Abstract
Background
Besides tobacco and alcohol, diet and inflammation have been suggested to be important risk factors for laryngeal cancer. In this study, we examined the role of diet-associated inflammation, as estimated by dietary inflammatory index (DII) scores, in laryngeal cancer in a multicentre case-control study conducted between 1992 and 2000 in Italy.
Methods
This study included 460 cases with incident, histologically confirmed laryngeal cancer, and 1088 controls hospitalized for acute nonneoplastic diseases unrelated to tobacco and alcohol consumption. DII scores were computed from a reproducible and valid 78-item food frequency questionnaire.
Results
Logistic regression models controlling for age, sex, study center, education, body mass index, tobacco smoking, alcohol drinking, and nonalcohol energy intake, were used to estimate odds ratios (ORs), and the corresponding 95% confidence intervals (CIs). Subjects with higher DII scores (i.e., with a more pro-inflammatory diet) had a higher risk of laryngeal cancer. The OR was 3.30 (95% CI 2.06, 5.28; p for trend < 0.0001) for the highest versus the lowest DII quartile. When DII was considered as a continuous variable, the OR was 1.27 (95 % CI 1.15, 1.40) for a one unit (9% of the DII range) increase. Stratified analyses produced slightly stronger associations between DII and laryngeal cancer risk among current smokers (ORQuartile4vs1= 4.16), moderate drinkers (ORQuartile4vs1= 4.86), overweight subjects (ORQuartile4vs1= 3.62), and among those with higher education (ORQuartile4vs1= 3.92). We also observed a strong combined effect of higher DII and tobacco smoking or alcohol consumption on risk of laryngeal cancer. Compared with non-smokers having low DII scores, the OR was 6.64 for smokers with high DII scores. Likewise, compared with non/moderate drinkers with low DII, the OR was 5.82 for heavy drinkers with high DII.
Conclusion
These results indicate that a pro-inflammatory diet is associated with increased risk of laryngeal cancer.
Introduction
Tobacco smoking and alcohol consumption are the main risk factors for laryngeal cancer (1). In addition, there is an emerging role of inflammation and diet in the development of this cancer (2, 3). The World Cancer Research Fund (WCRF) report in 2007, which combined other head and neck cancers and reviewed results from several epidemiological studies, concluded a probable protective role of non-starchy vegetables, fruits, and carotenoid-rich foods, and a strong carcinogenic role for alcohol (4).
Inflammation is the body’s response to any kind of tissue injury or insult, in the presence of inflammatory stimulants including cytokines (5, 6). Chronic inflammation – which is characterised by the presence of inflammatory cytokines – is known to play a role in the development of various epithelial neoplasms, including laryngeal cancer (2, 7).
Diet represents a complex set of exposures that often interact, and whose cumulative effect modifies both inflammatory responses and health outcomes. A literature-derived, population-based dietary inflammatory index (DII) was developed to assess the inflammatory potential of an individual’s diet (8). The DII has been validated with various inflammatory markers, including C-reactive protein (9, 10), interleukin-6 (11, 12), and homocysteine (10). The DII has been associated with esophageal squamous cell cancer risk in case-control studies from Italy (13) and Iran (14), as well as colorectal (15), pancreatic (16), hepatocellular (17), prostate (18), and endometrial (19) cancers.
Using a case-control study conducted in Italy (20), this is the first attempt to examine the association between the DII and laryngeal cancer risk. Our working hypothesis is that increasing inflammatory potential of diet is associated with increased risk of laryngeal cancer.
