Abstract
Takotsubo cardiomyopathy is a syndrome mimicking an acute myocardial infarction in absence of obstructive epicardial coronary artery disease to explain the degree of the wall motion abnormalities. Typically more common in the elderly women, this condition is usually triggered by unexpected emotional or physical stress situations, and is associated with electrocardiogram abnormalities and slight elevation of cardiac biomarkers. The pathophysiological mechanism is not clear yet, but it is believed that a high circulating concentration of catecholamines causes an acute dysfunction of the coronary microcirculation and metabolism of cardiomyocytes, leading to a transient myocardial stunning. Typically, it presents with acute left ventricular systolic dysfunction that in most cases is completely resolved at short term. Recurrences are rare and it is thought that the long-term prognosis is good. We present here a review of the clinical features, pathophysiology and management of this enigmatic condition.
Keywords: Takotsubo cardiomyopathy, Stress, Review, Myocardial stunning, Left ventricle systolic dysfunction
Core tip: Takotsubo cardiomyopathy is a syndrome mimicking an acute myocardial infarction in absence of obstructive epicardial coronary artery disease to explain the degree of the wall motion abnormalities. Typically more common in the elderly women, this condition is usually triggered by unexpected emotional or physical stress situations, and presents with acute left ventricular systolic dysfunction that in most cases is completely resolved at short term. Recurrences are rare and it is thought that the long-term prognosis is good. We present here a review of the clinical features, pathophysiology and management of this enigmatic condition.
INTRODUCTION
Takotsubo cardiomyopathy (TTC) was first described in Japan at the beginning of 90’s[1]. Patients with this condition present signs and symptoms resembling those with an acute coronary syndrome (ACS), but the angiographic appearance of the epicardial coronary arteries do not explain neither the grade of the left ventricle systolic dysfunction (LVSD) nor the wall motion abnormalities typically observed in this syndrome[2]. The term “takotsubo” was used to remind the octopus trap form of the left ventricle during systole in the acute phase of disease, as result of the wall motion abnormalities in the mid-apical segments with hyperkinetic motion of the base. Along the first years of its description, it was observed that most affected people were postmenopausal women after suffering a stress situation. However, cases in men and young people have been progressively reported. Although the left ventricle mid-apical dysfunction is the pattern most frequently found, transient abnormalities in other myocardial segments have been described, such as mid-ventricular and “inverted” forms. On the other hand, there is a significant percentage of patients in whom a trigger is not identified. Therefore, currently it is recognized that TTC is a multifaceted disease with a wide spectrum[3].
Many names have been used for calling this syndrome, including stress cardiomyopathy, transient apical dyskinesia, broken heart syndrome, apical ballooning or transient cardiomyopathy, but a consensus to define an universal name is lacking. Due to different forms of presentation, it seems more appropriate to use the term “takotsubo cardiomyopathy”[4]. Recently, it has been proposed include TTC as part of the so-called syndrome of “acute myocardial infarction without obstructive coronary atherosclerosis”[5].
Throughout this review, based in our experience and that of the other authors, we will discuss the clinical, epidemiological and electrocardiographic features of this syndrome with an approach to the pathophysiological hypotheses and advances in the understanding of this enigmatic disease.
CLINICAL FEATURES AND EPIDEMIOLOGY
TTC has been increasingly recognized along last two decades[6-8], but it is still a rare condition. The true incidence of this syndrome is unknown. Several studies have estimated an incidence ranging 1.2%-2% among patients undergoing coronary angiography with a presumptive diagnosis of ACS[9,10]. Near 90% of patients with TTC are women, most of them at postmenopausal period with a mean age around 70 years[11]. Hypertension is the predominant cardiovascular risk factor (CVRF), while prevalence of diabetes is low[12], specially compared with those patients with ACS in whom diabetes is present at least as twice (30%)[13]. In Spain, most patients with TTC have no more than 2 CVRF (68.7%)[14]. The high predominance of female gender and the cardiovascular risk profile support the notion that coronary atherosclerosis in this syndrome does not seem to play a key role in the primary mechanisms, as in fact it happens in ACS.
Regarding clinical picture, chest pain is the most common presentation symptom, affecting 54%-80% of patients, followed by dyspnea[14-18]; among patients presenting with chest pain, typical rest angina is by far the most common symptom (59%)[14]. A triggering factor can be identified in 70%-86% of cases, being the distribution between emotional and physical stressful situations very variable among different case series studies. Within emotional triggers, the unexpected death of a loved one and family matters are very frequent, while severe acute illness and post-operative states are very common within physical triggers[14,18]. Psychological factors may play a key role in the triggering mechanisms of TTC. A high prevalence of psychiatric disorders (acute or chronic) has been recently reported, being the affective disorders, specially depression and chronic anxiety, a common finding[18,19]. TTC patients suffer psychiatric disorders more than twice than patients with ACS. These observations may lead to propose the chronic affective disorders as predisposing factors to develop TTC.
Of note, some epidemiological features such as the incidence, the most prevalence in women, the average age around 70 years, the low prevalence of diabetes, and the chest pain as the most common symptom are concordant between the published series along worldwide (Table 1). However, concerning other epidemiological data such as frequency of triggering factors, differences between those emotional and physical triggers, and incidence of specific electrocardiographic (ECG) abnormalities are very variable, which might suggest some ethnic variations of the disease or a more aggressive diagnostic approach in some countries.
Table 1.
Epidemiological and clinical features of takotsubo cardiomyopathy
| Tsuchihashi et al[15] | Núñez et al[14] | Kurowski et al[13] | Eshtehardi et al[16] | Parodi et al[11] | Ahmed et al[17] | Templin et al[18] | |
| Country | Japan | Spain | Germany | Swiss | Italy | United States | Europe and United States |
| Year of publication | 2001 | 2015 | 2007 | 2009 | 2007 | 2013 | 2015 |
| Subjects, n | 88 | 202 | 35 | 41 | 36 | 620 (systematic review) | 1750 (international registry) |
| Age (yr) | 67 ± 13 | 70 ± 12.5 | 72 ± 9 | 65 ± 11 | 75 ± 7 | 67 | 66.8 ± 13 |
| In percentage (%) | |||||||
| Reported incidence1 | --- | 1.2 | 1.2 | 1.7 | 2 | --- | --- |
| Women | 86 | 90 | 94 | 85 | 1006 | 91 | 89.8 |
| Hypertension | 48 | 67 | 74 | 56 | 50 | --- | 65 |
| Diabetes | 12 | 15 | 23 | 5 | 5.5 | --- | 14 |
| Hyperlipidemia | 24 | 41 | 34 | 39 | 39 | --- | 31 |
| Current smoking | --- | 15 | 20 | 27 | 19 | --- | 20 |
| Apical type | 1003 | --- | 60 | --- | --- | --- | 81.7 |
| Emotional/psychological trigger | 20 | 50 | 43 | 46 | --- | 41 | 27.7 |
| Physical (acute diseases, exercise, surgery and medical procedures) trigger | 53 | 20 | 43 | 17 | --- | 45 | 36 |
| No identified triggering factor | 26 | 27 | 14 | 37 | 28 | 14 | 28.5 |
| Chest pain | 67 | 80 | --- | 76 | 1002 | 54 | 76 |
| Dyspnea | 7 | 45 | --- | 24 | --- | 26 | 47 |
| Syncope | --- | 9 | --- | --- | --- | --- | 7.7 |
| ST segment elevation | 90 | 62 | 69 | 39 | 1002 | 39 | 43.7 |
| T wave inversion | 97 | 94.4 | --- | 46 | --- | 31 | 415 |
| In hospital mortality | 1 | 2.44 | 9 | 0 | --- | 4 | 4.1 |
| Long term mortality from all causes | --- | --- | 8.6 (at 12 mo) | 2 (23 ± 10 mo) | 3 (at 6 mo) | --- | 5.6 (per patient-year) |
| Recurrences | 2.7 | 0 | 6 | 5 | --- | --- | 1.8 (per patient-year) |
Incidence is based on patients with acute coronary syndrome;
This case series included only patients with chest pain and ST segment elevation;
Included only the typical form (apical ballooning);
All from noncardiac causes;
On admission;
Only included women.
