Abstract
Trichloroethylene (TCE) is a widespread environmental toxicant with immunotoxic and neurotoxic potential. Previous studies have shown that continuous developmental exposure to TCE encompassing gestation and early life as well as postnatal only exposure in the drinking water of MRL+/+ mice promoted CD4+ T cell immunotoxicity, glutathione depletion and oxidative stress in the cerebellum, as well increased locomotor activity in male offspring. The purpose of this study was to characterize the effects of exclusively prenatal exposure on these parameters. Another goal was to investigate potential plasma oxidative stress/inflammatory biomarkers to possibly be used as predictors of TCE-mediated neurotoxicity. In the current study, 6 week old male offspring of dams exposed gestationally to 0, 0.01, and 0.1 mg/ml TCE in the drinking water were evaluated. Our results confirmed that the oxidized phenotype in plasma and cerebellum was maintained after exclusively prenatal exposure. A Phenotypic analysis by flow cytometry revealed that TCE exposure expanded the effector/memory subset of peripheral CD4+ T cells in association with increased production of pro-inflammatory cytokines IFN-γ and IL-17. Serum biomarkers of oxidative stress and inflammation were also elevated in plasma suggesting that systemic effects are important and may be used to predict neurotoxicity in our model. These results suggested that the prenatal period is a critical stage of life by which the developing CNS and immune system are susceptible to long-lasting changes mediated by TCE.
Keywords: trichloroethylene oxidative stress, cerebellum, behavior, glutathione, redox, inflammation
1. Introduction
The generation of reactive oxygen species and/or depletion of the glutathione anti-oxidant system as evidenced by a decrease in the active form of glutathione (GSH) and an increase in the inactive oxidized disulfide (GSSG), enhances susceptibility to oxidative stress leading to organ-specific cell damage (Biswas et al., 2006; Bobyn et al., 2002; Filomeni et al., 2002). This is especially evident during development and early life stages (Maffi et al., 2008; McLean et al., 2005; Noble et al., 2005), and may represent a key process by which environmental toxicants influence neurotoxicity (Costa et al, 2015). Glutathione, the major intracellular anti-oxidant in the brain, is derived from the transsulfuration pathway that intersects with the methionine cycle [reviewed in (Selhub et al., 2002)]. Methionine forms S-adenosylhomocysteine (SAM) which, through the transfer of its methyl group, is converted to S-adenosylmethionine (SAH) and homocysteine. Homocysteine has an alternative fate. It can regenerate methionine for an additional cycle, or it can be used to produce cysteine which can feed glutathione synthesis. Thus, from a functional standpoint, deficits in any of these metabolites could lead to neurotoxicity by promoting cellular differentiation, gene expression, DNA methylation, and oxidative stress.
Aside from oxidative stress, inflammation including peripheral immune activation and/or the cytokines they release can also adversely impact CNS responses (Anthony et al., 2011; Deledi et al., 2015). These cells and their mediators apparently cross the blood-brain-barrier made more permeable by inflammation (Stolp et al., 2005) to promote neuroinflammation and alter behavior (Wilson et al., 2002). Increased maternal cytokine release occurring during the perinatal period has been shown to increase the risk of offspring developing neurologic disease in adulthood (Hagberg et al., 2015). Similarly, rodent models of maternal infection resulting in increased cytokines have been experimentally linked with adverse neurologic outcomes in offspring (Malkova et al., 2012). Immune activation can also activate the kynurenine pathway (i.e., a by-product of the amino acid tryptophan) implicated in major depressive disorder (Hufner et al., 2015), as well as the release of oxidative stress/inflammatory mediators such as 3-Chlorotyrosine, by certain immune cells (Knutson et al., 2013). Thus, systemic inflammation immune activation, and oxidative stress are closely linked and may act in concert to promote neurotoxicity.
Environmental toxicants with the ability to promote oxidative stress and inflammatory responses may be important from a neurotoxicological standpoint. One such toxicant is the organic solvent and environmental pollutant trichloroethylene (TCE). TCE is a widespread contaminant that has been used for several decades in many industrial, commercial, medical, and consumer applications. TCE use has declined in the US in recent years. However, due to improper disposal practices, this chemical has become a persistent soil and water pollutant, and areas of TCE contamination are still being revealed. Based on likelihood of exposure together with negative health impact TCE is consistently ranked 16th out of 275 pollutants on the Superfund list of hazardous chemicals (ASTDR, 1997). Thus, human exposure to TCE remains a significant public health concern, and there is clearly a need to study the potential adverse health effect of TCE (Chiu et al., 2013).
Several studies have implicated the brain and immune system, namely CD4+ T cells, as targets of developmental TCE toxicity. Children exposed to TCE beginning in utero to a TCE-contaminated water supply had altered ratios of T cell subsets indicating T cell hyperactivity (Byers et al., 1988). We and others have shown that TCE promoted expansion of activated/memory CD4+ T cells with a Th1-like pro- inflammatory phenotype (Peden-Adams et al., 2006; Blossom and Doss, 2007; Blossom et al., 2008; Gilbert et al., 2014). In terms of neurotoxicity, studies by our lab and others have shown cerebellar and hippocampal neurotoxicity with exposure during the perinatal and postnatal periods of development associated with decreased learning and increased locomotor and exploratory activity (Blossom et al., 2012; Blossom et al., 2013; Taylor et al., 1985; Isaccson et al., 1990). Children of mothers working with TCE during pregnancy had poorer visual acuity, as well as impaired motor coordination and behavior characterized by inattention and hyperactivity (Laslo-Baker et al., 2004; Till et al., 2001). The mechanism by which developmental exposure to TCE imparts neurotoxicity is largely understudied. Several studies have shown that TCE induces oxidative stress (Ogino et al., 1991; Channel et al., 1998; Wang et al., 2009). We have focused on oxidative stress responses in the brain associated with developmental TCE exposure.
Before mechanism can be addressed, it is important to determine the most sensitive window of susceptibility for developmental TCE toxicity. Development of the immune system and the CNS in both humans and rodents spans throughout gestation and postnatally until adulthood suggesting that these systems, due to their extended period of maturation, may be particularly vulnerable to environmental stressors (Dietert et al., 2006; Rice et al, 2000). Although many important neurodevelopmental processes are shaped prenatally (Lindahl et al., 2008), the brain undergoes significant development postnatally, including transient periods of rapid or slow growth between birth and young adulthood (Gottlieb et al., 1977; Clancy et al., 2007; Dumas et al., 2005). Thus, we hypothesized that the impact of TCE after prenatal-only exposure would be less robust than observed at other developmental periods. In contrast to our expectations, the findings of this study revealed that many effects associated with postnatal and/or early life exposure were maintained with prenatal TCE exposure. This study also revealed several potential plasma biomarkers that may potentially be used to monitor TCE-induced effects in the brain.
2. Materials and Methods
2.1 Mice and TCE exposure
Gestational exposure to TCE in MRL+/+ mice has been described in detail (Blossom et al., 2008; Gilbert et al., 2014). Pregnant females were assigned to 3 groups by stratified randomization and given ultrapure water with 0 (control), 0.01, or 0.1 mg/ml TCE (purity 99+% purchased from Sigma, St. Louis, MO). Water with 0 (control), 0.01, or 0.1 mg/ml TCE also contained 1% Alkamuls EL-620, an emulsifier consisting of ethoxylated castor oil (Rhone-Poulenc, Cranbury, NJ), a reagent used to solubilize the TCE. Maternal exposure to TCE-containing drinking water ended at birth [i.e., postnatal day zero (PND0)]. Litters were standardized at birth to consist of no more than 8 pups per litter. At ~ 6 weeks of age, one randomly selected male mouse from each litter was used for all assays described below. All studies were approved by the Animal Care and Use Committee at the University of Arkansas for Medical Sciences.
2.2 Open field activity
All open field tests were conducted and analyzed using EthoVision XT 8.0 video- tracking software (Noldus Information Technology, Inc., Leesburg, VA). This system digitizes the video signal obtained from a color LCD camera mounted on the ceiling to determine the spatial coordinates of the mouse’s location within the testing arena. From these coordinates, the distance traveled per unit time, the number of times a defined zone is entered and the amount of time within a zone was calculated. The behavioral tests were conducted under standard lighting conditions in an isolated room to minimize the interference of noise. For locomotor activity, mice were placed in an unfamiliar testing arena (45 × 45 cm square) and the distance traveled (cm) was measured in a 20 min testing period. Other outcomes in the open field included total time in the center, number of times in the center, latency to enter the center zone, and velocity. Each behavior was calculated by the EthoVision software for each individual mouse.
