Abstract
Type 1 diabetes (T1D) is an autoimmune disease characterized by pancreatic β cell destruction induced by islet reactive T cells that have escaped central tolerance. Many physiological and environmental triggers associated with T1D result in β cell endoplasmic reticulum (ER) stress and dysfunction, increasing the potential for abnormal post-translational modification (PTM) of proteins. We hypothesized that β cell ER stress induced by environmental and physiological conditions generates abnormally-modified proteins for the T1D autoimmune response. To test this hypothesis we exposed the murine CD4+ diabetogenic BDC2.5 T cell clone to murine islets in which ER stress had been induced chemically (Thapsigargin). The BDC2.5 T cell IFNγ response to these cells was significantly increased compared to non-treated islets. This β cell ER stress increased activity of the calcium (Ca2+)-dependent PTM enzyme tissue transglutaminase 2 (Tgase2), which was necessary for full stress-dependent immunogenicity. Indeed, BDC2.5 T cells responded more strongly to their antigen after its modification by Tgase2. Finally, exposure of non-antigenic murine insulinomas to chemical ER stress in vitro or physiological ER stress in vivo caused increased ER stress and Tgase2 activity, culminating in higher BDC2.5 responses. Thus, β cell ER stress induced by chemical and physiological triggers leads to β cell immunogenicity through Ca2+-dependent PTM. These findings elucidate a mechanism of how β cell proteins are modified and become immunogenic, and reveal a novel opportunity for preventing β cell recognition by autoreactive T cells.
Keywords: Type 1 diabetes, β cell, ER stress, post-translational modification, autoimmunity
1. Introduction
Type 1 diabetes (T1D) is an autoimmune disease in which pancreatic islet β cells are targeted and destroyed by the immune system, with autoreactive T cells largely mediating pathogenesis. In individuals genetically predisposed to autoimmunity, autoreactive T cells enter the periphery as a result of failed central tolerance and recognize β cell autoantigens [1, 2]. As disease progresses, additional β cell proteins become immunogenic due to failures in peripheral tolerance [3, 4]. While the identification of β cell autoantigens is of great importance, elucidating how physiological mechanisms lead to defective peripheral tolerance is crucial to designing therapeutic intervention to reduce β cell immunogenicity and prevent T1D.
Islet β cells, like all professional secretory cells, contain a more fully developed endoplasmic reticulum (ER) than nonsecretory cells and are consequently more susceptible to ER stress due to their normal physiology and insulin biosynthesis [5–14]. β cells increase production of preproinsulin by 50-fold in response to heightened blood glucose concentrations, ultimately reaching a translation rate of 1 million molecules per minute [15]. These 1 million molecules of preproinsulin enter the ER lumen for folding and disulfide bond formation, heavily burdening the protein folding machinery of the ER and causing tremendous ER stress. This heightened ER stress due to insulin secretion is detected in the murine pancreas as early as 3 weeks of age [16]. In addition, ER stress is further increased by many physiological and environmental triggers associated with T1D, including viral infection [17–19], exposure to chemicals [20–23] or reactive oxygen species [24–26], dysglycemia [15], and pancreatic inflammation [27, 28]. Therefore, exposure to these known triggers may increase ER stress to levels above those inherent to the physiology of the secretory β cell. Thus, heightened ER stress may be an important factor in early T1D pathogenesis.
ER stress activates the unfolded protein response (UPR), which functions to restore ER homeostasis [29]. The UPR includes three signaling cascades that are initiated by protein sensors of ER stress, protein kinase RNA (PKR)-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol-requiring protein 1 (IRE1). These cascades initiate new chaperone synthesis to aid with folding of accumulated misfolded proteins [30, 31] and attenuate non-chaperone mRNA translation to reduce the protein burden in the ER [32, 33]. If ER stress is too great or too prolonged, the cytoprotective mechanisms of the UPR fail, and pro-apoptotic signaling pathways become activated [34–36]. However, even transient ER stress and UPR activation have significant consequences for the cell. For example, ER stress leads to the release of calcium (Ca2+) from the ER lumen into the cytosol. This efflux of Ca2+ negatively affects protein folding in the ER since many chaperones require Ca2+ [37–39] and also affects the function of Ca2+-dependent cytosolic enzymes. This Ca2+ flux is observed in β cells during insulin production and secretion [40–43], confirming the occurrence of ER stress during glucose-stimulated insulin production.
ER stress has been implicated in a number of autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus [15, 44–51]. In many such diseases, ER stress and dysfunction increase the potential for abnormal post-translational modification (PTM) of proteins. These modified proteins are processed and presented by antigen presenting cells (APC) in draining lymph nodes and, in the context of genetic susceptibility to autoimmunity, activate autoreactive T cells that have escaped central and peripheral tolerance to initiate or exacerbate pathology [52–55]. However, the mechanisms by which ER stress leads to abnormal PTM in these disease models have not been elucidated.
The release of Ca2+ from the ER lumen during ER stress increases the activity of cytosolic Ca2+-dependent enzymes. Tissue transglutaminase 2 (Tgase2) is a ubiquitously expressed cytosolic Ca2+-dependent PTM enzyme [56] that is activated during ER stress [50, 57–60]. Active Tgase2 translocates to the ER [50, 59, 61] and to secretory granules [62] to modify proteins through the formation of ε(γ-glutamyl) isopeptide bonds between glutamine and lysine residues that crosslink and aggregate proteins, and through the deamidation of glutamine to glutamic acid [63]. Tgase2 is involved in normal cellular functions including cell growth and inhibition of apoptosis [64, 65]. However, Tgase2 modifications are also associated with breaks in peripheral tolerance in several autoimmune disorders [44, 50, 51, 66, 67], although the mechanisms by which Tgase2 becomes activated in these models has not been explored.
The diabetogenic CD4+ T cell clone BDC2.5 recognizes WE14, a naturally occurring cleavage product of Chromogranin A (CHgA) [68]. However, WE14 is significantly less immunogenic than more physiologically relevant forms of antigen, such as whole β cells [68], suggesting that physiological expression of CHgA in β cells is necessary for immunogenicity. WE14 was later shown to elicit stronger BDC2.5 T cell responses after in vitro modification by Tgase2 [69]. However, whether Tgase2 is active in β cells, or whether this activity is relevant for β cell immunogenicity, was not explored. Indeed, although there is precedent to suggest that many murine and human β cell peptides, in addition to WE14, elicit stronger T cell responses after PTM [69–75], these studies have not explored or addressed the cellular and physiological processes that lead to PTM.
We hypothesized that β cell ER stress induced by physiological conditions generates abnormally-modified proteins that activate the autoimmune response in T1D. Here, we demonstrate that β cell ER stress induced by a chemical trigger in vitro or by physiological triggers in vivo increased the recognition of these β cells by diabetogenic BDC2.5 T cells. Furthermore, ER stress increased cytosolic Ca2+ concentrations and Tgase2 activity, both of which were crucial for ER stress-dependent β cell immunogenicity. Together, these results demonstrate that exposure to triggers of ER stress increases the activity of Ca2+-dependent PTM enzymes (such as Tgase2) in β cells. These findings also provide a mechanism to explain how and why the β cell peptides described by others [69–75] may undergo PTM and come to be recognized by autoreactive T cells. Therefore, the normal physiology of the β cell, which predisposes the cell to ER stress, may contribute to its own immune-mediated destruction in T1D.
2. Materials and methods
2.1. Mice
Mice were bred and housed under specific pathogen-free conditions at Rangos Research Center of Children’s Hospital of Pittsburgh of University of Pittsburgh Medical Center. All experiments were approved by Institutional Animal Care and Use Committee of the University of Pittsburgh.
In 4 experiments, 13 NOD.scid mice were transplanted with 5×106 NIT-1 cells under the kidney capsule, and 10 nonrecipient mice were used as controls. Starting at day 2 post-transplant, blood glucose was monitored for hypoglycemia and serum was collected to measure insulin levels. Hypoglycemia was defined as 2 consecutive blood glucose readings ≤ 40 mg/dl. At onset, mice were sacrificed, the kidney was harvested, and the NIT-1 cells were explanted for further analysis.
2.2. Cell culture
Primary murine islets were harvested from NOD.scid, C57BL/6, or BALB/c pancreata as described [76–78]. Islet cells were 90% viable as determined by trypan blue (Gibco) exclusion assay. Islet cell-conditioned media was harvested from islet cultures after 1 hr at 37°C.
The NIT-1 insulinoma cell line was a gift from Clayton Mathews (University of Florida) and CD4+, MHC class II-restricted BDC2.5 T cells were a gift from Kathryn Haskins (University of Colorado). NIT-1 cells were maintained at 37°C in a 5% CO2 humid air incubator, in DMEM (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Mediatech), 10 mM HEPES buffer (Gibco), 4 mM L-glutamine (Gibco), 200 µM nonessential amino acids (Gibco), 1 mM sodium pyruvate (Gibco), 61.5 µM β-mercaptoethanol (Sigma-Aldrich), and 100 µg/ml gentamicin (Gibco). NIT-1 cell-conditioned media was harvested from NIT-1 cultures after 1 hr at 37°C.
BDC2.5 T cell clones were maintained in supplemented DMEM as described [79].
2.3. BDC2.5 T cell assay
BDC2.5 T cells (2×104), NOD.scid splenocytes as APC (4×105), and antigen (1×103 dispersed islet cells/ml, 50 µl islet cell conditioned media, or 90 µM CHgA351–370 peptide) were combined in 200 µl supplemented DMEM in triplicate in 96-well flat-bottom tissue culture plates (Greiner Bio-One) and incubated at 37°C for 72 hr. TH1 effector function was determined by measuring IFNγ secretion by enzyme-linked immunosorbent assay (ELISA).
2.4. ELISA
IFNγ from T cell assays was measured with murine IFNγ ELISA antibody pairs (BD Biosciences) as described [80, 81]. Insulin in mouse serum was measured by Mouse Insulin Ultrasensitive ELISA (Alpco Immunoassays) following the manufacturer’s instructions. Absorbances were measured at 450 nm with a SpectraMax M2 microplate reader (Molecular Devices). Data were analyzed with SoftMax Pro (Molecular Devices).
2.5. Induction of ER stress
Primary murine islets were incubated in low-binding tissue culture dishes (Corning) with 5 µM Thapsigargin (Thaps; Sigma-Aldrich) or control for 1 hr at 37°C. Prior to BDC2.5 T cell assay or other downstream analysis, the islets were washed extensively (50,000× original volume) to remove residual Thaps, and dispersed in Cell Dissociation Buffer (Gibco) at 37°C for 15 min with periodic vortexing.
NIT-1 cells were cultured in 25 cm2 tissue culture flasks (Greiner Bio-one) with 5 µM Thaps or control for 1 hr at 37°C. Prior to BDC2.5 T cell assay or other downstream analysis, the cells were washed extensively (50,000× original volume) to remove residual Thaps, and removed from the flask with 0.05% Trypsin-EDTA (Gibco).
2.6. Preparation of Cell Lysates
Cells were lysed by sonication in 50 mM Tris pH 8.0, 137 mM NaCl, 10% glycerol, 1% NP-40, 1 mM NaF, 10 µg/ml leupeptin, 10 µg/ml aprotinin, 2 mM Na3VO4, and 1 mM PMSF. Protein concentration was determined by bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific).
2.7. Western blotting
Lysates were separated by SDS-PAGE with 4–20% polyacrylamide gels and transferred to PVDF membranes. Membranes were blocked in 4% milk in TBST for 1 hr, and probed with antibodies to phosphorylated PERK (Cell Signaling Technology; 1:200), phosphorylated eIF2α (Cell Signaling Technology; 1:1,000), and β-actin (Sigma-Aldrich; 1:10,000) overnight at 4°C. Membranes were washed and incubated with HRP-conjugated goat anti-rabbit (Cell Signaling Technology; 1:2,000) or horse anti-mouse (Jackson ImmunoResearch 1:10,000) for 1 hr. Chemiluminescence was detected with Luminata Crescendo Western HRP Substrate (Millipore) and analyzed with Fujifilm LAS-4000 imager and Multi Gauge Software (Fujifilm Life Science).
2.8. Calcium tracing
Primary islets were dispersed and incubated in glass-bottom plates (MatTek) for 2 days until adherent. NIT-1 cells were seeded in glass-bottom plates overnight. Adherent cells were labeled with 1 µM Fluo-4 (Invitrogen) at 37°C for 1 hr. The cells were washed and the intensity of Fluo-4 at 488 nm was monitored by live imaging with the 40× objective lens of an Olympus Fluoview FV1000 microscope for 350 sec at room temperature. At 70 sec, the cells were exposed to 5 µM Thaps or control in supplemented DMEM. Data were analyzed with FV10-ASW imaging software.
2.9. Calcium chelation
Cells were incubated with 2.5 µM Bapta-AM (Invitrogen) or control for 1 hr at 37°C before the addition of 5 µM Thaps for 1 hr. Prior to BDC2.5 T cell assay or other downstream analysis, the cells were washed extensively (50,000× original volume) to remove residual Bapta-AM and Thaps. Conditioned media was obtained after 1 hr incubation at 37°C.
