Abstract
Polycomb repressive complex 2 (PRC2) is an essential regulator of cell physiology. While there have been numerous studies on PRC2 function in somatic tissue development and stem cell control, these have focused on the loss of a single PRC2 subunit. Recent studies, however, have shown that PRC2 subunits may function independently of the PRC2 complex. To investigate the function of PRC2 in the control of skin development, we generated and analysed three conditional knockout mouse lines, in which the essential PRC2 subunits EED, Suz12, or Ezh1/2 are conditionally ablated in the embryonic epidermal progenitors that give rise to the epidermis, hair follicles, and Merkel cells. Our studies showed that the observed loss-of-function phenotypes are shared between the three knockouts, indicating that in the skin epithelium, EED, Suz12, and Ezh1/2 function largely as subunits of the PRC2 complex. Interestingly, the absence of PRC2 results in dramatically different phenotypes across the different skin lineages: premature acquisition of a functional epidermal barrier, formation of ectopic Merkel cells, and defective postnatal development of hair follicles. The strikingly different roles of PRC2 in the formation of three lineages exemplify the complex outcomes that the lack of PRC2 can have in a somatic stem cell system.
Keywords: Polycomb complex, Chromatin, Epigenetics, Epidermis, Skin
Introduction
Polycomb repressive complex (PRC) 2 is a major chromatin repressor (Schwartz and Pirrotta, 2013) that regulates tissue development and fate control, and is implicated in a number of diseases, including cancer (Margueron and Reinberg, 2011; Perdigoto et al., 2014b; Sauvageau and Sauvageau, 2010; Schwartz and Pirrotta, 2008; Surface et al., 2010). PRC2 consists of the core subunits EED, Suz12, and the histone methyltransferases Ezh1 or Ezh2, which catalyse the tri-methylation of lysine 27 of histone H3 (H3K27me3), resulting in gene silencing by chromatin compaction (Margueron et al., 2008; Schwartz and Pirrotta, 2013; Simon and Kingston, 2009).
Recent studies have suggested that PRC2 subunits might have PRC2-independent phenotypes, complicating the assessment of the functional significance of PRC2. Indeed, in prostate cancer, the catalytic subunit Ezh2 mediates the methylation of the androgen receptor to control its activity independently of the other PRC2-subunits (Xu et al., 2012). Similarly, in breast cancer cells, Ezh2 is able to mediate the crosstalk between Wnt signalling and its effectors (Shi et al., 2007), and to activate NF-κB target genes through the formation of a ternary complex, independently of the PRC2 complex (Lee et al., 2011). Furthermore, recent loss-of-function studies in mice have shown that EED is required for proper haematopoiesis during development, while Ezh2 is dispensable (Xie et al., 2014). Thus, despite the vast number of studies reporting the results of the loss of a single PRC2 subunit in stem cells (Aloia et al., 2013), it is still unclear which of the observed phenotypes are indeed due to the loss of PRC2 function.
Ezh1/2 have been shown to play a critical role in skin development by restricting epidermal and Merkel cell differentiation programs and promoting hair follicle cell proliferation (Bardot et al., 2013; Ezhkova et al., 2011; Ezhkova et al., 2009). Since the loss of Ezh1/2 in the skin epithelium displayed strong yet different developmental alterations in these skin lineages (Bardot et al., 2013; Ezhkova et al., 2011; Ezhkova et al., 2009), and considering that Ezh2 can have PRC2-independent roles, we wanted to determine the role of PRC2 in skin development. To this end, we conditionally ablated Ezh1/2, EED, or Suz12 from the embryonic epidermal progenitor cells and performed comparative analysis between the three different mutants to analyse the formation of the epidermis, hair follicles, and Merkel cells. We found that the loss of each subunit resulted in the same phenotypes, with the different mutants being indistinguishable from each other. Furthermore, our analysis revealed that loss of PRC2 resulted in distinct phenotypes for each of the epidermal lineages: premature formation of a functional epidermal barrier, an expansion in the number of Merkel cells, and defective postnatal hair follicle development. Our study illustrates how epigenetic regulation can have dramatically different functions in the development and differentiation of different cell lineages.
Results
Loss of PRC2 leads to premature epidermal barrier formation
In order to conditionally ablate EED and Suz12 in the skin epithelium (EEDcKO and Suz12cKO, respectively), we crossed EED floxed or Suz12 floxed mice with mice expressing Cre recombinase under the control of the Keratin (Krt) 14 promoter, which is active in embryonic epidermal progenitors starting at embryonic day (E) 12 (Bardot et al., 2013; Dassule et al., 2000). As in mice where Ezh1/2 were conditionally ablated in the epidermis (Ezh1/2 2KO) (Bardot et al., 2013; Ezhkova et al., 2011), EED-null and Suz12-null epidermal cells lacked H3K27me3 (Supplementary Figure 1a,b), while dermal cells, which are not targeted by Krt14-Cre ablation, retained this histone mark (Supplementary Figure 1a). We next analysed the formation of the interfollicular epidermis (IFE), the Merkel cells, and the hair follicles in EEDcKO and Suz12cKO mice compared to Ezh1/2 2KO to determine the common phenotypes, as these would likely be caused by a loss of PRC2 function, rather than PRC2-independent roles.
