Abstract
Background
Pathologic nodal stage is a key prognostic factor for patients with surgically resected lung cancer. We previously described the extent of missed intrapulmonary nodal metastasis in a cohort of patients treated at metropolitan Memphis, TN institutions. With long-term follow-up, we now quantify the survival impact of missed nodal metastasis.
Methods
We conducted a prospective cohort study to evaluate inadvertently discarded lymph nodes in re-dissected remnant lung resection specimens from lung cancer patients. Retrieved material was histologically examined and classified as lymph nodes with and without metastasis. Survival information was obtained from hospital cancer registries. We plotted survival distributions using the Kaplan Meier method and evaluated them with proportional hazards models controlling for significant demographic and clinical factors.
Results
The study included 110 patients who were 54% female and 69% Caucasian. Discarded lymph nodes with metastasis were found in 25 (23%) patients. Patients with missed lymph node metastasis had an increased risk of death with an unadjusted hazard ratio of 2.0 (p-value= 0.06) and an adjusted hazard ratio of 1.4 (p-value=0.45) compared with those without missed lymph node metastasis. Patients with >2 missed lymph nodes with metastasis had 4.8 (p-value=0.0005) times the hazard of death compared to patients without missed lymph node metastasis (adjusted hazard ratio =6.5, p-value=0.0001).
Conclusions
Metastasis to inadvertently discarded intrapulmonary lymph nodes from lung cancer resection specimens was associated with reduced survival. A more rigorous gross dissection protocol for lung cancer resection specimens may provide prognostically useful information.
Keywords: lymph nodes, metastases, lung cancer, quality improvement, survival
Lung cancer accounts for 27% of all US cancer deaths [1]. Most long-term survivors of lung cancer are patients with non-small cell lung cancer (NSCLC) who have undergone curative-intent surgical resection. However, most such patients die within five years of surgery [2]. The pathologic nodal stage is the most powerful prognostic factor in the curative-intent resection population, with 5-year survival rates of 56%, 38%, 22%, and 6% for patients with pathologic N0, N1, N2 and N3 respectively [3]. In acknowledgement of this, the Association of Directors of Anatomic and Surgical Pathology has recommended examination of all lymph nodes in lung resection specimens as standard pathology practice [4]. Unfortunately, this recommendation has not been widely implemented as standard practice [5, 6].
The gap in quality of pathologic (p) nodal staging is illustrated in the fact that 13% of all resections, and 18% of pathologic node-negative lung cancer resections in the US Surveillance, Epidemiology and End-Results (SEER) database have no lymph nodes examined (pNX) [6]. Furthermore, the median number of lymph nodes examined in pN0 resections is approximately 6, which is significantly lower than the median of 18–21 nodes associated with the best survival [7]. Failure to retrieve and examine intrapulmonary lymph nodes is a major contributor to the nodal staging quality gap. In a hypothesis-confirming experiment, we previously demonstrated that 60% of intrapulmonary lymph nodes were routinely discarded without examination, discarded lymph nodes were found in 90% of lobectomy specimens, and 29% of discarded lymph nodes had metastasis, including up to 12% of pN0 specimens [8].
However, our initial report did not include the survival impact of missed lymph node metastasis [9]. In the current report, we compared the survival of patients with and without discarded lymph node metastasis, to determine if missed lymph node metastasis has any prognostic value.
Material and Methods
We hypothesized that the low number of hilar and intrapulmonary (N1) lymph nodes routinely examined in lung resection specimens suggested that a significant number of lymph nodes were being discarded without examination, and further hypothesized that a clinically significant proportion of such discarded lymph nodes have metastasis. We have previously reported the details of the design and implementation of this study [8, 10]. Briefly, we collected consecutive lobectomy (or greater) lung cancer resection specimens, earmarked for permanent destruction after completion of routine pathology examination, in two hospital pathology departments in metropolitan Memphis, TN. Only specimens from patients who did not receive pre-operative chemotherapy or radiation therapy were examined. We applied a fastidious thin-section gross re-dissection protocol to retrieve all material that grossly appeared to be lymph nodes and processed all retrieved material for histologic examination by standard hemotoxylin and eosin-staining light microscopy. The histologic examination included only newly discovered lymph node material, and did not re-examine lymph nodes discovered by routine pathologic examination. We have previously provided details of the bench protocol for the fastidious re-dissection method [8, 10].
