Abstract
Objectives
Vein graft adaptation is characterized by loss of expression of the tyrosine kinase receptor Eph-B4, the embryonic determinant of venous identity, without increased expression of its ligand Ephrin-B2, the embryonic determinant of arterial identity. eNOS is an important mediator of vessel remodeling. We hypothesized that the mechanism of action of Eph-B4 during vein graft adaptation might be via regulation of downstream eNOS activity.
Methods
Mouse lung endothelial cells (MLEC) were stimulated with Ephrin-B2/Fc, without and with preclustering, without and with the eNOS inhibitor L-NAME or the Eph-B4 inhibitor NVP-BHG712, and assessed by Western blot and immunofluorescence for eNOS and Eph-B4 phosphorylation. Nitric oxide (NO) production was assessed using a NO-specific chemiluminescence analyzer. Cell migration was assessed using a transwell assay. Human and mouse vein graft specimens were examined for eNOS activity by Western blot, and vessel remodeling was assessed in vein grafts in wild-type (WT) or eNOS-knockout (KO) mice.
Results
Ephrin-B2/Fc stimulated both Eph-B4 and eNOS phosphorylation in a bimodal temporal distribution (n=4; p<.05), with preclustered Ephrin-B2/Fc causing prolonged peak Eph-B4 and eNOS phosphorylation as well as altered subcellular localization (n=4; p<.05). Ephrin-B2/Fc increased NO release (n=3; p<.01) as well as increased endothelial cell migration (n=6; p<.05) in an eNOS-dependent fashion. Both human and mouse vein grafts showed increased eNOS phosphorylation compared with normal veins (n=3; p<.05). Vein grafts from eNOS-KO mice showed less dilation and less wall thickening compared with WT vein grafts (n=7; p<.05).
Conclusions
eNOS is a mediator of vein graft adaptation to the arterial environment. Eph-B4 stimulates eNOS phosphorylation in vitro and may mediate vein graft adaptation by regulation of eNOS activity in vivo.
Introduction
Autologous saphenous vein grafts are the preferred conduit for revascularization of advanced peripheral arterial disease.1, 2 Vein grafts placed in the arterial circulation are subjected to higher arterial pressure and shear stress and remodel to this environment by wall thickening and lumen dilatation that normalizes shear stress on the endothelium.3 In addition to this adaptive response to the change in the hemodynamic environment, vein grafts also respond to the injury of the procedure and the new environment with an inflammatory response.4 In some patients, the early adaptive remodeling and inflammatory responses become excessive and lead to neointimal hyperplasia with subsequent late vein graft failure.5 Understanding the mechanisms of how venous adaptive remodeling is finely regulated to balance adaptation without excessive thickening may improve long-term vein graft patency.4
The tyrosine kinase receptor Eph-B4, and its cognate ligand Ephrin-B2 determine the molecular distinction between veins and arteries, respectively, in the early embryo.6 Despite the reciprocal distribution of venous Eph-B4 and arterial Ephrin-B2 during early vascular development, the function of Eph-B4 and Ephrin-B2 in normal adult vessels is not well understood. Eph-B4 has been previously thought to be a passive marker of venous identity in adult veins; however, we have previously shown that Eph-B4 is functional during vein graft adaptation by inhibiting vein wall thickening, suggesting that Eph-B4 is functional in adult veins.7, 8 Stimulation of Eph-B4 inhibits neointimal hyperplasia in adult human veins, further suggesting the potential utility of this pathway for therapeutic modulation.9 However, the mechanisms by which Eph-B4 regulates vein graft adaptation are not well understood.
Nitric oxide (NO) production by endothelial nitric oxide synthase (eNOS) regulates both physiological and pathological vascular functions.10 Impairment of eNOS derived NO leads to less vasorelaxation and accelerated smooth muscle cell proliferation and migration that contribute to pathological neointimal hyperplasia.11, 12 Despite data that eNOS plays a critical role in arterial remodeling,12 little is known about its function in venous adaptive remodeling. Therefore we hypothesized that eNOS may be a downstream mediator of Eph-B4 signaling during venous adaptive remodeling such as occurs during vein graft adaptation to the arterial environment.
Methods
Antibodies and Reagents
Primary antibodies to the following antigens were obtained as follows: Eph-B4 (R&D systems; Catalog: AF446; 1:500 for western blot and immuopercipitation; NOVUS Biologicals; Catalog: NBP1-49607; 1:100 for immunofluorescence), phospho-tyrosine (Cell Signaling Technology; Catalog: #8954;1:100); phospho-tyrosine mouse mAb sepharose bead conjugate (Cell Signaling Technology; Catalog: #9419); GAPDH (Cell Signaling Technology; Catalog: #2118; 1:2000), phospho-eNOS (peNOS) (Santa Cruz Biotechnology, Inc; Catalog: 612392; 1:1000 for Western blot, Abcam; Catalog: ab87750; 1:100 for immunofluorescence), and eNOS (Santa Cruz Biotechnology, Inc; Catalog: 610297). Secondary antibodies were: Donkey anti goat IgG-HRP (Santa Cruz Biotechnology, Inc; Catalog: sc-2020; 1:10000); Donkey anti rabbit IgG-HRP (Santa Cruz Biotechnology, Inc; Catalog: sc-2313; 1:5000); Anti-mouse IgG, HRP linked antibody (Cell Signaling Technology; Catalog: #7076; 1:2000); and Alexa Fluor 488-, 568- conjugated IgG (Invitrogen; 1:200). Other reagents included: SlowFade Gold antifade reagent with DAPI, Prolong Gold antifade reagent with DAPI (Invitrogen), recombinant mouse Ephrin-B2/Fc chimera (R&D Systems; Catalog: 496-EB-200), goat anti-human IgG Fc fragment specific (Jackson immunoresearch laboratories, Inc; Code Number: 109-001-008), NVP-BHG712 (Sigma-Aldrich; Catalog: SML0333) and Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME; Sigma-Aldrich; Catalog: N5751).