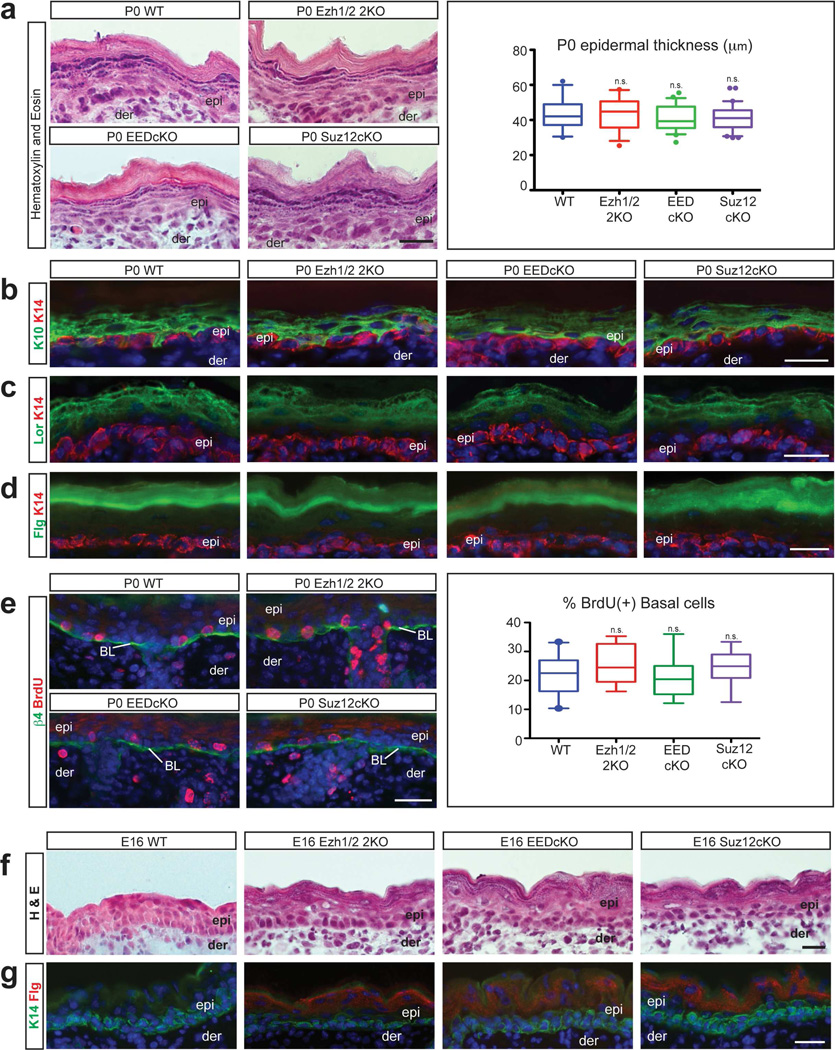
As in Ezh1/2 2KO mice (Ezhkova et al., 2011), formation of the suprabasal epidermal layers was not grossly affected in new-born (P0) EEDcKO or Suz12cKO mice, as shown by histological analysis (Fig. 1a). Immunofluorescence staining with antibodies against epidermal differentiation proteins of spinous (Krt10) (Figure 1b), granular (Loricrin) (Figure 1c), and stratum corneum (Filaggrin) layers (Figure 1d) confirmed proper formation of the different epidermal layers. Importantly, expression of Krt10, Loricrin, and Filaggrin was excluded from the Krt14-expressing basal layer, which contains epidermal progenitor cells (Figure 1b–d). A whole-mount dye-exclusion epidermal barrier assay (Hardman et al., 1998) was performed on new-born EEDcKO and Suz12cKO mice and confirmed the formation of a functional epidermal barrier (Supplementary Figure 1c). Analysis of proliferation and apoptosis in PRC2-null basal and suprabasal cells did not reveal any abnormalities (Figure 1e; Supplementary Figure 1d).
Figure 1. Loss of PRC2 leads to premature epidermis formation.
(a) Hematoxylin and Eosin staining showing similar epidermal structure in P0 WT and PRC2-null mice; quantification of epidermal thickness (n≥2; p=0.6475). (b–d) Immunofluorescence for Krt10 (K10) (b), Loricrin (Lor) (c), and Filaggrin (Flg) (d) showing that the differentiated suprabasal layers are unaffected in P0 PRC2-null mice. (e) Immunofluorescence for BrdU showing no changes in proliferation in the integrin β4 (β4)-labeled basal layer (BL) in P0 PRC2-null mice; quantification of percentage of BrdU(+) cells (n=3; p=0.1460). (f,g) Hematoxylin and Eosin staining (f) and immunofluorescence for Filaggrin (g) showing premature formation of the stratum corneum in E16 PRC2-null mice when compared to WT. epi: epidermis, der: dermis; HF: Hair Follicle. Scale bars: 25µm.
During embryogenesis, Ezh2 represses epidermal differentiation and E16 Ezh2 skin conditional knockout (Ezh2cKO) embryos have premature formation of the stratum corneum (Ezhkova et al., 2009). If Ezh1/2, EED, and Suz12 are all functioning as part of the PRC2 complex to control epidermal barrier formation, we would expect that EEDcKO and Suz12cKO mice would also have premature stratum corneum development. We analysed Suz12cKO and EEDcKO embryos at E16, when WT embryos lack Filaggrin(+) stratum corneum layers. Both histological analysis and immunofluorescence staining for Filaggrin revealed premature acquisition of the stratum corneum in E16 Ezh1/2 2KO, Suz12cKO, and EEDcKO embryos (Figure 1f,g). Taken together, these data indicate that while the cornified layers of the IFE are acquired prematurely in PRC2-null mice, the neonate PRC2-null epidermis is fully functional and indistinguishable from WT epidermis.
Loss of PRC2 results in ectopic formation of Merkel cells
Recent studies have shown that embryonic epidermal progenitors give rise to Merkel cells (Bardot et al., 2013; Morrison et al., 2009; Van Keymeulen et al., 2009), but the mechanisms controlling Merkel cell lineage specification and differentiation are largely unknown. Merkel cell differentiation is a temporally regulated process during which early specified Merkel cells start to express transcription factors essential for Merkel differentiation (Atoh1, Sox2, and Isl1), cytoskeletal proteins (Krt8, Krt18, and Krt20), and components of the synaptic machinery (Rab3c), and undergo innervation by sensory neurons expressing neurofilament NF200 (Haeberle et al., 2004; Owens and Lumpkin, 2014; Perdigoto et al., 2014a; Vielkind et al., 1995).
