Abstract
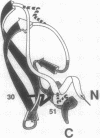
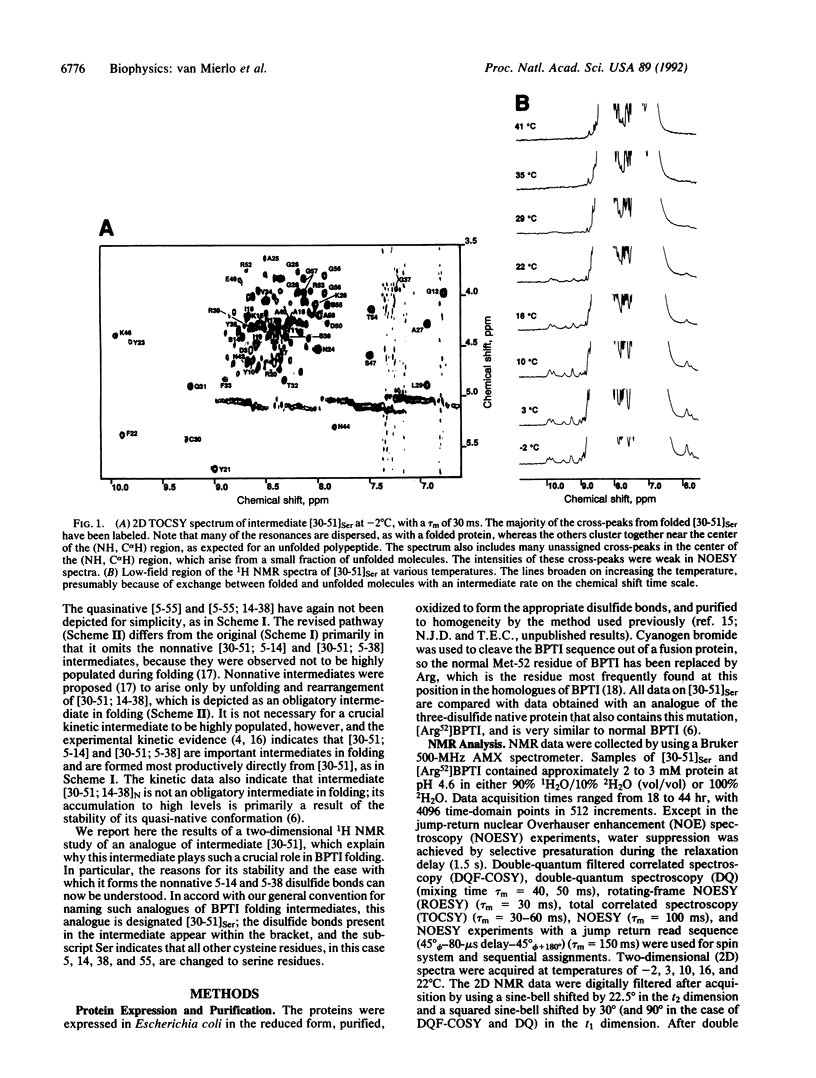
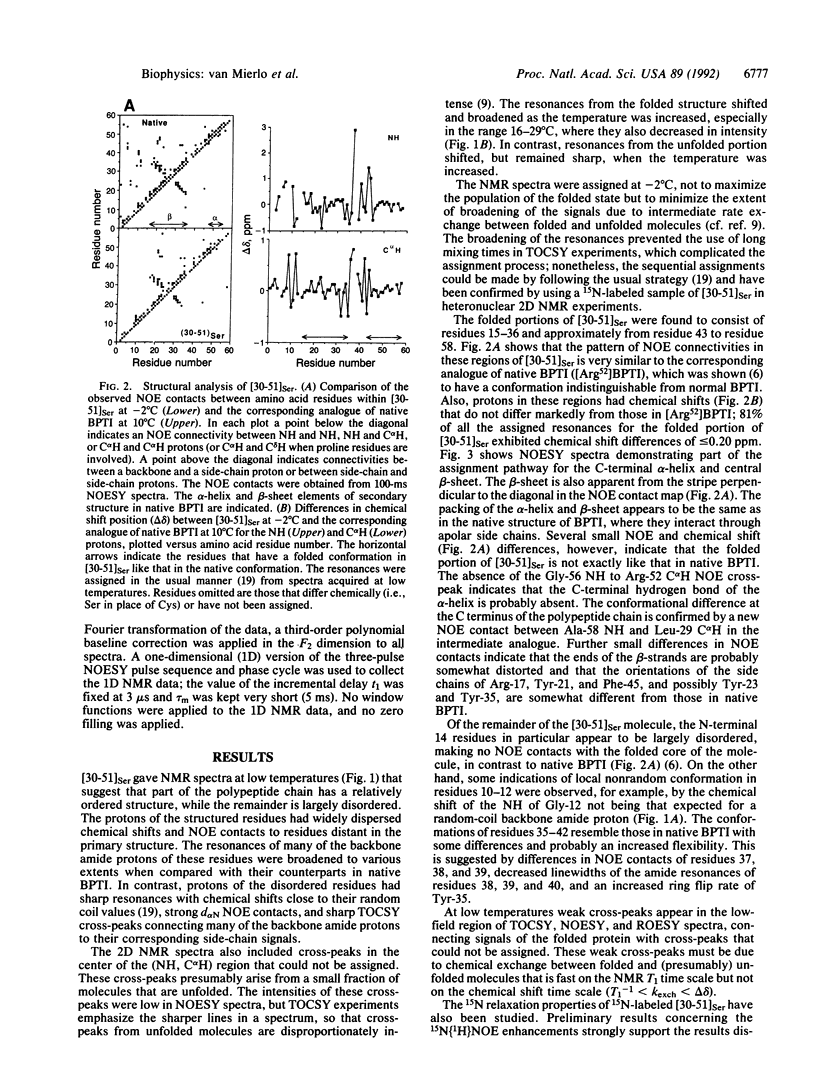
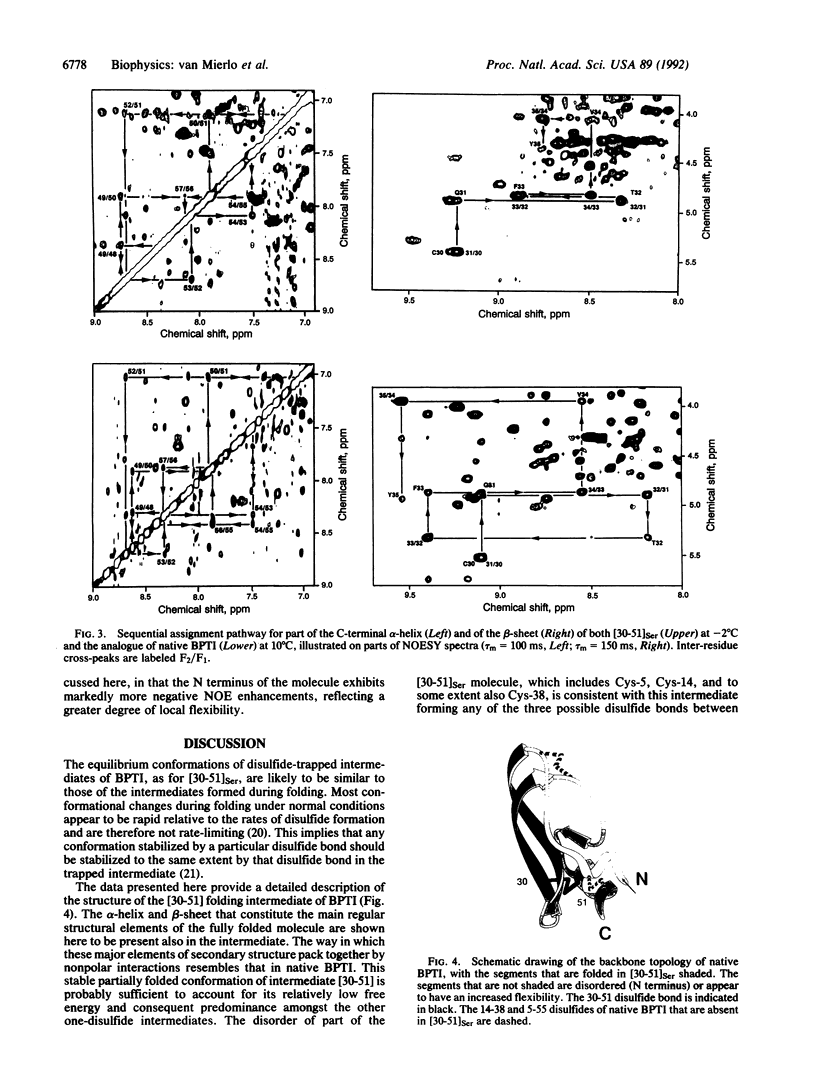
The best-characterized protein folding pathway is that of bovine pancreatic trypsin inhibitor, which folds from the reduced form through a series of disulfide bond intermediates. The crucial one-disulfide intermediate of bovine pancreatic trypsin inhibitor with the disulfide bond between Cys-30 and Cys-51 is shown here to have a partially folded conformation in which the major elements of secondary structure interact via a core of apolar side chains, which resembles part of the native conformation. The stability of this structure can account for the predominance of this one-disulfide intermediate during folding. Much of the remaining one-third of the polypeptide chain, in particular the N-terminal 14 residues, is largely disordered; this accounts for the ability of this intermediate to form readily any of the three possible second disulfide bonds involving Cys-5, -14, and -38. The partially folded conformation of this intermediate provides direct evidence for the importance of native-like interactions between elements of secondary structure in directing protein folding, which is assumed in many studies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Creighton T. E., Charles I. G. Biosynthesis, processing, and evolution of bovine pancreatic trypsin inhibitor. Cold Spring Harb Symp Quant Biol. 1987;52:511–519. doi: 10.1101/sqb.1987.052.01.058. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Conformational restrictions on the pathway of folding and unfolding of the pancreatic trypsin inhibitor. J Mol Biol. 1977 Jun 25;113(2):275–293. doi: 10.1016/0022-2836(77)90142-5. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Disulfide bonds as probes of protein folding pathways. Methods Enzymol. 1986;131:83–106. doi: 10.1016/0076-6879(86)31036-x. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Energetics of folding and unfolding of pancreatic trypsin inhibitor. J Mol Biol. 1977 Jun 25;113(2):295–312. doi: 10.1016/0022-2836(77)90143-7. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Creighton T. E., Goldenberg D. P. Kinetic role of a meta-stable native-like two-disulphide species in the folding transition of bovine pancreatic trypsin inhibitor. J Mol Biol. 1984 Nov 5;179(3):497–526. doi: 10.1016/0022-2836(84)90077-9. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Protein folding. Biochem J. 1990 Aug 15;270(1):1–16. doi: 10.1042/bj2700001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. The disulfide folding pathway of BPTI. Science. 1992 Apr 3;256(5053):111–114. doi: 10.1126/science.1373519. [DOI] [PubMed] [Google Scholar]
- Darby N. J., van Mierlo C. P., Creighton T. E. The 5-55 single-disulphide intermediate in folding of bovine pancreatic trypsin inhibitor. FEBS Lett. 1991 Feb 11;279(1):61–64. doi: 10.1016/0014-5793(91)80251-w. [DOI] [PubMed] [Google Scholar]
- Darby N. J., van Mierlo C. P., Scott G. H., Neuhaus D., Creighton T. E. Kinetic roles and conformational properties of the non-native two-disulphide intermediates in the refolding of bovine pancreatic trypsin inhibitor. J Mol Biol. 1992 Apr 20;224(4):905–911. doi: 10.1016/0022-2836(92)90458-v. [DOI] [PubMed] [Google Scholar]