With the passage of time, we have now retrieved survival information from the participating institutions’ tumor registries, which are obtained through state vital records. We measured survival time from the date of surgery to the date of death or last follow-up. Patients currently alive were censored on March 31, 2015. The median duration of follow up for the study cohort was 44 months (range: 0 – 62 months). This study was approved by the Institutional Review Boards at each participating institution, with a waiver of informed consent.
Data and Statistical Analysis
Lymph node metastasis was evaluated per patient as ‘any additional lymph nodes with metastasis found’ (‘yes’ or ‘no’), and as the total number of additional lymph nodes with metastasis found (0, 1–2, >2). Sex, race (African-American or Caucasian), pathologic N-category (pN 0, 1 or 2), pathologic T-category (pT1/T2/Tx or pT3/T4), resection margin status (positive or negative), age, %DLC0, %FEV1, type of procedure, and Charlson comorbidity score were evaluated as potential confounders in the multiple variable analyses. The best multiple variable model (parsimonious model) was determined using a step-wise procedure based on confounding variables that impact the hazard ratio estimate by more than 10%. To evaluate the sensitivity of the model selection, full models adjusting for all potential confounders are reported in the Appendix.
Survival distributions were estimated and plotted using the Kaplan-Meier method and compared with the logrank test. Three-year survival estimates are presented with 95% confidence intervals. Multiple variable models were evaluated using cox proportional hazards models, and crude and adjusted hazard ratios are presented with 95% confidence intervals. All analyses were conducted in SAS Version 9.4 (Cary, NC).
Results
Clinical and demographic characteristics
The 110 patients available for this analysis were 54% female and 69% Caucasian. A lobectomy or bilobectomy was performed in 91% of patients, with the remaining 9% undergoing pneumonectomy. Sixty-nine percent of patients had private insurance, 24% had Medicaid or Medicare, and 7% were uninsured. A preoperative CT scan was performed in 99% of patients and 79% had preoperative PET scans. Forty-four percent of patients had T1 disease, while 35% were T2, 16% were T3, 4% were T4, and 1% were TX. Additional demographic and clinical information is reported overall and according to status of discovery of discarded lymph nodes with metastasis (Table 1).
Table 1.
Demographic and Clinical Variables of Patients Categorized by Discovery of Missed Lymph Node Metastasis
| Lymph Node Metastasis | |||
|---|---|---|---|
| No | Yes | ||
| Variable | Total | ||
| N (%) |
N (%) |
||
| Female | 46 (54) |
13 (52) |
59 |
| Male | 39 (46) |
12 (48) |
51 |
| African-American | 23 (27) |
11 (44) |
34 |
| Caucasian | 62 (73) |
14 (56) |
76 |
| Commercial | 60 (71) |
16 (64) |
76 |
| Medicaid | 9 (11) |
1 (4) |
10 |
| Medicare | 12 (14) |
4 (16) |
16 |
| None | 4 (5) |
4 (16) |
8 |
| Bi-lobectomy | 8 (9) |
2 (8) |
10 |
| Lobectomy | 74 (87) |
16 (64) |
90 |
| Pneumonectomy | 3 (4) |
7 (28) |
10 |
| N0 | 70 (82) |
6 (24) |
76 |
| N1 | 6 (7) |
12 (48) |
18 |
| N2 | 9 (11) |
7 (28) |
16 |
| T1 | 45 (52) |
3 (12) |
48 |
| T2 | 28 (33) |
11 (44) |
39 |
| T3 | 10 (12) |
8 (32) |
18 |
| T4 | 2 (2) |
2 (8) |
4 |
| Tx | 0 (0) |
1 (4) |
1 |
| Margin Negative | 82 (96) |
22 (88) |
104 |
| Margin Positive | 3 (4) |
3 (12) |
6 |
|
Mean (SD) |
Mean (SD) |
||
| Age | 66.3 (12.3) |
64.4 (9.9) |
65.8 (11.8) |
| Charlson Score | 1.8 (1.6) |
1.8 (1.7) |
1.8 (1.6) |
| Tumor Size (cm) | 3.2 (1.7) |
5.0 (2.1) |
3.6 (2.0) |
Discovery of missed lymph node metastasis
After routine pathology examination, 69%, 16% and 15% of patients were pN0, pN1, and pN2 respectively. After re-dissection of the discarded lung resection specimens, additional lymph nodes with metastasis were found in 25 (23%) patients. Of these 25 patients, 6 were pN0 after routine pathology examination, 12 were pN1, and 7 were pN2. Eleven of the 25 patients had >2 additional lymph nodes discovered with metastasis, 3 were pN0 after routine pathology examination, 4 were pN1, and 4 were pN2. Including information from the discarded lymph nodes, the number of patients with pN0 decreased from 77 (70%) to 71 (65%), and the pN1 population increased from 18 (16%) to 24 (22%) (Table 1).
