Abstract
Non-invasive Doppler ultrasonographic study of cerebral arteries [transcranial Doppler (TCD)] has been extensively applied on both outpatient and inpatient settings. It is performed placing a low-frequency (≤ 2 MHz) transducer on the scalp of the patient over specific acoustic windows, in order to visualize the intracranial arterial vessels and to evaluate the cerebral blood flow velocity and its alteration in many different conditions. Nowadays the most widespread indication for TCD in outpatient setting is the research of right to left shunting, responsable of so called “paradoxical embolism”, most often due to patency of foramen ovale which is responsable of the majority of cryptogenic strokes occuring in patients younger than 55 years old. TCD also allows to classify the grade of severity of such shunts using the so called “microembolic signal grading score”. In addition TCD has found many useful applications in neurocritical care practice. It is useful on both adults and children for day-to-day bedside assessment of critical conditions including vasospasm in subarachnoidal haemorrhage (caused by aneurysm rupture or traumatic injury), traumatic brain injury, brain stem death. It is used also to evaluate cerebral hemodynamic changes after stroke. It also allows to investigate cerebral pressure autoregulation and for the clinical evaluation of cerebral autoregulatory reserve.
Keywords: Transcranial Doppler ultrasonography, Lindegaard ratio, Paradoxical embolism, Microembolic signals, Middle cerebral artery, Patent foramen ovale, Cryptogenic STroke, Vasospasm, Acute subarachnoid hemorrhage, Ischemic stroke
Core tip: Non-invasive Doppler ultrasonographic study of cerebral arteries [transcranial Doppler (TCD)] has been extensively applied on both outpatient and inpatient settings. Nowadays the most widespread indication for TCD in outpatient setting is the research of right to left shunting, responsable of so called “paradoxical embolism”, most often due to a patency of foramen ovale which is responsable of the majority of cases of cryptogenic stroke occuring in patients younger than 55 years old. In addition TCD has found many useful applications in neurocritical care practice. It is useful on both adults and children for day-to-day bedside assessment of critical conditions including vasospasm in acute subarachnoid hemorrhage, traumatic brain injury, brain stem death.
INTRODUCTION
Non-invasive Doppler ultrasonographic study of cerebral arteries [transcranial Doppler (TCD)] was introduced in clinical practice in 1982[1], since then it has been extensively applied in both outpatient and inpatient settings.
TCD ultrasonography is performed placing a low-frequency (≤ 2 MHz) transducer on the scalp of the patient, in order to visualize the intracranial arterial vessels through specific acoustic windows, where bone is thinner, and evaluate cerebral blood flow velocity (CBFV) and its alteration in different cerebrovascular diseases and traumatic brain injuries.
It is inexpensive, repeatable, and can be used in neurocritical intensive care to continually monitor CBFV at bedside[2].
Nowadays the most widespread indication for TCD in an outpatient setting is the research of right to left shunting (RLS), responsible of so called “paradoxical embolism”, most often due to a patency of foramen ovale, mostly occurring in people younger than 55 years of age[3,4] with ischemic stroke or TIA of unknown origin.
For this purpose it is necessary to inject an ultrasonographic contrast medium in an upper limb vein. The finding of typical artifacts in middle cerebral artery (MCA) Doppler tracing after a provocative manoeuvre is diagnostic for RLS.
In addition TCD has found many useful applications in neurocritical care practice. In particular, its principal use is in the assessment of vasospastic reaction after subarachnoid haemorrhage[5] (caused by aneurysm rupture or traumatic injury)[6,7], both in adults and children. It is used also to evaluate cerebral hemodynamic changes after stroke. Moreover TCD is able to provide a non-invasive estimate intracranial pressure (ICP) and to study cerebral autoregulatory function, thus helping to adjust cerebral perfusion pressure and mechanical ventilation in the single patient. Finally it represents an adjunctive test for the confirmation of brain death.
In this review we will describe in the first place physical principles, scanning proceedings, acoustic windows used in standard TCD examination, then will be discussed flow indices most frequently used in clinical practice. Finally we will focus on the incremental diagnostic role of TCD in Cryptogenic Stroke and the main critical care indications for this imaging modality.
ANATOMY OF MAIN INTRACRANIAL ARTERIES
For better understanding of TCD findings and its applications in clinical setting, can be useful to make a brief description of the anatomy of intracranial arteries of major clinical interest: Internal carotid artery (ICA), MCA, anterior cerebral artery (ACA) and posterior cerebral artery (PCA).
The ICA, together with the external carotid artery is the terminal branch of the common carotid artery. It starts at C3 and C5 vertebral level, and it has been sub-divided into seven segments (named from C1 to C7): (1) cervical segment; (2) petrous (horizontal) segment; (3) lacerum segment; (4) cavernous segment; (5) clinoid segment; (6) ophthalmic (supraclinoid) segment; and (7) communicating (terminal) segmen. The ICA gives rise to two terminal branches which are the MCA and the ACA.
The MCA is the most frequently insonated artery during TCD examinations. It arises from the ICA and runs into the lateral sulcus where it then branches and gives blood to many parts of the lateral cerebral cortex. It can be subdivided into 4 tracts. The sphenoidal segment, M1 is also called the horizontal segment, because of its origin and its lateral course on sphenoid bone. The insular segment, M2 segment, is situated anteriorly on the insula. The opercular segments, M3 segment, extend laterally and exteriorly from the insula towards the cortex. The Cortical segments, the M4 terminal segments, irrigate cortex.
The ACA is smaller than MCA, and arches anteromedially to run anterior to genu of the corpus callosum, where the artery divides into its two major branches, pericallosal and callosomarginal.
The PCA represents the terminal branches of the basilar artery (BA) and irrigate the occipital lobes and posteromedial temporal lobes.
PROBE AND SCANNING PROCEDURES
In clinical practice the most frequently used transducer is a pulsed Doppler sectorial probe with a 2.0-3.5 MHz emission frequency capable of changing the size of the sample volume in order to adapt to the diameter of major intracranial arteries, moreover the angle and position of insonation should be adjusted to provide/determinated the highest quality Doppler signal.
The probe can then be fixed to the scalp with a headband so that the same angle of insonation for continuous flow velocity recordings is maintained throughout the exam. TCD can be conducted using two acquisition modalities.
The first is transcranial color-coded duplex sonography (TCCS), in which it is displayed a two-dimensional color-coded image[8] and, once the desired blood vessel is insonated, blood flow velocities may be measured using PW Doppler.
The second method is conventional TCD, using only Doppler probe function. The TCDS with combined ColorFlow and power Doppler provides more useful data than TCD since it allows direct imaging of the intracranial arteries, their anatomic course, diameter and relationships with the adjacent structures. Although the use of TCCS can be considered superior to TCD, no substantial differences were found when the two methods were compared in their accuracy to detect vasospasm in the setting of acute subarachnoidal haemorrhage (SAH)[1,9].
In order to get a better quality of the Doppler signal in spite of background noises, the TCD devices are equipped with a larger sample volume compared to other PW Doppler probe. Specific Doppler settings used in TCD examination include also the emission power between 10 and 100 mW/cm2 second and a pulse repetition frequency (PFR) up to 20 kHz with a focus depth between 40 and 60 mm[10].
In clinical practice can be found two channel TCD transducers with dual emission frequency (2.0 MHz and 2.5 MHz, Embo-Dop). In standard TCD examination should be recorded bilateral PW-Doppler tracing lasting at least 10 cardiac cycles after a 30-s stabilized recording period.
ACOUSTIC WINDOWS AND SCANNING PLANE
The transmission of an ultrasound beam through skull is influenced by structural characteristics of the diploe bone: The almost complete absence of bone spicules makes penetration of the ultrasound similar to conventional “acoustic windows” consenting the visualization of intracranial vessels. First of all the patient should be lying in supine position, with his head and shoulders on a pillow.
In general terms transcranial United States study is performed using two main scanning planes: The axial and coronal planes at a depth that allows to display also the contralateral vessels (14-16 cm depth), with the brain stem structures remaining in the middle of the scanning plane.
The axial scan is the one most commonly used and it allows two different types of imaging planes: The mesencephalic and diencephalic views. The mesencephalic plane is obtained by positioning the probe parallel to the zygomatic arch. At this level can be identified the hypoechogenic “butterfly-shaped midbrain”, located about half of the scanning plane. In the 75% of cases, can be also detected the posterior communicating arteries if they have enough relevant diameter. In the middle of the diencephalic plane, which is obtained by slightly tilting the transducer 10 degrees upwards, can be seen the III ventricle: Behind it can be identified hyperechogenic pineal gland, while the thalamus and internal capsule are located anteriorly to it. The lateral ventricles can be also detected.
The coronal scan is obtained by rotating the probe of 90° from the axial position. In this view are shown the III ventricle, the lateral ventricles, the thalamus and internal capsule. The examination carried out on this plane is mainly useful for assessment of the shift of the median line caused by space occupying lesions (ischaemic area, haemorrhage and tumors). For what concerns the Doppler Study of Intracranial arteries, in clinical practice there are four acoustic windows that can be used for TCD and TCDS.
The temporal window is situated above the zygomatic arch, anterior to the tragus, using an axial plane in order to obtain a mesencephalic view, with the patient’s head in the antero-posterior position (Figure 1). This window can be divided in an anterior, middle and posterior zone and allows to identify the MCA, in particular M1 and M2 tracts. From this approach can be also visualized A1 segment of the ACA, P1 and P2 segments of the PCA and C1 segment of the carotid siphon (CS) (Figure 2). In this temporal view can be also seen the communicating arteries - anterior and posterior - and the distal end of the BA. It should be noted that about 10%-20% of subjects have poor and unsuitable trans-temporal acoustic views, depending on patient age, female sex, and other factors affecting the temporal bone thickness[2,11,12].
Figure 1.
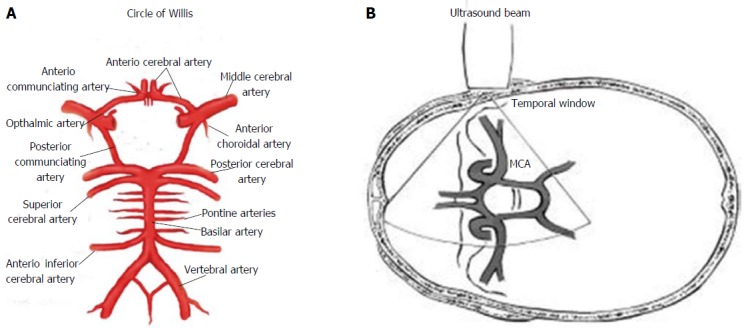
Circle of Willis and Ultrasonographic study by transcranial Doppler ultrasound. A: Circle of Willis; B: Transmission of ultrasound beam through skull using pulsed Doppler sectorial probe with a 2.0-3.5 MHz emission frequency. Probe is positioned on temporal window. MCA: Middle cerebral artery.
Figure 2.
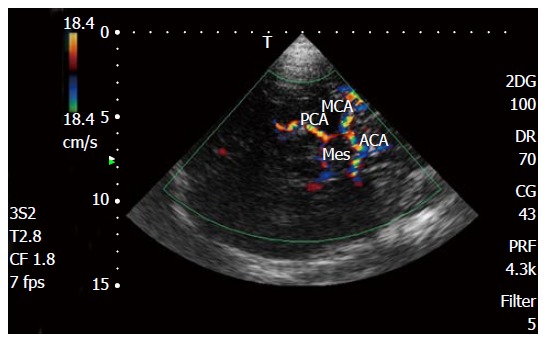
Transcranial Doppler color Doppler study of intracranial arteries. MCA: Middle cerebral artery; PCA: Posterior cerebral artery; ACA: Anterior cerebral artery; Mes: Mesencephalon.
In the occipital window, the probe must be positioned on the median sub-occipital line and the patient should be sitting or lying down with the head turned to opposite direction respect to the operator with the chin lowered toward the shoulder. With US beam passing through the foramen magnum in this window it can be visualized the intracranial segment of the two vertebral arteries (VA) and the basilar trunk. All these three vessels dispose in a Y shape with their flow, depicted in blue color, moving away from the probe. In this view, with slight lateral movements it is possible to display also both the inferior cerebellar arteries, the posterior and the anterior[13].