2.10 Antibody stimulation of BDC2.5 T cells
Stimulation of BDC2.5 T cells with plate-bound α-CD3 (BD Biosciences; 0.5 µg/ml) and α-CD28 (BD Biosciences; 1.0 µg/ml) was performed as described [82, 83], with the following modifications. BDC2.5 T cells (2×104) were combined with 50 µl conditioned media as indicated in 200 µl supplemented DMEM at 37°C for 72 hr. IFNγ secretion was measured by ELISA.
2.11. In Situ Tgase2 Activity Microtiter Plate Assay
The activity of Tgase2 was measured by the incorporation of biotinylated pentylamine into endogenous proteins as described [84, 85]. Briefly, primary murine islets or NIT-1 cells were incubated with 5 mM Pentylamine-Biotin (Thermo Fisher Scientific) or control for 2 hrs at 37°C prior to the addition of 5 µM Thaps or control for 1 hr. The cells were washed and lysed as described above. The concentration of the lysates was measured by BCA assay and adjusted to 40 µg/ml (islets) or 200 µg/ml (NIT-1) in coating buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 5 mM EGTA, 5mM EDTA). The lysates were plated in triplicate in 96-well microtiter plates (Corning) and incubated overnight at 4°C. The wells were blocked with 200 µl blocking buffer (5% BSA, 0.01% SDS, 0.01% Tween-20 in borate buffered saline) for 2 hrs at 37°C. After washing (1% BSA, 0.01% Tween-20 in borate buffered saline), streptavidin HRP (Invitrogen) was diluted in wash buffer following the manufacturer’s instructions and incubated at room temperature for 1 hr. After washing, TMB substrate (Dako) was added to the wells for 20 minutes at room temperature, and the reaction was stopped with 0.18 M H2SO4. Absorbance was measured at 450 nm. Values were normalized by subtracting the absorbance of cells not incubated with pentylamine-biotin (represented endogenous biotin). Data are reported as fold change in Tgase2 activity compared to cells incubated with pentylamine-biotin alone.
2.12. Synthesis of CHgA351–370
CHgA351–370 (REWEDKRWSRMDQLAKELTA) was synthesized at 95% purity and confirmed by HPLC analysis (Peptide2.0).
2.13. Expression of recombinant CHgA in E. coli
Full length murine CHgA was amplified from the pCMV6-Kan/Neo cDNA clone (Origene) with the following primers: F, 5’-TCTAGATCTTTCCGCACCGTCCG-3’, 5’-GTCAGAATTCCCTACTCGAGCAGCAGTC-3’. The cycling parameters were as follows: 94°C for 6 mins, followed by 39 cycles of 94°C for 30 sec, 56°C for 60 sec, 72°C for 60 sec, and 72°C for 10 mins. The PCR product was ligated into the pTrcHis2 vector (Invitrogen). The pTrcHis2 vector was transformed into E. coli strain NiCo21 (DE3) (New England BioLabs) and the expression of HIS-tagged proteins was induced with isopropyl β-D-1-thiogalactopyranoside (IPTG) at 37°C for 3.5 hr. The bacteria were lysed using B-PER (Thermo Scientific), following the manufacturer’s instructions. CHgA was purified using the NI-NTA column (Qiagen), following the manufacturer’s instructions.
2.14. In vitro modification with Tgase2
Tgase2 (Sigma-Aldrich;1.3 µM) was incubated with CHgA351–370 (0.2 mM) in 50 mM Tris, pH 8.0, 10 mM CaCl2, 1 mM DTT, 1 mM EDTA at 37°C for 3 hr. The reactions were repeated with 0 mM CaCl2 or 10 mM EDTA. Reactions were separated with 16.5% Tris-Tricine gels (Bio-Rad), and stained with Coomassie G250 (Bio-Rad). Gels were imaged with Fujifilm LAS-4000 imager and Multi Gauge Software.
Tgase2 (1.3 µM) was incubated with full length recombinant CHgA (3.9 µM) in 50 mM Tris, pH 8.0, 10 mM CaCl2, 1 mM DTT, 1 mM EDTA at 37°C for 3 hr.
2.15. Mass Spectrometry
Recombinant CHgA, incubated with Tgase2 or control, was separated by SDS-PAGE. The gel was stained with Gel Code Blue (Thermo Scientific). Gel samples were washed with 25 mM ammonium bicarbonate followed by acetonitrile. Samples were then reduced with 10 mM DTT at 60°C followed by alkylation with 50 mM iodoacetamide at room temperature. Samples were then digested with trypsin (Promega) at 37°C for 4 h. Following digestion, samples were quenched with formic acid, and the supernatant was analyzed directly by nano-LC/MS/MS with a Waters NanoAcquity HPLC system interfaced to a ThermoFisher Orbitrap Velos Pro mass spectrometer. Peptides were loaded on a trapping column and eluted over a 75 µm analytical column at 350 nL/min; both columns were packed with Jupiter Proteo resin (Phenomenex). The mass spectrometer was operated in data-dependent mode, with MS and MS/MS performed at 70,000 and 17,500 FWHM resolution, respectively. The 15 most abundant ions were selected for MS/MS. The MS/MS data were searched against the SwissProt database using Mascot search engine version 2.4 (Matrix Science, London, UK; version Mascot). Mascot was set up to search the SwissProt_Mouse_20150127 database assuming the digestion enzyme stricttrypsin. The precursor and product ion tolerances were 10 ppm and 0.5 Da, respectively. Carbamidomethylation of cysteines was set as a fixed modification. Gln->pyro-Glu of the N-terminus, deamidated of asparagine and glutamine, oxidation of methionine and acetylation of the N-terminus were specified as variable modification. Mascot DAT files were parsed into Scaffold (Proteome Software, Portland, OR) for visualization and validation using the Protein Prophet algorithm [86, 87]. Minimum protein probability was 90%, and peptide probability was 50%; a minimum of two unique peptides was necessary for protein identification. Putative modified peptides were confirmed by manual inspection of the raw MS/MS spectra.
2.16. Reduction of Tgase2 expression by shRNA
NIT-1 cells were incubated with lentiviral particles (multiplicity of infection 2.5) carrying vectors (pLKO.1) encoding control shRNA or Tgase2-targeting shRNA (Sigma-Aldrich) for 20 hr at 37°C. After 24 hrs in fresh supplemented DMEM, cells were selected with 7.5 µg/ml puromycin (Sigma-Aldrich). Five shRNA sequences were tested, and data shown were obtained with the sequence that most reduced Tgase2 expression: 5’–CCGGCCTGGAGAATCCCGAAATCAA–3’.
2.17. Quantitative Real Time PCR (qRT-PCR)
mRNA was isolated with RNeasy Kit (Qiagen) and cDNA was synthesized with RT2 First Strand Kit (Qiagen). Quantification of cDNA was performed by qRT-PCR (iCycler, BioRad) with the iQ SYBR Green Supermix (BioRad). Cycling parameters were 95°C for 15 min and 40 cycles of 95°C for 30 sec, 60°C for 30 sec, and 72°C for 30 sec. Murine Tgase2 primers (tgm2), F 5’–GACAATGTGGAGGAGGGATCT–3’, R 5’–CTCTAGGCTGAGACGGTACAG–3’ were generated by Primer Bank, ID# 6678329a1. Murine RPLO (rplo) primers were previously published [88]. Gene expression was normalized using the ΔΔCt method, where the amount of target, normalized to an endogenous reference and relative to a calibrator, is given by 2−ΔΔCt, where Ct is the cycle number of the detection threshold.
2.18. Glucose-stimulated insulin secretion assay
Glucose-stimulated insulin secretion assays were performed as described [89].
2.19. Ex Vivo Tgase2 Activity Assay
The activity of Tgase2 in explanted NIT-1 cell lysates was measured with Transglutaminase Colorimetric Assay Kit (Covalab) following the manufacturer’s instructions, and normalized to total lysate concentration (mg/ml) determined by BCA assay.
2.20. Statistical analysis
For ELISA, data are mean IFNγ or insulin secretion ± S.E.M. For SDS-PAGE and western blotting, data are representative of 3 experiments. Densitometry data are phosphorylation levels normalized by β-actin and relative to that in control treated cells. For Ca2+ tracing, images are representative of 3 experiments and combined data are change in fluorescent intensity ± S.E.M. For in situ Tgase2 activity, data are mean relative Tgase2 activity ± S.E.M. For qRT-PCR, data are relative Tgase2 expression, normalized to rplo expression, ± S.E.M. For ex vivo Tgase2 activity, data are mean normalized mU/ml, ± S.E.M. Statistical significance was determined by Student t test and statistically significant differences are shown for p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***).
For mass spectrometry data, data are representative of 3 independent experiments.
For NIT-1 transplant data, survival analysis was performed by Kaplan-Meier method with hypoglycemia as endpoint. Mice that did not become hypoglycemic were censored. Significance was determined by log-rank test. p < 0.001
3. Results
3.1. Primary islets from many mouse strains elicit effector responses from the BDC2.5 T cell clone
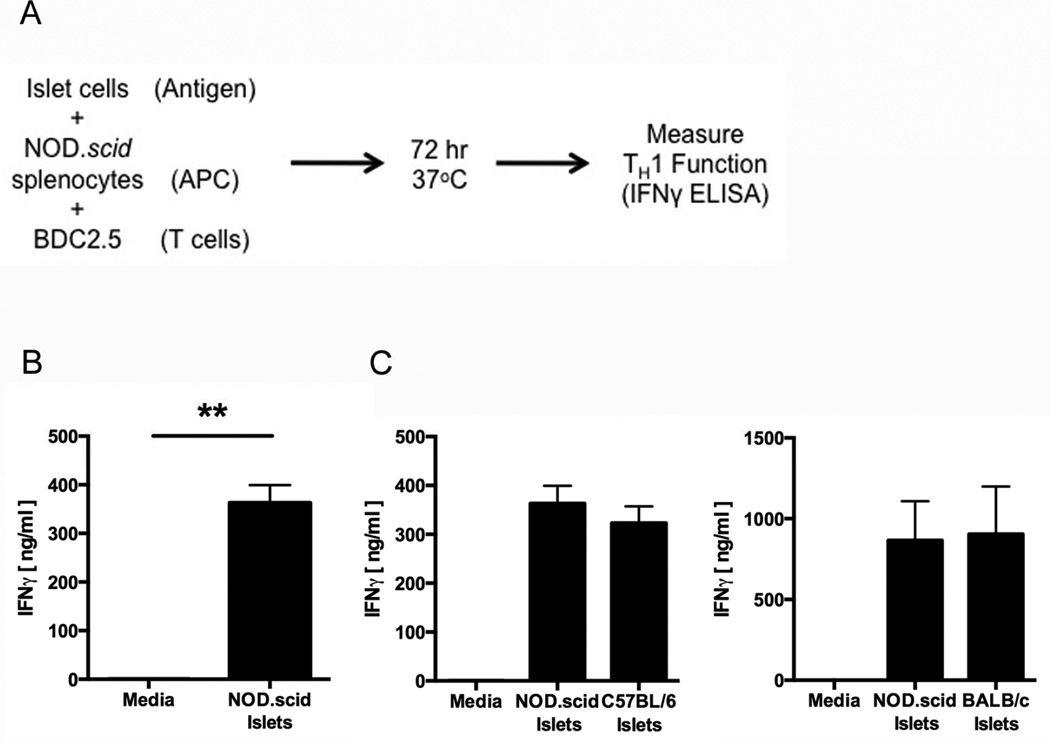
The diabetogenic murine CD4+ T cell clone BDC2.5 proliferates in response to primary NOD.scid islets presented in the context of the NOD-derived MHC class II I-Ag7 on antigen presentation cells (APC) [79]. To confirm that these T cells also exhibit effector function in response to primary NOD.scid islets, the immunogenicity of primary NOD.scid islets was measured by BDC2.5 T cell assay. Briefly, 2×104 BDC2.5 T cells, 4×105 NOD.scid splenocytes acting as APC, and 5×103 NOD.scid islet cells were combined in 200µl and incubated for 72 hrs (Fig. 1A). T cell effector function was measured by IFNγ secretion. BDC2.5 T cells secreted significant levels of IFNγ in response to primary NOD.scid islets presented by NOD-derived APC (p < 0.01) (Fig. 1B), confirming the immunogenicity of these islet cells. However, supernatants harvested from cultures of NOD.scid islets did not elicit significant levels of IFNγ from BDC2.5 T cells (Supplemental Fig. 1). These data suggest that primary islet immunogenicity is contained in the β cell, not secreted into the assay milieu.
Fig. 1. Primary islets from many mouse strains elicit effector responses from BDC2.5 T cell clones.
(A) Schematic of the BDC2.5 T cell assay protocol. Antigen presenting cell (APC). (B) The immunogenicity of NOD.scid islet cells was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM. ** p < 0.01. (C) The immunogenicity of NOD.scid and C57BL/6 (left) or BALB/c (right) islet cells was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM.