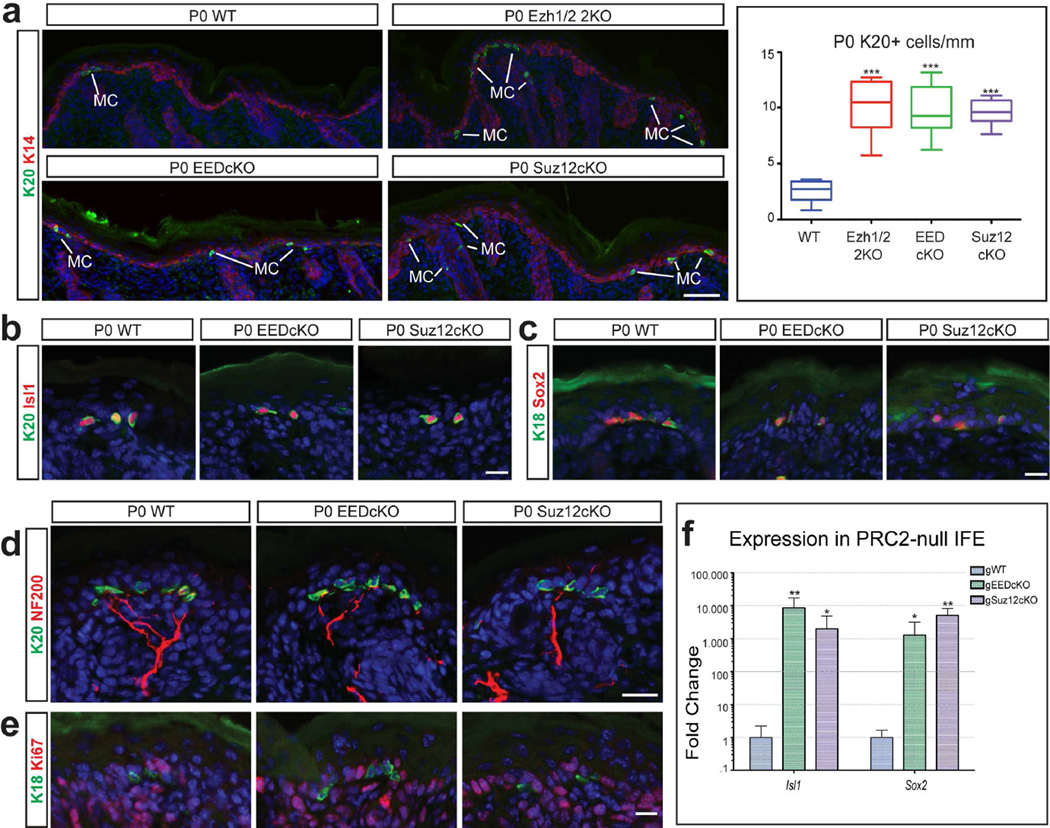
Our previous studies have shown that in P0 Ezh1/2-null skin, there is an increase in the number of Merkel cells due to the derepression of essential Merkel cell-specific genes and differentiation of Ezh1/2-null epidermal progenitors towards the Merkel cell lineage (Bardot et al., 2013). We analysed P0 Suz12-null and EED-null epidermis and observed a similar increase in the number of Merkel cells. Indeed, immunofluorescence analysis with antibodies against Krt20 and Krt18 revealed a significant increase in the number of Krt20(+) (Figure 2a) and Krt18(+) (Supplementary Figure 2a) cells in the back skin of P0 Ezh1/2 2KO, EEDcKO, and Suz12cKO mice compared to WT. An increase in the number of Merkel cells was also observed in other Merkel cell-rich areas, such as the whisker IFE (Supplementary Figure 2d,e) and the paws (Supplementary Figure 2f,g), while no increase in the number of Merkel cells was observed in the whisker follicles (Supplementary Figure 2b,c) of EEDcKO and Suz12cKO mice compared to WT. This is similar to what was previously seen in Ezh1/2 2KO mice (Bardot et al., 2013).
Figure 2. Loss of PRC2 results in Merkel cell expansion.
(a) Immunofluorescence for Krt20 (K20) showing a significant increase in the number of Merkel cells (MC) in P0 PRC2-null epidermis, compared to WT; quantification of number of Merkel cells (n≥3; p<0.0001). (b,c) Immunofluorescence for Merkel cell markers Krt20 (b), Isl1 (b), Krt18 (K18) (c), and Sox2 (c) showing co-expression of markers in PRC2-null Merkel cells. (d) Immunofluorescence for Krt20 and NF200 showing that the PRC2-null Merkel cells are innervated. (e) Ki67 staining showing that Merkel cells are not proliferating in P0 PRC2-null epidermis. (f) RT-qPCR in FACS-purified interfollicular epidermis (IFE) cells showing upregulation of Merkel genes Isl1 and Sox2 in P14 gWT and gPRC-null skin; (mean +/−SD; n=3; all significant, p<0.05). Scale bars: (a): 100µm: (b–e): 25µm.
Characterization of the EED-null and Suz12-null Merkel cells confirmed that they express key Merkel cell regulatory proteins such as Isl1 and Sox2 (Figure 2b,c) and are innervated by NF200(+) sensory neurons (Figure 2d). As with Ezh1/2-null epidermis, the increase in the number of Merkel cells was not due to their aberrant proliferation, as analysis of the proliferation marker Ki67 in P0 WT, EEDcKO, and Suz12cKO mice showed that, as in WT mice, the PRC2-null Merkel cells were Ki67-negative (Figure 2e). Finally, we confirmed that apoptosis was not altered in the Merkel cells of P0 WT, Ezh1/2 2KO, EEDcKO, or Suz12cKO skin (Supplementary Figure 2h).
In Ezh1/2 2KO mice, Merkel cell expansion is due to the derepression of key Merkel cell differentiation genes, Isl1 and Sox2, in epidermal progenitors (Bardot et al., 2013). We purified Suz12-null and EED-null epidermal progenitors by fluorescence-activated cell-sorting (FACS), performed semi-quantitative real-time PCR (RT-qPCR), and confirmed increased transcription of Isl1 and Sox2 in knockout cells (Figure 2f). Therefore, we concluded that PRC2 represses the Merkel cell differentiation program in epidermal progenitors.
Loss of PRC2 leads to defective postnatal development of hair follicles due to decreased proliferation and increased apoptosis
So far, our analysis has revealed that the loss of PRC2 from embryonic epidermal progenitors leads to premature epidermal development and ectopic formation of Merkel cells. During development, embryonic epidermal progenitors also give rise to hair follicles. Interestingly, and in contrast to the epidermal and Merkel cell lineage phenotypes, the hair follicles of Ezh1/2 2KO mice never reached their full length (Ezhkova et al., 2011). This striking difference between the roles of PRC2 in control of the epidermal/Merkel cell lineages and the hair follicle lineage led us to question whether defective formation of Ezh1/2-null hair follicles is indeed due to the loss of PRC2 or PRC2-independent functions of Ezh1/2. Therefore, we analysed hair follicle development in Suz12-null and EED-null skins at a time point when WT hair follicles are fully formed.