Methods
A case-control study of laryngeal cancer was conducted between 1992 and 2000 in the greater Milan area and the province of Pordenone in northern Italy (20). Cases were 460 subjects (415 men, 45 women; median age, 61 y; range, 30–80 y) diagnosed with incident, histologically confirmed squamous cell cancer of the larynx and admitted to major teaching and general hospitals in the study areas no longer than one year before the interview and with no history of cancer at other sites. Laryngeal cancer cases included 248 cases of cancer of the glottis [International Classification of Diseases (ICD)-IX, 161.0], 90 of the supraglottis (ICD-IX, 161.1), 4 of the subglottis (ICD-IX, 161.2), 4 of the laryngeal cartilage (ICD-IX, 161.3), 3 of overlapping lesions of larynx (ICD-IX, 161.8), and 111 cases of cancer from unspecified sites of the larynx (ICD-IX, 161.9). Controls were 1,088 subjects (863 men, 225 women; median age, 61 y; range, 31–79 y) admitted to the same hospitals as cases for a wide spectrum of acute non-neoplastic conditions unrelated to smoking or alcohol consumption, or to long-term modifications of diet. Controls were frequency matched with cases by age (5-y groups), sex, and area of residence. To compensate for the rarity of laryngeal cancer in women, a control-to-case ratio of ~5 was chosen for women as opposed to ~2 for men. Twenty-nine percent of the controls were admitted for traumas, 36% for other orthopedic disorders, 12% for acute surgical conditions, and 23% for miscellaneous other illnesses. Cases and controls were comparable in terms of age, sex, education, body mass index (BMI). However, cases reported higher consumption of tobacco and alcohol than controls. Less than 5% of both cases and controls contacted refused to participate. The study was approved by the appropriate ethics committees and all procedures have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
The same structured questionnaire and coding manual were used in each center, and all interviewers were centrally trained and routinely supervised. The questionnaire included information on socio-demographic characteristics, such as education and occupation, lifetime smoking and alcohol-drinking habits, anthropometric measures at various ages, a problem-oriented personal medical history, and family history of cancer. A reproducible (21) and valid (22) food frequency questionnaire (FFQ) was used to assess the patients’ usual diet in the two years preceding cancer diagnosis (for cases) or hospital admission (for controls). The FFQ included the average weekly consumption of 78 food items or food groups and of five alcoholic beverages. Intakes lower than once a week, but at least once per month were coded as 0.5 per week. Nutrients and energy intake were estimates using an Italian food composition database, supplemented with other sources (23).
FFQ-derived dietary data were used to calculate DII scores for each study subject. A complete description of the DII is available elsewhere (8). Briefly, to calculate DII for the subjects in this study, the dietary data were first linked to a world database that provided a robust estimate of a mean and standard deviation for each food parameter included in the DII. These parameters then became the multipliers to express an individual’s exposure relative to the “standard global mean” as a z-score. This was achieved by subtracting the “standard global mean” from the amount reported and dividing this value by the standard deviation. To minimize the effect of “right skewing,” this value was then converted to a centered percentile score (i.e., by doubling the percentile score and subtracting 1). The centered percentile score for each food parameter for each subject was then multiplied by the corresponding food parameter effect score, in order to obtain a food parameter-specific DII score. All of the food parameter-specific DII scores were then summed up to create the overall DII score for each study subject.
The DII scores computed based on this study’s FFQ include data on 30 of the 45 food parameters comprising the DII: carbohydrates, proteins, fats, fibers, cholesterol, saturated fatty acids, monounsaturated fatty acids, polyunsaturated fatty acids, omega 3, omega 6, niacin, thiamin, riboflavin, vitamin B6, iron, zinc, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, beta carotene, anthocyanidins, flavan3ols, flavonols, flavanones, flavones, isoflavones, caffeine, and tea. Alcohol was included as a covariate; hence, it was not included in the DII calculation. The DII was analysed both as a continuous variable, with each point corresponding to ≈9% of its range (5.00 to −5.48), and by quartiles of exposure computed among controls. Distributions of characteristics across quartiles of DII for controls and cases were computed and differences were analyzed using the chisquare test. Differences in food groups across quartiles of DII were determined using ANOVA. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were estimated using logistic regression models, including terms for age (quinquennia), sex, study center, education (<7, 7- 11, ≥12 years, categorically), BMI (<25, 25–30, ≥30 kg/m2, categorically), tobacco smoking (never smoker, ex-smoker, current smoker of <15, 15–24, and ≥25 cigarettes/day, categorically), alcohol drinking (<21, 21-<35, 35-<56, 56-<84, ≥84 drinks/week, categorically), and non-alcohol energy intake (quintiles among controls). Tests for linear trend were performed using the median value within each quartile as an ordinal variable. To investigate whether the effect of the DII was homogeneous across strata of selected covariates, we conducted analyses stratified by age, education, and BMI. We did not include results by sex because the number observations for women were too small. To test for homogeneity between strata, the difference between the −2 log(likelihood) of the models with and without the interaction terms was compared with the χ2 distribution with the same number of degrees of freedom as the number of interaction terms.
We then evaluated whether the actual interactions between alcohol or smoking and DII differed from either additive or multiplicative joint effects. In order to estimate the departure from additivity of effects we used the relative excess risk due to interaction (RERI) and the synergy index (S) (24). CI for the indices were obtained using the delta method (25). In order to evaluate the significance of the multiplicative interaction we used the likelihood ratio tests of significance comparing models including versus excluding the interaction term.
Statistical tests were performed using SAS® 9.3 (SAS Institute Inc., Cary, NC).