Missed lymph node metastasis and survival
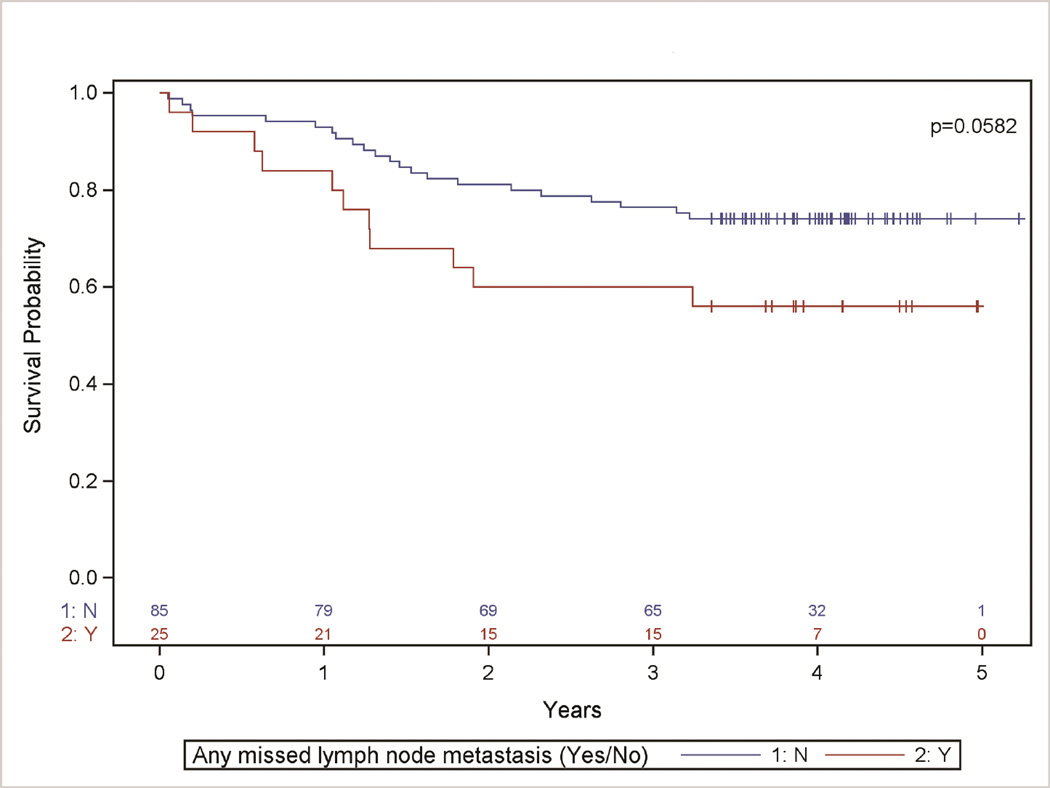
Patients with at least one missed lymph node with metastasis had decreased survival estimates when evaluated crudely (Fig 1), and after stratification for pathologic N-category and T-category (Table 2), with a strong trend towards statistical significance. The hazard of death in patients with missed lymph node metastasis was 2.0 times (95% CI: 1.0, 4.1) the hazard in those without missed lymph node metastasis (p-value= 0.0633).
Figure 1. Survival distribution stratified by discovery of any missed lymph node metastasis (Yes/No).
