Abstract
Chemotherapy-induced emergence of drug resistant cells is frequently observed and is exemplified by the expression of family of drug resistance proteins including, multidrug resistance protein 1 (MDR1). However, a concise mechanism for chemotherapy-induced MDR1 expression is unclear. Mechanistically, mutational selection, epigenetic alteration, activation of the Wnt pathway or impaired p53 function have been implicated. The present study describes that the surviving fraction of cisplatin resistant cells co-upregulate MDR1, BMI1 and acetyl transferase activity of TIP60. Using complementary gain and loss of function approaches, we demonstrate that the expression of MDR1 is positively regulated by BMI1, a stem-cell factor classically known as a transcriptional repressor. Our study establishes a functional interaction between TIP60 and BMI-1 resulting in upregulation of MDR1 expression. Chromatin immunoprecipitation (ChIP) assays further establish that the proximal MDR1 promoter responds to cisplatin in a BMI1 dependent manner. BMI1 interacts with a cluster of E-box elements on the MDR1 promoter and recruits TIP60 resulting in acetylation of histone H2A and H3. Collectively, our data establish a hitherto unknown liaison among MDR1, BMI1 and TIP60 and provide mechanistic insights into cisplatin-induced MDR1 expression resulting in acquired cross-resistance against paclitaxel, doxorubicin and likely other drugs. In conclusion, our results advocate utilizing anti-BMI1 strategies to alleviate acquired resistance to chemotherapy.
Keywords: BMI1, MDR, TIP60, Chemoresistance
Graphical abstract
Introduction
The inevitable development of resistance to chemotherapy is the primary cause for therapy failure in several malignancies [1–3]. The proposed mechanisms for cellular resistance include activation of alternate survival pathways, DNA repair pathways, alteration of the drug target or enhanced expression of detoxification proteins [4–6]. Of these, one of the best molecularly characterized mechanisms of cellular resistance constitute expression of the ABC-transporter family proteins, particularly ATP binding cassette subfamily B member 1 (ABCB1)/MDR1 [7–10]. Several structurally divergent drugs of clinical significance such as paclitaxel and doxorubicin are pumped out of the cell by MDR1, thereby conferring a multi-drug resistant phenotype [11]. In cancer patients, chemotherapy induces MDR1 expression that correlates with increased chemoresistance and therapy failure, a phenomenon that can be readily recapitulated in several cell culture systems [8, 10, 12]. Mechanistically, the expression of MDR1 correlates with upregulation of the Wnt (wingless-type MMTV integration site family member)/β-catenin (CTNNB1; cadherin-associated protein beta 1) pathway, is associated with epigenetic changes and concomitant methylation within the MDR1 locus [13–15]. Genome-wide histone acetylation studies in response to chemotherapy also implicate histone acetyl transferases (HATs) in the development of multi-drug resistance [16–20]. Thus, upregulation of MDR1 in response to chemotherapy appears to be multifactorial and the linked molecular mechanisms remain elusive.
BMI1, a member of the Polycomb Repressor Complex 1 (PRC1) is a proto-oncogene that represses transcription of the INK4A/ARF and HOX genes that is critical for cell cycle regulation and embryonic development respectively [21, 22]. BMI1 is frequently upregulated and its expression correlates with poor prognosis in several types of cancer [23–25]. Several studies have implicated BMI1 in chemotherapy resistance since it is preferentially expressed in stem cells where it supports self-renewal and clonal growth [21, 26, 27]. Several groups including ours have shown that targeted depletion of BMI1 sensitizes cancer cells to chemotherapeutics albeit via different plausible mechanisms that include induction of p53 or AKT mediated apoptotic pathways or through changes in Redox homeostatis [27–30]. Over the past decade our understanding of cytotoxic cancer therapy has evolved and it is now recognized that cells that survive chemotherapy or radiation exhibit resistance and enhanced expression of stem-like markers [31–33]. Since both BMI1 and MDR1 are integral components of the chemoresistant stem-like circuitry, the current study investigated their regulatory liaison.
Here we describe that in major types of cancer such as those of the breast, lung and ovary; cisplatin treatment confers resistance to doxorubicin and paclitaxel through BMI1 dependent upregulation of MDR1 that involves the histone acetyl transferase, TIP60. By binding to Enhancer (E) boxes on the proximal MDR1 promoter, BMI1, positively regulates the expression of MDR1. Thus exposure to frontline platinum therapy induces resistance to drugs given in combination such as paclitaxel or as second-line such as doxorubicin. Therefore recently described small molecule inhibitors of BMI1 [24, 29] have the potential to alleviate acquired resistance to chemotherapy when applied in combination with first- or second-line drugs.
Results
Cisplatin induces cross-chemoresistance through MDR1
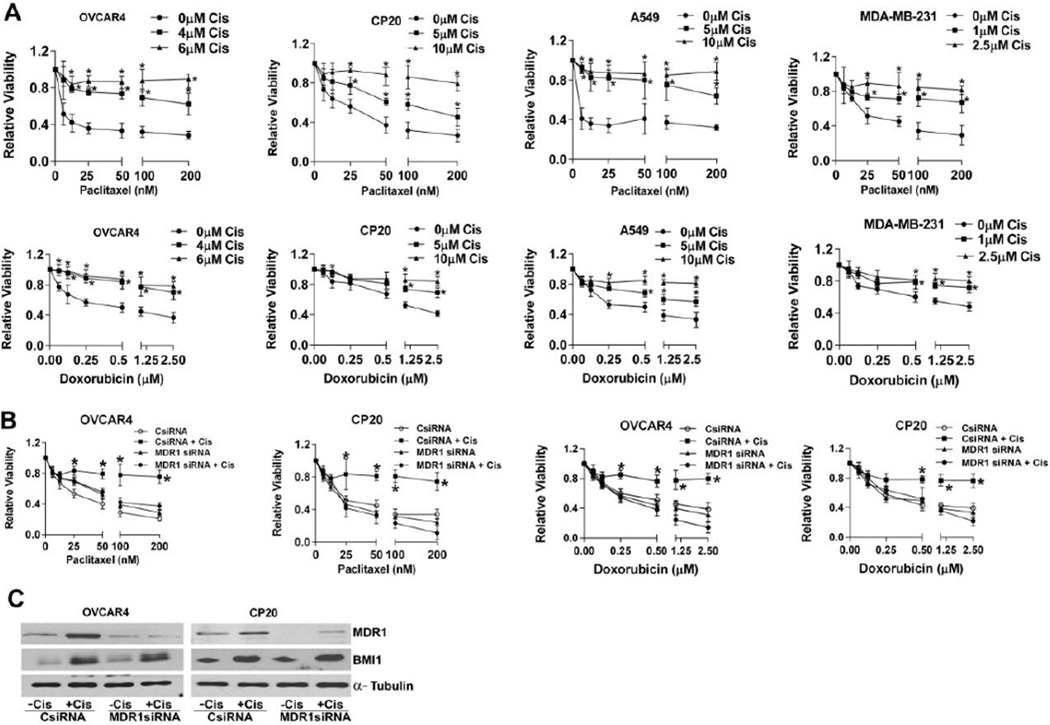
Frontline chemotherapy for major solid tumors involves platinum treatment either singly or in combination with other drugs [34–38]. Several reports corroborate that cancer cells that survive platinum onslaught develop cross-resistance to diverse drugs [39–41]. In order to delineate the mechanisms involved in such cross-resistance we treated ovarian, lung and breast cancer cell lines with different concentrations of cisplatin for 48h. The concentrations of cisplatin chosen were either at or sub IC50 levels for the respective cell lines (data not shown). Irrespective of their p53 status (see supplementary Table 1), all the cell lines significantly induced BMI1 and MDR1 expression both at the protein and mRNA level (Fig 1 A, B) as determined by immunoblotting and quantitative real time PCR (RTqPCR) respectively. Since MDR1 is a major efflux transporter for several anticancer drugs we posited that cisplatin pre-treatment would likely enhance resistance of cancer cells towards paclitaxel and doxorubicin. To this end, we pre-treated, OVCAR4 and CP20 (ovarian cancer cell line), A549 (lung carcinoma cell line) and MDA-MB-231 (breast carcinoma cell line) respectively with different concentrations of cisplatin for 48h. Equal numbers of the cisplatin surviving fraction, or cells without any cisplatin treatment were re-plated and treated with increasing concentrations of doxorubicin or paclitaxel for another 48h and cell viability determined. Compared to the untreated controls, a significant increase in respective IC50 values for both paclitaxel and doxorubicin was observed for all the cisplatin pre-treated cell lines (Fig 2A and Supplementary Table 2). While relevant to other cancers this is especially significant in high grade serous ovarian cancer since MDR1 overexpression has recently been reported to be one of the molecular events contributing to acquired chemotherapy resistance [7].