In P0 mice, when hair follicle formation is still ongoing, no alterations in hair follicle formation were observed in Ezh1/2 2KO, EEDcKO, and Suz12cKO mice compared to WT (Supplementary Figure 3a). Like Ezh1/2 2KO (Ezhkova et al., 2011), EEDcKO and Suz12cKO mice were born alive, but were unable to eat and died soon after birth. To analyse hair follicle development, which is completed postnatally, we performed full-thickness grafting of P0 skins onto immuno-compromised Nude mice (Supplementary Figure 3b), as was done for the analysis of Ezh1/2-null hair follicles (Ezhkova et al., 2011). Whenever possible, male donor skins were grafted onto female Nude hosts, and fluorescence in situ hybridisation for the Y-chromosome was used to detect the grafted male donor skins (Supplementary Figure 3c), as previously described (Ezhkova et al., 2011; Nowak et al., 2008).
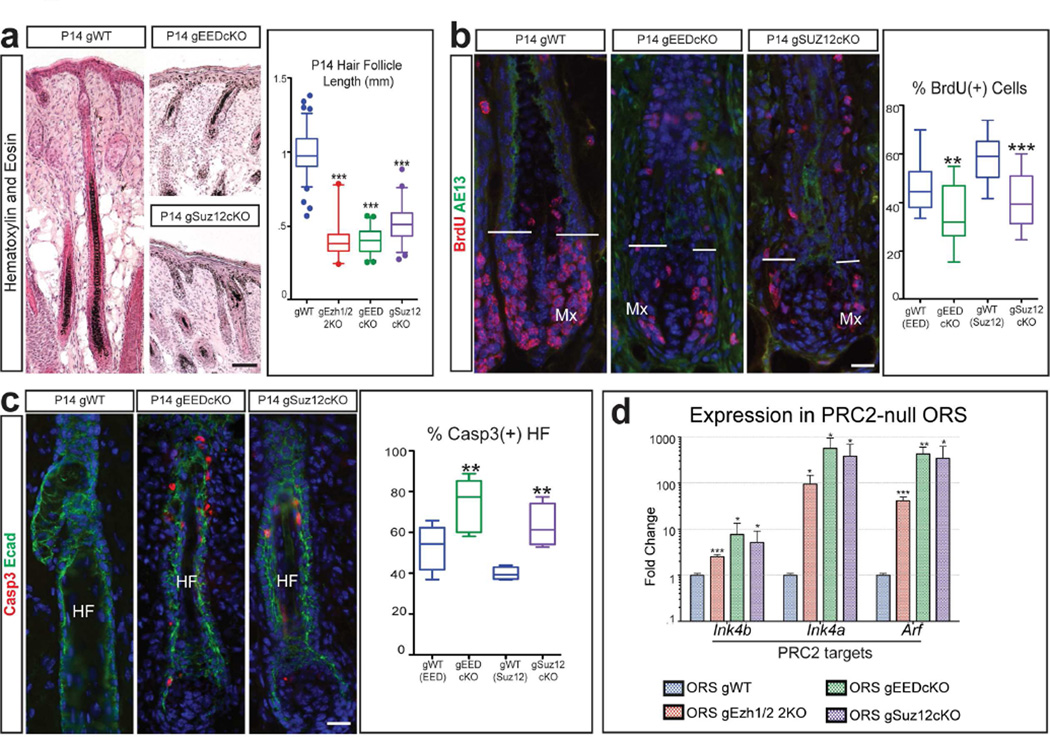
The hair follicles in grafted Suz12cKO and EEDcKO (gSuz12cKO and gEEDcKO) skin were significantly shorter than those in grafted WT (gWT) skin and lacked hair shafts 14 days post-grafting (P14) (Figure 3a). Incomplete formation of the gSuz12-null and gEED-null hair follicles was confirmed by immunofluorescence analysis of Krt6 protein, which is expressed in the differentiated layers of the hair follicle. While in both grafted gPRC2-null and gWT skin the hair follicles contained the early, Krt6(+), differentiated companion layer, the terminally differentiated medulla layer located at the centre of the hair shaft was missing in the PRC2-null follicles (Supplementary Figure 3d). Additional characterization revealed that PRC2-null hair follicles were not cycling properly. By analysing a later time point after grafting (P56), we observed that the gWT hair follicles had undergone one hair cycle, as shown by the presence of club hairs (Supplementary Figure 3g). In contrast, gEED-null hair follicles were arrested, and lacked club hairs (Supplementary Figure 3g), as was previously observed in the Ezh1/2 2KO (Ezhkova et al., 2011). These data further confirm the inability of PRC2-null hair follicles to fully develop.
Figure 3. Loss of PRC2 causes reduced proliferation and increased apoptosis in hair follicles.
(a) Hematoxylin and Eosin showing that P14 gPRC2-null mice have shorter hair follicles (HF) than gWT; quantification of HF length (n≥2; p<0.0001). (b) BrdU staining showing reduced proliferation in the matrix (Mx) of P14 gPRC2-null HFs; quantification of percentage of BrdU(+) matrix cells (n=3; gEEDcKO, p=0.0083; gSuz12cKO, p<0.0001). AE13 marks matrix limit. (c) Activated Caspase 3 (Casp3) staining showing increased apoptosis in P14 gPRC2-null HFs, labelled with E-Cadherin (Ecad); quantification of percentage of Casp3(+) HFs (n≥2; gEEDcKO, p=0.0055; gSuz12cKO, p=0.0055). (d) RT-qPCR of FACS-purified outer root sheath (ORS) cells showing p15(INK4B), p16(INK4A), and p19(ARF) upregulation in P14 gPRC2-null mice (mean +/− SD; n=3; all significant, p<0.05). Scale bars: (a): 100µm; (b,c): 25µm.