When TCD examination is performed from the orbital window, transducer is put perpendicularly to the eyelid, with patient’s eye closed and looking on the opposite side respect to the probe. This approach allows to insonate the ophthalmic artery and the C2, C3 and C4 segments of the carotid syphon, through the foramen of the ocular cavity. The limitation of this approach is represented by the potential retinal injuries caused by the US beam: It is advisable to reduce 10%-15% power of the device respect to transtemporal scan.
In addition to the above mentioned views it can be also used the submandibular window, putting the transducer underneath the angle of the mandible, in front of the masseter muscle, inclinating the probe toward the skull. This window allows only the detection of the terminal segment (C5-C6) of the ICA (CI) and of the C1 segment of the CS. So, this approach is employed in case of impossibility to realize the TCD examination using the other standard windows for hemodynamic assessment of the Circle of Willis.
MCA
The most frequently examined intracranial vessel in clinical practice is the MCA, it is easily delineated through the temporal window above the zygomatic arch. The 60%-70% of the ICA blood flow is directed to MCA, so its TCD evaluation can be taken to represent almost total blood flow to ipsilateral hemisphere. MCA is detected at a depth of 45-60 mm, and the blood flow is directed toward the probe[14]. The identification of the sphenoid bone, through the “butterfly wing sign”, leads to a easy MCA visualization in almost all patients, with a constant depth of 59 ± 3 mm[15]. The time to achieve an adequate echographic image of MCA is about 50 ± 20 s[15].
TCD: PHYSICAL PRINCIPLES AND TCD INDICES
TCD examination, as explained above, is executed placing on the surface of the skull a probe of a range-gated ultrasound Doppler instrument, which allows to determine flow velocities in the intracranial arteries[16]. The attenuation of US beam due to bone and soft tissues requires a low emission frequency in order to provide satisfactory recordings of intracranial CBFVs, usually a 2-MHz frequency is adopted[16].
In physical terms, the probe trasmits an ultrasonic beam that crosses the skull and is reflected back from the erythrocytes flowing in blood vessels, when a sound wave hits a moving object, the wave of reflection shows a shift in its frequency (the Doppler shift f ) that proportionally corrrelated to the velocity (V) of the same object. The Doppler shift represents the difference between the transmitted and received signal frequency while the time interval from pulse emission and reception determines the depth at which any Doppler frequency shift is detected[16].
In the intracranial vessels, as in the arteries of other vital organs (liver, kidney, and heart), the Doppler signal shows a prominent diastolic component of blood flow.
The following equation derived from Doppler principles described above, is used for estimation of CBFV with TCD:
v = [(c × f)/(2 × fo × costheta)]
Where c represents the speed of the US Wave emitted from probe, fo represents the emitted Wave pulse frequency, theta represents the angle of formed by reflected wave relatively to the initial US emission beam[17].
When performing the TCD examination the operator should keep a Theta angle of 15° or less, because the cosine remains 0.96 or more so that any error caused by changes in the angle is less than 4%.
Mean CBFV is derived through the spectral envelope of Doppler Signal, as indicated by following formula:
Mean CBFV = [PSV + (EDV × 2)]/3,
Where PSV is peak systolic velocity, and EDV is end-diastolic blood flow velocity[18,19] (Figure 3).
Figure 3.
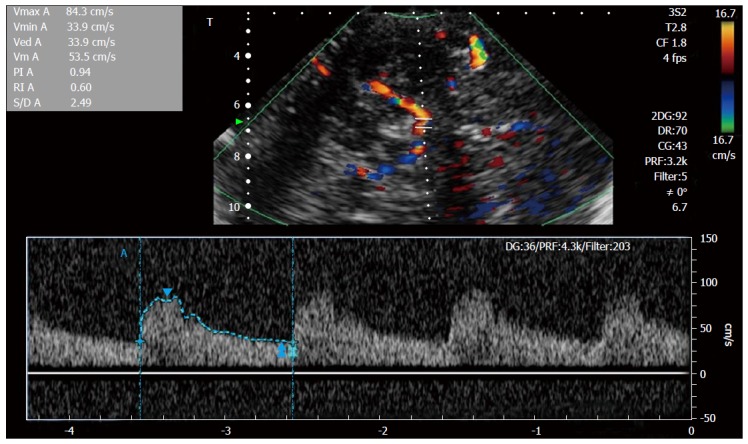
Transcranial Doppler spectral Doppler study of intracranial middle cerebral artery.
By the Bernoulli principle, the correlation between velocity and pressure exerted by blood flowing, is characterized by a decrease of pressure exerted by the fluid as the velocity of flow increases. Moreover, it should be remembered that by the continuity principle the CBFV in a given artery is inversely related to the cross-sectional area of the same artery[19,20]. So, TCD gives an indirect evaluation of the diameter of intracranial vessel through the analysis of blood flow velocity[19]. It should be also considered that there are many physiologic factors affecting CBFV: Age, hematocrit, gender, fever, metabolic factors, pregnancy, menstruation, exercise, and brain activity[21-24] (Tables 1 and 2).
Table 1.
Factors influencing cerebral blood flow velocit
| Factor change in CBFV | |
| Age | Increase up 6-10 yr then decrease |
| Sex | Women > men |
| Pregnancy | Decrement in the III Trimester |
| Hematocrit | Increase with decreasing Hct |
| PCO2 | Increase with increasing PCO2 |
| Main | Arterial pressure increase with increasing MAP |
CBFV: Cerebral blood flow velocity; MAP: Mean arterial pressure.
Table 2.
Mean cerebral blood flow velocity (cm/s) related to age
| Artery | Age 20-40 yr | Age 40-60 yr | Age > 60 yr |
| Anterior cerebral artery | 56-60 | 53-61 | 44-51 |
| Middle cerebral artery | 74-81 | 72-73 | 58-59 |
| Posterior cerebral artery P1 | 48-57 | 41-56 | 37-47 |
| Posterior cerebral artery P2 | 43-51 | 40-57 | 37-47 |
| Vertebral artery | 37-51 | 29-50 | 30-37 |
| Basilar artery | 39-58 | 27-56 | 29-47 |
In clinical practice an higher mean CBFV is suggestive of hyperdynamic flow, stenotic arterial disease or vasospastic reaction. On the other hand, a decreased value of this parameter could be suggestive of low intracranial perfusional pressure, or increased ICP or even brain stem death[21]. Stenosis or vasospasm in an arterial segment is defined as an increase in mean CBFV of more than 30 cm/s, within a tract 5 to 10 mm long on one side, if confronted with the healthy corresponding contralateral arterial tract[25].
The Lindegaard ratio (LR) permits to differentiate between hyperdynamic arterial blood flow and vasospasm. It is obtained by the following equation:
LR = MCA mean CBFV/extracranial ICA mean CBF-V[26].
This ratio tends to increase in relation to the severity of symptomatic vasospasm (VSP). Normal reference range is from 1.1 to 2.3 and in the absence of vasospasm is lower than 3[26]. When the CBFV is elevated but the LR ratio is lower than 3, the elevation is considered to be caused by hyperemia, because patients after acute subarachnoidal haemorrhage (aSAH) are often treated following so called triple-H therapy: Hypertension, hypervolemia, hemodilution. In case of a ratio more than 6, there is a severe VSP[20,27,28]. So, in summary, LR defines the severity of vasospasm: MCA mean CBFV/extracranial ICA mean CBFV > 3 mild to moderate VSP; MCA MEan CBFV/extracranial ICA mean CBFV > 6 severe VSP.
Moreover, for detecting the severity of BA vasospasm it is calculated the modified LR: BA mean CBFV/left or right extracranial VA Mean CBFV; LR modified: 2 to 2.49 possible VSP; LR modified: 2.5 to 2.99 moderate VSP; LR modified: > 3 severe VSP (Table 3).
Table 3.
Intracranial arteries: Severity of vasospasm
| MFV (cm/s) | LR | LR modified | |
| MCA or ICA vasospasm | |||
| Mild (< 25%) | 120-149 | 3-6 | |
| Moderate (25%-50%) | 150-199 | 3-6 | |
| Severe (> 50%) | > 200 | > 6 | |
| BA vasospasm | |||
| Possible vasospasm | 70-85 | 2-2.49 | |
| Moderate (25%-50%) | > 85 | 2.5-2.99 | |
| Severe (> 50%) | > 85 | > 3 |
MCA: Middle cerebral artery; ICA: Internal carotid artery; LR: Lindegaard ratio; BA: Basilar artery; MFV: Mean flow velocity. MCA: Middle cerebral artery; ICA: Internal carotid artery; LR: Lindegaard ratio; BA: Basilar artery; MFV: Mean flow velocity.
Incremental diagnostic role in cryptogenic stroke
The American Academy of Neurology states that TCD main clinical indications include ischaemic cerebrovascular disease, neurointensive care and periprocedural applications in the setting of carotid and intracranial vascular interventions[29].
In this section we shall focus on the role of TCD ultrasonography for the research of the so-named “paradoxical embolism” through patent foramen ovale (PFO) which has been recognized as a relevant aetiologic factor for cryptogenic stroke, mainly when occurring in patients younger than 55 years old[30,31]. In fact TCD can be used to detect a cardiac source of embolism due to right-left intracardiac or pulmonary shunts (e.g., patency of foramen ovale or pulmonary arterio-venous malformations). It also allows to classify the grade of severity of such shunts using the so called “microembolic signals (MES) grading score”[32,33].
PARADOXICAL EMBOLISM: PFO AND CRYPTOGENIC STROKE
PFO can be considered a remnant of the fetal circulation. During the fetal life, it allows the transit of blood flow from the right cardiac chambers to the left cardiac chambers, determining a so-called right-left shunt. The presence of a PFO in adult life can be considered persistence of such fetal communication between right and left atrium, it usually appears as an oblique, slit-shaped defect which looks like a tunnel. The cause of its incomplete closure after birth is not known, but it appears to be associated with multifactorial inheritance. In some patients such interatrial communication can be associated with a thinner and redundant interatrial septum which shows mono or bidirectional movement during cardiac cycle [atrial septal aneurysm (ASA)].
Frequency of such lesion in general adult population varies between 25% to 30%: The prevalence and size of the defect are similar for males and females[33-35] and decrease progressively with age. In detail, PFO is diagnosed into 34% of patients 30’s old, into 25% between 30’s and 80’s old, and finally into 20% over 80’s old and this trend is inversely related to the dimensions of the defect. Moreover the average dimensions increase progressively from 3.4 mm in the first decade of life, to 5.8 mm after the ninth decade[36]. The explanation of this phenomenon is probably that larger defects tend to persist while those of smaller dimensions go towards spontaneous closure with time[36].
Most individuals with a PFO remain asymptomatic, but in some cases it has been associated with several clinical manifestations due to transient RLS, such as decompression sickness in scuba divers[37] or platypnea-orthodeoxia syndrome[38]. But, the most important potential manifestations related to PFO are represented by cryptogenic stroke due to paradoxical embolism, and migraine and vascular headache, although the causal relationship between PFO and migraine is not yet completely understood and is still object of research.
The clinical significance and the pathogenic role of PFO in patients with cryptogenic stroke is still a matter of debate: About 40% of ischemic strokes that occur in people under the age of 55 are cryptogenic[31,39]. Cryptogenic stroke is defined as an ischemic stroke which takes place without any clearly identifiable etiology from cardioembolic source or large vessel atheromasia. This kind of cerebrovascular accident has an embolic origin and typically shows a distribution pattern that is not consistent with small vessel involvement.
Prevalence of PFO is higher among subjects hit by a cryptogenic stroke: In a prospective study (the PFO-ASA study) were included 581 patients with a cryptogenic cerebrovascular ischemic accident of less than 55 years of age (mean 42), 37% had PFO and 9% had PFO associated with ASA[39].
