Abstract
Acute myelopathy is increasingly being recognized as a common neurological complication of schistosomiasis. Schistosome eggs reach the spinal cord either as egg emboli or as eggs produced by ectopic worms. This leads to inflammatory reaction and granuloma formation around the eggs. Patients with spinal schistosomiasis may not have clinical evidence of schistosomiasis. The typical clinical picture is that of lumbar pain preceded by other symptoms by hours or up to 3 weeks. Patients may present with paraparesis, urinary retention or paraplegia. Definitive diagnosis of spinal cord schistosomiasis is by detection of the eggs in a spinal cord biopsy or at autopsy. However, most cases are diagnosed based on a presumptive diagnosis that depends on a suggestive clinical picture, history or evidence of active schistosomiasis and exclusion of other conditions. Investigations include stools and urine examination for schistosome eggs, blood tests, magnetic resonance imaging (MRI) and examination of the cerebrospinal fluid. Treatment of cases is mainly by praziquantel, corticosteroids, surgical intervention and rehabilitation.
Keywords: Schistosomiasis, Spinal cord, Paralysis, Urinary retention, Diagnosis, Treatment
Introduction
Acute myelopathy is increasingly being recognized as a common neurological complication of schistosomiasis. One half of the non-traumatic spinal cord injury patients consecutively admitted to a rehabilitation hospital in Malawi were presumed to be cases of schistosomal myelopathy[1]. In Brazil, 5.6% of cases presenting with an inflammatory myelopathy were due to Schistosoma mansoni infection [2]. It has been recommended that neuroschistosomiasis should be included in the differential diagnosis of acute childhood paraplegia in areas where infection by Schistosoma mansoni is endemic [3–6].
Adult schistosome worms inhabit the portal veins where the female worms lay large numbers of eggs. The prevalence of oviposition in the spinal cord varies among studies, ranging from 0.3% to 13% [3]. In spinal schistosomiasis, eggs may reach the spinal veins via the valveless venous plexus of Batson, which connects the deep iliac veins and inferior vena cava with the veins of the spinal cord. Schistosoma ova may gain entrance into the veins when the intraabdominal pressure rises, e.g. during defecation or coughing. This could explain the greater incidence of myelopathy in lumbosacral regions. Schistosome eggs have been described in foci of large quantities in the spinal cord and this has been attributed to ectopic migration of adult worms through leptomeningeal veins.
Diagnosis
Definitive Diagnosis
Conclusive diagnosis of spinal cord schistosomiasis is only possible with a histopathological examination of biopsy showing Schistosoma eggs (Figure 1). However, this procedure should generally be avoided because of the risk of additional damage to the injured nervous tissue.
Figure 1.
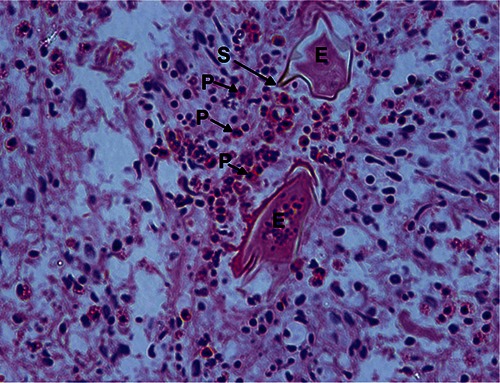
Spinal cord biopsy in a case of spinal schistosomiasis. Note the schistosome eggs in section (E), with a lateral spine (S) characteristic of Schistosoma mansoni. There is cellular infiltration with eosinophils (P).
Presumptive diagnosis
Most cases are treated for spinal schistosomiasis based on a presumptive diagnosis which is based on three parameters (Figure 2) [7]:
Figure 2.
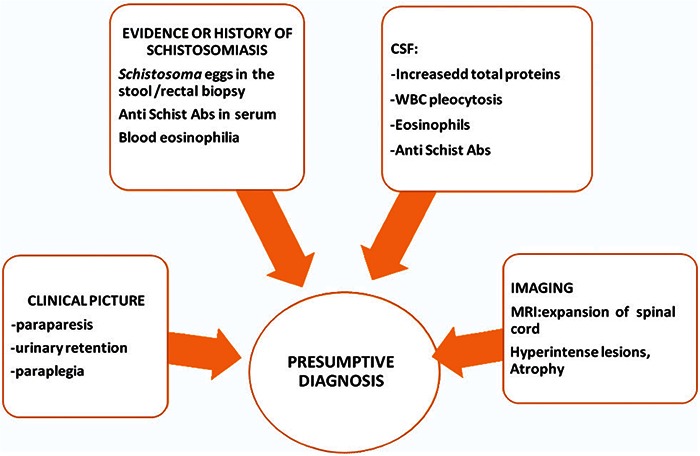
Presumptive diagnosis of spinal cord schistosomiasis.
