Abstract
Acute chest pain is an important clinical challenge and a major reason for presentation to the emergency department. Although multiple imaging techniques are available to assess patients with suspected acute coronary syndrome (ACS), considerable interest has been focused on the use of non-invasive imaging options as coronary computed tomography angiography (CCTA) and cardiac magnetic resonance (CMR). According to several recent evidences, CCTA has been shown to represent a useful tool to rapidly and accurately diagnose coronary artery disease (CAD) in patients with low to intermediate cardiovascular risk. CCTA examination has the unique ability to non-invasively depict the coronary anatomy, not only allowing visualization of the lumen of the arteries in order to detect severe stenosis or occlusion responsible of myocardial ischemia, but also allows the assessment of coronary artery wall by demonstrating the presence or absence of CAD. However, routine CCTA is not able to differentiate ischemic from non-ischemic chest pain in patients with known CAD and it does not provide any functional assessment of the heart. Conversely, CMR is considered the gold standard in the evaluation of morphology, function, viability and tissue characterization of the heart. CMR offers a wide range of tools for diagnosing myocardial infarction (MI) at least at the same time of the elevation of cardiac troponin values, differentiating infarct tissue and ischemic myocardium from normal myocardium or mimicking conditions, and distinguishing between new and old ischemic events. In high-risk patients, with acute and chronic manifestations of CAD, CMR may be preferable to CCTA, since it would allow detection, differential diagnosis, prognostic evaluation and management of MI.
Keywords: Acute chest pain, acute coronary syndrome (ACS), cardiac magnetic resonance (CMR), coronary computed tomography angiography (CCTA), myocardial infarction (MI)
Introduction
Acute coronary syndromes (ACSs) encompass acute forms of ischemic heart disease-unstable angina (UA) and myocardial infarction (MI) with or without ST elevation—with a broad spectrum of clinical presentations (1,2).
Patients with ACS frequently present with acute chest pain complaints, but among 8–10 million patients presenting with this symptom to an Emergency Department (ED) annually in the United States, only less than 20% of them are ultimately diagnosed with ACS (1,3,4).
Chest pain may be cardiac-related or may be due to non-cardiac causes representing a common diagnostic dilemma in ED (5). In order to adequately diagnose, manage and treat patients with suspected ACS, the initial approach entails risk stratification based on a clinical evaluation and ancillary testing including electrocardiography (ECG) and cardiac biomarkers (6). When patients present typical ACS symptoms with significant ECG abnormalities and/or positive cardiac troponins, they are rapidly admitted for immediate catheter angiography with the aim of achieving a prompt restoring of an effective perfusion. Conversely, when patients have a very low probability of ACS based on clinical evaluation, atypical symptoms, negative values of cardiac troponins and a normal ECG they can be triaged to home, for further workup as an outpatient if needed (2,4,7). However, fewer than 20% of hospitalized patients have subsequent confirmation of ACS, and 0.4–4% of patients are inappropriately discharged and are reported to have a high mortality (7). Unfortunately, the initial evaluation of patients who present to the ED with chest pain often fails to provide a firm enough diagnosis to allow a prompt triage decision to be made. Most of them have intermediate risk for having underlying ACS, negative values of biomarkers of cardiac injury and non-diagnostic ECG changes, thus their management is very challenging (2,7,8).
Given the limitations of the clinical history, physical examination, and ECGs, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines suggested that patients with possible ACS (including low-risk patients) may be considered for an early stress test to provoke ischemia or advanced cardiac imaging (3,7,9,10). Among cardiac imaging modalities, coronary computed tomography angiography (CCTA) and cardiac magnetic resonance (CMR) imaging, are increasingly becoming clinically validated and applied as possible alternative or supplementary imaging tools for assessing patients presenting with chest pain syndromes (3,9,10).