- Eigenbrot C., Randal M., Kossiakoff A. A. Structural effects induced by removal of a disulfide-bridge: the X-ray structure of the C30A/C51A mutant of basic pancreatic trypsin inhibitor at 1.6 A. Protein Eng. 1990 Jul;3(7):591–598. doi: 10.1093/protein/3.7.591. [DOI] [PubMed] [Google Scholar]
- Goldenberg D. P. Kinetic analysis of the folding and unfolding of a mutant form of bovine pancreatic trypsin inhibitor lacking the cysteine-14 and -38 thiols. Biochemistry. 1988 Apr 5;27(7):2481–2489. doi: 10.1021/bi00407a034. [DOI] [PubMed] [Google Scholar]
- Karplus S., Snyder G. H., Sykes B. D. A nuclear magnetic resonance study of bovine pancreatic trypsin inhibitor. Tyrosine titrations and backbone NH groups. Biochemistry. 1973 Mar 27;12(7):1323–1329. doi: 10.1021/bi00731a012. [DOI] [PubMed] [Google Scholar]
- Kosen P. A., Creighton T. E., Blout E. R. Circular dichroism spectroscopy of bovine pancreatic trypsin inhibitor and five altered conformational states. Relationship of conformation and the refolding pathway of the trypsin inhibitor. Biochemistry. 1981 Sep 29;20(20):5744–5754. doi: 10.1021/bi00523a017. [DOI] [PubMed] [Google Scholar]
- Kosen P. A., Creighton T. E., Blout E. R. Circular dichroism spectroscopy of the intermediates that precede the rate-limiting step of the refolding pathway of bovine pancreatic trypsin inhibitor. Relationship of conformation and the refolding pathway. Biochemistry. 1983 May 10;22(10):2433–2440. doi: 10.1021/bi00279a020. [DOI] [PubMed] [Google Scholar]
- Oas T. G., Kim P. S. A peptide model of a protein folding intermediate. Nature. 1988 Nov 3;336(6194):42–48. doi: 10.1038/336042a0. [DOI] [PubMed] [Google Scholar]
