Abstract
Cable bacteria are long, multicellular filaments that can conduct electric currents over centimeter-scale distances. All cable bacteria identified to date belong to the deltaproteobacterial family Desulfobulbaceae and have not been isolated in pure culture yet. Their taxonomic delineation and exact phylogeny is uncertain, as most studies so far have reported only short partial 16S rRNA sequences or have relied on identification by a combination of filament morphology and 16S rRNA-targeted fluorescence in situ hybridization with a Desulfobulbaceae-specific probe. In this study, nearly full-length 16S rRNA gene sequences of 16 individual cable bacteria filaments from freshwater, salt marsh, and marine sites of four geographic locations are presented. These sequences formed a distinct, monophyletic sister clade to the genus Desulfobulbus and could be divided into six coherent, species-level clusters, arranged as two genus-level groups. The same grouping was retrieved by phylogenetic analysis of full or partial dsrAB genes encoding the dissimilatory sulfite reductase. Based on these results, it is proposed to accommodate cable bacteria within two novel candidate genera: the mostly marine “Candidatus Electrothrix”, with four candidate species, and the mostly freshwater “Candidatus Electronema”, with two candidate species. This taxonomic framework can be used to assign environmental sequences confidently to the cable bacteria clade, even without morphological information. Database searches revealed 185 16S rRNA gene sequences that affiliated within the clade formed by the proposed cable bacteria genera, of which 120 sequences could be assigned to one of the six candidate species, while the remaining 65 sequences indicated the existence of up to five additional species.
Keywords: Long distance electron transport (LDET), Cable bacteria, 16S rRNA phylogeny, dsrAB phylogeny, Fluorescence in situ hybridization (FISH)
Introduction
The term “cable bacteria” is collectively used for long, multicellular filamentous bacteria affiliated to the deltaproteobacterial family Desulfobulbaceae that can mediate electric currents over centimeter-scale distances in marine, freshwater, and salt-marsh sediments [16], [20], [36], [39], [41], [44]. Cable bacteria have been proposed to perform electrogenic sulfur oxidation via long-distance electron transport. Thereby they electrically couple the oxidation of sulfide in anoxic layers with the reduction of oxygen at the sediment surface [29], [31]. This unique type of microbial metabolism creates distinct geochemical signals that can be detected by depth profiling of O2, pH, H2S, and electric potential [8], [29], [35], and drastically changes the geochemistry of cable bacteria-populated sediments [24], [28], [37].
Cable bacteria have so far evaded cultivation in axenic culture, although all specimens reported to date are morphologically conspicuous, cm-long filaments with distinct longitudinal ridges on their surface [31] and belong to an apparently monophyletic sister lineage of the genus Desulfobulbus [31], [36]. However, their taxonomic delineation and diversity are uncertain, as most studies have only reported short partial sequences that covered different regions of the 16S rRNA gene [16], [21], [23], [46]. Molecular identification of cable bacteria therefore currently relies on matching short environmental sequences with high sequence identity (>97%) to “confirmed” cable bacteria sequences (i.e. 16S rRNA gene sequences obtained from single filaments) [31], [36], [39]. Alternatively, filament morphology and the general Desulfobulbaceae-specific, 16S rRNA-targeted probe DSB706 [18] are combined to detect and quantify cable bacteria by fluorescence in situ hybridization (FISH) without further identification [20], [41], [44].
The aim of the current study was to establish a robust phylogenetic framework for cable bacteria, which could be used for their taxonomic delineation and the reliable identification of cable bacteria environmental sequences.
Therefore, individual filaments of cable bacteria were picked from freshwater and marine sediments for whole genome amplification, sequencing, and assembly of nearly full-length 16S rRNA and dsrAB gene sequences in order to complement the 16S rRNA phylogeny with that of a faster evolving marker gene [25]. The data were used to reconstruct a robust cable bacteria phylogeny, which, together with the available morphological, physiological and ecological information, led to the proposal of six candidate species within two novel candidate sister genera. Using this taxonomic framework, cable bacterial 16S rRNA gene sequences were identified in public databases, and a comprehensive overview of their environmental diversity and distribution is presented together with the design of novel oligonucleotide probes for their specific detection.
Materials and methods
Sites and sample collection
Aarhus Bay, Denmark
Sediment was collected from Aarhus Bay, Denmark (56°8′20.004″N, 10°12′51.084″E) in April 2012 and cable bacteria enrichment cultures were prepared as described previously [31]. In February 2013, sediment from Aarhus Bay was incubated under anoxic, nitrate-amended conditions, according to Marzocchi et al. [23], and incubations were subsampled for cable bacteria collection.
Buzzards Bay, MA, USA
Samples from intertidal salt marsh sediment, Little Sippewissett, MA, USA (41°34′33.9″N, 70°38′04.9″W) were obtained from laboratory incubations during the study of Larsen et al. [16] in August 2012.
Tokyo Bay, Japan
Sediment cores from Tokyo Bay, Japan (35°32′12.11″N, 139°55′0.12″E; water depth, 20 m) were collected by Scuba diving in November 2013, and the uppermost 20 cm were preserved in 50% (v/v) ethanol and later used for cable bacteria extraction without prior enrichment. The site was seasonally hypoxic and bioturbated with brittle stars and polychaetes.
Giber Å, Denmark
For freshwater cable bacteria, four recently published full-length 16S rRNA gene sequences (GenBank acc. no. KP728462-65) were used, which had been retrieved from freshwater sediment enrichments of the lowland stream Giber Å (56°3′53.9″N, 10°10′20.3″E), at a site located approximately 5 km inland from the coast of Aarhus Bay, Denmark [36].
Filament preparation, genome amplification, sequencing, and genome reconstruction
Cable bacteria were extracted from sediments as described previously [31]. Briefly, single filaments of cable bacteria were picked from sediments with custom-made capillary glass hooks under dissection microscope guidance (Leica M125; Leica, Wetzlar, Germany). All filaments were washed in sterile artificial seawater (salinity: 25‰, pH 8; Reef Crystal) and lysed by ultrasonic bead-beating (Sonoplus HD2070; Bandelin, Berlin, Germany; sonication parameters: 3 min, continuous mode, amplitude setting: 30% ≈ 21 W). Genomes were amplified using the GenomePlex® Single Cell Whole Genome Amplification Kit (Sigma–Aldrich). Sequencing of the amplified genomic material was performed on an Ion Torrent PGM™ sequencer (Life Technologies, USA) using 316v1 chips and 200 or 400 bp chemistry according to the manufacturer's protocols. Read trimming and adapter clipping were carried out as described earlier [36]. The trimmed and clipped reads were assembled using (i) gsAssembler version 2.6 (Roche 454 Life Sciences, Branford, CT) with 10 different configurations: minimum overlap settings of 50 or 100 bp, respectively, and minimum similarity values of 96–100% with 1% steps; and (ii) SPAdes version 2.2.1 (Aarhus Bay MCF) or 3.5 (all other filaments) [2]. Additionally, a set of 10 reduced-complexity assemblies was generated, where 500,000 randomly-selected reads were assembled using gsAssembler 2.6 with a minimum overlap of 100 bp and a minimum sequence identity of 98%. The resulting 21 assemblies were combined, and contigs shorter than 1000 bp were excluded from further analysis. Cable bacterial 16S rRNA genes were extracted from the assemblies as described previously [36]. Open reading frames (ORFs) were predicted on the extracted contigs using FragGeneScan version 1.19 [34]. ORFs coding for DsrA and DsrB were identified and extracted using MEGAN version 4.70.4 [13] based on BLASTp hits to NCBI's nr database (January 25th, 2015 version). Due to the multiple assemblies, this resulted in multiple dsrA and dsrB ORFs extracted from most single filaments, which were aligned and merged using ARB [49], yielding the final dsrA and dsrB sequences used for further analysis. Several of the reconstructed dsrAB sequences were incomplete, or even absent in filaments F1 and F5 (Table S2), most likely due to incomplete and biased genome recovery after the genome amplification step.