In the PFO in Cryptogenic Stroke study was found an analogous prevalence of PFO (39%) in 250 patients with a mean age of 59 years[40]. Moreover patients with cryptogenic stroke showed significantly higher rate of a large PFO compared to patients with a stroke of known cause (20% vs 9.7%)[40]. The pathophysiological mechanism underlying stroke of cryptogenic origin in PFO carriers probably consists in a paradoxical embolism in the setting of a transient right to left shunt. In detail when the right atrial pressure is higher than the pressure in the left atrium, a transient right-to-left shunt possibly occurs through a PFO that becomes a pathway for the passage of emboli from venous to arterial circulation (paradoxical embolism).
Thus, a transitory occurrence of interatrial right-to-left pressure gradient can cause paradoxical shunting and can commonly be elicitated using specifical maneuvers in patients with no baseline RLS (including both subjects without net shunt at all or with a left-to-right shunt). In particular a short-lived right-to-left gradient can be present in normal individuals during early ventricular systole and after release of maneuvers which raise intra-abdominal pressure (such as Valsalva maneuver, defecation, cough, lifting or pushing heavy objects). In a community based study of 148 subjects carriers of a PFO, 57% showed resting right-to-left shunt, and 92% showed elicitable RLS after Valsalva maneuver or cough[41]. In summary PFO represents a possible cardioembolic source responsible of cryptogenic stroke and a risk factor for neurological events.
ROLE OF THE TCD METHODOLOGY AND DIAGNOSTIC ACCURACY
The diagnosis of PFO, in order to achieve a clinical significance, should provide both an anatomic description and a physiologic assessment of a potential RLS. The first is usually obtained by transesophageal echocardiography (TEE) or by intracardiac echocardiography while the physiologic assessment of an RLS is usually obtained using contrast transthoracic echocardiography (TTE) or TCD. A definite ultrasonographic diagnosis of temporary right-left shunting requires the use of contrast enhancement. In clinical practice the most frequently used ultrasonographic contrast medium is represented by agitated saline solution. In fact the different density present at the interface separating gas-containing microbubbles from sorrounding tissue modifies the “acoustic impedance” of such interface: The higher impedance the higher echogenicity at the same level. Moreover gas microbubbles work very effectively as contrast medium, since they are 100000 times less dense than blood[42].
Traditionally, TEE supported by agitated saline contrast-enhancement has always been considered the gold standard techinque both for demonstration of a right-to-left shunt through a PFO and for morphological description of interatrial septum. It should be noted that micro-bubbles with a diameter smaller than 9 μm are not able to pass through pulmonary capillary network, so the finding of any micro-bubble after intravenous contrast administration is diagnostic for RLS.
Contrast enhancement for the research of paradoxical interatrial shunting has been applied also to TTE (Figure 4), with a reported sensitivity and specificity similar to that of c-TEE[43,44]. This was also due to the introduction of harmonic imaging, which improved image quality of TTE[45]. In recent times contrast enhanced TCD (c-TCD) has gained a growing role for the diagnosis of transient RLS, which allows to recognize the passage of intravenously injected micro-bubbles directly in cerebral circulation. As stated above about TEE, also with c-TCD the finding of a single microbubble in cerebral arterial circulation (usually MCA) is considered diagnostic of RLS. C-TCD represents a low cost, widely available, non-invasive imaging techinque, of easy interpretation, which also permits to semiquantitatively estimate severity of venous-arterial shunt[46].
Figure 4.
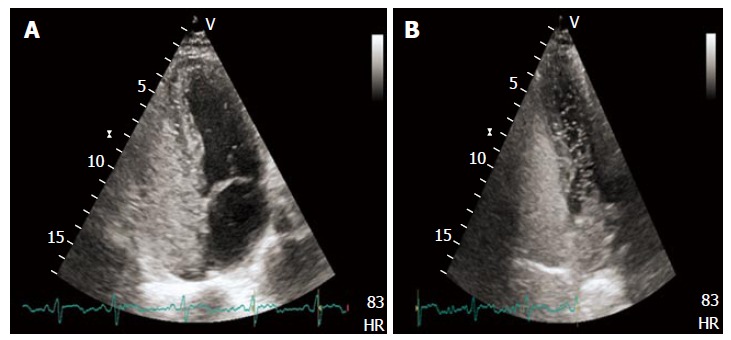
Transthoracic echocardiography showing high grade right to left shunt with evident micro-bubbles in the left heart after intravenous contrast administration (A and B).
In order to highlight RLS a contrast medium, usually agitated saline is injected into a peripheral vein, usually right antecubital vein in three boluses, at the same time the Doppler signal is recorded while the patient performs a Valsalva maneuver. The contrast agent is obtained by combining 9 mL of normal saline solution with 1 mL of air and then it is usually shaken up about 10 times through a system constituted by two 10 mL syringes linked by a 3-way stopcock. The agitated solution is then administrated into the antecubital vein by an 18-gauge. The patient is then invited to perform a forced expiration against the closed glottis for a minimum of 10 s (Valsalva Maneuver). When a right to left shunt is present the air microbubbles constituting ultrasonographic contrast medium will directly pass from venous to systemic circulation and will be visualized in cerebral arterial vessels as so called MES.
In addition it is possible to evaluate the entity and functional relevance of a paradoxical RLS through the MES grading score, based on the number of Doppler signals provoked by microbubbles that reach MCA (Figure 5 and Table 4). Moreover the entity of right to left shunt is directly associated with the risk of stroke[33,47]. It should be noted that when the number of microbubbles passing through a RLS is very low, they may not be able to reach the MCA giving a false negative result of absent RLS. But on the other hand clinical relevance of such small entity of shunt is uncertain. A very large amount of microbubbles reaching MCA is responsible on the Doppler Spectrum of the so called “Curtain effect”, characterized by impossibility to identify on Doppler spectrum a single MES. In the work of Serena et al[48], “Curtain effect” is characteristically found in patient hit by cryptogenic stroke, so the identification of this Doppler aspect in a subject could denote a higher risk of cerebrovascular events, thus providing useful information for the clinician in order to differentiate “innocent” from “harmful” shunts information[48].
Figure 5.
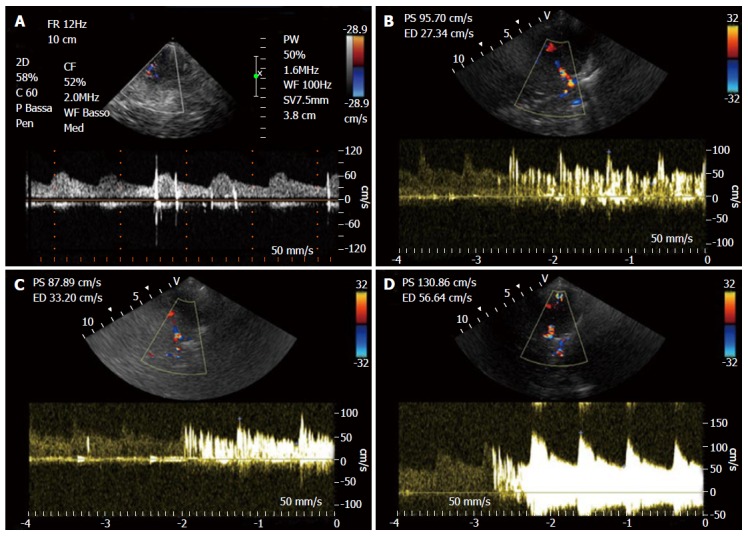
Right to left shunt with microembolic signals. A: Low grade shunt; B: Moderate grade shunt; C: High grade shunt (shower); D: Curtain effect.
Table 4.
Grade of transient right to left shunting based on microembolic signals grading score
| Grade transient shunt | MES |
| No shunt | 0 |
| Low grade shunt | 1-10 |
| Moderate grade shunt | 11-25 |
| High grade shunt | > 25 (shower) or uncountable (curtain effect) |
MES: Microembolic signals.
Nowadays there is no consensus about a definite time interval from contrast administration until recording of the first MES on MCA Doppler spectrum. In a recent work, twenty-six patients with stroke (16 with PFO vs 10 without PFO, diagnosed by cTEE) after a positive cTCD test were evaluated for three parameters: The amount of MES, latency time (LT) before the first MES and the duration time of MES, looking for any difference between PFO carriers and no-PFO. The presence of more than 9 MES with a LT of less than 9 s (so called rule of nine) can be considered a marker for PFO diagnosis by cTEE, providing a specificity and positive predictive value (PPV) of 100%[49].
To increase the test sensitivity for identification of the right to left shunt [PFO detection can be increased by asking patient to cough or by releasing a sustained Valsalva manoeuvre (VM)] the patient may be asked to cough or to perform a prolonged VM, since in the release phase of these strain maneuvres a RLS can be elicitated when the right atrial chamber is filled with blood from the abdominal cavity, while the left atrial chamber is still volume depleted before passage of increased blood return through pulmonary circulation[50]. VM should be always performed for the research of RLS, it is started 5 s after agitated saline administration (because it represents the avarage time interval required for the injected solution to reach right atrium from the cubital vein).
Effectiveness of VM strength can be assessed through peak flow velocity of the MCA Doppler Spectrum, which tends to decrease during a well executed VM[44]. Mojadidi et al[51] have published an extensive bivariate meta-analysis of 27 prospective studies with a total of 1968 patients comparing PFO detection with TCD to the c-TEE as gold standard. Starting from these data they could determine sensitivity in FOP identification for TCD (index test) and TEE (considered reference test) according to type of contrast medium, different provocative maneuvers, different quantitative microembolic cutoffs, different time of onset of provocation maneuver, and insonation of a single or both MCA. No difference in sensitivity and specificity was found between each contrast medium (agitated saline, Echovist, and gelatin-based solutions, P > 0.05). No significant difference between cough or Valsalva as provocative maneuvre was evident (P > 0.7). When cut-off number of 10 microbubbles instead of 1 was chosen to define TCD positivity study specificity was showed a significant improvement from 89% to 100% (P = 0.04), nevertheless this approach did not result in a substantial improvement in sensitivity (from 98% to 97%, P = 0.29).
Duration of Valsalva strain, more or less than 5 s, did not show a significant influence sensitivity or specificity of TCD (P > 0.50). Finally a not significant trend towards an improvement of specificity when a single MCA was insonated instead of both (95% specificity vs 89% respectively, P = 0.09), while no significant difference was seen regarding sensitiviy (P = 0.15).
In conclusion Mojadidi found an overall sensitivity of 97% and a specificity of 93% for detection of RLS with c-TCD compared with c-TEE[51]. Increasing the number of microbubbles needed for a positive TCD from 1 to 10 resulted in a predictable significant improvement in specificity. TCD showed a good diagnostic performance with an overall LR+ of 13.51 and LR- of 0.04 and a disease probability of 93%-94% after a positive test and of 4% after a negative test[51].
In Table 5 are summarized sensitivity, specificity and diagnostic accuracy of c-TCD for the research of RLS in patients with cryptogenic stroke in different studies, which adopted TEE as a gold standard. So in the context of a cryptogenic stroke, the clinician is called to choose the best diagnostic technique between c-TCD, c-TEE or c-TTE in order to detect a RLS.
Table 5.
Diagnostic role of transcranial Doppler and its accuracy
| Ref. | No. of patients | Sensitivity (%) | Specificity (%) | Accuracy (%) | Cut-off for RLS |
| Serena et al[48], 1998 | 55 | 100 | 100 | 100 | ≥ 1 MES1 |
| Lange et al[49], 2010 | 26 | 31 | 100 | 65.5 | ≥ 9 MES |
| González-Alujas et al[52], 2011 | 93 | 97 | 98 | 97.5 | ≥ 1 MES |
| Mojadidi et al[51], 2014 | 1968 | 97 | 93 | 95 | Meta-analysis2 |
In this study c-TCD performs better than c-TEE;
This study is a large meta-analysis comprising 27 studies. TCD: Transcranial Doppler; c-TCD: Contrast enhanced TCD; TEE: Transesophageal echocardiography; RLS: Right-to-left shunting; MES: Microembolic signals.
