Abstract
Spasmolytic polypeptide-expressing metaplasia (SPEM) and intestinal metaplasia are considered neoplastic precursors of gastric adenocarcinoma in humans. Loss of parietal cells causes the development of SPEM in the gastric corpus and then chronic inflammation drives SPEM toward a more proliferative lineage. Mongolian gerbils infected with Helicobacter pylori (H. pylori) develop chronic gastritis and metaplasia, mimicking aspects of human gastritis with H. pylori infection. We therefore examined metaplastic lineages in the gastric corpus mucosa of gerbils infected by H. pylori strain 7.13, which produces rapid onset of severe inflammation. Six weeks following H. pylori infection, Griffonia simplicifolia lectin II (GSII)-positive SPEM developed in the base of oxyntic glands in association with parietal cell loss and inflammation. In association with severe inflammation, SPEM glands evolved into aberrant phenotypes, including branched lesions, dilated lesions and penetrating invasive glands. Mucin 4 (MUC4) was upregulated in SPEM and progressive SPEM. Clusterin was expressed in the tips of branched and dilated lesions and throughout regions of invasive glands. Intriguingly, clusterin-positive regions in these lesions expressed Ki67 and matrix metalloproteinase 7 (MMP-7). These same regions were also positive for expression of phospho-IκBα, suggestive of activated NFκB signalling. These findings suggest that clusterin-positive regions in progressive phenotypes of SPEM have invasive characteristics. Thus, H. pylori infection in gerbils induces SPEM, which then can progress to further aberrant and invasive metaplastic phenotypes.
Keywords: SPEM, MUC4, clusterin, GSII, gastritis, intestinal metaplasia, MMP-7, IκBα
Introduction
Gastric cancer is the third leading cause of cancer-related death worldwide [1]. Helicobacter pylori (H. pylori) infection is the most common predisposing factor of gastric cancer [2,3]. Intestinal types of gastric cancers typically generate from H. pylori-infected gastric mucosa with chronic gastritis, atrophy and metaplastic changes [4]. Metaplastic lesions in H. pylori-infected gastric mucosa have two phenotypes: intestinal metaplasia and spasmolytic polypeptide-expressing metaplasia (SPEM). Intestinal metaplasia displays the characteristics of intestinal mucosa with the expression of TFF3 and MUC2. On the other hand, SPEM is a metaplastic mucous cell lineage in the corpus with characteristics of deep antral gland cells, including the expression of TFF2 (spasmolytic polypeptide) and MUC6 [5,6]. Numerous studies have shown that these metaplastic lesions exist adjacent to dysplastic or cancerous lesions, suggesting that these metaplastic lesions can be pre-neoplastic [7–9].
Recently we have determined that SPEM develops through the transdifferentiation of chief cells following parietal cell loss in mice [10]. The administration of the drugs DMP-777 or L635 induces acute parietal cell loss, resulting in the emergence of SPEM in the corpus. In the mouse model with Helicobacter felis (H. felis) infection, SPEM evolves into “SPEM with intestinal characteristics” (SPEM-IC), which expresses some markers for intestinal metaplasia observed in humans following parietal cell loss and chronic inflammation [11]. The H. pylori-infected Mongolian gerbil is a powerful model for human gastritis and gastric cancer development. After H. pylori infection, gerbils gradually develop chronic gastritis, parietal cell loss and metaplasia, as observed in humans [12–14]. At longer times following infection, SPEM is observed and then, after at least a year of infection, mixed glands expressing both SPEM and intestinal metaplasia can be observed, suggesting that SPEM gives rise to intestinal metaplasia [15]. Recently, we have established an in vivo-adapted H. pylori 7.13 strain that can induce more rapidly severe gastritis and pre-neoplastic lesions in the gerbil gastric mucosa [16,17]. Development of invasive lesions in this gerbil model is dependent on the bacterial effector CagA as well as severe inflammation [17,18]. Additionally, in gerbils fed an iron-depleted diet, mucosal changes develop even faster after H. pylori 7.13 strain infection. Therefore, the H. pylori 7.13-infected gerbil is a good model to investigate the process of metaplasia caused by H. pylori infection. In the current study, to evaluate how metaplastic lesions evolve, we have utilized immunostaining to examine metaplastic lineages and characterize the progressive phenotypes of metaplasia in gerbils following H. pylori infection.
Materials and Methods
Animals and H. pylori challenge
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Vanderbilt University. All gastric samples from Mongolian gerbils in this study were obtained from archival paraffin-embedded material from previous studies [16,18]. In those studies, all male Mongolian gerbils were housed in the Animal Care Facility of Vanderbilt University in a room with a 12-hour light:dark cycle. The gerbil-passaged H. pylori strain 7.13 isolate used for these experiments was originally isolated from a gastric ulcer patient. H. pylori infection was performed as described previously [16,18]. We examined corpus stomach sections from archival samples of gerbils fed either an iron-depleted diet [18] or a standard commercial rodent chow [16]. Stomachs from all gerbils were fixed in neutral-buffered 10% formalin. Tissues were paraffin-embedded and stained with haematoxylin and eosin. The severity of gastric inflammation was evaluated on a 6-point scale, which graded acute and chronic inflammation in the corpus based on a score of 0 to 3 for each of these parameters, as described previously [16,18]. Colonization density of H. pylori was determined by quantitative culture and all animals were successfully colonized [18]. Since the iron-depleted diet leads to more rapid progression of mucosal changes in H. pylori 7.13 infected animals, these gerbils were examined at 6 or 8 wk following inoculation. To evaluate changes in more advanced invasive lesions, we also examined specimens from H. pylori 7.13-infected gerbils fed a standard diet and euthanized at 12 or 16 wk after infection (Table 1).
Table 1.
Histological findings in gerbil gastric corpus following H. pylori 7.13 infection.
| Duration of H. pylori infection | 6 weeks | 8 weeks | 12 weeks | 16 weeks |
|---|---|---|---|---|
| Number of gerbils (Iron-depleted diet/Standard diet) |
7 (7/0) | 6 (6/0) | 2 (0/2) | 2 (0/2) |
| SPEM | 7/7 | 6/6 | 2/2 | 2/2 |
| Branched lesion | 3/7 | 3/6 | 2/2 | 2/2 |
| Dilated lesion | 3/7 | 3/6 | 2/2 | 2/2 |
| Invasive gland | 2/7 | 3/6 | 2/2 | 2/2 |
| Intestinal metaplasia | 0/7 | 0/6 | 1/2 | 0/2 |
Immunohistochemistry
For all immunohistochemistry studies, 5 µm thick sections of paraffin-embedded material were used. For serial section analyses, 30 sections per sample were cut consecutively. For evaluation of MUC4 staining in humans, sections of an archival tissue array constructed as previously described were evaluated [19]. Sections were deparaffinised, rehydrated and submitted to antigen retrieval using Target Retrieval solution (Dako North America, Inc., Carpinteria, CA) in a pressure cooker. Blocking was performed using Protein Block Serum-Free (Dako North America, Inc.) during 90 min at room temperature. The primary antibody incubation was performed in Antibody Diluent with Background Reducing Components (Dako North America, Inc.) overnight at 4oC. Primary antibodies used were as follows: rabbit anti-MUC2 (1:500, cat. sc-15334; Santa Cruz, Dallas, TX), mouse anti-MUC4 (1:100, cat. sc-33654; Santa Cruz), goat anti-clusterin α (1:2000, cat. sc-6420; Santa Cruz), rabbit anti-Ki67 (1:1000, cat. 12202; Cell Signaling, Danvers, MA), mouse anti-phospho-IκBα (1:150, cat. 9246; Cell Signaling) and rat anti-MMP-7 (1:500, Vanderbilt Antibody and Protein Resource, Nashville, TN). Detection for immunohistochemistry was performed with 3,3’-diaminobenzidine using DAKO Envision + System-HRP DAB (Dako North America, Inc.). Samples were counterstained with hematoxylin. For immunofluorescence, secondary antibodies (1:500) conjugated with Cy2, Cy3 and Cy5 (Jackson ImmunoResearch Laboratories, West Grove, PA) and Alexa-488, Alexa-594, and Alexa-647 (Invitrogen, Carlsbad, CA), and Alexa-488 or 647-conjugated Griffonia simplicifolia lectin II (GSII lectin) (1:2000, Molecular Probes, Eugene, OR) were incubated for 1 h at room temperature. After incubation with DAPI for 5 min, slides were mounted with ProLong Gold Antifade Reagent (Invitrogen). Fluorescence imaging was analysed using an Axio Imager 2 microscope (Carl Zeiss AG, Oberkochen, Germany) or an Ariol SL-50 automated slide scanner (Leica Biosystems, Buffalo Grove, IL) in the Vanderbilt Digital Histology Shared Resource.