Results
The mean DII value was 0.44 (standard deviation, SD=1.41) among cases and −0.17 (SD=1.41) among controls, indicating a more pro-inflammatory diet among for cases. Control characteristics across quartiles of DII are provided in Table 1. There were significant differences in socio-demographic, anthropometric, and lifestyle habits across DII quartiles. Participants in the highest DII quartile were more often females, elderly, normal weight, and more likely to be less drinkers.
Table 1.
Participants’ characteristics across quartiles of dietary inflammatory index (DII) among 1088 controls. Italy, 1992–2000.
| Characteristics | DII quartiles | ||||
|---|---|---|---|---|---|
| −5.48, −2.19 | −2.18, −1.09 | −1.08,0.26 | 0.27, 5.00 | p valuea | |
| N (%) | N (%) | N (%) | N (%) | ||
| Sex | 0.01 | ||||
| Male | 234 (86.0) | 211 (77.6) | 212 (77.9) | 206 (75.7) | |
| Female | 38 (14.0) | 61 (22.4) | 60 (22.1) | 66 (24.3) | |
| Age (years) | 0.0001 | ||||
| <50 | 40 (14.7) | 26 (9.6) | 24 (8.8) | 25 (9.2) | |
| 50–54 | 29 (10.7) | 45 (16.5) | 36 (13.2) | 28 (10.3) | |
| 55–59 | 69 (25.4) | 54 (19.8) | 53 (19.5) | 37 (13.6) | |
| 60–64 | 62 (22.8) | 59 (21.7) | 57 (21.0) | 53 (19.5) | |
| 65–69 | 48 (17.6) | 43 (15.8) | 56 (20.6) | 72 (26.5) | |
| ≥70 | 24 (8.8) | 45 (16.5) | 46 (16.9) | 57 (21.0) | |
| Education (years)b | 0.42 | ||||
| <7 | 153 (56.3) | 160 (58.8) | 164 (60.3) | 172 (63.2) | |
| 7–11 | 88 (43.3) | 87 (32.4) | 77 (28.3) | 68 (25.0) | |
| >11 | 31 (11.4) | 24 (8.8) | 31 (11.4) | 32 (11.8) | |
| Body mass index(kg/m2) | 0.04 | ||||
| <25 | 80 (29.4) | 109 (40.1) | 94 (34.6) | 112 (41.2) | |
| 25 to <30 | 145 (53.3) | 132 (48.5) | 135 (49.6) | 128 (47.1) | |
| ≥30 | 47 (17.3) | 31 (11.4) | 43 (15.8) | 32 (11.8) | |
| Tobacco smoking | 0.78 | ||||
| Never smokers | 98 (36.0) | 107 (39.4) | 98 (36.0) | 101 (37.1) | |
| Ex-smokers | 102 (37.5) | 107 (39.4) | 96 (35.3) | 94 (34.6) | |
| Current smokers | |||||
| <15 cigarettes/day | 30 (11.0) | 26 (9.6) | 40 (14.7) | 32 (11.8) | |
| 15–24 cigarettes/day | 28 (10.3) | 24 (8.8) | 26 (9.6) | 33 (12.1) | |
| ≥25 cigarettes/day | 14 (5.1) | 8 (2.9) | 12 (4.4) | 12 (4.4) | |
| Alcohol drinking (drinks/week)b | <0.0001 | ||||
| <21 | 82 (30.3) | 139 (51.1) | 131 (48.2) | 144 (52.9) | |
| 21–<35 | 79 (29.1) | 70 (25.7) | 72 (26.5) | 69 (25.4) | |
| 35–<56 | 48 (17.7) | 31 (11.4) | 43 (15.8) | 29 (10.7) | |
| 56–<84 | 44 (16.2) | 23 (8.5) | 22 (8.1) | 23 (8.5) | |
| ≥84 | 18 (6.6) | 9 (3.3) | 4 (1.5) | 7 (2.6) | |
p value for Chi-square test
The sum does not add up to the total because of some missing values
Table 2 shows the distribution of 10 food groups across DII quartiles among controls. Servings of fruit, vegetables, fish and poultry decreased significantly across quartiles, whereas servings of pork, sugar, cheese, and desserts increased significantly.
Table 2.