TEE provides detailed morphological description of interatrial septum and is able to identify anatomic characteristics of a PFO. In particular a diameter greater than 4 mm or the coexistence of an aneurysm of interatrial septum is associated with recurrent ischaemic cerebrovascular accidents. These c-TEE may be useful in guiding management towards an interventional strategy instead antithrombotic treatment in patient hit by cryptogenic stroke[52]. On the other hand recent published data suggest that TEE should not be considered the true gold standard imaging technique for the detection of RLS. In fact in the case of really small shunts (of 1 to 3 bubbles), c-TCD may show a better sensitivity, because such a small number of microbubbles may me missed on a single tomographic echocardiographic plain[53]. Moreover TEE is an high cost, semi-invasive technique characterized by poor patient’s compliance, it is not always available and contrast administration may be inconclusive or be followed by falsely negative results[53], mainly due to inability of the patient to carry out an effective Valsalva maneuvre[54-57].
A lower sensitivity of c-TEE compared with c-TTE and c-TCD was reported by the work of González-Alujas et al[52] (86% sensitivity for TEE, vs 100% for TTE and 97% for TCD, P < 0.001), while here was no significant difference in sensitivity between TTE and TCD. These results may have a clinical impact, because they confirm that TEE is not the most accurate diagnostic technique as it was commonly considered in the past years.
Higher sensitivity shown by c-TCD is also due to its positive results also in presence of extracardiac shunts, such as pulmonary arterio-venous malformations. TCD is not able to show the exact anatomic position of the RLS, although LT from contrast injection in antecubital vein to the appearance of MES in the setting of an intracardiac shunt is about 11 s, while in presence of a pulmonary artero-venous malformation is about 14 s[58]. Interestingly as reported in the work of Gonzalez-Aluja[52], c-TTE performed simultaneously with TCD was able to confirm presence of an artero-venous pulmonary malformation in a positive TCD, showing the entrance of microbubbles in left atrium from a pulmonary vein.
RECOMMENDATIONS
American Academy of Neurology confers a class II indication for both c-TCD and TEE for interatrial shunt detection[29,58]. On the other hand Italian stroke guidelines (SPREAD) consider TCD a better screening tool than TEE in the population of patient with suspect shunt through a foramen ovale[59].
In a consensus document published on behalf of Italian society of interventional cardiology by Pristipino et al[60] in 2010 TCD was proposed as first-choice screening tool for RLS in the setting of a cryptogenic stroke in subjects 55 years old or younger, while in patients older than 55 TEE was recommended as first-line test.
In conclusion our suggestion in the setting of a cryptogenic ischemic stroke is to use c-TCD as a first line screening tool, due to its higher sensitivity and its better tolerability. TEE may be considered as a complementary imaging technique for a more detailed anatomic definition of interatrial septum, especially when PFO closure is contemplated (Figure 6). Moreover TCD is also useful for follow-up of patients after PFO closure in order to identify those with residual shunting[61] due also to its repeatability and its sensitivity for the detection of small entity residual shunts[62].
Figure 6.
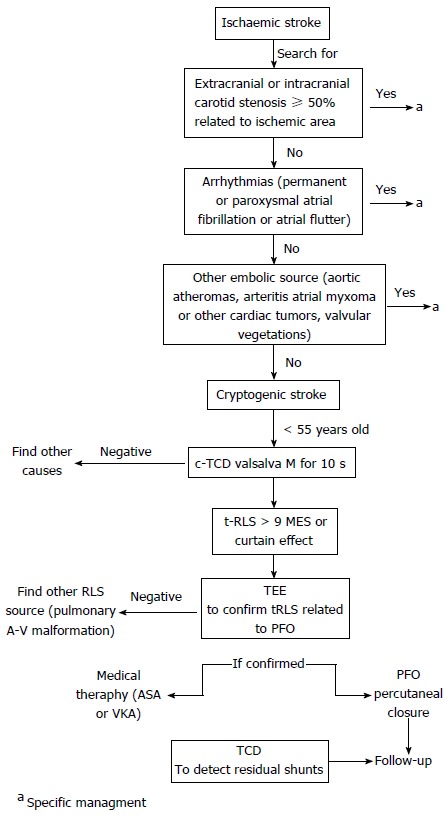
Contrast enhanced transcranial Doppler as a first line screening tool in the setting of a cryptogenic ischemic stroke. TCD: Transcranial Doppler; c-TCD: Contrast enhanced TCD; TEE: Transesophageal echocardiography; RLS: Right-to-left shunting; PFO: Patent foramen ovale; ASA: Atrial septal aneurysm; MES: Microembolic signals; VKA: Vitamin K antagonist.
Principal applications in neurocritical care unit
TCD examinations have gained an important role in the very early phase of critical cerebral pathologies, as well during follow-up of patients with chronic cerebrovascular diseases.
In neurocritical care bedside TCD examination provides the clinician useful information to guide the management of patients with SAH, allowing to recognize vasospasm both in adult and paediatric patients. Moreover TCD represents an additional non-invasive tool for cerebral hemodynamic monitoring, which is particularly of interest in the follow-up of patients with ischemic stroke. It allows to investigate cerebral pressure autoregulation and for the clinical evaluation of cerebral autoregulatory reserve[63]. TCD has important clinical application in the management of patients with sickle-cell disease, traumatic brain injury (TBI), brain stem death[64], raised ICP[65].
VASOSPASM AFTER SAH: DIAGNOSIS AND MONITORING ON TCD
Symptomatic vasospasm (VSP) is a frequent complication of aSAH, secondary to intracranial aneurysm rupture (aSAH). It should be considered that 25% of patients affected by aSAH develops clinical delayed ischemic deficits due to vasospasm[6,11,66-68].
The retarded vasospasm of intracranial arteries is reported by angiographic studies to occur in about 70% of patients affected by SAH and in most cases it develops between 4-17 d following the acute episode[20,69]. When it’s still present up to day 20 by TCD[70], morbidity and mortality are considered to increase significantly up to 20%[8,71,72].
VSP is characterized by a decrease in blood flow through cerebral regions after aSAH secondary to reflex vasoconstriction of intracranial arteries[20]. The exact mechanism causative of delayed cerebral ischemia (DCI) is not clearly understood, and several theories have been proposed[73]. Clinically, the terms “delayed ischemic neurologic deficit and DCI” have been introduced to describe symptomatic VSP.
Angiographic study is considered as the gold standard for the detection of intracranial vasospastic reaction but it is invasive diagnostic exam and cannot be used for continuous monitorization[2,74]. Angiographic VSP, identified by digital subtraction angiography and computed tomography angiography (CTA) has been diagnosed up to 50% to 70% of patients affected by aSAH and about half of them showed clinical symptoms[73,75].
TCD ultrasonography is a noninvasive, repeatable, and relatively inexpensive imaging test and it could be used in patients affected by aSAH for diagnosing and monitoring of VSP[16,76]. It can identify cerebral hemodynamic changes, diagnosing VSP before appeareance of clinical neurologic deficits, and can suggest earlier intervention[77].
So, in NCCU daily TCD monitoring is warranted for the management of patient affected by aSAH: The timing of the development and resolution of VSP can guide therapeutic strategies such as triple-H therapy (hypertension, haemodilution, and hypervolaemia). TCD also can monitor the efficacy of interventional procedures such as transluminal balloon angioplasty[78] and can identify patients at higher risk of developing DCI.
TCD is able to recognize vasospastic reactions in MCA and BA with a good sensitivity and specificity. A systematic analisys collecting 26 works, which compared TCD with angiographic exam has shown that a Mean CBFV > 120 cm/s in MCA detected by TCD carries 99% specificity and 67% sensitivity for identification of angiographic vasospasm of ≥ 25%[79]. For MCA vasospasm it is calculated as MCA mean CBFV/extracranial ICA mean CBFV (Table 3). MCA mean CBFV/extracranial ICA mean CBFV > 3 indicates mild to moderate VSP. MCA mean CBFV/extracranial ICA mean CBFV > 6 indicates severe VSP. Thus TCD, compared with angiography as gold standard, showed high specificity and high PPV for MCA vasospasm detection, making it a very useful diagnostic tool in this setting[79].
TCD criteria for BA VSP have not been universally defined yet (Table 3). Sviri et al[75] argued that the CBFV ratio (LR BA/VA) between the BA and the extracranial VA is related to the degree BA narrowing (0.648, P < 0.0001). A BA/VA ratio (LR BA/VA) over 2.5 with BA velocity higher than 85 cm/s was 86% sensitive and 97% specific for BA narrowing of more than 25%. A BA/VA ratio over 3.0 with BA velocities higher than 85 cm/s was 92% sensitive and 97% specific for BA narrowing of more than 50%. The investigators so concluded that the BA/VA ratio increases the sensitivity and specificity of BA VSP diagnosis by TCD. Therefore, the reported evidences indicate that TCD is highly predictive of angiographically demonstrated VSP in the MCA, but its diagnostic accuracy is lower to identify VSP in the BA[80,81]. For VSP detection after aSAH in ACA and PCA territory, TCD’s diagnostic performance has revealed quite insufficient. In a study involving 57 patients undergone TCD study within 24 h of cerebral angiographic exam, a mean CBFV superior to 120 cm/s in ACA showed a 18% sensitivity and 65% specificity to detect VSP and a CBFV superior to ≥ 90 cm/s in PCA had 48% sensitivity and 69% specificity to detect VSP[82]. Therefore, caution should be used to make therapeutic decisions based only on the absence of VSP of ACA or PCA by TCD. So, an increased mean CBFV on TCD is highly predictive of VSP of main intracranial arteries after aSAH. It is of critical importance to evaluate day-to-day changes in CBFV: Mean CBFV raising of 50 cm/s or more within 24-h[83] or mean CBFV increases of > 65 cm/s per day from day 3 to 7[11] indicates high risk for DCI (delayed cerebral ischaemia DCI), which is related to adverse outcome.
In conclusion, the association of clinical examination and different imaging techniques such as computed tomography and TCD should be used for diagnosis of VSP after aSAH instead of the single independent tests[84].
The American Heart Association states that TCD could be considered a valid diagnostic tool to identify and to monitor the development of vasospasm on the management of aSAH[85].
TCD STUDY OF CEREBRAL AUTOREGULATION: IT’S APPLICATION IN ASAH, CAROTID DISEASE, AND SYNCOPE
Cerebral autoregulatory mechanism is a homeostatic function of local brain circulation which keeps CBF constant throughout a wide range of Cerebral Perfusion Pressure (estimated between 50 to 150 mmHg)[28]. Dysfunction of cerebrovascular autoregulation was shown in TBI[86], ischemic cerebrovascular accidents[87], carotid atherosclerosis[88], and in syncope, although for the latter there is still uncertainity about its pathophysiological role[89]. Evaluation of cerebrovascular autoregulation can give useful prognostic information in these conditions[90]. The first evidences regarding physiologic cerebral circulatory autoregulation cam from works which adopted a static approach measuring CBF after a pharmacologic modification of[90]. Following the introduction of TCD, CBFV could be used as an estimate of CBF, allowing dynamic monitoring of local cerebral blood flow.
TCD performed simultaneously with thigh cuff deflation was used for the first times by Aaslid[91] in 1989, after this many different nonpharmacologic stimuli were adopted in order to provoke a pressure modification, like pressure over carotid artery[92], Valsalva manoevre[93], head-up tilting[94], and application of negative pressure to lower portion of the body[89,95]. In particular the static autoregulatory index (sARI), which is calculated as the percent of change in cerebrovascular resistence (CVR) divided by the percent of change in cerebral perfusional pressure (CPP).
sARI[96] = % change in CVR/% change in CPP
This index is used to classify autoregulatory function going from 0 (no response) to 1 (full response). Anyway it should be kept in mind that static methods need pharmacologic or mechanical stimulations which may not be allowed in critically ill patients[87,90,97]. Regarding dynamic study a cerebral autoregulatory function, there is no index which can be considered as gold standard[98]. The Mx index expresses the relationship among CPP and m CBF-V: A positivity of this index means that cerebro-vascular flow is pressure-dependent and absent autoragulation, a negative correlation is found when autoregulary function is preserved[97,99].
Tiecks et al[96] introduced the dynamic autoregulatory index (dARI), a parameter which is obtained constructing,through graphic representations, a CBFV response curve following pressure modifcation and adapting it to 10 of ipothetical models CBFV, ranging from curve 0 (no autoregulatory function) to curve 9 (fully unaffected autoregulation)[96].