Some study has found a relation between seasonal variation and incidence of TTC, with a higher frequency in winter[16], but this finding has not been confirmed in other series[14]. Typically, TTC mimics an anterior-ST-segment elevation myocardial infarction (STEMI); some of the main clinical differences between these are listed in Table 2.
Table 2.
Clinical comparison between takotsubo cardiomyopathy and STEMI
| TTC | STEMI | |
| Predominant gender | Women | Men |
| Myocardial segments involved | Extent beyond one coronary artery | Corresponding to culprit vessel |
| Peak of troponin | Lower | Higher |
| Left ventricle dysfunction recovery | Complete and at short term | Variable |
| Long term mortality | Lower | Higher |
TTC: Takotsubo cardiomyopathy; STEMI: ST-segment elevation myocardial infarction.
PATHOPHYSIOLOGY
Different hypotheses have been proposed to explain the pathophysiological mechanisms in TTC, but no one seems to be conclusive[9]. Studies in animals and humans using cardiac magnetic resonance (CMR), nuclear testing, endomyocardial biopsy, advanced echocardiography techniques, biochemical testing, intracoronary imaging, physiological studies of coronary microcirculation and pharmacological tests have attempted to elucidate the origin of the ventricular dysfunction and the selective impairment of myocardial segments without reaching a definitive conclusion. TTC is an enigmatic disease and very little is known yet about its primary mechanism.
The cause seems to be multifactorial and probably a single way is not enough to explain all findings. However, it is accepted that an intense release of catecholamines could be the initial trigger that finally leads to myocardial stunning, although the mechanisms that occur into the halfway are not clear yet.
A significant proportion of TTC patients have a stressor condition (emotional or physical) shortly before the appearance of symptoms. On the other hand, patients with pheochromocytoma are susceptible to suffer similar cardiomyopathy during catecholamine crisis[20-22]. Together, those observations suggest an exaggerated response of the sympathetic system, causing a high serum catecholamine levels that initiate the cascade of events that ultimately hit the cardiomyocytes. In fact, higher levels of catecholamines have been demonstrated in TTC compared with ACS[23]. Moreover, the absence of permanent late gadolinium enhancement on CMR and the complete recovery of the ventricular dysfunction support the myocardial-stunning phenomenon in TTC patients.
One of the first hypotheses, which emerged after ruling out obstructive coronary artery disease, was the spasm of multiple epicardial coronary arteries triggered by high levels of catecholamines[1]. This theory has not been demonstrated or reproduced reliably[24]. Attempts to induce vasospasm with acetylcholine in patients with TTC have been successful in a proportion of patients that is not enough to draw definitive conclusions[25]. Also, it is well known that some patients with definitive TTC have shown persistent ST-segment elevation without simultaneous evident coronary spasm at the angiography. Furthermore, a significant proportion of patients does not report symptoms such as chest pain or syncope that would be expected to find if epicardial coronary vasospasm would be involved.
The rupture of an atherosclerotic plaque in a long and recurrent left anterior descending (LAD) coronary artery, with thrombus formation and spontaneous lysis early aborting myocardial infarction[26], also seems unlikely, since most patients have normal both coronary angiography and intracoronary imaging. Optical coherence tomography (OCT) have ruled out any suspicion of plaque rupture and other injuries that may go unnoticed on angiography[27]. In fact, the extent of the left ventricle wall motion abnormalities exceeds the subtended myocardial territory of a recurrent LAD.
Myocarditis was another hypothesis. The strongest argument to rule out myocarditis is the absence of both clinical signs and permanent late enhancement on CMR demonstrated in the majority of patients with TTC.
Otherwise, nuclear studies have been of outstand relevance to investigate the potential mechanisms at metabolic level. PET studies have found a markedly reduced uptake of F-18 fluorodeoxyglucose (an analogue of glucose) at the apical segments in patients with typical TTC[28]. Moreover, it has been found a concordance between the myocardial wall motion abnormalities and the myocardial region with an impairment of glucose uptake[13]. However, this latter seems to be more severe and extensive than the corresponding myocardial perfusion defect, which is called a mismatch between metabolism and perfusion abnormalities[29]. Similar results have been obtained with fatty acids, another energy source, in terms of reduced uptake and mismatch in the apical zone of TTC patients[30,31]. Why this reduced uptake of energy sources is produced is not well understood, but a metabolic disorder, derived from myocardiocytes injury by the cathecolamines storm probably plays a key role in the mechanisms of TTC, causing a metabolic stunned myocardium.
On the other hand, coronary microvascular dysfunction (CMD) has been strongly highlighted as a key pathophysiologic mechanism. A decreased coronary flow velocity reserve and a short diastolic deceleration time, measured with intracoronary Doppler, have been found in TTC patients[32]. This findings have been supported by non-invasive studies, such as the assessment of coronary flow reserve through transthoracic Doppler, finding that in TTC patients, there is a transient impairment of the microcirculation at the acute phase, demonstrated by a reduced CFR[33]. Other studies have documented indirect signs of CMD, such as abnormal myocardial blush grade and TIMI frame count[13,34-36]. Such abnormalities have been found not only in the LAD subtended myocardial territory but also in the other main epicardial vessels, which may suggest that CMD may occur at multivessel level. Therefore, it seems that the coronary microvascular integrity is impaired, but what is not clear yet is if myocardial stunning is consequence of metabolic disorder or CMD[23]. Another relevant question is why other people subjected to stress conditions do not develop this syndrome. Some argue that TTC patients are unprotected at molecular level to facing the acute storm of cathecolamines within context of stress situation. Recently, d’Avenia et al[37] have found that mutation of BAG3, a gene involved in the epinephrine-induced apoptosis of altered cardiomyocytes, may play a role in the impaired response of myocardium to supraphysiological levels of cathecolamines.
There are many questions still unanswered. Nowadays, we do not know for sure why this disease predominantly affects postmenopausal women, but epidemiological data invite us to think that estrogens in women may play a protective role. Why the left ventricle apical segments are the most affected and why the basal segments behave hyperkinetic are issues not clearly answered today, but it is believed that heterogeneous distribution and variable response of beta-receptors along myocardial segments are involved[38,39].
Based on the myocardial dysfunction beyond one single coronary artery and the absence of concordant abnormalities in the epicardial arteries, the mechanism of myocardial stunning in TTC seems to overstep the frontiers of the epicardial coronary vessels, which lead us to search the cause in the coronary microcirculation or even at a molecular level.
