have all data available, such as proteinuria and specific medications. Another confounder in the present study is that patients with diabetes also are prone to proteinuria. Finally, a recent study showed that the calculation of eGFR using MDRD formula compared to an iothalamate examination may not be as reliable in patients with RAS [42].
The present study highlights the importance of stratifying patients based on pre-interventional comorbidities including KDOQI CKD stage and proteinuria for patient selection when considering renal artery revascularization. Based on this analysis, patients with atherosclerotic RAS who undergo RA stenting who have high-grade proteinuria and CKD stage 3B, 4 or 5 have an increased risk of progression to RRT. Patients with highgrade proteinuria, CKD stage 3B, 4 or 5, carotid disease or diabetes have an increased risk for all-cause mortality after renal artery stenting. ACEi/ARB use in this patient population has a decreased risk of progression to RRT and patients on statins have a decreased risk of all-cause mortality.
Acknowledgments
AK has received a Medical Student grant from the Society of Interventional Radiology Foundation. This work was funded by a HL098967 (SM) from the National Heart, Lung, And Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have none.
References
- 1.Mailloux LU, Napolitano B, Bellucci AG, Vernace M, Wilkes BM, Mossey RT. Renal vascular disease causing end-stage renal disease, incidence, clinical correlates, and outcomes: a 20-year clinical experience. Am J Kidney Dis. 1994;24:622–629. doi: 10.1016/s0272-6386(12)80223-x. [DOI] [PubMed] [Google Scholar]
- 2.Safian RD, Textor SC. Renal-Artery Stenosis. N Engl J Med. 2001;44:431–442. doi: 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 3.Thatipelli M, Misra S, Johnson CM, et al. Renal artery stent placement for restoration of renal function in hemodialysis recipients with renal artery stenosis. J Vasc Interv Radiol. 2008;19:1563–1568. doi: 10.1016/j.jvir.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Zeller T, Frank U, Muller C, et al. Stent-supported angioplasty of severe atherosclerotic renal artery stenosis preserves renal function and improves blood pressure control: long-term results from a prospective registry of 456 lesions. J Endovasc Ther. 2004;11:95–106. doi: 10.1583/03-1062.1. [DOI] [PubMed] [Google Scholar]
- 5.Beutler JJ, Van Ampting JM, Van De Ven PJ, et al. Long-term effects of arterial stenting on kidney function for patients with ostial atherosclerotic renal artery stenosis and renal insufficiency. J Am Soc Nephrol. 2001;12:1475–1481. doi: 10.1681/ASN.V1271475. [DOI] [PubMed] [Google Scholar]
- 6.Zeller T, Frank U, Muller C, et al. Predictors of improved renal function after percutaneous stent-supported angioplasty of severe atherosclerotic ostial renal artery stenosis. Circulation. 2003;108:2244–2249. doi: 10.1161/01.CIR.0000095786.44712.2A. [DOI] [PubMed] [Google Scholar]
- 7.Wheatley K, Ives N, Gray R, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962. doi: 10.1056/NEJMoa0905368. [DOI] [PubMed] [Google Scholar]
- 8.Bax L, Woittiez AJ, Kouwenberg HJ, et al. Stent placement in patients with atherosclerotic renal artery stenosis and impaired renal function: a randomized trial. Ann Intern Med. 2009;150:840–848. W150–W151. doi: 10.7326/0003-4819-150-12-200906160-00119. [DOI] [PubMed] [Google Scholar]
- 9.van de Ven P, Kaatee R, Beutler J, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: a randomised trial. Lancet. 1999;353:282–286. doi: 10.1016/S0140-6736(98)04432-8. [DOI] [PubMed] [Google Scholar]