Distribution of servings of food groups across quartiles of dietary inflammatory index (DII) among 1088 controls (mean ± standard deviation). Italy, 1992–2000.
| DII quartiles | p valuea | ||||
|---|---|---|---|---|---|
| Servings/week | −5.48, −2.19 |
−2.18, −1.09 |
−1.08,0.26 | 0.27, 5.00 | |
| Fruit | 24.2±10.6 | 19.3±8.5 | 15.4±8.0 | 9.4±8.6 | <0.0001 |
| Vegetables | 19.8±8.1 | 17.0±5.6 | 14.0±9.0 | 8.9±5.2 | <0.0001 |
| Fish | 2.0±1.4 | 1.8±1.1 | 1.5±1.0 | 1.3±0.9 | <0.0001 |
| Egg | 1.8±1.4 | 2.1±2.1 | 1.8±1.5 | 1.7±1.8 | 0.20 |
| Coffee | 17.8±12.4 | 17.9±10.8 | 18.6±11.8 | 17.8±12.9 | 0.81 |
| Poultry | 1.9±1.4 | 2.1±1.4 | 1.8±1.4 | 1.6±1.4 | 0.0002 |
| Pork | 2.3±1.8 | 2.8±2.5 | 2.6±2.0 | 2.9±3.2 | 0.03 |
| Sugar | 27.9±23.6 | 33.3±25.4 | 37.6±29.2 | 35.2±28.6 | 0.0003 |
| Cheese | 4.2±2.2 | 5.0±2.9 | 5.4±3.4 | 4.9±3.9 | 0.007 |
| Desserts | 4.7±4.5 | 6.1±5.4 | 6.1±7.6 | 5.8±9.4 | 0.008 |
p values were obtained from ANOVA
Table 3 gives the distribution of cases and controls according to quartiles of DII score, and the corresponding multivariable ORs, together with the continuous one. Subjects in the highest quartile had greater than a 3-fold excess risk of laryngeal cancer compared to subjects in the lowest quartile (ORQuartile4vs1= 3.30, 95% CI 2.06,5.28, p trend <0.0001). When analyses were carried out using DII as a continuous variable, the OR was 1.27 (95%CI 1.15,1.40) for a one unit (9% of the DII range) increase in DII.
Table 3.
Odds ratios (OR) of laryngeal cancer and corresponding 95% confidence intervals (CI) according to quartiles of dietary inflammatory index (DII) among 460 cases and 1088 controls. Italy, 1992–2000.
| Cases | Controls | ORa (95% CI) | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| DII quartiles | |||||
| −5.48, −2.19 | 100 | 21.7 | 272 | 25.0 | 1b |
| −2.18,−1.09 | 114 | 24.8 | 272 | 25.0 | 1.69 (1.13, 2.52) |
| −1.08,0.26 | 113 | 24.6 | 272 | 25.0 | 1.81 (1.19, 2.75) |
| 0.27,5.00 | 133 | 28.9 | 272 | 25.0 | 3.30 (2.06, 5.28) |
| p-value for trend | <0.0001 | ||||
| ORa in continuousc | 460 | 1088 | 1.27 (1.15, 1.40) | ||
Estimated from multiple logistic regression models adjusted for age, sex, centre, education, body mass index, tobacco smoking, alcohol consumption, and non-alcohol energy intake.
One unit increase equals to approximately 9% increase of its global range (5.00 to –5.48).
Reference category.
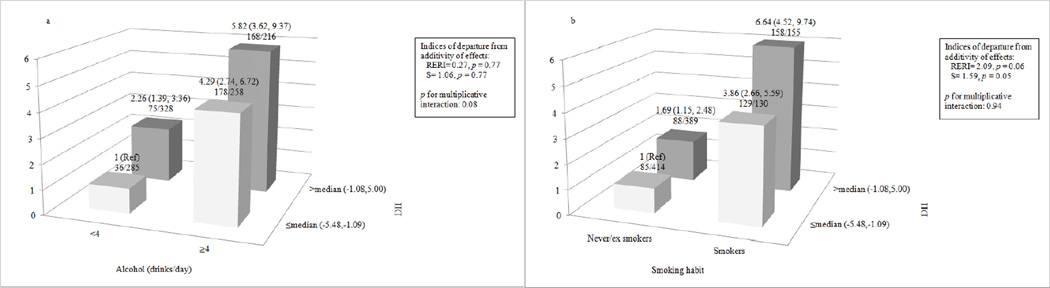
Table 4 shows multivariable ORs of laryngeal cancer in strata of selected covariates. Stronger associations were observed among subjects <60 years (ORQuartile4vs1= 4.68, 95% CI 2.26, 9.67) with BMI ≥25 kg/m2 (ORQuartile4vs1= 3.62, 95% CI 1.96, 6.69), and with more education (ORQuartile4vs1= 3.92, 95% CI 1.88, 8.19). However, results were not significantly heterogeneous (p values >0.10). Figure 1 shows the joint effects of DII and alcohol drinking or tobacco smoking on laryngeal cancer risk, controlling for centre and known confounders. Compared to the lowest-risk category; i.e., never to moderate drinkers (<4 drinks/day) with lower DII scores (≤median), the OR for laryngeal cancer was 5.82 (95% CI 3.62,9.37) for heavy drinkers with the higher DII category (> median). RERI was 0.27 (p = 0.77), S was 1.06 (p = 0.77), and p-value for multiplicative interaction was 0.08, suggesting a (borderline significant) negative multiplicative interaction. With reference to smoking, compared to never/ex smokers with the lower DII category, the OR for laryngeal cancer was 6.64 (95% CI 4.52,9.74) for current smokers with the higher DII category. RERI was 2.09 (p = 0.06), S was 1.59 (p = 0.05), and p-value for multiplicative interaction was 0.94, suggestive of (borderline significant) positive additive interaction of a pro-inflammatory diet with smoking in the association with laryngeal cancer.