Cell culture
Mouse lung endothelial cells (MLEC) were isolated from C57Bl/6 or eNOS knockout (KO) mice as previously described;13 primary EC were immortalized by infection with retrovirus encoding the middle T antigen and were maintained with endothelial basal medium 2/endothelial cell growth media-2 MV SingleQuot Kit Supplement & Growth Factors (Lonza), supplemented with 20% fetal bovine serum (FBS; Gibco by Life technology), penicillin/streptomycin (Corning Life Science, 5ml in total 500ml medium), and L-glutamine (Corning Life Science, 5ml in total 500ml medium). Culture medium was changed every 2 days. When cells reached ~90% confluence, cells were passaged and split with the use of 0.25% trypsin (Gibco by Life technology). Where indicated, cells were pre-incubated with NVP-BHG712 (1 μM) for 1 hour prior to Ephrin-B2/Fc stimulation.
Ephrin-B2/Fc and clustering treatments in vitro
Confluent MLEC were cultured with serum-free endothelial basal medium 2 (Lonza) for 24 hours and then treated with Ephrin-B2/Fc (2 μg/ml) for the indicated time. Ephrin-B2/Fc multimers were generated by pre-incubation of Ephrin-B2/Fc with Anti-Fc at a ratio of 1:5 (10 μg/ml of Anti-Fc with 2 μg/ml of Ephrin-B2/Fc dimer) for 30 minutes at room temperature.
Nitric Oxide release analysis
WT and eNOS KO MLEC were placed in serum-free medium for 24 hours after they reached ~90% confluence, and pretreated with control or L-NAME (1 mM, 30 min) prior to stimulation with Ephrin-B2/Fc (2 μg/ml; 60 min). Conditioned medium was then collected and processed with an NO-specific chemiluminescence analyzer as previously described.14
Cell migration assay
EC migration was measured using 8 μm transwell inserts (Corning Life Science) as previously described.15 Briefly, WT and eNOS KO cells were starved for 15 hours and placed in a 0.1% Gelatin (Sigma-Aldrich) coated transwell upper chamber. Cells were pretreated with L-NAME (1 mM, 30 mins) as indicated. Serum-free medium, Ephrin-B2/Fc (2 μg/ml) or 10% FBS was placed in the lower chamber; FBS was used as a positive control. After 8 hours, cells were fixed, stained and counted in 5 representative high power fields.
Western Blot
Cells were lysed in buffer containing RIPA lysis buffer, 10% SDS buffer, protease inhibitor (Roche) and phosphatase inhibitor I&II (Calbiochem). After sonication for 5 sec and centrifugation at 13500 rpm for 15 min, equal amounts of protein from each group were loaded into a 10% SDS-polyacrylamide gel and then transferred to a polyvinylidene difluoride membrane (Millipore). The membrane was incubated with appropriate primary and secondary antibodies as described above. Membrane signals were detected using the ECL detection reagent (Perkin Elmer) and film processor SRX - 101A (Konica Minolta).
Immunoprecipitation
After scraping cells with lysis buffer, the protein concentration was measured using the protein assay reagent (Bio-Rad) and normalized to 1.25 mg/ml. Samples were incubated with phospho-tyrosine mouse mAb (Sepharose bead conjugate) overnight at 4°C. Samples were processed for analysis by western blot.
Immunofluorescence
Cells were grown on a square 18 × 18 mm sterile microscope cover glass (Fisherbrand) placed in the well of six-well-plate that was coated with 0.1% gelatin (Sigma-Aldrich). After stimulation, cells were fixed with Acetone (J.T.Baker) at −20°C for 15 min, and then permeabilized in 0.1% PBS-T (PBS with 0.1% Triton X-100) for 15 min. Cells were blocked with 10% goat serum (Vector Laboratories) for 1 hour, then incubated with primary antibody diluted in blocking buffer overnight in 4°C. Secondary detection was performed using Alexa Fluor 488 and 568 for 2 hours and counterstained with DAPI.
Vein graft tissue processed for immunofluorescence was fixed with formalin, and embedded in paraffin, cut into 5-mm sections, and placed on slides. Tissue sections were deparaffinized using xylene and a graded series of alcohols. Antigen retrieval was conducted by boiling with 10 mM citric acid (pH 6) for 30 minutes. Slides were blocked with 1% bovine serum albumin before incubation with primary antibody overnight in 4°C. Alexa Fluor 488 and 568 were used for fluorescence.
Human specimens
The principles outlined in the Declaration of Helsinki were followed, and approval of the institutional Human Investigation Committee was obtained. De-identified patent saphenous vein grafts were explanted from human patients undergoing cardiac transplantation, or from patients with a patent peripheral bypass undergoing amputation for distal ischemia without infection, as previously described.7 Lysis buffer was added to samples to extract protein and processed for Western blot.
Mouse vein graft model
All protocols were approved by Yale University's Institutional Animal Care and Use Committee, and were performed and administered within National Institutes of Health and ethical guidelines. The mouse vein graft model was performed as previously described.8 Briefly, 12-wk-old C57BL/6 wild type (WT) and eNOS knockout (eNOS KO) mice (The Jackson Laboratory) were purchased. An approximately 2.0-mm segment of the intrathoracic inferior vena cava (WT or eNOS KO) was isolated and implanted into the infrarenal abdominal aorta of a recipient WT mouse. The abdominal aorta was temporarily occluded with atraumatic micro-clamps and a segment corresponding to the length of the vein graft was excised. The vein was sutured into the arterial circulation using 10–0 nylon in continuous fashion. Vein grafts were followed postoperatively using the Vevo 770 High-Resolution Imaging System (VisualSonics). At 3 weeks after surgery, mice were sacrificed and vein grafts were fixed by transcardial perfusion of phosphate-buffered saline (PBS), followed by 10% formalin. Vein grafts were removed and fixed overnight in 10% formalin, followed by 70% alcohol for 24 hours. Tissue was embedded in paraffin and sectioned (5 μm thick). Tissue sections were deparaffinized and either stained with hematoxylin and eosin or processed for immunofluorescence.