To determine the mechanisms involved in the arrested postnatal hair follicle phenotype, we first sought to analyse whether there were defects in the hair follicle stem cells. During normal postnatal development, hair follicle stem cells express critical transcriptional regulators Sox9 and Lhx2 (Blanpain and Fuchs, 2009; Mardaryev et al., 2011; Nowak et al., 2008; Rhee et al., 2006; Vidal et al., 2005). Immunofluorescence analysis revealed that both gWT and gPRC2-null hair follicles contained Sox9(+) and Lhx2(+) cells (Supplementary Figure 3e,f), suggesting that the hair follicle stem cells are not lost in the P14 gPRC2-null skin. We next analysed the proliferation and apoptosis in hair follicle cells. Analysis of gSuz12-null and gEED-null hair follicles compared to the corresponding WT follicles revealed a drastic reduction in the percentage of BrdU(+) cells in the matrix, the lower portion of the hair follicle, which typically contains highly proliferative cells (Figure 3b). Additionally, we observed an increased percentage of Activated Caspase 3(+) hair follicles in gSuz12-null and gEED-null back skin (Figure 3c), as was previously observed in the gEzh1/2-null skin (Ezhkova et al., 2011). We concluded that the defective formation of PRC2-null hair follicles is due to decreased proliferation and increased apoptosis.
RT-qPCR analyses of RNAs purified from FACS-isolated P14 gWT and gPRC-null hair follicle progenitors, localized in the outer root sheath, revealed strong upregulation of the INK4A/ARF/INK4B locus in knockout hair follicles (Figure 3d). This locus encodes the critical G1-S cell cycle inhibitors p15 (INK4b) and p16 (INK4a), and the apoptosis regulator p19 (ARF) (Sherr, 2012); it is also a direct target of PRC2 repression (Bracken et al., 2007; Chen et al., 2009). Immunofluorescence staining for p19 confirmed its expression in gEED-null and gSuz12-null hair follicles (Supplementary Figure 3h). Importantly, these findings are consistent with the previously identified role for this locus in inducing defective proliferation and apoptosis in gEzh1/2-null hair follicles (Ezhkova et al., 2011). In this study, proliferation defects in Ezh1/2-null hair follicle progenitor cells could be rescued, in vitro, by knocking down the INK4A/ARF/INK4B locus, suggesting that the derepression of this locus was responsible for the defective proliferation in vivo (Ezhkova et al., 2011). We therefore concluded that PRC2 is required for proper hair follicle development due to its regulation of proliferation and apoptosis in the developing hair follicles. This regulation is carried out, at least in part, by repression of the INK4A/ARF/INK4B locus.
Discussion
While PRC2 was first identified several decades ago, the role of this complex in the regulation of stem cell fate and differentiation of somatic tissues in vivo is still not well understood. Understanding how this complex functions in stem cells in vivo is of paramount importance, as a wide variety of human genomic studies have revealed the importance of the Polycomb proteins for different human diseases (Perdigoto et al., 2014b; Sauvageau and Sauvageau, 2010).
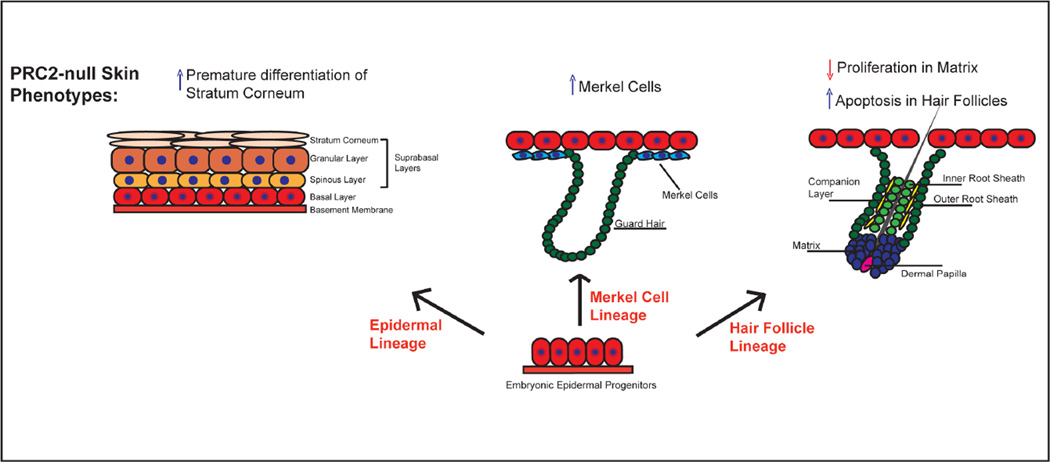
To define the role of PRC2 in the skin epithelium, we provided a systematic study comparing the function of all core PRC2 subunits in a mammalian somatic stem cell system. Our analysis revealed that the absence of any of the PRC2 components resulted in a complete loss of H3K27me3 in the epidermis. This indicates that all core PRC2 subunits are essential for the establishment of this histone mark and is in accordance with previous observations in embryonic stem (ES) cells (Leeb et al., 2010; Pasini et al., 2007). Our data further show that the conditional knockouts for any core PRC2 subunits are indistinguishable from each other, indicating that EED, Suz12, and Ezh1/2 all function as PRC2 subunits in the skin epithelium. Finally, we show that the loss of PRC2 results in strikingly different outcomes across different skin lineages: premature acquisition of epidermal stratum corneum, ectopic Merkel cell formation, and defective postnatal hair follicle development (Figure 4). Additionally, while the epidermal and Merkel cell lineage phenotypes are evident during embryogenesis and at birth, alterations in hair follicle development are observed postnatally, showing a difference in the timing of phenotype onset. The drastically different roles of PRC2 in the formation of the three lineages exemplify the complex outcomes that the lack of PRC2 can have in any given stem cell system.
Figure 4. Loss of PRC2 results in three distinct skin phenotypes.
Schematic diagram showing how loss of PRC2 affects the development of different skin lineages. Embryonic epidermal progenitors give rise to the epidermis, Merkel cells, and hair follicles. In mice in which either Ezh1/2, EED, or Suz12 are deleted from the epidermal progenitors, there is premature formation of the stratum corneum, an increase in the number of Merkel cells, and defective postnatal hair follicle development due to decreased matrix proliferation and increased apoptosis of the hair follicles.