Quantitation
To perform the quantitation of the percentage of MUC4 or clusterin-positive glands in SPEM, all corpus glands were analysed in each section of all samples used in this study. To perform the quantitation of the percentage of Ki67 or MMP-7 positive cells in SPEM and clusterin-positive regions of SPEM, 10 corpus glands randomly chosen each from 3 sections in the normal stomach and gastritis without architectural aberration, and 21 dilated/branched lesions and 12 invasive glands from representative gastritis sections with architectural aberration were analysed. Counting was performed in slides digitally imaged by the Ariol SL-50 scanner or in fluorescence images overlaid using Adobe Photoshop. For all cells counted, nuclei were present as detected by DAPI. The Mann Whitney U test was used to calculate statistical significance.
Results
Morphological changes in gerbil gastric corpus mucosa with H. pylori 7.13 infection
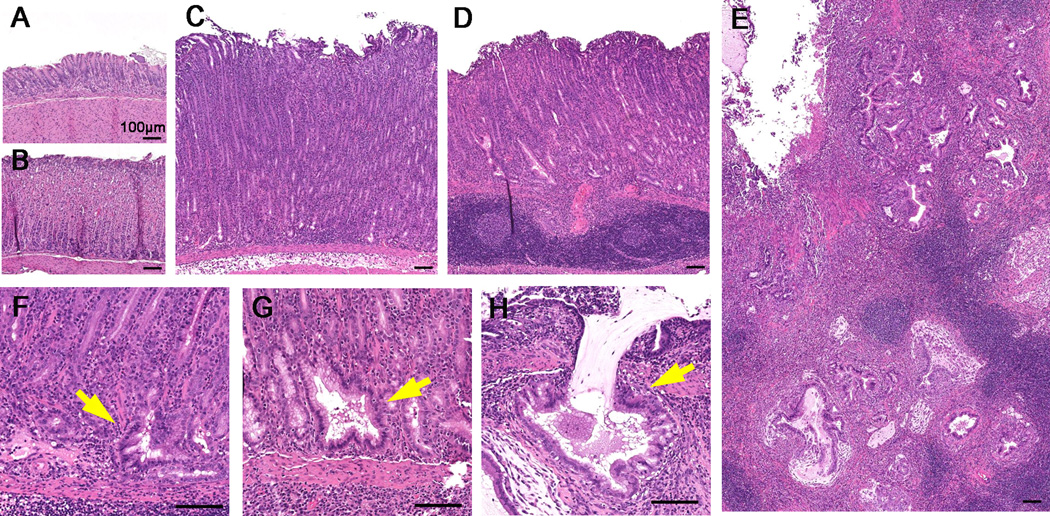
We and others previously demonstrated that inflammatory cell infiltrates, foveolar hyperplasia and parietal cell loss were observed in the intermediate zone and the corpus of gerbil gastric mucosa during H. pylori infection [15]. We have now sought to re-examine the classification of metaplastic changes in the corpus of the stomach within the Helicobacter-infected gerbil stomach. In these investigations, we focused on metaplastic changes in the corpus, because identification of SPEM is not possible in the antrum, due to the similarities of the metaplastic lineages to deep antral mucosal cells. First, we investigated morphological changes within the gastric corpus mucosa of gerbils with the infection of H. pylori strain 7.13, a strain that can rapidly induce severe inflammation and pre-neoplastic lineage changes. Inflammatory cell infiltrates were observed in the corpus early after the infection, and then atrophic gastritis (oxyntic atrophy) progressed with development of metaplastic mucous gland phenotypes (Figure 1). To analyse the process of the alteration of glands, we identified glands as “gastritis without architectural aberration” (Figure 1C) when they maintained a single linear lumen, while “gastritis with architectural aberration” was associated with glands with branched or cystic glands (Figure 1D). All 13 gerbils at 6 or 8 wk post infection had prominent inflammation in the corpus or the intermediate zone (Supplementary Figure 1). Among them, 6 gerbils had gastritis with architectural aberration in the gastric corpus mucosa, and 7 had only gastritis without architectural aberration. In this study, we investigated gastric tissues of the latter 7 gerbils as typical of gastritis without architectural aberration. In addition to the former 6 gerbils, we examined corpus tissues with gastritis with more severe architectural aberrations in 4 gerbils sacrificed at 12 or 16 wk post infection (see Table 1). Gastritis with architectural aberration demonstrated specific phenotypes, including branched lesions, dilated lesions and penetrating invasive glands (Figure 1F, G, H). At 16 wk after H. pylori infection, extensive lesions with invasive glands were seen (Figure 1E). To further characterize the spectrum of metaplastic phenotypes, we performed immunostaining analyses on sections of corpus mucosa from gerbils with the range of metaplastic changes.
Figure 1. Morphological changes in gerbil gastric corpus mucosa following H. pylori 7.13 infection.
(A) Uninfected antrum. (B) Uninfected corpus. (C) Gastritis without architectural aberration in the corpus mucosa of a representative animal at 6 wk post. Inflammatory cell infiltrates were observed, but gastric glands still showed normal morphologies. (D) Gastritis with architectural aberration in the corpus mucosa of a representative animal at 8 wk post infection. Aberrant tortuous and cystic glands were observed. (E) Gastritis with architectural aberration in the corpus mucosa of a representative animal at 16 wk post infection. Prominent invasive gland lesions were observed. (F) Branched lesions (arrow), (G) dilated lesions (arrow), and (H) invasive glands (arrow) were observed in the corpus mucosa of a representative animal at 12 wk post infection. Bar=100 µm.