To determine whether BDC2.5 T cells respond to islets from other mouse strains, not only those from the autoimmune-prone NOD background, the immunogenicity of islets from non-autoimmune-prone C57BL/6 mice and BALB/c mice was measured by BDC2.5 T cell assay. BDC2.5 T cells also proliferated [79] and secreted IFNγ (Fig. 1C) in response to primary islets from the C57BlL6 and BALB/c murine backgrounds. These data demonstrate that islets from all backgrounds (both autoimmune prone and non-autoimmune prone) are equally immunogenic and capable of eliciting an immune response from autoreactive diabetogenic T cells. These data suggest that immunogenicity is, at least in part, the consequence of a factor that is common to islet cells from all individuals.
3.2. ER stress increases β cell recognition by BDC2.5 T cells
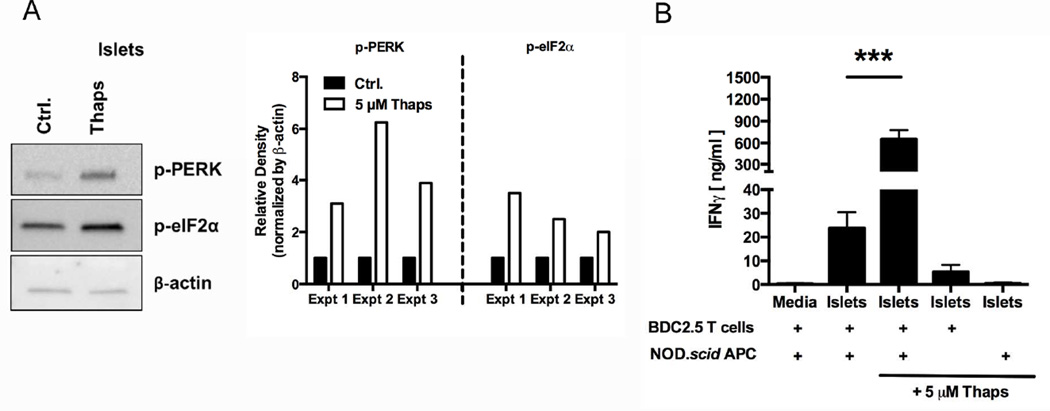
β cells from all individuals, regardless of genetic predisposition to autoimmunity, exhibit increased ER stress due to their normal secretory physiology [5–14]. Therefore, ER stress is one factor that all islets have in common. Since ER stress has been implicated in autoimmune pathology in a number of diseases such as multiple sclerosis, rheumatoid arthritis, and systemic lupus erythematosus [15, 44–51], we hypothesized that ER stress may cause β cell immunogenicity, leading to autoreactive T cell activation. To test this hypothesis, ER stress was induced in primary islets with Thapsigargin (Thaps), which is a widely accepted chemical trigger of ER stress [50, 57]. To measure ER stress induction, the phosphorylation of UPR proteins PERK and eIF2α was assessed by Western blot. As discussed above, the UPR is activated during ER stress [29], and so the phosphorylation of these proteins is a reliable measure of ER stress levels. As expected, incubation with 5 µM Thaps for 1 hr increased phosphorylation of PERK (4.4-fold) and eIF2α (2.7-fold) compared to control-treated cells (Fig. 2A). Importantly, incubation with 5 µM Thaps for 1 hr did not induce apoptosis as determined by trypan blue exclusion (data not shown).
Fig. 2. β cell ER stress increases recognition by diabetogenic T cells.
(A) Primary NOD.scid islets were incubated with 5 µM Thaps or control (Ctrl.) for 1 hr, washed extensively, and dispersed. Cell lysates were analyzed for the phosphorylation of UPR proteins PERK and eIF2α. Data are representative of 3 independent experiments. Densitometry data are phosphorylation levels normalized by β-actin and relative to that in control (Ctrl.) treated cells. (B) The immunogenicity of NOD.scid islet cells treated with 5 µM Thaps or control for 1 hr was measured by BDC2.5 T cell assay. Either APC or T cells were omitted from assay to demonstrate the necessity of both cell types for full IFNγ secretion. Data are mean IFNγ secretion ± SEM. *** p < 0.001.
To determine whether Thaps-induced ER stress increased immunogenicity, these cells were analyzed by BDC2.5 T cell assay. Thaps-induced ER stress significantly increased the BDC2.5 T cell response to islets (27.6-fold, p < 0.001) (Fig. 2B), confirming a role for ER stress in β cell immunogenicity. Full IFNγ secretion required APC-mediated β cell antigen processing and presentation to BDC2.5 T cells (Fig. 2B). Without APC, any residual Thaps remaining in the β cells after washing did not elicit significant IFNγ.
These data suggest that BDC2.5 T cells recognize a β cell antigen that is altered due to ER stress-modified β cell physiology. Since incubation with 5 µM Thaps for 1 hr did not induce apoptosis, apoptosis is not likely the cause of this increased immunogenicity.
3.3. ER stress-induced immunogenicity is Ca2+ dependent
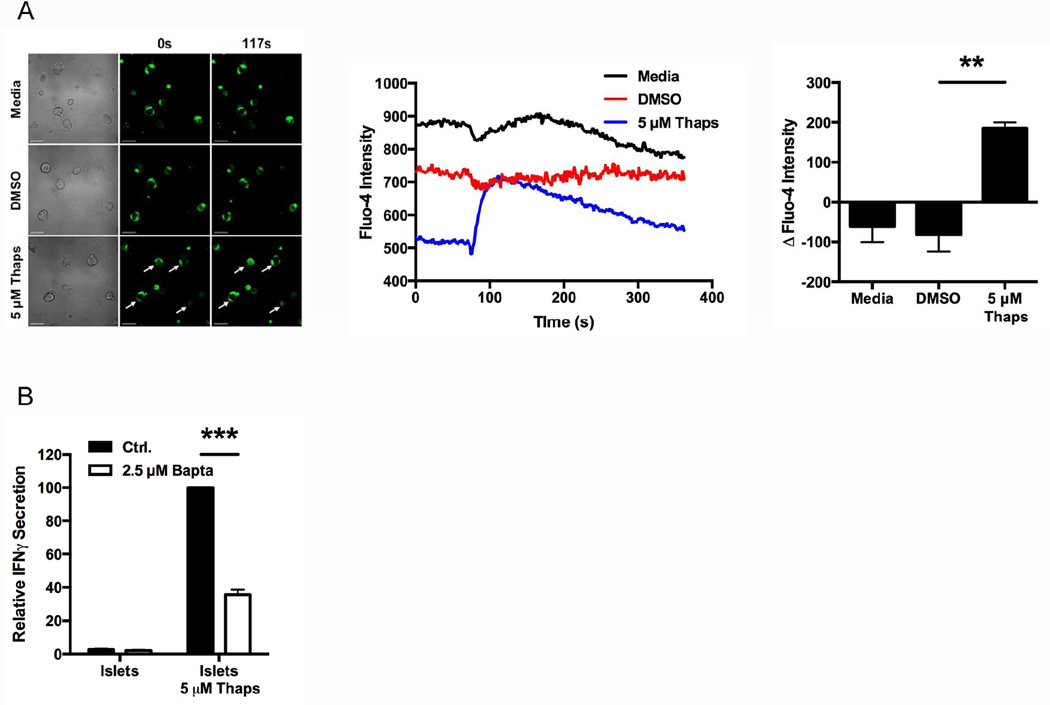
ER stress causes a release of Ca2+ stores from the ER [50, 57, 58, 90, 91], which increases the activity of cytosolic Ca2+-dependent enzymes. To confirm the occurrence of this Ca2+ flux in Thaps-induced β cell ER stress, the cytosolic Ca2+ concentration was monitored with the Ca2+ indicator Fluo-4. As is evident from the images, Thaps significantly increased Fluo-4 fluorescence, indicating increased cytosolic Ca2+ (p < 0.01), while the fluorescence of control-treated cells remained constant (Fig. 3A).
Fig. 3. ER stress-induced immunogenicity is Ca2+ dependent.
(A) Dispersed NOD.scid islet cells were labeled with Fluo-4 and analyzed by live imaging with an Olympus Fluoview FV1000 microscope for 350 sec at room temperature. At 70 sec, the cells were exposed to 5 µM Thaps or controls. Scale bars, 50 µm. Arrows indicate cells with greater Fluo-4 intensity after exposure to Thaps. Line graphs represent the intensity of Fluo-4 in each sample over time. Data are representative of 3 independent experiments. Quantified data are change in Fluo-4 intensity from baseline to peak, with mean ± SEM. ** p < 0.01. (B) The immunogenicity of primary NOD.scid islet cells treated with 2.5 µM Bapta or control (Ctrl.) prior to incubation with 5 µM Thaps or control was measured by BDC2.5 T cell assay. Data are relative IFNγ secretion compared to 5 µM Thaps, with mean ± SEM. *** p < 0.001
To determine whether ER stress-induced immunogenicity depends on cytosolic Ca2+, primary islets were incubated with 2.5 µM of the Ca2+ chelator Bapta-AM or control for 1 hr before incubation with 5 µM Thaps for 1 hr. Chelation of cytosolic Ca2+ significantly diminished ER stress-induced immunogenicity by 64% (p < 0.001) (Fig. 3B). Importantly, any residual Bapta-AM remaining in the β cells after extensive washing did not directly affect the BDC2.5 T cells, as conditioned media harvested from Bapta-AM-treated islets did not reduce IFNγ secretion from antibody-stimulated T cells (Supplemental Fig. 2). Together, these data demonstrate the crucial role of ER stress-induced Ca2+ release in β cell immunogenicity.
3.4. Tgase2 activity is increased during ER stress
Tgase2 is a Ca2+-dependent cytosolic PTM enzyme that is associated with breaks in peripheral tolerance in several autoimmune disorders [44, 50, 51, 66, 67]. Also, recent findings suggest that Tgase2-mediated PTM increase the immunogenicity of synthetic peptides of known T1D autoantigens [69, 70, 72, 74]. But whether Tgase2 is relevant to whole β cell immunogenicity, or how Tgase2 becomes activated in β cells, remains unknown.
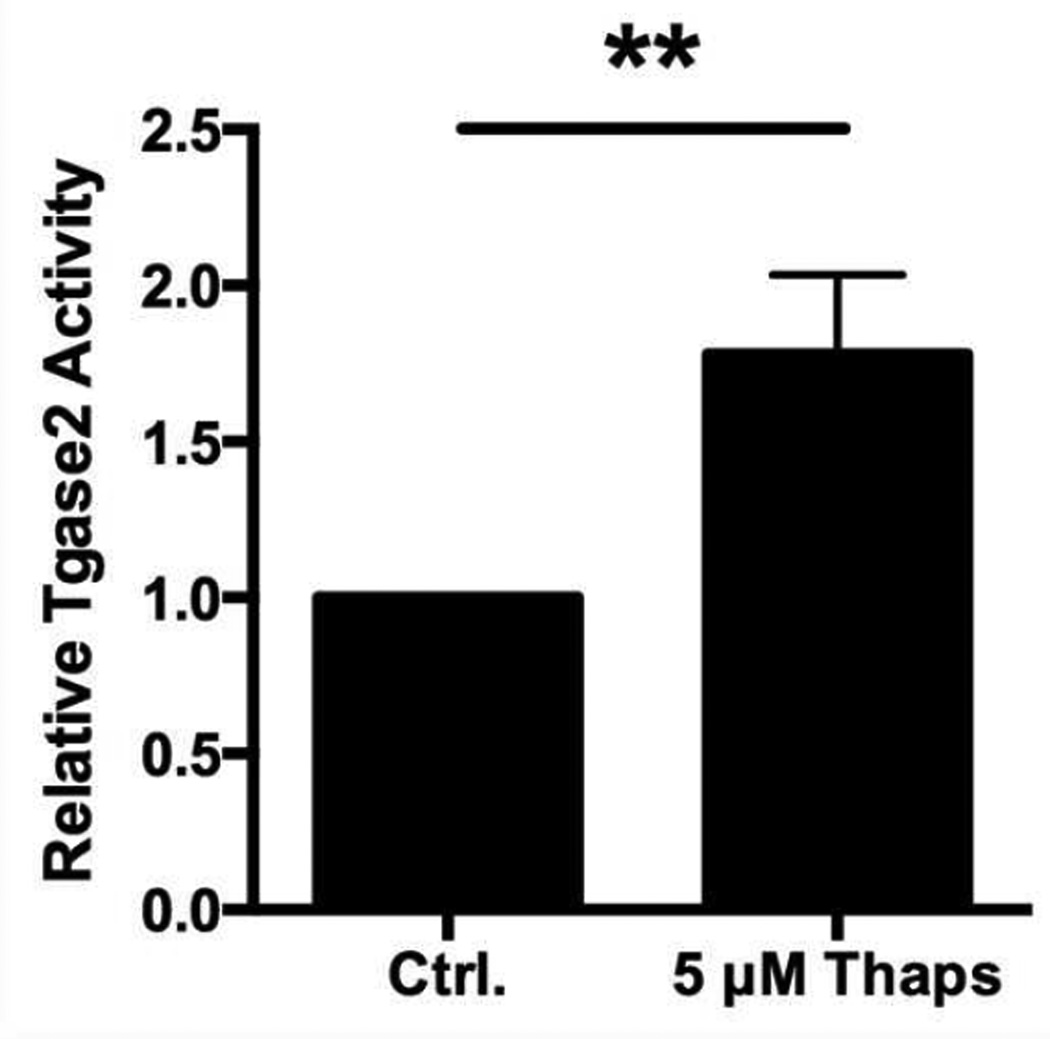
To determine whether Tgase2 is active in β cells undergoing ER stress, Tgase2 activity was measured in primary murine islets. Islets incubated with 5 µM Thaps for 1 hr contained 1.8-fold higher Tgase2 activity than control islet cells (p < 0.01) (Fig. 4). These data demonstrate that Tgase2 activity corresponds with high ER stress and immunogenicity in islets (Fig. 2) and suggest a possible role for Tgase2 in ER stress-induced immunogenicity.