Statistics
Western blot results were quantitated with Image J software. Data are expressed as mean ± SEM. The statistical significance was determined using the t-test or analysis of variance (ANOVA) with appropriate post-hoc testing between groups. Differences were considered to be significant at P < 0.05 (Prism 6.0, Graphpad Software).
Results
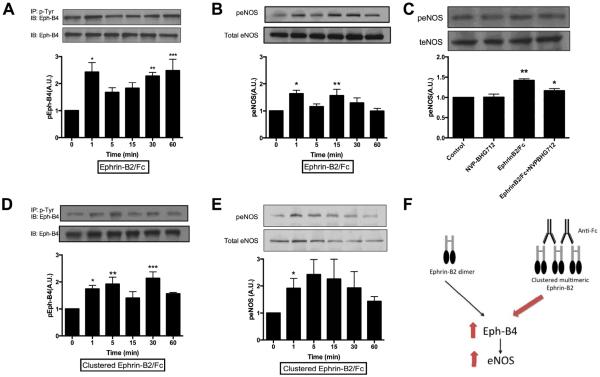
Since both Eph-B4 and eNOS regulate vessel remodeling,8, 12 we determined if stimulation of Eph-B4 activates eNOS in MLEC in vitro. We previously showed that MLEC express both Eph-B4 and Ephrin-B2, consistent with a venous phenotype.16 As expected, stimulation of endothelial cells with Ephrin-B2/Fc stimulated Eph-B4 phosphorylation, in a bimodal temporal distribution, with an early peak at approximately 1 minute and a later peak at approximately 30–60 minutes (Figure 1A). In addition to Ephrin-B2/Fc stimulating Eph-B4 phosphorylation, Ephrin-B2/Fc also stimulated eNOS phosphorylation, in a similar bimodal pattern (Figure 1B), that was inhibited in cells pretreated with the Eph-B4 inhibitor NVP-BHG712 (Figure 1C). Since Eph receptor signaling is triggered by clustered ligands,17 and multimeric forms may have increased magnitude or temporal pattern of receptor activation,18 we examined the effect of clustered Ephrin-B2/Fc on Eph-B4 phosphorylation. Multimeric Ephrin-B2/Fc was generated by clustering Ephrin-B2/Fc with anti-Fc antibodies,18 using an Ephrin-B2/Fc: anti-Fc ratio of 1:5 to maximally enhance Eph-B4 phosphorylation (data not shown). Clustered Ephrin-B2/Fc also stimulated Eph-B4 phosphorylation in a bimodal pattern similar to unclustered Ephrin-B2/Fc, although the early peak of phosphorylation appeared to be sustained to 5 minutes, potentially consistent with increased Eph-B4 activation with the clustered ligand (Figure 1D). Clustered Ephrin-B2/Fc also stimulated eNOS phosphorylation, but in a unimodal distribution, of greater maximal magnitude compared to unclustered Ephrin-B2/Fc (Figure 1E). These results suggest that activation of Eph-B4 with Ephrin-B2/Fc stimulates eNOS phosphorylation in vitro (Figure 1F), eg, eNOS may be a downstream mediator of Eph-B4 signaling in endothelial cells.
Figure 1.
Ephrin-B2/Fc stimulates Eph-B4 and eNOS phosphorylation. A–B. Bar graphs show time course of Eph-B4 phosphorylation (A) and eNOS phosphorylation (B) following Ephrin-B2/Fc (2 μg/ml). IP, immunoprecipitation; IB: immunoblot. A, n=4; *, P=.0041; **, P=.0097; ***, P=.0029. B, n=5; *, P=.0161; **, P=.0366. C. Bar graphs show eNOS phosphorylation in response to EphrinB2/Fc (2 μg/ml, 15 min), without or with NVP-BHG712 (1 μmol, 1 hour). n=3,*, P=.0313; **, P=.0018. D–E. Bar graph show time course of Eph-B4 phosphorylation (D) and eNOS phosphorylation (E) following preclustered Ephrin-B2/Fc (2 μg/ml, Ephrin-B2/Fc: Anti-Fc ratio, 1:5) stimulation. D, n=3; *, P=.0496; **, P=.0146; ***, P=.0032. E, n=4; *, p=.0484. (F) Schematic of the Eph-B4-eNOS pathway in vitro. In isolated endothelial cells, treatment of Eph-B4 with Ephrin-B2/Fc stimulates Eph-B4 and eNOS phosphorylation; these effects are enhanced (red arrows) in the presence of the clustered multimeric Ephrin-B2/Fc.
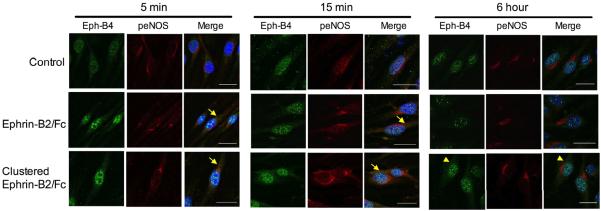
To confirm the interaction between Eph-B4 and eNOS in MLEC in vitro, we used immunofluorescence to determine if Eph-B4 and peNOS colocalized in endothelial cells after Ephrin-B2/Fc stimulation. Prior to stimulation, Eph-B4 immunoreactivity was primarily detected in the endothelial cell nucleus; after Ephrin-B2/Fc stimulation, Eph-B4 immunoreactivity was enhanced in the cytoplasm within 5 min (Figure 2). peNOS immunoreactivity was enriched in the perinuclear region and also found diffusely in the cytoplasm, and maximal enhancement of peNOS immunoreactivity was seen 15 min after Ephrin-B2/Fc stimulation (Figure 2). Colocalization of Eph-B4 with peNOS was observed in the cytoplasm following Ephrin-B2/Fc stimulation (Figure 2, yellow arrows). Stimulation with preclustered Ephrin-B2/Fc showed a similar pattern of phosphorylation and colocalization at 5 and 15 min compared to Ephrin-B2/Fc, but with sustained localization of Eph-B4 in the cytoplasm at 6 hours (Figure 2, yellow arrowheads). As expected, total eNOS expression was similar in all conditions (data not shown), consistent with similar total eNOS expression detected with Western blot (Figures 1C, 1D). Since colocalization of Eph-B4 and peNOS suggests that Eph-B4 and peNOS may interact with each other in endothelial cells, we performed co-immunoprecipitation of Eph-B4 and peNOS; however, immunoprecipitation using a peNOS-specific antibody was unable to co-precipitate any Eph-B4 protein (data not shown), suggesting only indirect interaction between Eph-B4 and peNOS. In toto, these data suggest that activation of Eph-B4 enhances endothelial cell eNOS phosphorylation in vitro.