DIAGNOSIS
Typical form of TTC affects the mid and apical segments of the left ventricle with compensatory hyperkinesis of the basal segments, but in any case, the myocardial wall motion abnormalities extend more than a single epicardial coronary artery distribution. Unlike what happens in ACS, the peak of troponin in TTC is disproportionately lower compared to the extent of the myocardial dysfunction. Ruling out severe obstructive coronary artery disease and acute plaque rupture must be a priority before diagnosing this syndrome. Currently, it is recognized that TTC is, by definition, a completely reversible disease. So, it is mandatory to confirm a full recovery of the ventricular wall motion abnormalities along follow-up. Table 3 shows the most recognized diagnosis criteria for this syndrome[15].
Table 3.
Diagnosis criteria for takotsubo cardiomyopathy
| Patients must satisfy all the following | |
| ECG | New abnormalities: ST-segment elevation and or T waves inversion |
| Blood test | Modest peak of troponin |
| Imaging | Transient wall motion abnormalities (with or without apical involvement) that extend beyond a single epicardial coronary artery |
| Angiography | Normal or near normal epicardial coronary arteries and no evidence of plaque rupture |
| Excluding other diseases | Pheochromocytoma, myocarditis |
Based on Mayo Clinic Criteria (2008). ECG: Electrocardiographic.
VARIANTS
The left ventricular dysfunction in TTC includes not only the classical apical ballooning form but also different angiographic patterns that have been increasingly reported along last decade (Figure 1). The “mid-ventricular shape” respects both the apex and base[40]. The “reversed takotsubo”, in which there are wall motion abnormalities of the base and mid segments with preserved motion/hiperkinesis of the apex, is very rare[41]. Furthermore, some case reports have documented simultaneous abnormalities at both left and right ventricles up to in third of cases[42-44], but the isolated involvement of the right ventricle is very uncommon[45,46]. Recently, it was described the first case of “double takotsubo”, in which the typical pattern was followed by the reversed type[47]. Thus, TTC may hit different myocardial walls, but in any case, this extends beyond a single epicardial coronary artery.
Figure 1.
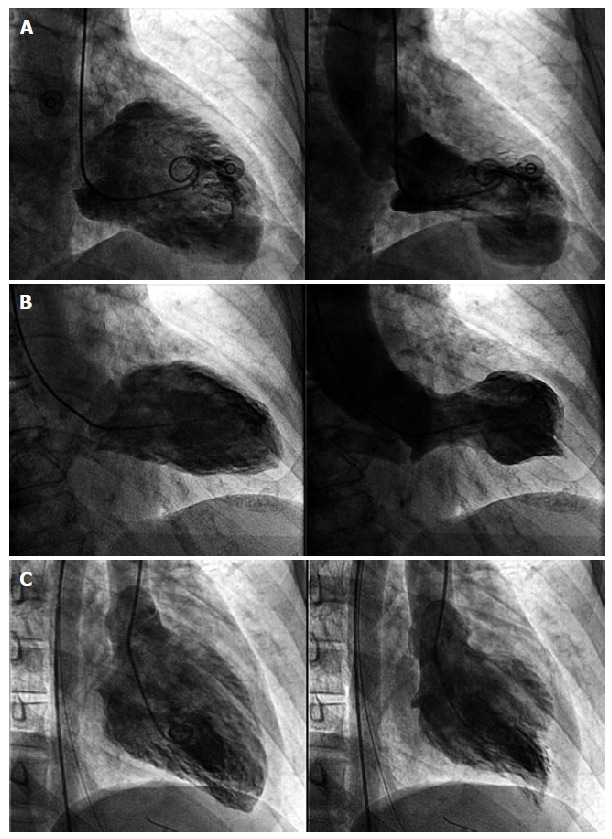
Left ventriculography showing different types of takotsubo cardiomyopathy. A: Takotsubo cardiomyopathy (TTC) typical form. On systole the left ventricle presents akinesis of the apex and hyperkinesis of the basal segments; B: Mid-ventricular variant. On systole the mid-segments are akinetic while the apical and basal segments are normal; C: “Inverted TTC”. Basal and mid-segments are akinetic on systole while the apex is hyperkinetic.
IMAGING TECHNIQUES IN TTC
Two-dimensional echocardiogram
This is an imperative imaging technique in the course of diagnosis and follow-up of TTC patients. Due to its ready availability, two-dimensional echocardiogram allows quantify the severity of the LVSD from the onset, which is usually not achieved with other imaging technique by time availability. This is key for supporting diagnosis, taking into account that sometimes the wall motion abnormalities improve very quickly (in some cases, it have been reported a complete recovery in less than 48 h)[48,49]. Dynamic left ventricular outflow tract obstruction (DLVOTO) due to systolic anterior motion (SAM) of mitral valve and intracavitary thrombi (mainly in the apex) are complications that can be early detected by this imaging technique, which determine specific strategies of treatment[50]. Moreover, advanced echocardiographic techniques, such as speckle-tracking and coronary flow assessment with transthoracic Doppler, are providing pathophysiologic insights about this syndrome[51].
Cardiac catheterization
Coronary angiography is warranted to exclude severe obstructive coronary disease as the cause of ventricular dysfunction. However, it is important to note that the presence of coronary atherosclerosis not exempt TTC. Indeed, near to 15% of patients has coronary artery disease[7,18]. Intracoronary imaging techniques, such as OCT, have been useful to definitively rule-out structural abnormalities in the epicardial vessels that may go unnoticed on angiography, including plaque rupture, eroded intimae, dissections or residual thrombus, supporting the need for searching an alternative pathophysiological mechanisms[27]. Left ventriculography has been traditionally used to describe the pattern of TTC (Figure 1).
CMR
This imaging technique has become an important tool to advance in understanding the pathophysiological mechanisms involved in TTC. The main contribution has been the demonstration of transient myocardial edema, mainly at the apex, which is related to the degree of ventricular dysfunction, even with the repolarization electrocardiographic abnormalities[52-56]. T2-weighted imaging has shown a non-coronary distributed apical edema without contrast enhancement, which confirms that myocardial abnormalities extend beyond one single coronary artery. In clinical practice CMR is key to exclude other differential diagnosis, such as myocarditis[57]. Recently, it has been found with CMR a profound diastolic dysfunction in the acute phase, that takes more time to resolve compared with the rapid recovery of the left ventricular systolic dysfunction[58].
Nuclear imaging: Single-photon emission computed tomography and positron emission tomography (PET) allow a precise assessment of myocardial perfusion and metabolism, ventricular function and even sympathetic innervations of the heart by using different radiotracers[59,60]. This techniques have been mainly used to study the pathophysiological mechanisms involved in TTC, showing that coronary flow reserve and myocardial blood flow are globally impaired, not only restricted to the dysfunctional segments, indicating a microcirculatory dysfunction at least in the acute phase[61].
ELECTROCARDIOGRAPHIC FEATURES OF TTC
T waves
In our experience, up to 90% of patients develop T waves inversion at some point of evolution. This is frequently seen from the onset (38.8%), either as single finding or with ST-segment abnormalities, but they typically appear when ST-segment begins to normalize. Compared with anterior-STEMI, negative T waves in TTC are usually deeper, wider and more diffuse, affecting a greater number of leads. In patients with inverted T waves from the onset without ST-segment elevation, the absence of negative T waves on V1 and positive T waves on aVR should raise suspicion of TTC[62]. T waves inversion is less frequently found on atypical forms[63].