2.3 HPLC quantification of plasma and cerebellum metabolites
Mice were deeply anesthetized with inhaled isoflurane (Fisher). A blood sample was collected using retro-orbital sampling, placed in heparinized tubes and processed to obtain plasma. Mice were then sacrificed and the cerebellum was dissected from the whole brain tissue and flash frozen in liquid nitrogen. Samples were stored at −80°C until extraction for metabolite detection. The methodological details have been described previously (Melnyk et al., 1999). The analyses were performed using HPLC with a Shimadzu solvent delivery system (ESA model 580) and a reverse phase C18 column (3 um; 4.6 × 150 mm, Phenomenex, Inc., Torrance, CA). Brain extracts were directly injected onto the column using a Beckman Autosampler (model 507E). All metabolites were quantified using a model 5200A Coulochem II and CoulArray electrochemical detection system (ESA, Inc., Chelmsford, MA) equipped with a dual analytical cell (model 5010), a 4 channel analytical cell (model 6210) and a guard cell (model 5020). The levels or concentrations of thiol metabolites, methyl group donor metabolites, and oxidative stress biomarkers in cerebellum or plasma were calculated from peak areas and standard calibration curves using HPLC software. Intracellular results are expressed as nmol/mg of protein using the BCA Protein Assay Kit (Pierce, Rockford, IL). Free reduced glutathione (GSH), oxidized glutathione (GSSG), cysteine (CyS) and cystine (CySSCy) are expressed as levels (moles) per milligram of protein in cerebellum or as molar concentrations in plasma. Plasma was evaluated for thiols and methyl donor metabolites, as well as for 3-nitrotyrosine (3-NT), 3-chlorotyrosine (3-CT), tyrosine and tryptophan. Levels were expressed as micromoles or nanomoles per liter. The percentage of oxidized glutathione is expressed in absolute glutathione equivalents and was calculated as [100 × 2 × GSSG/(GSH + 2GSSG)] where 2x GSSG is the glutathione equivalents in oxidized glutathione and GSH is the glutathione level or concentration (Melnyk et al., 2011). Total GSH concentrations (micromolar in plasma) or levels (nmol/mg cellular protein) were determined by calculation from free GSH concentrations or levels, i.e., total GSH=GSH + 2GSSG. Likewise, total CyS concentrations or levels were determined by calculation from free cysteine and cysteine concentrations or levels, i.e., total CyS=CyS + 2CySSCy.
2.4 Phenotypic Analysis of Spleen cells
The phenotypic analysis of 30,000 events per sample was carried out using a CyFlow ML (Partec GmbH, Munster, Germany) as described previously using monoclonal antibodies from BD Biosciences or eBioscience (Blossom et al., 2008). The data (percentage of cells) are presented as individual mice represented in scatter plots. Fluorescence Minus One controls and isotype Ig controls were included.
2.5 Cytokine Analysis
CD4+ T cells were isolated from spleen cell suspensions from individual mice using a Dynabeads FlowComp Mouse CD4 kit (Invitrogen). CD4+ T cells were stimulated with immobilized anti-CD3 antibody and anti-CD28 antibody for 18 hours as described (Gilbert et al., 2006). Culture supernatants were then collected for cytokine evaluation using Luminex multiplex kits for mouse IFN-γ, IL-2, IL-4, IL-10, IL-17, and TNF-α (Millipore, Billerica, Massachusetts).
2.6 Statistical Analysis
Statistical analysis for all data except for some behavioral comparisons was performed using GraphPad Prism 5.0 (LaJolla, CA). All data were expressed as mean ± standard deviation (SD) or ± standard error of the mean (SEM) where indicated. One way ANOVA with a Dunnett’s Multiple Comparison Test was used to determine significant differences between vehicle control vs. low- or high- dose TCE treatment groups for experimental outcomes. Treatment-related differences for those outcomes were considered to be significant at p≤0.05. When the means were not significantly different among treatment groups, Bartlett’s tests were employed to compare the variability within each exposure group. Variances were considered to be significantly different at p≤0.05. Pearson product-moment correlation coefficients were used to determine the relationship between levels of GSH in cerebellum and plasma. P values ≤0.05 were considered significant.
Behavioral outcomes were estimated from generalized linear mixed models (GLMM) accounting for the underlying distribution of each outcome. For continuous-type outcomes (i.e. distance traveled, time in the zone, number of times the mouse enters the center zone), a linear mixed model (LMM) assuming a normal distribution was utilized. For count-measuring variables (i.e. frequency to enter the zone), a GLMM assuming a Poisson distribution was utilized to account for the count data. The model also included TCE treatment and time as fixed effects, and a random intercept accounting for the correlation within each subject. A nominal significant level with P-value≤0.05 was considered to be statistically significant. Analysis of behavioral outcomes was completed using R version 3.0.2 (Vienna, Austria: R Development Core Team).
3. Results
3.1 Characteristics of dams and offspring
As shown in Table 1, prenatal exposure to TCE did not alter the weight of the dams or affect the average volume of water (ml) consumed per day. The amount of TCE (mg/kg/day) was based on average water intake, body weight, and a calculated average of ~20% TCE degradation in the water bottles. There were no differences in offspring birth and physical parameters including the number of litters, litter size, and age/weight at sacrifice.
Table 1.
Characteristics of dams and offspring. Data presented as mean (SD).
| Characteristics | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| Dams | |||
|
| |||
| Body weight (kg) | 0.047 (0.008) | 0.045 (0.007) | 0.045 (0.006) |
| Water consumption (ml) | 14.42 (3.06) | 15.74 (2.66) | 13.66 (2.55) |
| TCE consumption (mg/kg/day) | 0 | 2.96 (0.52) | 26.56 (7.93) |
|
| |||
| Offspring | |||
|
| |||
| Number of litters | 10 (100%) | 8 (80%) | 9 (90%) |
| Litter size (#) | 6.20 (2.25) | 6.62 (1.40) | 6.22 (2.99) |
| Male pups (%) | 54.40 (13.90) | 54.50 (12.24) | 58.33 (22.14) |
| Female pups (%) | 45.50 (13.90) | 45.50 (12.24) | 41.61 (22.14) |
| Age at sacrifice (PND) | 43.30 (3.02) | 43.86 (3.97) | 45.17 (3.86) |
| Weight at sacrifice (g) | 32.14 (3.03) | 31.90 (0.96) | 30.33 (2.22) |
Statistically significant differences (p<0.05) were not detected among the groups.
3.1.1. Prenatal TCE exposure increases locomotor activity in offspring
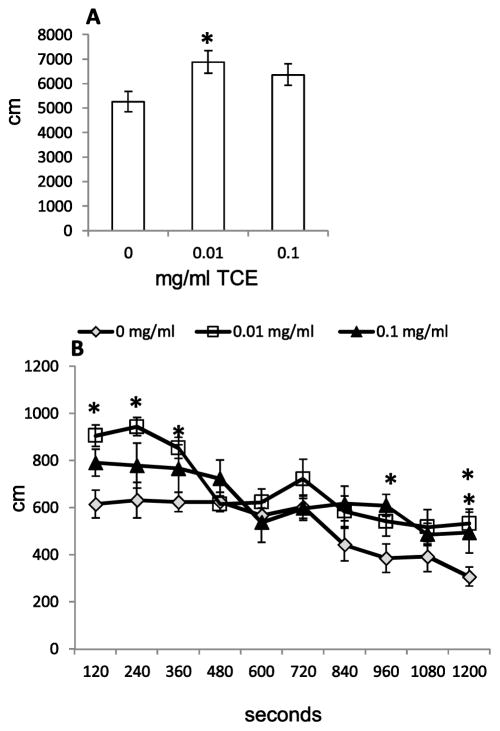
Open field tests were conducted to measure locomotor activity in control and TCE-exposed mice. As shown in Fig. 1A, mice exposed to TCE travelled an average distance of ~81 cm and ~55 cm greater than control mice (0.01 and 0.1 mg/ml respectively; overall p value=0.03). Mice exposed to 0.01 mg/ml TCE travelled a total distance of 6,883 cm which represented a 1,622 cm increase over that observed in mice from the control group (p=0.01). Mice treated with 0.1 mg/ml TCE traveled a total distance of 6,366 cm which represented a 1,105 cm increase over controls. This effect did not reach statistical significance (p=0.06).
Fig. 1. Prenatal TCE exposure increased spontaneous locomotor activity in an open field.
Presented in (A) is the cumulative distance (cm) traveled over time (20 min). Fig 1B represents distance traveled in 2 min epochs over 20 min. Behavioral tests were evaluated with one randomly selected male mouse per litter; n=10 for controls, n=8 for 0.01 mg/ml TCE, and n=9 for 0.1 mg/ml TCE. The mean ± SEM are presented in the graphs. *Statistically significant compared with control groups (p≤0.05).