Figure 2.
Colocalization of Eph-B4 and peNOS in EC. Representative immunofluorescence showing Eph-B4 (green), peNOS (red) and merge (yellow) in EC following control (top row), Ephrin-B2/Fc (2 μg/ml, middle row), or clustered Ephrin-B2/Fc (2 μg/ml, Ephrin-B2/Fc: Anti-Fc ratio, 1:5, bottom row) stimulation for 5 min (left columns), 15 min (middle columns) or 6 hours (right columns). Blue color is DAPI. Yellow arrows show merge of Eph-B4 and peNOS, yellow arrowheads show Eph-B4 in the cytoplasm. Scale bar, 20 μm.
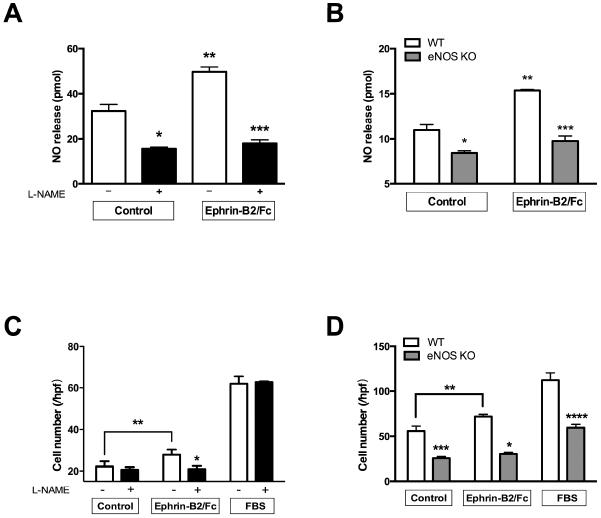
To determine whether stimulation of Eph-B4 signaling is linked to a functional eNOS pathway, we determined if eNOS is a mechanism of Eph-B4-mediated nitric oxide (NO) release and endothelial cell migration. Stimulation of endothelial cell Eph-B4 enhanced NO release into the medium that was inhibited in cells pretreated with L-NAME, both basally as well as after Eph-B4 stimulation (Figure 3A). Similarly, there was less NO release in eNOS KO endothelial cells compared to wild type endothelial cells, both basally as well as after Eph-B4 stimulation (Figure 3B). Stimulation of Eph-B4 similarly enhanced endothelial cell migration that was also inhibited with L-NAME (Figure 3C); eNOS KO endothelial cells also had less basal as well as less Eph-B4-stimulated migration (Figure 3D). These results suggest that some Eph-B4 mediated endothelial cell functions are mediated by eNOS signaling.
Figure 3.
Eph-B4 functions in endothelial cells are mediated by eNOS. A. Bar graph showing mean NO release in response to Ephrin-B2/Fc (2 μg/ml, 60 min), without or with L-NAME pretreatment (1 mM, 30 min). n=3; *, P=.0018; **, P=.0013; ***, P<.0001. B. Bar graph showing mean NO release in response to Ephrin-B2/Fc (2 μg/ml, 30 min) in WT or eNOS KO EC. n=4; *, P=.0076; **, P<.0001; ***, P<.0001. C. Bar graph showing EC migration in response to Ephrin-B2/Fc (2 μg/ml, 8 hr) or 10% fetal bovine serum (FBS), without or with L-NAME pretreatment (1 mmol, 30 min). n=6; *, P=0.0376; **, P<.0001. D. Bar graph showing EC migration in response to Ephrin-B2/Fc (2 μg/ml, 8 hr) or 10% FBS in WT or eNOS KO EC. n=5; *, p<.0001; **, p=.0217; ***, p<.0001; ****, p<.0001.
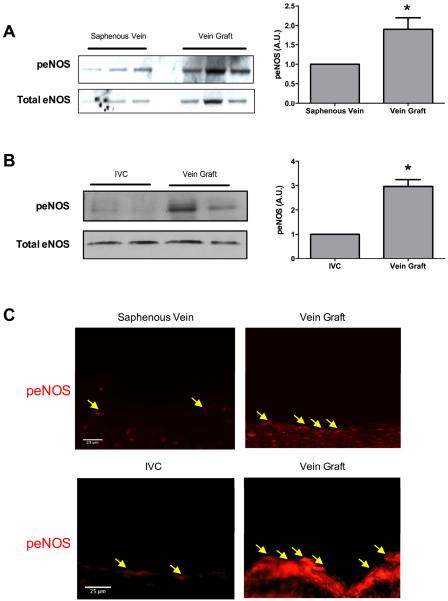
Since vein graft adaptation is associated with reduced Eph-B4 expression,7 we determined if eNOS is phosphorylated during vein graft adaptation in both human as well as mouse vein grafts. 8 In patent human vein grafts, eNOS phosphorylation was increased 1.9-fold in vein grafts compared to saphenous veins (Figure 4A). Similarly, eNOS phosphorylation was increased 3-fold in vein grafts after 21 days compared to the mouse IVC (Figure 4B). Immunofluorescence confirmed increased expression of phosphorylated eNOS in the endothelial cells of both human and mouse vein grafts compared to veins (Figure 4C). Since we have previously reported that Eph-B4 expression was decreased in vein grafts compared to saphenous veins,7 these results suggest that diminished Eph-B4 expression during vein graft adaptation is associated with increased eNOS phosphorylation in vivo, eg Eph-B4 is an early upstream regulator of later downstream eNOS activity.
Figure 4.