Phylogenetic analyses
All 16 cable bacteria 16S rRNA gene sequences were aligned using the SINA online tool [32], added to the SILVA Release 119 SSU Ref database [33] using ARB [49], and the alignment was manually inspected. Phylogenetic trees were calculated by maximum likelihood (ML; RAxML [43]) and maximum parsimony (MP; Phylip [9]) methods implemented in ARB with 1000 bootstraps and a 50% base frequency filter. Full-length 16S rRNA gene sequences from 36 Desulfobulbaceae isolates, extracted from RDP [http://rdp.cme.msu.edu/] were included as reference sequences (Table S1). A consensus tree was constructed from ML and MP trees with multifurcations at nodes that differed between the two treeing methods, indicating that the tree topology at these nodes could not be unambiguously resolved. Pairwise 16S rRNA gene sequence similarities were calculated using the ARB neighbor-joining tool.
The dsrA and dsrB sequences (Table S2) were concatenated and added into a published reference alignment [25] using ARB. Nucleotide identities between aligned dsrAB sequences were calculated using MEGA version 6.06 [45]. Nucleotide dsrAB sequences were translated to amino acid sequences and aligned based on the corresponding nucleotide alignment using ARB. Phylogenetic trees were calculated based on almost full-length DsrAB sequences (≥788 amino acids long) by ML analysis with RAxML version 8.2.4, and MP analysis with Phylip version 3.696 (http://evolution.genetics.washington.edu/phylip.html). ML analysis was performed using the Γ model of rate heterogeneity and the WAG amino acid substitution matrix. Node stability of ML and MP phylogenies was evaluated by 1000 bootstrap replicates. Short DsrAB sequences (<788 amino acids long) were added to the ML phylogeny using an evolutionary placement algorithm (EPA) [4] implemented in RAxML version 8.2.4.
Sequences obtained in the present study were deposited in GenBank under accession numbers KR912338 to KR912349 (16S rRNA) and KU844004 to KU844028 (dsrAB).
Database mining for 16S rRNA sequences of cable bacteria and phylogenetic placement
The GenBank (Release May 2015) [3] and SILVA SSU Ref (Release 119) [33] databases were screened for sequences with nucleotide identities ≥90% to any of the full-length cable bacterial 16S rRNA sequences using BLASTn [1]. Partial sequences were added separately to the consensus tree by parsimony criteria using the quick-add tool in ARB. Sequences affiliating within the cable bacteria cluster were retained for further analysis. The VAMPS database [12] and the Sequence Read Archive (SRA) [17] were searched using the key words “metagenome” and “sediment”. Relevant VAMPS data sets were downloaded manually from the database server, while SRA data sets were retrieved via their accession numbers using the fastq-dump function of the SRA software toolkit [17] (May 2015). Reads featuring a sequence identity of ≥90% to one of the full-length 16S rRNA sequences of cable bacteria were extracted using BBmap version 34.94 (http://sourceforge.net/projects/bbmap/). Extracted reads were taxonomically classified using the classify.seqs function integrated in Mothur version 1.25 [40]. Classification was carried out using the “Wang” function with 1000 bootstrap iterations, and was based on the Desulfobulbaceae phylogenetic tree created in this study. Only classifications featuring a bootstrap support of at least 80% were retained.
The retained partial sequences were phylogenetically placed using the EPA [4] implemented in RAxML 8.2.4 [43]. In brief, the EPA assigned the environmental sequences to the edges of the full-length 16S rRNA consensus tree (see above) under the maximum likelihood model. RAxML was called with an alignment of 280 query and reference sequences to place 227 partial sequences into the 103 branches of the consensus tree with 53 taxa. The algorithm returned multifurcating nodes if two or more query sequences were assigned to the same edge. Only sequences placed in the cable bacteria cluster were retained (Table S3) and displayed in the final tree.
Fluorescence in situ hybridization (FISH)
Probe design and evaluation
Based on the 16S rRNA gene diversity observed in the phylogenetic analyses, a set of five new cable bacteria-specific probes, including competitor (C) and helper (H) oligonucleotides, as needed, were developed using ARB (Table 3). Probes were tested for sensitivity and specificity in silico with the ARB probe match tool (SILVA database 119 SSU Ref Nr 99). In addition, TestProbe 3.0, the SILVA probe match and evaluation tool, was used to check probes against the current SILVA Release 119 SSU Ref and Parc database for specificity and group coverage. mathFISH [55] was applied for in silico evaluations of the new probes, including hybridization efficiency calculations [53], formamide curve simulation [54], mismatch analysis [52], and competitor model [11]. The predicted hybridization conditions were tested in situ with cable bacteria-enriched sediment from Aarhus Harbor and Giber Å. EUB338 probe mixture [7] and probe NON338 [48] were used as positive and negative controls, respectively, and probe DSB706 [18] was used to identify filamentous Desulfobulbaceae. Stringency was adjusted as described by Manz et al. [22] in order to discriminate single mismatch differences between co-occurring filaments of clusters I and IV (in Aarhus Harbor enrichments), and between clusters V and VI (in Giber Å enrichments).
Table 3.
16S rRNA-targeted oligonucleotide probes developed in this study.
| Probe | Probe sequence (5′–3′) | Specificity | E. coli position no.a | % FA FISHb |
|---|---|---|---|---|
| EXma1271 | GCT TTC AGG GAT TTG CGC CT | Cluster I | 1271–1290 | 45 |
| H1229 | TTG TAC CGG CCA TTG TAG TAC G | 1229–1250 | ||
| H1251 | CTC GCG AAG TGG CTG CCC G | 1251–1269 | ||
| EXaa430 | TTT CTT CCC TTC TGA CAG GGT TT | Cluster II | 430–452 | 45 |
| H409 | TAC GAC CCG AAA GCC TTC CTC | 409–429 | ||
| C432 | CTT CTT CCC TTC TGA CAG GGC | 432–452 | ||
| C444 | CCC ATA AGC ACT TCT TCC C | 444–462 | ||
| EXco1016 | CTC TCA AAG AGA GCA CTT CCC TA | Cluster III, IV | 1016–1038 | 45 |
| H994 | TTT CTA GGG CTT TTT CGG GAT G | 994–1015 | ||
| ENni1437 | CCC GAA GGT CCG CCC AGC T | Cluster V | 1437–1455 | 50 |
| ENpa1421c | CCA GCT GCT TCT GGT GCA ATC G | Cluster VI | 1421–1442 | 45 |
Probe target position on E. coli 16S rRNA according to [6].