In subjects affected by ICA stenosis, derangement of autoregulatory function can represent a marker of high risk of stroke and so it can be used to guide treatment decision making towards revascularization[88,100]. In fact significant decrease in dARI and increase in Mx indexes have been reported in patients with ipsilateral steno-occlusion of ICA, with a significant correlation with the severity of stenotic lesions[88,101]. On the other hand altered dARI and Mx indexes were only found in subjects with severely (> 80%-90%) stenotic carotid arteries and Mx index wasn’t significantly different in symptomatic confronted with asymptomatic subjects[88,101].
In the setting of severe SAH, Lang et al[100] studied cerebral autoregulation through continuous monitoring of BP and CBFV recording in 12 patients, confronted with 40 controls. Autoregulatory function was impaired when compared with control subjects (P < 0.01 for days 106, and P < 0.001 for days 7013). They suggested that TCD could evaluate the entity of autoregulatory dysfunction in patients SAH and a derangement of autoregulation foretells VSP. Moreover the presence of VSP was associated with worsening of autoregulatory response and the degree of cerebral autoregulatory dysfunction in the first days after the event (days 1-6) has a negative prognostic value.
In stroke patients TCD showed a consistent ipsilateral cerebral autoregulation dysfunction, which was associated with the need of surgical decompression, the severity of neurological damage and poor outcome[101]. Many methodological issues of TCD, limit the application of this technique in clinical practice for the evaluation of cerebrovascular autoregulation.
The presence of many different static and dynamic stimuli used in many different studies of this subject, without a reference gold standard methodology to confront with and the absence of a single reference value to define an impairment autoregulatory function impede the comparison and synthesis of different study results[87,89,102]. Moreover many published works have been conducted with small samples and are statistically underpowered[89].
In addiction since the majority of TCD studies is focused on MCA, alterations of autoregulatory function of posterior cerebral vasculature or in regional cortical vessels may be overlooked[87].
In conclusion, TCD imaging represents a promising technique for the study of cerebral autoregulatory function, thanks to its good temporal resolution, non invasive approach, and good cost-benefit ratio.
TCD IN ACUTE ISCHAEMIC STROKE: DIAGNOSIS AND PROGNOSIS
The American Academy of Neurology Report of the Therapeutics and Technology Assessment Subcommittee states that TCD can accurately identify acute MCA occlusions with a sensitivity, specificity, PPV and NPV higher than 90%[29], while for occlusion of ICA siphon, Vertebral Artery (VA) and BA shows 70% to 90% sensitivity and PPV and very high specificity and NPV[29].
In the setting of acute stroke TCD has been confronted with magnetic resonance angiography (MRA) and CTA[103-105]: It has been especially used to assess steno-occlusive pathology of intracranial vessels, such as the terminal ICA, ICA siphon, and MCA. TCD is 100% specific and 93% sensitive for identification of MCA lesions, while MRA had a sensitivity of 46% and a specificity of 74% in the assessment of intracranial arteries. In the emergency department in patients with suspected acute cerebral ischemia, bedside TCD can give real-time information about cerebral blood flow adjunctive to that obtained by CTA[105].
In ischemic stroke, TCD evidence of complete intracranial arterial occlusions predicted worse neurologic outcome, disability, or death after 90 d in 2 studies[106,107]. Normal TCD findings instead predicted early neurological improvement[29,108].
Performing a TCD examination in the first 24 h of stroke symptom onset greatly increases the accuracy of early stroke subtype diagnosis (hemorrhagic vs ischemic). Moreover early and accurate detection of arterial occlusion guides emergency management in patients with acute ischemic cerebrovascular accident. It is universally recognized that clinical course of stroke may present either spontaneous improvements or worsening in relation to dynamic changes in cerebral blood flow. Thus the detection of such haemodynamic changes with the use of TCD may have an important prognostic role.
Cerebral blood flow before and after the administration of thrombolytic agents in ischemic cerebrovascular accident, is described by the thrombolysis in brain ischaemia (TIBI) score[108]. Post-trombolysis flow is classified ranging from 0: Absent flow to 5: Normal flow[109].
TIBI grade and its increase post-thrombolysis correlate with severity, survival, and clinical recovery in ischemic stroke[11,109-112]. As shown by a meta-analysis, reopening of the occluded vessel within a time window of 6 h from stroke symptoms onset, assessed by TCD imaging, portends a better clinical outcome at 48 h (OR = 4.31, 95%CI: 2.67-6.97) and better functional status at 3 mo (OR = 6.75, 95%CI: 3.47-13.12)[113].
Moreover a sudden improvement of TIBI score or its gradual improvement over 30 min denotes more effective vessel recanalization and has been correlated to a better early outcome, whereas those in whom flow restoration takes place after more than 30 min show a significantly worse clinical outcome[111].
Furthermore, applying TIBI score to TCD, early re-occlusion (flow decrease ≥ 1 TIBI grade, within 2 h) after thrombolysis can be recognized. It has been found in about 34% of cases of initial reperfusion[112] and has been associated with a worse outcome at 3 mo and a reduction of survival when confronted with patients experiencing stable reperfusion of occluded artery[112].
So, daily TCD examinations can be useful to recognize dynamic changes in cerebral circulation more time-effectively than a single neuroradiological study. Seriated evaluation of cerebral hemodynamics in patients with acute cerebral ischemia improves the diagnostic accuracy and gives valuable information about monitoring and decision making.
In conclusion, TCD represents a low-cost and readily repeatable diagnostic imaging test characterized by sensitivity and specificity > 80% for ICA and MCA occlusion[99,101].
It also gives useful information about prognosis in MCA occlusion[99,103,104]. However, CTA and MRA should still be used as first-line imaging tests in ischaemic stroke because TCD is operator dependent and has low diagnostic accuracy for posterior circulation occlusive pathology[114].
Sickle cell disease and ischemic stroke
Subjects affected by sickle cell anemia carry a high risk for brain cerebrovascular injuries including stroke and subclinical infarction and haemorrhagic accidents. The rate of ischemic cerebrovascular accidents in this setting is 600 for 100000 patient years[115].
More frequently involved intracranial arteries are ICA, proximal MCA and ACA, adhesion of sickle cells to the vascular endothelium of these vessels results in progressive stenotic or occlusive phenomena.
Asymptomatic children with CBFV > 200 cm/s show an higher rate of stroke events reported as 10000 per 100000 patient-years[116]. Blood transfusions can effectively lower the rate of stroke by > 90%[117]. So for children between 2- and 6-year-old affected by sickle cell anaemia it is recommended to perform a screening by TCD on semestral or annual basis.
On TCD screening peak mean CBFV among major intracranial vessels is measured[118]. Subjects showing a peak time averaged CBFV in all the above mentioned vessels lower than 170 cm/s are considered at low risk[118]. Whereas a CBFV higher than 200 cm/s in any artery demandates blood transfusion aiming to obtain a rate of pathologic haemoglobin lower than 30% in order to decrease the risk of stroke[118].
The Stroke Prevention Trial in Sickle Cell Anemia (STOP Trial) showed that chronic red-cell transfusion reduced the risk of a first stroke by 90% and TCD can be used to screen and identify children at greatest risk of cerebrovascular disease.
TBI AND BRAIN STEM DEATH
Trauma represents, among neurological conditions, the principal cause of morbidity and mortality in people under 45 years of age[119]. It is characterized by thriphasic pattern in cerebral blood flow: Hypoperfusion at time 0, hyperperfusion between 24 to 72 h, vasospasm from days 4 to days 15, and finally by raised ICP[119,120].
Final outcome of patients depends on two main causes: (1) the initial traumatic injury, which takes place at time of accident; and (2) the secondary consecutive pathogenic responses which represents consecutive pathologic processes starting at the moment of trauma and leads to late clinical manifestations (e.g., DCI due to VSP and intracranial hypertension are the most important secondary injuring factors).
TCD allows non invasive and repeatable bedside assessment of post-traumatic cerebrovascular hemodynamic alterations, providing useful prognostic information and has relevant implications for management of TBI patients[8,29].
Moreover TCD in this setting may be useful as a noninvasive mean of calculating of CPP. Czosnyka et al[102] studied the reliability of CPP using TCD-measured CBFV in MCA (mean and diastolic) in 96 patients with TBI (Glasgow Coma Scale < 13). The CPP measured by TCD and the calculated CPP (MAP minus ICP, measured using an intraparenchymal sensor) were compared. The results showed that in 71% of the studies, the estimation error was less than 10 mmHg and in 84% of the examinations, the error was less than 15 mmHg. The TCD method had a high positive predictive power (94%) for detecting low CPP (< 60 mmHg).
Although TCD study allows non-invasive estimation of ICP and CPP, and is widely considered a valuable alternative to invasive monitoring[2], too many formulae have been proposed for this application, often carrying too wide confidence intervals and in many cases without full validation[2,8]. Thus TCD is more properly used to monitor dynamic changes in CPP instead of its real value in the setting of TBI[2].
Cerebral hypoperfusion is correlated with outcome at 6 mo after TBI, so non invasive measurement of CBF through TCD has proven to give information about prognosis similar to invasive CBF assessment[121].
During the 72 h post TBI, a reduced cerebral blood flow state, characterized by an MCA mean-CBFV lower than 35 cm/s has been associated with unfavourable outcome at 6 mo evaluated by Glasgow Outcome Score.
In addition, a worse outcome at 6 mo (GOS 1–3) was demonstrated in 50 patients with head injury in which TCD monitoring showed vasospasm and hyperaemia identified by interrogation of the MCA, ACA, and BA within 7 d from traumatic brain event, respect to the absence of alterations in blood flow velocity[122].
Peak mean-CBFV was also an independent predictor of outcome with higher CBFV values carrying an increased risk for worse outcome evaluated by Glasgow Outcome Score[123].
Diagnosis of brain stem death is usually derived from physical examination and prolonged monitoring[124]. It can be confirmed with the use of ancillary diagnostic modalities, such as EEG, radionuclide scans, and angiography. TCD ultrasonography can be also used to support diagnosis of brain death. In addition it may be of great value in this indication, as it is portable, less time consuming, and can be performed at bedside. Arrest in cerebral circulation is a condition before the terminal state of brain stem death, and it can be evidenced by TCD if one of specific Doppler spectra listed below is obtained insonating BA and ICA or MCA of both sides in two different studies performed at least 30 min apart[125]: (1) an oscillating waveshape (equal systolic anterograde flow and diastolic retrograde flow, i.e., zero net flow); (2) small systolic spikes of lasting less than 200 ms and with a PSV of less than 50 cm/s with no diastolic flow; or (3) The absence of intracranial flow not with concomitant specific findings in extracranial arteries. These peculiar findings come after the progressive increase in ICP which occurs after necrosis of a critically large amount of cerebral tissue.
In detail, when ICP reaches the level of diastolic arterial pressure, then cerebral perfusion will happen exclusively during systole, while with the increase of ICP at the level of systolic arterial pressure there will be no net cerebral blood flow. In this phase TCD will show an oscillatory Doppler signal, as mentioned above, with equalization of area under the envelope of forward and backward Doppler spectra, so that resulting net flow is zero: This pattern has been correlated with angiographic evidence of brain circulatory arrest. Fourteen Later ICP will continue to rise above the level of systolic arterial pressure, at this stage only systolic spikes can be recorded on Doppler spectrum and absence of diastolic flow.
Successively the amplitude of systolic those Doppler signals will progressively decrease, so that in the final stage blood flow will be completely abolished and no Doppler signal can be recorded. In this case the diagnosis of brain death needs to be confirmed by Doppler exploration of extracranial arteries (Common Carotid, ICA and VA). Compared with arteriography as gold standard TCD showed a 100% agreement for diagnosis of brain stem death[126]. A meta-analysis performed by the American Academy of Neurology have demonstrated for this technique a sensitivity of 89%-100% and a specificity of 97%-100%[29,127].