Increased eNOS phosphorylation in human and mouse vein grafts. A. Western blot showing eNOS phosphorylation in human saphenous veins and vein grafts. Bar graph shows the ratio of densitometry values of phosphorylated to total eNOS in veins and vein grafts. n=3; *, P=.0138. B. Western blot showing eNOS phosphorylation in mouse inferior vena cava (IVC) and vein grafts, 3 weeks after implantation. Bar graph shows the ratio of densitometry values of phosphorylated to total eNOS in mouse IVC and vein grafts. n=2; *, P=.0198. C. Representative immunofluorescence showing eNOS phosphorylation in human saphenous vein and vein graft (first row), and mouse IVC and vein graft (second row). Yellow arrows show peNOS positive cells. Scale bar: 25 μm.
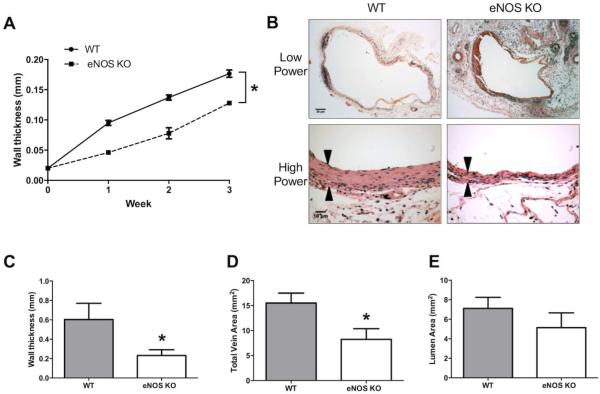
To determine if eNOS is a mechanism that mediates vein graft adaptation, we compared eNOS KO vein grafts with WT vein grafts after implantation into WT host mice. Serial ultrasonography of the vein grafts showed that eNOS KO vein grafts developed less thick walls over time compared to WT vein grafts (Figure 5A), consistent with previous data showing increased wall thickness over time in WT vein grafts.8 Histology similarly showed that eNOS KO vein grafts had thinner walls compared to WT vein grafts at 21 days after implantation (Figures 5B, 5C). eNOS KO vein grafts also showed smaller vessel area (Figure 5D), consistent with less outward remodeling. Although eNOS KO vein grafts showed less wall thickness and less outward remodeling compared to WT vein grafts, there was no significant difference in lumen area (Figure 5E). These results are consistent with a mechanistic role for eNOS during vein graft adaptation.
Figure 5.
eNOS mediates venous remodeling during mouse vein graft adaptation. A. Line graph showing time course of venous wall thickness of wild type (WT) and eNOS KO mice, measured using Doppler ultrasound. Solid line represents WT mice, dashed line represents eNOS KO mice. n=5; *, P<.0001. B. Representative photomicrographs showing vein grafts in WT mice (left column) and eNOS KO mice (right column), stained with H&E. Black arrowheads show the venous wall thickness. Scale bar: low power 20μm, high power 10μm. C-E. Bar graphs showing (C) mean wall thickness, (D) total vein area, and (E) lumen area in WT and eNOS KO mice. C, n=7; *, P=.0462. D, n=7; *, P=.028. E, n=7; P=.3273.
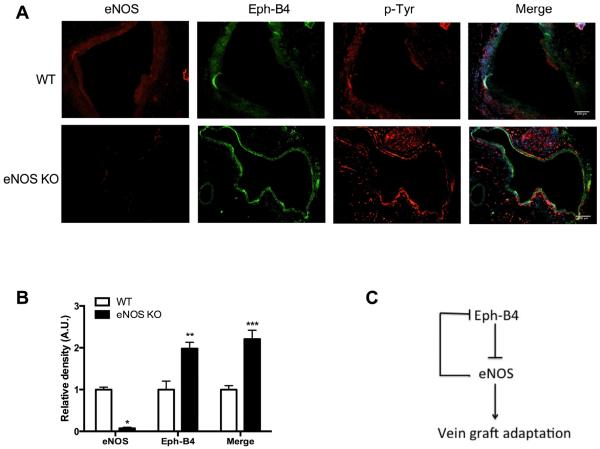
To confirm a mechanistic role for eNOS, since eNOS is downstream from Eph-B4, we hypothesized that eNOS KO vein grafts would have increased expression of Eph-B4. As expected, control vein grafts showed diffuse eNOS immunoreactivity in endothelial cells, whereas eNOS immunoreactivity was not detected in eNOS KO vein grafts (Figure 6A, first column; Figure 6B). Also as expected, in control vein grafts, Eph-B4 immunoreactivity was barely detectable; however, in eNOS KO vein grafts Eph-B4 immunoreactivity was comparatively increased (Figure 6A, second column; Figure 6B). Furthermore, there was more phospho-tyrosine immunoreactivity in eNOS KO vein grafts (Figure 6A, third column), as well as increased colocalization of phospho-tyrosine with Eph-B4 in eNOS KO vein grafts compared to control vein grafts (Figure 6A, last column; Figure 6B). These data suggest that reduced vessel remodeling and wall thickness in eNOS KO vein grafts (Figure 5) is associated with increased or persistent expression of upstream Eph-B4. These results are consistent with feedback regulation of Eph-B4 (Figure 6C), suggesting that the Eph-B4 and eNOS pathways may be functionally linked during vein graft adaptation.
Figure 6.
Increased Eph-B4 expression in eNOS knockout vein grafts. A. Representative immunofluorescence showing eNOS (red, left column), Eph-B4 (green, second column), p-Tyr (red, third column) and merge of Eph-B4 and p-Tyr (yellow, fourth column) in WT (top row) and eNOS KO (bottom row) vein grafts. n=3; scale bar, 100μm. B. Bar graph showing mean densitometry of eNOS, Eph-B4 and merge of Eph-B4 and p-Tyr. n=3; *, P=.0017; **, P=.0011; ***, P=.0002. C. Schematic of the Eph-B4-eNOS pathway during vein graft adaptation in vivo. During vein graft adaptation, Eph-B4 expression is decreased, allowing eNOS activity to promote adaptive venous remodeling. eNOS negatively regulates Eph-B4 activity in a feedback loop; when eNOS is not present, venous remodeling is reduced and Eph-B4 activity is enhanced.