Abs - antibodies, CSF - cerebrospinal fluid, MRI - magnetic resonance imaging, WBC - white blood cells.
-
Clinical Picture
Patients with acute schistosomal myelopathy may not have clinical evidence of systemic schistosomiasis. This has been reported in travellers to endemic areas [8]. Spinal cord schistosomiasis, however, typically presents as an acute/subacute low cord syndrome generally associated with the involvement of the cauda equina roots. The diagnosis is largely based on clinical evidence. Typically, lumbar pain precedes other symptoms by hours or up to 3 weeks. Patients may present with paraparesis, urinary retention or paraplegia. The clinical picture is characterized by:- the low localization in the spinal cord,
- acute/subacute progression of the symptoms
- presence of manifestations due to both medullary and radicular (most frequently the cauda equina roots)
-
History or evidence of active schistosomiasis
-
▪
Stools and Urine examination:
Stools testing fails to demonstrate S. mansoni eggs in 60% of the cases, even when three or more samples are examined on different days. The rectal biopsy has a higher sensitivity allowing the identification of the eggs considered the procedure of choice for establishing the diagnosis of active schistosomal infection. However, in endemic areas the finding of eggs in stool or a positive serology provide supportive but not direct evidence of schistosomal myelopathy. These patients should be seen as having probable neuroschistosomiasis.
-
▪
Blood Examination:
In endemic areas the serological diagnosis of schistosomiasis is limited by cross-reaction with other antigens (the difficulty in distinguishing between active and previous infection due to the persistence of some antibodies for long periods of time even after successful treatment of the infection). Serologic tests may be more helpful for diagnosis of schistosomiasis in travellers. Blood eosinophilia is also a suggestive, but nonspecific, finding which is not observed in all cases.
-
▪
-
Exclusion of Other Causes
-
▪
Magnetic resonance imaging (MRI):
MRI demonstrates inflammatory myelopathy in virtually all patients with spinal cord schistosomiasis [9,10]. The most frequent findings are enlargement of the conus medullaris and thickening of the cauda equina roots with a heterogeneous pattern of contrast enhancement. Typically there is edema of the spinal cord, conus medullaris and cauda equina. Intramedullary schistosomal granuloma may show moderate expansion of the distal cord, isointense relative to the cord, a heterogeneous hyperintense lesion with an unclear boundary, or multiple patchy nodular lesions resembling a string of beads mainly in the ventral spinal cord which may be significantly enhanced. Atrophy of the spinal cord may also be found in longstanding cases.
-
▪
Cerebrospinal fluid examination (CSF)
CSF generally shows a non-specific inflammatory pattern with mild/moderate increase in both total protein concentration and cell count. Most cells are mononuclear cells, whereas eosinophils can be found in <50%. There are newer tests being evaluated to detect antischistosomal antibodies in CSF [11].Schistosoma ova have never been detected in the CSF of cases. CSF glucose levels are usually normal.
-
▪
A system of classification that does not take into account CSF alterations nor image studies for the probability of spinal schistosomiasis is as follows [12]:
POSSIBLE: low thoracic or lumbar/ sacral spinal cord compromise + positive epidemiology for schistosomiasis;
PROBABLE : when besides the aforementioned criteria there are demonstration of schistosomal infection by parasitological methods and exclusion of other possible causes;
CONFIRMED: histopathological evidence of the CNS demonstrating the presence of Schistosoma ova or worm.
Treatment
There is no consensus and no randomized clinical trials regarding therapy in neuroschistosomiasis. Schistosomicidal drugs, steroids and surgery are currently the available treatments for neuroschistosomiasis.
Praziquatel (PZQ)
Praziquantel is a safe and effective chemotherapy against all species of schistosomes [13]. Observations in animal models suggest that PZQ kills most S. mansoni eggs in host tissues when administered in higher doses than are routinely recommended for treatment of intestinal schistosoma mansoni infestation. In order to reduce the lifespan of metabolically active eggs in sensitive tissues, prolonged courses of PZQ could be used when treating central nervous system schistosomiasis [14]. It has been successfully used in the treatment of schistosomal myelopathy. The therapeutic range has not been established but courses between 1 day and 14 days have been used [15]. The recommended a dose of praziquantel for the treatmentof neuroschistosomiasis is 50 mg/kg/day divided in two daily doses for 5 days [16]. Patients should be followed-up by stools and urine re-examined 1 month after treatment to assess the efficacy of chemotherapy.