- Stassinopoulou C. I., Wagner G., Wüthrich K. Two-dimensional 1H NMR of two chemically modified analogs of the basic pancreatic trypsin inhibitor. Sequence-specific resonance assignments and sequence location of conformation changes relative to the native protein. Eur J Biochem. 1984 Dec 3;145(2):423–430. doi: 10.1111/j.1432-1033.1984.tb08571.x. [DOI] [PubMed] [Google Scholar]
- States D. J., Creighton T. E., Dobson C. M., Karplus M. Conformations of intermediates in the folding of the pancreatic trypsin inhibitor. J Mol Biol. 1987 Jun 5;195(3):731–739. doi: 10.1016/0022-2836(87)90192-6. [DOI] [PubMed] [Google Scholar]
- States D. J., Dobson C. M., Karplus M. A new two-disulphide intermediate in the refolding of reduced bovine pancreatic trypsin inhibitor. J Mol Biol. 1984 Apr 5;174(2):411–418. doi: 10.1016/0022-2836(84)90345-0. [DOI] [PubMed] [Google Scholar]
- Weissman J. S., Kim P. S. Reexamination of the folding of BPTI: predominance of native intermediates. Science. 1991 Sep 20;253(5026):1386–1393. doi: 10.1126/science.1716783. [DOI] [PubMed] [Google Scholar]
- van Mierlo C. P., Darby N. J., Neuhaus D., Creighton T. E. (14-38, 30-51) double-disulphide intermediate in folding of bovine pancreatic trypsin inhibitor: a two-dimensional 1H nuclear magnetic resonance study. J Mol Biol. 1991 Nov 20;222(2):353–371. doi: 10.1016/0022-2836(91)90216-s. [DOI] [PubMed] [Google Scholar]
- van Mierlo C. P., Darby N. J., Neuhaus D., Creighton T. E. Two-dimensional 1H nuclear magnetic resonance study of the (5-55) single-disulphide folding intermediate of bovine pancreatic trypsin inhibitor. J Mol Biol. 1991 Nov 20;222(2):373–390. doi: 10.1016/0022-2836(91)90217-t. [DOI] [PubMed] [Google Scholar]