Fig. 4. β cell ER stress increases the activity of Tgase2.
Primary NOD.scid islets were incubated with 5 µM Thaps or control (Ctrl.) for 1 hr. The cells were lysed and Tgase2 activity was measured by microtiter plate assay. Data are relative Tgase2 activity compared to control (Ctrl.) islet cells, with mean ± SEM. ** p < 0.01.
3.5. Tgase2-mediated PTM increases immunogenicity of CHgA
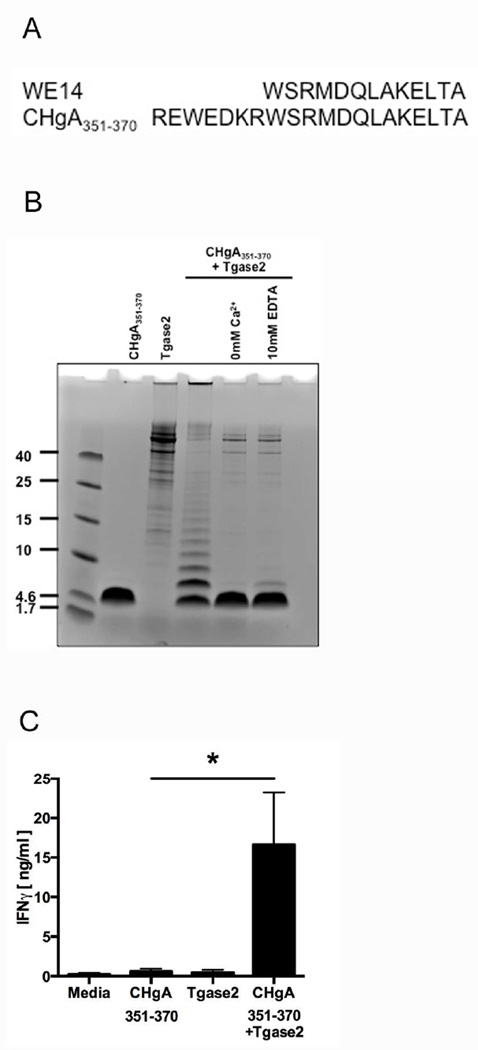
Treatment of the CHgA-derived peptide WE14 with Tgase2 increased the BDC2.5 response to the peptide [69]. However, WE14 is a nontraditional T cell antigen since it contains only 14 amino acids and is predicted to bind MHC molecules outside the binding groove [68]. We studied a peptide, CHgA351–370, that contains WE14 but is 20 amino acids long and includes traditional MHC binding motifs (Fig. 5A).
Fig. 5. Modification of CHgA by Tgase2 increases immunogenicity.
(A) Alignment of the sequences of WE14 and CHgA351–370. (B) Synthetic CHgA351–370 was incubated with purified Tgase2 at 37°C for 3 hr. To confirm the dependence of these reactions on Ca2+, the reactions were repeated without CaCl2 (0 mM Ca2+) or with increased EDTA (10 mM EDTA). The reaction products were analyzed by SDS-PAGE. Lane 1: CHgA351–370 alone; Lane 2: Tgase2 alone; Lane 3: CHgA351–370 incubated with Tgase2; Lane 4: CHgA351–370 incubated with Tgase2 in 0 mM Ca2+; Lane 5, CHgA351–370 incubated with Tgase2 in 10 mM EDTA. (C) The immunogenicity of CHgA351–370 incubated with Tgase2 was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM. * p < 0.05.
We first sought to determine whether Tgase2 would modify CHgA351–370. Tgase2 modifies substrates through two mechanisms: the formation of ε(γ-glutamyl) isopeptide bonds between glutamine and lysine residues that crosslink and aggregate proteins, and through the deamidation of glutamine to glutamic acid [63]. The crosslinking of CHgA351–370 was measured by SDS-PAGE analysis. Indeed, Tgase2 modified CHgA351–370 by crosslinking and aggregation (Fig. 5B). Importantly, this modification was Ca2+-dependent, since aggregation did not occur when Ca2+ was omitted or chelated (Fig. 5B). In addition, recombinant full length CHgA was incubated with Tgase2 and analyzed by mass spectrometry. Mass spectrometry confirmed that Tgase2 modified CHgA by deamidation (Supplemental Fig. 3 and Supplemental Table 1). Tgase2-mediated deamidation was found throughout the protein, including within the CHgA351–370 region. Together, these data demonstrate that Tgase2 is able to modify CHgA351–370 by both crosslinking and deamidation.
To determine whether Tgase2 modification of CHgA351–370 increases its immunogenicity, the native and modified peptides were analyzed by BDC2.5 T cell assay. Tgase2-modified CHgA351–370 elicited significantly higher (27.9-fold) BDC2.5 T cell responses than untreated CHgA351–370 (p < 0.05) (Fig. 5C). These data support the findings of Delong et al. [69] and demonstrate that Tgase2-mediated PTM increases immunogenicity of the more traditional CHgA351–370 peptide.
3.6. ER stress in NIT-1 insulinomas increases immunogenicity through Ca2+-dependent PTM
Thus far, the experiments described were performed with primary murine islets. These primary cells are the ideal cell type for elucidating the cellular mechanisms by which β cell immunogenicity occurs. However, primary murine islet cells are not ideal for experiments requiring longer in vitro culture, or for experiments requiring high cell numbers. For such experiments, we surmised that NIT-1 insulinomas, which are derived from NOD β cell tumors [92], would be better suited both for longer culture and for the collection of greater cell numbers. Although other studies have demonstrated that insulinomas are a useful model of β cell processes [93], we sought to confirm in NIT-1 cells the observations made in primary islet cells.
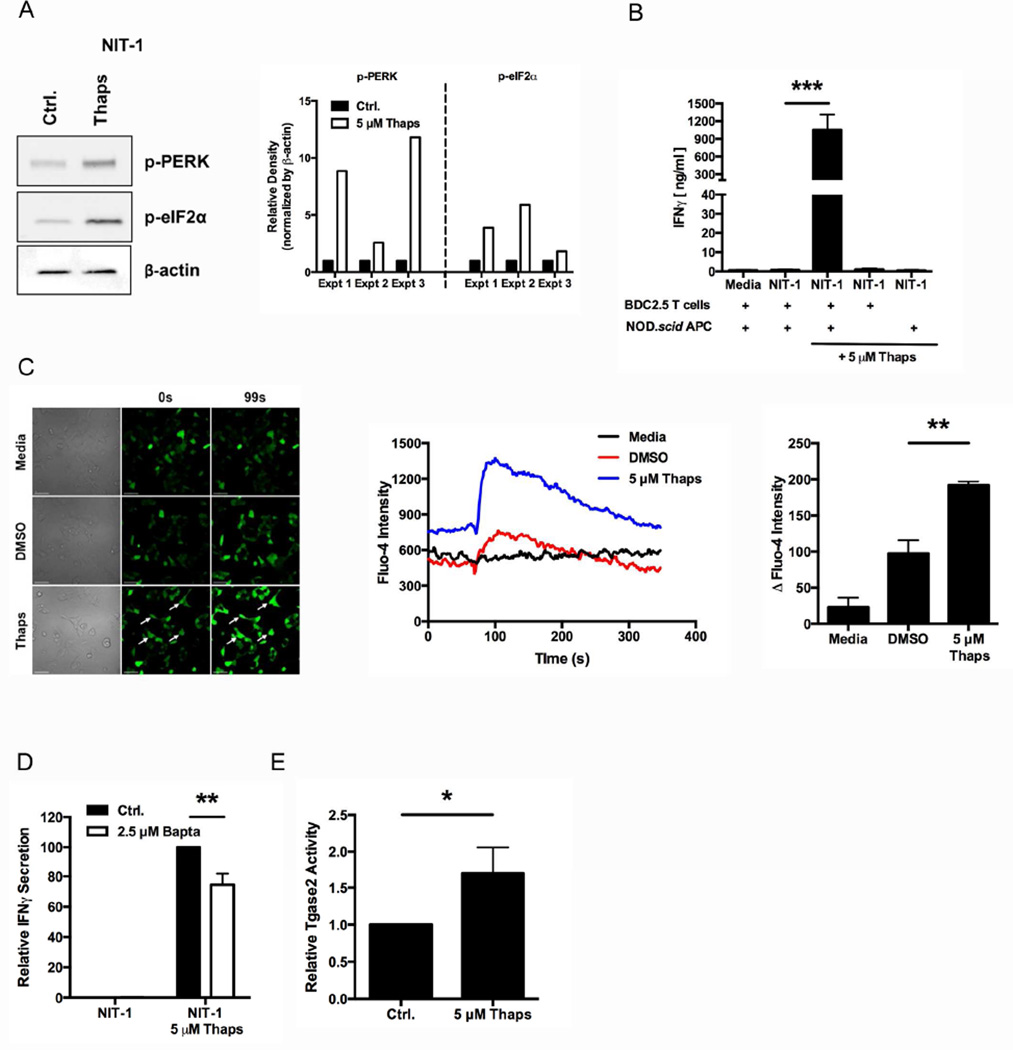
First, to determine whether ER stress increases NIT-1 immunogenicity, NIT-1 cells were incubated with 5 µM Thaps for 1 hr. Thaps increased phosphorylation of UPR proteins PERK (7.8-fold) and eIF2α (3.9-fold) compared to control-treated cells (Fig. 6A). As in primary islet cells, this Thaps-induced ER stress significantly increased the BDC2.5 T cell response to NIT-1 insulinomas (1451.1-fold, p < 0.001) (Fig. 6B). In addition, the response to Thaps-treated NIT-1 cells also required APC-mediated β cell antigen processing and presentation to BDC2.5 T cells (Fig. 6B).
Fig. 6. ER stress in NIT-1 insulinomas increases immunogenicity in a Ca2+-dependent manner.
(A) NIT-1 insulinoma cells were incubated with 5 µM Thaps or control (Ctrl.) for 1 hr and washed extensively. Cell lysates were analyzed for the phosphorylation of UPR proteins PERK and eIF2α. Data are representative of 3 independent experiments. Densitometry data are phosphorylation levels normalized by β-actin and relative to that in control (Ctrl.) treated cells. (B) The immunogenicity of NIT-1 cells treated with 5 µM Thaps or control was measured by BDC2.5 T cell assay. Either APC or T cells were omitted from assay to demonstrate the necessity of both cell types for full IFNγ secretion. Data are mean IFNγ secretion ± SEM. *** p < 0.001. (C) NIT-1 cells were labeled with Fluo-4 and analyzed by live imaging with an Olympus Fluoview FV1000 microscope for 350 sec at room temperature. At 70 sec, the cells were exposed to 5 µM Thaps or controls. Scale bars, 50 µm. Arrows indicate cells with greater Fluo-4 intensity after exposure to Thaps. Line graphs represent intensity of Fluo-4 in each sample over time. Data are representative of 3 independent experiments. Quantified data are change in Fluo-4 intensity from baseline to peak, with mean ± SEM. ** p < 0.01. (D) The immunogenicity of NIT-1 cells treated with 2.5 µM Bapta or control (Ctrl.) prior to incubation with 5 µM Thaps or control was measured by BDC2.5 T cell assay. Data are relative IFNγ secretion compared to 5 µM Thaps, with mean ± SEM. ** p < 0.01 (E) NIT-1 cells were incubated with 5 µM Thaps or control (Ctrl.) for 1 hr. The cells were lysed and Tgase2 activity was measured by microtiter plate assay. Data are relative Tgase2 activity compared to control (Ctrl.) NIT-1 cells, with mean ± SEM. * p < 0.05.
Second, to assess whether Ca2+ stores are released from the ER into the cytosol in NIT-1 cells undergoing ER stress, the cytosolic Ca2+ concentration was monitored with Fluo-4. As in primary islet cells, Thaps significantly increased Fluo-4 fluorescence (p < 0.01), indicating a significant increase in cytosolic Ca2+ concentrations, while the fluorescence of control-treated cells remained constant (Fig. 6C). In addition, pre-incubation with Bapta-AM significantly reduced Thaps-induced immunogenicity in NIT-1 cells (27%, p < 0.01) (Fig. 6D) without directly affecting the BDC2.5 T cells (Supplemental Fig. 2), demonstrating that, as in primary islets, increased cytosolic Ca2+ is important for ER stress-induced NIT-1 immunogenicity.