Aretemether
Praziquantel is ineffective against immature developing larvae in acute schistosomiasis. Artemether, an antimalarial drug, can kill schistosomula during the first 3 weeks of life. Accordingly, it has been used for schistosomiasis chemoprophylaxis in China [17, 18]. Oxamniquine is another schistosomicidal drug effective against S. mansoni [19].
Corticosteroids
The minimally symptomatic or asymptomatic individual may be treated with praziquantel alone. Corticosteroids are used to suppress the inflammatory response and granuloma formation, thereby preventing further tissue destruction, and reduce ova deposition [15,20]. Prednisone and dexamethasone are used in conjunction with schistosomicidal agents in schistosomal encephalopathy during the oviposition stage.
There have been no double-blind randomised studies on the effects of steroids in spinal schistosomiasis. Nevertheless, rapid improvement of acute schistosomal myelitis has been reported after use of corticosteroids [21–23].
Acute neuroschistosomiasis should initially be treated with corticosteroids rather than praziquantel to avoid potential neurological complications [24]. Patients usually receive intravenous methylprednisolone (15-20 mg/kg; maximum dose1 g) over 5-7 days followed by oral prednisone for 2-6 weeks. Some authors use oral prednisone (1-1.5 mg/kg/day for 3 weeks) followed by a progressive and gradual reduction [15].
Surgical interventions
These include decompressive laminectomy, mass exeresis and liberation of roots which might be considered when acute S. mansoni myelitis deteriorates despite clinical treatment. There are no clinical data in the literature regarding timing of treatment, results of surgery and combination of therapies. Complete recovery is seen in only 30% of S. mansoni myeloradiculopathy patients. A less favourable outcome is observed in patients affected by transverse myelitis.
Rehabilitation and multidisciplinary team care are necessary in severely disabled and paraplegic patients. Wheel chair devices, bladder intermittent catheterisation, re-education pertaining to intestinal and sexual dysfunction, prevention of pressure sores and venous thrombosis; and treatment of spasticity, neurogenic and musculoskeletal pain and urinary tract infections may be needed.
Complications are common following spinal schistosomiasis [15]. Social, family and psychological support are necessary since neuroschistosomiasis affects mainly young and middle aged people although it can manifest as early as 5 years of age [25].
References
- 1.Naus CW, Chipwete J, Visser LG, Zijlstra EE, and pathologic. The contribution made by Schistosoma infection tonon-traumatic disorders of the spinal cord in Malawi. Ann Trop Med Parasitol 2003; 97:711–721. [DOI] [PubMed] [Google Scholar]
- 2.Carod-Artal FJ, Vargas AP, Horan TA, Marinho PB, Coelho Costa PH. Schistosoma mansoni myelopathy: clinical and pathologic findings. Neurology 2003;63:388–391. [DOI] [PubMed] [Google Scholar]
- 3.CarodArtal FJ. Neurological complications of Schistosoma infection. Trans Roy Soc Trop Med Hyg 2008: 102;107–116. [DOI] [PubMed] [Google Scholar]
- 4.Koul R, Alexander P, Scrimgeour E, Idris M, Joseph K. Schistosoma mansoni myeloradiculopathy in an 8-year-old Omani boy. J Trop Pediatr 2002; 48:183–186. [DOI] [PubMed] [Google Scholar]
- 5.Nascimento-Carvalho CM, Moreno-Carvalho OA. Clinical and cerebrospinal fluid findings in patients less than 20 years old with a presumptive diagnosis of neuroschistosomiasis. J TropPediatr 2004; 50:98–100. [DOI] [PubMed] [Google Scholar]
- 6.Rasamoelisoa JM, Tovone XG, Rakotovao E, Razafimandimby D, Andriambao D. Bilharzian meningomyeloradiculopathy in children [in French]. Arch Inst Pasteur Madagascar 2000;66:36–38. [PubMed] [Google Scholar]