The distance travelled was summed for each mouse per group in 2 min (i.e., 120 sec) epochs during the 20 min testing period. Fig. 1B showed that on average, mice exposed to 0.01 mg/ml TCE travelled significantly further than controls (p=0.02, 0.03, and 0.02 for 120, 240, and 360 sec epochs, respectively). Mice exposed to 0.1 mg/ml TCE traveled further compared to controls towards the end of the testing period (i.e., at 960 sec; p=0.02). Mice in both TCE exposure groups travelled a greater distance as compared to control mice during the last two minutes of the testing period (p=0.01 for 0.01 and 0.1 mg/ml TCE).
Table 2 summarized the results of additional behavioral tasks evaluated in the open field. The average speed at which the mice travelled (i.e., velocity) did not differ significantly among the groups of mice. The average time spent in the center of the testing arena and the number of times the test mouse entered the virtual center zone of the testing arena during the testing period did not significantly differ among the groups of mice. In contrast, latency to enter the virtual center zone was decreased in mice exposed to 0.01 mg/ml TCE (p=0.01).
Table 2.
Open field behavior. Results represent the mean (SD).
| Outcome | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| Time in center (s) | |||
| Average | 1.32 (0.39) | 1.42 (0.43) | 1.13 (0.41) |
| Cumulative | 28.65 (34.92) | 28.66 (12.99) | 22.94 (12.99) |
| Frequency in center (#) | |||
| Average | 1.26 (1.12) | 1.50 (0.58) | 1.37 (0.61) |
| Cumulative | 24.302 (22.13) | 29.63 (12.14) | 26.56 (11.27) |
| Latency (s) | 131.37 (35.25) | 40.74 (39.41)* | 95.06 (37.16) |
| Velocity (cm/s) | 6.28 (0.26) | 7.53 (0.19) | 7.23 (0.62) |
Statistically significant from the results of controls (p≤0.05).
3.1.2. Prenatal TCE exposure alters brain redox homeostasis in the cerebellum
The data presented in Table 3 depict the concentrations of reduced GSH, GSSG, the glutathione redox (GSH/GSSG) ratio, and the percentage of oxidized glutathione equivalents in the cerebellum. Compared to vehicle-treated control mice, mean GSH levels in cerebellum from mice treated with 0.1 mg/ml TCE were decreased (p=0.006). In contrast, the average concentration of GSSG among groups was not significantly different. The percent oxidized glutathione, expressed as glutathione equivalents (100 × 2 GSSG/[GSH + 2GSSG]) is an indicator of intracellular glutathione redox potential (Lenton et al., 1999). The percent oxidized glutathione was significantly increased by 41% (p=0.02) in mice treated with 0.1 mg/ml TCE, relative to controls. This difference was similarly reflected by a 44% decrease in the GSH/GSSG ratio (p=0.04).
Table 3.
Comparison of transsulfuration metabolites in cerebellum of control and TCE-treated mice. Results represent the mean (SD).
| Glutathione and cysteine redox status in cerebellum (nmol/mg/protein) | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| GSH | 17.63 (1.14) | 17.23 (0.98) | 14.57 (2.17)* |
| GSSG | 0.45 (0.10) | 0.56 (0.20) | 0.68 (0.22) |
| GSH/GSSG ratio | 41.03 (11.56) | 34.29 (13.67) | 23.12 (8.64)* |
| Oxidized GSH (%) | 5.22 (0.91) | 6.15 (2.16) | 8.73 (2.58)* |
| CyS | 2.73 (0.42) | 1.43 (0.28)* | 1.51 (0.22)*** |
| CySSCy | 9.71 (1.32) | 9.10 (1.55) | 8.24 (1.24) |
| CyS/CySSCy ratio | 0.29 (0.08) | 0.16 (0.04)* | 0.18 (0.05)* |
| Total CyS | 22.14 (2.43) | 19.63 (3.21) | 17.99 (2.51)* |
Total Cysteine (CyS + 2xCySSy) was calculated as described in the Methods section. Results are statistically significant compared to results obtained from controls
p≤0.05,
p≤0.005,
p≤0.0005.
Also shown in Table 3 are concentrations of the major extracellular redox couple, CyS, the rate limiting precursor for glutathione synthesis, and the oxidized form of CyS (i.e., CySSCy) and the ratio of the pair (CyS/CySSCy). The levels of CyS were significantly reduced in with both concentrations of TCE as compared to controls by 52% (p=0.011) and 55% (p=0.0004) for 0.01 and 0.1 mg/ml TCE, respectively. While the mean concentration of CySSCy was not significantly different among the treatment groups, the ratio of CyS to CySSCy was significantly decreased by 45% (p=0.01) and 38% (0.014) for 0.01 mg/ml and 0.1 mg/ml TCE, respectively. Compared to controls, total CyS (CyS + 2xCySS) was also significantly lower in the 0.1 mg/ml TCE group (p=0.02).
3.1.3. Prenatal TCE exposure alters transsulfuration and transmethylation metabolites in plasma
Plasma levels of redox and methyl metabolites in the interrelated transmethylation pathway were also examined. Free reduced GSH is a measurement of unbound GSH remaining after protein precipitation and an indication of readiness to neutralize free radicals. As shown in Table 4 there was a significant ~46% decrease (p=0.0002) in free reduced GSH in the plasma of mice exposed prenatally to 0.1 mg/ml TCE. The mean concentration of GSSG, was significantly increased by 43% relative to controls in the 0.1 mg/ml TCE dose group (p=0.02). Correspondingly, free plasma GSH/GSSG ratios were significantly lower in mice treated with 0.1 mg/ml TCE compared to controls (by 65%; p=0.005). The percent oxidized glutathione was significantly increased in mice treated with 0.1 mg/ml TCE compared to controls (p=0.004). Statistical significance was not reached with the 0.01 mg/ml dose of TCE. Calculated total GSH (i.e., GSH + 2GSSG) was not significantly increased with TCE exposure.
Table 4.
Comparison of transsulfuration metabolites in plasma of control and TCE-treated mice. Results represent the mean (SD).
| Plasma thiols | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| GSH (μM)f | 2.52 (0.17) | 2.14 (0.33) | 1.37 (0.50)*** |
| GSSG (μM) | 0.63 (0.12) | 0.91 (0.41) | 1.42 (0.65)* |
| GSH/GSSG | 4.12 (0.75) | 2.77 (1.34) | 1.44 (1.35)** |
| % oxidized GSH | 33.05 (3.84) | 44.64 (12.27) | 62.35 (18.25)** |
| Total GSH | 3.77 (0.34) | 3.96 (0.64) | 4.20 (0.99) |
|
| |||
| CyS (μM)f | 17.33 (2.71) | 20.90 (7.35) | 15.43 (2.15) |
| CySSCy (μM) | 21.70 (2.59) | 27.67 (6.31) | 29.02 (3.52)* |
| Cys/CySSCy | 0.80 (0.87) | 0.74 (0.12) | 0.54 (0.09)** |
| Total CyS | 60.73 (7.37) | 76.23 (19.7)+ | 73.46 (7.77)* |
Total glutathione (GSH) and cysteine (CyS) was calculated as described in Materials and Methods.
Reflects free or unbound thiols remaining after protein precipitation. Results are statistically different from results obtained from controls
p≤0.05,
p≤0.005,
p≤0.0005.
Variance ratios are statistically different (p=0.02).
Free CyS levels were not statistically different among the groups of mice. In contrast, CySSCy was significantly elevated in mice exposed to 0.1 mg/ml TCE (~26%; p=0.02). The CyS/CySSCy thiol redox couple ratio was significantly different (p=0.009) with 0.1 mg/ml TCE exposure compared to controls. Mean total CyS levels were significantly higher with exposure to the highest dose of TCE (p=0.01). For the 0.01 mg/ml group, the means were not statistically significant (p=0.28). However, when a Bartlett’s test for variance was conducted, there was an increase in the variation (p=0.02) with exposure to this dose. Selected plasma methyl metabolites are shown in Table 5. There was no effect of TCE on plasma methionine levels. However, mean levels of SAM, were decreased by ~29% with the highest dose of TCE (p=0.01). In contrast, SAH was unchanged. However, there was a significant decrease in the SAM/SAH ratio with exposure to the highest dose of TCE (p=0.02).
Table 5.
Comparison of methyl metabolites in plasma of control and TCE-treated mice. Results represent the mean (SD).
| Plasma transmethylation metabolites | 0 mg/ml TCE | 0.01 mg/ml TCE | 0.1 mg/ml TCE |
|---|---|---|---|
| methionine (μM) | 52.90 (8.13) | 48.92 (6.88) | 44.42 (3.27) |
| SAM (nM) | 268.40 (22.66) | 257.00 (39.70) | 191.33 (55.74)* |
| SAH (nM) | 52.40 (15.35) | 49.25 (7.49) | 58.58 (16.03) |
| SAM/SAH ratio | 5.44 (1.41) | 5.32 (1.13) | 3.40 (0.99)* |
Statistically different from the results obtained from controls (p≤0.05).