CCTA
CCTA represents a variation of the standard multidetector (MD)-CT angiography, which allows the non-invasive visualization of coronary arteries after injection of intravenous contrast medium (rates of 4.5–6.5 mL/s), preferably with dual-headed pump. CCTA requires ECG-gating/triggering to assure the appropriate timing of scanning according to the patient’s heart rhythm. Because slow regular heart rate considerably improves image quality, the use of beta-blockers should be considered in patients with heart rates above 65 beats per min (bpm), providing the usual contra-indications are observed (7). Patients with irregular and/or high rhythms can be scanned, but at greater radiation doses and with lower diagnostic rates than would be otherwise expected. The advantages of CCTA examination are good to excellent resolution (approximately 0.6 mm) of coronary artery anatomy and short study time (single breath hold), while its main drawbacks are represented by radiation dose (8–24 mSv), contrast dye exposure, and necessity to achieve a slow, regular heart rate (10). However, CCTA technique has seen massive technological advances over the past 5 years, with growing temporal and spatial resolution associated to radiation doses decrease. Patients with rapid rhythms that cannot be controlled with beta-blockade can now benefit from the increased temporal resolution of dual-source CT systems (7).
CCTA examination has the unique ability to non-invasively depict coronary anatomy, thus allowing the visualization of the lumen of the arteries, as does catheter angiography, in order to detect severe stenosis or occlusion responsible of myocardial ischemia. Nevertheless, this technique also allows the assessment of coronary artery wall, coronary atherosclerotic plaque and the likelihood of an ACS by demonstrating the presence or absence of coronary artery disease (CAD) (4,7) (Figure 1). For the evaluation of potential UA/non—ST-segment elevation MI (NSTEMI), coronary artery calcium (CAC) is typically assessed by performing low-dose calcium scoring prior to CCTA in the same sitting. The total volume of CAC deposits is a good indicator of overall plaque burden and of future coronary events, so that it is used as a reliable marker of atherosclerotic disease and of cardiovascular risk. However, localization of CAC does not correlate well with the severity or vulnerability of coronary lesions, especially in older patients. Indeed, plaques rich in collagen and calcium are widely considered rigid and stable, whereas highly vascularized atheromas containing a core of lipids and necrotic debris are “soft” and more likely to be biologically “unstable” (11). In this regard, in addition to defining coronary anatomy and luminal stenosis severity, CCTA can provide information on atherosclerotic plaque morphology and composition (Figure 2). Plaques can hence be readily classified as calcified, partially calcified (<50%), or noncalcified. When assessing plaque volume, CCTA tends to underestimate the size of noncalcified plaques and overestimate calcified plaque because of blooming artifact. Fibrous plaques display high attenuation on CCTA, whereas low attenuation occurs in relation to necrotic core and fibrofatty tissue (12). Multiple studies have attempted to determine distinct Hounsfield unit (HU) ranges corresponding to different histological plaque types. Mean HU densities for lipid-rich soft plaques range from 14–75 HU, fibrous plaques range from 67–149 HU, and calcified plaques range from 135–1,089 HU (13). HU <30 on CCTA has been proposed as a cut-off for identification of lipid rich plaque, with 30 to 150 HU for fibrous and >220 HU for calcific ones. However, using absolute CT attenuation values to determine plaque composition is challenging due to the influence of various factors, including size of necrotic core, wall thickness, measurement point, density of intraluminal contrast medium, slice thickness and reconstruction filter (12).
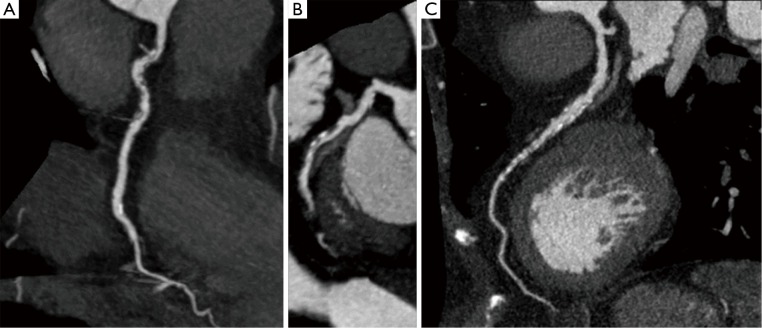
Figure 1.