ST-segment abnormalities and comparison with AMI
ST-segment elevation is the second most common finding observed in our large registry (62%)[14]. However, as it can be seen on Table 1, there is a different incidence along worldwide, which may be explained by ethnic variations or a more aggressive diagnostic approach in some countries[64]. Because the apical region of the left ventricle is the most affected, the ST-segment elevation is more frequently found on the LAD subtended myocardial territories[65], while it is uncommon in V1 because the right ventricle is respected mostly times. On the other hand, ST-segment reciprocal depression in the inferior leads is uncommon compared with anterior-STEMI[66]. Moreover, ST-segment depression as the unique find is the least frequent ECG abnormality on TTC, and is very uncommon compared with ACS[18].
Evolution of the ECG abnormalities
The repolarization changes follow a pattern very similar to STEMI, but the normalization of ST-segment and the appearance of T waves inversion usually occur more rapidly in TTC. Therefore, one can not rule out that patients presenting with T waves inversion in the first ECG, have previously had a short unnoticed phase with ST-segment elevation. In general, the evolution of the main ECG abnormalities described in TTC patients is, in order: ST-segment elevation, development of negative T waves while ST-segment is normalizing, and prolongation of QT-interval. The time to resolve both T waves inversion and prolonged QT-interval is highly variable, it could be take few weeks or several months[67]. Interestingly, the ECG abnormalities take more time to resolve than the wall motion impairment. Other electrocardiographic abnormalities have been described, including a high prevalence of low QRS voltage and attenuation of the amplitude of QRS complexes, which might help to support the suspicion of TTC[68].
QT-interval
Although a prolonged QT-interval is very common (47.7% by Templin et al[18]; 78.8% by Núñez et al[14]), the incidence of ventricular arrhythmias is very low, which highlight a benign prognosis of this form of acquired long QT-interval. Interestingly, Gopalakrishnan et al[69] found a strong correlation between prolonged-QTc interval at presentation and overall outcome.
Q-waves
Given the full recovery of myocardial damage, it is exceptional to find permanent pathological Q waves on previously normal hearts in patients with TTC[70].
The similarities between TTC and anterior-STEMI have aroused a great interest on scientific community in searching of electrocardiographic features that help to distinguish them from the onset[65,66,71,72]. Although some ECG signs have been described in patients with TTC (Table 4), more studies are needed, particularly prospective, rigorously comparing the electrocardiographic findings with anterior-STEMI[9]. Currently, there are no ECG signs which alone may rule out a culprit coronary artery stenosis[73]. Figure 2 shows an example of the most common ECG findings in TTC patients.
Table 4.
Electrocardiographic findings in takotsubo cardiomyopathy
| T waves inversion | ST-segment | QRS complex | Q waves |
| Are the most frequent finding along ECG evolution | Makes priority rule out obstructive coronary artery disease | aVR lead is especially sensible to changes in voltage because it "faces" the apex | Permanent pathological Q waves are exceptional |
| Appear mainly in precordial leads (V2-V6) | More frequent on precordial leads, except V1 | ||
| Negative T waves are deep, symmetrical and widespread | Reciprocal depression is less frequent than in STEMI | ||
| Progressive QT-interval prolongation | Suspicious combinations: | ||
| ST-depression in aVR plus no elevation in V1 (91% sensitivity, 96% specificity)[87] | |||
| The sum of elevation in V4-V6/V1-V3 ≥ 1 (77% sensitivity, 80% specificity)[65] | |||
| No negative T wave in V1 plus positive T wave in aVR must raise suspicion (95% sensitivity, 97% specificity)[62] | Level of ST segment elevation lesser than in anterior STEMI |
ECG: Electrocardiogram; STEMI: ST-segment elevation myocardial infarction.
Figure 2.
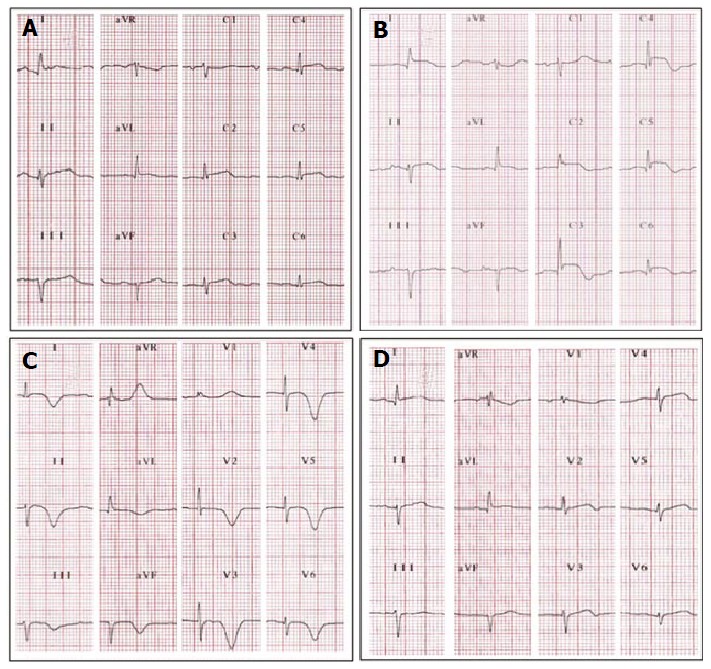
Electrocardiographic evolutionary changes in a 65-year-old woman with typical takotsubo cardiomyopathy. A: Initial electrocardiographic (ECG) after 3 h of symptoms. There is diffuse ST-segment elevation (DI, aVL and all precordial leads except on V1); B: ECG after 24 h of symptoms. The ST-segment elevation seems to be more prominent. Note ST-segment depression on aVR. The T waves start to invert on leads with ST-segment elevation, except on V1 where there is a more prominent positive T wave; C: Third day. The ST-segment is almost normal. The T waves are now inverted, deep, wide and symmetrical on all leads except on aVR and V1 where they are positive. The corrected QT-interval is prolonged (520 milliseconds); D: ECG 3 wk later, outpatient. The T waves are almost normal and the QT-interval is not prolonged.
Arrhythmias
Incidence and type of arrhythmias observed in different TTC series varies widely, but it has in common that usually resolve after overcoming the acute setting. In our registry, paroxysmal atrial fibrillation is the more common sustained tachyarrhythmia (11%). Sinus bradycardia and different degrees of atrioventricular block have been observed. Ventricular arrhythmias such as ventricular tachycardia (4.8%) and ventricular fibrillation (VF) (0.7%) are uncommon in the acute phase in our experience[14], which is concordant with other observational studies[18]. Torsades de pointes have rarely been reported. However, among patients with TTC who present prolonged QT-interval, male sex has been associated with more risk of Torsades de pointes, as well as severe left ventricular systolic dysfunction, bradycardia, hypokalemia and use of QT-prolonging agents[74]. Some patients debut with sudden death due to VF[75,76], although in these cases it is unclear if VF is a trigger or consequence of TTC[77].