Formamide concentration (v/v) in FISH hybridization buffer at 46 °C.
Use Probe ENpa1421 together with probe DSB706 for specific detection.
FISH
Cable bacteria filaments were extracted and separated from Aarhus Bay sediment, as described by Kallmeyer et al. [14], with samples sonicated for 3 × 10 s with 10 s between cycles (Sonopuls HD 2070; Bandelin). Freshwater sediments were diluted and spotted onto slides without cell extraction, as described previously [31]. FISH was performed according to the standard protocol [30] with formamide concentrations in the hybridization buffer and with helper and competitor oligonucleotides added as listed in Table 3. Probes were 5′-labeled with either 6-carboxyfluorescein (6-FAM), the sulfoindocyanine dye Cy3, or Atto542, and they were all purchased from biomers.net (Ulm, Germany). Samples were counterstained with the general DNA stain 4′,6-diamidino-2-phenylindole (DAPI) and evaluated using a Zeiss Axiovert 200M epifluorescence microscope (Carl Zeiss, Göttingen, Germany). Pictures were taken with a black and white camera (AxioCam MRm; Carl Zeiss). Digital image analysis was performed using the Zeiss imaging software AxioVision Rel.4.8.2 (Carl Zeiss).
Electron and atomic force microscopy analysis
Bundles of cable bacteria were collected with glass hooks as described above from sediment incubations with “Candidatus Electronema nielsenii” (identified by sequencing and FISH), washed in water to remove sediment particles, and either used directly or stored in 50% (v/v) ethanol at −20 °C. Transmission electron microscopy (TEM) carbon support films on copper grids were made hydrophilic using a Pelco easiGlow™ glow discharger. Cable bacteria bundles were pipetted onto the grids and allowed to settle for 45 min. The grids were then washed in water before staining with 2% phosphotungstic acid (pH 7) for 2–3 s. TEM were acquired on a Tecnai G2 Spirit microscope (FEI, Eindhoven, The Netherlands) with a side-mounted Veleta CCD camera (Olympus Soft Imaging Solutions, Münster, Germany). For scanning electron microscopy (SEM), bundles were pipetted onto 0.2 μm pore size track-etched polycarbonate filters and allowed to dry. Dry filters were mounted on SEM specimen mounts using conductive carbon adhesive tabs and coated with 5 nm gold using a Leica EM SCD500 sputter coater. Imaging was performed on a FEI Nova 600 NanoSEM in high vacuum at 5 kV.
Native, hydrated cable bacteria were analyzed by atomic force microscopy (AFM) to avoid artifacts potentially introduced by sample preparation (dehydration, staining, or coating) or the vacuum environment necessary for EM imaging. AFM images were captured on a Dimension Icon® AFM (Bruker, Santa Barbara, USA) in tapping mode with ultra-sharp probes (OMCL-AC160TS-C2; Olympus, Tokyo, Japan) in air. The spring constant of the probe was 26.1 N m−1, with a resonant frequency of approximately 300 kHz. Analysis of AFM images was carried out with Scanning Probe Image Processor software (SPIP™; Image Metrology ApS, Lyngby, Denmark).
Results and discussion
Refined phylogeny of cable bacteria
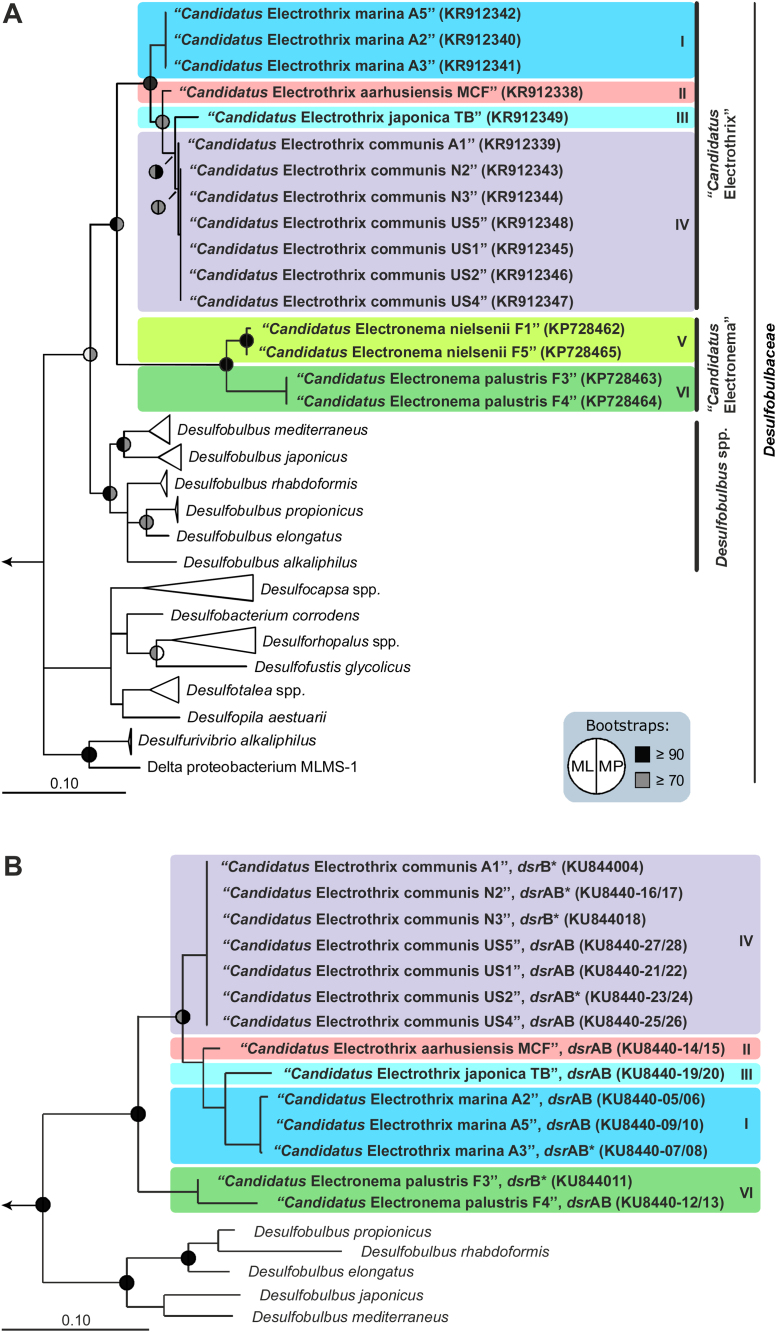
Picking of individual cable bacteria filaments with subsequent whole genome amplification and 16S rRNA sequence reconstruction enabled direct correlation of sequence information with the phenotypic property of cable bacteria and their environmental origin, in line with earlier studies of large sulfur bacteria [19], [26], [38]. All 16 full-length 16S rRNA gene sequences retrieved from single filaments in this study clustered together as a distinct, monophyletic sister group to the genus Desulfobulbus within the family Desulfobulbaceae (Fig. 1A). This cable bacteria clade displayed two major, genus-level branches with a 16S rRNA gene sequence identity of <94.5% (Table 1), which is the cut-off for discriminating different genera [51]. One branch contained the twelve sequences originating from marine and salt marsh sites, whereas the other comprised the four freshwater sequences, separated into two distinct species-level clusters, below the 98.7% 16S rRNA gene sequence identity threshold proposed for discriminating different species [42]. Overall, the analysis thus reproduced the cable bacteria phylogeny reported previously [36]. However, due to the full-length sequences, it was also possible to differentiate four species that had a <98.7% 16S rRNA gene sequence identity (Table 1) and were clearly phylogenetically distinct (Fig. 1A) in the hitherto unresolved marine group [16], [21], [23], [31], [39], [46].