Figure 1. Cisplatin treatment co-upregulates BMI1 and MDR1 across different types of cancer.
(A) Immunoblots represent expression of MDR1 and BMI1 after 48h treatment with different concentrations of cisplatin as indicated below. α-Tubulin was used as internal loading control. (B) mRNA expression of BMI1 and MDR1 was determined by RTqPCR and then expressed as fold change + SD compared to the control cells without cisplatin. Cisplatin treatment was for 48h and at a concentration of 4µM for OVCAR4, 5µM for CP20, A549, and NC1-H1299, 1µM for MDA-MB231 and 10µM for MCF7 respectively. Data represent mean ± S.D. of three independent experiments performed in triplicate. Student’s t test was performed for statistical analysis and * denotes p-value<0.05 in comparison to control.
Figure 2. Cisplatin induced cross-chemoresistance is through upregulation of MDR1.
(A) Relative viability was determined in ovarian (OVCAR4 and CP20), lung (A549) and breast (MDA-MB-231) cancer cells that were pretreated with indicated concentrations of cisplatin (cis) for 48h and then equal number of the surviving fraction of cells was replated and subsequently challenged with increasing concentration of paclitaxel and doxorubicin for another 48h. Data represent mean ± S.D. of three independent experiments performed in triplicate. Data was analyzed using ANNOVA with Dunnett’s multiple comparison test, * denotes p-value<0.05. (B) Relative viability was determined in cancer cells that were first transfected with scrambled-control (CsiRNA) or MDR1 siRNA, then treated with a single dose of cisplatin (4µM OVCAR4, 10µM CP20) for 48h or left untreated; subsequently the surviving fractions were replated and challenged with increasing concentrations of paclitaxel or doxorubicin. Data represent mean ± S.D. of three independent experiments performed in triplicate. Data was analyzed using ANOVA with Dunnett’s method for multiple comparisons, and * denotes p-value<0.05. (C) Immunoblot represent expression of BMI1 and MDR1 in ovarian cancer cells that were first transfected with scrambled-control or MDR1 siRNA, then treated with a cisplatin (4µM OVCAR4, 10µM CP20) for 48h or left untreated. α-Tubulin was used to indicate equal protein loading.
To further confirm that the enhanced resistance to paclitaxel and doxorubicin was due to upregulation of MDR1 we employed a knockdown approach by siRNA. Ovarian cancer cells transfected with scrambled-control or MDR1 siRNA were pre-treated with cisplatin for 48h. Equal numbers of the surviving fraction of cells were re-plated and treated with increasing concentrations of doxorubicin or paclitaxel for another 48h and cell viability determined. As expected both OVCAR4 and CP20 control cells that were pre-treated with cisplatin demonstrated significant resistance to paclitaxel or doxorubicin (Fig 2B). However, silencing MDR1 significantly abrogated this acquired resistance (Fig 2B) suggesting a critical role for MDR1 in conferring resistance to paclitaxel and doxorubicin. In addition, efficient knockdown of MDR1 by siRNA both in presence or absence of cisplatin was confirmed in OVCAR4 and CP20 cells (Fig 2C). Since BMI1 and MDR1 were co-induced by cisplatin (Fig 1A) we determined whether knockdown of MDR1 influenced the protein expression of BMI1. Silencing MDR1 by siRNA did not alter cisplatin-induced BMI1 levels (Fig 2C) suggesting that expression of BMI1 was independent of MDR1. Similar results were obtained by using an additional set of MDR1 siRNA thereby ruling out any possible off target effects. (Supplementary Figure S1). Thus cisplatin mediated induction of acquired resistance to secondary drugs through MDR1, appears to be a more generalized phenomenon exhibited by multiple types of cancer cells.
BMI1 positively regulates expression of MDR1
To determine if BMI1 was required for cisplatin-mediated induction of MDR1, OVCAR4 or CP20 cells transfected with scrambled-control or BMI1 siRNA were treated with or without cisplatin for 48h. Silencing BMI1 significantly decreased cisplatin-mediated induction of MDR1 both at the protein and the mRNA levels (Fig 3 A, B, Supplementary Figure S1) as determined by immunoblotting and RTqPCR. Furthermore, when BMI1 was silenced in CP20 cells, the cisplatin induced resistance to doxorubicin as determined by the viability assay and induction of MDR1 was decreased to near control levels (Supplementary Figure 2A and 2B). Together these data suggest that BMI1 serves as a critical link between cisplatin mediated induction of MDR1 and chemoresistance. To further determine if the cisplatin mediated induction of MDR1 is a sustained effect, CP20 and OVCAR4 cells were treated with cisplatin for 48h and the surviving cells were subcultured for two more passages without any further treatment. Interestingly the cisplatin surviving fraction maintained upregulated MDR1 for at least up to two passages (Fig 3C). Qualitatively, compared to the untreated control, cisplatin treated CP20 cells demonstrated distinct staining for MDR1 at the cellular membrane that was significantly reduced in BMI1 silenced cells as determined by immunofluorescence. (Fig 3D). For quantitative evaluation we utilized Rhodamine123, a fluorescent substrate that specifically allows measurement of the efflux activity of MDR1. Cisplatin dose-dependently enhanced efflux of rhodamine123 and maximum efflux was observed at 4h (Fig 3E). Compared to the untreated control, efflux activity was ~6 fold and ~11 fold higher for 5µM and 10 µM cisplatin treated cells respectively (Fig 3E). We next determined if silencing BMI1 affected MDR1 efflux activity. Compared to the ~8 fold increase in efflux in the control cisplatin-treated cells, the BMI1 silenced cisplatin-treated cells were similar to that of cisplatin-untreated control cells (Fig 3F). In addition to loss of function (Fig 3A), we performed gain of BMI1 function experiments in the non-malignant immortalized ovarian surface epithelial (OSE) and fallopian tube epithelial cells (FTE 188). Mere over-expression of MYC-tagged BMI1 (MYC-BMI1 WT) in these cell lines phenocopied cisplatin treatment and significantly upregulated the expression of MDR1 (Fig 3G), as well as increased resistance to paclitaxel and doxorubicin (Fig 3H and Supplementary Table 3) thereby suggesting a more direct regulation by BMI1. Together these results suggest that BMI1 positively and likely directly regulates the expression of MDR1.
Figure 3. BMI1 positively regulates expression of MDR1.
(A) Immunoblot represent expression of BMI1 and MDR1 in ovarian cancer cells that were first transfected with scrambled-control (CsiRNA) or BMI1 siRNA, then treated with 4µM (OVCAR4) or 10µM (CP20) cisplatin (Cis) for 48h or left untreated. α-Tubulin was used to indicate equal protein loading. The numbers denote densitometric quantitation of MDR1, normalized with respect to α-Tubulin by NIH Image J (B) mRNA expression of BMI1 and MDR1 was determined by RTqPCR in cells treated as in (A) and then expressed as fold change ± SD compared to scrambled control cells without cisplatin treatment. At least three independent experiments were performed in triplicate. Data was analyzed using ANOVA with Dunnett’s method for multiple comparisons and * denotes p-value<0.05. (C) Immunoblot represent expression of MDR1 in cells that were untreated or treated with 4µM (OVCAR4) or 10µM (CP20) cisplatin (Cis) for 48h denoted as P0 and subsequently the surviving fraction was propagated for two more passages (P1 and P2; 48h on plate before trypsinization) without any further cisplatin treatment. α-Tubulin was used to indicate equal protein loading. A schematic representation is provided in the lower panel. (D) CP20 cells were transfected and treated as in (A) followed by immunofluorescent confocal microscopy for MDR1 (green). The nuclei and cytoskeleton were counterstained with DAPI (blue) and Phalloidin (red) respectively and is presented in the merged panel. The white scale bar represents 10µM. (E) As a measure of the activity of MDR1, efflux of the florescent dye Rhodamine 123 over time (h) was determined from an equal number of the surviving fraction of cisplatin pre-treated CP20 cells and represented as mean RFU ± SD. Data was analyzed using ANOVA with Dunnett’s method for multiple comparisons,* denotes p-value< 0.05. (F) Rhodamine 123 efflux after 4h, from equal number of surviving fraction of CP20 cells treated as in (A) were expressed as mean fold change ± SD compared to scrambled control cells without cisplatin treatment (-Cis). Data was analyzed using ANOVA with Dunnett’s method for multiple comparisons,* denotes p-value<0.05. (G) Immunoblot represents expression of MDR1 in OSE and FTE 188, cells that were transfected with empty vector (EV) or the MYC-BMI1WT construct. MYC tag and α-Tubulin were immunoblotted for determining transfection efficiency and equal protein loading respectively. (H) Empty vector (EV) or MYC-BMI1WT transfected FTE 188 cells were challenged with increasing concentrations of paclitaxel and doxorubicin for 48h and relative viability determined. Data represent mean ± S.D. of three independent experiments performed in triplicate. Data was analyzed using ANOVA with Dunnett’s method for multiple comparisons,* denotes p-value<0.05.