- 10.Webster J, Marshall F, Abdalla M, et al. Randomised comparison of percutaneous angioplasty vs continued medical therapy for hypertensive patients with atheromatous renal artery stenosis. Scottish and Newcastle Renal Artery Stenosis Collaborative Group. J Hum Hypertens. 1998;12:329–335. doi: 10.1038/sj.jhh.1000599. [DOI] [PubMed] [Google Scholar]
- 11.Plouin PF, Chatellier G, Darne B, Raynaud A. Blood pressure outcome of angioplasty in atherosclerotic renal artery stenosis: a randomized trial. Essai Multicentrique Medicaments vs Angioplastie (EMMA) Study Group. Hypertension. 1998;31:823–829. doi: 10.1161/01.hyp.31.3.823. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg MD, Olin JW. Stenting for atherosclerotic renal artery stenosis: one poorly designed trial after another. Cleve Clin J Med. 2010;77:164–171. doi: 10.3949/ccjm.77a.10001. [DOI] [PubMed] [Google Scholar]
- 13.White CJ. Kiss my astral: one seriously flawed study of renal stenting after another. Catheter Cardiovasc Interv. 2010;75:305–307. doi: 10.1002/ccd.22416. [DOI] [PubMed] [Google Scholar]
- 14.Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703. doi: 10.1161/hy0202.103782. [DOI] [PubMed] [Google Scholar]
- 15.Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal-artery stenosis. N Engl J Med. 2001;344:410–417. doi: 10.1056/NEJM200102083440603. [DOI] [PubMed] [Google Scholar]
- 16.Korsakas S, Mohaupt MG, Dinkel HP, et al. Delay of dialysis in end-stage renal failure: prospective study on percutaneous renal artery interventions. Kidney Int. 2004;65:251–258. doi: 10.1111/j.1523-1755.2004.00353.x. [DOI] [PubMed] [Google Scholar]
- 17.Modrall JG, Timaran CH, Rosero EB, et al. Predictors of outcome for renal artery stenting performed for salvage of renal function. J Vasc Surg. 2011;54:1414–1421. e1. doi: 10.1016/j.jvs.2011.04.042. discussion 20–21. [DOI] [PubMed] [Google Scholar]
- 18.Rocha-Singh KJ, Ahuja RK, Sung CH, Rutherford J. Long-term renal function preservation after renal artery stenting in patients with progressive ischemic nephropathy. Catheter Cardiovasc Interv. 2002;57:135–141. doi: 10.1002/ccd.10296. [DOI] [PubMed] [Google Scholar]
- 19.Watson PS, Hadjipetrou P, Cox SV, Piemonte TC, Eisenhauer AC. Effect of renal artery stenting on renal function and size in patients with atherosclerotic renovascular disease. Circulation. 2000;102:1671–1677. doi: 10.1161/01.cir.102.14.1671. [DOI] [PubMed] [Google Scholar]
- 20.Harden P, MacLeod M, Rodger R, et al. Effect of renal-artery stenting on progression of renovascular renal failure. The Lancet. 1997;349:1133–1136. doi: 10.1016/s0140-6736(96)10093-3. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sadjadi SA, Jaipaul N. Correlation of random urine protein creatinine (P-C) ratio with 24-hour urine protein and P-C ratio, based on physical activity: a pilot study. Therapeutics and clinical risk management. 2010;6:351–357. doi: 10.2147/tcrm.s12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 24.Ivanovic V, McKusick MA, Johnson CM, 3rd, et al. Renal artery stent placement: complications at a single tertiary care center. J Vasc Interv Radiol. 2003;14:217–225. doi: 10.1097/01.rvi.0000058324.82956.2a. [DOI] [PubMed] [Google Scholar]
- 25.van Jaarsveld BC, Krijnen P, Pieterman H, et al. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group.[see comment] New England Journal of Medicine. 2000;342:1007–1014. doi: 10.1056/NEJM200004063421403. [DOI] [PubMed] [Google Scholar]