Curved multiplanar reformatted images of the (A) right coronary artery, (B) circumflex artery, (C) left anterior descending coronary artery. CCTA depicts both the lumen, enhanced by the contrast medium, and the wall of the coronary arteries. CCTA, coronary computed tomography angiography.
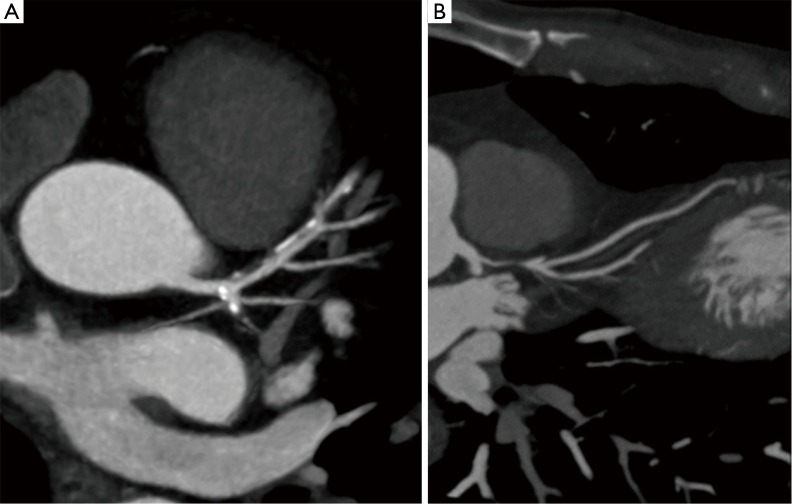
Figure 2.
Curved multiplanar reformatted images of the left anterior descending coronary artery. (A) Partially calcified plaque in the proximal-medium segment causing a mild (<50%) stenosis; (B) soft eccentric plaque in the proximal segment causing severe (>70%) stenosis.
The most important histological predictors of plaque rupture are cap thickness and necrotic core. Unstable lesions imaged with CCTA in patients with ACS tend be non-calcified, with low attenuation and spotty calcification, larger plaque volume, and higher remodeling index compared with stable lesions in patients with chronic stable UA. Positive (outward) remodeling (cross-sectional area >10%) occurs because of compensatory enlargement of the vessel wall, leading to high-volume plaque with often little luminal narrowing, a feature associated with large lipid core and high macrophage count (12). When a lipid-rich necrotic core and fibrous component are present in the atheroma, they result as an area of low attenuation adjacent to the vessel lumen, with surrounding higher-attenuation ring (napkin-ring sign). Low attenuation plaque, positive remodeling, and napkin-ring sign on CT are prognostic indicators linked to increased risk of MI.
CCTA has been reported to have 90% to 95% or greater sensitivity and specificity for occlusive CAD, with negative predictive value for CAD of approximately 99% (3,10). Moreover, several studies demonstrated that the negative predictive value for ACS remains high for up to 2 years (3). Unlike stress modalities, which require at least two negative cardiac injury biomarkers to be obtained before imaging, CCTA can be performed in parallel with serial cardiac biomarker evaluation. These features makes CCTA ideally suited for investigating patients with low to moderate probability of coronary disease presenting with acute chest pain, and its use in this context is supported by the recent AAC/AHA guidelines and National Institute for Health and Clinical Excellence (NICE) documentation (7,10,14). In particular, although the most recent AHA/ACC guidelines for management of patients with NSTEMI and UA do not provide a specific recommendation for the use of CCTA in patients with acute chest pain and suspected ACS, CCTA has been judged to be useful for the evaluation of obstructive CAD in symptomatic patients and appropriate for acute chest pain evaluation for those with intermediate and possibly low pretest probability of CAD when serial ECG and biomarkers are negative (4,10).