TIP60 and BMI1 are required for expression of MDR1
BMI1 is known as a transcriptional repressor therefore it’s positive regulation of MDR1 is intriguing. In this context, several lines of evidence suggest the involvement of histone acetyl transferases (HAT) in acquired resistance to drugs [6, 42, 43]. Importantly in a yeast two-hybrid screen, BMI1 was reported to interact with the histone acetyl transferase TIP60 [44]. Furthermore, overexpression of TIP60 has been reported in human lung cancer and it’s knockdown rendered cells sensitive to chemotherapy [43]. Therefore we investigated the relationship between BMI1 and TIP60 in the context of regulation of MDR1. Expression of TIP60 was unaltered, however acetylation of histone 2A (H2AK5) was increased by cisplatin in OVCAR4 and CP20 cells respectively (Fig 4A). Because H2AK5 is a known substrate of TIP60 [45], we measured the acetyl transferase activity of TIP60 after cisplatin treatment. Using antibody, endogenous TIP60 was immunoprecipitated from ovarian cancer cells that were either untreated or treated with cisplatin for 48h. Acetyl transferase activity towards histone4 (H4) was determined using immunoprecipitated TIP60 and radiolabeled acetyl CoA. Interestingly, treatment with cisplatin stimulated the acetyl transferase activity of TIP60 by ~1.7 to 1.9 fold in both OVCAR4 and CP20 (Fig 4B). FLAG tagged TIP60 (250ng) was overexpressed and used as positive control to assess HAT activity and efficient immunoprecipitation was confirmed by immunoblotting (Fig 4B). To determine if BMI1 interacts with TIP60, we performed reciprocal co-precipitation of endogenous BMI1 with TIP60 (Fig 4C). After confirming that BMI1 and TIP60 co-precipitated with one another, we then tested if cisplatin affected the interaction. After cisplatin treatment more BMI1 co-precipitated with TIP60, likely due to induction of BMI1 by cisplatin as evident from the input lanes (Fig 4D). This interaction was further confirmed by performing reciprocal co-immunoprecipitation from CP20 cells expressing FLAG tagged TIP60 and MYC tagged BMI1 (Fig 4E). Since BMI1 is known to preferentially associate with the chromatin [46], we further tested if the BMI1-TIP60 interaction was DNA dependent. Co-immunoprecipitation performed in presence of increasing concentration of DNASE1 revealed that BMI1-TIP60 interaction is stabilized by DNA and loss of the chromosomal DNA from the lysate negatively impacted the ability of TIP60 to co-purify with BMI1 (Fig 4F). By using deletion analysis, we then determined that both the wild-type (BMI1WT) and the PEST domain deleted (BMI1ΔPS) BMI1 co-precipitated with TIP60WT (wild type), whereas the ring finger deleted BMI1 (BMIΔR) did not (Fig 4G). It is notable that the ring finger domain of BMI1 is critical for interaction with other PRC1 complex partners as well, like RING1B [47]. Reciprocally, deletion of the histone acetyl transferase domain of TIP60 (TIP60ΔHAT) significantly reduced the interaction with BMI1WT (Fig 4H). Thus the interaction between BMI1 and TIP60 involves their respective ring finger and acetyl transferase domains.
Figure 4. TIP60 is required for BMI1 induced expression of MDR1.
(A) Immunoblot represents expression of TIP60 and AcH2AK5 in OVCAR4 and CP20 cells treated with (Cis) or without (C) 4µM and 10µM cisplatin respectively for 48h. H2A and α-Tubulin were used to indicate equal protein loading. (B) HAT activity of TIP60 was determined in OVCAR4 and CP20 cells and expressed as counts per minute (CPM) ± SD. Endogenous TIP60 was immunoprecipitated from untreated control (C) or 48h cisplatin treated OVCAR4 (4µM,Cis) and CP20 (10µM,Cis) cells and incubated with radiolabeled acetyl CoA and H4 in HAT buffer. Overexpressed TIP60 was used as positive control. Radioactivity of acetylated H4 was measured. Data was analyzed using ANOVA with Dunnett’s multiple comparison test * denotes p-value<0.05. The panel on the right confirms expression and immunoprecipitation of TIP60 in both the cell lines. (C) Reciprocal co-immunoprecipitation (IP) of endogenous TIP60 and BMI1 was performed from CP20 cellular lysates. IgG served as the negative control and 10% of total cellular lysate represent “input” (D) Immunoprecipitation of TIP60 was performed from CP20 cells that were either treated with 10µM cisplatin for 48h or left untreated and then immunoblotted with BMI1 or TIP60. IgG served as the negative control and 10% of total cellular lysate represent “input” (E) FLAG/MYC was immunoprecipitated from CP20 cells expressing FLAG-TIP60WT (wild type) or MYC-BMI1WT (wild type), as indicated and immunoblot performed to probe for the MYC or FLAG tag. Arrow heads denote the IgG heavy chain and 10% of total cellular lysate represent “input” (F) IP with antibody against FLAG was performed from CP20 cells co-expressing FLAG-TIP60WT and the MYC-BMI1WT constructs. IP was performed for 6h in absence or presence of the indicated concentrations of DNASE1 and probed with antibodies against MYC or the FLAG tag. Lower panel represents DNA samples from the cell lysates after immunoprecipitation (supernatant) run on an agarose gel and stained with ethidium–bromide. (G–H) Domain structure of the wild-type or different deletion mutants of BMI1 or TIP60 are shown in the top panels respectively. Interaction between specific domains of TIP60 and BMI1 was established by co-immunoprecipitation. FLAG/MYC was immunoprecipitated from CP20 cells expressing FLAG-TIP60WT (wild type), FLAG-TIP60 ΔHAT (histone acetyl transferase domain deleted), MYC-BMI1WT (wild type), MYC-BMI1ΔPS (PEST domain) and/or BMI1ΔR (RING Finger domain deleted) as indicated and immunoblot performed to probe for the MYC or FLAG tag. 10% of the total lysate is denoted as “Input” (I–K) Immunoblots represent expression of MDR1, MYC tag, FLAG tag, AcH2AK5 and monoubiquitinated H2AK119 in FTE188 cells transfected as indicated. Immunoblotting with MYC/FLAG, H2A and α-Tubulin antibodies was performed to indicate transfection efficiency and equal protein loading respectively.
Having established the interaction domains between BMI1-TIP60, we next determined their effect on the expression of MDR1. Both WT and ΔPS but not BMI1ΔR increased expression of MDR1 in FTE188 cells (Fig 4I). To confirm if the acetylation activity of TIP60 was required for induction of MDR1, we co-expressed BMI1WT with TIP60WT or TIP60ΔHAT that acts in a dominant negative (DN) manner [48]. As observed in Fig 3G and Fig 4J, expression of BMI1 itself enhanced expression of MDR1 and co-expression of TIP60WT did not further affect MDR1 levels (Fig 4J). However co-expression of TIP60ΔHAT or treatment with NU9056 [49] significantly reduced expression of MDR1 (Fig 4J). The ability of NU9056 to specifically inhibit TIP60 activity was determined by reduction in acetylated H2AK5 (Supplementary Figure S3). Additionally, since H2A is a monoubiquitination (K119) and acetylation (K5) target for BMI1 and TIP60 respectively, we determined if these modifications of H2A were mutually exclusive or linked to one another. While monoubiquitination of H2A was independent of TIP60 and dependent on BMI1, acetylation was induced by TIP60 or BMI1 itself suggesting that BMI1 is sufficient in recruiting endogenous TIP60 to H2A for acetylation (Fig 4K). Together these results indicate that the interaction of TIP60 and BMI1 occurs through their respective HAT and ring finger domains resulting in induction of MDR1.