Finally, Tgase2 activity was measured in NIT-1 insulinomas. NIT-1 cells incubated with 5 µM Thaps for 1 hr contained 1.7-fold higher Tgase2 activity than control NIT-1 cells (p < 0.05) (Fig. 6E). These data confirm that ER stress increases the activity of this cytosolic PTM enzyme in NIT-1 cells.
Taken together, these data demonstrate that ER stress contributes to NIT-1 immunogenicity through Ca2+- and Tgase2-dependent mechanisms. These data confirm that, in these limited respects, NIT-1 cells are sufficiently similar to primary murine islets to be used for additional mechanistic studies.
3.7. Tgase2 contributes to ER stress-induced β cell immunogenicity
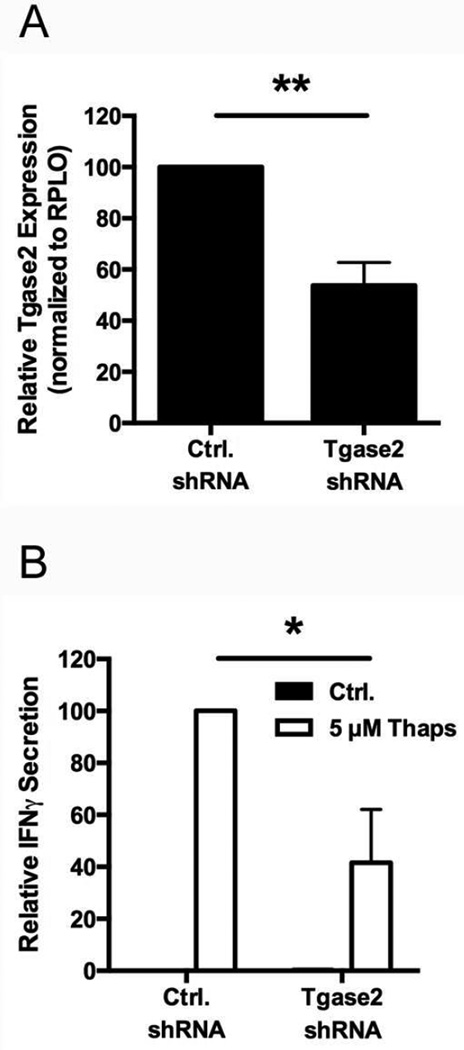
Tgase2 activity corresponds to heightened ER stress and β cell immunogenicity (Fig. 4 and 6E). To confirm the importance of Tgase2 in ER stress-induced immunogenicity, NIT-1 cells were stably transduced with either control shRNA or Tgase2-targeting shRNA. Tgase2-targeting shRNA reduced Tgase2 expression by 46% compared to control shRNA (p < 0.01) (Fig. 7A). Furthermore, Tgase2-deficient NIT-1 cells incubated with Thaps elicited significantly lower BDC2.5 T cell responses compared to control cells incubated with Thaps (59%, p < 0.05) (Fig. 7B). Therefore, even a modest decrease in Tgase2 expression significantly impacted ER stress-induced β cell immunogenicity. These data confirm the importance of Tgase2 during ER stress-induced immunogenicity, and suggest that Tgase2-mediated PTM of β cell proteins, like CHgA, contributes to the recognition of β cells by diabetogenic T cells.
Fig. 7. Tgase2 activity contributes to β cell immunogenicity.
(A) NIT-1 cells were transduced with control shRNA (Ctrl. shRNA) or Tgase2-targeting shRNA (Tgase2 shRNA). Tgase2 expression was measured by qRT-PCR. Data are relative Tgase2 expression compared to cells transduced with control shRNA, with mean ± SEM. ** p < 0.01. (B) The immunogenicity of NIT-1 cells transduced with control shRNA (Ctrl. shRNA) or Tgase2-targeting shRNA (Tgase2 shRNA) prior to incubation with 5 µM Thaps or control (Ctrl.) for 1 hr was measured by BDC2.5 T cell assay. Data are relative IFNγ secretion compared to NIT-1 cells transduced with Ctrl. shRNA and incubated with 5 µM Thaps, with mean ± SEM. * p < 0.05.
3.8. Physiological cues in vivo increase β cell ER stress and immunogenicity
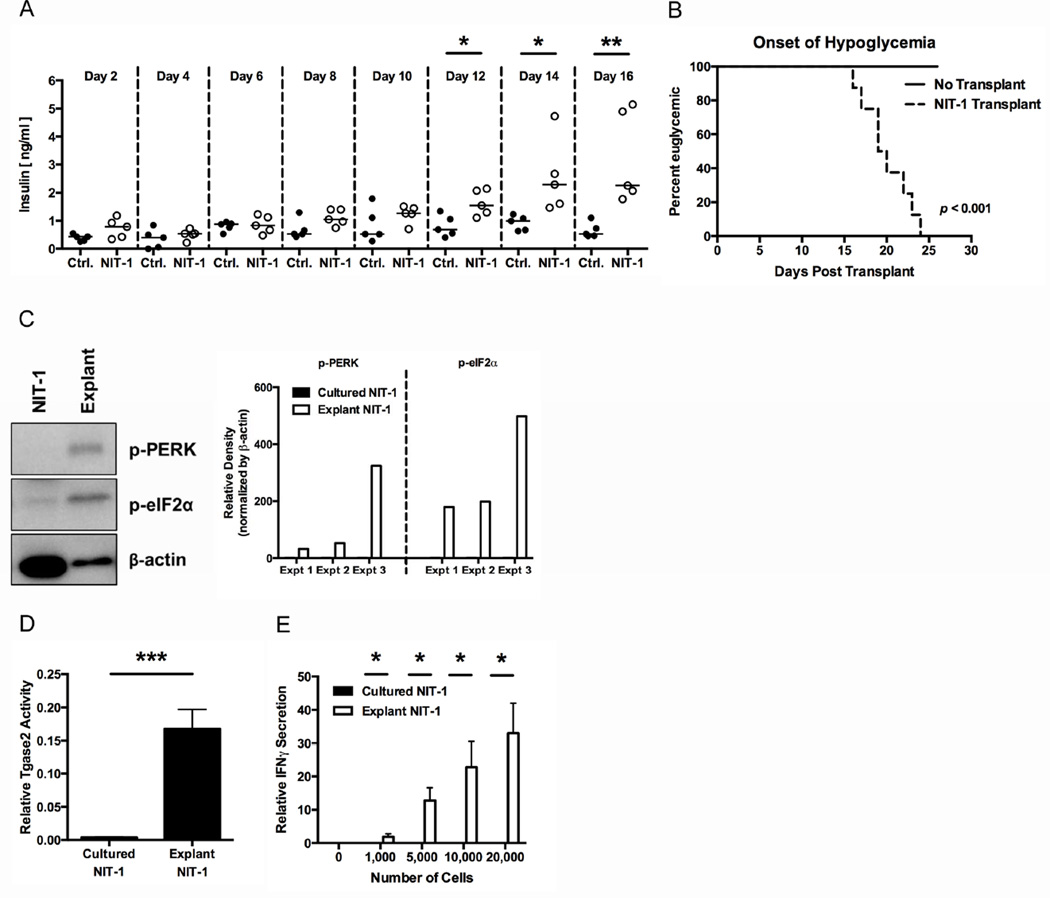
Islet β cells undergo high ER stress due to their normal physiology [5–14]. Indeed, primary islets exhibit high ER stress and elicit strong BDC2.5 T cell responses immediately after harvest (Fig. 1B and C) [79]. We therefore hypothesized that the normal physiology of β cells in vivo is sufficient to generate ER stress-dependent PTM and cause β cell recognition by autoreactive T cells. To test this hypothesis, NIT-1 cells, which exhibit low ER stress and immunogenicity (Fig. 6A and B), were transplanted under the kidney capsule of NOD.scid recipients and thus exposed to physiological triggers of ER stress (such as changes in glucose concentration). Similar to previous findings with mice injected with NIT-1 cells [94], NIT-1 recipient mice had 4.9-fold higher serum insulin than control mice (p < 0.01) (Fig. 8A) and became hypoglycemic (blood glucose levels ≤ 40 mg/dl) beginning at day 16 post-transplant (p < 0.001) (Fig. 8B). These data show that, unlike NIT-1 cells that do not secrete insulin in response to static glucose concentrations in culture (Supplemental Fig. 4), NIT-1 cells exposed to physiological cues (including, but limited to, dynamic changes in blood glucose levels) regain the ability to secrete insulin. Importantly, the grafted NIT-1 cells did not increase in number during the course of these experiments (Supplemental Fig. 5). This indicates that the increased insulin secretion was due to physiological changes within the NIT-1 cells, rather than proliferation of the transplanted cells.
Fig. 8. Physiological cues increase β cell ER stress and immunogenicity.
Non-immunogenic NIT-1 cells (2–5×106) were transplanted under the kidney capsule of NOD.scid mice. (A) Serum insulin levels in control mice (Ctrl.) or mice transplanted with NIT-1 cells (NIT-1) were measured by ELISA. Medians are indicated with a line. Data are representative of 4 independent experiments. * p < 0.05, ** p < 0.01. (B) The onset of hypoglycemia in control mice (No Transplant) or mice transplanted with NIT-1 cells (NIT-1 Transplant) was monitored over time. p < 0.001 (C) At the onset of hypoglycemia, the mice were sacrificed and the NIT-1 cells were explanted. Cell lysates of cultured NIT-1 cells (NIT-1) or explanted NIT-1 cells (Explant) were analyzed for the phosphorylation of UPR proteins PERK and eIF2α. Data are representative of 3 independent experiments. Densitometry data are phosphorylation levels normalized by β-actin and relative to that in cultured NIT-1 cells. (D) Cultured NIT-1 cells or explanted NIT-1 cells were lysed, and Tgase2 activity was measured by ex vivo Tgase2 activity assay. Data are mean Tgase2 activity, with mean ± SEM. *** p < 0.001. (E) The immunogenicity of cultured NIT-1 cells or explanted NIT-1 cells was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM. * p < 0.05.
At the onset of hypoglycemia, transplanted NIT-1 cells were explanted from the kidney and analyzed by Western blot. Since explanted NIT-1 cells exhibit increased insulin secretory capacity, we surmised that these cells, like primary islets, would experience heightened ER stress. Indeed, compared to cultured NIT-1 cells, explanted NIT-1 cells showed greater phosphorylation of UPR proteins PERK (136.4-fold) and eIF2α (292.1-fold), confirming higher ER stress (Fig. 8C). This heightened ER stress further supports the hypothesis that physiological cues increase ER stress in β cells and β cell insulinomas.
Tgase2 activity correlates with ER stress (Fig. 4 and 6E) and is necessary for ER stress-induced β cell immunogenicity (Fig. 7B). We hypothesized that ER stress triggered by physiological cues would increase Tgase2 activity. To test this hypothesis, Tgase2 activity was measured in explanted and cultured NIT-1 cells. Explanted NIT-1 cells exhibited 42.0-fold higher Tgase2 activity than cultured NIT-1 cells (p < 0.001, Fig. 8D). These data confirm that physiological ER stress increases Tgase2 activity in β cells.
Finally, we hypothesized that increased ER stress and Tgase2 activity would increase immunogenicity in NIT-1 cells exposed to physiological cues. Explanted and cultured NIT-1 cells were analyzed by BDC2.5 T cell assay. As observed in Fig. 6B, cultured NIT-1 cells did not elicit IFNγ secretion from BDC2.5 T cells (Fig 8E). As a control, we confirmed that that these cultured NIT-1 cells did not directly inhibit BDC2.5 T cell activation, as conditioned media harvested from cultured NIT-1 cells did not reduce IFNγ secretion from antibody-stimulated T cells (Supplemental Fig. 6). However, explanted NIT-1 cells elicited 33.0-fold higher BDC2.5 T cell responses than cultured NIT-1 cells (p < 0.05) (Fig. 8E). These data demonstrate that physiological ER stress is sufficient to render β cells immunogenic.
Importantly, these observations were only made when NIT-1 cells were explanted at the onset of hypoglycemia. When NIT-1 cells were explanted after only 2 days in vivo (a time point at which NIT-1 recipient mice have similar serum insulin levels to control mice and are not hypoglycemic (Fig. 8A and B)), neither ER stress nor immunogenicity was different from that in cultured NIT-1 cells (Supplemental Fig. 7). These data suggest that the increased ER stress and immunogenicity observed in explanted NIT-1 cells (Fig. 8C and E) was not due to technical manipulation of transplantation and explantation. Rather, these data demonstrate that increased ER stress, Tgase2 activity, and immunogenicity, occur as the NIT-1 cells begin to secrete insulin in response to normal physiological cues.
Together, the data presented in Fig. 8 demonstrate that NIT-1 cells with low ER stress and low immunogenicity (Fig. 6A and B) can secrete insulin when exposed to similar physiological cues as those to which primary islets are exposed in vivo. Furthermore, insulin secretion is accompanied by high ER stress, high Tgase2, and high immunogenicity. These data implicate the normal physiology of the β cell in ER stress- and PTM-dependent β cell immunogenicity. Therefore, normal β cell physiology, and the ER stress inherent to this normal physiology, may predispose β cells to immunogenicity and immune-mediated destruction. In the context of genetic susceptibility to autoimmunity and the presence of autoreactive T cells in the periphery, these β cell processes lead to activation of the autoreactive immune response and T1D.