Fig. 1.
(A) Consensus tree of full-length 16S rRNA gene sequences of cable bacteria and all isolated members of the Desulfobulbaceae. Phylogenetic trees were calculated using a 50% base frequency filter. The nomenclature is based on the proposal of Candidatus taxa made in this study. Roman numbers indicate identified species within the genera “Candidatus Electrothrix” and “Candidatus Electronema”, which are indicated with different background colors. Corresponding accession numbers of cable bacteria are shown in parentheses. Scale bar represents 10% estimated sequence divergence. Bootstrap support (1000 re-samplings) of meaningful nodes is indicated by split circles (left: maximum likelihood (ML); right: maximum parsimony (MP)), with black, gray and white/absence indicating ≥90%, 70–90% and <70% support, respectively. Accession numbers of reference sequences are listed in Table S1. (B) DsrAB-based phylogeny of cable bacteria and closely related Desulfobulbaceae, as reconstructed by ML analysis. Scale bar represents 10% sequence divergence. Bootstrap support (1000 re-samplings) of meaningful nodes is indicated by split circles (left: ML; right: MP), with black, gray and white/absence indicating ≥90%, 70–90% and <70% support, respectively. Short sequences (<788 amino acids, marked with asterisks) were added to the tree without changing its topology. DsrAB sequences of distantly related Desulfobulbaceae were used as an outgroup (not shown). No dsrAB ORFs were identified in the partial genomes of “Ca. Electronema nielsenii”. Accession numbers of reference sequences are listed in Table S2. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
16S rRNA gene sequence identities among the proposed candidate species of cable bacteria.
| Cluster | Name of species within the cluster | 16S rRNA gene identity |
|||||
|---|---|---|---|---|---|---|---|
| With cluster I [%] | With cluster II [%] | With cluster III [%] | With cluster IV [%] | With cluster V [%] | With cluster VI [%] | ||
| I | “Ca. Electrothrix marina” | 100 | 97.4 | 96.6 | 96.9–97.1 | 90.4–90.8 | 89.7 |
| II | “Ca. Electrothrix aarhusiensis”a | 97.4 | 100 | 97.2 | 98.3 | 90.3–90.5 | 89.6 |
| III | “Ca. Electrothrix japonica”1 | 96.6 | 97.2 | 100 | 98.3–98.4 | 89.9–90.2 | 89.6 |
| IV | “Ca. Electrothrix communis” | 96.9–97.1 | 98.3 | 98.3–98.4 | 99.9–100 | 90.7–91 | 89.9 |
| V | “Ca. Electronema nielsenii” | 90.4–90.8 | 90.3–90.5 | 89.9–90.2 | 90.7–91 | 99.7 | 95.1 |
| VI | “Ca. Electronema palustris” | 89.7 | 89.6 | 89.6 | 89.9 | 95.1 | 100 |
| Desulfobulbus | D. mediterraneus 86FS1 | 92.2 | 92.2 | 92.1 | 91.9–92 | 89.7 | 88.6 |
| Desulfobulbus | D. propionicus DSM 2032 | 91.1 | 90.9 | 90.7 | 91.3–91.4 | 89.7 | 88.6 |
Only one sequence available.
The same grouping was retrieved by phylogenetic analysis of DsrAB amino acid sequences (Fig. 1B). The dsrA and dsrB genes encode the alpha and beta subunits of the dissimilatory sulfite reductase and are widely used functional and phylogenetic marker genes for sulfate-reducing and sulfide oxidizing-prokaryotes [25], [47]. The identities of the dsrAB genes between 16S rRNA-defined species were ≤92%, <82% between members of the two cable bacteria genera, and <80% between cable bacteria and the genus Desulfobulbus (Table 2, Table S4). These values were within the 90–92% dsrAB identity range proposed for species delineation [15], [25], or the 75–82% identity range for genus delineation inferable from the data set of Müller et al. [25], and thus supported the 16S rRNA-based analysis.
Table 2.
dsrAB gene sequence identities among the proposed candidate species of cable bacteria.
| Cluster | Name of species within the cluster |
dsrAB gene identity |
|||||
|---|---|---|---|---|---|---|---|
| With cluster I [%] | With cluster II [%] | With cluster III [%] | With cluster IV [%] | With cluster V [%] | With cluster VI [%] | ||
| I | “Ca. Electrothrix marina”a | 99.6–99.9 | 91.3–91.9 | 88.5–88.6 | 89.3–90.6 | n. d. | 80.2–81.6 |
| II | “Ca. Electrothrix aarhusiensis” | 91.3–91.9 | 100 | 89.4 | 91.4–92.4 | n. d. | 78.3–80.2 |
| III | “Ca. Electrothrix japonica” | 88.5–88.6 | 89.4 | 100 | 87.5–88.6 | n. d. | 76.9–79.1 |
| IV | “Ca. Electrothrix communis”1 | 89.3–90.6 | 91.4–92.4 | 87.5–88.6 | 100 | n. d. | 75.5–79.8 |
| V | “Ca. Electronema nielsenii” | n. d. | n. d. | n. d. | n. d. | n. d. | n. d. |
| VI | “Ca. Electronema palustris”1 | 80.2–81.6 | 78.3–80.2 | 76.9–79.1 | 75.5–79.8 | n. d. | 100 |
| Desulfobulbus | D. mediterraneus 86FS1 | 78.9–79.5 | 78.7 | 78.9 | 77.5–79.8 | n. d. | 73.6–76.7 |
| Desulfobulbus | D. propionicus DSM 2032 | 78.6–79.4 | 77.9 | 76.8 | 75.5–77.4 | n. d. | 73.2–78.3 |
Includes partial sequences.
n. d., no data; for further details see Table S2.