Discussion
We show that Ephrin-B2/Fc stimulates Eph-B4 and eNOS phosphorylation in endothelial cells in a time-dependent manner in vitro (Figure 1), with colocalization of Eph-B4 and eNOS in the EC cytoplasm (Figure 2). Compared to the Ephrin-B2/Fc dimer, the multimeric form of Ephrin-B2/Fc shows sustained activation of Eph-B4 and eNOS. Eph-B4 activation promotes NO release and endothelial cell migration, and this stimulation is eliminated in the absence of eNOS (Figure 3). eNOS phosphorylation is increased in both human and mouse vein grafts in vivo (Figure 4). Lack of eNOS expression prevents adaptive thickening and remodeling of the venous wall (Figure 5) and increases Eph-B4 expression (Figure 6), suggesting that eNOS negatively feeds back on Eph-B4 expression during vein graft adaptation. In toto, these results suggest that eNOS is a downstream mediator of Eph-B4 that regulates venous adaptive remodeling to the arterial environment.
It is established that Eph-B4 is an embryonic determinant of the venous endothelium whereas its ligand Ephrin-B2 is a determinant of arterial fate during development of the vascular system.19–22 We have previously shown that Eph-B4 expression is diminished during vein graft adaptation in adults, suggesting that successful vein graft adaptation, characterized by increased venous wall thickness and adaptive outward remodeling, is associated with loss of venous identity.7, 8 Our data also suggests that Eph-B4 is a negative regulator of eNOS during vein graft adaptation in vivo, with reduced Eph-B4 expression during vein graft adaptation associated with enhanced eNOS phosphorylation after 3 weeks (Figure 4), although not at 1 week postoperatively,15 consistent with successful vein graft adaptation at 3 weeks in this model. Improving vein graft adaptation with increased NO production has led to the use of eNOS as a potential therapeutic agent.11, 23 However, the regulation of successful vein graft adaptation to the arterial circulation, with just the “right amount” of wall thickening and dilation, without progression to neointimal hyperplasia and/or aneurysmal dilation, is not currently understood.24
Downstream regulators of Eph-B4 signaling in the endothelial cell include Akt, ERK1/2 25, 26 and caveolin-1.8 Our finding that eNOS is phosphorylated with stimulation of Eph-B4 in adult venous endothelial cells is consistent with previous reports that have shown that activation of Eph-B4 enhances NO production in endothelial cells in vitro.8, 25 We previously showed that reduced endothelial Eph-B4 signaling in endothelial cells derived from heterozygous Eph-B4 mice 27 was associated with diminished basal levels of eNOS protein expression and NO production but increased tube formation,15 consistent with our data in vitro (Figure 3), but emphasizing the differences between endothelial cell function in vitro and in 3-dimensions, especially during vein graft adaptation in vivo.
eNOS is regulated by phosphorylation,28, 29 and Akt phosphorylates eNOS and enhances the ability of eNOS to generate NO.14 We have previously shown that reduced Eph-B4 function is associated with increased basal Akt phosphorylation in endothelial cells but not in whole vein grafts,15 suggesting a link from Eph-B4 to eNOS via the Akt pathway, at least in endothelial cells. In addition, caveolin-1 interacts with eNOS, and negatively regulates eNOS phosphorylation,30, 31 and we have previously shown that caveolin-1 is a mediator of vein graft adaptation,8 eg the Eph-B4-eNOS pathway is quite complex,10 and the activity of this pathway during vein graft adaptation is likely to be have additional layers of regulation in vivo. It is likely that differences between Eph-B4-eNOS pathway activity in vitro, e.g. Eph-B4 activation is associated with increased eNOS phosphorylation (Figures 1 and 2) and function (Figure 3), and pathway activity in vivo, e.g. diminished Eph-B4 expression is associated with increased eNOS activity (Figures 4 and 5), is likely due to several factors. First, the in vitro data reflects rapid interaction between Eph-B4 and eNOS over minutes, whereas the in vivo data reflects results after weeks. Second, the in vitro data is derived solely from endothelial cells, whereas the in vivo data is derived from whole vessels that have other cell types; of note, Eph-B4 is present predominantly in venous endothelial cells, but is present to a lesser extent in smooth muscle cells.8 Thirdly, the in vitro data may reflect differences between MLEC and the in vivo large vessel endothelial cells; in addition, MLEC are immortalized and may have higher basal expression of Akt and eNOS compared to primary cells. There are likely to be other important differences as well; nonetheless, our in vitro data suggests a link between Eph-B4 and eNOS signaling in endothelial cells that is also present in vein grafts in vivo.
NO is produced by three isoforms of NO synthase: neuronal (nNOS), inducible (iNOS) and endothelial (eNOS). iNOS has been previously reported to be involved in vein graft adaptation to the arterial environment. Veins produce less NO compared to arteries,32 and after vein graft implantation, endothelial injury and denudation is associated with further reduced eNOS-derived NO.33 Early reports of iNOS expression after arterial injury 34 led several groups to show that iNOS delivery to vein grafts can lead to improved nitrite release 35 and less neointimal hyperplasia.36, 37 However, iNOS may be associated with increased superoxide and peroxynitrite production, and thus may not be optimal for human treatment to prevent graft failure 38 despite its positive effects.39 eNOS is typically thought to be protective for vessels, and reduced eNOS is associated with vein graft neointimal hyperplasia.40 eNOS is induced in veins exposed to arterial shear stress 41 and delivery of eNOS to vein grafts inhibits neointimal hyperplasia.11, 23 Interestingly, the vein graft vasa vasorum may be a source of eNOS promoting vein graft adaptation.42 We reported that Eph-B4 regulates vein graft adaptation via caveolin-1,8 and our data suggests that Eph-B4-caveolin-1 signaling may regulate downstream eNOS, a potential target for therapeutic modulation.