Metaplastic lesions in gerbil gastric corpus mucosa with H. pylori 7.13 infection
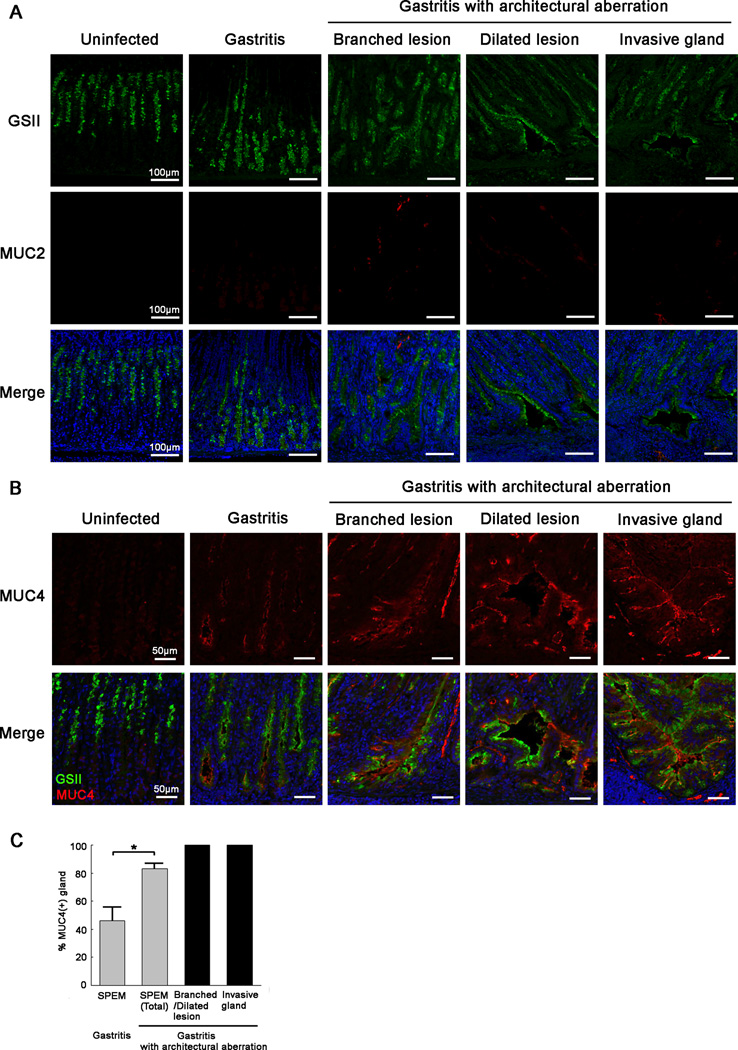
To detect metaplastic lesions in gastric mucosa after H. pylori 7.13 infection, we stained for GSII and MUC2, typical markers for SPEM and intestinal metaplasia, respectively (Figure 2). In the normal corpus, GSII was expressed in mucous neck regions. In gastritis without architectural aberration, GSII expression was localized in the cytoplasm of cells of the base regions of the corpus glands (Figure 2A), with morphologies characteristic of SPEM. Gastritis with architectural aberration, such as branched lesions, dilated lesions and invasive glands also showed GSII expression (Figure 2A), consistent with the concept that these gastritis lesions were progressive phenotypes of SPEM. GSII expression was seen in the sub-apical regions and cytoplasm of cells in dilated lesions and invasive glands. On the other hand, MUC2 was absent in the normal corpus and in gastritis without architectural aberration (Figure 2A).
Figure 2. Progression of metaplastic lesions in gerbil gastric corpus mucosa following H. pylori 7.13 infection.
(A) Immunofluorescence staining of metaplastic glands with GSII lectin (green) and antibodies against MUC2 (red). All sections were from the corpus or intermediate zone of gerbils. Gastritis sections shown, containing glands without architectural aberration, were from representative gerbils at 6 wk post infection. Gastritis sections showing glands with architectural aberration, including branched lesions, dilated lesions and invasive glands were from representative gerbils in the corpus mucosa of a representative animal at 8 wk post infection. In uninfected gerbils, GSII expression was seen in mucous neck cells. GSII was expressed not only in cells at the base of gastritis glands without architectural aberration, but also in branched lesions, dilated lesions and invasive glands of gastritis glands with architectural aberration. MUC2 was not expressed in the normal corpus or in the corpus of animals with gastritis. Bar=100 µm.
(B) Immunofluorescence staining of SPEM with antibodies against MUC4 (red) and GSII lectin (green). All sections were from the corpus or intermediate zone of gerbils. Representative gastritis sections without architectural aberration are shown from gerbils in the corpus mucosa of a representative animal at 6 wk post infection. Branched lesions and dilated lesions were analysed from in the corpus mucosa of a representative gerbil at 12 wk post infection. Invasive glands were examined in the gastric corpus mucosa of a representative gerbil at 16 wk post infection. In uninfected gerbils, MUC4 expression was not observed. In gastritis without architectural aberration, MUC4 was weakly expressed in the sub-apical region of cells at the base of SPEM glands. In gastritis with architectural aberration, MUC4 was strongly expressed in progressive phenotypes of SPEM, including dilated lesions, branched lesions and invasive glands. Bar=50 µm.
(C) Quantitation of the percentage of MUC4-positive glands in SPEM. All gastric corpus glands were analysed in each section of all samples used in this study. SPEM was detected by GSII staining. In the presence of more severe inflammation, the percentage of MUC4-positive glands increased significantly. All glands with architectural aberration expressed MUC4 expression. All values are shown as mean±SEM (*p<0.05 vs gastritis without architectural aberration.).
While gerbils infected with H. pylori 7.13 show a rapid onset of mucosal changes, they do not tend to develop significant amounts of goblet cell intestinal metaplasia. Indeed, we identified only one gerbil sample displaying a focal lesion of MUC2-expressing goblet cells indicative of intestinal metaplasia. In this gerbil (with gastritis with architectural aberration), MUC2-positive goblet cells were observed in the bottom of glands with SPEM (Supplementary Figure 2A). In these glands, some cells showed the co-expression of GSII and MUC2. Overall, these results suggest that the pathological mucosal changes in the H. pylori 7.13 strain infection model are dominated by SPEM rather than intestinal metaplasia.
MUC4 is strongly expressed in progressive SPEM in gerbil stomach corpus
We have previously examined markers related with the emergence and progression of SPEM by comparing microarray data from SPEM regions in three different mouse models of parietal cell loss [11]. In the current study, to investigate possible markers related with the progression of SPEM following chronic inflammation, we focused on transcripts that were specifically upregulated in the mouse model of SPEM with chronic inflammation from this previous microarray analysis. Among these transcripts, we examined the expression of MUC4 in the gastric mucosa based on antibody availability for gerbils. MUC4 is a membrane mucin that is abundantly expressed in many epithelia, and acts through anti-adhesive or signalling mechanisms, including as a ligand/modulator of the receptor tyrosine kinase ErbB2 [20]. First, we examined the expression of MUC4 in human gastric tissues. In normal human gastric mucosa, MUC4 was detected only in the cytoplasm of some scattering cells in the antrum and not in the corpus (Supplementary Figure 3). In human duodenum, MUC4 was expressed in the sub-apical membrane of intestinal epithelial cells and goblet cells. In metaplasias of the human stomach, MUC4 was expressed in the sub-apical regions and goblet cells of intestinal metaplasia, but not in SPEM. These results suggest that MUC4 is a marker of intestinal metaplasia in humans. Next, we examined the expression of MUC4 in the gerbil gastric mucosa. In normal gerbil gastric mucosa, MUC4 expression was observed only in the cytoplasm of some scattering cells in the antrum (Supplementary Figure 4) and not in the corpus (Figure 2B), as observed in humans (Supplementary Figure 3). However, in gastritis without architectural aberration, MUC4 was weakly expressed in the sub-apical membrane of cells at the base of SPEM glands (Figure 2B,C). Interestingly, MUC4 was strongly upregulated in progressive phenotypes of SPEM, including branched lesions, dilated lesions and invasive glands (Figure 2B,C). In addition, in the one gerbil exhibiting intestinal metaplasia, MUC4 expression was observed in MUC2-positive goblet cells as well as adjacent SPEM (Supplementary Figure 2A). These data suggest that MUC4 is expressed in both SPEM and intestinal metaplasia in gerbils.