The consensus document of Neurosonology Research Group of the World Federation of Neurology on diagnosis of cerebral circulatory arrest using Doppler-sonography confirms that extracranial and intracranial Doppler sonography is useful as a confirmatory test to establish irreversibility of cerebral circulatory arrest. Although optional, TCD is of special value when the therapeutic use of sedative drugs renders EEG unreliable[128]. This statement also mentions that the absence of flow in MCA precedes complete loss of brain stem functions. The AAN considers TCD a confirmatory test of brain death along with clinical testing and other allied tests[129].
CONCLUSION
To conclude, in NCCU TCD examination should be routinely recommended as a non invasive tool, which allows early identification of patients progressing to VSP secondary to aSAH and TBI. Moreover TCD can be used in NCCU for bed side assessment of CPP with acceptable reliability. The frequency with which TCD should be performed may be guided by patient clinical presentation, risk factors for VSP, and early clinical course. The presence and temporal profile of CBFVs in all available vessels must be detected and serially monitored. The high sensitivity of TCD to identify abnormally high CBFVs due to the onset of VSP demonstrates that TCD is an excellent first-line examination to identify those patients who may need urgent aggressive treatment. Several features of TCD assessment of VSP are similar to cerebral angiography. Most likely, validation of new TCD criteria for VSP and combination of different physiologic monitoring modalities that includes TCD, electroencephalography, brain tissue oxygen monitoring, cerebral microdialysis, and near-infrared spectroscopy will improve TCD accuracy to predict clinical deterioration and infarction from DCI.
Footnotes
Conflict-of-interest statement: The authors report no relevant conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 7, 2016
First decision: April 15, 2016
Article in press: May 27, 2016
P- Reviewer: Razek AAKA, Zhang ZH S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 2.Saqqur M, Zygun D, Demchuk A. Role of transcranial Doppler in neurocritical care. Crit Care Med. 2007;35:S216–S223. doi: 10.1097/01.CCM.0000260633.66384.FB. [DOI] [PubMed] [Google Scholar]
- 3.Di Tullio M, Sacco RL, Gopal A, Mohr JP, Homma S. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992;117:461–465. doi: 10.7326/0003-4819-117-6-461. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh S, Ghosh AK, Ghosh SK. Patent foramen ovale and atrial septal aneurysm in cryptogenic stroke. Postgrad Med J. 2007;83:173–177. doi: 10.1136/pgmj.2006.051094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arenillas JF, Molina CA, Montaner J, Abilleira S, González-Sánchez MA, Alvarez-Sabín J. Progression and clinical recurrence of symptomatic middle cerebral artery stenosis: a long-term follow-up transcranial Doppler ultrasound study. Stroke. 2001;32:2898–2904. doi: 10.1161/hs1201.099652. [DOI] [PubMed] [Google Scholar]
- 6.Christou I, Felberg RA, Demchuk AM, Grotta JC, Burgin WS, Malkoff M, Alexandrov AV. A broad diagnostic battery for bedside transcranial Doppler to detect flow changes with internal carotid artery stenosis or occlusion. J Neuroimaging. 2001;11:236–242. doi: 10.1111/j.1552-6569.2001.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 7.Rigamonti A, Ackery A, Baker AJ. Transcranial Doppler monitoring in subarachnoid hemorrhage: a critical tool in critical care. Can J Anaesth. 2008;55:112–123. doi: 10.1007/BF03016323. [DOI] [PubMed] [Google Scholar]
- 8.White H, Venkatesh B. Applications of transcranial Doppler in the ICU: a review. Intensive Care Med. 2006;32:981–994. doi: 10.1007/s00134-006-0173-y. [DOI] [PubMed] [Google Scholar]
- 9.Bogdahn U, Becker G, Winkler J, Greiner K, Perez J, Meurers B. Transcranial color-coded real-time sonography in adults. Stroke. 1990;21:1680–1688. doi: 10.1161/01.str.21.12.1680. [DOI] [PubMed] [Google Scholar]
- 10.Antignani PL, Benedetti-Valentini F, Aluigi L, Baroncelli TA, Camporese G, Failla G, Martinelli O, Palasciano GC, Pulli R, Rispoli P, et al. Diagnosis of vascular diseases. Ultrasound investigations--guidelines. Int Angiol. 2012;31:1–77. [PubMed] [Google Scholar]
- 11.Tsivgoulis G, Alexandrov AV, Sloan MA. Advances in transcranial Doppler ultrasonography. Curr Neurol Neurosci Rep. 2009;9:46–54. doi: 10.1007/s11910-009-0008-7. [DOI] [PubMed] [Google Scholar]
- 12.Marinoni M, Ginanneschi A, Forleo P, Amaducci L. Technical limits in transcranial Doppler recording: inadequate acoustic windows. Ultrasound Med Biol. 1997;23:1275–1277. doi: 10.1016/s0301-5629(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 13.Babikian V, Sloan MA, Tegeler CH, DeWitt LD, Fayad PB, Feldmann E, Gomez CR. Transcranial Doppler validation pilot study. J Neuroimaging. 1993;3:242–249. doi: 10.1111/jon199334242. [DOI] [PubMed] [Google Scholar]
- 14.Bouzat P, Oddo M, Payen JF. Transcranial Doppler after traumatic brain injury: is there a role? Curr Opin Crit Care. 2014;20:153–160. doi: 10.1097/MCC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 15.Paulus J, Cinotti R, Hamel O, Buffenoir K, Asehnoune K. The echographic “butterfly wing” aspect of the sphenoid bone is a critical landmark to insonate the middle cerebral artery. Intensive Care Med. 2014;40:1783–1784. doi: 10.1007/s00134-014-3447-9. [DOI] [PubMed] [Google Scholar]
- 16.Aaslid R, Huber P, Nornes H. Evaluation of cerebrovascular spasm with transcranial Doppler ultrasound. J Neurosurg. 1984;60:37–41. doi: 10.3171/jns.1984.60.1.0037. [DOI] [PubMed] [Google Scholar]
- 17.Aaslid R. The Doppler principle applied to measurement of blood flow velocity in cerebral arteries. in Vienna RA Transcranial Doppler Sonography, New York, Springer. 1986. pp. 22–38. [Google Scholar]
- 18.Tegeler CH, Crutchfield K, Katsnelson M, Kim J, Tang R, Passmore Griffin L, Rundek T, Evans G. Transcranial Doppler velocities in a large, healthy population. J Neuroimaging. 2013;23:466–472. doi: 10.1111/j.1552-6569.2012.00711.x. [DOI] [PubMed] [Google Scholar]
- 19.Nicoletto HA, Burkman MH. Transcranial Doppler series part II: performing a transcranial Doppler. Am J Electroneurodiagnostic Technol. 2009;49:14–27. [PubMed] [Google Scholar]
- 20.Arnolds BJ, von Reutern GM. Transcranial Doppler sonography. Examination technique and normal reference values. Ultrasound Med Biol. 1986;12:115–123. doi: 10.1016/0301-5629(86)90016-5. [DOI] [PubMed] [Google Scholar]
- 21.Moppett IK, Mahajan RP. Transcranial Doppler ultrasonography in anaesthesia and intensive care. Br J Anaesth. 2004;93:710–724. doi: 10.1093/bja/aeh205. [DOI] [PubMed] [Google Scholar]
- 22.Droste DW, Harders AG, Rastogi E. A transcranial Doppler study of blood flow velocity in the middle cerebral arteries performed at rest and during mental activities. Stroke. 1989;20:1005–1011. doi: 10.1161/01.str.20.8.1005. [DOI] [PubMed] [Google Scholar]
- 23.Patel PM, Drummond JC. 2009. Cerebral physiology and the effects of anesthetic drugs. In Miller’s Anesthesia 7th edition. New York: Churchill Livingstone, 2009: 305-340. [Google Scholar]
- 24.Shahlaie K, Keachie K, Hutchins IM, Rudisill N, Madden LK, Smith KA, Ko KA, Latchaw RE, Muizelaar JP. Risk factors for posttraumatic vasospasm. J Neurosurg. 2011;115:602–611. doi: 10.3171/2011.5.JNS101667. [DOI] [PubMed] [Google Scholar]
- 25.Kaps M, Stolz E, Allendoerfer J. Prognostic value of transcranial sonography in acute stroke patients. Eur Neurol. 2008;59 Suppl 1:9–16. doi: 10.1159/000114455. [DOI] [PubMed] [Google Scholar]
- 26.Lindegaard KF, Nornes H, Bakke SJ, Sorteberg W, Nakstad P. Cerebral vasospasm after subarachnoid haemorrhage investigated by means of transcranial Doppler ultrasound. Acta Neurochir Suppl (Wien) 1988;42:81–84. doi: 10.1007/978-3-7091-8975-7_16. [DOI] [PubMed] [Google Scholar]
- 27.Martin PJ, Evans DH, Naylor AR. Transcranial color-coded sonography of the basal cerebral circulation. Reference data from 115 volunteers. Stroke. 1994;25:390–396. doi: 10.1161/01.str.25.2.390. [DOI] [PubMed] [Google Scholar]
- 28.Rasulo FA, De Peri E, Lavinio A. Transcranial Doppler ultrasonography in intensive care. Eur J Anaesthesiol Suppl. 2008;42:167–173. doi: 10.1017/S0265021507003341. [DOI] [PubMed] [Google Scholar]
- 29.Sloan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, Wechsler LR, Newell DW, Gomez CR, Babikian VL, et al. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004;62:1468–1481. doi: 10.1212/wnl.62.9.1468. [DOI] [PubMed] [Google Scholar]
- 30.Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, Chedru F, Guérin F, Bousser MG, de Recondo J. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke. 1993;24:1865–1873. doi: 10.1161/01.str.24.12.1865. [DOI] [PubMed] [Google Scholar]
- 31.D'Andrea A, Calabrò R. The diagnosis of cryptogenic stroke: is the combined ultrasound approach the right choice? J Cardiovasc Med (Hagerstown) 2011;12:527–529. doi: 10.2459/JCM.0b013e32834976d6. [DOI] [PubMed] [Google Scholar]
- 32.Sarkar S, Ghosh S, Ghosh SK, Collier A. Role of transcranial Doppler ultrasonography in stroke. Postgrad Med J. 2007;83:683–689. doi: 10.1136/pgmj.2007.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kerut EK, Norfleet WT, Plotnick GD, Giles TD. Patent foramen ovale: a review of associated conditions and the impact of physiological size. J Am Coll Cardiol. 2001;38:613–623. doi: 10.1016/s0735-1097(01)01427-9. [DOI] [PubMed] [Google Scholar]
- 34.Hara H, Virmani R, Ladich E, Mackey-Bojack S, Titus J, Reisman M, Gray W, Nakamura M, Mooney M, Poulose A, et al. Patent foramen ovale: current pathology, pathophysiology, and clinical status. J Am Coll Cardiol. 2005;46:1768–1776. doi: 10.1016/j.jacc.2005.08.038. [DOI] [PubMed] [Google Scholar]
- 35.Wu LA, Malouf JF, Dearani JA, Hagler DJ, Reeder GS, Petty GW, Khandheria BK. Patent foramen ovale in cryptogenic stroke: current understanding and management options. Arch Intern Med. 2004;164:950–956. doi: 10.1001/archinte.164.9.950. [DOI] [PubMed] [Google Scholar]
- 36.Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17–20. doi: 10.1016/s0025-6196(12)60336-x. [DOI] [PubMed] [Google Scholar]
- 37.Knauth M, Ries S, Pohimann S, Kerby T, Forsting M, Daffertshofer M, Hennerici M, Sartor K. Cohort study of multiple brain lesions in sport divers: role of a patent foramen ovale. BMJ. 1997;314:701–705. doi: 10.1136/bmj.314.7082.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godart F, Rey C, Prat A, Vincentelli A, Chmaït A, Francart C, Porte H. Atrial right-to-left shunting causing severe hypoxaemia despite normal right-sided pressures. Report of 11 consecutive cases corrected by percutaneous closure. Eur Heart J. 2000;21:483–489. doi: 10.1053/euhj.1999.1944. [DOI] [PubMed] [Google Scholar]