In high-risk patients with typical symptoms, the role of catheter-based angiography is well established and without question, and also in patients with atypical symptoms but worrisome ST segment elevation, urgent angiography is a useful tool to identify potential lesions that require intervention and allows treatment at the same time. However, for intermediate- and low-risk patients, the majority of whom do not have an ACS, catheter angiography is controversial and may result in delayed discharge even in those with normal coronary arteries due to bed stay required after the procedure. In this group, non-invasive coronary artery imaging with CCTA holds great promise (7). Thanks to the high negative predictive value of CCTA, if no evidence of either calcified or non-calcified (soft/fibrous) plaque is found, then it is highly unlikely that the patients’ symptoms may be attributable to UA/NSTEMI of atherosclerotic origin. Accordingly, CCTA is reported to be associated with a decreased time-to-diagnosis and an increase in the number of patients that can be discharged directly from the ED (3).
Multi-detector CT technology development has allowed the recent emergence of a related angiographic protocol, the triple-rule-out (TRO) scan, which is a tailored ECG-gated test, requiring more individualized attention, designed to evaluate the aorta, coronary circulation, pulmonary arteries, and the adjacent structures with a single scan (15,16). TRO CT may be valuable if overlapping symptoms occur in patients with acute chest pain, and is reported to be a powerful tool to investigate non-coronary causes of chest pain (e.g., acute aortic syndromes, pulmonary embolism), as well as providing potential other causes (e.g., hiatal hernia), all within a single breath-hold examination (7,16). TRO CT may be appropriate when the clinical impression favors pulmonary embolism or acute aortic syndrome in selected patients who do not display significantly increased values of cardiac biomarkers, or when is necessary to exclude ACS without immediate intention of address the patient to invasive cardiac catheterization. When clinical suspicion is truly limited to ACS, a dedicated CCTA is preferred, as it will require less contrast material and expose the patient to a lower radiation dose (16).
Actually, with substantial technical advances and wide availability registered in the last few years, CCTA seems to be a viable alternative to standard of care management in patients presenting to the ED with acute chest pain and suspected ACS (4).
CMR imaging
The role of CMR imaging continues to expand, supported by ongoing technological advances that have shortened acquisition times while maintaining and often improving image quality. New applications of CMR in cardiovascular diseases continue to emerge, but the role of CMR in the assessment of ACS remains not well established (7,17).
CMR is a non-invasive imaging tool which has the unique ability to comprehensively evaluate cardiac morphology together with ventricular function, myocardial perfusion, tissue characterization, and potentially anatomical coronary artery visualization (4,18,19). Its advantages include an excellent resolution (approximately 1 mm) of cardiac structures and avoidance of exposure to radiation and iodinated contrast (9,10). However, CMR imaging is complex and requires substantial technical expertise and the specific skills for its execution and interpretation, which are not widely available. Access to the magnetic resonance scanner is typically limited in many hospitals while comprehensive CMR imaging typically takes between 30 min and 1 h. Other downsides of CMR include patient intolerance due to claustrophobia, contraindications associated with metallic objects like pacemakers, defibrillators or implanted pumps. Nevertheless, CMR imaging seems to be able to provide unique information in chest pain syndromes that can aid in diagnosis and improve risk stratification after an event (2-4,7).
CMR images are usually obtained with breath-hold, their acquisition is synchronized to the patient’s ECG and obtained throughout the cardiac cycle. The individual images or movies (cine loops) that are acquired over several cardiac cycles are then gated using patient’s ECG. If the patient’s rhythm is irregular or there are frequent ectopics, real-time acquisition can be used. Nevertheless, the spatial resolution of these images is lower. The basic protocol for ACS assessment includes morphologic black blood images acquired with T1 or T2 weighting (w) with or without fat suppression (T2w images are used to assess cardiac anatomy, T2w fat-suppressed images are used to assess myocardial edema), cine imaging (for the evaluation of regional wall motion and global functioning parameters, ventricular end-systolic and end-diastolic volumes, stroke volume, ejection fraction and myocardial mass), first-pass contrast-enhanced imaging after intravenous administration of gadolinium contrast medium (to assess myocardial perfusion), and delayed contrast enhancement imaging (to evaluate the presence of late gadolinium enhancement (LGE) (7). LGE is the key strength of CMR imaging being considered the most accurate and best validated criterion for the assessment of MI and scarring. LGE images are obtained between 7 and 15 min (mean 10 min) after gadolinium injection (2,7). The mechanism of enhancement is likely based on the principle that while normal myocardiocytes are densely packed excluding gadolinium chelates diffusion (which are extracellular agents that cannot cross intact cell membranes), when acute myocyte necrosis occurs (as in acute MI or myocarditis), the membrane rupture allows gadolinium chelates to diffuse into the cells. This results in increased gadolinium concentration and shortened T1 relaxation, corresponding to signal enhancement. Interestingly, the scar replaces necrotic tissue and the expansion of the interstitial space leads to increased gadolinium concentration and signal enhancement in chronic condition (2).