Clusterin is strongly expressed in specific regions of metaplasia in gerbil stomach corpus
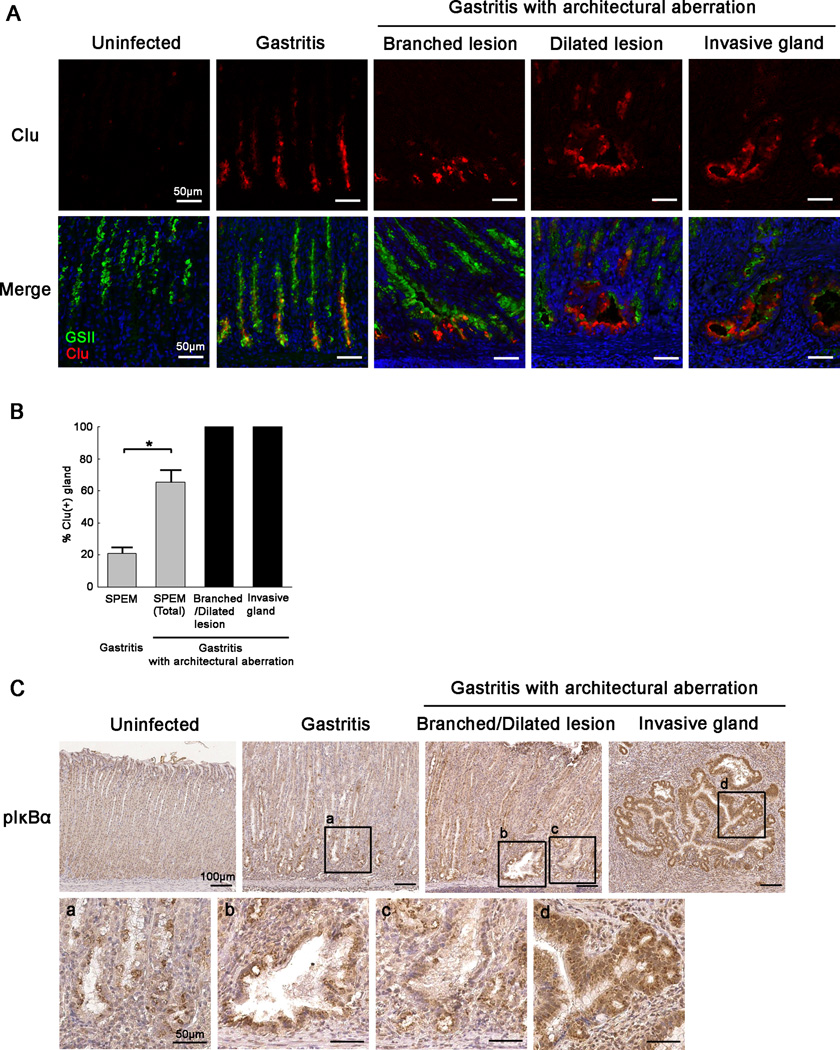
Clusterin is a stress-induced, multifunctional secreted and cytoplasmic molecular chaperone. Levels of cytoplasmic clusterin increase with retrotranslocation against various ER stressors [21]. We demonstrated previously that clusterin was upregulated throughout regions of SPEM in humans and three different mouse models of parietal cell loss [11]. In addition, clusterin expression was correlated with poor prognosis in human gastric cancers [11,22]. To investigate protein expression profiles in the progression of metaplasia, we examined clusterin expression in gerbil gastric mucosa. In the normal gastric corpus of gerbils, clusterin was expressed only in scattered cells (Figure 3A). In gastritis without architectural aberration, clusterin expression was detected at high levels in the cytoplasm of cells in the deep regions of SPEM glands (Figure 3A,B). Interestingly, progressive phenotypes of SPEM, including branched and dilated lesions, showed strong expression of clusterin, in particular in the tips of branched glands (Figure 3A,B). In addition, invasive glands also strongly expressed clusterin throughout (Figure 3A,B). In these progressive lesions, clusterin was localized in the sub-apical regions strongly as well as weakly in the cytoplasm. In the one animal with intestinal metaplasia, clusterin expression was also observed in some MUC2-positive goblet cells (Supplementary Figure 2B). These findings suggest that clusterin is a possible marker of progressive regions of metaplasia in the gerbil gastric corpus mucosa.
Figure 3. Expression of clusterin and phospho-IκBα in gerbil gastric corpus mucosa following H. pylori 7.13 infection.
(A) Immunofluorescence staining of SPEM with antibodies against clusterin (Clu) (red) and GSII (green). All sections were from the corpus or intermediate zone of gerbil stomach. Representative gastritis sections without architectural aberration shown were from gerbils at 6 wk post infection. Representative branched lesions and dilated lesions shown were from gerbils at 12 wk post infection. Invasive glands were examined from gerbils at 16 wk post infection. In uninfected gerbils, Clu was detected only in some scattered cells. In gastritis without architectural aberration, Clu was expressed strongly in the cytoplasm of cells at the base regions of SPEM glands. In gastritis with architectural aberration, Clu was strongly expressed in the sub-apical regions as well as in the cytoplasm of progressive phenotypes of SPEM, in particular in the cells at the tips of branched and dilated lesions and throughout regions of invasive glands. Bar=50 µm.
(B) Quantitation of the percentage of Clu-positive glands in SPEM. All corpus glands were analysed in each section of all samples used in this study. SPEM was detected by GSII staining. In the presence of more severe inflammation, the percentage of Clu-positive glands increased significantly. All glands with architectural aberration showed Clu expression. All values are shown as mean±SEM (*p<0.05 vs gastritis without architectural aberration.).
(C) Immunohistochemistry of SPEM with antibodies against phospho-IκBα. All sections were from the gastric corpus or intermediate zone of gerbil stomachs. Branched lesions and dilated lesions were obtained from gerbils at 8 wk post infection. Invasive glands were examined from gerbils at 16 wk post infection. a. the base regions of gastritis without architectural aberration. b. a dilated lesion. c. a branched lesion. d. an invasive gland. No phospho-IκBα expression was observed in uninfected gerbils. In gastritis without architectural aberration, phospho-IκBα was expressed in the cytoplasm of cells in the base regions of glands. In gastritis with architectural aberration, phospho-IκBα was strongly expressed in the cytoplasm of progressive phenotypes of SPEM, in particular throughout regions of invasive glands. Bar=100 µm or 50 µm (high magnification).
To evaluate this hypothesis, we examined the expression of phospho-IκBα as a marker of activation of NFκB [23,24]. NFκB activation has been associated with progressive phenotypes in a number of malignancies [23]. In the normal mucosa, expression of phospho-IkBα was not observed (Figure 3C). In gastritis without architectural aberration, phospho-IκBα was detected in the cytoplasm of cells in the base regions of glands in a similar distribution as clusterin-expressing regions (Figure 3C). In gastritis with architectural aberration, phospho-IκBα was strongly expressed in progressive phenotypes of SPEM, particularly in invasive glands. These results suggest that activation of the NFκB pathway is associated with the progression of metaplasia.