Unlike other receptor tyrosine kinases, Eph receptor activation in vitro requires membrane attachment or clustered ligands.17 Therefore, to activate Eph-B4 receptors, we used the homodimer Ephrin-B2/Fc that consists of the extracellular domain of mouse Ephrin-B2 fused to the Fc region of human IgG. In addition, different oligomerization states of the ligands can be discriminated by Eph receptors and contribute to downstream cellular responses.18 Therefore we also used a pre-clustered multimeric Ephrin-B2/Fc to stimulate Eph-B4, and showed that both dimeric and pre-clustered multimeric Ephrin-B2/Fc stimulated Eph-B4 phosphorylation (Figures 1, 2), as expected.18 However, the multimeric Ephrin-B2/Fc showed a longer duration of Eph-B4 phosphorylation, and increased eNOS phosphorylation; these results are consistent with activation of Eph-B1 by multimeric Ephrin-B1/Fc,18 eg the multimeric form of Eph receptor ligands enhances receptor activation and signaling (Figure 1F). The bimodal distribution of Eph-B4 and eNOS phosphorylation following Ephrin-B2/Fc stimulation (Figures 1A and 1B) suggests the presence of downstream fast and slow pathways of receptor activation and signal transduction. Stimulation of Eph-B4 with the pre-clustered multimeric Ephrin-B2/Fc enhanced the activity of the fast pathway (Figures 1D and 1E) and allowed sustained detection of Eph-B4 in the endothelial cell (Figure 2); however the significance of these two pathways in not yet known.
Conclusion
Increased eNOS phosphorylation in both human and mouse vein grafts is consistent with an active response to the hemodynamic changes that occur during vein graft adaptation. Since diminished Eph-B4 expression actively regulates vein graft adaptation,7, 8 our results linking Eph-B4 with eNOS suggest that eNOS phosphorylation is an essential mechanism of Eph-B4 function during venous adaptive remodeling. As such, the Eph-B4-eNOS pathway may be a potential specific target for therapeutic manipulation to enhance vein graft patency.
Clinical relevance.
To date, despite a large number of clinical trials, no strategy appears effective in improving long-term vein graft patency. Eph-B4 activity regulates vein graft adaptation, and this study shows that Eph-B4 stimulates eNOS phosphorylation, suggesting that Eph-B4 activity during vein graft adaptation is mediated by eNOS. Modulation of eNOS activity may provide a potential new therapeutic target to improve vein graft patency.
Acknowledgement
We thank Dr. Akihito Muto for his expert work in the laboratory generating some of the data, as well as Drs. Clinton Protack, Tai Yi, Caroline Jadlowiec, Nirvana Sadaghianloo, Chenzi Yang, Kota Yamamoto, Go Kuwahara, Roland Assi, Michael Hall, Kirstyn Brownson, Hao He, Haidi Hu, and Jesse Hanisch for their assistance and advice. We sincerely thank Dr. Bill Sessa and his many laboratory members in the Yale Vascular Biology and Therapeutic program who have provided invaluable advice and suggestions to enhance this work.
This study was supported by the National Institutes of Health (R01-HL095498 and R56-HL095498 [AD]); the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Program (Merit Review Award I01-BX002336 [AD]); a Sarnoff Cardiovascular Foundation Fellowship (JMS); as well as with the resources and the use of facilities at the VA Connecticut Healthcare System, West Haven, CT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented at the 2015 Eastern Vascular Society Annual Meeting, Baltimore, Maryland, September 24–25, 2015.
Disclosures No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3(1):104–14. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330(20):1431–8. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 4.Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: clinical implications. J Vasc Surg. 2010;51(3):736–46. doi: 10.1016/j.jvs.2009.07.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan S, George SJ, Berry C, Baker AH. Vein graft failure: current clinical practice and potential for gene therapeutics. Gene Ther. 2012;19(6):630–6. doi: 10.1038/gt.2012.29. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465(7297):483–6. doi: 10.1038/nature09002. [DOI] [PubMed] [Google Scholar]
- 7.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, et al. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27(7):1562–71. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 8.Muto A, Yi T, Harrison KD, Davalos A, Fancher TT, Ziegler KR, et al. Eph-B4 prevents venous adaptive remodeling in the adult arterial environment. J Exp Med. 2011;208(3):561–75. doi: 10.1084/jem.20101854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong DJ, Lu DY, Protack CD, Kuwahara G, Bai H, Sadaghianloo N, et al. Ephrin type-B receptor 4 activation reduces neointimal hyperplasia in human saphenous vein in vitro. J Vasc Surg. 2014 doi: 10.1016/j.jvs.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33(7):829–37. 37a–37d. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugimoto M, Yamanouchi D, Komori K. Therapeutic approach against intimal hyperplasia of vein grafts through endothelial nitric oxide synthase/nitric oxide (eNOS/NO) and the Rho/Rho-kinase pathway. Surgery today. 2009;39(6):459–65. doi: 10.1007/s00595-008-3912-6. [DOI] [PubMed] [Google Scholar]
- 12.Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101(4):731–6. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–27. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jadlowiec CC, Feigel A, Yang C, Feinstein AJ, Kim ST, Collins MJ, et al. Reduced adult endothelial cell EphB4 function promotes venous remodeling. Am J Physiol Cell Physiol. 2013;304(7):C627–35. doi: 10.1152/ajpcell.00333.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang C, Guo Y, Jadlowiec CC, Li X, Lv W, Model LS, et al. Vascular endothelial growth factor-A inhibits EphB4 and stimulates delta-like ligand 4 expression in adult endothelial cells. J Surg Res. 2013;183(1):478–86. doi: 10.1016/j.jss.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, et al. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266(5186):816–9. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- 18.Stein E, Lane AA, Cerretti DP, Schoecklmann HO, Schroff AD, Van Etten RL, et al. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12(5):667–78. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93(5):741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 20.Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Developmental biology. 2001;230(2):151–60. doi: 10.1006/dbio.2000.0112. [DOI] [PubMed] [Google Scholar]