Table 4.
Odds ratios (OR) of laryngeal cancer and corresponding 95% confidence intervals (CI) according to quartiles of dietary inflammatory index (DII), among 460 cases and 1088 controls, in strata of selected covariates. Italy, 1992–2000.
| Cases/Cont rols |
DII quartiles, OR (95% CI)a |
p value for trend |
p value for homogeneity |
||||
|---|---|---|---|---|---|---|---|
| −5.48, − 2.19 |
−2.18,−1.09 | −1.08,0.26 | 0.27, 5.00 | ||||
| Age (years) | 0.18 | ||||||
| <60 | 193/465 | 1b | 1.77 (0.98, 3.2) | 1.88 (1.00, 3.54) | 4.68 (2.26, 9.67) | <0.0001 | |
| ≥60 | 264/622 | 1b | 1.57 (0.92, 2.66) | 1.66 (0.97, 2.86) | 2.31 (1.27, 4.19) | 0.007 | |
|
Education (years)c |
0.25 | ||||||
| <7 | 289/649 | 1b | 1.46 (0.86, 2.46) | 1.25 (0.72, 2.18) | 3.09 (1.62, 5.91) | 0.001 | |
| ≥7 | 171/438 | 1b | 2.05 (1.05, 4.01) | 2.99 (1.52, 5.87) | 3.92 (1.88, 8.19) | <0.0001 | |
| BMI (kg/m2)c | 0.90 | ||||||
| <25 | 207/395 | 1b | 1.09 (0.57, 2.14) | 1.30 (0.63, 2.69) | 2.93 (1.34, 6.43) | 0.002 | |
| ≥25 | 251/693 | 1b | 2.21 (1.32, 3.69) | 2.24 (1.32, 3.79) | 3.62 (1.96, 6.69) | 0.0001 | |
Estimated from multiple logistic regression models adjusted for age, sex, centre, education, body mass index, tobacco smoking, alcohol consumption, and non-alcohol energy intake.
Reference category.
The sum does not add up to the total because of some missing values
Figure 1.
Joint effect of dietary inflammatory index (DII) and alcohol (Figure 1a) and tobacco (Figure 1b) on laryngeal cancer risk. Italy, 1992–2000.
Footnote:
Abbreviation: RERI: Relative excess risk due to interaction; S: Synergy index.
Odds ratios were estimated from multiple logistic regression models, including terms for age, sex, centre, education, body mass index, non-alcohol energy intake, and tobacco smoking and alcohol consumption when appropriate.
Discussion
In our Italian case-control study, a pro-inflammatory diet, as reflected in high DII scores, was strongly associated with an increased risk of laryngeal cancer. These results are in accordance with our previous results which showed protective effects of flavanones and flavonols (26), fibre (27), and various micronutrients (28), all of which have anti-inflammatory properties and are components of the DII. In addition, a diet rich in animal products and animal fats was positively associated with laryngeal cancer, whereas a diet rich in fruit and vegetables was negatively associated with laryngeal cancer (20). In the pooled analyses within the International Head and Neck Cancer Epidemiology Consortium, vitamin C was found to be protective against laryngeal cancer (29), whereas no association was observed with coffee and tea intake (30).
The effect of DII scores on the risk of laryngeal cancer appears to be multiplicative with tobacco smoking, but approximatively additive with alcohol. Previous results have been reported for other dietary components, such as vitamin C (31) and vitamin E (32) − thus leading to a 6-fold excess risk for subjects with high DII and tobacco smokers/heavy alcohol drinkers.