In ED patients, resting CMR has been shown to be highly sensitive for early signs of ischemia, especially in patients with UA and NSTEMI, thus allowing to detecting infarction before serum cardiac troponin elevation occurs (3,7). The ability of CMR imaging to show regional changes in myocardial blood flow (by means of perfusion imaging) and regional variation in systolic function (with cine imaging) allows for the identification of myocardial ischemia in patients even without ECG changes (4). In patients with suspected ACS with low-risk for CAD or in the presence of concomitant medical problems that increase the risk of complications from cardiac catheterization, an initial non-invasive test may be preferred. In this context, CMR presents an attractive alternative to established diagnostic methods (17). Detection of regional wall motion abnormalities may remain abnormal for several hours after transient ischemia because of myocardial stunning, and is reported to be the most powerful and sensitive element of CMR assessment in this setting, in which perfusion abnormalities may be normal between episodes of pain and MI may not yet be established (7,17). In this context, the use of stress CMR imaging to evaluate ED patients with chest pain for inducible ischemia represents a relatively recent adaption of this technology (3,8). High-dose dobutamine stress CMR has high diagnostic accuracy to identify inducible ventricular wall motion abnormalities indicative of flow-limiting coronary stenosis (17). Compared with radionuclide imaging, CMR imaging has the advantage of higher spatial resolution, sensitivity and freedom from radiation exposure. However, lengthy examination times and lack of availability both limit its use in the diagnostics of ACS (7).
Both wall-motion abnormalities and resting perfusion defects may be seen in patients with MI and UA (2). By definition, UA is not associated with myocardial necrosis and therefore is not detected by LGE, thus regional wall-motion abnormalities without LGE in that region would effectively rule out MI and suggest this diagnosis in the absence of baseline abnormalities. Unfortunately, in the ED setting, it is often not known if baseline wall-motion abnormalities existed. In addition, wall motion abnormalities are not specific to ACS and can be seen in non-ischemic conditions such as cardiomyopathies as well as myocarditis or infiltrative diseases (2). CMR imaging has the great advantage to provide complimentary information in a single examination, which is useful for differential diagnosis of ACS, particularly in the context of a normal coronary angiogram (2,17). There is a small but significant number of patients presenting with chest pain, increased cardiac troponin values, with normal coronary artery angiography or non-flow-limiting CAD. Potential causes of this presentation include acute myocarditis, MI with coronary artery recanalization due to thrombolytic therapy, takotsubo cardiomyopathy, coronary artery embolism and non-cardiac causes of increased cardiac troponin values. These patients have a poorer prognosis than patients with ACS receiving revascularization therapies, in part due to the lack of accurate diagnosis and appropriate treatment in this difficult group of patients. CMR imaging has the ability to identify areas of inflammation and myocardial damage and can be used to differentiate ACS conditions from their mimics (20). Standard CMR sequences can provide quantitative information about ventricular function and support to identification of myocardial areas affected. Additional sequences using T2w imaging and LGE are used to delineate the underlying etiology (2,17).