- 7.Nascimento-Carvalho CM, Moreno-Carvalho OA. Neuroschistosomiasis due to schistosoma mansoni: a review of pathogenesis, clinical syndromes and diagnostic approaches. Rev Inst Med trop S Paulo 2005; 47:179–184. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control Prevention (CDC). Schistosomiasis in U.S. Peace Corps volunteers-Malawi 1992. Morb Mortal Wkly Rep 1993; 42:565–570. [Erratum. MMWR Morb MortalWkly Rep 42:615.] [PubMed] [Google Scholar]
- 9.Saleem S, Belal AI, El-Ghandour NM. Spinal cord schistosomiasis: MR imaging appearance with surgical and pathologic correlation. Am J Neuroradiol 2005; 26:1646–1654. [PMC free article] [PubMed] [Google Scholar]
- 10.Ross A, McManus D, Farrar J, Hunstman R, Gray D, Li Y. Neuroschistosomiasis. J Neurol 2012; 259:22–32. DOI . [DOI] [PubMed] [Google Scholar]
- 11.Ferrari TCA. A laboratory test for the diagnosis of neuroschistosomiasis. Neurological Research 2010; 32:252–262. [DOI] [PubMed] [Google Scholar]
- 12.Santos EC, Campos GB, Diniz, AC, Leal JC, Rocha MO. Clinical profile and criteria for the diagnosis of schistosomal myeloradiculopathy. Arq Neuropsiquiat 2001;59:772–777. [PubMed] [Google Scholar]
- 13.Doenhoff MJ, Pica-Mattoccia L. Praziquantel for thetreatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev Anti Infect Ther 2006; 4:199–210. [DOI] [PubMed] [Google Scholar]
- 14.Richards F, Sullivan J, Ruiz-Tiben E, Eberhard M, Bishop H. Effect of praziquantel on the eggs of Schistosoma mansoni, with a note on the implications for managing central nervous system schistosomiasis. Ann Trop Med Parasitol 1989; 83:465–472. [DOI] [PubMed] [Google Scholar]
- 15.Carod-Artal FJ. Neurological complications of Schistosoma infection. Trans R Soc Trop Med Hyg 2008; 102:107–116. [DOI] [PubMed] [Google Scholar]
- 16.CarodArtal FJ, Vargas AP, Horan TA, Marinho PB, Coelho Costa PH. Schistosoma mansoni myelopathy: clinical and pathologic findings. Neurology 2004; 63:388–391. [DOI] [PubMed] [Google Scholar]
- 17.Utzinger J, Xiao SH, Tanner M, et al. Artemisininsforschistosomiasis and beyond. CurrOpin Invest Drugs 2007; 8:105–116. [Google Scholar]
- 18.Xiao SH. Development of antischistosomal drugs in China, with particularconsideration to praziquantel and the artemesinins. Acta Trop 2005; 96:153–167. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari MLA, Coelho PMZ, Antunes CMF, Tavares CAP, da Cunha AS. Efficacy of oxamniquine and praziquantel in the treatment of Schistosoma mansoni infection: a controlled trial. Bull World Health Organ 2003; 81:190–196. [PMC free article] [PubMed] [Google Scholar]
- 20.Fowler R, Lee C, Keytone JS. The role of corticosteroids in the treatment of cerebral schistosomiasis caused by Schistosoma mansoni: case report and discussion. Am J Trop Med Hyg 1999; 61:47–50. [DOI] [PubMed] [Google Scholar]
- 21.Gellido CL, Onesti S, Llena J, Suarez M. Spinal schistosomiasis. Neurology 2000; 54: 527. [DOI] [PubMed] [Google Scholar]
- 22.Silva LC, Maciel PE, Ribas JG, Souza-Pereira SR, Antunes CM, Lambertucci JR. Treatment of schistosomalmyeloradiculopathy with praziquantel and corticosteroids and evaluation by magnetic resonance imaging: a longitudinal study. Clin Infect Dis 2004; 39:1618–1624. [DOI] [PubMed] [Google Scholar]
- 23.van Leusen H, Perquin WVM. Spinal cord schistosomiasis. J Neurol Neurosurg Psychiatry 2000; 69:690–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jauréguiberry S, Ansart S, Perez L, Danis M, Bricaire F, Caumes E. Acute neuroschistosomiasis: two cases associated with cerebral vasculitis. Am J Trop Med Hyg 2007; 76:964–966. [PubMed] [Google Scholar]
- 25.El Malik EB. Spinal cord schistosomiasis: A treatable cause of childhood paralysis in Sudan (Abstract). Sudan J Paediatr 2015; 15(1):102–105. [Google Scholar]