Kaplan-Meier Survival Analysis comparing patients with and without the discovery of missed lymph nodes with metastasis (Yes or No) (p-value=0.0582). After controlling for age, procedure, and pathologic N-category, patients with missed lymph node metastasis had 1.4 times the hazard of death compared to those with no missed lymph node metastasis (p-value=0.45).
Table 2.
Kaplan Meier Survival Estimates of Patients Categorized by Discovery of Missed Lymph Node Metastasis, T-Stage, and N-Stage.
| 3-Year Overall Survival (95% Confidence Interval) | |||||
|---|---|---|---|---|---|
| Missed Lymph Node Metastasis? | |||||
| No | Yes | P-Value | |||
| Overall | 76% (67%, 85%) | 60% (41%, 78%) | 0.0582 | ||
| T1/T2 | 77% (66%, 86%) | 67% (42%, 87%) | 0.30 | ||
| T3/T4 | 75% (48%, 94%) | 50% (21%, 79%) | 0.14 | ||
| N0 | 79% (68%, 87%) | 83% (46%, 100%) | 0.70 | ||
| N1 | 67% (28%, 95%) | 58% (31%, 83%) | 0.72 | ||
| N2 | 67% (35%, 92%) | 43% (11%, 78%) | 0.10 | ||
| Number of missed lymph node metastasis | |||||
| 0 | 1–2 | >2 | P-Value | ||
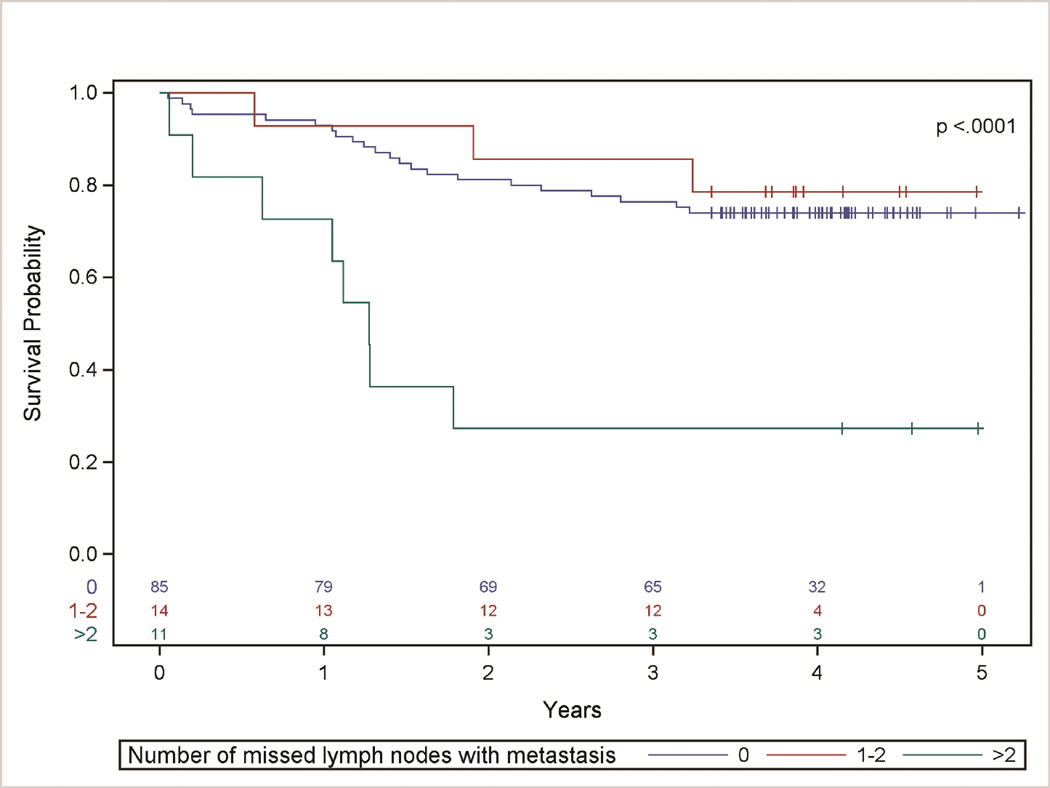
| Overall | 76% (67%, 85%) | 86% (63%, 98%) | 27% (6%, 56%) | <0.0001 | |
| T1/T2 | 77% (66%, 86%) | 82% (55%, 98%) | 25% (0%, 72%) | 0.0426 | |
| T3/T4 | 75% (48%, 94%) | 100% (NA) | 29% (4%, 65%) | 0.0125 | |
| N0 | 79% (68%, 87%) | 100% (NA) | 67% (14%, 100%) | 0.60 | |
| N1 | 67% (28%, 95%) | 75% (42%, 97%) | 25% (0%, 72%) | 0.13 | |
| N2 | 67% (35%, 92%) | 100% (NA) | 0% (NA) | 0.0006 | |
After evaluating sex, race, pathologic N-category, pathologic T-category, margin status, age, %DLC0, %FEV1, type of procedure, and Charlson comorbidity score as potential confounding variables, age, procedure, and pathologic N-category were retained in the final adjusted model. After controlling for confounding, we still observed decreased survival times in patients with missed lymph node metastasis (hazard ratio = 1.4 (95% CI: 0.6, 3.7); p-value=0.45).