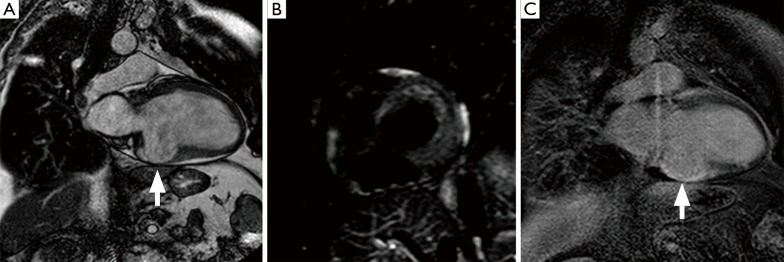
CMR imaging also enables the distinction between new and old MI, combining LGE with T2w imaging, which will delineate the edema, an early marker of ischemia, associated with acute infarction (Figure 3) (3,17). Moreover, T2w images offer a potentially attractive alternative for the non-invasive measurement of area at risk (19). The subtle increase in water content of the myocardium after acute ischemia/reperfusion injury can be detectable using T2w imaging, although black-blood T2w techniques are challenged by relatively low contrast-to-noise ratio as well as intra-cavitary flow artifacts in regions of slow flow adjacent to wall motion abnormalities. The high signal on T2w images combined with LGE allows the delineation of areas that are injured but not infarcted after reperfusion (2,17). The areas of T2w enhancement are invariably transmural and subsequently larger than the regions of LGE, and the difference between them likely represents the extent of salvageable myocardium (2,17). Similarly, on LGE images, a border zone of intermediate signal can be observed between the infarct and surrounding tissue. This peri-infarct zone may reflect partial volume or partial myocardial necrosis and edema. After acute MI, low-dose dobutamine cine CMR can be used to predict viability and functional recovery. The evaluation of the extension of infarcted myocardium has prognostic implications because the infarct size measured by LGE is directly associated with outcome after MI event (17,19). The extension and distribution of scar by LGE are related to the extent of coronary obstruction leading to infarction (7,17). MI caused by occlusion of small or distal coronary branches not amenable to revascularisation will cause small, well-circumscribed but transmural enhancement. Proximal coronary occlusion that has been successfully revascularised will produce a large area of subendocardial enhancement corresponding to the arterial territory. Microvascular obstruction (MVO) is another major prognostic factor after reperfusion therapy for AMI. MVO results in poor tissue perfusion between 2 and 9 days following AMI, due to myocardial microcirculation damage within an infarcted area following restoration of epicardial coronary flow. Contrast-enhanced CMR imaging is very sensitive to detecting MVO: on first-pass imaging, MVO appears as an area of hypo-enhancement of varying transmurality while on LGE, MVO can be observed as an area of non-enhancement within the area of late enhancement (Figure 4) (7,17). In addition to supporting management decision making in ACS, CMR reliably reveals important complications of acute MI such as papillary muscle dysfunction/infarction causing mitral regurgitation, ventricular pseudo-aneurysm formation, ventricular thrombus and pericardial effusion (7,17,21).
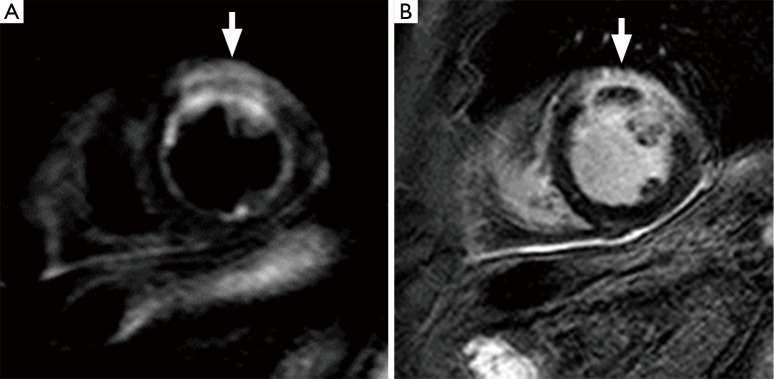
Figure 3.
CMR images of an acute myocardial infarction; (A) T2w fat-suppressed image and (B) LGE image: region of T2w hyperintensity (A, arrow) of the anterior wall, which appears thickened, associated to LGE (B, arrow). CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
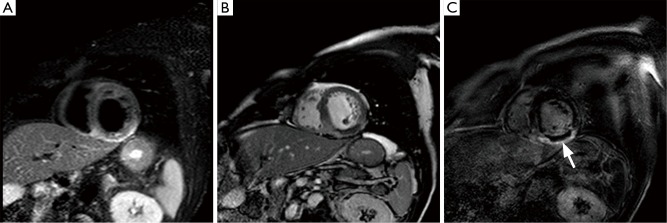
Figure 4.