3.1.4. Positive association between oxidative endpoint GSH in plasma and cerebellum
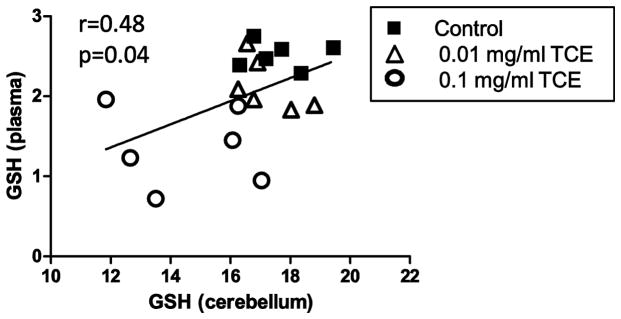
Fig 2 illustrates the significant positive correlation found between levels of GSH in cerebellum and plasma with notable clustering of TCE-treated mice in the lower corner of the plot (more oxidized) with TCE exposure (correlation coefficient = 0.48; p=0.04).
Fig. 2. Positive correlation between GSH in plasma and cerebellum.
Presented is a correlation plot of the levels of plasma and cerebellar GSH. Each symbol represents an individual mouse in each treatment group.
3.1.5. 3-nitrotyrosine increased in cerebellum and plasma
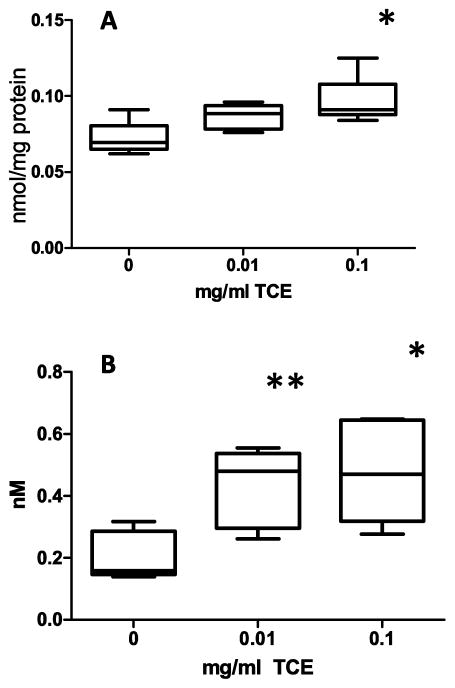
Fig 3 presents the concentrations of 3-NT, a protein nitrosative/oxidative stress biomarker in plasma and cerebellum. In cerebellum (Fig 3A), 3-NT levels were significantly increased in mice exposed prenatally to 0.1 mg/ml TCE relative to controls (p=0.02). 3-NT concentrations were significantly increased in plasma by 54% (p=0.005) for 0.01 mg/ml TCE and 57% (p=0.006) for 0.1 mg/ml TCE (Fig 3B).
Fig 3. 3-Nitrotyrosine levels in the cerebellum and plasma were elevated with prenatal TCE exposure.
3-Nitrotyrosine were measured in the cerebellum (A) and plasma (B) of one randomly selected male mouse per litter (n=6 for all treatment groups) using HPLC with electrochemical detection as described in the Materials and Methods section. Results are reported as nmol/mg protein (cerebellum) or nM (plasma). The mean ± SD and range are presented in the graphs. Results are statistically significant compared with control groups (*p≤0.05 and **p≤0.005).
3.1.6. Prenatal TCE exposure increased plasma inflammatory biomarkers
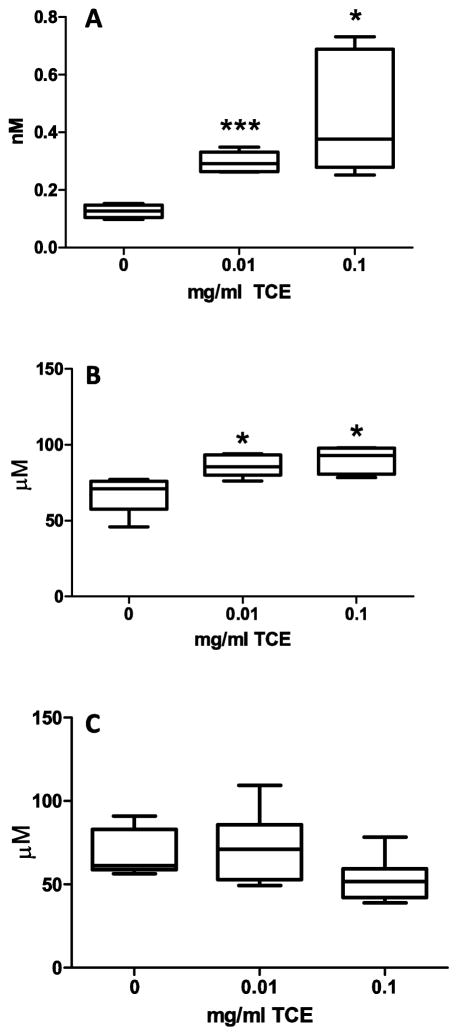
The increase in 3-NT with TCE exposure appeared to be more robust in plasma than in cerebellum. Thus, we investigated an additional inflammatory/oxidative stress-related biomarker, 3-CT, in plasma. As shown in Fig 4A. The mean concentration of 3-CT in the plasma was increased by 52% (p=0.0001) for 0.01 mg/ml TCE and 72% (p=0.006) for 0.1 mg/ml TCE relative to controls. We also examined plasma levels of tryptophan, an amino acid that is a precursor to serotonin with known immunomodulatory properties associated with neurological disorders (Oxenkrug et al., 2010). Prenatal TCE exposure significantly increased plasma levels of tryptophan (p=0.01 for 0.01 mg/ml TCE and p=0.006 for 0.1 mg/ml TCE (Fig. 4B). As a control, the amino acid tyrosine was evaluated. Levels of tyrosine were not statistically different among the groups (Fig 4C).
Fig 4. TCE exposure increased biomarkers associated with oxidative stress and inflammation in plasma.
Plasma samples were evaluated from one randomly selected male mouse per litter (n=6 for all groups) for the concentrations of 3-CT (A), tryptophan (B), and tyrosine (C) using HPLC with electrochemical detection as described in the Materials and Methods section. Results are reported as nM (3-CT) or μM (tyrosine and tryptophan). The mean + SD and range are presented in the graphs. Results are statistically significant compared with control groups (*p<0.05 and ***p<0.0005.
3.1.7. Prenatal TCE exposure increases population of memory CD4+ T cells in adult mice
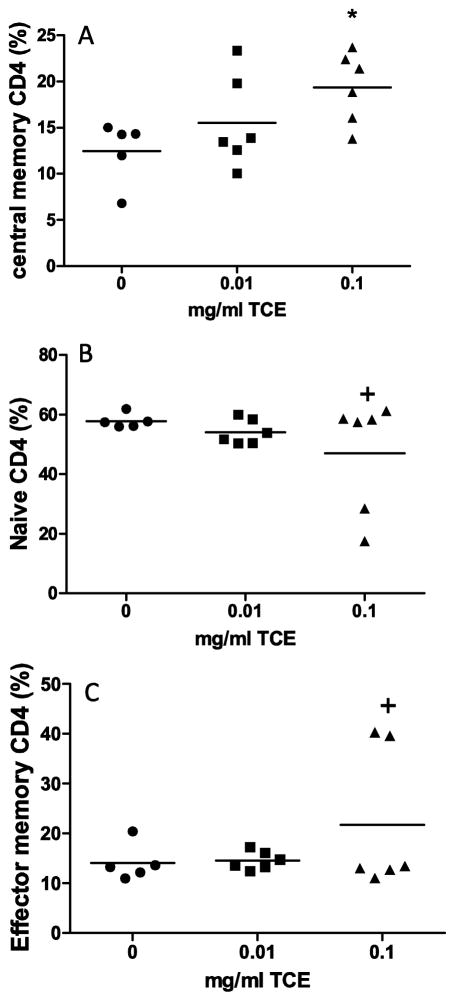
Results from the plasma evaluation indicated that prenatal TCE exposure induced a systemic pro-oxidative and pro-inflammatory state. We therefore evaluated phenotypic and functional characteristics of CD4+ T cells that are associated with TCE-induced immunotoxicity. Based on differential cell surface expression of CD62L and CD44 we, in addition to naïve/resting CD4+ T cells, evaluated two memory CD4+ T cell populations that are important in inflammatory responses (Pepper and Jenkins et al., 2011). The phenotypic analysis of the CD4+ T cells revealed an interesting expression pattern. The graph in Fig 5A showed a statistically significant increase in the mean percentage of central memory CD4+ T cells in the spleen with exposure to the highest dose of TCE (12.4% vs. 19.4%; control vs. 0.1 mg/ml TCE exposure, respectively; p=0.04). As shown in Fig. 5B. TCE exposure did not significantly alter the mean percentage of naïve CD4+T cells or effector memory CD4+ T cells in the spleen (Fig 5C) relative to controls. However, the variance was significantly different for these cell populations.