Clusterin-positive regions of progressive SPEM in gerbil stomach show increased proliferation and invasive characteristics
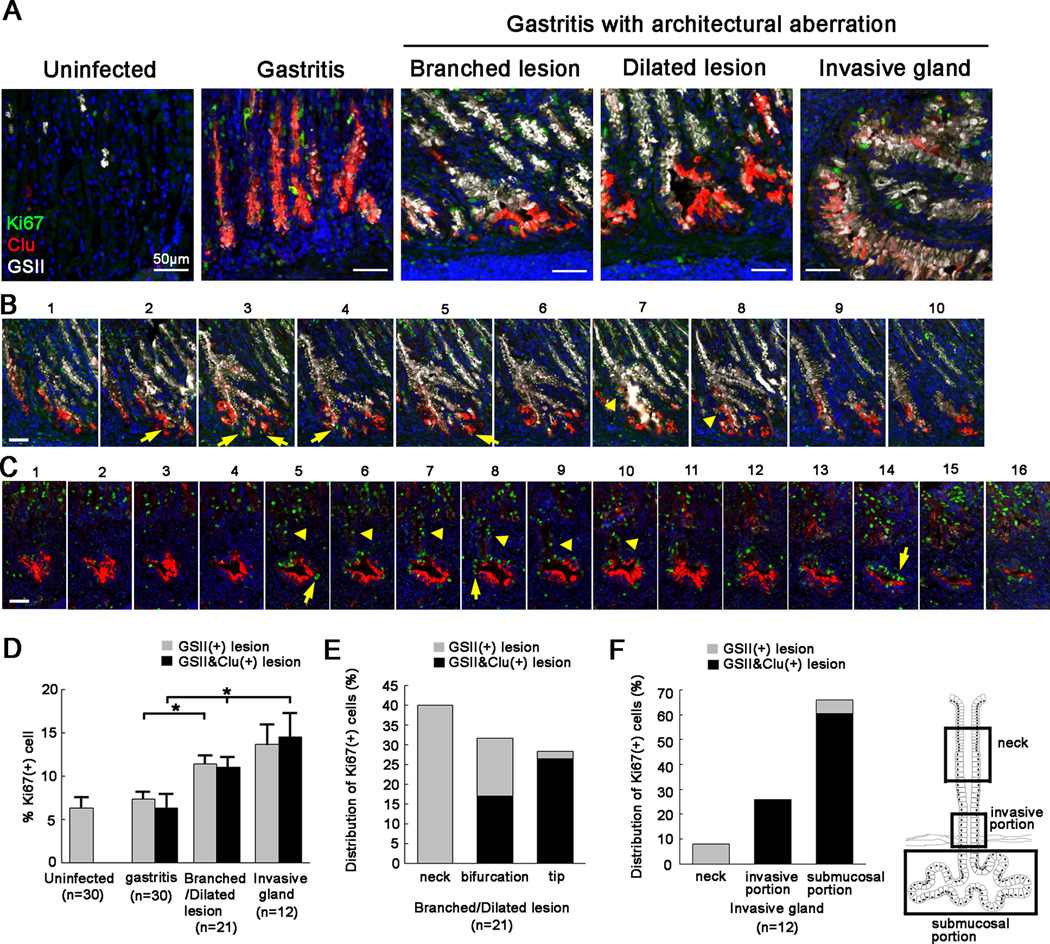
To characterize progressive phenotypes of SPEM with clusterin expression, we performed Ki67 staining in H. pylori-infected gastric corpus mucosa. In normal corpus, Ki67 expression was limited to the region close to the progenitor zone in the neck, but was not seen in the base regions of the glands (Figure 4A). In gastritis without architectural aberration, Ki67-positive cell number increased not only in the progenitor zone of the neck regions of glands, but also in the base regions of glands (Figure 4A). These findings suggest that some SPEM cells have proliferative function, whereas chief cells are not proliferative [10]. In addition to progenitor zones in the neck regions of glands, Ki67 expression was observed in the tips and bifurcations of branched and dilated lesions. Clusterin and Ki67 double-positive cells were frequently seen in the tips of these lesions (Figure 4B,D,E). In invasive glands, Ki67-positive cells were present in submucosal regions with strong clusterin expression as well as in invasive portions through the muscularis mucosa (Figure 4C,F, Supplementary Figure 5). These findings suggest that clusterin-positive regions in progressive phenotypes of SPEM have proliferative behaviour.
Figure 4. Ki67 expression in metaplastic lesions of gerbil gastric corpus mucosa following H. pylori 7.13 infection.
(A) Immunofluorescence staining of SPEM with antibodies against Ki67 (green) and clusterin (Clu) (red) with GSII lectin (white). All sections were from the gastric corpus or intermediate zone of gerbil stomachs. Representative gastritis sections without architectural aberration were from gerbils at 6 wk post infection. Representative specimens with branched lesions and dilated lesions were from gerbils at 12 wk post infection. Invasive glands were examined in sections from gerbils at wk post infection. In uninfected gerbils, Ki67 expression was limited to the progenitor zone in the neck region. In gastritis without architectural aberration, Ki67 was expressed in the neck regions of glands as well as in some Clu-positive cells at the bases of glands. In gastritis with architectural aberration, Ki67 expression was observed in the tips and bifurcations of branched and dilated lesions and in submucosal regions of invasive glands, in addition to the neck regions of mucosal glands. Bar=50 µm.
(B) Serial section analyses of branched lesions. Serial sections were obtained from a representative gerbil corpus at 12 wk post infection. Immunofluorescence staining of SPEM with antibodies against Ki67 (green), Clu (red) and GSII (white) was performed. Ki67 expression was observed in the tips (arrows) and bifurcations (arrowheads) of branched lesions. Bar=50 µm.
(C) Serial section analyses of invasive glands. Serial sections were obtained from a representative gerbil corpus at 16 wk post infection. Immunofluorescence staining of SPEM with antibodies against Ki67 (green) and Clu (red) with GSII lectin (white) was performed. Ki67 expression was detected in the submucosal region (arrows) and invasive portions (arrowheads). Bar=50 µm.
(D) Quantitation of the percentage of Ki67-positive cells in SPEM and Clu-positive regions of SPEM. In the normal stomach and gastritis without architectural aberration, 10 corpus glands were analysed each from 3 sections from 3 different gerbils. In gastritis with architectural aberration, 21 dilated/branched lesions and 12 invasive glands were analysed. SPEM was detected by GSII staining. In uninfected gerbils, GSII-positive cells represent mucous neck cells. In the presence of more-severe inflammation, the percentage of Ki67-positive cells increased in both SPEM in general and in Clu-positive SPEM. All values are shown as means±SE of the mean (*p<0.05 vs gastritis without architectural aberration.).
(E) Distribution of Ki67-positive cells in branched or dilated lesions. The percentage of Ki67-positive cells in the upper (neck) regions, bifurcations and tips are shown (grey bar). In addition, the percentage of Clu-positive cells in these Ki67-positive cells are also shown (black bar).
(F) Distribution of Ki67-positive cells in invasive glands. The percentage of Ki67-positive cells in the upper (neck) regions, invasive portions and submucosal portions are shown (grey bar). The percentage of Clu-positive cells in these Ki67-positive cells are also shown (black bar).
Next, to investigate invasive characteristics in progressive phenotypes of SPEM, we examined the expression of matrix metalloproteinase 7 (MMP-7), a member of a family of enzymes associated with tumour-initiating properties, in H. pylori-infected gerbil gastric corpus mucosa. We and others previously demonstrated that MMP-7 was overexpressed in human gastric cancer tissues and that H. pylori CagA-positive strains selectively increased MMP-7 levels in gastric epithelial cells [25–27]. In gerbil gastric mucosa, MMP-7 expression was not observed in the normal corpus. However, MMP-7 was present in the base of glands of gastritis without architectural aberration, particularly in clusterin-positive cells (Figure 5A). Furthermore, MMP-7 was strongly expressed in branched lesions, dilated lesions and invasive glands; in particular, in the tips, bifurcations and submucosal regions of these lesions (Figure 5A). Clusterin-positive cells showed MMP-7 expression increasingly with the severity of inflammation (Figure 5B). These findings suggest that clusterin and MMP-7 expressing regions in progressive phenotypes of SPEM have invasive properties.