One of the possible mechanisms for the positive association between the DII and laryngeal cancer might be through the excess production of protooncogenic cytokines like IL-6, IL-8, platelet-derived growth factor, and vascular endothelial growth factor in the tumor microenvironment which are then responsible for carcinogenic activities like anti-apoptosis, tumor angiogenesis and metastasis (33). Hypoxia is a common state in cancers and inflamed tissues are more likely to experience DNA damage and induction of tumorigenic factors. Furthermore, tissue vasculature is a vital part of its microenvironment, supplying oxygen, nutrients, and growth factors to rapidly dividing cells and providing a mechanism for metastatic spread (2).
Among the limitations of our study, there are the possible biases of hospital-based case-control studies, including selection and information bias. However, the same interview setting and catchment areas for cases and controls, and the almost complete participation, are reassuring with reference to selection bias. Moreover, only patients admitted to hospital for acute conditions unrelated to major changes in diet and other lifestyle factors were included, whereas subjects with admission diagnosis related to tobacco smoking, alcohol drinking, and diet modifications were not eligible controls. With reference to information, bias in the recall of food intake by cases should be small, given the limited knowledge and attention paid in this population at the time of the study to the possible relationship between diet and laryngeal cancer. The FFQ was satisfactorily reproducible (21) and valid (22). With reference to confounding, we were able to carefully adjust for socio-demographic factors, tobacco smoking and alcohol drinking − whose information was satisfactorily reproducible (34, 35) −, as well as for energy intake. Among the strengths of the study are the large sample size, the high and variable consumption and diversity of fruits and vegetables in this Mediterranean population (36).
Using the DII, a unique approach was taken by focusing on the inflammatory effects of 30 foods and nutrients. As such, it relies on reviewing and scoring of the peer-reviewed literature on the subject of diet and inflammation. Also, it standardizes individuals’ dietary intakes of pro- and antiinflammatory food constituents to world referent values that results in values that are not dependent on units of consumption and can be used for comparison across studies.
In conclusion, subjects who consumed a pro-inflammatory diet had an increased risk of laryngeal cancer compared to those who consumed an antiinflammatory diet in this Italian population. This is the first study to examine this association and the results suggest that increasing intake of antiinflammatory dietary factors, such as plant-based foods rich in fiber and phytochemicals, and reducing intake of pro-inflammatory factors, such as fried foods or processed foods rich in saturated fat or animal protein, may be a strategy for reducing risk of laryngeal cancer, besides the key measures of avoiding tobacco and limiting alcohol consumption. It appears that the interaction of a proinflammatory diet with tobacco or heavy alcohol consumption leads to appreciably high risks of laryngeal cancer. This should help to provide focusing leads for the prevention of this neoplasm.
Acknowledgments
Funding: This study was supported by the Italian Foundation for Research on Cancer (FIRC) and by the Italian Ministry of Health, General Directorate of European and International Relations. Drs. Shivappa and Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Valentina Rosato was supported by a fellowship from the Italian Foundation for Cancer Research (FIRC #18107).
Footnotes
Disclosure: Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. Dr. Nitin Shivappa is an employee of CHI.
References
- 1.Hashibe M, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Feb;18(2):541–550. doi: 10.1158/1055-9965.EPI-08-0347. PubMed PMID: 19190158. Pubmed Central PMCID: 3051410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonomi M, Patsias A, Posner M, Sikora A. The role of inflammation in head and neck cancer. Advances in experimental medicine and biology. 2014;816:107–127. doi: 10.1007/978-3-0348-0837-8_5. PubMed PMID: 24818721. [DOI] [PubMed] [Google Scholar]