Fig. 5. TCE enhanced the percentage of memory CD4+ T cells following prenatal exposure.
Spleen cell suspensions from of one randomly selected male mouse per litter (n=6 for all groups) were examined by flow cytometry to determine the percentage of CD4+ T cells that expressed (A) central memory CD4+ T cells expressing low levels of CD62L and high levels of CD44; (B) naïve CD4+ T cells expressing high levels of CD62L and low levels of CD44; (C) Effector memory CD4+ T cells expressing high levels of CD62L and high levels of CD44. Each symbol in the graphs represents the staining pattern in individual mice. *Means are statistically different (p≤0.05) relative to controls. +Variance ratios are statistically different relative to controls (p≤0.005).
3.1.8. Prenatal TCE exposure alters CD4+ T cell cytokine production
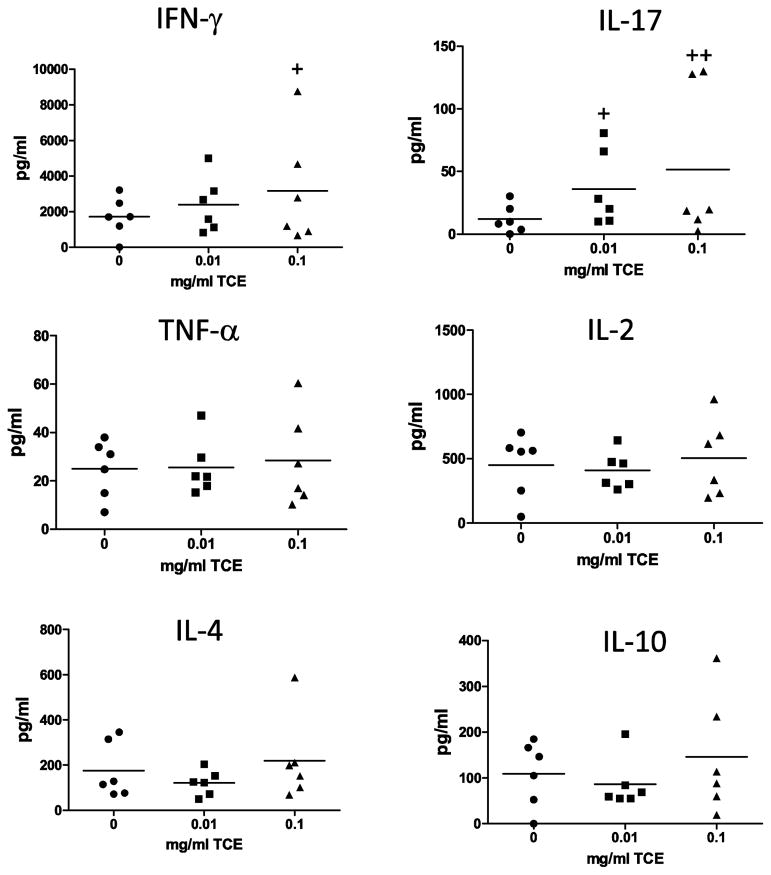
CD4+ T cell production of cytokines including IFN-γ, IL-2, IL4, IL-10, IL-17, and TNF-α were examined as functional biomarkers of prenatal TCE exposure. Prenatal TCE exposure alone was not sufficient to alter the mean cytokine production by CD4+ T cells over control mice. However, as shown in Fig. 6 the variance in CD4+ T cell-secreted IFN-γ levels were statistically significant comparing 0.1 mg/ml TCE vs. controls (p=0.05). As for IL-17, variability was markedly increased with both concentrations of TCE (p=0.03 and 0.002 with 0.01 and 0.1 mg/ml TCE, respectively). The mean and variance ratios of CD4+ T cell derived TNF-α, IL-2, IL-10, and IL-4 were not statistically different among the treatment groups.
Fig. 6. TCE increased the variance of IFN-γ and IL-17 production by CD4+ T cells.
CD4+ T cells were isolated from of one randomly selected male mouse per litter (n=6 for all groups) and stimulated as described in the methods section to elicit a cytokine response. The culture supernatants were harvested at 18h and assessed for cytokines using a multiplex assay and Luminex technology. Each symbol in the graphs represents cytokine secretion (pg/ml) from individual mice. +Variance ratios are statistically different compared to controls (p≤0.05).
4. Discussion
The goal of this study was to determine if TCE-induced neurotoxicity was sustained in after a period of exclusively prenatal exposure. The cerebellum was selected for neurochemical analysis to compare our previous findings using identical doses of TCE with postnatal/early life TCE exposure. In addition, the cerebellum is reportedly sensitive to oxidative stress/redox impairment with toxicant exposure (Giordano et al., 2008; LeDuc et al., 2015). This study provides novel evidence that the effects of prenatal TCE exposure are maintained later in life.
The doses used in the current study were relevant to actual human exposure. The lowest concentrations of TCE used (0.01 mg/ml or 10 ppm) were considerably lower than acceptable human occupational exposure. It has been estimated that approximately 3.5 million workers are exposed to TCE in the workplace and it can be presumed that a significant proportion of these workers are women of childbearing age (Wu and Schaum, 2000). Although our study used doses higher than the EPA’s Maximum Contaminant Level for public drinking water (5 ppb), TCE has been detected in contaminated well water at levels up to 1.4 ppm (ATSDR, 1997). Since the mice were exposed prenatally, TCE metabolism by the pregnant dams may be enhanced thereby reducing the bioavailability of TCE and its metabolites to the developing fetus as shown with other toxicants (Legraverend et al., 1984). Thus, the mice in utero may have been exposed to doses of TCE even lower than estimated.
The results implied that the time of gestation may be important risk consideration for TCE-exposed human populations. As such, human contact with TCE can occur at all stages of life. Exposure to the fetus has been demonstrated by the finding that TCE and its metabolites cross the placenta (Laham S, 1970). Exposure to TCE during infancy can be implied due to its detection in breast milk samples (Beamer et al., 2012). Very little is known about developmental TCE toxicity in humans. Most epidemiological studies of developmental TCE exposure have been confined to analysis of birth outcomes including cardio-teratogenicity, eye anomalies, perinatal deaths, gestational size, and neural tube defects (Bove et al., 2012). While these investigations are important, there is a need to document additional health effects that may manifest from exposure during different developmental windows.
We hypothesized that the effects observed in 6 week old male mice after exclusively prenatal exposure would be modest, or even absent after a considerable period of exposure cessation. While not all of the results were statistically significant, the overall direction of our results showed an effect with prenatal TCE exposure. The increase in locomotor activity was sustained with prenatal TCE exposure. This became more apparent when the time intervals were broken into 2 minute bins. In contrast, other open-field behaviors measured in the present study were not significantly different between TCE-exposed mice and controls. One notable exception was the decreased latency to approach the center of the testing arena observed in mice exposed to the highest dose of TCE. Open field behaviors were more robust with postnatal/early life exposure to identical doses of TCE (Blossom et al., 2013). The reason for the disparity between the two results is not clear, but it is possible that behavioral responses are enhanced during exposure periods when brain is reportedly more sensitive to environmental stressors.
Impaired motor activity has been associated with glutathione depletion (Diaz-Hung et al., 2014), and nearly all sulfur compounds measured were significantly lower in cerebellum in TCE treated mice indicating a generalized oxidized state. Especially interesting were the TCE-mediated alterations in the major extracellular redox couple, CyS and CySSCy. The synthesis of glutathione in the brain is unique in that cells in the brain cannot generate CyS. Thus the brain is dependent on the liver to synthesize and export CyS for uptake and utilization by astrocytes for glutathione synthesis (Atawa et al., 1995; Lu, 1998). CyS is converted to CySSCy in the oxidizing environment in the plasma where it is converted to CyS and transported to the brain for intracellular glutathione synthesis. Because CyS is the precursor for glutathione synthesis, the availability of extracellular CySSCy and CyS determines intracellular glutathione concentrations and resistance to toxic compounds in astrocytes and neurons, respectively. Indeed, toxicants, like methyl mercury, have been shown to inhibit the biosynthesis of GSH by inhibiting the uptake of CyS by astrocytes (Shanker, et al., 2002). Whether TCE and/or its metabolites promote oxidative stress by a similar manner is not known.