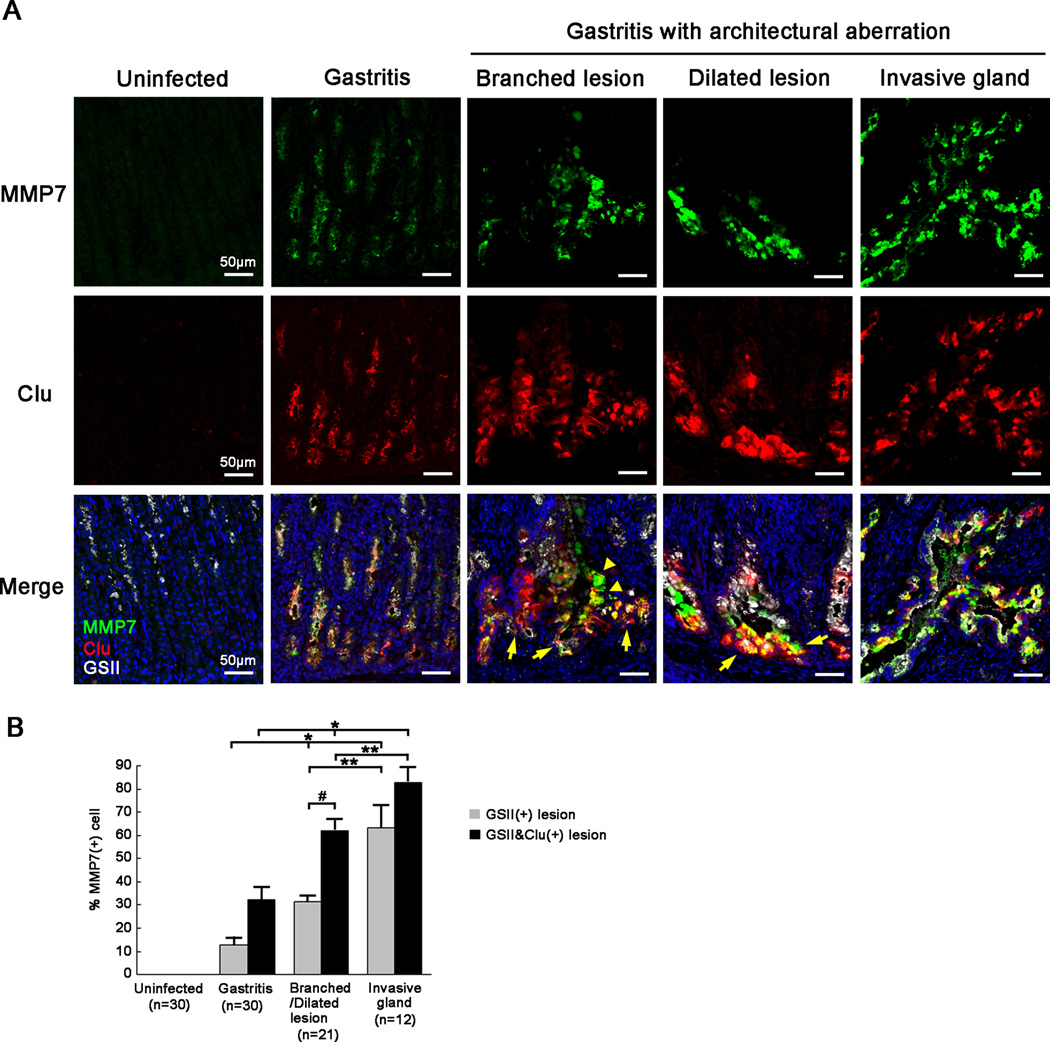
Figure 5. MMP-7 expression in metaplastic lesions of gerbil gastric corpus mucosa following H. pylori 7.13 infection.
(A) Immunofluorescence staining of SPEM with antibodies against MMP-7 (green), clusterin (Clu) (red) and GSII (white). All sections were from the corpus or intermediate zone of gerbil stomachs. Representative gastritis sections without architectural aberration were from gerbils at 6 wk post infection. Representative branched lesions and dilated lesions were from gerbils at 12 wk post infection. Invasive glands were examined in gerbils at 16 wk post infection. In uninfected gerbils, MMP-7 expression was not detected. In gastritis without architectural aberration, MMP-7 was expressed in Clu-positive cells at the base of glands. In gastritis with architectural aberration, MMP-7 was strongly expressed in branched lesions, dilated lesions and invasive glands, in particular in the tips (arrows), bifurcations (arrowheads) and submucosal regions of these lesions. Bar=50 µm.
(B) The percentage of MMP-7-positive cells in SPEM and Clu-positive regions of SPEM. In the normal stomach and gastritis without architectural aberration, 10 corpus glands were analysed from 3 sections from 3 different gerbils. In gastritis with architectural aberration, 21 dilated/branched lesions and 12 penetrating heterotopic glands were analyzed. SPEM was detected by GSII staining. In gastritis without architectural aberration, less than half of Clu-positive cells in SPEM had MMP-7 expression. In gastritis with architectural aberration, the percentage of MMP-7-positive cells in SPEM increased, in particular, in Clu-positive regions. All values are shown as mean±SEM (*p<0.05 vs gastritis without architectural aberration. **p<0.05 vs branched/dilated lesions. #p<0.05 vs GSII(+) lesions.).
Discussion
Gastric cancer develops ion humans after decades of chronic H. pylori infection. Chronic inflammation as well as virulence factors of H. pylori, are required for gastric cancer development [28]. Metaplastic changes, such as SPEM and intestinal metaplasia, are considered neoplastic precursors, but it is difficult to examine the progression from metaplastic lesions to dysplasia in humans, because inflammation persists for decades. In the current study, we investigated how metaplasia progresses into advanced phenotypes using a gerbil model infected with H. pylori strain 7.13. The characteristics of this model, including persistent and chronic H. pylori-induced inflammation and the ability of H. pylori strains to maintain CagA translocation, allow examination of the process of metaplastic progression [16,17]. In this model, SPEM developed after H. pylori infection, and then dynamically evolved into aberrant gland phenotypes, including branched lesions, dilated lesions and invasive glands. These findings suggest that the disturbance of gland morphology emerges in SPEM during chronic gastritis with H. pylori infection. These changes resemble pre-neoplastic progression in human lesions. In humans, SPEM frequently forms at the bases of corpus glands after chronic H. pylori infection (Supplementary Figure 3). These findings are consistent with our previous studies demonstrating the evolution of SPEM through transdifferentiation of chief cells following parietal cell loss [10].
There are differing viewpoints as to whether invasive lesions observed in association with H. pylori infection in gerbils truly represents gastric adenocarcinoma [16,29]. Unlike humans, invasive lesions frequently emerge in gerbils several months after H. pylori infection and expand in the submucosa as well as subserosa. These lesions have been classified as adenocarcinoma based upon previously published criteria defining morphological characteristics of neoplasia arising in the setting of inflammation in mouse models [30]. However, some reports have suggested that these lesions are not adenocarcinoma, because they dramatically diminish with the eradication of H. pylori [29,31]. Regression of invasive glands may relate to the duration of H. pylori infection, indicating a point of no return after longer infection [32]. Since the gerbil model does not develop metastatic disease, definition of dysplasia or adenocarcinoma has relied on haematoxylin and eosin morphological criteria. In the current study, detailed characterization of metaplastic biomarkers indicates a process of lineage expression patterns associated with increased architectural aberrations that are consistent with neoplastic or dysplastic transitions. Interestingly, we showed that branched and cystic glands as well as invasive glands detected in this study had the characteristics of SPEM. Invasive glands may represent a pre-neoplastic lesion derived from progressive evolution of SPEM into more invasive phenotypes. These findings are consistent with a view that SPEM represents a key precursor to development of gastric neoplasia.