BMI1 occupies regulatory regions on the MDR1 promoter
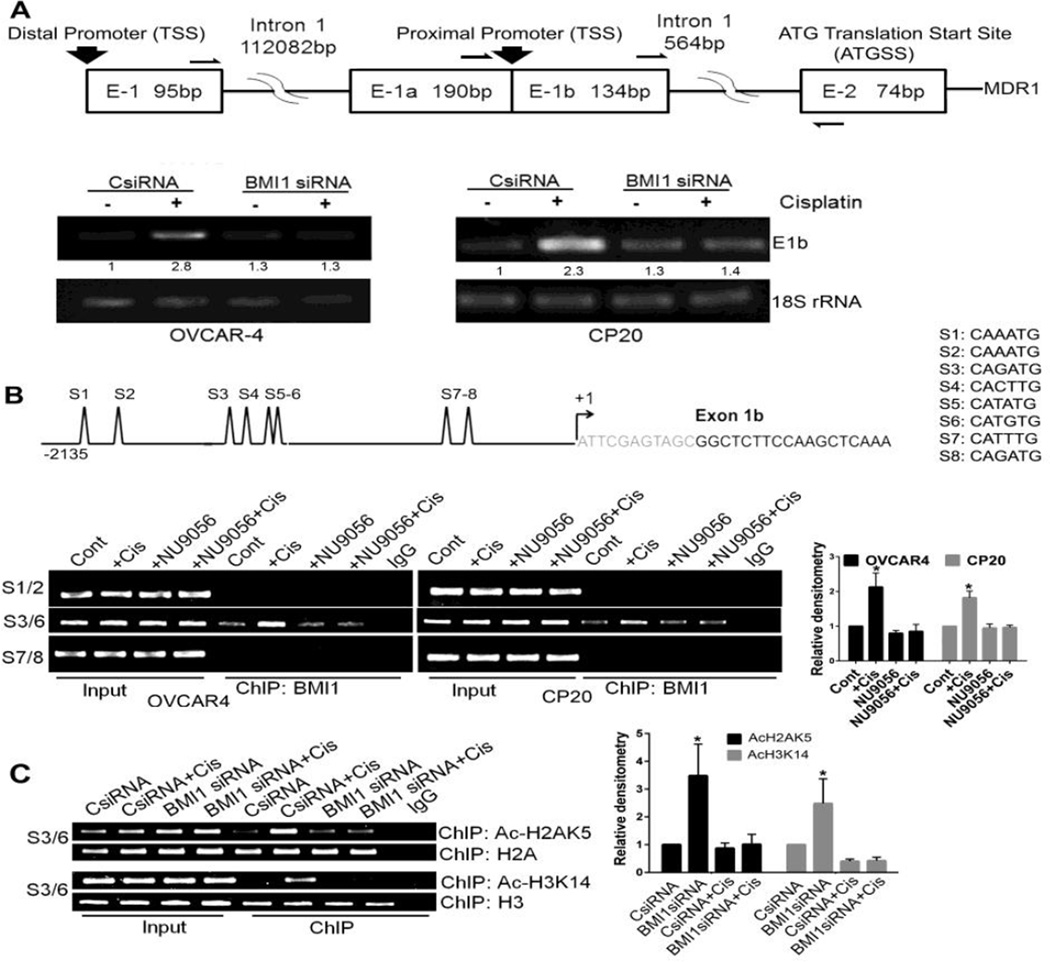
In literature, a proximal and a distal promoter have been described for MDR1 (Fig 5A) [50]. Using a single reverse primer and three different forward primers we determined that both in OVCAR4 and CP20 cells only the proximal promoter was actively transcribed. Transcription from the proximal promoter was induced by cisplatin and was reduced to control levels upon silencing BMI1 (Fig 5A). Examining nucleotide sequences within the proximal promoter region revealed eight E boxes conforming to the consensus sequence CANNTG (Fig 5B). To determine if BMI1 occupied these regions we performed ChIP assays using PCR primers encompassing the three different clusters of E boxes on the proximal MDR1 promoter. BMI1 bound only to the second cluster comprising of four E boxes (Fig 5B). While cisplatin treatment increased the association of BMI1 (~2.2 fold for OVCAR4 and ~1.8 fold for CP20 compared to respective controls), treatment with NU9056 decreased association to control levels (Fig 5B). These results suggest that BMI1 directly regulates expression of MDR1 likely through activation by TIP60. We then investigated if blocking the HAT activity of TIP60 could be compensated by inhibiting the deacetylase activity of HDACs using a broad spectrum inhibitor like TSA [51]. ChIP assay for BMI1 showed that HDAC inhibition (1µM TSA-12h) could not restore BMI1 recruitment to the MDR1 promoter that was blocked by inactivating TIP60 (24 µM NU9056-48h) (Supplementary Figure S4).
Figure 5. MDR1 promoter is regulated by Cisplatin and BMI1.
(A) Schema of the proximal and distal promoter region of MDR1 is shown in the top panel. The arrows represent a single reverse primer and three different forward primer binding sites used to amplify the E1–E2, E1a–E2 or the E1b–E2 amplicon. Lower panel depicts the E1b–E2 or the 18S rRNA (internal control) PCR amplicon. OVCAR4 and CP20 cells were first transfected with scrambled-control (CsiRNA) or BMI1siRNA treated with 4µM (OVCAR4) or 10µM (CP20) cisplatin for 48h or left untreated. RNA isolated from treated and transfected cells were reverse transcribed into cDNA and PCR performed using primers as indicated. Numbers below the lane represent relative densitometry of the E1b–E2 amplicon determined using NIH ImageJ. (B) A representative schema of the E-box clusters (S1–S8) within the proximal MDR1 promoter is shown in the upper panel. The major transcription start site (+1) is indicated by an arrow. Lower left panel represents the sites of BMI1 association within the regulatory regions of the MDR1 promoter as determined by the ChIP Assay. OVCAR4 and CP20 cells were treated with cisplatin (4µM OVCAR4, 10µM CP20) and 24µM NU9056 (TIP60 inhibitor) either alone or in combination for 48h. Immunoprecipitation of the sheared DNA was performed with BMI1 antibody and DNA was extracted to perform PCR with primers that specifically amplify either S1–S2, S3–S6 or S7–S8 regions as indicated in the schema. In the lower right panel, densitometry was performed using S3/6 amplicon signals from ChIP BMI1 (normalized to respective input) and graphically presented with mean values ± SD. Values were obtained from three independent experiments and data was analyzed using ANOVA with Dunnett’s method for multiple comparisons,* denotes p-value<0.05. (C) Association of AcH2AK5, H2A, AcH3K14 and H3 with the S3–S6 cluster of E-Box within the MDR1 promoter was determined by ChIP after transfection and treatment in CP20 cells as in (A) and is presented in the left panel. In the right panel, densitometry was performed using S3/6 amplicon signals from ChIP AcH2AK5 or AcH3K14 (normalized to respective input) and graphically presented with mean values ± SD. Values were obtained from three independent experiments and data was analyzed using ANOVA with Dunnett’s method for multiple comparisons,* denotes p-value<0.05.