- 39.Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, Coste J, Mas JL. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA Study. Atrial Septal Aneurysm. Stroke. 2002;33:706–711. doi: 10.1161/hs0302.104543. [DOI] [PubMed] [Google Scholar]
- 40.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 41.Woods TD, Patel A. A critical review of patent foramen ovale detection using saline contrast echocardiography: when bubbles lie. J Am Soc Echocardiogr. 2006;19:215–222. doi: 10.1016/j.echo.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 42.Meltzer RS, Tickner EG, Sahines TP, Popp RL. The source of ultrasound contrast effect. J Clin Ultrasound. 1980;8:121–127. doi: 10.1002/jcu.1870080205. [DOI] [PubMed] [Google Scholar]
- 43.Lefèvre J, Lafitte S, Reant P, Perron JM, Roudaut R. Optimization of patent foramen ovale detection by contrast transthoracic echocardiography using second harmonic imaging. Arch Cardiovasc Dis. 2008;101:213–219. doi: 10.1016/s1875-2136(08)73695-7. [DOI] [PubMed] [Google Scholar]
- 44.Van Camp G, Franken P, Melis P, Cosyns B, Schoors D, Vanoverschelde JL. Comparison of transthoracic echocardiography with second harmonic imaging with transesophageal echocardiography in the detection of right to left shunts. Am J Cardiol. 2000;86:1284–1287, A9. doi: 10.1016/s0002-9149(00)01224-8. [DOI] [PubMed] [Google Scholar]
- 45.Kühl HP, Hoffmann R, Merx MW, Franke A, Klötzsch C, Lepper W, Reineke T, Noth J, Hanrath P. Transthoracic echocardiography using second harmonic imaging: diagnostic alternative to transesophageal echocardiography for the detection of atrial right to left shunt in patients with cerebral embolic events. J Am Coll Cardiol. 1999;34:1823–1830. doi: 10.1016/s0735-1097(99)00412-x. [DOI] [PubMed] [Google Scholar]
- 46.Jauss M, Zanette E. Detection of right-to-left shunt with ultrasound contrast agent and transcranial Doppler sonography. Cerebrovasc Dis. 2000;10:490–496. doi: 10.1159/000016119. [DOI] [PubMed] [Google Scholar]
- 47.Rajamani K, Gorman M. Transcranial Doppler in stroke. Biomed Pharmacother. 2001;55:247–257. doi: 10.1016/s0753-3322(01)00063-4. [DOI] [PubMed] [Google Scholar]
- 48.Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Dávalos A. The need to quantify right-to-left shunt in acute ischemic stroke: a case-control study. Stroke. 1998;29:1322–1328. doi: 10.1161/01.str.29.7.1322. [DOI] [PubMed] [Google Scholar]
- 49.Lange MC, Zétola VF, deSouza AM, Novak FM, Piovesan EJ, Werneck LC. Intracranial embolism characteristics in PFO patients: a comparison between positive and negative PFO by transesophageal echocardiography: the rule of nine. J Neurol Sci. 2010;293:106–109. doi: 10.1016/j.jns.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Meier B, Lock JE. Contemporary management of patent foramen ovale. Circulation. 2003;107:5–9. doi: 10.1161/01.cir.0000046073.34261.c1. [DOI] [PubMed] [Google Scholar]
- 51.Mojadidi MK, Roberts SC, Winoker JS, Romero J, Goodman-Meza D, Gevorgyan R, Tobis JM. Accuracy of transcranial Doppler for the diagnosis of intracardiac right-to-left shunt: a bivariate meta-analysis of prospective studies. JACC Cardiovasc Imaging. 2014;7:236–250. doi: 10.1016/j.jcmg.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 52.González-Alujas T, Evangelista A, Santamarina E, Rubiera M, Gómez-Bosch Z, Rodríguez-Palomares JF, Avegliano G, Molina C, Alvarez-Sabín J, García-Dorado D. Diagnosis and quantification of patent foramen ovale. Which is the reference technique? Simultaneous study with transcranial Doppler, transthoracic and transesophageal echocardiography. Rev Esp Cardiol. 2011;64:133–139. doi: 10.1016/j.recesp.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Zoghbi WA. Patent foramen ovale: going beyond the bubbles. JACC Cardiovasc Imaging. 2014;7:251–253. doi: 10.1016/j.jcmg.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Johansson MC, Eriksson P, Guron CW, Dellborg M. Pitfalls in diagnosing PFO: characteristics of false-negative contrast injections during transesophageal echocardiography in patients with patent foramen ovales. J Am Soc Echocardiogr. 2010;23:1136–1142. doi: 10.1016/j.echo.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Souteyrand G, Motreff P, Lusson JR, Rodriguez R, Geoffroy E, Dauphin C, Boire JY, Lamaison D, Cassagnes J. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr. 2006;7:147–154. doi: 10.1016/j.euje.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Clarke NR, Timperley J, Kelion AD, Banning AP. Transthoracic echocardiography using second harmonic imaging with Valsalva manoeuvre for the detection of right to left shunts. Eur J Echocardiogr. 2004;5:176–181. doi: 10.1016/S1525-2167(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 57.Daniëls C, Weytjens C, Cosyns B, Schoors D, De Sutter J, Paelinck B, Muyldermans L, Van Camp G. Second harmonic transthoracic echocardiography: the new reference screening method for the detection of patent foramen ovale. Eur J Echocardiogr. 2004;5:449–452. doi: 10.1016/j.euje.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Inzitari D. The Italian Guidelines for stroke prevention. The Stroke Prevention and Educational Awareness Diffusion (SPREAD) Collaboration. Neurol Sci. 2000;21:5–12. doi: 10.1007/s100720070112. [DOI] [PubMed] [Google Scholar]
- 59.Inzitari D, Carlucci G. Italian Stroke Guidelines (SPREAD): evidence and clinical practice. Neurol Sci. 2006;27 Suppl 3:S225–S227. doi: 10.1007/s10072-006-0622-y. [DOI] [PubMed] [Google Scholar]
- 60.Pristipino C, Anzola GP, Ballerini L, Bartorelli A, Cecconi M, Chessa M, Donti A, Gaspardone A, Neri G, Onorato E, et al. [Multidisciplinary position paper on the management of patent foramen ovale in the presence of cryptogenic cerebral ischemia - Italian version 2013] G Ital Cardiol (Rome) 2013;14:699–712. doi: 10.1714/1335.14838. [DOI] [PubMed] [Google Scholar]
- 61.Anzola GP, Morandi E, Casilli F, Onorato E. Does transcatheter closure of patent foramen ovale really “shut the door?” A prospective study with transcranial Doppler. Stroke. 2004;35:2140–2144. doi: 10.1161/01.STR.0000137764.07815.de. [DOI] [PubMed] [Google Scholar]
- 62.Sorensen SG, Aguilar H, McKnight WK, Thomas H, Muhlestein JB. Transcranial Doppler quantification of residual shunt after percutaneous patent foramen ovale closure. Comparison of two devices. J Interv Cardiol. 2010;23:575–580. doi: 10.1111/j.1540-8183.2010.00587.x. [DOI] [PubMed] [Google Scholar]
- 63.Ursino M, Giulioni M. Quantitative assessment of cerebral autoregulation from transcranial Doppler pulsatility: a computer simulation study. Med Eng Phys. 2003;25:655–666. doi: 10.1016/s1350-4533(02)00251-5. [DOI] [PubMed] [Google Scholar]
- 64.Chang JJ, Tsivgoulis G, Katsanos AH, Malkoff MD, Alexandrov AV. Diagnostic Accuracy of Transcranial Doppler for Brain Death Confirmation: Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2016;37:408–414. doi: 10.3174/ajnr.A4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moreno JA, Mesalles E, Gener J, Tomasa A, Ley A, Roca J, Fernández-Llamazares J. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. 2000;8:e8. doi: 10.3171/foc.2000.8.1.1702. [DOI] [PubMed] [Google Scholar]
- 66.Velat GJ, Kimball MM, Mocco JD, Hoh BL. Vasospasm after aneurysmal subarachnoid hemorrhage: review of randomized controlled trials and meta-analyses in the literature. World Neurosurg. 2011;76:446–454. doi: 10.1016/j.wneu.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 67.Dorsch N. A clinical review of cerebral vasospasm and delayed ischaemia following aneurysm rupture. Acta Neurochir Suppl. 2011;110:5–6. doi: 10.1007/978-3-7091-0353-1_1. [DOI] [PubMed] [Google Scholar]
- 68.Papaioannou V, Dragoumanis C, Theodorou V, Konstantonis D, Pneumatikos I, Birbilis T. Transcranial Doppler ultrasonography in intensive care unit. Report of a case with subarachnoid hemorrhage and brain death and review of the literature. Greek ej Periop Med. 2008;6:95–104. [Google Scholar]
- 69.Biller J, Godersky JC, Adams HP. Management of aneurysmal subarachnoid hemorrhage. Stroke. 1988;19:1300–1305. doi: 10.1161/01.str.19.10.1300. [DOI] [PubMed] [Google Scholar]
- 70.Harders AG, Gilsbach JM. Time course of blood velocity changes related to vasospasm in the circle of Willis measured by transcranial Doppler ultrasound. J Neurosurg. 1987;66:718–728. doi: 10.3171/jns.1987.66.5.0718. [DOI] [PubMed] [Google Scholar]
- 71.Armonda RA, Bell RS, Vo AH, Ling G, DeGraba TJ, Crandall B, Ecklund J, Campbell WW. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215–1225; discussion 1225. doi: 10.1227/01.NEU.0000249190.46033.94. [DOI] [PubMed] [Google Scholar]
- 72.Keyrouz SG, Diringer MN. Clinical review: Prevention and therapy of vasospasm in subarachnoid hemorrhage. Crit Care. 2007;11:220. doi: 10.1186/cc5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rowland MJ, Hadjipavlou G, Kelly M, Westbrook J, Pattinson KT. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth. 2012;109:315–329. doi: 10.1093/bja/aes264. [DOI] [PubMed] [Google Scholar]
- 74.Topcuoglu MA, Pryor JC, Ogilvy CS, Kistler JP. Cerebral Vasospasm Following Subarachnoid Hemorrhage. Curr Treat Options Cardiovasc Med. 2002;4:373–384. doi: 10.1007/s11936-002-0017-1. [DOI] [PubMed] [Google Scholar]
- 75.Sviri GE, Ghodke B, Britz GW, Douville CM, Haynor DR, Mesiwala AH, Lam AM, Newell DW. Transcranial Doppler grading criteria for basilar artery vasospasm. Neurosurgery. 2006;59:360–366; discussion 360-366. doi: 10.1227/01.NEU.0000223502.93013.6E. [DOI] [PubMed] [Google Scholar]
- 76.Bederson JB, Connolly ES, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 77.McGirt MJ, Blessing RP, Goldstein LB. Transcranial Doppler monitoring and clinical decision-making after subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2003;12:88–92. doi: 10.1053/jscd.2003.10. [DOI] [PubMed] [Google Scholar]
- 78.Washington CW, Zipfel GJ. Detection and monitoring of vasospasm and delayed cerebral ischemia: a review and assessment of the literature. Neurocrit Care. 2011;15:312–317. doi: 10.1007/s12028-011-9594-8. [DOI] [PubMed] [Google Scholar]
- 79.Lysakowski C, Walder B, Costanza MC, Tramèr MR. Transcranial Doppler versus angiography in patients with vasospasm due to a ruptured cerebral aneurysm: A systematic review. Stroke. 2001;32:2292–2298. doi: 10.1161/hs1001.097108. [DOI] [PubMed] [Google Scholar]
- 80.Harders A, Gilsbach J. Transcranial Doppler sonography and its application in extracranial-intracranial bypass surgery. Neurol Res. 1985;7:129–141. doi: 10.1080/01616412.1985.11739711. [DOI] [PubMed] [Google Scholar]
- 81.Skjelland M, Krohg-Sørensen K, Tennøe B, Bakke SJ, Brucher R, Russell D. Cerebral microemboli and brain injury during carotid artery endarterectomy and stenting. Stroke. 2009;40:230–234. doi: 10.1161/STROKEAHA.107.513341. [DOI] [PubMed] [Google Scholar]