We further evaluated patients based on the number of missed intrapulmonary lymph node metastasis (0, 1–2, or >2) found on re-dissection, both overall and by stage (Table 2, Figure 2). Patients with >2 discarded intrapulmonary lymph nodes with metastasis had 4.8 times (95% CI: 2.1, 10.9) the hazard of death compared with those without missed lymph node metastasis, (unadjusted p-value=0.0005). This result was consistent after evaluating potential confounding, with the final model controlling for age, procedure, and pathologic N-category (adjusted hazard ratio =6.5 (95% CI: 2.3, 18.2), p-value=0.0001).
Figure 2. Survival distribution stratified by number of missed lymph nodes with metastasis.
Kaplan-Meier Survival Analysis of Patients Categorized by Number of Discovered Missed Lymph Nodes with Metastasis (p-value < 0.0001). After controlling for age, procedure, and pathologic N-category, patients with >2 missed lymph nodes with metastasis had 6.5 times the hazard of death compared to those with no missed lymph node metastasis (p-value= 0.0001).
Comment
We have previously demonstrated that current routine gross dissection of lung cancer resection specimens discards the majority of intrapulmonary lymph nodes, a significant minority of which have metastasis on H&E microscopy [8, 10]. After a median of 44 months’ follow-up, we now show that missed lymph node metastasis has prognostic implications, irrespective of patients’ stage. This finding provides one plausible explanation for the oft-described association between the number of lymph nodes examined and survival in patients with pathologic node-negative lung cancer [11–13], and affirms the connection between the number of lymph nodes with metastasis and survival in patients with node-positive disease [14–20]. It also provides one potential explanation for the dismal survival of patients who have no lymph nodes examined (pNX) [5, 6].
The Tumor, Node, Metastasis staging system is our most powerful prognostic tool in lung cancer. However, there are ongoing attempts to enhance its value [21, 22]. There is also an ongoing debate about the relative prognostic value of the number of lymph nodes involved with metastasis [19, 23]. Several investigators have proposed the number of lymph nodes involved as a more powerful prognostic factor than the location of lymph node metastasis, which is currently the basis of the pathologic nodal staging system [14, 16, 19]. Some have shown a link between the number of lymph nodes involved and the anatomic dispersal of nodal metastasis, suggesting that patients with more N1 nodal metastasis are more likely to have mediastinal nodal involvement [14, 16]. It is true that pathologists have no direct access to hilar and mediastinal lymph nodes, retrieval of which is heavily dependent on surgical practice, which is also highly variable [24, 25]. However, the volume-outcome relationship is maintained, even in clinical trials with very rigorous surgical hilar and mediastinal lymph node dissection, but no tight control of pathology examination practice [26].
Patients with nodal metastasis generally benefit from post-operative adjuvant chemotherapy, which significantly decreases their hazard for death or disease recurrence [27–29]. There is also ongoing interest in developing more effective post-operative adjuvant therapies. Obviously such studies benefit significantly from accurate categorization of patients into post-operative risk subsets. Mis-categorization of pathologic N-stage inhibits the successful testing of such novel adjuvant treatments, increases the effect-size needed for such novel treatments to be demonstrably effective, increases the probability of false negative results in clinical trials, and raises the sample size needed for adequate statistical power in clinical trials of such adjuvant therapies.