H3 is an acetylation target of TIP60 [45] and drug resistant breast cancer cells with enhanced MDR1 expression show preferential H3 acetylation at the proximal MDR1 locus [20]. Therefore we performed ChIP assays with acetylated H3K14 antibody and primers encompassing the second E box cluster that are occupied by BMI1. In cisplatin treated cells more acetylated H3K14 was associated with the E boxes that was decreased to control levels in BMI1 silenced cells (Fig 5C). Additionally ChIP assays for acetylated H2AK5 revealed enrichment of acetylated H2AK5 at E boxes in cisplatin treated cells that significantly decreased upon silencing BMI1 (Fig 5C). ChIP assays performed with antibodies to H2A and H3 confirmed that there was no significant change in the nucleosome density upon BMI1 knockdown (Fig 5C). Together these results suggest that the interaction between BMI1 and TIP60 is critical in regulating expression of MDR1 that likely involves recruitment of TIP60, association with BMI1 and acetylation of H2A and H3 at the MDR1 locus (Fig 6).
Figure 6. Mechanistic summary of cisplatin-induced multi-drug resistance.
Cancer cells treated with cisplatin activate the acetyl transferase TIP60 and induce expression of BMI1. TIP60 interacts with BMI1 and this complex deploys to the E box cluster on the proximal MDR1 promoter. Here TIP60 acetylates H2A, H3 and presumably H4 converting the chromatin into an open transcribing conformation. Following translation, MDR1 is translocated to the plasma membrane and serves as an energy dependent efflux pump for several drugs including doxorubicin, paclitaxel, leading to a multi-drug resistance phenotype.
Discussion
Here we report that platinum treatment enhances resistance to co-administered drugs through BMI1 dependent upregulation of MDR1 that involves the histone acetyl transferase, TIP60.
Combination of platinum compounds with various agents, e.g., paclitaxel and doxorubicin are the cornerstone of chemotherapy in major malignancies [34–38]. However, the patient population that acquire platinum resistance have significantly lower response rates to any other therapy and this remains a significant clinical challenge [6, 40]. Investigating the mechanistic link between cisplatin treatment and cross-chemoresistance, we describe that cisplatin induces co-expression of BMI1 and MDR1 in breast, lung and ovarian cancer cells independent of their p53 status. In all cancer types tested, the cisplatin resistant surviving fraction demonstrated increased resistance to paclitaxel and doxorubicin. Silencing MDR1 using genetic approaches abrogated the resistance to paclitaxel or doxorubicin of the cisplatin surviving fraction thus corroborating reports that MDR1 is a major efflux transporter of paclitaxel and doxorubicin [52, 53].
As a member of the PRC1, BMI1 complexes with Ring1B that modifies the chromatin, stimulates monoubiquitination of H2A and represses the INK4A/ARF locus [54, 55]. BMI1 is also instrumental in maintaining clonal self-renewal of normal and cancer stem-cells [56, 57]. As such, it is co-expressed along with other stem-markers, e.g., MDR1 in the stem compartment, however, its mechanistic function within this compartment remains unclear. At first, using genetic approaches in ovarian cancer cells, we confirmed that cisplatin-mediated induction of MDR1 was BMI1 dependent. Secondly, expression of BMI1, upregulated MDR1 even without cisplatin in FTE188 cells. It was intriguing as to why or how BMI1, a known repressor of transcription was positively regulating the expression of MDR1.
Several lines of evidence indicate increased genome-wide histone acetylation in response to chemotherapy, thus implicating histone acetyl transferases in the development of multi-drug resistance [16–20]. One such acetyl-transferase TIP60, has been reported to interact with BMI1 and is a component of a multiprotein complex that preferentially acetylates H2AK5, H3K14 and H4K5/8/12/16 [44, 45, 58, 59]. Like BMI1, TIP60 participates in double-strand break repair where it acetylates ataxia telangiectasia (ATM) [60–62]. TIP60 promotes tumorigenesis in lung cancer and androgen independent prostate cancer and silencing it sensitizes lung cancer cells to cisplatin [43, 63]. We observed that cisplatin treatment or overexpression stimulated the acetyl transferase activity of TIP60 corroborating previous reports that phosphorylation of TIP60 by drugs that cause G(2)/M arrest [64] such as cisplatin increase HAT activity. We also observe that the ring finger domain of BMI1 and the histone acetyl transferase domain of TIP60 are important for interaction with each other, and this interaction is DNA-dependent resulting in expression of MDR1. In this context, an acetylation-dependent nuclear arrangement and recruitment of BMI1 to the UV-damaged chromatin has been reported [65].
The mechanisms proposed for chemotherapy-mediated upregulation of MDR1 also include mutational selection, aberrant signaling, e.g., Wnt/β-catenin and impaired p53, epigenetic changes involving histone methylation or acetylation and altered chromatin structure [14, 15, 20, 42, 66–69]. Since BMI1 itself is a transcriptional target of activated β-catenin, thus regulation of MDR1 expression by β-catenin knockdown might still involve BMI1 [70–72], however, we have ruled out any role for p53 in our system. Regarding epigenetic changes, reports suggest that the basal MDR1 promoter is regulated through the interaction of NF-Y and the HAT, p300/CREB binding protein-associated factor (P/CAF) with an inverted CCAAT box (−75 to −79) [70–72]. Drug induced upregulation of MDR1 was also associated with increases in H3 acetylation [13]. Our results while consistent with epigenetic changes suggest involvement of BMI1 and TIP60 further upstream at E box elements located on the MDR1 promoter.
We observe that the cisplatin-induced transcription from the proximal MDR1 promoter was BMI1-dependent. BMI1 occupied a cluster of E boxes on the promoter and the association increased with cisplatin treatment that was reduced to control levels by inhibiting TIP60. This is not entirely unexpected since reports suggest association of BMI1 with E box elements on its own promoter positively regulating expression and on the E-cadherin promoter, repressing expression [31, 46, 70]. The associations of acetylated H2A and H3 within the same E box motifs that are occupied by BMI1 indicate involvement of TIP60. Interestingly while the interaction between TIP60 and BMI1 was DNA-dependent, chromatin immunoprecipitation of TIP60 did not amplify any E-box elements on the MDR1 promoter indicating the unlikelihood of TIP60 directly occupying the DNA (data not shown). Coupled with a basal level occupation of the S3/6 cluster by BMI1, these results suggest that the presence of DNA and possible post translational modification by TIP60 may induce a conformational change in BMI1 that further enriches its recruitment to the promoter leading to the upregulation of MDR1. This position is in line with reports that BMI1 can exist in two kinetically distinct pools of highly dynamic and less dynamic fractions, with a different binding affinity towards the chromatin [73].
In summary, we demonstrate that in major types of cancer the generalized mechanism of cisplatin-mediated MDR1 induction leads to resistance against paclitaxel, doxorubicin and likely other drugs. Hence the surviving fraction from front-line platinum therapy becomes resistant to drugs given in combination such as paclitaxel or as second-line such as doxorubicin. Thus utilizing anti-BMI1 strategies might alleviate acquired resistance to chemotherapy that is facilitated by the recent advent of small molecule inhibitors.
Materials and methods
Plasmids and Constructs
The plasmids MYC-BMI1WT (wild type), MYC-BMI1ΔPS (PEST domain deleted) and MYC-BMI1ΔR (Ring domain deleted) were a kind gift from Dr. Michael Hendzel, University of Alberta, Canada. FLAG-TIP60WT (wild type) and FLAG-TIP60ΔHAT (HAT domain deleted) plasmids were a kind gift from Dr. H. Zhao, University of Pennsylvania School of Medicine, Philadelphia.
Cell Culture and Transfection
CP20 cells were developed by sequential exposure of the A2780 parental cell line to increasing concentrations of cisplatin and was a kind gift from Dr. Anil Sood, MD Anderson Cancer Center. Ovarian epithelial cell line (OSE tsT/hTERT, henceforth OSE) was a kind gift from Dr. V. Shridhar, Mayo Clinic, Rochester, MN. OVCAR4 was a kind gift from Dr. Ronny Drapkin, formerly at the Dana-Farber Cancer Institute, Boston, MA. Immortalized normal fallopian tube epithelial cells (FTE-188) were a kind gift from Dr. Jinsong Liu, MD Anderson Cancer Center. OVKATE was purchased from JCRB, Japan and MD-MBA-231, MCF7, NCI-H1299, and A549 were a kind gift from Dr. Rajagopal Ramesh, University of Oklahoma. These were routinely cultured in RPMI/DMEM/MCDB-Med199 supplemented with 10% FBS and 1× penicillin-streptomycin (Gibco, NY, USA) in a 5% CO2 humidified atmosphere.