- 82.Wozniak MA, Sloan MA, Rothman MI, Burch CM, Rigamonti D, Permutt T, Numaguchi Y. Detection of vasospasm by transcranial Doppler sonography. The challenges of the anterior and posterior cerebral arteries. J Neuroimaging. 1996;6:87–93. doi: 10.1111/jon19966287. [DOI] [PubMed] [Google Scholar]
- 83.Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, Connolly ES, Mayer SA. Defining vasospasm after subarachnoid hemorrhage: what is the most clinically relevant definition? Stroke. 2009;40:1963–1968. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 84.Gonzalez NR, Boscardin WJ, Glenn T, Vinuela F, Martin NA. Vasospasm probability index: a combination of transcranial doppler velocities, cerebral blood flow, and clinical risk factors to predict cerebral vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2007;107:1101–1112. doi: 10.3171/JNS-07/12/1101. [DOI] [PubMed] [Google Scholar]
- 85.Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- 86.Puppo C, López L, Caragna E, Biestro A. One-minute dynamic cerebral autoregulation in severe head injury patients and its comparison with static autoregulation. A transcranial Doppler study. Neurocrit Care. 2008;8:344–352. doi: 10.1007/s12028-008-9069-8. [DOI] [PubMed] [Google Scholar]
- 87.Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010;41:2697–2704. doi: 10.1161/STROKEAHA.110.594168. [DOI] [PubMed] [Google Scholar]
- 88.Reinhard M, Roth M, Müller T, Czosnyka M, Timmer J, Hetzel A. Cerebral autoregulation in carotid artery occlusive disease assessed from spontaneous blood pressure fluctuations by the correlation coefficient index. Stroke. 2003;34:2138–2144. doi: 10.1161/01.STR.0000087788.65566.AC. [DOI] [PubMed] [Google Scholar]
- 89.Panerai RB. Transcranial Doppler for evaluation of cerebral autoregulation. Clin Auton Res. 2009;19:197–211. doi: 10.1007/s10286-009-0011-8. [DOI] [PubMed] [Google Scholar]
- 90.Panerai RB. Assessment of cerebral pressure autoregulation in humans--a review of measurement methods. Physiol Meas. 1998;19:305–338. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- 91.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 92.Giller CA. A bedside test for cerebral autoregulation using transcranial Doppler ultrasound. Acta Neurochir (Wien) 1991;108:7–14. doi: 10.1007/BF01407660. [DOI] [PubMed] [Google Scholar]
- 93.Tiecks FP, Douville C, Byrd S, Lam AM, Newell DW. Evaluation of impaired cerebral autoregulation by the Valsalva maneuver. Stroke. 1996;27:1177–1182. doi: 10.1161/01.str.27.7.1177. [DOI] [PubMed] [Google Scholar]
- 94.Schondorf R, Stein R, Roberts R, Benoit J, Cupples W. Dynamic cerebral autoregulation is preserved in neurally mediated syncope. J Appl Physiol (1985) 2001;91:2493–2502. doi: 10.1152/jappl.2001.91.6.2493. [DOI] [PubMed] [Google Scholar]
- 95.Levine BD, Giller CA, Lane LD, Buckey JC, Blomqvist CG. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation. 1994;90:298–306. doi: 10.1161/01.cir.90.1.298. [DOI] [PubMed] [Google Scholar]
- 96.Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- 97.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10:373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 98.Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng. 2008;8:42–59. doi: 10.1007/s10558-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 99.Czosnyka M, Smielewski P, Kirkpatrick P, Menon DK, Pickard JD. Monitoring of cerebral autoregulation in head-injured patients. Stroke. 1996;27:1829–1834. doi: 10.1161/01.str.27.10.1829. [DOI] [PubMed] [Google Scholar]
- 100.Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med. 2001;29:158–163. doi: 10.1097/00003246-200101000-00031. [DOI] [PubMed] [Google Scholar]
- 101.White RP, Markus HS. Impaired dynamic cerebral autoregulation in carotid artery stenosis. Stroke. 1997;28:1340–1344. doi: 10.1161/01.str.28.7.1340. [DOI] [PubMed] [Google Scholar]
- 102.Czosnyka M, Matta BF, Smielewski P, Kirkpatrick PJ, Pickard JD. Cerebral perfusion pressure in head-injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg. 1998;88:802–808. doi: 10.3171/jns.1998.88.5.0802. [DOI] [PubMed] [Google Scholar]
- 103.Demchuk AM, Christou I, Wein TH, Felberg RA, Malkoff M, Grotta JC, Alexandrov AV. Accuracy and criteria for localizing arterial occlusion with transcranial Doppler. J Neuroimaging. 2000;10:1–12. doi: 10.1111/jon20001011. [DOI] [PubMed] [Google Scholar]
- 104.Razumovsky AY, Gillard JH, Bryan RN, Hanley DF, Oppenheimer SM. TCD, MRA and MRI in acute cerebral ischemia. Acta Neurol Scand. 1999;99:65–76. doi: 10.1111/j.1600-0404.1999.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 105.Tsivgoulis G, Sharma VK, Lao AY, Malkoff MD, Alexandrov AV. Validation of transcranial Doppler with computed tomography angiography in acute cerebral ischemia. Stroke. 2007;38:1245–1249. doi: 10.1161/01.STR.0000259712.64772.85. [DOI] [PubMed] [Google Scholar]
- 106.Camerlingo M, Casto L, Censori B, Servalli MC, Ferraro B, Mamoli A. Prognostic use of ultrasonography in acute non-hemorrhagic carotid stroke. Ital J Neurol Sci. 1996;17:215–218. doi: 10.1007/BF01995686. [DOI] [PubMed] [Google Scholar]
- 107.Baracchini C, Manara R, Ermani M, Meneghetti G. The quest for early predictors of stroke evolution: can TCD be a guiding light? Stroke. 2000;31:2942–2947. doi: 10.1161/01.str.31.12.2942. [DOI] [PubMed] [Google Scholar]
- 108.Kushner MJ, Zanette EM, Bastianello S, Mancini G, Sacchetti ML, Carolei A, Bozzao L. Transcranial Doppler in acute hemispheric brain infarction. Neurology. 1991;41:109–113. doi: 10.1212/wnl.41.1.109. [DOI] [PubMed] [Google Scholar]
- 109.Demchuk AM, Burgin WS, Christou I, Felberg RA, Barber PA, Hill MD, Alexandrov AV. Thrombolysis in brain ischemia (TIBI) transcranial Doppler flow grades predict clinical severity, early recovery, and mortality in patients treated with intravenous tissue plasminogen activator. Stroke. 2001;32:89–93. doi: 10.1161/01.str.32.1.89. [DOI] [PubMed] [Google Scholar]
- 110.Christou I, Alexandrov AV, Burgin WS, Wojner AW, Felberg RA, Malkoff M, Grotta JC. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke. 2000;31:1812–1816. doi: 10.1161/01.str.31.8.1812. [DOI] [PubMed] [Google Scholar]
- 111.Alexandrov AV, Burgin WS, Demchuk AM, El-Mitwalli A, Grotta JC. Speed of intracranial clot lysis with intravenous tissue plasminogen activator therapy: sonographic classification and short-term improvement. Circulation. 2001;103:2897–2902. doi: 10.1161/01.cir.103.24.2897. [DOI] [PubMed] [Google Scholar]
- 112.Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. doi: 10.1212/wnl.59.6.862. [DOI] [PubMed] [Google Scholar]
- 113.Stolz E, Cioli F, Allendoerfer J, Gerriets T, Del Sette M, Kaps M. Can early neurosonology predict outcome in acute stroke?: a metaanalysis of prognostic clinical effect sizes related to the vascular status. Stroke. 2008;39:3255–3261. doi: 10.1161/STROKEAHA.108.522714. [DOI] [PubMed] [Google Scholar]
- 114.Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Qureshi AI, Rosenfield K, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 115.Platt OS. Prevention and management of stroke in sickle cell anemia. Hematology Am Soc Hematol Educ Program. 2006:54–57. doi: 10.1182/asheducation-2006.1.54. [DOI] [PubMed] [Google Scholar]
- 116.Adams RJ, McKie VC, Carl EM, Nichols FT, Perry R, Brock K, McKie K, Figueroa R, Litaker M, Weiner S, et al. Long-term stroke risk in children with sickle cell disease screened with transcranial Doppler. Ann Neurol. 1997;42:699–704. doi: 10.1002/ana.410420505. [DOI] [PubMed] [Google Scholar]
- 117.Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C, Abboud M, Gallagher D, Kutlar A, Nichols FT, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 118.Adams RJ. TCD in sickle cell disease: an important and useful test. Pediatr Radiol. 2005;35:229–234. doi: 10.1007/s00247-005-1409-7. [DOI] [PubMed] [Google Scholar]
- 119.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 120.Martin NA, Patwardhan RV, Alexander MJ, Africk CZ, Lee JH, Shalmon E, Hovda DA, Becker DP. Characterization of cerebral hemodynamic phases following severe head trauma: hypoperfusion, hyperemia, and vasospasm. J Neurosurg. 1997;87:9–19. doi: 10.3171/jns.1997.87.1.0009. [DOI] [PubMed] [Google Scholar]
- 121.Jaggi JL, Obrist WD, Gennarelli TA, Langfitt TW. Relationship of early cerebral blood flow and metabolism to outcome in acute head injury. J Neurosurg. 1990;72:176–182. doi: 10.3171/jns.1990.72.2.0176. [DOI] [PubMed] [Google Scholar]
- 122.van Santbrink H, Schouten JW, Steyerberg EW, Avezaat CJ, Maas AI. Serial transcranial Doppler measurements in traumatic brain injury with special focus on the early posttraumatic period. Acta Neurochir (Wien) 2002;144:1141–1149. doi: 10.1007/s00701-002-1012-8. [DOI] [PubMed] [Google Scholar]
- 123.Zurynski YA, Dorsch NW, Fearnside MR. Incidence and effects of increased cerebral blood flow velocity after severe head injury: a transcranial Doppler ultrasound study II. Effect of vasospasm and hyperemia on outcome. J Neurol Sci. 1995;134:41–46. doi: 10.1016/0022-510x(95)00178-x. [DOI] [PubMed] [Google Scholar]
- 124.Llompart-Pou JA, Abadal JM, Güenther A, Rayo L, Martín-del Rincón JP, Homar J, Pérez-Bárcena J. Transcranial sonography and cerebral circulatory arrest in adults: a comprehensive review. ISRN Critical Care. 2013 [Google Scholar]
- 125.Ducrocq X, Braun M, Debouverie M, Junges C, Hummer M, Vespignani H. Brain death and transcranial Doppler: experience in 130 cases of brain dead patients. J Neurol Sci. 1998;160:41–46. doi: 10.1016/s0022-510x(98)00188-9. [DOI] [PubMed] [Google Scholar]
- 126.Poularas J, Karakitsos D, Kouraklis G, Kostakis A, De Groot E, Kalogeromitros A, Bilalis D, Boletis J, Karabinis A. Comparison between transcranial color Doppler ultrasonography and angiography in the confirmation of brain death. Transplant Proc. 2006;38:1213–1217. doi: 10.1016/j.transproceed.2006.02.127. [DOI] [PubMed] [Google Scholar]
- 127.Monteiro LM, Bollen CW, van Huffelen AC, Ackerstaff RG, Jansen NJ, van Vught AJ. Transcranial Doppler ultrasonography to confirm brain death: a meta-analysis. Intensive Care Med. 2006;32:1937–1944. doi: 10.1007/s00134-006-0353-9. [DOI] [PubMed] [Google Scholar]
- 128.Ducrocq X, Hassler W, Moritake K, Newell DW, von Reutern GM, Shiogai T, Smith RR. Consensus opinion on diagnosis of cerebral circulatory arrest using Doppler-sonography: Task Force Group on cerebral death of the Neurosonology Research Group of the World Federation of Neurology. J Neurol Sci. 1998;159:145–150. doi: 10.1016/s0022-510x(98)00158-0. [DOI] [PubMed] [Google Scholar]
- 129.Wijdicks EF. Determining brain death in adults. Neurology. 1995;45:1003–1011. doi: 10.1212/wnl.45.5.1003. [DOI] [PubMed] [Google Scholar]