Biomarkers of methylation capacity were evaluated in the current study because of their close association to glutathione synthesis cellular methylation reactions. Methionine is an immediate precursor to SAM, the major methyl donor. Unlike with postnatal/early life TCE exposure, methionine was not altered in cerebellum or plasma with TCE exposure in the current study. However, in plasma SAM and the SAM/SAH ratio frequently used as an indicator of cellular methylation capacity was decreased significantly with TCE exposure which would predict reduced methylation potential. Since we did not evaluate DNA methylation in this study, the implication of this finding is not clear. A reduction in SAM together with the maintenance of effects after exposure cessation suggested that epigenetic factors might be involved.
Our results provided evidence of inflammation and immunomodulation with prenatal TCE exposure. TCE increased the mean percentage of central memory CD4+ T cells in offspring and, in at least 33% of the mice, the effector memory T cell subset was increased as reflected by the variance ratio. Effector memory subpopulations express homing receptors such as CD62L that help them migrate from the lymphoid organs to inflammatory sites (e.g., lung, liver or gut) to participate in immune responses. These cells produce cytokines including IL-17 and IFN-γ within several hours of activation. Central memory cells, unlike memory effector cells, are not required for immediate effector function. Instead these cells proliferate in spleen and lymph nodes until they receive homing signals to migrate to inflammatory sites. Thus, toxicants like TCE that expand these populations of cells might contribute to uncontrolled inflammation leading to tissue pathology including neuropathology. Since a pathologic examination was not conducted in this study, it was not possible to determine the homing location of these memory CD4+ T cells.
The mean levels of the pro-inflammatory cytokines, IFN-γ and IL-17 were not statistically different among exposure groups. However, when statistical tests to evaluate differences in the variance ratios were employed the results showed that TCE-exposed mice showed more variation in IFN-γ and IL-17 than controls. The biological relevance of this result is important to consider. This result might suggest that in certain mice within the TCE treatment groups, the CD4+ T cells may have converted to from a naïve to memory to a proinflammatory phenotype. Th1-like responses are known to be highly plastic and can be influenced by the local inflammatory cytokine milieu. Thus, conversion from a naïve phenotype to a pathogenic, disease-promoting Th1 phenotype may vary within individual mice in the same group as shown in some mouse models of autoimmune disease (Bending et al., 2014).
It is important to identify potential plasma biomarkers that may be used to predict toxicant-induced effects in the brain. Several studies have established linkages between plasma oxidative stress/altered glutathione redox state and inflammatory biomarkers in the blood with some neurological and psychiatric disorders including Alzheimer’s disease (Butterfield et al., 2006), Parkinson’s disease (Martin et al., 2009), mood disorders (Duffy et al., 2015; Andreazza et al., 2008), and autism spectrum disorders (ASDs; James, et al., 2004; Rose S et al., 2012). However, the brain has remained relatively understudied due to obvious difficulties associated with obtaining this tissue and technical issues with the use of post-mortem brain. There was a positive correlation between GSH in plasma and cerebellum with TCE exposure. Although this biomarker alteration is common to many toxicants, it is important to study measurable markers in the blood as a surrogate for possible toxicant-induced adverse effects in the brain. We emphasize that TCE itself has not been implicated in promoting human neurologic disease, however; identifying environmental stressors that may underlie biological processes common to many different neurologic diseases is important in understanding neurotoxicity of TCE.
Along this line, we observed increased 3-NT in both plasma and cerebellum. 3-NT is an oxidized protein tyrosine derivative that provides a stable biochemical footprint of oxidative protein damage. 3-NT is formed from peroxynitrite, a highly reactive free radical generated from nitric oxide and superoxide (Butterfield et al, 2008; Nakawaza et al, 2000). The presence of this biomarker has been detected in brain tissue from patients with neurologic disease (Mythri et al, 2011; Butterfield et al, 2006; Sajdel-Sulkowska et al., 2009). The tyrosine derivative, 3-CT, like 3-NT is a stable post-translational modification of protein tyrosine residues and a biomarker of inflammation. 3-CT is generated from hypochlorous acid, a potent chlorinating oxidant derived from myeloperoxidase in activated immune cells during inflammation (Knutson et al., 2013). Elevated myeloperoxidase is also associated with neurological and autoimmune disorders (Green et al., 2004; Choi et al., 2005; Nagra et al., 1997). Additionally, myeloperoxidase promotes Th1 (e.g., IL-17 and IFN-γ producing immune cells) associated with autoimmune disease (Gan et al., 2010).
Plasma levels of the amino acid tryptophan were included as a plasma biomarker based on its role in inflammation. Cytokines including T cell-derived IFN-γ are among the strongest inducers of indoleamine dioxygenase (IDO) which causes tryptophan to covert to kynurenine rather than serotonin. Kynurenine inhibits tryptophan transport into the brain and stimulates the production of additional IDO (Oxenkrug, 2010; Maes et al., 2007). Kynurenine metabolites have been associated with reduced striatal volume in the brain of depressed individuals (Savitz et al., 2015), and altered tryptophan metabolism promotes cognitive errors and anxiety in rats (Asor et al., 2015). In mice neonatally treated with kynurenine demonstrated increased locomotor activity as adults (Liu et al., 2014). Thus, the increased tryptophan observed with TCE exposure in our model may provide an interesting mechanistic link to the reported neurochemical and behavioral changes associated with the tryptophan/kynurenine pathway. Taken together these findings underscore the importance of investigating peripheral inflammatory biomarkers that may be used to predict neurotoxicity.
Among the four plasma biomarkers evaluated in the present study not directly linked to the transsulfuration/transmethylation pathway (i.e., 3-NT, 3-CT, tyrosine, and tryptophan) only 3-NT was evaluated in both plasma and cerebellum due to sample limitations. Our results revealed that 3-NT was significantly increased relative to controls with exposure to both high and low concentrations of TCE in plasma, as opposed to cerebellum in which the TCE effects was only seen with the highest dose. The meaning of this finding is not clear, but the significant increase in plasma biomarkers (3-NT, 3-CT, and tryptophan) to both doses of TCE might predict a direct peripheral effect (e.g., the liver or immune system) rather than serving as a specific biomarker of neurotoxicity. It is also possible that the cerebellum may not reflect responses that are occurring in other regions of the brain. Indeed open field behavior appeared to be more robust in the low dose group. This issue can be addressed in future studies by evaluating these biomarkers in other relevant brain regions to address correlation between brain and blood markers of neurotoxicity.
There is very little mechanistic information regarding developmental TCE exposure and neurotoxicity. The findings reported in the current study underscore the need for additional studies in this area. In addition to direct effects of TCE and or its metabolites in the brain, TCE may additionally exert its neurotoxic effects indirectly by activating CD4+ T cells to produce pro-inflammatory cytokines and other inflammatory mediators in the periphery to promote oxidative stress, neuroinflammation, and altered behavior. Studies to further characterize neuroinflammation in additional brain regions in association with a larger repertoire of behaviors with developmental exposure are currently underway. Possible maternal factors including epigenetic regulation of neurobehavior by TCE exposure are also important considerations for future study.
Highlights.
Prenatal TCE exposure enhanced oxidative stress biomarkers in the cerebellum.
Prenatal TCE exposure promoted increased locomotor activity.
Prenatal TCE exposure increased peripheral CD4+ T cell activated/memory cells.
Prenatal TCE exposure increased variance ratio of cytokines IL-17 and IFN-γ.
Prenatal TCE exposure increased plasma biomarkers of oxidative stress/inflammation.
Acknowledgments
Funding
Arkansas Biosciences Institute New Investigator Funds (Arkansas Children’s Hospital Research Institute) and the National Institutes of Health (5 R21ES017311 and K02ES024387) to S.J.B.