Lipofectamine 3000 (Invitrogen) was used for plasmid (1µg) transfection and cotransfection with siRNA. Gene silencing was performed using Hiperfect (Qiagen) and 10 picomoles siRNA (scrambled control, Dharmacon; BMI1 SASI_HS01_00175765 and MDR1 SASI_Hs01_00087519 from Sigma).
Cell Viability Assay
Transfected or untransfected cells were treated with cisplatin (Sigma) for 48h, equal number of surviving fraction of cells was replated in a 96 well plate and further treated with paclitaxel or doxorubicin (Sigma) for additional 48h. Cell viability was determined using the CellTiter 96® AQueous One Solution Cell Proliferation (MTS) assay (Promega) as per protocol.
RNA isolation, Reverse Transcription and Analysis of Gene expression using quantitative real time PCR (RTqPCR)
RNA extraction was performed using RNeasy Plus Mini kit, (Qiagen) following manufacturer’s protocol. RNA was first retro-transcribed using iScript cDNA Synthesis kit (Bio-Rad, Hercules, CA), and then either real-time PCR was carried out using iTaq SYBR Green Master Mix (Bio-Rad). The comparative Ct (2−ΔΔCT) method [74] was used to calculate the relative abundance of the mRNA and compared with that of housekeeping gene expression 18S rRNA as previously described [75]. For end point PCR, RNA was isolated, retro-transcribed into cDNA and then amplified using 2× Taq polemerase (NEB) and primers. Primer list is provided in the supplementary file.
Immunoblotting and immunoprecipitation
Total Cell Lysate was prepared in RIPA (Boston Bioproducts) or Cell Lytic M (Sigma) compatible with enzymatic assay and immunoprecipitation. Immunoprecipitation was performed using with Agarose A/G beads (SCBT-2003), and cell lystaes or immunoprecipitates were separated by SDS-PAGE and Western immunoblotting analysis was performed using standard protocol as described previously [75]. When required, DNASE1 (Sigma) digestion was done during immunoprecipitation with 150U and 250U DNASE1. Antibodies were purchased from following venders: CST (MDR1#12683, UbHistone2AK119#8240, MYC-tag#2276, Histone3 #4499; Histone 2A #2578); Proteintech (TIP60#10827-1-AP); EMD-Millipore (AcHistone3K14#07-353, BMI1#05637); Life-Technologies (BMI1#375400); ABCAM (α–Tubulin #ab4074) and Sigma (FLAG #F1804, secondary antibodies conjugated with horseradish peroxidase IgG Rabbit and Mouse).
Immunofluorescence
Localization of MDR1 was determined by immunostaining followed by confocal microscopy. The cells were fixed with 4% PFA, permeabilized with 0.1% TritonX-100 in PBS, blocked with 4% BSA in PBS, stained overnight with primary antibody in 1% BSA-PBS, washed and stained with Alexa Fluor 488-labelled goat anti-rabbit secondary antibody for 1h. DAPI (blue) was used to stain the nucleus, and cytoskeletal structure along with cell membrane was stained with Phalloidin (red). The images were acquired using Olympus FX-400 microscope and processed using ImageJ (NIH).
HAT activity
Briefly, transfected or untransfected cells were treated with cisplatin and immunoprecipitation was performed with anti-TIP60 antibody as described previously [76]. Pelleted agarose beads were resuspended in 30µl of HAT reaction buffer (50mMTris/HCl, pH8.0,10%glycerol, 1mMDTT, 1mMPMSF and 0.1 mM EDTA) containing 2.5µg of Histone 4 (New England BioLabs, Ipswich, MA), 10 mM sodium butyrate, and 50 nCi3H-labeled acetyl- CoA (Amersham Pharmacia,). The reaction mixture was incubated at 30°C for 60 minutes and was spotted onto Whatman P81 phosphocellulose squares and allowed to air dry. Dry filters were washed with 200 mM sodium bi-carbonate (pH 9.2) to remove unincorporated radioactive acetyl-CoA. The filters were placed in 2 ml of scintillation fluid containing vials for 10 minutes and incorporated radioactivity determined using a liquid scintillation counter.
Rhodamine 123 efflux assay
Transfected or untransfected cells were exposed to 0–20 µM of Rhodamine123 (1% methanol, HBSS, Sigma) for 10 minutes at 37°C, to allow loading of dye into cells. Following loading, cells were washed three times with cold PBS to remove excess dye, and efflux initiated by adding pre-warmed HBSS and incubation at 37°C. Fluorescence was determined at different time points within the linear phase of the assay using CLARIOstar (BMG Labtech).
ChIP Assay (Chromatin immunoprecipitation)
Transfected/untransfected cells that either received drug treatment as indicated or left untreated were subjected to Chromatin immunoprecipitation (ChIP) assays, performed using EZ-ChIP chromatin immunoprecipitation kit (Millipore #17-371) as per manufacturer’s instructions. Briefly, after in vivo cross-linking, cells were lysed and sonicated to shear the chromatin to a manageable size (200–1000 bop). Immunoselections of cross-linked protein-DNA were performed with anti-rabbit IgG (negative control), anti-BMI1, anti-AcHistone2AK5 anti-Histone2A; Anti-Histone 3 and anti-AcHistone3K14 antibody, and protein G-conjugated agarose beads. Protein-DNA complexes were washed, and then protein-DNA cross-links were reversed to free DNAs. The purified DNAs were analyzed by PCR using primers (sequence information provided in supplementary file) for E-box promoter clusters.
Data analysis and Statistics
All the experiments were repeated independently at least 3 times and in triplicate where applicable. Data are expressed as mean ± standard deviation (SD). Comparisons between two groups were evaluated using Student’s t-test with equal/unequal variances. For comparisons among multiple groups, we performed ANOVA. If the overall test was significant, we compared each treatment to control with Dunnett's method for multiple comparisons. P<0.05 was considered statistically significant. All tests were two-sided.
Supplementary Material
Highlights.
A mechanistic model of chemotherapy induced multi drug resistance is presented.
Independent of p53 status, cisplatin treatment elevates MDR1 in cancer cells.
Cisplatin-induced expression of MDR1 is positively regulated by BMI1.
BMI1-TIP60 interaction at the proximal promoter upregulates transcription of MDR1.