Our hypothesis-testing study is limited by a relatively small sample size and relatively short duration of follow up (3 years, rather than the customary 5 years), which restricted our statistical power in certain analyses. Therefore, the strong trends towards survival impact demonstrable in this limited study suggests that the negative survival impact of missed lymph node metastasis is probably even greater than we report. Another limitation is our evaluation of specimens from only 2 pathology groups in a single city. Therefore these findings might not reflect practice in other parts of the US. However, we have shown from analysis of the SEER database that the Memphis experience with the nodal staging quality gap accurately reflects US national practice [5–7, 30, 31]. Comparison of the lymph node staging quality gap from analyses of US national databases vs. the Memphis Metropolitan Area Quality of Surgical Resection cohort illustrates this fact. The rate of resections without lymph node examination is 13% in SEER [6] vs. 12% in the Memphis cohort [5]; resections without mediastinal lymph node examination in a 2001 American College of Surgeons patient care survey was 42% [32] vs. 42% in the Memphis cohort [30]; the median total number of lymph nodes examined in pN0 resections was 6 in a SEER analysis [7] vs. 5 in the Memphis cohort [5].
It is possible that this quality gap in gross retrieval and examination of intrapulmonary lymph nodes is limited to the types of community-based pathology practices included in our study. Much better results are probably achieved within academic institutions. However, one of the institutions included in our study is a teaching institution with a pathology residency training program. Moreover, the fact remains that 80% of surgical lung cancer care in the US occurs in community hospitals, similar to those included in our study [32]. Besides, there is also evidence of heterogeneity in quality of lung cancer care in academic centers. For example, Little et al found only slight improvement in nodal staging quality in academic centers compared to non-academic centers [32].
Furthermore, analysis of the landmark American College of Surgeons Oncology Group Z0030 trial, which involved predominantly academic centers in North America shows that the distribution of N1 lymph node counts is similarly weighted towards the low end, with a median of 5 nodes examined [26], identical to results in our cohort [8]. By comparison, a median of 11 N1 lymph nodes are examined when the discarded intrapulmonary lymph nodes are included in our cohort [8]. Finally, variation in the thoroughness of pathologic N1 nodal examination is a plausible hypothesis to explain the striking geographic differences in pN0 survival rates in Asian patients in the International Association for the Study of Lung Cancer database (5-year survival 79%), compared to patients from Australia, Europe and North/South America, with respective 5-year survival rates of 58%, 54%, and 67% [33].
Accurate pathologic nodal staging requires improvement in surgical processes to retrieve and accurately label the anatomic provenance of hilar and mediastinal nodes, and concurrent improvement in gross retrieval of intrapulmonary lymph nodes. Current recommendations, such as those of the Association of Directors of Anatomic and Surgical Pathology, require examination of all lymph nodes present in a resection specimen [4]. Two widely used manuals of gross dissection describe methods to achieve this, but may need to be modified in view of these findings, and reports of improved intrapulmonary lymph node yield from a novel gross dissection protocol [34–36]. The stage-migration possible with the combination of corrective interventions to improve surgical and pathology processes has also been reported, including the impact on post-operative adjuvant therapy eligibility rates [37]. If validated in prospective studies involving more heterogeneous practice settings, wide dissemination and implementation of such improved processes as the standard of care for curative-intent lung cancer resection may provide a practical means of improving lung cancer survival at the broad population level. Scientifically rigorous large-scale studies to definitively establish the population-level impact of these quality improvement projects are both ongoing, and in gestation.
Supplementary Material
Acknowledgments
Set acknowledgement: This project was supported by R01 CA172253 (Osarogiagbon). The authors wish to thank all past and present members of Dr. Osarogiagbon’s Thoracic Oncology Research Group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Sixteenth Annual World Conference on Lung Cancer, Denver, CO, Sept. 6–9, 2015.
Set MMC box: The Appendix can be viewed in the online version of this article [INSERT article doi] on http://www.annalsthoracicsurgery.org.