While ablation of the core PRC2 subunits in ES cells does not compromise their ability to self-renew (Chamberlain et al., 2008; Leeb et al., 2010; Pasini et al., 2007), the vast majority of in vivo phenotypes resulting from the lack of PRC2 subunits in somatic stem cells are associated with inhibited proliferation. For example, conditional ablation of Ezh2 from embryonic cardiomyocytes results in lethal congenital heart malformations due to cardiac hypoplasia (He et al., 2012). Similarly, in hepatic progenitors, islet β-cells, haematopoietic stem cells, and astrocytes in the subventricular zone, loss of Polycomb repression results in decreased proliferation and an inability of the somatic stem cells to self-renew (Aoki et al., 2010; Chen et al., 2009; Hidalgo et al., 2012; Hwang et al., 2014). In all cases, these in vivo phenotypes are associated with the activation of the INK4A/ARF/INK4B locus, which triggers cell death and apoptosis in the PRC2-null cells. Our transcriptional profiling of FACS-purified cells from PRC2-null mice revealed upregulation of the cell cycle inhibitor INK4A/ARF/INK4B locus in the hair follicle progenitors, which resulted in cell cycle arrest and apoptosis. These data underline the importance of PRC2 in proper tissue homeostasis as a regulator of proliferation and apoptosis via the repression of the INK4A/ARF/INK4B locus. Importantly, alterations of this locus are a common cytogenic alteration in human cancers, while its upregulation has been associated with aging (Kim and Sharpless, 2006). Therefore, it will be critical to better understand how PRC2 regulates the INK4A/ARF/INK4B locus in somatic stem cells.
Additionally, transcriptional profiling of PRC2-null epidermal cells revealed upregulation of key Merkel cell signature genes Isl1 and Sox2. These Merkel cell signature genes and the INK4A/ARF/INK4B locus are normal targets of PRC2 repression in wild type cells. However, the Merkel cell and the hair follicle phenotypes become evident at different developmental time points. It will be very interesting to further understand how the different cell signalling events and transcriptional programs specific to each lineage interact with PRC2-dependent regulation of gene repression to ensure proper cell fate specification during development.
Not only does PRC2 have essential functions in stem cells and during development, but alterations in PRC2 function have been found in multiple types of cancer (Perdigoto et al., 2014b; Sauvageau and Sauvageau, 2010). Understanding how PRC2 functions in the different systems and how a complex that regulates expression of a wide number of genes can be misregulated in different cancers will likely be extremely important to further understanding tumorigenesis and for the development of novel therapeutic approaches.
Materials & Methods
Mice
All mice were housed and cared for according to MSSM and IACUC approved protocols. At least two animals were used for each analysis. Ezh1/2 2KO mice were previously reported (Ezhkova et al., 2011). Eed floxed and Suz12 floxed mice were generously provided by Weipeng Mu and Terry Magnuson (Mu et al., 2014). As previously described with Ezh1/2 2KO mice, mice null for EED or Suz12 die shortly after birth, and all analysis of these mice after P0 was performed on grafted skin obtained using the previously described full-thickness grafting protocol (Ezhkova et al., 2011). Krt14-Cre and immuno-compromised Nude mice were obtained from The Jackson Laboratories. Mice were genotyped by PCR using DNA extracted from tail skin. BrdU was administered as previously reported (Ezhkova et al., 2009). Briefly, BrdU was administered (50µg BrdU per 1g mouse weight) to mice 3–5 h before sacrificing.
Immunofluorescence and Y-chromosome florescence in situ hybridisation
Tissues were collected from mice, embedded fresh into OCT, and subsequently cut into 5µm or 10µm sections. Slides were then fixed for 10 min in 4% PFA and blocked for 1 h or overnight in PBS-Triton with BSA/NGS/NDS. Primary antibodies were diluted in blocking solution and incubations were carried out for 1 h or overnight, followed by incubation in secondary antibodies for 1 h at room temperature. Slides were then counterstained with DAPI and mounted using anti-fade mounting media. Y-chromosome florescence in situ hybridisation (FISH) analysis was performed as previously described (Ezhkova et al., 2011; Nowak et al., 2008) on OCT sections using a Cy3 Star*FISH detection kit (Cambio).
Barrier Assay
Whole-mount dye-exclusion epidermal barrier assay was performed as described (Ezhkova et al., 2009; Hardman et al., 1998). Briefly, unfixed, untreated, freshly isolated P0 mice were immersed in standard X-gal solution (1 mg/ml Xgal substrates in PBS with 100mM NaPO4 1.3 mM MgCl2, 3 mM K3Fe(CN)6, and 3 mM K4Fe(CN)6; pH adjusted to 4.5) overnight, rotating, at 37°C.
Microscopy and Quantification
Slides were imaged using a Leica DM5500 upright microscope and either 10×, 20×, or 40× objectives or a Zeiss Axioplan2 microscope and 40× or 63× objectives. For each analysis, at least 2 mice per genotype were used for quantification (n≥2). Fluorescence intensity was calculated from at least three raw, single-channel greyscale images per condition using Leica LAS AF software. Fluorescence intensity was normalized to non-nuclear background. Quantifications of epidermal thickness were measured from the bottom of the basal layer to the top of the stratum corneum using the Leica LAS AF software. Quantifications of Merkel cells per mm of skin were performed as described (Bardot et al., 2013). Quantifications of hair follicle length were measured using the Leica LAS AF software; the numbers of hair follicles quantified were as follows: WT=91; Ezh1/2 2KO=24; EEDcKO=46; Suz12cKO=56. BrdU cells where quantified using Leica LAS AF software or ImageJ; nuclear DAPI staining was used to quantify total number of cells and the % of BrdU(+) cells was quantified. For the quantification of BrdU(+) matrix cells, the numbers of hair follicles quantified were as follows: EED WT=16; EEDcKO=17; Suz12 WT=19; Suz12cKO=18. The quantification of Activated Caspase 3(+) and (−) HFs was performed using a Leica DM5500 upright slide microscope and positive HFs were presented as a percentage of the total counted HFs; the numbers of hair follicles quantified were as follows: EED WT=466; EEDcKO=1052; Suz12 WT=454; Suz12cKO=395.