- 3.Edefonti V, Hashibe M, Ambrogi F, Parpinel M, Bravi F, Talamini R, et al. Nutrient-based dietary patterns and the risk of head and neck cancer: a pooled analysis in the International Head and Neck Cancer Epidemiology consortium. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012 Jul;23(7):1869–1880. doi: 10.1093/annonc/mdr548. PubMed PMID: 22123733. PubMed Central PMCID: 3387823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Mouth, pharyngeal and laryngeal cancer. World Cancer Research Fund International; 2007. Available at http://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/mouth-pharyngeal-and-laryngeal: [Google Scholar]
- 5.Keibel A, Singh V, Sharma MC. Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des. 2009;15(17):1949–1955. doi: 10.2174/138161209788453167. PubMed PMID: 19519435. [DOI] [PubMed] [Google Scholar]
- 6.Pan MH, Lai CS, Dushenkov S, Ho CT. Modulation of inflammatory genes by natural dietary bioactive compounds. J Agric Food Chem. 2009 Jun 10;57(11):4467–4477. doi: 10.1021/jf900612n. PubMed PMID: 19489612. [DOI] [PubMed] [Google Scholar]
- 7.Tezal M, Scannapieco FA, Wactawski-Wende J, Hyland A, Marshall JR, Rigual NR, et al. Local inflammation and human papillomavirus status of head and neck cancers. Archives of otolaryngology--head & neck surgery. 2012 Jul;138(7):669–675. doi: 10.1001/archoto.2012.873. PubMed PMID: 22710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014 Aug;17(8):1689–1696. doi: 10.1017/S1368980013002115. PubMed PMID: 23941862. PubMed Central PMCID: 3925198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR. A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS) Public Health Nutrition. 2013 Oct 10;10:1–9. doi: 10.1017/S1368980013002565. PubMed PMID: 24107546.Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, et al. Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med. 2014;56(9):986–989. doi: 10.1097/JOM.0000000000000213. PubMed PMID: 25046320. English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wood L, Shivappa N, Berthon BS, Gibson PG, Hebert JR. Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clinical & Experimental Allergy. 2014 doi: 10.1111/cea.12323. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Huybrecht I. Association between dietary inflammatory index and inflammatory markers in Asklepios study. British Journal of Nutrition. 2014 doi: 10.1017/S000711451400395X. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivappa N, Zucchetto A, Serraino D, Rossi M, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of esophageal squamous cell cancer in a case-control study from Italy. Cancer causes & control : CCC. 2015 Oct;26(10):1439–1447. doi: 10.1007/s10552-015-0636-y. PubMed PMID: 26208592. [DOI] [PubMed] [Google Scholar]
- 14.Shivappa N, Hebert JR, Rashidkhani B. Dietary Inflammatory Index and Risk of Esophageal Squamous Cell Cancer in a Case-Control Study from Iran. Nutrition and cancer. 2015 Nov-Dec;67(8):1253–1259. doi: 10.1080/01635581.2015.1082108. PubMed PMID: 26400625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivappa N, Zucchetto A, Montella M, Serraino D, Steck SE, La Vecchia C, et al. Inflammatory potential of diet and risk of colorectal cancer: a case-control study from Italy. The British journal of nutrition. 2015 Jul 14;114(1):152–158. doi: 10.1017/S0007114515001828. PubMed PMID: 26050563. [DOI] [PubMed] [Google Scholar]
- 16.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR. Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. The British journal of nutrition. 2014 Dec;17:1–7. doi: 10.1017/S0007114514003626. PubMed PMID: 25515552. PubMed Central PMCID: 4470878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shivappa N, Hebert JR, Polesel J, Zucchetto A, Crispo A, Montella M, et al. Inflammatory potential of diet and risk for hepatocellular cancer in a case-control study from Italy. The British journal of nutrition. 2016 Jan;115(2):324–331. doi: 10.1017/S0007114515004419. PubMed PMID: 26556602. [DOI] [PubMed] [Google Scholar]
- 18.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, et al. Association between dietary inflammatory index and prostate cancer among Italian men. The British journal of nutrition. 2014 Nov;17:1–6. doi: 10.1017/S0007114514003572. PubMed PMID: 25400225. PubMed Central PMCID: 4433863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shivappa N, Hebert JR, Zucchetto A, Montella M, Serraino D, La Vecchia C, et al. Dietary inflammatory index and endometrial cancer risk in an Italian case-control study. The British journal of nutrition. 2016 Jan;115(1):138–146. doi: 10.1017/S0007114515004171. PubMed PMID: 26507451. [DOI] [PubMed] [Google Scholar]
- 20.Edefonti V, Bravi F, Garavello W, La Vecchia C, Parpinel M, Franceschi S, et al. Nutrient-based dietary patterns and laryngeal cancer: evidence from an exploratory factor analysis. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010 Jan;19(1):18–27. doi: 10.1158/1055-9965.EPI-09-0900. PubMed PMID: 20056619. [DOI] [PubMed] [Google Scholar]