References
- 1.American Cancer Society. Cancer Facts and Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Pfannschmidt J, Muley T, Bulzebruck H, et al. Prognostic assessment after surgical resection for non-small cell lung cancer: Experiences in 2083 patients. Lung Cancer. 2007;55(3):371–377. doi: 10.1016/j.lungcan.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Rusch VW, Crowley J, Giroux DJ, et al. The iaslc lung cancer staging project: Proposals for the revision of the N descriptors in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2(7):603–612. doi: 10.1097/JTO.0b013e31807ec803. [DOI] [PubMed] [Google Scholar]
- 4.Association of Directors of A. Surgical P. Recommendations for processing and reporting of lymph node specimens submitted for evaluation of metastatic disease. Am J Clin Pathol. 2001;115(6):799–801. doi: 10.1309/685F-X6RF-6TBD-PY0P. [DOI] [PubMed] [Google Scholar]
- 5.Osarogiagbon RU, Allen JW, Farooq A, et al. Outcome of surgical resection for pathologic N0 and NX non-small cell lung cancer. J Thorac Oncol. 2010;5(2):191–196. doi: 10.1097/JTO.0b013e3181c8cc32. [DOI] [PubMed] [Google Scholar]
- 6.Osarogiagbon RU, Yu X. Nonexamination of lymph nodes and survival after resection of non-small cell lung cancer. Ann Thorac Surg. 2013;96(4):1178–1189. doi: 10.1016/j.athoracsur.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg. 2014;97(2):385–393. doi: 10.1016/j.athoracsur.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramirez RA, Wang CG, Miller LE, et al. Incomplete intrapulmonary lymph node retrieval after routine pathologic examination of resected lung cancer. J Clin Oncol. 2012;30(23):2823–2828. doi: 10.1200/JCO.2011.39.2589. [DOI] [PubMed] [Google Scholar]
- 9.Brzezniak C, Giaccone G. Intrapulmonary lymph node retrieval: Unclear benefit for aggressive pathologic dissection. Transl Lung Cancer Res. 2012;1(4):230–233. doi: 10.3978/j.issn.2218-6751.2012.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osarogiagbon RU, Ramirez RA, Wang CG, et al. Size and histologic characteristics of lymph node material retrieved from tissue discarded after routine pathologic examination of lung cancer resection specimens. Ann Diagn Pathol. 2014;18(3):136–139. doi: 10.1016/j.anndiagpath.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YC, Lin CF, Hsu WH, et al. Long-term results of pathological stage i non-small cell lung cancer: Validation of using the number of totally removed lymph nodes as a staging control. Eur J Cardiothorac Surg. 2003;24(6):994–1001. doi: 10.1016/s1010-7940(03)00567-0. [DOI] [PubMed] [Google Scholar]
- 12.Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest. 2005;128(3):1545–1550. doi: 10.1378/chest.128.3.1545. [DOI] [PubMed] [Google Scholar]
- 13.Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage ia non-small cell lung cancer. J Thorac Oncol. 2008;3(8):880–886. doi: 10.1097/JTO.0b013e31817dfced. [DOI] [PubMed] [Google Scholar]
- 14.Fukui T, Mori S, Yokoi K, et al. Significance of the number of positive lymph nodes in resected non-small cell lung cancer. J Thorac Oncol. 2006;1(2):120–125. [PubMed] [Google Scholar]
- 15.Cerfolio RJ, Bryant AS. Predictors of survival and disease-free survival in patients with resected n1 non-small cell lung cancer. Ann Thorac Surg. 2007;84(1):182–188. doi: 10.1016/j.athoracsur.2007.03.030. discussion 189–190. [DOI] [PubMed] [Google Scholar]
- 16.Lee JG, Lee CY, Park IK, et al. Number of metastatic lymph nodes in resected non-small cell lung cancer predicts patient survival. Ann Thorac Surg. 2008;85(1):211–215. doi: 10.1016/j.athoracsur.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer. 2009;66(3):365–371. doi: 10.1016/j.lungcan.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Jonnalagadda S, Smith C, Mhango G, et al. The number of lymph node metastases as a prognostic factor in patients with n1 non-small cell lung cancer. Chest. 2011;140(2):433–440. doi: 10.1378/chest.10-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei S, Asamura H, Kawachi R, et al. Which is the better prognostic factor for resected non-small cell lung cancer: The number of metastatic lymph nodes or the currently used nodal stage classification? J Thorac Oncol. 2011;6(2):310–318. doi: 10.1097/JTO.0b013e3181ff9b45. [DOI] [PubMed] [Google Scholar]