Proposal of novel candidate taxa
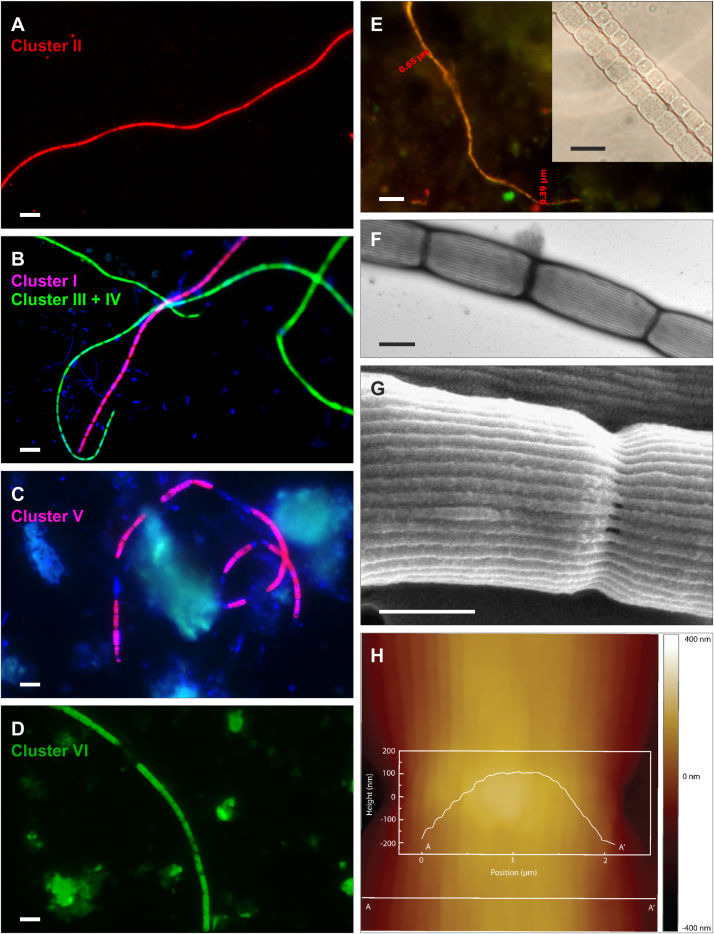
Based on these results, it is proposed to accommodate cable bacteria within two novel candidate genera: the preferably marine “Candidatus Electrothrix”, with four novel candidate species, “Ca. E. marina”, “E. aarhusiensis”, “E. communis”, and “E. japonica”, and the preferably freshwater “Candidatus Electronema”, with two novel candidate species, “Ca. E. nielsenii”, and “E. palustris”. The proposed genera and species can be distinguished based on their 16S rRNA and dsrAB gene sequences (Fig. 1; Table 1, Table 2), and can also be identified in situ by FISH with a suite of newly designed oligonucleotide probes (Table 3; Fig. 2A–D). In contrast, cell diameter was not a good predictor for taxonomic affiliation, due to the considerable size variation among cable bacteria of the same species [39] and even within a single filament (Fig. 2E). Cable bacteria diameters ranged from 0.4 to 8 μm, with an average cell length of 3 μm [16], [21], [23], [31], [39], [46] (Fig. 2). The total length of cable bacteria is difficult to determine, as they often form bundles and break when handling sediment samples. However, filaments up to 1.5 cm long have been recorded [31] (Fig. S1), suggesting that cable bacteria are long enough to span the entire suboxic zone.
Fig. 2.
Micrographs of cable bacteria. (A–D) FISH identification of cable bacteria: (A) “Ca. Electrothrix aarhusiensis”, hybridized with probe EXaa430-CY3 (red); (B) “Ca. Electrothrix marina”, hybridized with probe EXma1271-CY3 (magenta from overlay with DAPI), and “Ca. Electrothrix communis/japonica”, hybridized with probe EXco1016-FAM (blue-green from overlay with DAPI); (C) “Ca. Electronema nielsenii”, hybridized with probe ENni1437-CY3 (magenta from overlay with DAPI); (D) “Ca. Electronema palustris”, hybridized with probe ENpa1421-FAM (green). Scale bars for panels A–D, 5 μm. (E) FISH-identified cable bacteria filament (orange from overlay of DSB706-CY3 and EUB338-FAM) showing differences in filament diameter over a short distance. Scale bar, 5 μm. Insert: phase contrast image of two distant, differently sized sections of the same cable bacteria filament. Scale bar, 10 μm (for complete filament image see Fig. S1). (F) TEM micrograph of “Ca. Electronema nielsenii” after phosphotungstic acid staining to visualize the longitudinal ridges. Scale bar, 1 μm. (G) SEM micrograph of gold-sputtered “Ca. Electronema nielsenii”, showing that the longitudinal ridges are continuous over a cell-cell junction. Scale bar, 0.5 μm. (H) AFM height image of an unfixed, hydrated “Ca. Electronema nielsenii”. The height profile along line A–A′ is overlaid onto the image, illustrating the 3D structure of the ridges.
Unifying morphological characteristics for all members of the candidate genera Electrothrix and Electronema are continuous ridges that run along the entire length of a filament [31]. Depending on cell diameters, single filaments contain 15–71 ridges with diameters of 30–100 nm [21], [31] that are detectable by TEM, SEM, and AFM (Fig. 2F–H). Cells within a filament share a common outer membrane and periplasm [31]. Cable bacteria filaments are unbranched and do not store elemental sulfur in globules, as is characteristic for many other large sulfur oxidizing bacteria [27], [50], but polyphosphate inclusions have been observed in some cable bacteria cells [44]. They show gliding motility [5] and conduct electrons from sulfide to oxygen [31] and, at least in some cases, nitrate/nitrite [23].
Environmental distribution of cable bacteria
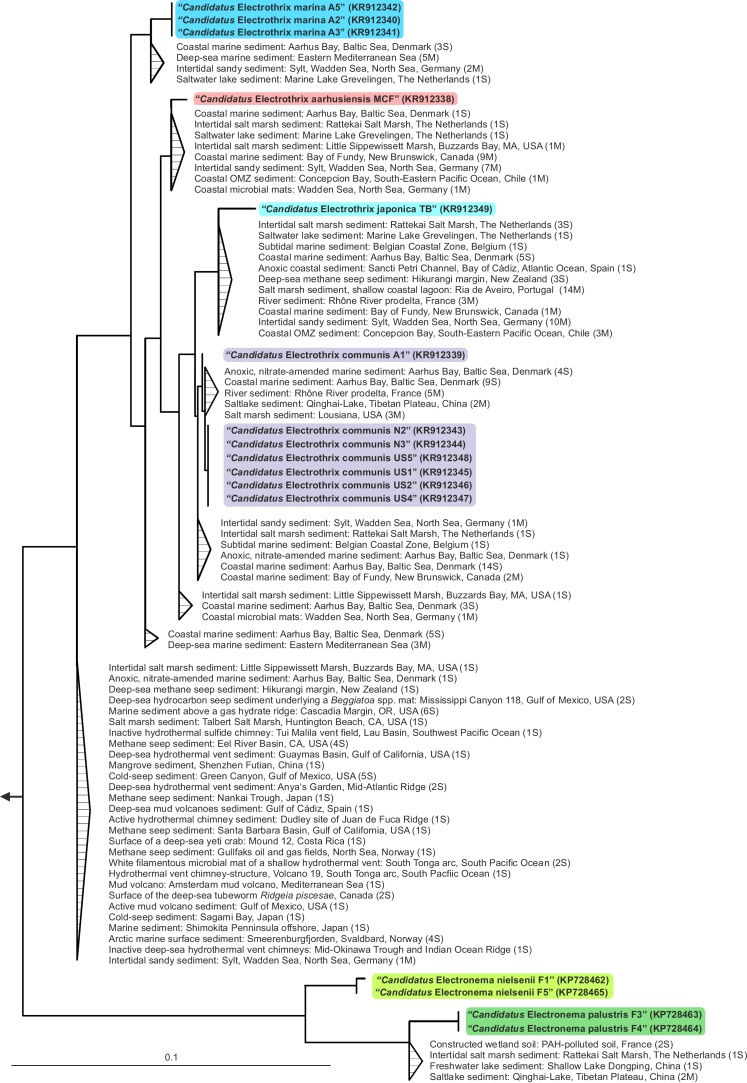
The robust phylogenetic and taxonomic framework for cable bacteria described above enabled a more informed database search in order to assess the environmental diversity and distribution of the candidate genera Electrothrix and Electronema. In total, 185 16S rRNA gene sequences were retrieved that affiliated with these cable bacteria (Fig. 3; Table S3); of these, 120 sequences could be assigned to the six candidate species, while the remaining 65 sequences indicated the existence of up to five additional species (Fig. 3; Table S3). The clear phylogenetic demarcation of the “Ca. Electrothrix/Electronema” cable bacteria clade also allowed a re-evaluation of the cable bacteria diversity suggested in earlier studies. Notably, this analysis revealed that nine of the twelve Desulfobulbaceae sequence types detected in a marine nitrate-enrichment [23], and all Desulfobulbus relatives detected at an anode in freshwater sediment [10], [39] affiliated outside the cable bacteria lineage, indicating most likely that they were not cable bacteria.