It is well recognized that intestinal metaplasia develops in the setting of chronic gastritis caused by H. pylori infection in humans [2]. Inflammatory signals are essential to the emergence of intestinal metaplasia [33], and SPEM is considered a possible origin of intestinal metaplasia [15,34], but detailed mechanisms are still unknown. In the current study, MUC4, an intestinal metaplasia marker in humans, was expressed in SPEM and a focal intestinal metaplasia lesion found in a single gerbil. Interestingly, MUC4 expression increased as SPEM progressed. Previous studies demonstrated that the inflammatory signal cytokine IL-6 can modulate MUC4 expression in gastric cancer cell lines via the STAT3 signalling pathway [35,36]. These findings suggest that MUC4 is a marker of progressive metaplasia with intestinal characteristics following chronic inflammation. In addition, a number of reports demonstrated that MUC4 expression in various malignant lesions, including gastric cancer, is correlated with poor outcome [37–41]. A recent report showed that MUC4-deficient mice are resistant to DSS-induced colitis and AOM/DSS-induced colorectal cancers [42]. These data suggest that MUC4 is not only a marker for intestinal characteristics in metaplasia, but also may participate in the development of inflammation-associated cancers.
The question of whether metaplastic lesions are direct origins of gastric neoplasia remains unclear. The expression pattern of clusterin in gerbil stomach shown in this study may indicate a link between metaplasia and neoplasia. In gastritis without architectural aberration, clusterin is expressed only in the bottom of SPEM glands. However, clusterin-positive regions already show the expression of MMP-7, which has been associated with the acquisition of invasive properties [43]. Clusterin-positive metaplastic cells show further elevations in MMP-7 expression as well as increased proliferation in association with more-severe inflammation. The increased expression of MMP-7 may contribute to the formation of more branched or invasive gland phenotypes. Previous studies showed that clusterin increases with retrotranslocation following many ER stressors and has specific roles in stress responses, including anti-apoptotic and cytoprotective functions [21]. These findings suggest that SPEM with clusterin expression may be more susceptible to transformation. Furthermore, these findings are supported by our previous results that clusterin is upregulated in the majority of intestinal-type and diffuse-type human gastric cancers and high levels of clusterin expression in late stage gastric cancers correlated with a poor outcome [11,22]. In humans, unlike in gerbils, clusterin is expressed in the isthmus of normal corpus and is upregulated throughout regions of SPEM and transitional zones between SPEM and intestinal metaplasia [11]. These transitional zones within metaplastic glands are also proliferative [44]. Considering our findings in gerbils, SPEM and transitional zone cells with clusterin expression might be a source of neoplasia in humans.
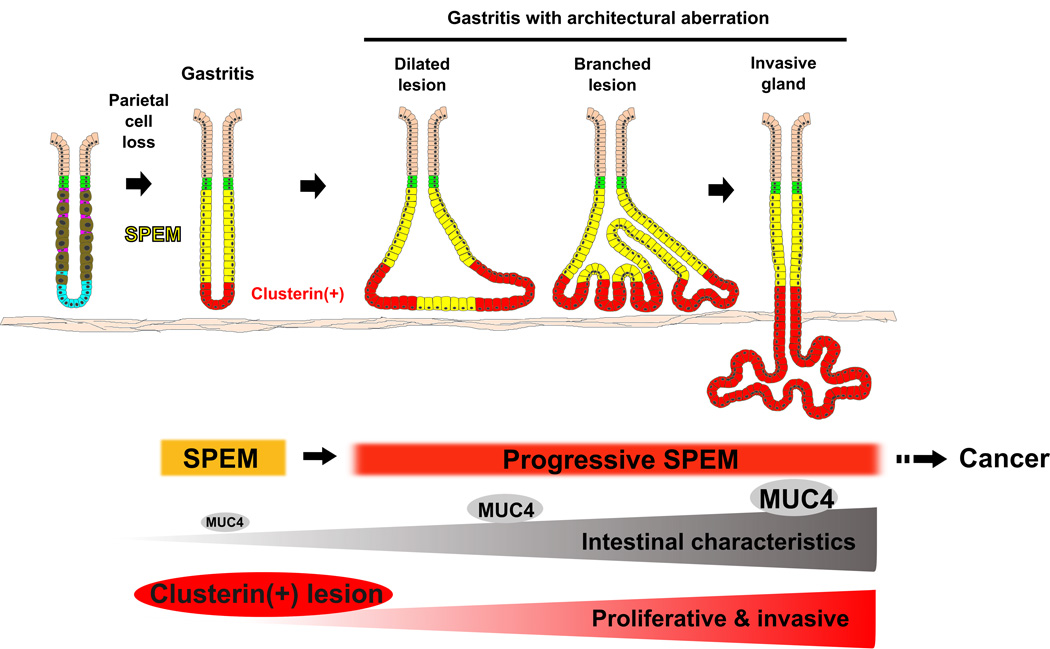
In summary, we have established that SPEM is generated after H. pylori infection and then progresses to more invasive phenotypes in Mongolian gerbils (Figure 6). Interestingly, our detailed characterization of biomarkers in metaplastic lesions using immunostaining methods demonstrates a clear progression of metaplasia from development of SPEM to progression to intramucosal cystic and branched phenotypes, which then progress to invasive glands. Intramucosal lesions showed similar proliferation levels compared with invasive glands. These advanced metaplastic phenotypes therefore define a progression of increasingly aberrant pre-neoplastic lesions derived from SPEM, which may define a paradigm for pre-cancerous evolution.
Figure 6. Schematic summary for progression of SPEM in gerbil gastric corpus following H. pylori 7.13 infection.
After H. pylori infection, SPEM is generated via transdifferentiation of chief cells following parietal cell loss, and then evolves into more progressive phenotypes with intestinal and invasive characteristics. MUC4 expression is upregulated in SPEM and then increases as SPEM progresses toward intestinal metaplasia. Clusterin is expressed in the bottom of SPEM glands, and the clusterin-positive regions give rise to aberrant gland phenotypes and invade into the submucosa.
Supplementary Material
Acknowledgments
These studies were supported by grants from a Department of Veterans Affairs Merit Review Award IBX000930 and NIH RO1 DK071590 and RO1 DK 101332, as well as a grant from the Martell Foundation (to J.R.G), as well as NIH grants RO1 DK58587, RO1 CA77955 and PO1 CA116087 (to R.M.P.). T.S. is the recipient of JSPS Postdoctoral Fellowships for Research Abroad. This work was supported by core resources of the Vanderbilt Digestive Disease Center, (P30 DK058404), the Vanderbilt-Ingram Cancer Center (P30 CA68485, Chemical Synthesis Core), and imaging supported by both the Vanderbilt Combined Imaging Shared Resource and the Vanderbilt Digital Histology Shared Resource.
Footnotes
Disclosures: The authors have no conflicts of interest.
Author contributions:
Takahiro Shimizu performed experiments and designed experiments, prepared figures, drafted and revised the manuscript.
Eunyoung Choi performed and designed experiments and revised the manuscript.
Christine P. Petersen performed and designed experiments and revised the manuscript.
Jennifer M. Noto performed and designed experiments and revised the manuscript.
Judith Romero-Gallo performed experiments and revised the manuscript.
Maria B. Piazuelo performed and designed experiments and revised the manuscript.
M. Kay Washington evaluated pathology and revised the manuscript.
Richard M. Peek Jr. designed experiments and revised the manuscript.
James R. Goldenring designed experiments and revised the manuscript.
SUPPORTING INFORMATION ON THE INTERNET
Figure S1. Inflammation in gerbil gastric corpus following H. pylori 7.13 infection.
Figure S2. Expression patterns in a focus of intestinal metaplasia in gerbil gastric corpus following H. pylori infection.
Figure S3. MUC4 expression in human tissues.
Figure S4. MUC4 expression in the antrum of uninfected gerbils.