Anti BMI1 strategies might sensitize platinum-refractory cancer to chemotherapy.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) CA 157481 (RB) and HL120585 (PM); the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 2.Luqmani YA. Mechanisms of drug resistance in cancer chemotherapy. Med Princ Pract. 2005;14(Suppl 1):35–48. doi: 10.1159/000086183. [DOI] [PubMed] [Google Scholar]
- 3.Vadlapatla RK, Vadlapudi AD, Pal D, Mitra AK. Mechanisms of drug resistance in cancer chemotherapy: coordinated role and regulation of efflux transporters and metabolizing enzymes. Curr Pharm Des. 2013;19:7126–7140. doi: 10.2174/13816128113199990493. [DOI] [PubMed] [Google Scholar]
- 4.Brown R, Curry E, Magnani L, Wilhelm-Benartzi CS, Borley J. Poised epigenetic states and acquired drug resistance in cancer. Nat Rev Cancer. 2014;14:747–753. doi: 10.1038/nrc3819. [DOI] [PubMed] [Google Scholar]
- 5.Gatti L, Cassinelli G, Zaffaroni N, Lanzi C, Perego P. New mechanisms for old drugs: Insights into DNA-unrelated effects of platinum compounds and drug resistance determinants. Drug Resist Updat. 2015;20:1–11. doi: 10.1016/j.drup.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Shen DW, Pouliot LM, Hall MD, Gottesman MM. Cisplatin resistance: a cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol Rev. 2012;64:706–721. doi: 10.1124/pr.111.005637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey PJ, Kassahn KS, Newell F, Quinn MC, Kazakoff S, Quek K, Wilhelm-Benartzi C, Curry E, Leong HS, G. Australian Ovarian Cancer Study. Hamilton A, Mileshkin L, Au-Yeung G, Kennedy C, Hung J, Chiew YE, Harnett P, Friedlander M, Quinn M, Pyman J, Cordner S, O'Brien P, Leditschke J, Young G, Strachan K, Waring P, Azar W, Mitchell C, Traficante N, Hendley J, Thorne H, Shackleton M, Miller DK, Arnau GM, Tothill RW, Holloway TP, Semple T, Harliwong I, Nourse C, Nourbakhsh E, Manning S, Idrisoglu S, Bruxner TJ, Christ AN, Poudel B, Holmes O, Anderson M, Leonard C, Lonie A, Hall N, Wood S, Taylor DF, Xu Q, Fink JL, Waddell N, Drapkin R, Stronach E, Gabra H, Brown R, Jewell A, Nagaraj SH, Markham E, Wilson PJ, Ellul J, McNally O, Doyle MA, Vedururu R, Stewart C, Lengyel E, Pearson JV, Waddell N, deFazio A, Grimmond SM, Bowtell DD. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521:489–494. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 8.Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res. 1999;5:3352–3356. [PubMed] [Google Scholar]
- 9.Clarke R, Leonessa F, Trock B. Multidrug resistance/P-glycoprotein and breast cancer: review and meta-analysis. Semin Oncol. 2005;32:S9–S15. doi: 10.1053/j.seminoncol.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst. 1997;89:917–931. doi: 10.1093/jnci/89.13.917. [DOI] [PubMed] [Google Scholar]
- 11.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science. 2009;323:1718–1722. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastan I, Gottesman M. Multiple-drug resistance in human cancer. N Engl J Med. 1987;316:1388–1393. doi: 10.1056/NEJM198705283162207. [DOI] [PubMed] [Google Scholar]
- 13.Baker EK, Johnstone RW, Zalcberg JR, El-Osta A. Epigenetic changes to the MDR1 locus in response to chemotherapeutic drugs. Oncogene. 2005;24:8061–8075. doi: 10.1038/sj.onc.1208955. [DOI] [PubMed] [Google Scholar]
- 14.Henrique R, Oliveira AI, Costa VL, Baptista T, Martins AT, Morais A, Oliveira J, Jeronimo C. Epigenetic regulation of MDR1 gene through post-translational histone modifications in prostate cancer. BMC Genomics. 2013;14:898. doi: 10.1186/1471-2164-14-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma D, Vertino PM. Epigenetic regulation of MDR1 gene in breast cancer: CpG methylation status dominates the stable maintenance of a silent gene. Cancer Biol Ther. 2004;3:549–550. doi: 10.4161/cbt.3.6.1041. [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manzo F, Tambaro FP, Mai A, Altucci L. Histone acetyltransferase inhibitors and preclinical studies. Expert Opin Ther Pat. 2009;19:761–774. doi: 10.1517/13543770902895727. [DOI] [PubMed] [Google Scholar]
- 18.Easton DF, Eeles RA. Genome-wide association studies in cancer. Hum Mol Genet. 2008;17:R109–R115. doi: 10.1093/hmg/ddn287. [DOI] [PubMed] [Google Scholar]
- 19.Archer SY, Hodin RA. Histone acetylation and cancer. Curr Opin Genet Dev. 1999;9:171–174. doi: 10.1016/s0959-437x(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 20.Toth M, Boros IM, Balint E. Elevated level of lysine 9-acetylated histone H3 at the MDR1 promoter in multidrug-resistant cells. Cancer Sci. 2012;103:659–669. doi: 10.1111/j.1349-7006.2012.02215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya R, Mustafi SB, Street M, Dey A, Dwivedi SK. Bmi-1: At the crossroads of physiological and pathological biology. Genes Dis. 2015;2:225–239. doi: 10.1016/j.gendis.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park IK, Morrison SJ, Clarke MF. Bmi1, stem cells, and senescence regulation. The Journal of clinical investigation. 2004;113:175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdouh M, Facchino S, Chatoo W, Balasingam V, Ferreira J, Bernier G. BMI1 sustains human glioblastoma multiforme stem cell renewal. J Neurosci. 2009;29:8884–8896. doi: 10.1523/JNEUROSCI.0968-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreso A, van Galen P, Pedley NM, Lima-Fernandes E, Frelin C, Davis T, Cao L, Baiazitov R, Du W, Sydorenko N, Moon YC, Gibson L, Wang Y, Leung C, Iscove NN, Arrowsmith CH, Szentgyorgyi E, Gallinger S, Dick JE, O'Brien CA. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20:29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S, Calin GA, Mukherjee P. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer research. 2009;69:9090–9095. doi: 10.1158/0008-5472.CAN-09-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddique HR, Saleem M. Role of BMI1, a stem cell factor, in cancer recurrence and chemoresistance: preclinical and clinical evidences. Stem cells. 2012;30:372–378. doi: 10.1002/stem.1035. [DOI] [PubMed] [Google Scholar]
- 27.Wang E, Bhattacharyya S, Szabolcs A, Rodriguez-Aguayo C, Jennings NB, Lopez-Berestein G, Mukherjee P, Sood AK, Bhattacharya R. Enhancing chemotherapy response with Bmi-1 silencing in ovarian cancer. PloS one. 2011;6:e17918. doi: 10.1371/journal.pone.0017918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crea F, Duhagon Serrat MA, Hurt EM, Thomas SB, Danesi R, Farrar WL. BMI1 silencing enhances docetaxel activity and impairs antioxidant response in prostate cancer. Int J Cancer. 2011;128:1946–1954. doi: 10.1002/ijc.25522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dey A, Mustafi SB, Saha S, Dwivedi S. Kumar Dhar, Mukherjee P, Bhattacharya R. Inhibition of BMI1 induces autophagy-mediated necroptosis. Autophagy. 2016;12:659–670. doi: 10.1080/15548627.2016.1147670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin L, Zhang X, Zhang L, Feng Y, Weng GX, Li MZ, Kong QL, Qian CN, Zeng YX, Zeng MS, Liao DF, Song LB. Downregulation of BMI-1 enhances 5-fluorouracil-induced apoptosis in nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2008;371:531–535. doi: 10.1016/j.bbrc.2008.04.117. [DOI] [PubMed] [Google Scholar]
- 31.Pisco AO, Brock A, Zhou J, Moor A, Mojtahedi M, Jackson D, Huang S. Non-Darwinian dynamics in therapy-induced cancer drug resistance. Nat Commun. 2013;4:2467. doi: 10.1038/ncomms3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmed N, Abubaker K, Findlay JK. Ovarian cancer stem cells: Molecular concepts and relevance as therapeutic targets. Mol Aspects Med. 2014;39:110–125. doi: 10.1016/j.mam.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 34.Silver DP, Richardson AL, Eklund AC, Wang ZC, Szallasi Z, Li Q, Juul N, Leong CO, Calogrias D, Buraimoh A, Fatima A, Gelman RS, Ryan PD, Tung NM, De Nicolo A, Ganesan S, Miron A, Colin C, Sgroi DC, Ellisen LW, Winer EP, Garber JE. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28:1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg B, VanCamp L, Trosko JE, Mansour VH. Platinum compounds: a new class of potent antitumour agents. Nature. 1969;222:385–386. doi: 10.1038/222385a0. [DOI] [PubMed] [Google Scholar]
- 36.Douillard JY, Rosell R, De Lena M, Carpagnano F, Ramlau R, Gonzales-Larriba JL, Grodzki T, Pereira JR, Le Groumellec A, Lorusso V, Clary C, Torres AJ, Dahabreh J, Souquet PJ, Astudillo J, Fournel P, Artal-Cortes A, Jassem J, Koubkova L, His P, Riggi M, Hurteloup P. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7:719–727. doi: 10.1016/S1470-2045(06)70804-X. [DOI] [PubMed] [Google Scholar]