- 26.Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. The New England journal of medicine. 2014;370:13–22. doi: 10.1056/NEJMoa1310753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies MG, Saad WE, Bismuth JX, Naoum JJ, Peden EK, Lumsden AB. Endovascular revascularization of renal artery stenosis in the solitary functioning kidney. Journal of Vascular Surgery. 2009;49:953–960. doi: 10.1016/j.jvs.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 28.Zierler RE, Bergelin RO, Davidson RC, Cantwell-Gab K, Polissar NL, Strandness DE., Jr A prospective study of disease progression in patients with atherosclerotic renal artery stenosis. American Journal of Hypertension. 1996;9:1055–1061. doi: 10.1016/0895-7061(96)00196-3. [DOI] [PubMed] [Google Scholar]
- 29.Zeller T, Muller C, Frank U, et al. Survival After Stenting of Severe Atherosclerotic Ostial Renal Artery Stenosis. J Endovasc Ther. 2003;10:539–545. doi: 10.1177/152660280301000320. [DOI] [PubMed] [Google Scholar]
- 30.Bates MC, Campbell JE, DStone PA, Jaff MR, Broce M, Lavigne PS. Factors Affecting Long-Term Survival Following Renal Artery Steneting. Catheter Cardiovasc Interv. 2007;69:1037–1043. doi: 10.1002/ccd.21121. [DOI] [PubMed] [Google Scholar]
- 31.Conley J, Tonelli M, Quan H, et al. Association between GFR, proteinuria, and adverse outcomes among White, Chinese, and South Asian individuals in Canada. Am J Kidney Dis. 2012;59:390–399. doi: 10.1053/j.ajkd.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 32.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–429. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 33.Wright JR, Shurrab AE, Cheung C, et al. A prospective study of the determinants of renal functional outcome and mortality in atherosclerotic renovascular disease. Am J Kidney Dis. 2002;39:1153–1161. doi: 10.1053/ajkd.2002.33384. [DOI] [PubMed] [Google Scholar]
- 34.Campo A, Boero R, Stratta P, Quarello F. Selective stenting and the course of atherosclerotic renovascular nephropathy. J Nephrol. 2002;15:525–529. [PubMed] [Google Scholar]
- 35.Cianci R, Martina P, Cianci M, et al. Ischemic nephropathy: proteinuria and renal resistance index could suggest if revascularization is recommended. Ren Fail. 2010;32:1167–1171. doi: 10.3109/0886022X.2010.516856. [DOI] [PubMed] [Google Scholar]
- 36.Makanjuola AD, Suresh M, Laboi P, Kalra PA, Scoble JE. Proteinuria in atherosclerotic renovascular disease. QJM. 1999;92:515–518. doi: 10.1093/qjmed/92.9.515. [DOI] [PubMed] [Google Scholar]
- 37.Chrysochou C, Cheung CM, Durow M, et al. Proteinuria as a predictor of renal functional outcome after revascularization in atherosclerotic renovascular disease (ARVD) QJM. 2009;102:283–288. doi: 10.1093/qjmed/hcp007. [DOI] [PubMed] [Google Scholar]
- 38.Perkovi V, Thomson KR, Becker GJ. Factors affecting outcome after percutaneous renal artery stent insertion. J Nephrol. 2002;15:649–654. [PubMed] [Google Scholar]
- 39.Murphy T, Dworkin L, Tobe S, et al. Relationship of albuminuria and renal artery stent outcomes in the CORAL study. J Vasc Interv Radiol. 2016;27:S70–S71. [Google Scholar]
- 40.Silva JA, Potluri S, White CJ, et al. Diabetes mellitus does not preclude stabilization or improvement of renal function after stent revascularization in patients with kidney insufficiency and renal artery stenosis. Catheter Cardiovasc Interv. 2007;69:902–907. doi: 10.1002/ccd.20980. [DOI] [PubMed] [Google Scholar]
- 41.Zeller T, Muller C, Frank U, et al. Stent Angioplasty of Severe Atherosclerotic Ostial Renal Artery Stenosis in Patients With Diabetes Meliitus and Nephrosclerosis. Catheter Cardiovasc Interv. 2003;58:510–515. doi: 10.1002/ccd.10435. [DOI] [PubMed] [Google Scholar]
- 42.Madder RD, Hickman L, Crimmins GM, et al. Validity of estimated glomerular filtration rates for assessment of baseline and serial renal function in patients with atherosclerotic renal artery stenosis: implications for clinical trials of renal revascularization. Circulation Cardiovascular interventions. 2011;4:219–225. doi: 10.1161/CIRCINTERVENTIONS.110.960971. [DOI] [PubMed] [Google Scholar]