Figure S5. Serial section analyses of mixed lesions of branched, dilated regions and invasive glands.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. A Human Model of Gastric Carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 3.Blaser MJ, Musser JM, Berg DE. Bacterial polymorphisms Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767–773. doi: 10.1172/JCI12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polk DB, Peek RM., Jr Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12:189–197. doi: 10.1007/s10120-009-0527-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldenring JR, Nam KT, Mills JC. The origin of pre-neoplastic metaplasia in the stomach: chief cells emerge from the Mist. Exp Cell Res. 2011;317:2759–2764. doi: 10.1016/j.yexcr.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rokkas T, Filipe MI, Sladen GE. Detection of an increased incidence of early gastric cancer in patients with intestinal metaplasia type III who are closely followed up. Gut. 1991;32:1110–1113. doi: 10.1136/gut.32.10.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zivny J, Wang TC, Yantiss R, et al. Role of therapy or monitoring in preventing progression to gastric cancer. J Clin Gastroenterol. 2003;36:S50–S60. doi: 10.1097/00004836-200305001-00009. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 10.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028.e9–2037.e9. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weis VG, Sousa JF, LaFleur BJ, et al. Heterogeneity in mouse spasmolytic polypeptide-expressing metaplasia lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirayama F, Takagi S, Yokoyama Y, et al. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996;31(Suppl 9):24–28. [PubMed] [Google Scholar]
- 13.Watanabe T, Tada M, Nagai H, et al. Helicobacter pylori infection induces gastric cancer in Mongolian gerbils. Gastroenterology. 1998;115:642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- 14.Honda S, Fujioka T, Tokieda M, et al. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998;58:4255–4259. [PubMed] [Google Scholar]
- 15.Yoshizawa N, Takenaka Y, Yamaguchi H, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 16.Franco AT, Israel DA, Washington MK, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci U S A. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco AT, Johnston E, Krishna U, et al. Regulation of gastric carcinogenesis by Helicobacter pylori virulence factors. Cancer Res. 2008;68:379–387. doi: 10.1158/0008-5472.CAN-07-0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noto JM, Gaddy JA, Lee JY, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest. 2013;123:479–492. doi: 10.1172/JCI64373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leys CM, Nomura S, Rudzinski E, et al. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37:1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Kim JK, Edwards CA, et al. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–915. doi: 10.1038/ncb1291. [DOI] [PubMed] [Google Scholar]
- 22.Bi J, Guo AL, Lai YR, et al. Overexpression of clusterin correlates with tumor progression, metastasis in gastric cancer: a study on tissue microarrays. Neoplasma. 2010;57:191–197. doi: 10.4149/neo_2010_03_191. [DOI] [PubMed] [Google Scholar]
- 23.Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-κB as the matchmaker. Nat Immunol. 2011;12:715–723. doi: 10.1038/ni.2060. [DOI] [PubMed] [Google Scholar]
- 24.Shibata W, Hirata Y, Maeda S, et al. CagA protein secreted by the intact type IV secretion system leads to gastric epithelial inflammation in the Mongolian gerbil model. J Pathol. 2006;210:306–314. doi: 10.1002/path.2040. [DOI] [PubMed] [Google Scholar]
- 25.Crawford HC, Krishna US, Israel DA, et al. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology. 2003;125:1125–1136. doi: 10.1016/s0016-5085(03)01206-x. [DOI] [PubMed] [Google Scholar]
- 26.Wroblewski LE, Noble PJ, Pagliocca A, et al. Stimulation of MMP-7 (matrilysin) by Helicobacter pylori in human gastric epithelial cells: role in epithelial cell migration. J Cell Sci. 2003;116:3017–3026. doi: 10.1242/jcs.00518. [DOI] [PubMed] [Google Scholar]
- 27.McDonnell S, Navre M, Coffey RJ, Jr, et al. Expression and localization of the matrix metalloproteinase pump-7 (MMP-7) in human gastric and colon carcinomas. Mol Carcinog. 1991;4:527–533. doi: 10.1002/mc.2940040617. [DOI] [PubMed] [Google Scholar]
- 28.Amieva M, Peek RM., Jr Pathobiology of Helicobacter pylori-induced Gastric Cancer. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatematsu M, Tsukamoto T, Mizoshita T. Role of Helicobacter pylori in gastric carcinogenesis: the origin of gastric cancers and heterotopic proliferative glands in Mongolian gerbils. Helicobacter. 2005;10:97–106. doi: 10.1111/j.1523-5378.2005.00305.x. [DOI] [PubMed] [Google Scholar]
- 30.Boivin GP, Washington K, Yang K, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–777. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 31.Nozaki K, Shimizu N, Tsukamoto T, et al. Reversibility of heterotopic proliferative glands in glandular stomach of Helicobacter pylori-infected Mongolian gerbils on eradication. Jpn J Cancer Res. 2002;93:374–381. doi: 10.1111/j.1349-7006.2002.tb01267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero-Gallo J, Harris EJ, Krishna U, et al. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328–336. doi: 10.1038/labinvest.3700719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen CP, Weis VG, Nam KT, et al. Macrophages promote progression of spasmolytic polypeptide-expressing metaplasia after acute loss of parietal cells. Gastroenterology. 2014;146:1727.e8–1738.e8. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam KT, Lee HJ, Mok H, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mejías-Luque R, Peiró S, Vincent A, et al. IL-6 induces MUC4 expression through gp130/STAT3 pathway in gastric cancer cell lines. Biochim Biophys Acta. 2008;1783:1728–1736. doi: 10.1016/j.bbamcr.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Mejías-Luque R, Lindén SK, Garrido M, et al. Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene. 2010;29:1753–1762. doi: 10.1038/onc.2009.467. [DOI] [PubMed] [Google Scholar]
- 37.Hwang I, Kang YN, Kim JY, et al. Prognostic significance of membrane-associated mucins 1 and 4 in gastric adenocarcinoma. Exp Ther Med. 2012;4:311–316. doi: 10.3892/etm.2012.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamura Y, Higashi M, Kitamoto S, et al. MUC4 and MUC1 expression in adenocarcinoma of the stomach correlates with vessel invasion and lymph node metastasis: an immunohistochemical study of early gastric cancer. PLoS One. 2012;7:e49251. doi: 10.1371/journal.pone.0049251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamada S, Shibahara H, Higashi M, et al. MUC4 is a novel prognostic factor of extrahepatic bile duct carcinoma. Clin Cancer Res. 2006;12:4257–4264. doi: 10.1158/1078-0432.CCR-05-2814. [DOI] [PubMed] [Google Scholar]
- 40.Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibahara H, Tamada S, Higashi M, et al. MUC4 is a novel prognostic factor of intrahepatic cholangiocarcinoma-mass forming type. Hepatology. 2004;39:220–229. doi: 10.1002/hep.20031. [DOI] [PubMed] [Google Scholar]
- 42.Das S, Rachagani S, Sheinin Y, et al. Mice deficient in Muc4 are resistant to experimental colitis and colitis-associated colorectal cancer. Oncogene. 2015 doi: 10.1038/onc.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ii M, Yamamoto H, Adachi Y, et al. Role of matrix metalloproteinase-7 (matrilysin) in human cancer invasion, apoptosis, growth, and angiogenesis. Exp Biol Med (Maywood) 2006;231:20–27. doi: 10.1177/153537020623100103. [DOI] [PubMed] [Google Scholar]
- 44.Sousa JF, Ham AJ, Whitwell C, et al. Proteomic profiling of paraffin-embedded samples identifies metaplasia-specific and early-stage gastric cancer biomarkers. Am J Pathol. 2012;181:1560–1572. doi: 10.1016/j.ajpath.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.