4. Discussion
While protein PTM has been implicated in pathology in other diseases [15, 44–51], this area of research has not been well explored in T1D [71]. Although recent literature shows that PTM of synthetic murine and human β cell peptides increases their recognition by autoreactive T cells [69–75], these studies did not explore the physiological relevance of cellular mechanisms that cause PTM. Here, for the first time, we demonstrate that β cell ER stress, such as that induced by physiological triggers, leads to PTM-dependent β cell recognition by diabetogenic T cells.
Primary murine islets analyzed immediately after harvest, but not islet-conditioned media, are highly immunogenic (Fig. 1B and C, Supplemental Fig. 1). In fact, primary islets from both autoimmune prone and non-autoimmune prone backgrounds are highly immunogenic (Fig. 1C). These data show that immunogenicity of some β cell antigens (such as CHgA) is not unique to islets from the autoimmune prone NOD mouse. Rather, these data suggest that islets from all mouse strains contain a common factor that may play an important role in generating β cell immunogenicity. β cells, as professional secretory cells, contain a more fully-developed ER than nonsecretory cells and are consequently more susceptible to ER stress due to their normal physiology [5–14]. Rises in blood glucose concentrations stimulate significant increases in insulin production by β cells [15] that, in turn, leads to heightened ER stress [16]. We hypothesized that normal β cell physiology, which includes heightened ER stress [5–16], contributes to β cell recognition by immune cells and breaks in peripheral tolerance.
ER stress has been linked to T1D through β cell malfunction and apoptosis [95–97]. However, β cell apoptosis may not be the only mechanism by which ER stress contributes to T1D. In other diseases, ER stress increases the generation of abnormally modified proteins that act as neo-antigens that, if presented by APC to autoreactive T cells, break peripheral tolerance [44, 50, 51]. Here, we show that β cell ER stress significantly increased BDC2.5 effector responses (Figs. 2B, 6B, and 8E). These data provide a novel link between β cell ER stress and β cell recognition by immune cells, namely that ER stress predisposes β cells to autoreactive T cell recognition.
ER stress releases Ca2+ from the ER lumen. In β cells, Ca2+ release occurs with insulin production and secretion [40–43]. As expected, Thaps-triggered ER stress in β cells significantly increased cytosolic Ca2+ (Fig. 3A and 6C). Higher Ca2+ concentrations increase the activity of Ca2+-dependent PTM enzymes that play important roles in cells under stress. For instance, Tgase2 modifies caspase 3 to delay apoptosis during stress [64]. However, Tgase2 has also been implicated in modifying other proteins to produce neo-antigens in several autoimmune diseases [44, 50, 51, 66]. Recently, Tgase2 has been implicated in the modification of T1D peptide antigens [72, 74] including WE14 [69, 72] (Fig. 5B, Supplemental Fig. 3, Supplemental Table 1). Together, these findings support the hypothesis that BDC2.5 T cells optimally recognize CHgA after PTM. However, these data alone do not address the physiological mechanisms that lead to CHgA PTM in β cells. Here, we show that ER stress-induced Ca2+ flux into the cytosol is necessary for ER stress-induced β cell immunogenicity (Fig. 3B and 6D). Also, Ca2+-dependent Tgase2 activity is significantly higher in β cells under ER stress (Fig. 4 and 6E), and is crucial for ER stress-induced immunogenicity (Fig. 7B). These data demonstrate for the first time the importance of Tgase2-mediated PTM in eliciting heightened effector responses from BDC2.5 T cells in response to whole β cells, and provide a mechanism to explain the cellular events that lead to Tgase2 activation and the Tgase2-mediated PTM of β cell proteins described by other groups [69, 70, 72, 74].
Finally, primary islet cells exhibit high ER stress [5–16] and are immunogenic immediately after harvest (Fig. 1B and C). We therefore hypothesized that normal physiological cues in vivo sufficiently raise ER stress to facilitate Ca2+-dependent PTM and immunogenicity. This hypothesis was supported by experiments in which non-immunogenic NIT-1 insulinoma cells were transplanted in NOD.scid mice and exposed to normal physiological cues. These NIT-1 cells regained the ability to secrete insulin (Fig. 8A, Supplemental Fig. 4) and the recipient mice suffered hypoglycemia (Fig. 8B). When examined ex vivo, NIT-1 cells exposed to normal physiological cues exhibited significantly higher ER stress and Tgase2 activity (Fig. 8C and D), both of which our studies showed to be necessary for β cell immunogenicity (Fig. 2, 4, 6A, 6B, 6E, and 7B). As with Thaps-induced ER stress, the physiologically heightened ER stress and Tgase2 activity led to increased responses from BDC2.5 T cells (Fig. 8E).
Interestingly, the BDC2.5 effector responses elicited by physiologically-stressed primary islets (Fig. 1B and C) or NIT-1 cells (Fig. 8E), were much lower than those elicited by Thaps-stressed β cells (Fig. 2B, 6B), demonstrating important differences between the two types of ER stress triggers. Thaps induces very strong and acute ER stress with strong Ca2+ fluxes, which cause apoptosis if exposure is prolonged beyond the 1 hr time point used in this study [98, 99]. In contrast, the physiological triggers of ER stress in vivo are less potent and induce less acute ER stress with smaller Ca2+ fluxes. This lower ER stress would be important for β cell survival and function in vivo, because lower stress would allow β cells in vivo to activate UPR, return to homeostasis, and avoid apoptosis. Although physiologically- and chemically-induced ER stress elicits different BDC2.5 T cell responses, these data still demonstrate that normal β cell physiology is sufficient to render β cells immunogenic through Ca2+-dependent PTM.
While identifying β cell autoantigens is important, elucidating mechanisms by which β cell proteins become immunogenic is crucial to identify therapeutic targets to reduce β cell immunogenicity and prevent immune-mediated β cell destruction in T1D. Thus, the aim of these studies was not to identify novel immunogenic β cell proteins or peptides, but rather to understand how and why β cells become immunogenic and come to be recognized by autoreactive T cells. Our data show that normal islet physiology sufficiently increases β cell ER stress and leads to β cell immunogenicity through PTM. Because islets are particularly susceptible to ER stress [5–16], these processes are likely occurring in all β cells in every individual, regardless of whether autoimmune pathology is occurring. Indeed, heightened ER stress is detected in islets from nonautoimmune mice as early as 3 weeks of age [16]. In addition, islets from autoimmune- and nonautoimmune-prone mouse strains elicit equal BDC2.5 T cell responses (Fig. 1C). Together, these data support the hypothesis that inherent β cell ER stress may provide the necessary trigger for protein modification [100] and lead to immunogenicity in all individuals, even in the absence of pancreatic inflammation. Therefore, T1D onset may not be determined by whether these ER stress-induced PTM are generated, but perhaps by genetic predisposition to autoimmunity. Individuals not genetically predisposed to autoimmunity would either not have autoreactive T cells in the periphery, or would have a higher frequency of circulating regulatory T cells. The presentation of abnormally-modified β cell proteins by APC would fail to elicit autoimmune β cell destruction. However, patients genetically predisposed to autoimmunity do have autoreactive T cells in the periphery due to failures in central and peripheral tolerance. In these individuals, the presentation of abnormally-modified β cell proteins may activate these autoreactive T cells, and lead to pathology.
In addition to inherent ER stress, exposure of β cells to additional triggers of ER stress (such as viral infection, exposure to chemicals or reactive oxygen species, dysglycemia, and inflammation) may increase ER stress above physiological levels, further increasing the activity of Ca2+-dependent PTM enzymes and the subsequent modification of β cell proteins. This could explain how environmental triggers lead to the progression of T1D [100, 101]. Once autoreactive immune cells invade the islet, the inflammatory environment likely further increases β cell ER stress, leading to modification of additional β cell proteins [75]. Thus, once immune pathology in the islet begins, other β cell antigens may undergo PTM, which may contribute to epitope spreading.
Although these studies focused on recognition of one autoantigen (CHgA) by one T cell clone (BDC2.5), the mechanisms by which tolerance is broken by ER stress would be easily applicable to other autoantigens in T1D and other autoimmune diseases [15, 44–51, 100]. For instance, ER stress may cause PTM of other endogenous proteins, or may facilitate recognition of non-modified proteins by autoreactive T cells by changing β cell physiology.
5. Conclusion
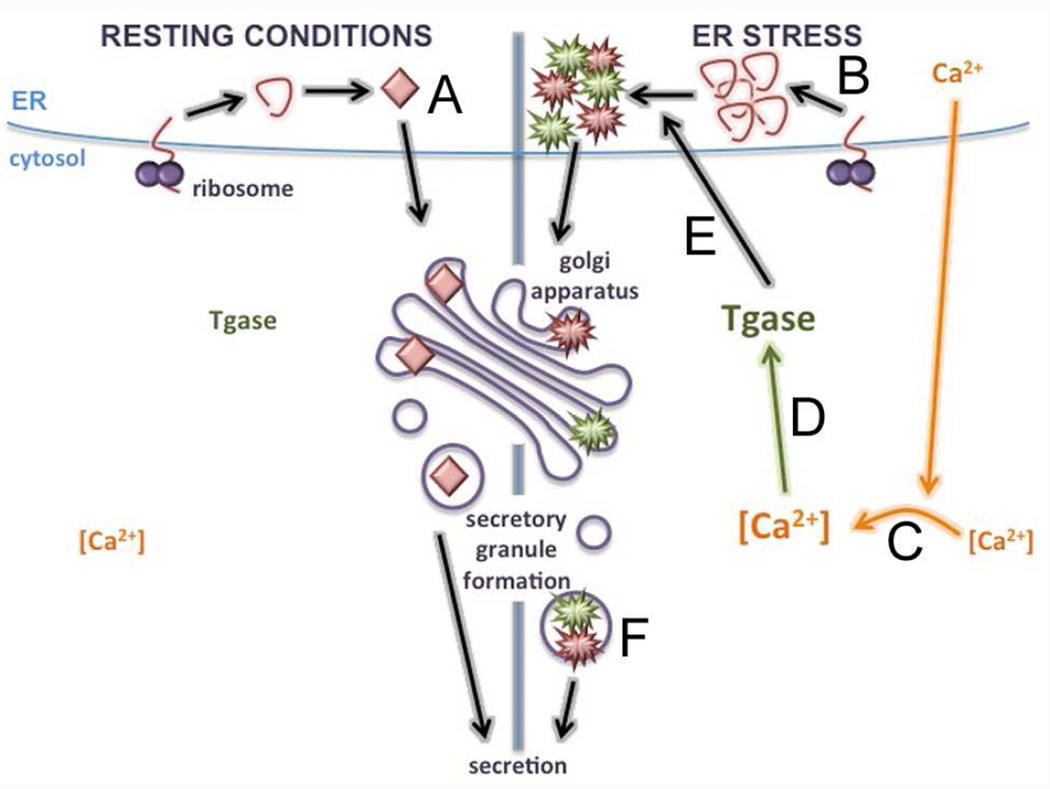
In summary, we have demonstrated two important novel findings. It is known that modification of β cell peptides (such as WE14) by PTM enzymes (such as Tgase2) increases the response of autoreactive T cells [69– 75]. Here, we show that these PTM enzymes are activated and contribute to β cell immunogenicity when ER stress releases Ca2+ into the cytosol. Second, we show that β cell ER stress and the resulting PTM-dependent immunogenicity occur due to normal β cell physiology. Therefore, the normal physiology and function of β cell insulin secretion is sufficient to generate immunogenicity and activate diabetogenic T cells. We therefore propose a model (Fig. 9) in which ER stress constantly leads to abnormal PTM of β cell proteins as a result of normal physiology. This process may be exacerbated when β cells are exposed to additional triggers of ER stress, such as the environmental triggers thought to accelerate T1D and the inflammatory environment created by islet-infiltrating immune cells. In patients with genetic susceptibility to autoimmunity, presentation of modified β cell proteins by APC to β cell-specific T cells causes a break in peripheral tolerance and, ultimately, to autoimmune T1D.
Fig. 9. Model.
(A) Under resting conditions, CHgA is translated, folded in the ER, and packaged in secretory granules. Cytosolic Ca2+ remains low, and Tgase2 activity remains low. (B) β cell exposure to physiological or environmental triggers of ER stress leads to increased ER burden. (C) Ca2+ stores are released from the ER, increasing cytosolic Ca2+, and (D) activating Ca2+-dependent PTM enzymes such as Tgase2. (E) Activated Tgase2 translocates to the ER to modify CHgA, (F) which is packaged into secretory granules. If presented to auto-reactive T cells by APC, modified CHgA breaks peripheral tolerance and facilitates immune recognition of β cells.
Supplementary Material
Primary NOD.scid islets were incubated in fresh media for 1 hr at 37°C. The immunogenicity of the conditioned media and of the islets themselves was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM.