- 21.Pasquale EB. Eph receptor signalling casts a wide net on cell behaviour. Nat Rev Mol Cell Biol. 2005;6(6):462–75. doi: 10.1038/nrm1662. [DOI] [PubMed] [Google Scholar]
- 22.Salvucci O, Tosato G. Essential roles of EphB receptors and EphrinB ligands in endothelial cell function and angiogenesis. Adv Cancer Res. 2012;114:21–57. doi: 10.1016/B978-0-12-386503-8.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu S, Wang X, Guo L, Zi J. Adenovirus mediated endothelial nitric oxide synthase gene transfer prevents restenosis of vein grafts. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2004;50(3):272–7. doi: 10.1097/01.mat.0000124842.79638.ce. [DOI] [PubMed] [Google Scholar]
- 24.Lu DY, Chen EY, Wong DJ, Yamamoto K, Protack CD, Williams WT, et al. Vein graft adaptation and fistula maturation in the arterial environment. J Surg Res. 2014;188(1):162–73. doi: 10.1016/j.jss.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steinle JJ, Meininger CJ, Forough R, Wu G, Wu MH, Granger HJ. Eph B4 receptor signaling mediates endothelial cell migration and proliferation via the phosphatidylinositol 3-kinase pathway. J Biol Chem. 2002;277(46):43830–5. doi: 10.1074/jbc.M207221200. [DOI] [PubMed] [Google Scholar]
- 26.Maekawa H, Oike Y, Kanda S, Ito Y, Yamada Y, Kurihara H, et al. Ephrin-B2 induces migration of endothelial cells through the phosphatidylinositol-3 kinase pathway and promotes angiogenesis in adult vasculature. Arterioscler Thromb Vasc Biol. 2003;23(11):2008–14. doi: 10.1161/01.ATV.0000096655.56262.56. [DOI] [PubMed] [Google Scholar]
- 27.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Molecular cell. 1999;4(3):403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]
- 28.Michel T, Li GK, Busconi L. Phosphorylation and subcellular translocation of endothelial nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(13):6252–6. doi: 10.1073/pnas.90.13.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Cardena G, Fan R, Stern DF, Liu J, Sessa WC. Endothelial nitric oxide synthase is regulated by tyrosine phosphorylation and interacts with caveolin-1. The Journal of biological chemistry. 1996;271(44):27237–40. doi: 10.1074/jbc.271.44.27237. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Bakhshi FR, Shajahan AN, Sharma T, Mao M, Trane A, et al. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol Biol Cell. 2012;23(7):1388–98. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, et al. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nature medicine. 2000;6(12):1362–7. doi: 10.1038/82176. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton CA, Berg G, McIntyre M, McPhaden AR, Reid JL, Dominiczak AF. Effects of nitric oxide and superoxide on relaxation in human artery and vein. Atherosclerosis. 1997;133(1):77–86. doi: 10.1016/s0021-9150(97)00114-7. [DOI] [PubMed] [Google Scholar]
- 33.Onan B, Erkanli K, Onan IS, Ersoy B, Canillioglu YE, Senturk GE, et al. The impact of vessel clamps on endothelial integrity and function of saphenous vein grafts. Annals of vascular surgery. 2014;28(5):1113–22. doi: 10.1016/j.avsg.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Hansson GK, Geng YJ, Holm J, Hardhammar P, Wennmalm A, Jennische E. Arterial smooth muscle cells express nitric oxide synthase in response to endothelial injury. The Journal of experimental medicine. 1994;180(2):733–8. doi: 10.1084/jem.180.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kibbe MR, Nie S, Yoneyama T, Hatakeyama K, Lizonova A, Kovesdi I, et al. Optimization of ex vivo inducible nitric oxide synthase gene transfer to vein grafts. Surgery. 1999;126(2):323–9. [PubMed] [Google Scholar]
- 36.Kibbe MR, Tzeng E, Gleixner SL, Watkins SC, Kovesdi I, Lizonova A, et al. Adenovirus-mediated gene transfer of human inducible nitric oxide synthase in porcine vein grafts inhibits intimal hyperplasia. Journal of vascular surgery. 2001;34(1):156–65. doi: 10.1067/mva.2001.113983. [DOI] [PubMed] [Google Scholar]
- 37.Mayr U, Zou Y, Zhang Z, Dietrich H, Hu Y, Xu Q. Accelerated arteriosclerosis of vein grafts in inducible NO synthase(−/−) mice is related to decreased endothelial progenitor cell repair. Circulation research. 2006;98(3):412–20. doi: 10.1161/01.RES.0000201957.09227.6d. [DOI] [PubMed] [Google Scholar]
- 38.Dashwood MR, Loesch A. Inducible nitric oxide synthase and vein graft performance in patients undergoing coronary artery bypass surgery: physiological or pathophysiological role? Current vascular pharmacology. 2014;12(1):144–51. doi: 10.2174/157016111201140327164409. [DOI] [PubMed] [Google Scholar]
- 39.Shears LL, Kawaharada N, Tzeng E, Billiar TR, Watkins SC, Kovesdi I, et al. Inducible nitric oxide synthase suppresses the development of allograft arteriosclerosis. The Journal of clinical investigation. 1997;100(8):2035–42. doi: 10.1172/JCI119736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buttery LD, Chester AH, Springall DR, Borland JA, Michel T, Yacoub MH, et al. Explanted vein grafts with an intact endothelium demonstrate reduced focal expression of endothelial nitric oxide synthase specific to atherosclerotic sites. The Journal of pathology. 1996;179(2):197–203. doi: 10.1002/(SICI)1096-9896(199606)179:2<197::AID-PATH587>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 41.Golledge J, Turner RJ, Harley SL, Springall DR, Powell JT. Circumferential deformation and shear stress induce differential responses in saphenous vein endothelium exposed to arterial flow. The Journal of clinical investigation. 1997;99(11):2719–26. doi: 10.1172/JCI119461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dreifaldt M, Souza D, Bodin L, Shi-Wen X, Dooley A, Muddle J, et al. The vasa vasorum and associated endothelial nitric oxide synthase is more important for saphenous vein than arterial bypass grafts. Angiology. 2013;64(4):293–9. doi: 10.1177/0003319712443729. [DOI] [PubMed] [Google Scholar]