- 21.Franceschi S, Negri E, Salvini S, Decarli A, Ferraroni M, Filiberti R, et al. Reproducibility of an Italian food frequency questionnaire for cancer studies: results for specific food items. European journal of cancer. 1993;29A(16):2298–2305. doi: 10.1016/0959-8049(93)90225-5. PubMed PMID: 8110502. [DOI] [PubMed] [Google Scholar]
- 22.Decarli A, Franceschi S, Ferraroni M, Gnagnarella P, Parpinel MT, La Vecchia C, et al. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Annals of epidemiology. 1996 Mar;6(2):110–118. doi: 10.1016/1047-2797(95)00129-8. PubMed PMID: 8775590. [DOI] [PubMed] [Google Scholar]
- 23.Salvini S, Parpinel M, Gnagnarella P, Maisonneuve P, Turrini A. Banca dati di composizione degli alimenti per studi epidemiologici in Italia. Istituto Europeo di Oncologia. Milano. 1998 [Google Scholar]
- 24.Rothman K. Measuring interactions. Oxford: Oxford University Press; 2002. Epidemiology: an introduction; pp. 168–180. [Google Scholar]
- 25.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992 Sep;3(5):452–456. doi: 10.1097/00001648-199209000-00012. PubMed PMID: 1391139. [DOI] [PubMed] [Google Scholar]
- 26.Garavello W, Rossi M, McLaughlin JK, Bosetti C, Negri E, Lagiou P, et al. Flavonoids and laryngeal cancer risk in Italy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2007 Jun;18(6):1104–1109. doi: 10.1093/annonc/mdm078. PubMed PMID: 17372161. [DOI] [PubMed] [Google Scholar]
- 27.Pelucchi C, Talamini R, Levi F, Bosetti C, La Vecchia C, Negri E, et al. Fibre intake and laryngeal cancer risk. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2003 Jan;14(1):162–167. doi: 10.1093/annonc/mdg032. PubMed PMID: 12488309. [DOI] [PubMed] [Google Scholar]
- 28.Bidoli E, Bosetti C, La Vecchia C, Levi F, Parpinel M, Talamini R, et al. Micronutrients and laryngeal cancer risk in Italy and Switzerland: a case-control study. Cancer causes & control : CCC. 2003 Jun;14(5):477–484. doi: 10.1023/a:1024991618398. PubMed PMID: 12946043. [DOI] [PubMed] [Google Scholar]
- 29.Edefonti V, Hashibe M, Parpinel M, Turati F, Serraino D, Matsuo K, et al. Natural vitamin C intake and the risk of head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. International journal of cancer Journal international du cancer. 2014 Dec 8; doi: 10.1002/ijc.29388. PubMed PMID: 25627906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galeone C, Tavani A, Pelucchi C, Turati F, Winn DM, Levi F, et al. Coffee and tea intake and risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010 Jul;19(7):1723–1736. doi: 10.1158/1055-9965.EPI-10-0191. PubMed PMID: 20570908. PubMed Central PMCID: 3047460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edefonti V, Hashibe M, Parpinel M, Turati F, Serraino D, Matsuo K, et al. Natural vitamin C intake and the risk of head and neck cancer: A pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. International journal of cancer Journal international du cancer. 2015 Jul 15;137(2):448–462. doi: 10.1002/ijc.29388. PubMed PMID: 25627906. PubMed Central PMCID: 4428957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edefonti V, Hashibe M, Parpinel M, Ferraroni M, Turati F, Serraino D, et al. Vitamin E intake from natural sources and head and neck cancer risk: a pooled analysis in the International Head and Neck Cancer Epidemiology consortium. British journal of cancer. 2015 Jun 30;113(1):182–192. doi: 10.1038/bjc.2015.149. PubMed PMID: 25989276. Pubmed Central PMCID: 4647526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pries R, Wollenberg B. Cytokines in head and neck cancer. Cytokine & growth factor reviews. 2006 Jun;17(3):141–146. doi: 10.1016/j.cytogfr.2006.02.001. PubMed PMID: 16540364. [DOI] [PubMed] [Google Scholar]
- 34.Ferraroni M, Decarli A, Franceschi S, La Vecchia C, Enard L, Negri E, et al. Validity and reproducibility of alcohol consumption in Italy. International journal of epidemiology. 1996 Aug;25(4):775–782. doi: 10.1093/ije/25.4.775. PubMed PMID: 8921456. [DOI] [PubMed] [Google Scholar]
- 35.D'Avanzo B, La Vecchia C, Katsouyanni K, Negri E, Trichopoulos D. Reliability of information on cigarette smoking and beverage consumption provided by hospital controls. Epidemiology. 1996 May;7(3):312–315. doi: 10.1097/00001648-199605000-00018. PubMed PMID: 8728449. [DOI] [PubMed] [Google Scholar]
- 36.Bosetti C, Gallus S, Trichopoulou A, Talamini R, Franceschi S, Negri E, et al. Influence of the Mediterranean diet on the risk of cancers of the upper aerodigestive tract. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2003 Oct;12(10):1091–1094. PubMed PMID: 14578148. [PubMed] [Google Scholar]