Fig. 3.
Environmental distribution of 16S rRNA gene sequences of cable bacteria. Partial environmental sequences were assigned to the consensus full-length 16S rRNA gene tree of Fig. 1A without changing its topology using EPA and RAxML. Only sequences placed within the proposed candidate genera are shown. The total number of 16S rRNA gene sequences in a cluster is given next to the clustered sequences. (M) and (S) indicate that sequences were retrieved from metagenome databases or from the SILVA SSU Ref 119/GenBank database, respectively. Triangles depict multifurcating nodes. Scale bar represents 10% estimated sequence divergence (not valid for multifurcation triangles). Accession numbers for all published sequences are given in Table S3. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Members of the “Ca. Electrothrix/Electronema” cable bacteria lineage were found in a wide range of marine, brackish, salt lake, and freshwater habitats (Fig. 3), confirming the global distribution of cable bacteria reported earlier [21]. Except for the salinity-based genus-level divide reported earlier (“Ca. Electrothrix” apparently prefers marine and saline habitats, “Ca. Electronema” freshwater habitats) [36], no habitat-specific distribution patterns of the six candidate species were observed. However, there was an overrepresentation of deep sea- and hydrothermal vent-derived cable bacteria sequences outside the six candidate species (Fig. 3, Table S3), suggesting a promising environment for the description of novel cable bacteria species.
A somewhat surprising result, given the cable bacteria's global distribution [21] (Fig. 3) and periodically high in situ biomass [41], [44], was the overall low number and biased distribution of cable bacteria sequences in public databases: reads were mostly obtained from next-generation sequencing projects (i.e. when thousands to millions of reads were analyzed), when Desulfobulbaceae-specific primers had been used, or when RNA had been extracted (Table S3). This may indicate a general problem with cell lysis and DNA extraction of cable bacteria, probably due to the rigid outer structures [31] (Fig. 2F–H), which needs to be considered when searching for cable bacteria in the environment.
In conclusion, the taxonomic framework presented here can be used to identify environmental sequences as cable bacteria, even without morphological information, and the newly developed FISH probes allow, in combination with the Desulfobulbaceae-specific probe DSB706, quick assignment of cable bacteria to one of the described candidate species.
Etymology of the Candidatus taxa
“Candidatus Electrothrix”
E.lec’tro.thrix. Gr. neut.n. electron, amber (which is the origin of the term electric); Gr. fem. n. thrix, hair; N.Gr. electric hair.
Multicellular filaments, up to several centimeters in length, with 15–71 characteristic longitudinal ridges and shared periplasm across cells; electron-conducting; typically spanning the suboxic zone in surface sediments; individual cells are 0.4–8 μm × 3 μm in size; polyphosphate inclusions; no sulfur inclusions; gliding motility; marine, including salt marsh and salt lake inhabiting.
“Candidatus Electronema”
E.lec.tro.ne’ma. Gr. neut.n. electron, amber; Gr. neut. n. nema, thread; N.Gr. electric thread.
Multicellular filaments, up to several centimeters in length, with 15–58 characteristic longitudinal ridges and shared periplasm across cells; electron-conducting; typically spanning the suboxic zone in surface sediments; individual cells are 0.4–3 μm × 3 μm in size; no sulfur inclusions; gliding motility; mostly freshwater-inhabiting.
“Candidatus Electrothrix marina”
ma.ri’na. L.fem.adj. marina of or belonging to the sea, marine, referring to its marine habitat.
Marine members of the candidate genus Electrothrix; distinguishable by their 16S rRNA and dsrAB sequences; identifiable by specific probe EXma430 (5′-TTTCTTCCCTTCTGACAGGGTTT-3′); accession numbers KR912340-42 (16S rRNA), KU844005-10 (dsrAB).
“Candidatus Electrothrix aarhusiensis”
aar.hu.si.en'sis. N.L.adj. aarhusiensis from Aarhus, referring to the place of the first discovery of cable bacteria.
Mostly coastal and intertidal members of the candidate genus Electrothrix; distinguishable by their 16S rRNA and dsrAB sequences; identifiable by specific probe EXaa1016 (5′-CTCTCAAAGAGAGCACTTCCCTA-3′); accession numbers KR912338 (16S rRNA), KU844014, KU844015 (dsrAB).
“Candidatus Electrothrix japonica”
ja.po’ni.ca. N.L.fem.adj. japonica Japanese, referring to its discovery in Tokyo Bay, Japan.
Coastal, intertidal, and marine members of the candidate genus Electrothrix; distinguishable by their 16S rRNA and dsrAB sequences; can be detected together with Ca. E. communis by specific probe EXco1271 (5′-GCTTTCAGGGATTTGCGCCT-3′); accession numbers KR912349 (16S rRNA), KU844019, KU844020 (dsrAB).
“Candidatus Electrothrix communis”
com.mu’nis. L.fem.adj. communis common, widespread, referring to its widespread distribution in diverse habitats.
Ubiquitous members of the candidate genus Electrothrix; distinguishable by their 16S rRNA and dsrAB sequences; can be detected together with Ca. E. japonica by specific probe EXco1271 (5′-GCTTTCAGGGATTTGCGCCT-3′); accession numbers KR912339; KR912343-48 (16S rRNA), KU844004; KU844016-18; KU844021-28 (dsrAB).
“Candidatus Electronema nielsenii”
niel.se’ni.i. N.L. gen.n. nielsenii of Nielsen, named in honor of Lars Peter Nielsen, the Danish microbial ecologist who started the cable bacteria studies by discovering electric currents in the seafloor; the species has been extracted from his backyard.
Members of the candidate genus Electronema; creeks, streams, and freshwater ponds; distinguishable by their 16S rRNA sequences; identifiable by specific probe ENni1437 (5′-CCCGAAGGTCCGCCCAGCT-3′); accession numbers KP728462, KP728465 (16S rRNA).
“Candidatus Electronema palustris”
pa.lus’tris. L. fem. adj. palustris, marshy, swampy, wetland-inhabiting.
Mostly freshwater members of the candidate genus Electronema; distinguishable by their 16S rRNA and dsrAB sequences; identifiable by specific probe ENpa1421 (5′-CCAGCTGCTTCTGGTGCAATCG-3′); accession numbers KP728463, KP728464 (16S rRNA), KU844011-13 (dsrAB).