We would like to gratefully acknowledge the excellent technical assistance of Jenny L. Rau and Grant R. Chandler.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATSDR Agency for Toxic Subjstances and Disease Registry. Toxicological Profile for Trichloroethylene. United States Department of Health and Human Services; Atlanta: 1997. [Google Scholar]
- Andreazza AC, Kauer-Sant-anna M, Frey BN, Bond DJ, Kapczinski F, Young LT, et al. Oxidative stress markers in bipolar disorder: a meta analysis. J Affect Disord. 2008;111:135–144. doi: 10.1016/j.jad.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Anthony D, Couch Y, Losey P, Evans M. The systemic response to brain injury and disease. Brain Behav Immun. 2011;26:534–540. doi: 10.1016/j.bbi.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Asor E, Stempler S, Avital A, Klein E, Ruppin E, Ben-Shachar D. The role of branched chain amino acid and tryptophan metabolism in rat’s behavioral diversity: Intertwined peripheral and brain effects. European neuropsychopharmacology. 2015;25:1695–1705. doi: 10.1016/j.euroneuro.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Atawa S, Nakayama K, Suzuki I, Sugahara K, Kodama H. Changes in cystathionine gamma-lyase in various regions of rat brain during development. Biochem Mol Int. 1995;25:1331–1338. [PubMed] [Google Scholar]
- Beamer PI, Luik CE, Abrell L, Campos S, Martinez ME, Saez AE. Correction to concentration of trichloroethylene in breast milk and household water from Nogales, Arizona. Environ Sci Technol. 2012;46:11483. doi: 10.1021/es301380d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2. 5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2015;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Chida AS, Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochem Pharmacol. 2006;71:551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Cooney CA, Melnyk SB, Rau JL, Swearingen CJ, Wessinger WD. Metabolic changes and DNA hypomethylation in cerebellum are associated with behavioral alterations in mice exposed to trichloroethylene postnatally. Toxicol Appl Pharmacol. 2013;269:263–269. doi: 10.1016/j.taap.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC. Trichloroethylene alters central and peripheral immune function in autoimmune-prone MRL(+/+) mice following continuous developmental and early life exposure. J Immunotoxicol. 2007;4:129–141. doi: 10.1080/15476910701337035. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Doss JC, Hennings LJ, Jernigan S, Melnyk S, James SJ. Developmental exposure to trichloroethylene promotes CD4 (+) T cell differentiation and hyperactivity in association with oxidative stress and neurobehavioral deficits in MRL+/+ mice. Toxicol Appl Pharmacol. 2008;231:344–353. doi: 10.1016/j.taap.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Blossom SJ, Melnyk S, Cooney CA, Gilbert KM, James SJ. Postnatal exposure to trichloroethylene alters glutathione redox homeostasis, methylation potential, and neurotrophin expression in the mouse hippocampus. Neurotoxicology. 2012;33:1518–1527. doi: 10.1016/j.neuro.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobyn PJ, Franklin JL, Wall CM, Thornhill JA, Juurlink BH, Paterson PG. The effects of dietary sulfur amino acid deficiency on rat brain glutathione concentration and neural damage in global hemispheric hypoxia-ischemia. Nutr Neurosci. 2002;5:407–15. doi: 10.1080/1028415021000055952. [DOI] [PubMed] [Google Scholar]
- Bove F, Shim Y, Zeitz P. Drinking water contaminants and adverse pregnancy outcomes: a review. Environ Health Perspect. 2002;110(Suppl 1):61–74. doi: 10.1289/ehp.02110s161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Sultana R. Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol. 2006;545:39–50. doi: 10.1016/j.ejphar.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Sultana R. Identification of 3-nitrotyrosine-modified brain proteins by redox proteomics. Methods Enzymol. 2008;440:295–308. doi: 10.1016/S0076-6879(07)00819-1. [DOI] [PubMed] [Google Scholar]
- Byers VS, Levin AS, Ozonoff DM, Baldwin RW. Association between clinical symptoms and lymphocyte abnormalities in a population with chronic domestic exposure to industrial solvent-contaminated domestic water supply and a high incidence of leukemia. Cancer Immunol Immunother. 1988;27:77–82. doi: 10.1007/BF00205762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channel SR, Latendresse JR, Kidneym JK, Grabaum JK, Lanem Jw, Steel-Goodwin L, Gothausm MC. A subchronic exposure to trichloroethylene causes lipid peroxidation and hepatocellular proliferation in male B6C3F1 mouse liver. Toxicol Sci. 1998;42:145–154. doi: 10.1006/toxs.1998.2456. [DOI] [PubMed] [Google Scholar]
- Chiu WA, Jinot J, Scott CS, Makris SL, Cooper GS, Dzubow RC, Bale AS, Evans MV, Guyton KZ, Keshava N, Lopscomb JC, Barone S, Fox JF, Gwinn MR, Schaum J, Caldwell JC. Human health effects of trichloroethylene: key findings and scientific issues. Environ Health Perspect. 2013;121:303–311. doi: 10.1289/ehp.1205879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DK, Pennathur S, Peier C, Tieu K, Teismann P, Wu DC. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci. 2005;25:6594–6600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2015 doi: 10.1016/j.neuro.2015.11.008. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deledi M, Jaggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci. 2015;9:172. doi: 10.3389/fnins.2015.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Hung ML, Blanco L, Pavon N, Leon R, Estupinan B, Orta E, Martinez K, Fernandez I. Sensory-motor performance after acute glutathione depletion by L-buthionine sufoximine injection into substantia nigra pars compacta. Behav Brain Res. 2014;271:286–93. doi: 10.1016/j.bbr.2014.05.066. [DOI] [PubMed] [Google Scholar]
- Dietert RR, Piepenbrink MS. Perinatal immunotoxicity: why adult exposure assessment fails to predict risk. Environ Health Perspect. 2006;114:477–483. doi: 10.1289/ehp.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy SL, Lagopoulos J, Cockayne N, Hermens DF, Hickie IB, Naismith SL. Oxidative stress and depressive symptoms in older adults: A magnetic resonance spectroscopy study. J Affect Disord. 2015;180:29–35. doi: 10.1016/j.jad.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Dumas TC. Late postnatal maturation of excitatory synaptic transmission permits adult-like expression of hippocampal-dependent behaviors. Hippocampus. 2005;15:562–578. doi: 10.1002/hipo.20077. [DOI] [PubMed] [Google Scholar]
- Filomeni G, Rotilio G, Ciriolo MR. Cell signaling and the glutathione redox system. Biochem Pharmacol. 2002;64:1057–64. doi: 10.1016/s0006-2952(02)01176-0. [DOI] [PubMed] [Google Scholar]
- Gan PY, Steinmetz OM, Tan DS, O’Sullivan KM, Ooi JD, Iwakura Y, Kitching AR, Holdsworth SR. Th17 cells promote autoimmune anti-myeloperoxidase glomerulonephritis. J Am Soc Nephrol. 2010;6:925–931. doi: 10.1681/ASN.2009070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert KM, Woodruff W, Blossom SJ. Differential immunotoxicity induced by two different windows of developmental trichloroethylene exposure. Autoimmune Dis. 2014;2014:982073. doi: 10.1155/2014/982073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Kavanaugh TJ, Costa LG. Neurotoxicity of a polybrominated diphenyl ether mixture (DE-71) in mouse neurons and astrocytes is modulated by intracellular glutathione levels. Toxicol Appl Pharmacol. 2008;232:161–168. doi: 10.1016/j.taap.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A, Keydar I, Epstein HT. Rodent brain growth stages: an analytical review. Biol Neonate. 1977;32:166–176. doi: 10.1159/000241012. [DOI] [PubMed] [Google Scholar]
- Green PS, Mendez AJ, Jacob JS, Crowley JR, Growdon W, Hyman BT. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J Neurochem. 2004;90:724–733. doi: 10.1111/j.1471-4159.2004.02527.x. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, et al. The role of inflammation in perinatal brain injury. Nat Rev Neruol. 2015;11:192–208. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufner K, Oberguggenberger A, Kohl C, Geisler S, Gamper E, Meraner V, Egeter J, Hubalek M, Beer B, Fuchs D, Sperner-Unterweger B. Levels in neurotransmitter precursor amino acids correlate with mental health in patients with breast cancer. Psychoneuroendocrinology. 2015;60:28–38. doi: 10.1016/j.psyneuen.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Isaacson LG, Spohler SA, Taylor DH. Trichloroethylene affects learning and decreases myelin in the rat hippocampus. Neurotoxicol Teratol. 1990;12:375–381. doi: 10.1016/0892-0362(90)90057-j. [DOI] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- Knutson CG, Mangerich A, Zeng Y, Raczynski AR, Liberman RG, Kang P, Ye W, Prestwich EG, Lu K, Wishnok JS, Korzenik JR, Wogan GN, Fox JG, Dedon PC, Tannenbaum SR. Chemical and cytokine features of innate immunity characterize serum and tissue profiles in inflammatory bowel disease. Proc Natl Acad Sci USA. 2013;110:E2332–41. doi: 10.1073/pnas.1222669110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laslo-Baker D, Barrera M, Knittel-Keren D, Kozer E, Wolpin J, Khattak S, Hackman R, Rovet J, Koren G. Child neurodevelopmental outcome and maternal occupational exposure to solvents. Arch Pediatr Adolesc Med. 2004;158:956–961. doi: 10.1001/archpedi.158.10.956. [DOI] [PubMed] [Google Scholar]