TREATMENT
Because pathophysiologic mechanisms are not clear yet, there is no consensus on specific treatment to this condition. In fact, treatment consists mainly on treatment of heart failure and its complications. Based on the theory of high catecholamine levels, use of beta-blockers seems reasonable, but caution must be taken due to the high frequency of heart failure on these patients[78]. However, it is striking that in some case series a significant percent of patients were on treatment with beta-blockers at the time of debut. Furthermore, in patients with recurrences, there are no differences regarding incidence among those who were being treated with beta-blockers and those without. Beta-blockers do not appear to have a protective effect for this syndrome based on these results[18,79].
Anticoagulation is indicated to the management of ventricular thrombus and should be maintained at least until confirm its resolution. it must be considered an early anticoagulation therapy irrespective of the presence of ventricular thrombus at admission, specially in patients with high risk of thromboembolic events[9,80].
Patients with hemodynamic instability may require positive inotropic drugs and circulatory support devices such as intra-aortic balloon pump counterpulsation or extracorporeal life support in case of refractory cardiogenic shock. However, it is not clear the benefit of exogenous catecholamines taking into account the pathophysiology of this syndrome, so positive inotropic drugs should be used with caution with the minimum dose required to maintaining an acceptable hemodynamic status[78,81].
Echocardiography is very useful to guide the treatment in patients with TTC. In presence of DLVOTO, SAM and hemodynamic instability, beta-blockers and/or intravenous fluids are preferred (in absence of significant pulmonary congestion) instead of positive inotropic drugs[9,82-84].
Typically, TTC patients are initially treated with the standard of care for ACS at the moment of presenting to the emergency department, due to the similarities among these two diseases. This decision implies that TTC patients must receive dual antiplatelet and anticoagulation therapy until coronary angiography is performed and culprit coronary obstructive disease is discarded.
Finally, angiotensin-converting-enzyme inhibitors and angiotensin-receptor blockers have been associated with better survival[18].
PROGNOSIS
By definition, TTC left ventricular dysfunction is completely reversible. The involvement of the right ventricle is occasional. Serious complications and recurrences are infrequent. So, TTC has been traditionally considered as a benign cardiac syndrome in absence of significant comorbid conditions[79,85,86]. However, this syndrome significantly contributes to morbidity and mortality. Some recent large observational studies have shown that TTC has a poorer prognosis than it was believed, comparable with ACS[7,18], and related to the patient’s risk profile such as frailty and associated comorbidities. Templin et al[18] found that elderly patients with emotional triggers have a low risk of significant cardiovascular events, while younger patients with physical triggers and acute neurologic or psychiatric diseases have an increased risk of acute complications. The risk of mortality seems to be higher in men and patients with underlying critical illness[8].
The recurrence of TTC is low; Elesber et al[79] have reported an average yearly recurrence rate of 2.9% in the first few years, decreasing later to 1.3% per year, which is similar to the recurrence rate reported by Templin et al[18] (1.8% per patient-year).
CONCLUSION
TTC is a wide spectrum syndrome that clinically mimics an ACS in absence of significant epicardial coronary artery disease to explain the extent of the wall motion abnormalities. Nowadays, there have not been clearly identified electrocardiographic signs to reliably differentiate TTC from ACS in the acute phase. Therefore, knowledge of the coronary anatomy is mandatory. The pathophysiological mechanism is not well understood, but it seems that an intense release of catecholamines triggers myocardial stunning. In the midway, CMD and abnormalities on myocardium metabolism have been highlighted as potential involved mechanisms. Treatment in the acute phase should be directed to treat complications, including heart failure, arrhythmias and ventricular thrombus, while long-term medical therapy remains empirical due to limited available data. Although it was thought that prognosis is good, it seems increasingly evident that TTC patients may have poorer outcomes, even similar with patients with ACS. Angiotensin-converting-enzyme inhibitors and angiotensin-receptor blockers have been associated with improved survival. We need more rigorous prospective studies to continue on the way of understanding this enigmatic disease.
Footnotes
Conflict-of-interest statement: The authors state that there is no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Peer-review started: March 27, 2016
First decision: April 15, 2016
Article in press: May 27, 2016
P- Reviewer: Bonanno C, Petretta M, Rauch B, Skobel E S- Editor: Ji FF L- Editor: A E- Editor: Li D
References
- 1.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases] J Cardiol. 1991;21:203–214. [PubMed] [Google Scholar]
- 2.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 3.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–341. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 4.Sharkey SW, Lesser JR, Maron MS, Maron BJ. Why not just call it tako-tsubo cardiomyopathy: a discussion of nomenclature. J Am Coll Cardiol. 2011;57:1496–1497. doi: 10.1016/j.jacc.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 5.Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475–481. doi: 10.1093/eurheartj/ehu469. [DOI] [PubMed] [Google Scholar]
- 6.Minhas AS, Hughey AB, Kolias TJ. Nationwide Trends in Reported Incidence of Takotsubo Cardiomyopathy from 2006 to 2012. Am J Cardiol. 2015;116:1128–1131. doi: 10.1016/j.amjcard.2015.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Redfors B, Vedad R, Angerås O, Råmunddal T, Petursson P, Haraldsson I, Ali A, Dworeck C, Odenstedt J, Ioaness D, et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction - A report from the SWEDEHEART registry. Int J Cardiol. 2015;185:282–289. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 8.Khera R, Light-McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53–63. doi: 10.1016/j.ahj.2015.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, et al. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 10.Akashi YJ, Nef HM, Lyon AR. Epidemiology and pathophysiology of Takotsubo syndrome. Nat Rev Cardiol. 2015;12:387–397. doi: 10.1038/nrcardio.2015.39. [DOI] [PubMed] [Google Scholar]