- 20.Nwogu CE, Groman A, Fahey D, et al. Number of lymph nodes and metastatic lymph node ratio are associated with survival in lung cancer. Ann Thorac Surg. 2012;93(5):1614–1619. doi: 10.1016/j.athoracsur.2012.01.065. discussion 1619–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rami-Porta R, Bolejack V, Giroux DJ, et al. The iaslc lung cancer staging project: The new database to inform the eighth edition of the tnm classification of lung cancer. J Thorac Oncol. 2014;9(11):1618–1624. doi: 10.1097/JTO.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 22.Rami-Porta R, Asamura H, Goldstraw P. Predicting the prognosis of lung cancer: the evolution of tumor, node and metastasis in the molecular age-challenges and opportunities. Transl Lung Cancer Res. 2015;4(4):415–423. doi: 10.3978/j.issn.2218-6751.2015.07.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rusch VW, Giroux DJ. Nodal staging in lung cancer: Lymph node location or number? J Thorac Oncol. 2011;6(2):237–238. doi: 10.1097/JTO.0b013e318207f79e. [DOI] [PubMed] [Google Scholar]
- 24.Osarogiagbon RU, Yu X. Mediastinal lymph node examination and survival in resected early-stage non-small-cell lung cancer in the surveillance, epidemiology, and end results database. J Thorac Oncol. 2012;7(12):1798–1806. doi: 10.1097/JTO.0b013e31827457db. [DOI] [PubMed] [Google Scholar]
- 25.Rami-Porta R. The achilles' heel of lung cancer resection in the united states. J Thorac Oncol. 2012;7(12):1742–1743. doi: 10.1097/JTO.0b013e3182767d52. [DOI] [PubMed] [Google Scholar]
- 26.Osarogiagbon RU, Decker PA, Ballman K, Wigle D, Allen M, Darling G. Survival Implications of Variation in the Lymph Node (LN) Count in ACOSOG Z0030 (Alliance) J Thorac Oncol. 2015;10(9 Suppl 2):S240. doi: 10.1016/j.athoracsur.2016.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer . N Engl J Med. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 28.Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. Observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi: 10.1056/NEJMoa043623. [DOI] [PubMed] [Google Scholar]
- 29.Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: A pooled analysis by the lace collaborative group. J Clin Oncol. 2008;26(21):3552–3559. doi: 10.1200/JCO.2007.13.9030. [DOI] [PubMed] [Google Scholar]
- 30.Allen JW, Farooq A, O'Brien TF, et al. Quality of surgical resection for nonsmall cell lung cancer in a US metropolitan area. Cancer. 2011;117(1):134–142. doi: 10.1002/cncr.25334. [DOI] [PubMed] [Google Scholar]
- 31.Osarogiagbon RU, Allen JW, Farooq A, et al. Pathologic lymph node staging practice and stage-predicted survival after resection of lung cancer. Ann Thorac Surg. 2011;91(5):1486–1492. doi: 10.1016/j.athoracsur.2010.11.065. [DOI] [PubMed] [Google Scholar]
- 32.Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051–2056. doi: 10.1016/j.athoracsur.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 33.Asamura H, Chanksy K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project. Proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:1675–1684. doi: 10.1097/JTO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 34.Lester SC. Manual of Surgical Pathology. 2. Elsevier Churchill Livingstone; 2006. pp. 497–516. [Google Scholar]
- 35.Westra WH, Hruban RH, Phelps TH, et al. Surgical Pathology Dissection. 2. Springer; 2002. [Google Scholar]
- 36.Osarogiagbon RU, Eke R, Sareen S, et al. The impact of a novel lung gross dissection protocol on intrapulmonary lymph node retrieval from lung cancer resection specimens. Ann Diagn Pathol. 2014;18(4):220–226. doi: 10.1016/j.anndiagpath.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osarogiagbon RU, Ramirez RA, Wang CG, et al. Dual intervention to improve pathologic staging of resectable lung cancer. Ann Thorac Surg. 2013;96(6):1975–1981. doi: 10.1016/j.athoracsur.2013.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.