- Legraverend C, Guenthner TM, Nebert DW. Importance of the route of administration for genetic differences in benzo[a]pyrene-induced in utero toxicity and teratogenicity. Teratology. 1984;29:35–47. doi: 10.1002/tera.1420290106. [DOI] [PubMed] [Google Scholar]
- Laham S. Studies on Placenta Transfer. IMS Ind Med Surg. 1970;39(1):46–49. [PubMed] [Google Scholar]
- LeDuc D, Spataru A, Ceanga M, Zagrean L, Schoneberg T, Toescu EC, Zagrean AM. Developmental exposure to ethanol increases the neuronal vulnerability to oxygen-glucose deprivation in cerebellar granule cell cultures. Brain Res. 2015;1614:1–13. doi: 10.1016/j.brainres.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Lenton KJ, Therriault H, Wagner JR. Analysis of glutathione and glutathione disulfide in whole cells and mitochondria by postcolumn derivatization high-performance liquid chromatography with ortho-phthaladehyde. Analytical Chemistry. 1999;274:125–130. doi: 10.1006/abio.1999.4258. [DOI] [PubMed] [Google Scholar]
- Lindahl JS, Kjellsen BR, Tigert J, Miskimins R. In utero PCP exposure alters oligodendrocyte differentiation and myelination in developing rat frontal cortex. Brain Res. 2008;1234:137–147. doi: 10.1016/j.brainres.2008.06.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XC, Holtze M, Powell SB, Terrando N, Larsson MK, Persson A, Olsson SK, Orhan F, Kegel M, Asp L, Goiny M, Schwieler L, Engberg G, Karlsson H, Erhardt S. Behavioral disturbances in adult mice following neonatal virus infection or kynurenine treatment-role of brain kynurenic acid. Brain Behav Immun. 2014;36:80–89. doi: 10.1016/j.bbi.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. Regulation of hepatic glutathione synthesis. Semin Liver Dis. 1998;18:331–343. doi: 10.1055/s-2007-1007168. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Ruyter MD, Kubera M, Bosmans E. The immune effects of TRYCATs (tryptophan catabolites along the IDO pathway): relevance for depression-and other conditions characterized by tryptophan depletion induced by inflammation. Neuro Endocrinol Lett. 2007;28:826–831. [PubMed] [Google Scholar]
- Maffi SK, Rathinam ML, Cherian PP, Pate W, Hamby-Mason R, Schenker S, Henderson GI. Glutathione content as a potential mediator of the vulnerability of cultured fetal cortical neurons to ethanol-induced apoptosis. J Neurosci Res. 2008;86:1064–1076. doi: 10.1002/jnr.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova NV, YU CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of three core symptoms of autism. Brain Behav Immun. 2012;24:607. doi: 10.1016/j.bbi.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HL, Teismann P. Glutathione-a review on its role and significance in Parkinson’s disease. FASEB J. 2009;23:3263–3272. doi: 10.1096/fj.08-125443. [DOI] [PubMed] [Google Scholar]
- McLean CW, Mirochnitchenko O, Claus CP, Noble-Haeusslein LJ, Ferriiero DM. Overexpression of glutathione peroxidase protects immature murine neurons from oxidative stress. Dev Neurosci. 2005;27:169–175. doi: 10.1159/000085989. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schultz E, Lopez M, Kahler SG, Fussell JJ, Bellando J, Pavliv O, Rose S, Seidel L, Gaylor DW, James SJ. Metabolic Imbalance Associated with Methylation Dysregulation and Oxidative Damage in Children with Autism. J Autism Dev Disord. 2011;42:367–77. doi: 10.1007/s10803-011-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnyk S, Pogribna M, Pogribny I, Hine RJ, James SJ. A new HPLC method for the simultaneous determination of oxidized and reduced plasma aminothiols using coulometric electrochemical detection. J Nutr Biochem. 1999;10:490–497. doi: 10.1016/s0955-2863(99)00033-9. [DOI] [PubMed] [Google Scholar]
- Mythri RB, Harish G, Dubey SK, Misra K, Bharath MM. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson’s disease. Mol Cell Biochem. 2011;347:135–143. doi: 10.1007/s11010-010-0621-4. [DOI] [PubMed] [Google Scholar]
- Nagra RM, Becher B, Tourtellotte WW, Antel JP, Gold D, Paladino T. Imunohistochemical and genetic evidence of myeloperoxidase involvement in multiple sclerosis. J Neruoimmunol. 1997;78:97–107. doi: 10.1016/s0165-5728(97)00089-1. [DOI] [PubMed] [Google Scholar]
- Nakazawa H, Fukuyama N, Takizawa S, Tsuji C, Yoshitake M, Ishida H. Nitrotyrosine formation and its role in various pathological conditions. Free Radic Res. 2000;33:771–784. doi: 10.1080/10715760000301291. [DOI] [PubMed] [Google Scholar]
- Noble M, Mayer-Proschel M, Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- Ogino K, Hobara T, Kobayashi H, Ishiyama H, Gotoh M, Imamura A, Egami N. Lipid peroxidation induced by trichloroethylene in rat liver. Bull Environ Contam Toxicol. 1991;46:471–421. doi: 10.1007/BF01688941. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Tryptophan-Kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: the serotonin hypothesis revisited 40 years later. Isr J Psychiatry Relat Sci. 2010;47:56–63. [PMC free article] [PubMed] [Google Scholar]
- Peden-Adams MM, Eudaly JG, Heesemann LM, Smythe J, Miller J, Gilkeson GS, Keil DE. Developmental immunotoxicity of trichloroethylene (TCE): studies in B6C3F1 mice. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2006;41:249–271. doi: 10.1080/10934520500455289. [DOI] [PubMed] [Google Scholar]
- Pepper M, Jenkins MK. Origins of CD4+effector and central memory T cells. Nat Immunol. 12:467–71. doi: 10.1038/ni.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, James SJ. Evidence of oxidative damage and inflammation associated with low glutathione redox status in autism brain. Trans Psych. 2012;2:e134. doi: 10.1038/tp.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajdel-Sulkowska EM, Xu M, Koibuchi N. Increase in Cerebellar Neurotrophin-3 and Oxidative Stress Markers in Autism. Cerebellum. 2009;8:366–72. doi: 10.1007/s12311-009-0105-9. [DOI] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Meier TB, Wurfel BE, Victor TA, McIntosh SA, Ford BN, Morris HM, Bodurka J, Teague TK, Drevets WC. Activation of the kynurenine pathway is associated with striatal volume in major depressive disorder. Psychoneuroendocrinology. 2015;62:54–58. doi: 10.1016/j.psyneuen.2015.07.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J Nutr Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- Shanker G, Mutkus LA, Walker SJ, Aschner M. Methylmercury enhances arachidonic acid release and cytosolic phospholipase A2 expression in primary cultures of neonatal astrocytes. Mol Brain Res. 2002;106:1–11. doi: 10.1016/s0169-328x(02)00403-5. [DOI] [PubMed] [Google Scholar]
- Stolp HB, Dziegielwska KM, Ek CJ, Habgood MD, Lane MA, Potter AM, et al. Breakdown of the blood-brain-barrier to proteins in white matter of the developing brain following systemic inflammation. Cell Tisssue Res. 2005;320:369–378. doi: 10.1007/s00441-005-1088-6. [DOI] [PubMed] [Google Scholar]
- Taylor DH, Lagory KE, Zaccaro DJ, Pfohl RJ, Laurie RD. Effect of trichloroethylene on the exploratory and locomotor activity of rats exposed during development. Sci Total Environ. 1985;47:415–20. doi: 10.1016/0048-9697(85)90345-6. [DOI] [PubMed] [Google Scholar]
- Teismann P. Myeloperoxidase in the neurodegenerative process of Parkinson’s disease. Dtsch Med Wochenschr. 2014;139:99–102. doi: 10.1055/s-0033-1359907. [DOI] [PubMed] [Google Scholar]
- Till C, Koren G, Rovet JF. Prenatal exposure to organic solvents and child neurobehavioral performance. Neurotoxicol Teratol. 2001;23:235–245. doi: 10.1016/s0892-0362(01)00141-6. [DOI] [PubMed] [Google Scholar]
- Wang G, Wang J, Ma H, Khan MF. Increased nitration and carbonylation of proteins in MRL+/+ mice exposed to trichloroethylene: potential role of protein oxidation in autoimmunity. Toxicol Appl Pharmacol. 2009;237:188–195. doi: 10.1016/j.taap.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Finch CE, Cohen HJ. Cytokines and cognition-the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Wu C, Schaum J. Exposure assessment of trichloroethylene. Environ Health Perspect. 2000;108:359–363. doi: 10.1289/ehp.00108s2359. [DOI] [PMC free article] [PubMed] [Google Scholar]