Primary NOD.scid islets or NIT-1 cells were incubated with 2.5 µM Bapta-AM for 1 hr and washed extensively. The cells were then incubated in fresh media for 1 hr at 37°C. This conditioned media was harvested and added to BDC2.5 T cells stimulated with 0.5 µg/ml α-CD3 and 1 µg/ml α-CD28 for 72 hrs. IFNγ secretion was measured by ELISA. Data are mean IFNγ secretion ± SEM.
Recombinant CHgA was incubated with Tgase2 (Tgase2-modified) or control (Ctrl.) for 3 hr at 37°C. The reaction products were analyzed by mass spectrometry to identify sites of deamidation. Residues highlighted in yellow indicate coverage, residues highlighted in green indicate deamidation, and residues in bold underline indicate the sequence of CHgA351–370.
Intact primary islets (3 per well) or NIT-1 cells (3×103 per well) were incubated in 0 mM, 2.8 mM, or 20 mM glucose for 1 hr. Insulin secretion was measured by ELISA. Data are mean insulin secretion ± SEM. *** p < 0.001.
Non-immunogenic NIT-1 cells (2–5×106) were transplanted under the kidney capsule of NOD.scid mice. At onset of hypoglycemia, the mice were sacrificed and the NIT-1 cells were explanted. The NIT-1 cells were counted to determine whether the graft had proliferated during the in vivo incubation.
Cultured NIT-1 cells were incubated in fresh media for 1 hr at 37°C. This conditioned media was harvested and added to BDC2.5 T cells stimulated with 0.5 µg/ml α-CD3 and 1 µg/ml α-CD28 for 72 hrs. IFNγ secretion was measured by ELISA. Data are mean IFNγ secretion ± SEM.
NIT-1 cells (2–5×106) were transplanted under the kidney capsule of NOD.scid mice. After only 2 days (when the mice remained euglycemic), the mice were sacrificed and the cells were explanted for analysis. (A) Cell lysates of cultured NIT-1 cells (NIT-1) or explanted NIT-1 cells (Explant) were analyzed for the phosphorylation of UPR proteins PERK and eIF2α. Data are representative of 3 independent experiments. Densitometry data are phosphorylation levels normalized by β-actin and relative to that in cultured NIT-1 cells. (B) The immunogenicity of cultured NIT-1 cells or NIT-1 cells explanted after 2 days was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM.
Highlights.
β cells undergo high levels of ER stress due to normal physiology
ER stress increases activity of cytosolic Ca2+-dependent PTM enzymes
PTM enzyme activity is necessary for full β cell immunogenicity
Physiological ER stress predisposes β cells to immune-mediated destruction in T1D
Acknowledgments
Funding
This work was supported in part by Children’s Hospital of Pittsburgh of the UPMC Health System (Research Advisory Committee Fellowship and Cochrane-Weber Endowed Fund to M.M.), National Institutes of Health (5T32AI089443-04 to M.M.) the American Diabetes Association (CDA 7.07 CD-16 and 1-12-BS-161 to J.D.P.), and the Juvenile Diabetes Research Foundation (3-PDF-2014-213-A-N to M.M. and 5-2013-91, 47-2013-517, and 2-SRA-2014-296-Q-R to J.D.P.). These funding sources had no role in the design of the study, in the collection, analysis, or interpretation of the data, or in the writing of this manuscript.
The authors thank the Piganelli laboratory (University of Pittsburgh) and Dr. Hubert M. Tse (University of Alabama at Birmingham) for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geenen V. Thymus and type 1 diabetes: an update. Diabetes Res Clin Pract. 2012;98:26–32. doi: 10.1016/j.diabres.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 2.Fan Y, Rudert WA, Grupillo M, He J, Sisino G, Trucco M. Thymus-specific deletion of insulin induces autoimmune diabetes. EMBO J. 2009;28:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallone R and van Endert P. T cells in the pathogenesis of type 1 diabetes. Curr Diab Rep. 2008;8:101–106. doi: 10.1007/s11892-008-0019-9. [DOI] [PubMed] [Google Scholar]
- 4.Grupillo M, Gualtierotti G, He J, Sisino G, Bottino R, Rudert WA, Trucco M, Fan Y. Essential roles of insulin expression in Aire+ tolerogenic dendritic cells in maintaining peripheral self-tolerance of islet beta-cells. Cell Immunol. 2012;273:115–123. doi: 10.1016/j.cellimm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006;13:374–384. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 6.Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F. Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab. 2006;4:245–254. doi: 10.1016/j.cmet.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca SG, Lipson KL, Urano F. Endoplasmic reticulum stress signaling in pancreatic beta-cells. Antioxid Redox Signal. 2007;9:2335–2344. doi: 10.1089/ars.2007.1790. [DOI] [PubMed] [Google Scholar]
- 8.Lipson KL, Fonseca SG, Urano F. Endoplasmic reticulum stress-induced apoptosis and auto-immunity in diabetes. Curr Mol Med. 2006;6:71–77. doi: 10.2174/156652406775574613. [DOI] [PubMed] [Google Scholar]
- 9.Kim MK, Kim HS, Lee IK, Park KG. Endoplasmic reticulum stress and insulin biosynthesis: a review. Exp Diabetes Res. 2012;2012:509437. doi: 10.1155/2012/509437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teodoro T, Odisho T, Sidorova E, Volchuk A. Pancreatic beta-cells depend on basal expression of active ATF6alpha-p50 for cell survival even under nonstress conditions. Am J Physiol Cell Physiol. 2012;302:C992–C1003. doi: 10.1152/ajpcell.00160.2011. [DOI] [PubMed] [Google Scholar]
- 11.Ortsater H, Sjoholm A. A busy cell--endoplasmic reticulum stress in the pancreatic beta-cell. Mol Cell Endocrinol. 2007;277:1–5. doi: 10.1016/j.mce.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern Med. 2003;42:7–14. doi: 10.2169/internalmedicine.42.7. [DOI] [PubMed] [Google Scholar]
- 13.Volchuk A, Ron D. The endoplasmic reticulum stress response in the pancreatic beta-cell. Diabetes Obes Metab. 2010;12(Suppl 2):48–57. doi: 10.1111/j.1463-1326.2010.01271.x. [DOI] [PubMed] [Google Scholar]
- 14.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 15.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwawaki T, Akai R, Kohno K, Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nat Med. 2004;10:98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 17.van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, Galama JM, Melchers WJ. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J. 1997;16:3519–3532. doi: 10.1093/emboj/16.12.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Kuppeveld FJ, Melchers WJ, Willems PH, Gadella TW., Jr Homomultimerization of the coxsackievirus 2B protein in living cells visualized by fluorescence resonance energy transfer microscopy. J Virol. 2002;76:9446–9456. doi: 10.1128/JVI.76.18.9446-9456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kuppeveld FJ, de Jong AS, Melchers WJ, Willems PH. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends Microbiol. 2005;13:41–44. doi: 10.1016/j.tim.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Heikkila RE, Winston B, Cohen G. Alloxan-induced diabetes-evidence for hydroxyl radical as a cytotoxic intermediate. Biochem Pharmacol. 1976;25:1085–1092. doi: 10.1016/0006-2952(76)90502-5. [DOI] [PubMed] [Google Scholar]
- 21.Takasu N, Komiya I, Asawa T, Nagasawa Y, Yamada T. Streptozocin- and alloxan-induced H2O2 generation and DNA fragmentation in pancreatic islets. H2O2 as mediator for DNA fragmentation. Diabetes. 1991;40:1141–1145. doi: 10.2337/diab.40.9.1141. [DOI] [PubMed] [Google Scholar]
- 22.Bedoya FJ, Solano F, Lucas M. N-monomethyl-arginine and nicotinamide prevent streptozotocin-induced double strand DNA break formation in pancreatic rat islets. Experientia. 1996;52:344–347. doi: 10.1007/BF01919538. [DOI] [PubMed] [Google Scholar]
- 23.Sandler S, Swenne I. Streptozotocin, but not alloxan, induces DNA repair synthesis in mouse pancreatic islets in vitro. Diabetologia. 1983;25:444–447. doi: 10.1007/BF00282526. [DOI] [PubMed] [Google Scholar]
- 24.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 26.Favero TG, Zable AC, Abramson JJ. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 27.Lee H, Park MT, Choi BH, Oh ET, Song MJ, Lee J, Kim C, Lim BU, Park HJ. Endoplasmic reticulum stress-induced JNK activation is a critical event leading to mitochondria-mediated cell death caused by beta-lapachone treatment. PLoS One. 2011;6:e21533. doi: 10.1371/journal.pone.0021533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Zhang H, Zhao B, Fei H. IL-1beta caused pancreatic beta-cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the c-Jun N-terminal kinase pathway. Mol Cell Biochem. 2009;324:183–190. doi: 10.1007/s11010-008-9997-9. [DOI] [PubMed] [Google Scholar]
- 29.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 30.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 33.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 34.Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang XZ, Lawson B, Brewer JW, Zinszner H, Sanjay A, Mi LJ, Boorstein R, Kreibich G, Hendershot LM, Ron D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153) Mol Cell Biol. 1996;16:4273–4280. doi: 10.1128/mcb.16.8.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 38.Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. doi: 10.1016/j.jchemneu.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman MY. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca(2+)-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- 40.Curry DL, Bennett LL, Grodsky GM. Requirement for calcium ion in insulin secretion by the perfused rat pancreas. Am J Physiol. 1968;214:174–178. doi: 10.1152/ajplegacy.1968.214.1.174. [DOI] [PubMed] [Google Scholar]
- 41.Milner RD, Hales CN. The role of calcium and magnesium in insulin secretion from rabbit pancreas studied in vitro. Diabetologia. 1967;3:47–49. doi: 10.1007/BF01269910. [DOI] [PubMed] [Google Scholar]
- 42.Grodsky GM, Bennett LL. Cation requirements for insulin secretion in the isolated perfused pancreas. Diabetes. 1966;15:910–913. doi: 10.2337/diab.15.12.910. [DOI] [PubMed] [Google Scholar]
- 43.Dolensek J, Spelic D, Klemen MS, Zalik B, Gosak M, Rupnik MS, Stozer A. Membrane Potential and Calcium Dynamics in Beta Cells from Mouse Pancreas Tissue Slices: Theory, Experimentation, and Analysis. Sensors (Basel) 2015;15:27393–27419. doi: 10.3390/s151127393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doyle HA, Mamula MJ. Posttranslational modifications of self-antigens. Ann N Y Acad Sci. 2005;1050:1–9. doi: 10.1196/annals.1313.001. [DOI] [PubMed] [Google Scholar]
- 45.Todd DJ, Lee AH, Glimcher LH. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 46.Rath E, Haller D. Inflammation and cellular stress: a mechanistic link between immune-mediated and metabolically driven pathologies. Eur J Nutr. 2011;50:219–233. doi: 10.1007/s00394-011-0197-0. [DOI] [PubMed] [Google Scholar]
- 47.Cunard R, Sharma K. The endoplasmic reticulum stress response and diabetic kidney disease. Am J Physiol Renal Physiol. 2011;300:F1054–F1061. doi: 10.1152/ajprenal.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niederreiter L, Kaser A. Endoplasmic reticulum stress and inflammatory bowel disease. Acta Gastroenterol Belg. 2011;74:330–333. [PubMed] [Google Scholar]
- 49.Harding HP, Ron D. Endoplasmic reticulum stress and the development of diabetes: a review. Diabetes. 2002;51(Suppl 3):S455–S461. doi: 10.2337/diabetes.51.2007.s455. [DOI] [PubMed] [Google Scholar]
- 50.Verhaar R, Drukarch B, Bol JG, Jongenelen CA, Musters RJ, Wilhelmus MM. Increase in endoplasmic reticulum-associated tissue transglutaminase and enzymatic activation in a cellular model of Parkinson's disease. Neurobiol Dis. 2012;45:839–850. doi: 10.1016/j.nbd.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 51.Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr Opin Immunol. 2012;24:112–118. doi: 10.1016/j.coi.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosen A, Casciola-Rosen L. Clearing the way to mechanisms of autoimmunity. Nat Med. 2001;7:664–665. doi: 10.1038/89034. [DOI] [PubMed] [Google Scholar]
- 54.Skriner K, Adolph K, Jungblut PR, Burmester GR. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54:3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 55.Law SC, Street S, Yu CH, Capini C, Ramnoruth S, Nel HJ, van Gorp E, Hyde C, Lau K, Pahau H, Purcell AW, Thomas R. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther. 2012;14:R118. doi: 10.1186/ar3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lesort M, Attanavanich K, Zhang J, Johnson GV. Distinct nuclear localization and activity of tissue transglutaminase. J Biol Chem. 1998;273:11991–11994. doi: 10.1074/jbc.273.20.11991. [DOI] [PubMed] [Google Scholar]