CMR images of an acute myocardial infarction; (A) T2w fat-suppressed image and (B) fisrts passage image and (C) LGE image: region of T2w hyperintensity (A) of the inferior wall and septum consistent with myocardial edema, associated to a region of subendocardial hypoperfusion (B), related to microvascular obstruction, appearing as an area of non-enhancement within the area of late enhancement (C, arrow). CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Actually, the abilities of CMR imaging in differentiating infarcted tissue and ischemic myocardium from normal myocardium, in diagnosing MI before elevation of cardiac troponin values, in distinguishing between new and old MI and in determining prognosis, ultimately make the CMR technique particularly attractive in patients with known CAD or prior MI, who may not benefit as much from CCTA or ECG or perfusion imaging-based stress testing (Figure 5) (4).
Figure 5.
CMR (A) cine, (B) T2w fat-suppressed and (C) LGE imaging depicts a left ventricular aneurysm (A, arrow) due to an old myocardial infarction, without edema (B) and lined by LGE (C, arrow). CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement.
Conclusions
One rationale for cardiac non-invasive imaging in patients presenting with acute chest pain and suspected ACS is to directly image the coronary arteries by CCTA, to investigate severe stenosis or occlusion that is causing the myocardial ischemia. Otherwise, another promising approach entails searching the effects of ischemia on myocardium by CMR (7).
The ability to rapidly image the coronary arteries with a non-invasive technique as CCTA having strong performance characteristics is a potentially very attractive option in the setting of evaluating patients with suspected ACS in the ED (4). However, while CCTA accurately determines if coronary disease is present, it does not provide any functional assessment of the heart. Moreover, routine CCTA is not able to differentiate ischemic from non-ischemic chest pain in patients with known CAD. In these patients, especially those with high-risk, other diagnostic imaging modalities such as CMR or nuclear imaging may be preferable to CCTA (3). CMR may be a useful and accurate test to detect the presence of ACS, and can be considered an additional diagnostic tool to differentiate ACS from chronic MI, and from disease entities which can mimic similar clinical presentations such as myocarditis (22). Furthermore, CMR offers a wide range of tools that can be used for the detection, differential diagnosis, prognostic evaluation and management of patients with acute and chronic manifestations of CAD (17).
Acknowledgements
The authors would like to thank MD Giuseppe Russo for his assistance during data collection.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Lang O. ScienceDirect Radionuclide imaging in acute coronary syndromes. Cor Vasa [Internet]. The Czech Society of Cardiology 2014;56:e354-61. Available online: http://dx.doi.org/ 10.1016/j.crvasa.2014.04.008 [DOI]
- 2.Farzaneh-Fara A, Kwong RY. Detecting acute coronary syndromes by magnetic resonance imaging. Met Imaging 2011;50:15-9. [Google Scholar]
- 3.Mahler SA, Miller CD. Diagnostic Imaging to Exclude Acute Coronary Syndrome. Curr Emerg Hosp Med Rep 2013;1:37-42. 10.1007/s40138-012-0002-2 [DOI] [Google Scholar]
- 4.Dave DM, Ferencic M, Hoffmann U, et al. Imaging techniques for the assessment of suspected acute coronary syndromes in the emergency department. Curr Probl Cardiol 2014;39:191-247. 10.1016/j.cpcardiol.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippi G, Sanchis-Gomar F, Cervellin G. Chest pain, dyspnea and other symptoms in patients with type 1 and 2 myocardial infarction. A literature review. Int J Cardiol 2016;215:20-2. 10.1016/j.ijcard.2016.04.045 [DOI] [PubMed] [Google Scholar]