- 11.Parodi G, Del Pace S, Carrabba N, Salvadori C, Memisha G, Simonetti I, Antoniucci D, Gensini GF. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am J Cardiol. 2007;99:182–185. doi: 10.1016/j.amjcard.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 12.Madias JE. Low prevalence of diabetes mellitus in patients with Takotsubo syndrome: A plausible ‘protective’ effect with pathophysiologic connotations. Eur Heart J Acute Cardiovasc Care. 2016;5:164–170. doi: 10.1177/2048872615570761. [DOI] [PubMed] [Google Scholar]
- 13.Kurowski V, Kaiser A, von Hof K, Killermann DP, Mayer B, Hartmann F, Schunkert H, Radke PW. Apical and midventricular transient left ventricular dysfunction syndrome (tako-tsubo cardiomyopathy): frequency, mechanisms, and prognosis. Chest. 2007;132:809–816. doi: 10.1378/chest.07-0608. [DOI] [PubMed] [Google Scholar]
- 14.Núñez Gil IJ, Andrés M, Almendro Delia M, Sionis A, Martín A, Bastante T, Córdoba Soriano JG, Linares Vicente JA, González Sucarrats S, Sánchez-Grande Flecha A. Characterization of Tako-tsubo Cardiomyopathy in Spain: Results from the RETAKO National Registry. Rev Esp Cardiol (Engl Ed) 2015;68:505–512. doi: 10.1016/j.rec.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchihashi K, Ueshima K, Uchida T, Oh-mura N, Kimura K, Owa M, Yoshiyama M, Miyazaki S, Haze K, Ogawa H, et al. Transient left ventricular apical ballooning without coronary artery stenosis: a novel heart syndrome mimicking acute myocardial infarction. Angina Pectoris-Myocardial Infarction Investigations in Japan. J Am Coll Cardiol. 2001;38:11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 16.Eshtehardi P, Koestner SC, Adorjan P, Windecker S, Meier B, Hess OM, Wahl A, Cook S. Transient apical ballooning syndrome--clinical characteristics, ballooning pattern, and long-term follow-up in a Swiss population. Int J Cardiol. 2009;135:370–375. doi: 10.1016/j.ijcard.2008.03.088. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed S, Ungprasert P, Ratanapo S, Hussain T, Riesenfeld EP. Clinical characteristics of takotsubo cardiomyopathy in north america. N Am J Med Sci. 2013;5:77–81. doi: 10.4103/1947-2714.107520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N Engl J Med. 2015;373:929–938. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 19.Summers MR, Lennon RJ, Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J Am Coll Cardiol. 2010;55:700–701. doi: 10.1016/j.jacc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Recalde A, Costero O, Oliver JM, Iborra C, Ruiz E, Sobrino JA. Images in cardiovascular medicine. Pheochromocytoma-related cardiomyopathy: inverted Takotsubo contractile pattern. Circulation. 2006;113:e738–e739. doi: 10.1161/CIRCULATIONAHA.105.581108. [DOI] [PubMed] [Google Scholar]
- 21.Naderi N, Amin A, Setayesh A, Pouraliakbar H, Mozaffari K, Maleki M. Pheochromocytoma-induced reverse tako-tsubo with rapid recovery of left ventricular function. Cardiol J. 2012;19:527–531. doi: 10.5603/cj.2012.0097. [DOI] [PubMed] [Google Scholar]
- 22.Kimura S, Mitsuma W, Ito M, Suzuki H, Hosaka Y, Hirayama S, Hanyu O, Hirono S, Kodama M, Aizawa Y. Inverted Takotsubo contractile pattern caused by pheochromocytoma with tall upright T-waves, but not typical deep T-wave inversion. Int J Cardiol. 2010;139:e15–e17. doi: 10.1016/j.ijcard.2008.06.073. [DOI] [PubMed] [Google Scholar]
- 23.Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- 24.Madias JE. Coronary vasospasm is an unlikely cause of Takotsubo syndrome, although we should keep an open mind. Int J Cardiol. 2014;176:1–5. doi: 10.1016/j.ijcard.2014.06.069. [DOI] [PubMed] [Google Scholar]
- 25.Kurisu S, Sato H, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Kono Y, Umemura T, Nakamura S. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: a novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143:448–455. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 26.Ibáñez B, Navarro F, Farré J, Marcos-Alberca P, Orejas M, Rábago R, Rey M, Romero J, Iñiguez A, Córdoba M. [Tako-tsubo syndrome associated with a long course of the left anterior descending coronary artery along the apical diaphragmatic surface of the left ventricle] Rev Esp Cardiol. 2004;57:209–216. doi: 10.1016/s0300-8932(04)77092-x. [DOI] [PubMed] [Google Scholar]
- 27.Alfonso F, Núñez-Gil IJ, Hernández R. Optical coherence tomography findings in Tako-Tsubo cardiomyopathy. Circulation. 2012;126:1663–1664. doi: 10.1161/CIRCULATIONAHA.112.122200. [DOI] [PubMed] [Google Scholar]
- 28.Bybee KA, Murphy J, Prasad A, Wright RS, Lerman A, Rihal CS, Chareonthaitawee P. Acute impairment of regional myocardial glucose uptake in the apical ballooning (takotsubo) syndrome. J Nucl Cardiol. 2006;13:244–250. doi: 10.1007/BF02971249. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida T, Hibino T, Kako N, Murai S, Oguri M, Kato K, Yajima K, Ohte N, Yokoi K, Kimura G. A pathophysiologic study of tako-tsubo cardiomyopathy with F-18 fluorodeoxyglucose positron emission tomography. Eur Heart J. 2007;28:2598–2604. doi: 10.1093/eurheartj/ehm401. [DOI] [PubMed] [Google Scholar]
- 30.Ito K, Sugihara H, Kinoshita N, Azuma A, Matsubara H. Assessment of Takotsubo cardiomyopathy (transient left ventricular apical ballooning) using 99mTc-tetrofosmin, 123I-BMIPP, 123I-MIBG and 99mTc-PYP myocardial SPECT. Ann Nucl Med. 2005;19:435–445. doi: 10.1007/BF02985570. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo S, Nakajima K, Kinuya S, Yamagishi M. Diagnostic utility of 123I-BMIPP imaging in patients with Takotsubo cardiomyopathy. J Cardiol. 2014;64:49–56. doi: 10.1016/j.jjcc.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 32.Kume T, Akasaka T, Kawamoto T, Yoshitani H, Watanabe N, Neishi Y, Wada N, Yoshida K. Assessment of coronary microcirculation in patients with takotsubo-like left ventricular dysfunction. Circ J. 2005;69:934–939. doi: 10.1253/circj.69.934. [DOI] [PubMed] [Google Scholar]
- 33.Meimoun P, Malaquin D, Benali T, Boulanger J, Zemir H, Tribouilloy C. Transient impairment of coronary flow reserve in tako-tsubo cardiomyopathy is related to left ventricular systolic parameters. Eur J Echocardiogr. 2009;10:265–270. doi: 10.1093/ejechocard/jen222. [DOI] [PubMed] [Google Scholar]
- 34.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Umemura T, Nakamura S, Yoshida M, Sato H. Myocardial perfusion and fatty acid metabolism in patients with tako-tsubo-like left ventricular dysfunction. J Am Coll Cardiol. 2003;41:743–748. doi: 10.1016/s0735-1097(02)02924-8. [DOI] [PubMed] [Google Scholar]