- 57.Ientile R, Caccamo D, Griffin M. Tissue transglutaminase and the stress response. Amino AcidS. 2007;33:385–394. doi: 10.1007/s00726-007-0517-0. [DOI] [PubMed] [Google Scholar]
- 58.Kojima S, Kuo TF, Tatsukawa H, Hirose S. Induction of cross-linking and silencing of Sp1 by transglutaminase during liver injury in ASH and NASH via different ER stress pathways. Dig Dis. 2010;28:715–721. doi: 10.1159/000324278. [DOI] [PubMed] [Google Scholar]
- 59.Wilhelmus MM, Verhaar R, Andringa G, Bol JG, Cras P, Shan L, Hoozemans JJ, Drukarch B. Presence of tissue transglutaminase in granular endoplasmic reticulum is characteristic of melanized neurons in Parkinson's disease brain. Brain Pathol. 2011;21:130–139. doi: 10.1111/j.1750-3639.2010.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuo TF, Tatsukawa H, Matsuura T, Nagatsuma K, Hirose S, Kojima S. Free fatty acids induce transglutaminase 2-dependent apoptosis in hepatocytes via ER stress-stimulated PERK pathways. J Cell Physiol. 2012;227:1130–1137. doi: 10.1002/jcp.22833. [DOI] [PubMed] [Google Scholar]
- 61.Orru S, Caputo I, D'Amato A, Ruoppolo M, Esposito C. Proteomics identification of acyl-acceptor and acyl-donor substrates for transglutaminase in a human intestinal epithelial cell line. Implications for celiac disease. J Biol Chem. 2003;278:31766–31773. doi: 10.1074/jbc.M305080200. [DOI] [PubMed] [Google Scholar]
- 62.Russo L, Marsella C, Nardo G, Massignan T, Alessio M, Piermarini E, La Rosa S, Finzi G, Bonetto V, Bertuzzi F, Maechler P, Massa O. Transglutaminase 2 transamidation activity during first-phase insulin secretion: natural substrates in INS-1E. Acta Diabetol. 2013;50:61–72. doi: 10.1007/s00592-012-0381-6. [DOI] [PubMed] [Google Scholar]
- 63.Facchiano F, Facchiano A, Facchiano AM. The role of transglutaminase-2 and its substrates in human diseases. Front Biosci. 2006;11:1758–1773. doi: 10.2741/1921. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi H, Wang HG. Tissue transglutaminase serves as an inhibitor of apoptosis by cross-linking caspase 3 in thapsigargin-treated cells. Mol Cell Biol. 2006;26:569–579. doi: 10.1128/MCB.26.2.569-579.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fok JY, Mehta K. Tissue transglutaminase induces the release of apoptosis inducing factor and results in apoptotic death of pancreatic cancer cells. Apoptosis. 2007;12:1455–1463. doi: 10.1007/s10495-007-0079-3. [DOI] [PubMed] [Google Scholar]
- 66.Sollid LM, Jabri B. Celiac disease and transglutaminase 2: a model for posttranslational modification of antigens and HLA association in the pathogenesis of autoimmune disorders. Curr Opin Immunol. 2011;23:732–738. doi: 10.1016/j.coi.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Molberg O, Mcadam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–717. doi: 10.1038/nm0698-713. [DOI] [PubMed] [Google Scholar]
- 68.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delong T, Baker RL, He J, Barbour G, Bradley B, Haskins K. Diabetogenic T-Cell Clones Recognize an Altered Peptide of Chromogranin A. Diabetes. 2012;61:3239–3246. doi: 10.2337/db12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gottlieb PA, Delong T, Baker RL, Fitzgerald-Miller L, Wagner R, Cook G, Rewers MR, Michels A, Haskins K. Chromogranin A is a T cell antigen in human type 1 diabetes. J Autoimmun. 2014;50:38–41. doi: 10.1016/j.jaut.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunne JL, Overbergh L, Purcell AW, Mathieu C. Posttranslational modifications of proteins in type 1 diabetes: the next step in finding the cure? Diabetes. 2012;61:1907–1914. doi: 10.2337/db11-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, Zaldumbide A, Gomez-Tourino I, Arif S, Peakman M, Drijfhout JW, Roep BO. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes. 2014;63:237–247. doi: 10.2337/db12-1214. [DOI] [PubMed] [Google Scholar]
- 73.Mannering SI, Harrison LC, Williamson NA, Morris JS, Thearle DJ, Jensen KP, Kay TW, Rossjohn J, Falk BA, Nepom GT, Purcell AW. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J Exp Med. 2005;202:1191–1197. doi: 10.1084/jem.20051251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McGinty JW, Chow IT, Greenbaum C, Odegard J, Kwok WW, James EA. Recognition of Posttranslationally Modified GAD65 Epitopes in Subjects With Type 1 Diabetes. Diabetes. 2014;63:3033–3040. doi: 10.2337/db13-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rondas D, Crevecoeur I, D'Hertog W, Ferreira GB, Staes A, Garg AD, Eizirik DL, Agostinis P, Gevaert K, Overbergh L, Mathieu C. Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes. 2015;64:573–586. doi: 10.2337/db14-0621. [DOI] [PubMed] [Google Scholar]
- 76.Bertera S, Crawford ML, Alexander AM, Papworth GD, Watkins SC, Robbins PD, Trucco M. Gene transfer of manganese superoxide dismutase extends islet graft function in a mouse model of autoimmune diabetes. Diabetes. 2003;52:387–393. doi: 10.2337/diabetes.52.2.387. [DOI] [PubMed] [Google Scholar]
- 77.Sklavos MM, Bertera S, Tse HM, Bottino R, He J, Beilke JN, Coulombe MG, Gill RG, Crapo JD, Trucco M, Piganelli JD. Redox modulation protects islets from transplant-related injury. Diabetes. 2010;59:1731–1738. doi: 10.2337/db09-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sklavos MM, Coudriet GM, Delmastro M, Bertera S, Coneybeer JT, He J, Trucco M, Piganelli JD. Administration of a negative vaccination induces hyporesponsiveness to islet allografts. Cell Transplant. 2013;22:1147–1155. doi: 10.3727/096368912X657233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–1448. doi: 10.2337/diab.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 80.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol. 2007;178:908–917. doi: 10.4049/jimmunol.178.2.908. [DOI] [PubMed] [Google Scholar]
- 81.Delmastro MM, Styche AJ, Trucco MM, Workman CJ, Vignali DA, Piganelli JD. Modulation of redox balance leaves murine diabetogenic TH1 T cells "LAG-3-ing" behind. Diabetes. 2012;61:1760–1768. doi: 10.2337/db11-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delmastro-Greenwood MM, Tse HM, Piganelli JD. Effects of metalloporphyrins on reducing inflammation and autoimmunity. Antioxid Redox Signal. 2014;20:2465–2477. doi: 10.1089/ars.2013.5257. [DOI] [PubMed] [Google Scholar]
- 83.Tse HM, Thayer TC, Steele C, Cuda CM, Morel L, Piganelli JD, Mathews CE. NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. J Immunol. 2010;185:5247–5258. doi: 10.4049/jimmunol.1001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JH, Jeong J, Jeong EM, Cho SY, Kang JW, Lim J, Heo J, Kang H, Kim IG, Shin DM. Endoplasmic reticulum stress activates transglutaminase 2 leading to protein aggregation. Int J Mol Med. 2014;33:849–855. doi: 10.3892/ijmm.2014.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang J, Lesort M, Guttmann RP, Johnson GV. Modulation of the in situ activity of tissue transglutaminase by calcium and GTP. J Biol Chem. 1998;273:2288–2295. doi: 10.1074/jbc.273.4.2288. [DOI] [PubMed] [Google Scholar]
- 86.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 87.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 88.Neal MD, Sodhi CP, Jia H, Dyer M, Egan CE, Yazji I, Good M, Afrazi A, Marino R, Slagle D, Ma C, Branca MF, Prindle T, Jr, Grant Z, Ozolek J, Hackam DJ. Toll-like receptor 4 is expressed on intestinal stem cells and regulates their proliferation and apoptosis via the p53 up-regulated modulator of apoptosis. J Biol Chem. 2012;287:37296–37308. doi: 10.1074/jbc.M112.375881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bottino R, Balamurugan AN, Bertera S, Pietropaolo M, Trucco M, Piganelli JD. Preservation of human islet cell functional mass by anti-oxidative action of a novel SOD mimic compound. Diabetes. 2002;51:2561–2567. doi: 10.2337/diabetes.51.8.2561. [DOI] [PubMed] [Google Scholar]
- 90.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 92.Hamaguchi K, Gaskins HR, Leiter EH. NIT-1, a pancreatic beta-cell line established from a transgenic NOD/Lt mouse. Diabetes. 1991;40:842–849. doi: 10.2337/diab.40.7.842. [DOI] [PubMed] [Google Scholar]
- 93.van der Torren CR, Zaldumbide A, Roelen DL, Duinkerken G, Brand-Schaaf SH, Peakman M, Czernichow P, Ravassard P, Scharfmann R, Roep BO. Innate and adaptive immunity to human beta cell lines: implications for beta cell therapy. Diabetologia. 2015 doi: 10.1007/s00125-015-3779-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stephens LA, Thomas HE, Kay TW. Protection of NIT-1 pancreatic beta-cells from immune attack by inhibition of NF-kappaB. J Autoimmun. 1997;10:293–298. doi: 10.1006/jaut.1997.0133. [DOI] [PubMed] [Google Scholar]
- 95.Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ron D. Proteotoxicity in the endoplasmic reticulum: lessons from the Akita diabetic mouse. J Clin Invest. 2002;109:443–445. doi: 10.1172/JCI15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nozaki J, Kubota H, Yoshida H, Naitoh M, Goji J, Yoshinaga T, Mori K, Koizumi A, Nagata K. The endoplasmic reticulum stress response is stimulated through the continuous activation of transcription factors ATF6 and XBP1 in Ins2+/Akita pancreatic beta cells. Genes Cells. 2004;9:261–270. doi: 10.1111/j.1356-9597.2004.00721.x. [DOI] [PubMed] [Google Scholar]
- 98.Furuya Y, Lundmo P, Short AD, Gill DL, Isaacs JT. The role of calcium, pH, and cell proliferation in the programmed (apoptotic) death of androgen-independent prostatic cancer cells induced by thapsigargin. Cancer Res. 1994;54:6167–6175. [PubMed] [Google Scholar]
- 99.Kaneko Y, Tsukamoto A. Thapsigargin-induced persistent intracellular calcium pool depletion and apoptosis in human hepatoma cells. Cancer Lett. 1994;79:147–155. doi: 10.1016/0304-3835(94)90253-4. [DOI] [PubMed] [Google Scholar]
- 100.Marre ML, James EA, Piganelli JD. beta cell ER stress and the implications for immunogenicity in type 1 diabetes. Front Cell Dev Biol. 2015;3:67. doi: 10.3389/fcell.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McGinty JW, Marre ML, Bajzik V, Piganelli JD, James EA. T cell epitopes and post-translationally modified epitopes in type 1 diabetes. Curr Diab Rep. 2015;15 doi: 10.1007/s11892-015-0657-7. 90,015-0657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary NOD.scid islets were incubated in fresh media for 1 hr at 37°C. The immunogenicity of the conditioned media and of the islets themselves was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM.
Primary NOD.scid islets or NIT-1 cells were incubated with 2.5 µM Bapta-AM for 1 hr and washed extensively. The cells were then incubated in fresh media for 1 hr at 37°C. This conditioned media was harvested and added to BDC2.5 T cells stimulated with 0.5 µg/ml α-CD3 and 1 µg/ml α-CD28 for 72 hrs. IFNγ secretion was measured by ELISA. Data are mean IFNγ secretion ± SEM.
Recombinant CHgA was incubated with Tgase2 (Tgase2-modified) or control (Ctrl.) for 3 hr at 37°C. The reaction products were analyzed by mass spectrometry to identify sites of deamidation. Residues highlighted in yellow indicate coverage, residues highlighted in green indicate deamidation, and residues in bold underline indicate the sequence of CHgA351–370.
Intact primary islets (3 per well) or NIT-1 cells (3×103 per well) were incubated in 0 mM, 2.8 mM, or 20 mM glucose for 1 hr. Insulin secretion was measured by ELISA. Data are mean insulin secretion ± SEM. *** p < 0.001.
Non-immunogenic NIT-1 cells (2–5×106) were transplanted under the kidney capsule of NOD.scid mice. At onset of hypoglycemia, the mice were sacrificed and the NIT-1 cells were explanted. The NIT-1 cells were counted to determine whether the graft had proliferated during the in vivo incubation.
Cultured NIT-1 cells were incubated in fresh media for 1 hr at 37°C. This conditioned media was harvested and added to BDC2.5 T cells stimulated with 0.5 µg/ml α-CD3 and 1 µg/ml α-CD28 for 72 hrs. IFNγ secretion was measured by ELISA. Data are mean IFNγ secretion ± SEM.
NIT-1 cells (2–5×106) were transplanted under the kidney capsule of NOD.scid mice. After only 2 days (when the mice remained euglycemic), the mice were sacrificed and the cells were explanted for analysis. (A) Cell lysates of cultured NIT-1 cells (NIT-1) or explanted NIT-1 cells (Explant) were analyzed for the phosphorylation of UPR proteins PERK and eIF2α. Data are representative of 3 independent experiments. Densitometry data are phosphorylation levels normalized by β-actin and relative to that in cultured NIT-1 cells. (B) The immunogenicity of cultured NIT-1 cells or NIT-1 cells explanted after 2 days was measured by BDC2.5 T cell assay. Data are mean IFNγ secretion ± SEM.