- 6.Cervellin G, Mattiuzzi C, Bovo C, et al. Diagnostic algorithms for acute coronary syndrome-is one better than another? Ann Transl Med 2016;4:193. 10.21037/atm.2016.05.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roobottom C, Mitchell G, Iyengar S. The role of non-invasive imaging in patients with suspected acute coronary syndrome. Br J Radiol 2011;84 Spec No 3:S269-79. 10.1259/bjr/57084479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pontone G, Andreini D, Baggiano A, et al. Functional relevance of coronary artery disease by cardiac magnetic resonance and cardiac computed tomography: myocardial perfusion and fractional flow reserve. Biomed Res Int 2015;2015:297696. [DOI] [PMC free article] [PubMed]
- 9.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA Guideline Revision ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidel. Circulation 2007;148-305. Available online: http://circ.ahajournals.org/content/circulationaha/116/7/e148.full.pdf 10.1161/CIRCULATIONAHA.107.181940 [DOI] [Google Scholar]
- 10.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA Practice Guideline 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction A Report of the American College of Cardiology Found. Circulation 2011;2022-60. Available online: http://circ.ahajournals.org/content/circulationaha/123/18/e426.full.pdf21444889 [Google Scholar]
- 11.Kantor B, Nagel E, Schoenhagen P, et al. Coronary computed tomography and magnetic resonance imaging. Curr Probl Cardiol 2009;34:145-217. 10.1016/j.cpcardiol.2008.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarkin JM, Dweck MR, Evans NR, et al. Imaging Atherosclerosis. Circ Res 2016;118:750-69. 10.1161/CIRCRESAHA.115.306247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwan AC, Cater G, Vargas J, et al. Beyond Coronary Stenosis: Coronary Computed Tomographic Angiography for the Assessment of Atherosclerotic Plaque Burden. Curr Cardiovasc Imaging Rep 2013;6:89-101. 10.1007/s12410-012-9183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeeth L, Skinner JS, Ashcroft J, et al. NICE clinical guideline: chest pain of recent onset. Br J Gen Pract 2010;60:607-10. 10.3399/bjgp10X515124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ropp A, Lin CT, White CS. Coronary computed tomography angiography for the assessment of acute chest pain in the emergency department: evidence, guidelines, and tips for implementation. J Thorac Imaging 2015;30:169-75. 10.1097/RTI.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 16.Halpern EJ. Triple-rule-out CT angiography for evaluation of acute chest pain and possible acute coronary syndrome. Radiology 2009;252:332-45. 10.1148/radiol.2522082335 [DOI] [PubMed] [Google Scholar]
- 17.Lockie T, Nagel E, Redwood S, et al. Use of cardiovascular magnetic resonance imaging in acute coronary syndromes. Circulation 2009;119:1671-81. 10.1161/CIRCULATIONAHA.108.816512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Filippo M, Sudberry JJ, Borgia D, et al. The use of magnetic resonance in myocardial ischaemia. Acta Biomed 2005;76:137-51. [PubMed] [Google Scholar]
- 19.Guaricci AI, Brunetti ND, Marra MP, et al. Diagnosis and prognosis of ischemic heart disease: the framework of cardiac magnetic resonance. J Cardiovasc Med (Hagerstown) 2015;16:653-62. 10.2459/JCM.0000000000000267 [DOI] [PubMed] [Google Scholar]
- 20.Saba L, Fellini F, De Filippo M. Diagnostic value of contrast-enhanced cardiac magnetic resonance in patients with acute coronary syndrome with normal coronary arteries. Jpn J Radiol 2015;33:410-7. 10.1007/s11604-015-0440-3 [DOI] [PubMed] [Google Scholar]
- 21.Capasso R, Panelo M, Fiorelli A, et al. Diverticulum, or not Diverticulum, That Is the Question! Discussing About a Case of Left Ventricular Outpouching Associated With Bicuspid Aortic Valve Assessed by Cardiac Magnetic Resonance. J Cardiovasc Thorac Res 2015;7:72-4. 10.15171/jcvtr.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu G, Zhang G, Zhu M, et al. Acute One-Stop Cardiovascular Magnetic Resonance Imaging for Differential Diagnosis in Patients with Acute Coronary Syndrome and Unobstructed Coronary Arteries. Med Princ Pract 2015;24:325-31. 10.1159/000381856 [DOI] [PMC free article] [PubMed] [Google Scholar]