- 35.Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, Wright RS, Rihal CS. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–346. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Elesber A, Lerman A, Bybee KA, Murphy JG, Barsness G, Singh M, Rihal CS, Prasad A. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152:469.e9–469.13. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 37.d'Avenia M, Citro R, De Marco M, Veronese A, Rosati A, Visone R, Leptidis S, Philippen L, Vitale G, Cavallo A, et al. A novel miR-371a-5p-mediated pathway, leading to BAG3 upregulation in cardiomyocytes in response to epinephrine, is lost in Takotsubo cardiomyopathy. Cell Death Dis. 2015;6:e1948. doi: 10.1038/cddis.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyon AR, Rees PS, Prasad S, Poole-Wilson PA, Harding SE. Stress (Takotsubo) cardiomyopathy--a novel pathophysiological hypothesis to explain catecholamine-induced acute myocardial stunning. Nat Clin Pract Cardiovasc Med. 2008;5:22–29. doi: 10.1038/ncpcardio1066. [DOI] [PubMed] [Google Scholar]
- 39.Tranter MH, Wright PT, Sikkel MB, Lyon AR. Takotsubo cardiomyopathy: the pathophysiology. Heart Fail Clin. 2013;9:187–96, viii-ix. doi: 10.1016/j.hfc.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 40.Hurst RT, Askew JW, Reuss CS, Lee RW, Sweeney JP, Fortuin FD, Oh JK, Tajik AJ. Transient midventricular ballooning syndrome: a new variant. J Am Coll Cardiol. 2006;48:579–583. doi: 10.1016/j.jacc.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 41.Manzanal A, Ruiz L, Madrazo J, Makan M, Perez J. Inverted Takotsubo cardiomyopathy and the fundamental diagnostic role of echocardiography. Tex Heart Inst J. 2013;40:56–59. [PMC free article] [PubMed] [Google Scholar]
- 42.Angelini P, Monge J, Simpson L. Biventricular takotsubo cardiomyopathy: case report and general discussion. Tex Heart Inst J. 2013;40:312–315. [PMC free article] [PubMed] [Google Scholar]
- 43.Daoko J, Rajachandran M, Savarese R, Orme J. Biventricular takotsubo cardiomyopathy: case study and review of literature. Tex Heart Inst J. 2013;40:305–311. [PMC free article] [PubMed] [Google Scholar]
- 44.Koo N, Yoon BW, Song Y, Lee CK, Lee TY, Hong JY. Biventricular Takotsubo Cardiomyopathy Associated with Epilepsy. J Cardiovasc Ultrasound. 2015;23:262–265. doi: 10.4250/jcu.2015.23.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stähli BE, Ruschitzka F, Enseleit F. Isolated right ventricular ballooning syndrome: a new variant of transient cardiomyopathy. Eur Heart J. 2011;32:1821. doi: 10.1093/eurheartj/ehr079. [DOI] [PubMed] [Google Scholar]
- 46.Burgdorf C, Hunold P, Radke PW, Schunkert H, Kurowski V. Isolated right ventricular stress-induced (“Tako-Tsubo”) cardiomyopathy. Clin Res Cardiol. 2011;100:617–619. doi: 10.1007/s00392-011-0293-4. [DOI] [PubMed] [Google Scholar]
- 47.Ehl NF, Zurek M, Rickli H, Maeder MT. “Double takotsubo”: first description of the sequence of classical followed by inverted type in a young woman. Int J Cardiol. 2014;174:e36–e37. doi: 10.1016/j.ijcard.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 48.Eitel I, Lücke C, Behrendt F, Sareban M, Gutberlet M, Schuler G, Thiele H. Full recovery of Takotsubo cardiomyopathy (apical ballooning) in two days. Int J Cardiol. 2010;143:e51–e53. doi: 10.1016/j.ijcard.2008.12.044. [DOI] [PubMed] [Google Scholar]
- 49.Shimokawahara H, Sonoda M, Tanaka H, Kashima K, Nagayoshi S, Kawasaki D, Ikeda D, Nagano S, Tanaka Y, Nakamura K. Case of transient mid-ventricular ballooning syndrome with a rapid and uncommon recovery. J Cardiol. 2009;54:311–316. doi: 10.1016/j.jjcc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Citro R, Piscione F, Parodi G, Salerno-Uriarte J, Bossone E. Role of echocardiography in takotsubo cardiomyopathy. Heart Fail Clin. 2013;9:157–66, viii. doi: 10.1016/j.hfc.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 51.Citro R, Lyon AR, Meimoun P, Omerovic E, Redfors B, Buck T, Lerakis S, Parodi G, Silverio A, Eitel I, et al. Standard and advanced echocardiography in takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28:57–74. doi: 10.1016/j.echo.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 52.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 53.Abdel-Aty H, Cocker M, Friedrich MG. Myocardial edema is a feature of Tako-Tsubo cardiomyopathy and is related to the severity of systolic dysfunction: insights from T2-weighted cardiovascular magnetic resonance. Int J Cardiol. 2009;132:291–293. doi: 10.1016/j.ijcard.2007.08.102. [DOI] [PubMed] [Google Scholar]
- 54.Joshi SB, Chao T, Herzka DA, Zeman PR, Cooper HA, Lindsay J, Fuisz AR. Cardiovascular magnetic resonance T2 signal abnormalities in left ventricular ballooning syndrome. Int J Cardiovasc Imaging. 2010;26:227–232. doi: 10.1007/s10554-009-9515-5. [DOI] [PubMed] [Google Scholar]
- 55.Tada H. Unraveling the riddle of transient T-wave inversion (Wellens’ ECG pattern): T2-weighted magnetic resonance imaging identifies myocardial edema. Heart Rhythm. 2011;8:1635–1636. doi: 10.1016/j.hrthm.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 56.Migliore F, Zorzi A, Marra MP, Basso C, Corbetti F, De Lazzari M, Tarantini G, Buja P, Lacognata C, Thiene G, et al. Myocardial edema underlies dynamic T-wave inversion (Wellens’ ECG pattern) in patients with reversible left ventricular dysfunction. Heart Rhythm. 2011;8:1629–1634. doi: 10.1016/j.hrthm.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 57.Dastidar AG, Frontera A, Palazzuoli A, Bucciarelli-Ducci C. TakoTsubo cardiomyopathy: unravelling the malignant consequences of a benign disease with cardiac magnetic resonance. Heart Fail Rev. 2015;20:415–421. doi: 10.1007/s10741-015-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahtarovski KA, Iversen KK, Christensen TE, Andersson H, Grande P, Holmvang L, Bang L, Hasbak P, Lønborg JT, Madsen PL, et al. Takotsubo cardiomyopathy, a two-stage recovery of left ventricular systolic and diastolic function as determined by cardiac magnetic resonance imaging. Eur Heart J Cardiovasc Imaging. 2014;15:855–862. doi: 10.1093/ehjci/jeu004. [DOI] [PubMed] [Google Scholar]
- 59.Testa M, Feola M. Usefulness of myocardial positron emission tomography/nuclear imaging in Takotsubo cardiomyopathy. World J Radiol. 2014;6:502–506. doi: 10.4329/wjr.v6.i7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mena LM, Martín F, Melero A, Ramos A, Jiménez IR. [Takotsubo syndrome. Usefulness of nuclear medicine studies] Rev Esp Med Nucl. 2011;30:104–106. doi: 10.1016/j.remn.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Feola M, Chauvie S, Rosso GL, Biggi A, Ribichini F, Bobbio M. Reversible impairment of coronary flow reserve in takotsubo cardiomyopathy: a myocardial PET study. J Nucl Cardiol. 2008;15:811–817. doi: 10.1007/BF03007363. [DOI] [PubMed] [Google Scholar]