Fluorescence Activated Cell Sorting Analysis
FACS analysis was performed as previously described (Ezhkova et al., 2011). Briefly, for gWT, gEzh1/2 2KO, gEEDcKO, and gSuz12cKO analysis, grafts were collected and subjected to 0.25% collagenase digestion for 90 min at 37 °C, followed by 0.25% trypsin for 14 min at 37 °C with moderate shaking. Cell suspension was passed through a cell strainer and 2 million cells were then stained with Ephrin B1-Biotin-SAV-PE (1:20), EpCAM-APC (1:200), α6-integrin-FITC (1:100) and Sca1-PreP-Cy5.5 (1:200). IFE was sorted as EpCAM(+), Sca1(+), α6-integrin(+); ORS was sorted as EpCAM(+), Sca1(−), α6-integrin(+), Ephrin(−). The dead cell population was excluded by DAPI staining. Sorting was performed on DB-FACS Aria II at 35 psi with a 100 µm nozzle, and 50,000 cells where collected directly into RLT+ buffer (Qiagen) with β-mercaptoethanol.
RNA Purification and RT-qPCR
Sorted cells were lysed in RLT+ buffer (Qiagen) with β-mercaptoethanol, and RNA was isolated using the Qiagen RNeasy Mini kit with DNaseI treatment. Reverse transcription was performed using qScript (Quanta) Superscript Supermix. All RT-qPCR was performed using Roche SYBR green reagents and a Lightcycler480 machine. All primers are listed in Supplementary Table 1.
Statistics
In RT-qPCR column bar graphs, mean value ± standard deviation is presented. Comparisons were made between three cKO and at least three Cre(−) WT siblings, using the Student’s t-test (GraphPad Prism 5). Box-and-whisker plots show first to third quartiles around the median, with whiskers showing 5%−95% range and outliers presented as individual data points. All quantifications were performed on multiple cell populations from at least 2 different animals (n≥2). To determine the significance between WT and EEDcKO or WT and Suz12cKO in the quantifications of BrdU(+) matrix cells (Figure 3b) and Caspase 3(+) hair follicles (Figure 3b), comparisons were made using the Student’s t-test (GraphPad Prism 5). To determine the significance between groups in all other quantification experiments (as indicated in the figures by parentheses), comparisons were made using One-way ANOVA with Bonferroni Correction (GraphPad Prism 5). For all statistical tests, the p<0.05 level of confidence was accepted for statistical significance.
Antibodies
Antibodies were used as follows: Krt14 (generous gift of Dr. Segre, National Human Genome Research Institute, MD, USA, 1:20,000); H3 (abcam, ab1791); H3K27me3 (Millipore, 07-449, 1:300); Krt18 (abcam, ab668, 1:100); Krt20 (Dako, M7019, 1:70); Sox2 (Stemgent, 09-0024, 1:150); Isl1 (abcam, ab109517, 1:250); NF200 (abcam, ab8135, 1:1000); Krt6 (generous gift of Dr. Fuchs, The Rockefeller University, NY, USA, 1:250); Lhx2 (generous gift of Dr. Fuchs, 1:5000); Sox9 (generous gift of Dr. Fuchs, 1:1000); BrdU (abcam, ab6326, 1:250; abcam, ab1893, 1:250); AcCasp3 (R&D, AF835, 1:250); Krt10 (Covance, PRB-159P, 1:500); Loricrin (Covance, PRB-145P, 1:250); Filaggrin (generous gift of Dr. Segre, 1:500); Integrin β4/ CD104 (BD Biosciences, 553745, 1:500); Ki67 (Novocastra, NCL-L-Ki67-MM1, 1:250); AE13 (Abcam, ab16113, 1:100); E-Cadherin (Invitrogen, 131900, 1/2000); p19/Arf (Abcam, Ab80, 1/200). For IF, secondary Abs coupled to FITC, Alexa488, 549, 649, RRX, or Cy5 were from Jackson Laboratories (1:1000). For FACS: anti-mEphrin-B1 (BAF473 R&D), FITC-Streptavidin (554061 BD), Ep-CAM-APC (118214, BioLegend), Ly-A6 Sca1-Cy5.5 (45-5981-82, eBioscience), CD49f-α6 integrin PE (11-0495-82, eBioscience). For Western blot, TrueBlot Anti-Rabbit IgG HRP (Rockland, 18-8816-33, 1:10,000) was used as a secondary Ab.
Supplementary Material
Acknowledgments
For help, advice, critical suggestions, experimental input, and reagents, we would like to thank Evan Bardot, Jose Silva, Julie Segre, and Elaine Fuchs. We would like to thank Weipeng Mu and Terry Magnuson for their generous gift of the Suz12 floxed and EED floxed mice (Mu et al., 2014). The Ezh1 mutant mice were generated at the Research Institute of Molecular Pathology (IMP, Vienna) by Donal O'Carroll (laboratory of Thomas Jenuwein) with the help of Maria Sibilia (laboratory of Erwin Wagner). We would also like to thank Alexander Tarakhovsky for previously providing us with Ezh2 floxed mice. Microscopy was performed at the Microscopy CORE at the Icahn School of Medicine at Mount Sinai. Katherine L. Dauber is a trainee of the NIDCR-Interdisciplinary Training Program in Systems and Developmental Biology and Birth Defects T32HD075735. Carolina N. Perdigoto was supported by EMBO fellowship ALTF 552-2012. Victor J. Valdes is a Pew Latin American Fellow in the Biomedical Sciences, supported by The Pew Charitable Trusts. Elena Ezhkova is an Ellison Medical Foundation New Scholar in Aging. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01 AR063724 and an NIH/NIAMS K99/R00 Pathway to Independence Award (R00AR057817). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by a New York State Stem Cell Science (NYSTEM) IDEA grant through New York State Department of Health (N11G-152).
Abbreviations
- IFE
Interfollicular epidermis
- Krt
Keratin
- PRC
Polycomb repressive complex
- H3K27me3
tri-methylation on lysine 27 of histone H3
- FACS
fluorescence-activated cell-sorting
- RT-qPCR
semi-quantitative real-time PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors state no conflict of interest.
Author Contributions
K.L.D., C.N.P., and E.E. designed the study. K.L.D., C.N.P., V.J.V., F.J.S., and I.C. performed the experiments. K.L.D., C.N.P., V.J.V., F.J.S., I.C., and E.E. analysed the data. K.L.D., C.N.P., and E.E. wrote the manuscript with input from all other authors.