- 37.Berek JS, Bertelsen K, du Bois A, Brady MF, Carmichael J, Eisenhauer EA, Gore M, Grenman S, Hamilton TC, Hansen SW, Harper PG, Horvath G, Kaye SB, Luck HJ, Lund B, McGuire WP, Neijt JP, Ozols RF, Parmar MK, Piccart-Gebhart MJ, van Rijswijk R, Rosenberg P, Rustin GJ, Sessa C, Thigpen JT, Trope C, Tuxen MK, Vergote I, Vermorken JB, Willemse PH. Epithelial ovarian cancer (advanced stage): consensus conference (1998) Gynecol Obstet Fertil. 2000;28:576–583. [PubMed] [Google Scholar]
- 38.Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J G. International Adjuvant Lung Cancer Trial Collaborative. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- 39.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 40.Hamaguchi K, Godwin AK, Yakushiji M, O'Dwyer PJ, Ozols RF, Hamilton TC. Cross-resistance to diverse drugs is associated with primary cisplatin resistance in ovarian cancer cell lines. Cancer Res. 1993;53:5225–5232. [PubMed] [Google Scholar]
- 41.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 42.Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miyamoto N, Izumi H, Noguchi T, Nakajima Y, Ohmiya Y, Shiota M, Kidani A, Tawara A, Kohno K. Tip60 is regulated by circadian transcription factor clock and is involved in cisplatin resistance. J Biol Chem. 2008;283:18218–18226. doi: 10.1074/jbc.M802332200. [DOI] [PubMed] [Google Scholar]
- 44.Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksoz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–968. doi: 10.1016/j.cell.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 45.Kimura A, Horikoshi M. Tip60 acetylates six lysines of a specific class in core histones in vitro. Genes Cells. 1998;3:789–800. doi: 10.1046/j.1365-2443.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 46.Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, Wu KJ. Bmi1 is essential in Twist1-induced epithelialmesenchymal transition. Nature cell biology. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 47.Buchwald G, van der Stoop P, Weichenrieder O, Perrakis A, van Lohuizen M, Sixma TK. Structure and E3-ligase activity of the Ring-Ring complex of polycomb proteins Bmi1 and Ring1b. EMBO J. 2006;25:2465–2474. doi: 10.1038/sj.emboj.7601144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MY, Ann EJ, Kim JY, Mo JS, Park JH, Kim SY, Seo MS, Park HS. Tip60 histone acetyltransferase acts as a negative regulator of Notch1 signaling by means of acetylation. Mol Cell Biol. 2007;27:6506–6519. doi: 10.1128/MCB.01515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coffey K, Blackburn TJ, Cook S, Golding BT, Griffin RJ, Hardcastle IR, Hewitt L, Huberman K, McNeill HV, Newell DR, Roche C, Ryan-Munden CA, Watson A, Robson CN. Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS One. 2012;7:e45539. doi: 10.1371/journal.pone.0045539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B, Ngoi S, Wang J, Chong SS, Lee CG. The promoter region of the MDR1 gene is largely invariant, but different single nucleotide polymorphism haplotypes affect MDR1 promoter activity differently in different cell lines. Mol Pharmacol. 2006;70:267–276. doi: 10.1124/mol.105.019810. [DOI] [PubMed] [Google Scholar]
- 51.Vigushin DM, Ali S, Pace PE, Mirsaidi N, Ito K, Adcock I, Coombes RC. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin Cancer Res. 2001;7:971–976. [PubMed] [Google Scholar]
- 52.Mechetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, Garcia R, Parker RJ, Fruehauf JP. Levels of multidrug resistance (MDR1) P-glycoprotein expression by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin Cancer Res. 1998;4:389–398. [PubMed] [Google Scholar]
- 53.Duan Z, Brakora KA, Seiden MV. Inhibition of ABCB1 (MDR1) and ABCB4 (MDR3) expression by small interfering RNA and reversal of paclitaxel resistance in human ovarian cancer cells. Mol Cancer Ther. 2004;3:833–838. [PubMed] [Google Scholar]
- 54.Cao R, Tsukada Y, Zhang Y. Role of Bmi-1 and Ring1A in H2A ubiquitylation and Hox gene silencing. Mol Cell. 2005;20:845–854. doi: 10.1016/j.molcel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 55.Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- 56.Molofsky AV, He S, Bydon M, Morrison SJ, Pardal R. Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes & development. 2005;19:1432–1437. doi: 10.1101/gad.1299505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 58.Ikura T, Ogryzko VV, Grigoriev M, Groisman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell. 2000;102:463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs M, Gerber J, Drapkin R, Sif S, Ikura T, Ogryzko V, Lane WS, Nakatani Y, Livingston DM. The p400 complex is an essential E1A transformation target. Cell. 2001;106:297–307. doi: 10.1016/s0092-8674(01)00450-0. [DOI] [PubMed] [Google Scholar]
- 60.Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 2005;102:13182–13187. doi: 10.1073/pnas.0504211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun Y, Jiang X, Price BD. Tip60: connecting chromatin to DNA damage signaling. Cell Cycle. 2010;9:930–936. doi: 10.4161/cc.9.5.10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun Y, Jiang X, Xu Y, Ayrapetov MK, Moreau LA, Whetstine JR, Price BD. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat Cell Biol. 2009;11:1376–1382. doi: 10.1038/ncb1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Halkidou K, Gnanapragasam VJ, Mehta PB, Logan IR, Brady ME, Cook S, Leung HY, Neal DE, Robson CN. Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene. 2003;22:2466–2477. doi: 10.1038/sj.onc.1206342. [DOI] [PubMed] [Google Scholar]
- 64.Lemercier C, Legube G, Caron C, Louwagie M, Garin J, Trouche D, Khochbin S. Tip60 acetyltransferase activity is controlled by phosphorylation. J Biol Chem. 2003;278:4713–4718. doi: 10.1074/jbc.M211811200. [DOI] [PubMed] [Google Scholar]
- 65.Sustackova G, Kozubek S, Stixova L, Legartova S, Matula P, Orlova D, Bartova E. Acetylation-dependent nuclear arrangement and recruitment of BMI1 protein to UV-damaged chromatin. J Cell Physiol. 2012;227:1838–1850. doi: 10.1002/jcp.22912. [DOI] [PubMed] [Google Scholar]
- 66.Xia Z, Guo M, Liu H, Jiang L, Li Q, Peng J, Li JD, Shan B, Feng P, Ma H. CBP-dependent Wnt/beta-catenin signaling is crucial in regulation of MDR1 transcription. Curr Cancer Drug Targets. 2015;15:519–532. doi: 10.2174/1568009615666150506093643. [DOI] [PubMed] [Google Scholar]
- 67.Katayama K, Noguchi K, Sugimoto Y. Regulations of P-Glycoprotein/ABCB1/MDR1 in Human Cancer Cells. New Journal of Science. 2014;2014:10. [Google Scholar]
- 68.Johnson RA, Shepard EM, Scotto KW. Differential regulation of MDR1 transcription by the p53 family members. Role of the DNA binding domain. J Biol Chem. 2005;280:13213–13219. doi: 10.1074/jbc.M414646200. [DOI] [PubMed] [Google Scholar]
- 69.Blanc E, Goldschneider D, Ferrandis E, Barrois M, Le Roux G, Leonce S, Douc-Rasy S, Benard J, Raguenez G. MYCN enhances P-gp/MDR1 gene expression in the human metastatic neuroblastoma IGR-N-91 model. Am J Pathol. 2003;163:321–331. doi: 10.1016/S0002-9440(10)63656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cho JH, Dimri M, Dimri GP. A positive feedback loop regulates the expression of polycomb group protein BMI1 via WNT signaling pathway. J Biol Chem. 2013;288:3406–3418. doi: 10.1074/jbc.M112.422931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herbst A, Jurinovic V, Krebs S, Thieme SE, Blum H, Goke B, Kolligs FT. Comprehensive analysis of beta-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/beta-catenin signaling. BMC Genomics. 2014;15:74. doi: 10.1186/1471-2164-15-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu T, Chen X, Zhang W, Colon D, Shi J, Napier D, Rychahou P, Lu W, Lee EY, Weiss HL, Evers BM, Liu C. Regulation of the potential marker for intestinal cells, Bmi1, by beta-catenin and the zinc finger protein KLF4: implications for colon cancer. J Biol Chem. 2012;287:3760–3768. doi: 10.1074/jbc.M111.316349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hernandez-Munoz I, Taghavi P, Kuijl C, Neefjes J, van Lohuizen M. Association of BMI1 with polycomb bodies is dynamic and requires PRC2/EZH2 and the maintenance DNA methyltransferase DNMT1. Molecular and cellular biology. 2005;25:11047–11058. doi: 10.1128/MCB.25.24.11047-11058.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 75.Banerjee Mustafi S, Grose JH, Zhang H, Pratt GW, Sadoshima J, Christians ES, Benjamin IJ. Aggregate-prone R120GCRYAB triggers multifaceted modifications of the thioredoxin system. Antioxidants & redox signaling. 2014;20:2891–2906. doi: 10.1089/ars.2013.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bannister AJ, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.