Acknowledgements
We thank Britta Poulsen and Trine Bech Søgaard for excellent technical assistance, as well as Steffen Larsen, Lars Riis Damgaard, and Nils Risgaard-Petersen for providing sediment samples. We thank Zegao Wang for assistance with acquiring AFM micrographs. Filip Meysman is acknowledged for suggesting the genus name Electrothrix.
This research was financially supported by the Danish National Research Foundation, the Danish Council for Independent Research|Natural Sciences, and the European Research Council (ERC Advanced Grant no 291650).
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.syapm.2016.05.006.
Contributor Information
Daniela Trojan, Email: trojan@microbial-ecology.net.
Andreas Schramm, Email: andreas.schramm@bios.au.dk.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Altschul S.F., Madden T.L., Schaffer A.A., Zhang J., Zhang Z., Miller W., Lipman D.J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., Pyshkin A.V., Sirotkin A.V., Vyahhi N., Tesler G., Alekseyev M.A., Pevzner P.A. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benson D.A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D.J., Ostell J., Sayers E.W. GenBank. Nucleic Acids Res. 2013;41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berger S.A., Krompass D., Stamatakis A. Performance, accuracy, and web server for evolutionary placement of short sequence reads under maximum likelihood. Syst. Biol. 2011;60:291–302. doi: 10.1093/sysbio/syr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjerg J.T., Damgaard L.R., Holm S.A., Schramm A., Nielsen L.P. Motility of electric cable bacteria. Appl. Environ. Microbiol. 2016;82 doi: 10.1128/AEM.01038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosius J., Dull T.J., Sleeter D.D., Noller H.F. Gene organization and primary structure of a ribosomal-RNA operon from Escherichia coli. J. Mol. Biol. 1981;148:107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- 7.Daims H., Brühl A., Amann R., Schleifer K.-H., Wagner M. The domain-specific probe EUB338 is insufficient for the detection of all bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 1999;22:434–444. doi: 10.1016/S0723-2020(99)80053-8. [DOI] [PubMed] [Google Scholar]
- 8.Damgaard L.R., Risgaard-Petersen N., Nielsen L.P. Electric potential microelectrode for studies of electro-biogeophysics. J. Geophys. Res. Biogeosci. 2014;119:1906–1917. [Google Scholar]
- 9.Felsenstein J. Phylip-phylogeny inference package (version 3.2) Cladistics. 1989;5:163–166. [Google Scholar]
- 10.Holmes D.E., Bond D.R., O’Neil R.A., Reimers C.E., Tender L.R., Lovley D.R. Microbial communities associated with electrodes harvesting electricity from a variety of aquatic sediments. Microb. Ecol. 2004;48:178–190. doi: 10.1007/s00248-003-0004-4. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino T., Yilmaz L.S., Noguera D.R., Daims H., Wagner M. Quantification of target molecules needed to detect microorganisms by fluorescence in situ hybridization (FISH) and catalyzed reporter deposition-FISH. Appl. Environ. Microbiol. 2008;74:5068–5077. doi: 10.1128/AEM.00208-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huse S., Mark Welch D., Voorhis A., Shipunova A., Morrison H., Eren A., Sogin M. VAMPS: a website for visualization and analysis of microbial population structures. BMC Bioinf. 2014;15:41. doi: 10.1186/1471-2105-15-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huson D.H., Mitra S., Ruscheweyh H.-J., Weber N., Schuster S.C. Integrative analysis of environmental sequences using MEGAN4. Genome Res. 2011;21:1552–1560. doi: 10.1101/gr.120618.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kallmeyer J., Smith D.C., Spivack A.J., D’Hondt S. New cell extraction procedure applied to deep subsurface sediments. Limnol. Oceanogr. Methods. 2008;6:236–245. [Google Scholar]
- 15.Kjeldsen K.U., Loy A., Jakobsen T.F., Thomsen T.R., Wagner M., Ingvorsen K. Diversity of sulfate-reducing bacteria from an extreme hypersaline sediment, Great Salt Lake (Utah) FEMS Microbiol. Ecol. 2007;60:287–298. doi: 10.1111/j.1574-6941.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 16.Larsen S., Nielsen L.P., Schramm A. Cable bacteria associated with long-distance electron transport in New England salt marsh sediment. Environ. Microbiol. Rep. 2015;7:175–179. doi: 10.1111/1758-2229.12216. [DOI] [PubMed] [Google Scholar]
- 17.Leinonen R., Sugawara H., Shumway M. The sequence read archive. Nucleic Acids Res. 2011;39:D19–D21. doi: 10.1093/nar/gkq1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loy A., Lehner A., Lee N., Adamczyk J., Meier H., Ernst J., Schleifer K.-H., Wagner M. Oligonucleotide microarray for 16S rRNA gene-based detection of all recognized lineages of sulfate-reducing prokaryotes in the environment. Appl. Environ. Microbiol. 2002;68:5064–5081. doi: 10.1128/AEM.68.10.5064-5081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacGregor B.J., Biddle J.F., Siebert J.R., Staunton E., Hegg E.L., Matthysse A.G., Teske A. Why orange Guaymas Basin Beggiatoa spp. are orange: single-filament-genome-enabled identification of an abundant octaheme cytochrome with hydroxylamine oxidase, hydrazine oxidase, and nitrite reductase activities. Appl. Environ. Microbiol. 2013;79:1183–1190. doi: 10.1128/AEM.02538-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malkin S.Y., Meysman F.J.R. Rapid redox signal transmission by “cable bacteria” beneath a photosynthetic biofilm. Appl. Environ. Microbiol. 2015;81:948–956. doi: 10.1128/AEM.02682-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malkin S.Y., Rao A.M.F., Seitaj D., Vasquez-Cardenas D., Zetsche E.-M., Hidalgo-Martinez S., Boschker H.T.S., Meysman F.J.R. Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor. ISME J. 2014;8:1843–1854. doi: 10.1038/ismej.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manz W., Amann R., Ludwig W., Wagner M., Schleifer K.H. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria – problems and solutions. Syst. Appl. Microbiol. 1992;15:593–600. [Google Scholar]
- 23.Marzocchi U., Trojan D., Larsen S., Meyer R.L., Revsbech N.P., Schramm A., Nielsen L.P., Risgaard-Petersen N. Electric coupling between distant nitrate reduction and sulfide oxidation in marine sediment. ISME J. 2014;8:1682–1690. doi: 10.1038/ismej.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meysman F.J.R., Risgaard-Petersen N., Malkin S.Y., Nielsen L.P. The geochemical fingerprint of microbial long-distance electron transport in the seafloor. Geochim. Cosmochim. Acta. 2015;152:122–142. [Google Scholar]