- 62.Kosuge M, Ebina T, Hibi K, Tsukahara K, Iwahashi N, Gohbara M, Matsuzawa Y, Okada K, Morita S, Umemura S, et al. Differences in negative T waves among acute coronary syndrome, acute pulmonary embolism, and Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care. 2012;1:349–357. doi: 10.1177/2048872612466790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song BG, Chun WJ, Park YH, Kang GH, Oh J, Lee SC, Park SW, Oh JK. The clinical characteristics, laboratory parameters, electrocardiographic, and echocardiographic findings of reverse or inverted takotsubo cardiomyopathy: comparison with mid or apical variant. Clin Cardiol. 2011;34:693–699. doi: 10.1002/clc.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Núñez-Gil IJ, Luaces M, Garcia-Rubira JC, Zamorano J. Electrocardiographic criteria in Takotsubo cardiomyopathy and race differences: Asians versus Caucasians. J Am Coll Cardiol. 2010;56:1433–1444; author reply 1434. doi: 10.1016/j.jacc.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 65.Ogura R, Hiasa Y, Takahashi T, Yamaguchi K, Fujiwara K, Ohara Y, Nada T, Ogata T, Kusunoki K, Yuba K, et al. Specific findings of the standard 12-lead ECG in patients with ‘Takotsubo’ cardiomyopathy: comparison with the findings of acute anterior myocardial infarction. Circ J. 2003;67:687–690. doi: 10.1253/circj.67.687. [DOI] [PubMed] [Google Scholar]
- 66.Jim MH, Chan AO, Tsui PT, Lau ST, Siu CW, Chow WH, Lau CP. A new ECG criterion to identify takotsubo cardiomyopathy from anterior myocardial infarction: role of inferior leads. Heart Vessels. 2009;24:124–130. doi: 10.1007/s00380-008-1099-9. [DOI] [PubMed] [Google Scholar]
- 67.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakamura S, Yoshida M, Mitsuba N, Hata T, Sato H. Time course of electrocardiographic changes in patients with tako-tsubo syndrome: comparison with acute myocardial infarction with minimal enzymatic release. Circ J. 2004;68:77–81. doi: 10.1253/circj.68.77. [DOI] [PubMed] [Google Scholar]
- 68.Madias JE. Transient attenuation of the amplitude of the QRS complexes in the diagnosis of Takotsubo syndrome. Eur Heart J Acute Cardiovasc Care. 2014;3:28–36. doi: 10.1177/2048872613504311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116:1586–1590. doi: 10.1016/j.amjcard.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 70.Looi JL, Wong CW, Lee M, Khan A, Webster M, Kerr AJ. Usefulness of ECG to differentiate Takotsubo cardiomyopathy from acute coronary syndrome. Int J Cardiol. 2015;199:132–140. doi: 10.1016/j.ijcard.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 71.Inoue M, Shimizu M, Ino H, Yamaguchi M, Terai H, Fujino N, Sakata K, Funada A, Tatami R, Ishise S, et al. Differentiation between patients with takotsubo cardiomyopathy and those with anterior acute myocardial infarction. Circ J. 2005;69:89–94. doi: 10.1253/circj.69.89. [DOI] [PubMed] [Google Scholar]
- 72.Kosuge M, Kimura K. Electrocardiographic findings of takotsubo cardiomyopathy as compared with those of anterior acute myocardial infarction. J Electrocardiol. 2014;47:684–689. doi: 10.1016/j.jelectrocard.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 73.Vervaat FE, Christensen TE, Smeijers L, Holmvang L, Hasbak P, Szabó BM, Widdershoven JW, Wagner GS, Bang LE, Gorgels AP. Is it possible to differentiate between Takotsubo cardiomyopathy and acute anterior ST-elevation myocardial infarction? J Electrocardiol. 2015;48:512–519. doi: 10.1016/j.jelectrocard.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Samuelov-Kinori L, Kinori M, Kogan Y, Swartzon M, Shalev H, Guy D, Ferenidou F, Mashav N, Sadeh B, Atzmony L, et al. Takotsubo cardiomyopathy and QT interval prolongation: who are the patients at risk for torsades de pointes? J Electrocardiol. 2009;42:353–357.e1. doi: 10.1016/j.jelectrocard.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Gasparetto N, Zorzi A, Perazzolo Marra M, Migliore F, Napodano M, Corrado D, Iliceto S, Cacciavillani L. Atypical (mid-ventricular) Takotsubo syndrome in a survival of out-of-hospital ventricular fibrillation: cause or consequence? Int J Cardiol. 2014;172:e51–e53. doi: 10.1016/j.ijcard.2013.12.064. [DOI] [PubMed] [Google Scholar]
- 76.Liang JJ, Cha YM, Oh JK, Prasad A. Sudden cardiac death: an increasingly recognized presentation of apical ballooning syndrome (Takotsubo cardiomyopathy) Heart Lung. 2013;42:270–272. doi: 10.1016/j.hrtlng.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Madias JE. Ventricular fibrillation and Takotsubo syndrome: which one was first? Int J Cardiol. 2014;173:506. doi: 10.1016/j.ijcard.2014.03.143. [DOI] [PubMed] [Google Scholar]
- 78.Madhavan M, Rihal CS, Lerman A, Prasad A. Acute heart failure in apical ballooning syndrome (TakoTsubo/stress cardiomyopathy): clinical correlates and Mayo Clinic risk score. J Am Coll Cardiol. 2011;57:1400–1401. doi: 10.1016/j.jacc.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 79.Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50:448–452. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 80.de Gregorio C. Cardioembolic outcomes in stress-related cardiomyopathy complicated by ventricular thrombus: a systematic review of 26 clinical studies. Int J Cardiol. 2010;141:11–17. doi: 10.1016/j.ijcard.2009.09.468. [DOI] [PubMed] [Google Scholar]
- 81.Angue M, Soubirou L, Vandroux D, Cordier C, Martinet O, Gauzere BA, Braunberger E. Beneficial effects of intravenous beta-blockers in Tako-Tsubo syndrome with dynamic left ventricular outflow tract obstruction and severe haemodynamic impairment. Int J Cardiol. 2014;177:e56–e57. doi: 10.1016/j.ijcard.2014.09.162. [DOI] [PubMed] [Google Scholar]
- 82.Bonacchi M, Maiani M, Harmelin G, Sani G. Intractable cardiogenic shock in stress cardiomyopathy with left ventricular outflow tract obstruction: is extra-corporeal life support the best treatment? Eur J Heart Fail. 2009;11:721–727. doi: 10.1093/eurjhf/hfp068. [DOI] [PubMed] [Google Scholar]
- 83.Shah BN, Curzen NP. Dynamic left ventricular outflow tract obstruction and acute heart failure in tako-tsubo cardiomyopathy. J Am Coll Cardiol. 2011;58:1195–1196; author reply 1196. doi: 10.1016/j.jacc.2011.03.062. [DOI] [PubMed] [Google Scholar]
- 84.Migliore F, Bilato C, Isabella G, Iliceto S, Tarantini G. Haemodynamic effects of acute intravenous metoprolol in apical ballooning syndrome with dynamic left ventricular outflow tract obstruction. Eur J Heart Fail. 2010;12:305–308. doi: 10.1093/eurjhf/hfp205. [DOI] [PubMed] [Google Scholar]
- 85.Sharkey SW, Lesser JR, Zenovich AG, Maron MS, Lindberg J, Longe TF, Maron BJ. Acute and reversible cardiomyopathy provoked by stress in women from the United States. Circulation. 2005;111:472–479. doi: 10.1161/01.CIR.0000153801.51470.EB. [DOI] [PubMed] [Google Scholar]
- 86.Prasad A. Apical ballooning syndrome: an important differential diagnosis of acute myocardial infarction. Circulation. 2007;115:e56–e59. doi: 10.1161/CIRCULATIONAHA.106.669341. [DOI] [PubMed] [Google Scholar]
- 87.Kosuge M, Ebina T, Hibi K, Morita S, Okuda J, Iwahashi N, Tsukahara K, Nakachi T, Kiyokuni M, Ishikawa T, et al. Simple and accurate electrocardiographic criteria to differentiate takotsubo cardiomyopathy from anterior acute myocardial infarction. J Am Coll Cardiol. 2010;55:2514–2516. doi: 10.1016/j.jacc.2009.12.059. [DOI] [PubMed] [Google Scholar]