References
- Aloia L, Di Stefano B, Di Croce L. Polycomb complexes in stem cells and embryonic development. Development. 2013;140:2525–2534. doi: 10.1242/dev.091553. [DOI] [PubMed] [Google Scholar]
- Aoki R, Chiba T, Miyagi S, Negishi M, Konuma T, Taniguchi H, et al. The polycomb group gene product Ezh2 regulates proliferation and differentiation of murine hepatic stem/progenitor cells. J Hepatol. 2010;52:854–863. doi: 10.1016/j.jhep.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Bardot ES, Valdes VJ, Zhang J, Perdigoto CN, Nicolis S, Hearn SA, et al. Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells. Embo J. 2013;32:1990–2000. doi: 10.1038/emboj.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature reviews Molecular cell biology. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev. 2007;21:525–530. doi: 10.1101/gad.415507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SJ, Yee D, Magnuson T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008;26:1496–1505. doi: 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Gu X, Su IH, Bottino R, Contreras JL, Tarakhovsky A, et al. Polycomb protein Ezh2 regulates pancreatic beta-cell Ink4a/Arf expression and regeneration in diabetes mellitus. Genes Dev. 2009;23:975–985. doi: 10.1101/gad.1742509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Ezhkova E, Lien WH, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Pasolli HA, Parker JS, Stokes N, Su IH, Hannon G, et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell. 2009;136:1122–1135. doi: 10.1016/j.cell.2008.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, et al. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci U S A. 2004;101:14503–14508. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman MJ, Sisi P, Banbury DN, Byrne C. Patterned acquisition of skin barrier function during development. Development. 1998;125:1541–1552. doi: 10.1242/dev.125.8.1541. [DOI] [PubMed] [Google Scholar]
- He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, et al. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ Res. 2012;110:406–415. doi: 10.1161/CIRCRESAHA.111.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo I, Herrera-Merchan A, Ligos JM, Carramolino L, Nunez J, Martinez F, et al. Ezh1 is required for hematopoietic stem cell maintenance and prevents senescence-like cell cycle arrest. Cell Stem Cell. 2012;11:649–662. doi: 10.1016/j.stem.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Hwang WW, Salinas RD, Siu JJ, Kelley KW, Delgado RN, Paredes MF, et al. Distinct and separable roles for EZH2 in neurogenic astroglia. Elife. 2014;3:e02439. doi: 10.7554/eLife.02439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Lee ST, Li Z, Wu Z, Aau M, Guan P, Karuturi RK, et al. Context-specific regulation of NF-kappaB target gene expression by EZH2 in breast cancers. Mol Cell. 2011;43:798–810. doi: 10.1016/j.molcel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Leeb M, Pasini D, Novatchkova M, Jaritz M, Helin K, Wutz A. Polycomb complexes act redundantly to repress genomic repeats and genes. Genes Dev. 2010;24:265–276. doi: 10.1101/gad.544410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardaryev AN, Meier N, Poterlowicz K, Sharov AA, Sharova TY, Ahmed MI, et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development. 2011;138:4843–4852. doi: 10.1242/dev.070284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, et al. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Dev Biol. 2009;336:76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu W, Starmer J, Fedoriw AM, Yee D, Magnuson T. Repression of the soma-specific transcriptome by Polycomb-repressive complex 2 promotes male germ cell development. Genes Dev. 2014;28:2056–2069. doi: 10.1101/gad.246124.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DM, Lumpkin EA. Diversification and specialization of touch receptors in skin. Cold Spring Harb Perspect Med. 2014;4 doi: 10.1101/cshperspect.a013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Mol Cell Biol. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigoto CN, Bardot ES, Valdes VJ, Santoriello FJ, Ezhkova E. Embryonic maturation of epidermal Merkel cells is controlled by a redundant transcription factor network. Development. 2014a;141:4690–4696. doi: 10.1242/dev.112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdigoto CN, Valdes VJ, Bardot ES, Ezhkova E. Epigenetic regulation of epidermal differentiation. Cold Spring Harb Perspect Med. 2014b;4 doi: 10.1101/cshperspect.a015263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H, Polak L, Fuchs E. Lhx2 maintains stem cell character in hair follicles. Science. 2006;312:1946–1949. doi: 10.1126/science.1128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7:299–313. doi: 10.1016/j.stem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. Polycomb complexes and epigenetic states. Curr Opin Cell Biol. 2008;20:266–273. doi: 10.1016/j.ceb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Schwartz YB, Pirrotta V. A new world of Polycombs: unexpected partnerships and emerging functions. Nat Rev Genet. 2013;14:853–864. doi: 10.1038/nrg3603. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Ink4-Arf locus in cancer and aging. Wiley Interdiscip Rev Dev Biol. 2012;1:731–741. doi: 10.1002/wdev.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, et al. Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol. 2007;27:5105–5119. doi: 10.1128/MCB.00162-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nature reviews Molecular cell biology. 2009;10:697–708. doi: 10.1038/nrm2763. [DOI] [PubMed] [Google Scholar]
- Surface LE, Thornton SR, Boyer LA. Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell. 2010;7:288–298. doi: 10.1016/j.stem.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol. 2009;187:91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, Lutzkendorf S, Cotsarelis G, Mill P, Hui CC, et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol. 2005;15:1340–1351. doi: 10.1016/j.cub.2005.06.064. [DOI] [PubMed] [Google Scholar]
- Vielkind U, Sebzda MK, Gibson IR, Hardy MH. Dynamics of Merkel cell patterns in developing hair follicles in the dorsal skin of mice, demonstrated by a monoclonal antibody to mouse keratin 8. Acta anatomica. 1995;152:93–109. doi: 10.1159/000147688. [DOI] [PubMed] [Google Scholar]
- Xie H, Xu J, Hsu JH, Nguyen M, Fujiwara Y, Peng C, et al. Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental-stage-specific manner. Cell Stem Cell. 2014;14:68–80. doi: 10.1016/j.stem.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 Oncogenic Activity in Castration-Resistant Prostate Cancer Cells Is Polycomb-Independent. Science. 2012;338:1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.