- 25.Müller A.L., Kjeldsen K.U., Rattei T., Pester M., Loy A. Phylogenetic and environmental diversity of DsrAB-type dissimilatory (bi) sulfite reductases. ISME J. 2015;9:1152–1165. doi: 10.1038/ismej.2014.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mussmann M., Hu F.Z., Richter M., de Beer D., Preisler A., Jørgensen B.B., Huntemann M., Glöckner F.O., Amann R., Koopman W.J., Lasken R.S., Janto B., Hogg J., Stoodley P., Boissy R., Ehrlich G.D. Insights into the genome of large sulfur bacteria revealed by analysis of single filaments. PLoS Biol. 2007;5:e230. doi: 10.1371/journal.pbio.0050230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelson D.C., Jannasch H.W. Chemoautotrophic growth of a marine Beggiatoa in sulfide-gradient cultures. Arch. Microbiol. 1983;136:262–269. [Google Scholar]
- 28.Nielsen L.P., Risgaard-Petersen N. Rethinking sediment biogeochemistry after the discovery of electric currents. Annu. Rev. Mar. Sci. 2015;7:425–442. doi: 10.1146/annurev-marine-010814-015708. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen L.P., Risgaard-Petersen N., Fossing H., Christensen P.B., Sayama M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature. 2010;463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- 30.Pernthaler J., Glöckner F.O., Schönhuber W., Amann R. Fluorescence in situ hybridization (FISH) with rRNA-targeted oligonucleotide probes. Methods Microbiol. 2001;30:207–226. [Google Scholar]
- 31.Pfeffer C., Larsen S., Song J., Dong M., Besenbacher F., Meyer R.L., Kjeldsen K.U., Schreiber L., Gorby Y.A., El-Naggar M.Y., Leung K.M., Schramm A., Risgaard-Petersen N., Nielsen L.P. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491:218–221. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- 32.Pruesse E., Peplies J., Glöckner F.O. SINA: accurate high-throughput multiple sequence alignment of ribosomal RNA genes. Bioinformatics. 2012;28:1823–1829. doi: 10.1093/bioinformatics/bts252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rho M., Tang H., Ye Y. FragGeneScan: predicting genes in short and error-prone reads. Nucleic Acids Res. 2010;38:e191. doi: 10.1093/nar/gkq747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risgaard-Petersen N., Damgaard L.R., Revil A., Nielsen L.P. Mapping electron sources and sinks in a marine biogeobattery. J. Geophys. Res. Biogeosci. 2014;119:1475–1486. [Google Scholar]
- 36.Risgaard-Petersen N., Kristiansen M., Frederiksen R.B., Dittmer A.L., Bjerg J.T., Trojan D., Schreiber L., Damgaard L.R., Schramm A., Nielsen L.P. Cable bacteria in freshwater sediments. Appl. Environ. Microbiol. 2015;81:6003–6011. doi: 10.1128/AEM.01064-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Risgaard-Petersen N., Revil A., Meister P., Nielsen L.P. Sulfur, iron-, and calcium cycling associated with natural electric currents running through marine sediment. Geochim. Cosmochim. Acta. 2012;92:1–13. [Google Scholar]
- 38.Salman V., Amann R., Girnth A.-C., Polerecky L., Bailey J.V., Høgslund S., Jessen G., Pantoja S., Schulz-Vogt H.N. A single-cell sequencing approach to the classification of large, vacuolated sulfur bacteria. Syst. Appl. Microbiol. 2011;34:243–259. doi: 10.1016/j.syapm.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Schauer R., Risgaard-Petersen N., Kjeldsen K.U., Bjerg J.J.T., Jørgensen B.B., Schramm A., Nielsen L.P. Succession of cable bacteria and electric currents in marine sediment. ISME J. 2014;8:1314–1322. doi: 10.1038/ismej.2013.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., Sahl J.W., Stres B., Thallinger G.G., Van Horn D.J., Weber C.F. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seitaj D., Schauer R., Sulu-Gambari F., Hidalgo-Martinez S., Malkin S.Y., Burdorf L.D.W., Slomp C.P., Meysman F.J.R. Cable bacteria generate a firewall against euxinia in seasonally hypoxic basins. Proc. Natl. Acad. Sci. U.S.A. 2015;112:13278–13283. doi: 10.1073/pnas.1510152112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stackebrandt E., Ebers J. Taxonomic parameters revisited: tarnished gold standards. Microbiol. Today. 2006;33:152–155. [Google Scholar]
- 43.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulu-Gambari F., Seitaj D., Meysman F.J.R., Schauer R., Polerecky L., Slomp C.P. Cable bacteria control iron-phosphorus dynamics in sediments of a coastal hypoxic basin. Environ. Sci. Technol. 2016;50:1227–1233. doi: 10.1021/acs.est.5b04369. [DOI] [PubMed] [Google Scholar]
- 45.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasquez-Cardenas D., van de Vossenberg J., Polerecky L., Malkin S.Y., Schauer R., Hidalgo-Martinez S., Confurius V., Middelburg J.J., Meysman F.J.R., Boschker H.T.S. Microbial carbon metabolism associated with electrogenic sulphur oxidation in coastal sediments. ISME J. 2015;9:1966–1978. doi: 10.1038/ismej.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wagner M., Roger A.J., Flax J.L., Brusseau G.A., Stahl D.A. Phylogeny of dissimilatory sulfite reductases supports an early origin of sulfate respiration. J. Bacteriol. 1998;180:2975–2982. doi: 10.1128/jb.180.11.2975-2982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wallner G., Amann R., Beisker W. Optimizing fluorescent in situ hybridization with ribosomal-RNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry. 1993;14:136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]
- 49.Westram R., Bader K., Pruesse E., Kumar Y., Meier H., Glöckner F.O., Ludwig W. Arb: a software environment for sequence data. In: de Bruijn F.J., editor. Handbook of Molecular Microbial Ecology I: Metagenomics and Complementary Approaches. John Wiley & Sons, Inc.; 2011. pp. 399–406. [Google Scholar]
- 50.Winogradsky S.N. Über Schwefelbakterien. Bot Zeitung. 1887;45:489–610. [Google Scholar]
- 51.Yarza P., Yilmaz P., Pruesse E., Glöckner F.O., Ludwig W., Schleifer K.-H., Whitman W.B., Euzeby J., Amann R., Rosselló-Móra R. Uniting the classification of cultured and uncultured Bacteria and Archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014;12:635–645. doi: 10.1038/nrmicro3330. [DOI] [PubMed] [Google Scholar]
- 52.Yilmaz L.S., Bergsven L.I., Noguera D.R. Systematic evaluation of single mismatch stability predictors for fluorescence in situ hybridization. Environ. Microbiol. 2008;10:2872–2885. doi: 10.1111/j.1462-2920.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 53.Yilmaz L.S., Noguera D.R. Mechanistic approach to the problem of hybridization efficiency in fluorescent in situ hybridization. Appl. Environ. Microbiol. 2004;70:7126–7139. doi: 10.1128/AEM.70.12.7126-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yılmaz L.Ş., Noguera D.R. Development of thermodynamic models for simulating probe dissociation profiles in fluorescence in situ hybridization. Biotechnol. Bioeng. 2007;96:349–363. doi: 10.1002/bit.21114. [DOI] [PubMed] [Google Scholar]
- 55.Yilmaz L.S., Parnerkar S., Noguera D.R. MathFISH, a web tool that uses thermodynamics-based mathematical models for in silico evaluation of oligonucleotide probes for fluorescence in situ hybridization. Appl. Environ. Microbiol. 2011;77:1118–1122. doi: 10.1128/AEM.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.